Are Advanced Glycation End-Products and Skin Autofluorescence Associated with E-Selectin and Pulse Wave Velocity as Markers of Atherosclerosis Risk in Children with Obesity?
Abstract
1. Introduction
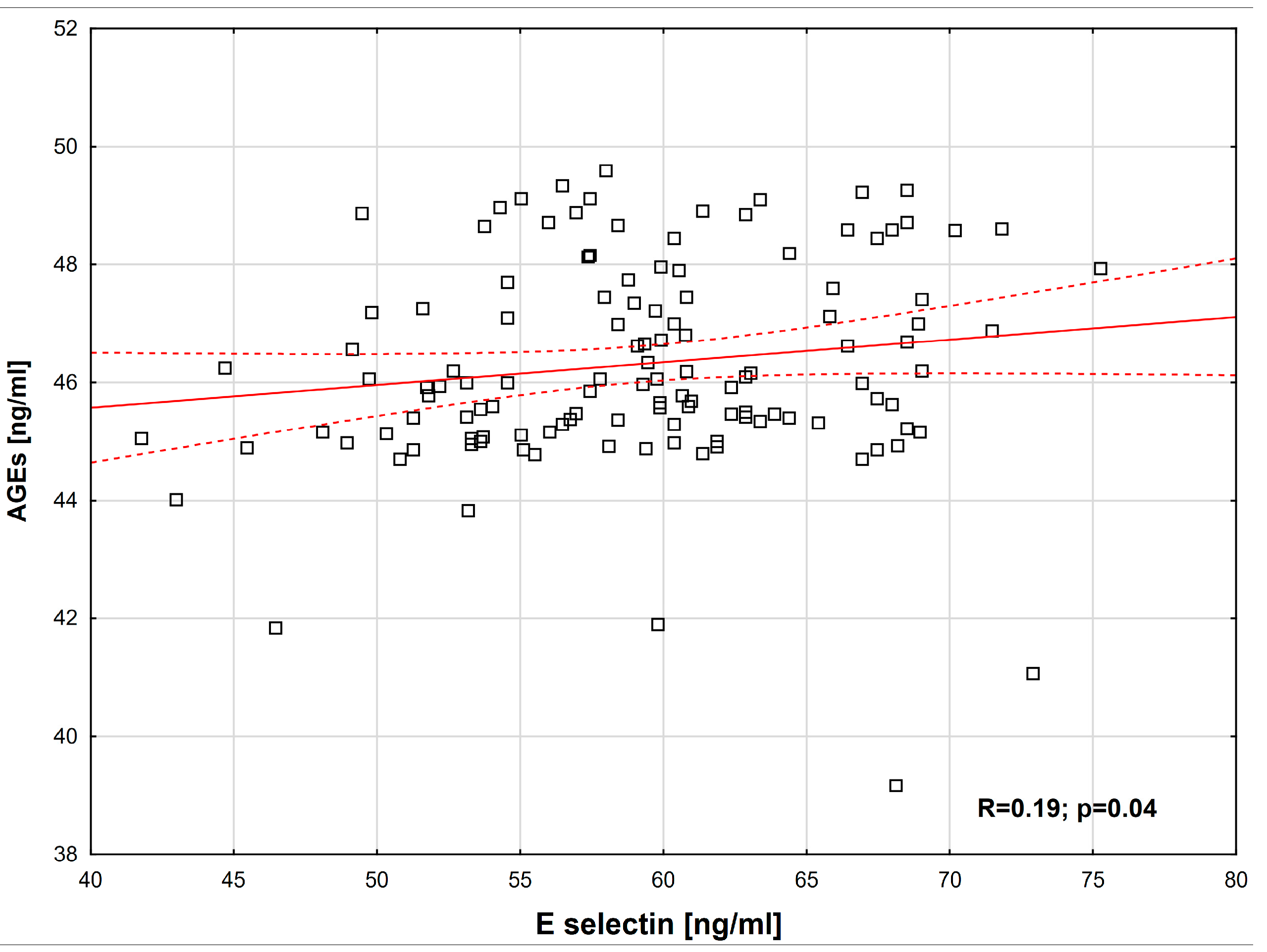
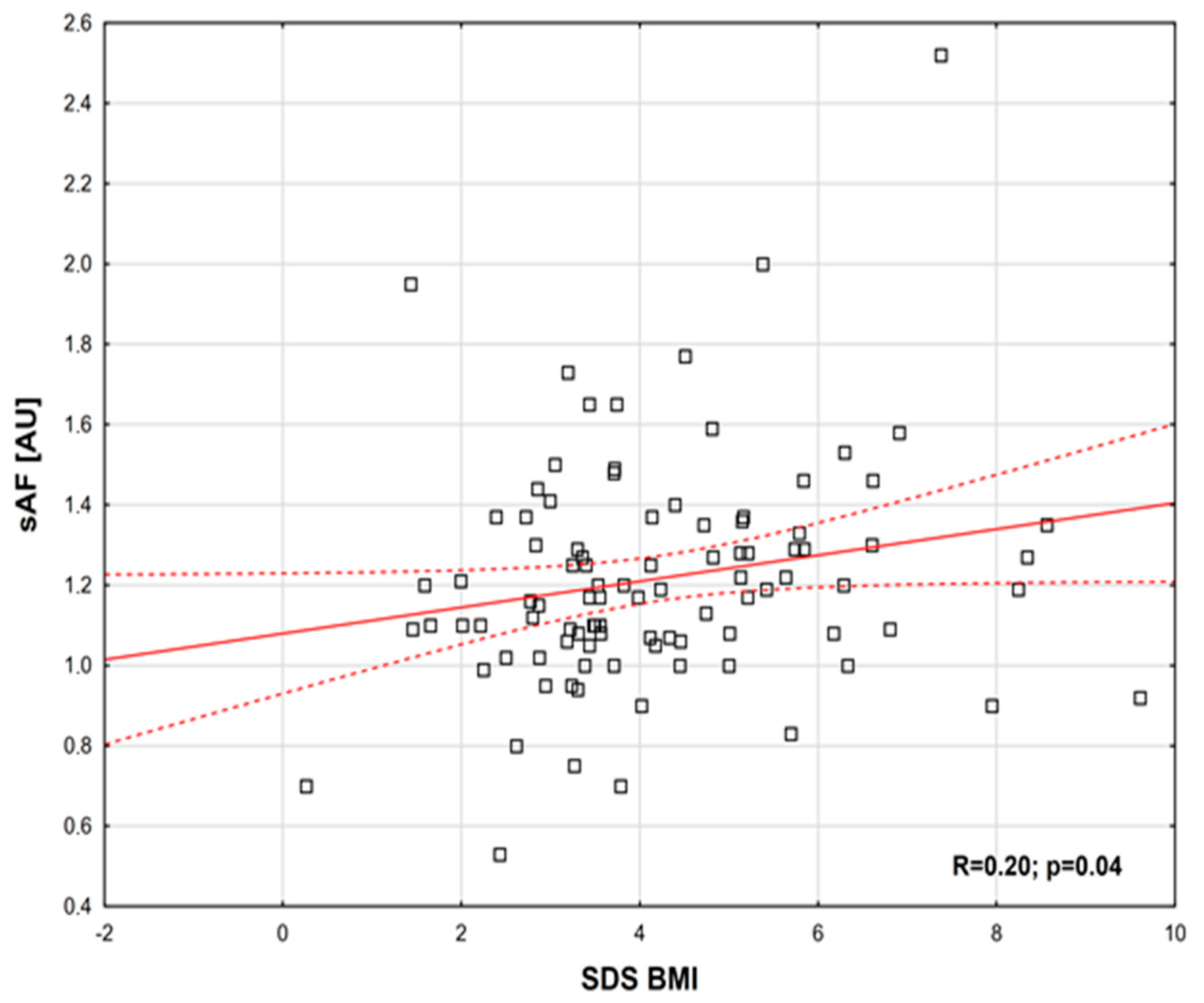
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Overview
4.2. Subjects
4.3. Skin Autofluorescence Measurements
4.4. Pulse Wave Velocity (PWV) Measurement
4.5. Statistical Analysis
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pakhare, M.; Anjankar, A. Critical Correlation Between Obesity and Cardiovascular Diseases and Recent Advancements in Obesity. Cureus 2024, 16, e51681. [Google Scholar] [CrossRef]
- Greenlund, K.J.; Kiefe, C.I.; Gidding, S.S.; Lewis, C.E.; Srinivasan, S.R.; Williams, O.D.; Berenson, G.S. Differences in cardiovascular disease risk factors in black and white young adults: Comparisons among five communities of the CARDIA and the Bogalusa heart studies. Coronary Artery Risk Development In Young Adults. Ann. Epidemiol. 1998, 8, 22–30. [Google Scholar] [CrossRef]
- Gupta, A.; Uribarri, J. Dietary Advanced Glycation End Products and Their Potential Role in Cardiometabolic Disease in Children. Horm. Res. Paediatr. 2016, 85, 291–300. [Google Scholar] [CrossRef]
- Hauser Ch Lona, G.; Köchli, S.; Streese, L.; Infanger, D.; Faude, O.; Hanssen, H. Subcutaneous advanced glycation end products, cardiovascular risk factors and vascular health during childhood development in a Swiss population. Front. Physiol. 2024, 15, 1371618. [Google Scholar] [CrossRef]
- Accacha, S.; Rosenfeld, W.; Jacobson, A.; Michel, L.; Schnurr, F.J.; Shelov, S.; Ten, S.; Boucher-Berry, C.; Carey, D.E.; Speiser, P.W.; et al. Plasma advanced glycation end products (AGEs), receptors for AGEs and their correlation with inflammatory markers in middle school-age children. Horm. Res. Paediatr. 2013, 80, 318–327. [Google Scholar] [CrossRef]
- Matafome, P.; Rodrigues, T.; Sena, C.; Seiça, R. Methylglyoxal in Metabolic Disorders: Facts, Myths, and Promises. Inc. Med. Res. Rev. 2017, 37, 368–403. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple non-invasive as-sessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Mulder, D.J.; Water, T.V.; Lutgers, H.L.; Graaff, R.; Gans, R.O.; Zijlstra, F.; Smit, A.J. