HSP90 Inhibition Disrupts 27-Hydroxycholesterol-Induced Inflammatory Signaling in Monocytic Cells
Abstract
1. Introduction
2. Results