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: An overview of current clinical studies, evidence, and limitations. Diabetes Technol. Ther. 2006, 8, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Kulsum-Mecci, N.; Goss, C.; Kozel, B.A.; Garbutt, J.M.; Schechtman, K.B.; Dhar-nidharka, V.R. Effects of Obesity and Hypertension on Pulse Wave Veloc-ity in Children. J. Clin. Hypertens. 2017, 19, 221–226. [Google Scholar] [CrossRef]
- Hudson, L.; Kinra, S.; Wong, I.; Cole, T.J.; Deanfield, J.; Viner, R. Is arterial stiffening associated with adiposity, severity of obesity and other contemporary cardiometabolic markers in a community sample of adolescents with obesity in the UK? BMJ Paediatr. Open 2017, 11, e000061. [Google Scholar] [CrossRef]
- Mihuta, M.S.; Paul, C.; Borlea, A.; Cepeha, C.M.; Velea, I.P.; Mozos, I.; Stoian, D. The oscillometric pulse wave analysis is useful in evaluating the arterial stiffness of obese children with relevant cardiometabolic risks. J. Clin. Med. 2022, 11, 5078. [Google Scholar] [CrossRef]
- Glowinska, B.; Urban, M.; Peczynska, J.; Florys, B. Soluble adhesion molecules (sICAM-1, sVCAM-1) and selectins (sE selectin, sP selectin, sL selectin) levels in children and adolescents with obesity, hypertension, and diabetes. Metabolism 2005, 54, 1020–1026. [Google Scholar] [CrossRef]
- Burns, S.F.; Lee, S.; Bacha, F.; Tfayli, H.; Hannon, T.S.; Arslanian, S.A. Pre-diabetes in overweight youth and early atherogenic risk. Metabolism 2014, 63, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Nobili, V.; Alisi, A.; Panera, N.; Handberg, A. Arterial Stiffness, Thickness and Association to Suitable Novel Markers of Risk at the Origin of Cardiovascular Disease in Obese Children. Int. J. Med. Sci. 2017, 14, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Woodward, M.; Tripp, E.; Goldberg, L.; Pyzik, R.; Yee, K.; Tansman, L.; Chen, X.; Mani, V.; et al. Elevated serum ad-vanced glycation endproducts in obese indicate risk for the metabolic syndrome: A link between healthy and unhealthy obesity? J. Clin. Endocrinol. Metabol. 2015, 100, 1957–1966. [Google Scholar] [CrossRef]
- Turki Jalil, A.; Alameri, A.A.; Iqbal Doewes, R.; El-Sehrawy, A.A.; Ahmad, I.; Ramaiah, P.; Kadhim, M.M.; Kzar, H.H.; Sivaraman, R.; Romero-Parra, R.M.; et al. Circulating and dietary advanced glycation end products and obesity in an adult population: A paradox of their detrimental effects in obesity. Front. Endocrinol. 2022, 13, 966590. [Google Scholar] [CrossRef]
- Coppola, S.; Paparo, L.; Trinchese, G.; Rivieri, A.M.; Masino, A.; De Giovanni Di Santa Severina, A.F.; Cerulo, M.; Escolino, M.; Turco, A.; Esposito, C.; et al. Increased dietary intake of ultraprocessed foods and mitochondrial metabolism alterations in pediatric obesity. Sci. Rep. 2023, 13, 12609. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Tripp, E.; Pyzik, R.; Yee, K.; Goldberg, L.; Tansman, L.; Chen, X.; Mani, V.; Fayad, Z.A.; et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: A randomised controlled trial. Diabetologia 2016, 59, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Mosa, A.A.; El-Shishtawy, M.M. Clinical study of advanced glycation end products in egyptian diabetic obese and non-obese patients. Int. J. Biomed. Sci. 2011, 7, 191–200. [Google Scholar] [CrossRef]
- Davis, K.E.; Prasad, C.; Vijayagopal, P.; Juma, S.; Imrhan, V. Serum soluble receptor for advanced glycation end products correlates inversely with measures of adiposity in young adults. Nutr. Res. 2014, 34, 478–485. [Google Scholar] [CrossRef]
- Luft, V.C.; Duncan, B.B.; Schmidt, M.I.; Chambless, L.E.; Pankow, J.S.; Hoogeveen, R.C.; Couper, D.J.; Heiss, G. Carboxymethyl lysine, an advanced glycation end product, and incident diabetes: A case-cohort analysis of the ARIC study. Diabetes 2015, 33, 1392–1398. [Google Scholar] [CrossRef]
- Foroumandi, E.; Alizadeh, M.; Kheirouri, S.; Jafarabadi, M.A. Exploring the role of body mass index in relationship of serum nitric oxide and advanced glycation end products in apparently healthy subjects. PLoS ONE 2019, 14, e0213307. [Google Scholar] [CrossRef]
- Garay-Sevilla, M.G.; Torres-Graciano, S.; Villegas-Rodríguez, M.E.; Rivera-Cisneros, A.E.; Wrobel, K.; Uribarri, J. Advanced glycation end products and their receptors did not show any association with body mass parameters in metabolically healthy adolescents. Acta Paediatr. 2018, 107, 2146–2151. [Google Scholar] [CrossRef]
- Sebeková, K.; Somoza, V.; Jarcusková, M.; Heidland, A.; Podracká, L. Plasma advanced glycation end products are decreased in obese children compared with lean controls. Int. J. Pediatr. Obes. 2009, 4, 112–118. [CrossRef]
- Medyńska, A.; Chrzanowska, J.; Zubkiewicz-Kucharska, A.; Zwolińska, D. New Markers of Early Kidney Damage in Children and Adolescents with Simple Obesity. Int. J. Mol. Sci. 2024, 25, 10769. [Google Scholar] [CrossRef] [PubMed]
- Lentferink, Y.E.; van Teeseling, L.; Knibbe, C.A.J.; van der Vorst, M.M.J. Skin autofluorescence in children with and without obesity. J. Pediatr. Endocrinol. Metab. 2019, 32, 41–47. [Google Scholar] [CrossRef]
- van Waateringe, R.P.; Slagter, S.N.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Lutgers, H.L.; Wolffenbuttel, B.H. Lifestyle and clinical determinants of skin autofluorescence in a population-based cohort study. Eur. J. Clin. Investig. 2016, 46, 481–490. [Google Scholar] [CrossRef]
- van Waateringe, R.P.; Slagter, S.N.; van Beek, A.P.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Lutgers, H.L.; Wolffenbuttel, B.H.R. Skin autofluorescence, a non-invasive biomarker for advanced glycation end products, is associated with the metabolic syndrome and its individual components. Diabetol. Metab. Syndr. 2017, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Huang, Q.-F.; Cheng, Y.-B.; Guo, Q.-H.; Chen, Q.; Li, Y.; Wang, J.-G. A Comparative Study on Skin and Plasma Advanced Glycation End Products and Their Associations with Arterial Stiffness. Pulse 2017, 4, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Apaydın, T.; Yavuz, D.G. Morbid obesity leads to increased skin autofluorescence independent of metabolic syndrome components. Turk. J. Med. Sci. 2022, 52, 1085–1092. [Google Scholar] [CrossRef]
- den Engelsen, C.; van den Donk, M.; Gorter, K.J.; Salomé, P.L.; Rutten, G.E. Advanced glycation end products measured by skin autofluorescence in a population with central obesity. Derm.-Endocrinol. 2012, 4, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kułaga, Z.; Litwin, M.; Tkaczyk, M.; Palczewska, I.; Zajączkowska, M.; Zwolińska, D.; Krynicki, T.; Wasilewska, A.; Moczulska, A.; Morawiec-Knysak, A.; et al. Polish 2010 growth references for school-aged children and adolescents. Eur. J. Pediatr. 2011, 170, 599–609. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef]
- Kułaga, Z.; Litwin, M.; Grajda, A.; Kułaga, K.; Gurzkowska, B.; Góźdź, M.; Pan, H. Oscillometric blood pressure percentiles for Polish normal-weight school-aged children and adolescents. J. Hypertens. 2012, 30, 1942–1954. [Google Scholar] [CrossRef]
- Viner, R.M.; White, B.; Barrett, T.; Candy, D.C.; Gibson, P.; Gregory, J.W.; Matyka, K.; Ong, K.; Roche, E.; Rudolf, M.C.; et al. Assessment of childhood obesity in secondary care: OSCA consensus statement. Arch. Dis. Child. Educ. Pract. Ed. 2012, 97, 98–105. [Google Scholar] [CrossRef]
- Keskin, M.; Kurtoglu, S.; Kendirci, M.; Atabek, M.E.; Yazici, C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005, 115, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Haycock, G.B.; Edelmann, C.M., Jr.; Spiter, A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinin. Pediatrics 1976, 58, 259–263. [Google Scholar] [CrossRef] [PubMed]

| Variable | Control Group F/M 18/15 | Children with Obesity F/M 68/57 | p | |
|---|---|---|---|---|
| Age [years] | mean ± SD | 12.9 ± 3.0 | 13.7 ± 2.84 | 0.172 |
| range (min–max) | 7.6–17.8 | 8.0–17.9 | ||
| Body weight [kg] | range (min–max) | 47.7 ± 11.9 | 85.7 ± 23.8 | 0.0001 * |
| median | 48.1 | 82.7 | ||
| quartile (25–75Q) | 38.6–55.4 | 72.7–100 | ||
| BMI | range (min–max) | 19.2 ± 2.3 | 32.1 ± 5.8 | 0.0001 * |
| median | 19 | 30.8 | ||
| quartile (25–75Q) | 17.7–20.3 | 28.4–35.2 | ||
| SDS BMI | range (min–max) | 0.061 ± 0.633 | 4.02 ± 1.7 | 0.0001 * |
| median | 0.061 | 3.55 | ||
| quartile (25–75Q) | (−0.547)–0.563 | 2.88–5.13 | ||
| SBP [mmHg] | mean ± SD | 106.3 ± 8.9 | 117.2 ± 9.9 | 0.0001 |
| range (min–max) | 85–120 | 98–140 | ||
| DBP [mmHg] | mean ± SD | 65.3 ± 7.2 | 71.8 ± 8.0 | 0.0001 |
| range (min–max) | 48–76 | 50–92 | ||
| Total cholesterol [mg/dL] | mean ± SD | 164.1 ± 14.8 | 179.2 ± 134.9 | 0.537 |
| range (min–max) | 133–188 | 111–1611 | ||
| HDL-cholesterol [mg/dL] | mean ± SD | 59 ± 9.6 | 42.2 ± 8.4 | 0.0001 |
| range (min–max) | 34–78 | 27–65 | ||
| LDL-cholesterol [mg/dL] | mean ± SD | 94.1 ± 14.9 | 100.4 ± 24.4 | 0.175 |
| range (min–max) | 65–121 | 49–184 | ||
| Triglycerides [mg/dL] | range (min–max) | 57–120 | 39–469 | 0.0021 * |
| median | 94 | 107 | ||
| quartile (25–75Q) | 74–105 | 83.5–141 | ||
| Creatinine [mg/dL] | mean ± SD | 0.736 ± 0.144 | 0.629 ± 0.123 | 0.0001 |
| range (min–max) | 0.54–1.19 | 0.37–0.89 | ||
| eGFR [mL/min/1.73 m2] | mean ± SD | 125 ± 13.2 | 154 ± 25.1 | 0 |
| range (min–max) | 96.0–160.0 | 109–235 | ||
| Fasting glucose [mg/dL] | range (min–max) | 75–94 | 56–153 | 0.0118 * |
| median | 88 | 82 | ||
| quartile (25–75Q) | 85–91 | 77–82 |
| Control Group | Children with Obesity | p | ||
|---|---|---|---|---|
| E-selectin [ng/mL] | range (min–max) | 22.3–37.4 | 41.8–75.3 | 0.0001 * |
| median | 31.5 | 59.4 | ||
| quartile (25–75Q) | 29.4–34.9 | 54.6–63.4 | ||
| AGEs [ng/mL] | range (min–max) | 22.1–26 | 39.2–49.6 | 0.0001 * |
| median | 24.3 | 46 | ||
| quartile (25–75Q) | 23.8–25.1 | 45.2–47.5 | ||
| MG [ng/mL] | range (min–max) | 41.2–85.9 | 156.4–302.5 | 0.0001 * |
| median | 69.3 | 227.2 | ||
| quartile (25–75Q) | 50.7–78.7 | 211.9–253.1 | ||
| hs-CRP [µg/mL] | range (min–max) | 1.02–1.45 | 2.36–4.46 | 0.0001 * |
| median | 1.2 | 3.22 | ||
| quartile (25–75Q) | 1.14–1.32 | 2.86–3.54 | ||
| sAF [AU] | mean ± SD | 1.02 ± 0.23 | 1.21 ± 0.29 | 0.00072 |
| range (min–max) | 0.7–1.7 | 0.53–2.52 | ||
| SDS PWV | mean ± SD | −1.54 ± 1.46 | –0.55 ±1.97 | 0.0106 |
| range (min–max) | (−4.08)–0.97 | (−9.43)–2.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medyńska, A.; Noczyńska, A.; Zwolińska, D. Are Advanced Glycation End-Products and Skin Autofluorescence Associated with E-Selectin and Pulse Wave Velocity as Markers of Atherosclerosis Risk in Children with Obesity? Int. J. Mol. Sci. 2025, 26, 9966. https://doi.org/10.3390/ijms26209966
Medyńska A, Noczyńska A, Zwolińska D. Are Advanced Glycation End-Products and Skin Autofluorescence Associated with E-Selectin and Pulse Wave Velocity as Markers of Atherosclerosis Risk in Children with Obesity? International Journal of Molecular Sciences. 2025; 26(20):9966. https://doi.org/10.3390/ijms26209966
Chicago/Turabian StyleMedyńska, Anna, Anna Noczyńska, and Danuta Zwolińska. 2025. "Are Advanced Glycation End-Products and Skin Autofluorescence Associated with E-Selectin and Pulse Wave Velocity as Markers of Atherosclerosis Risk in Children with Obesity?" International Journal of Molecular Sciences 26, no. 20: 9966. https://doi.org/10.3390/ijms26209966
APA StyleMedyńska, A., Noczyńska, A., & Zwolińska, D. (2025). Are Advanced Glycation End-Products and Skin Autofluorescence Associated with E-Selectin and Pulse Wave Velocity as Markers of Atherosclerosis Risk in Children with Obesity? International Journal of Molecular Sciences, 26(20), 9966. https://doi.org/10.3390/ijms26209966






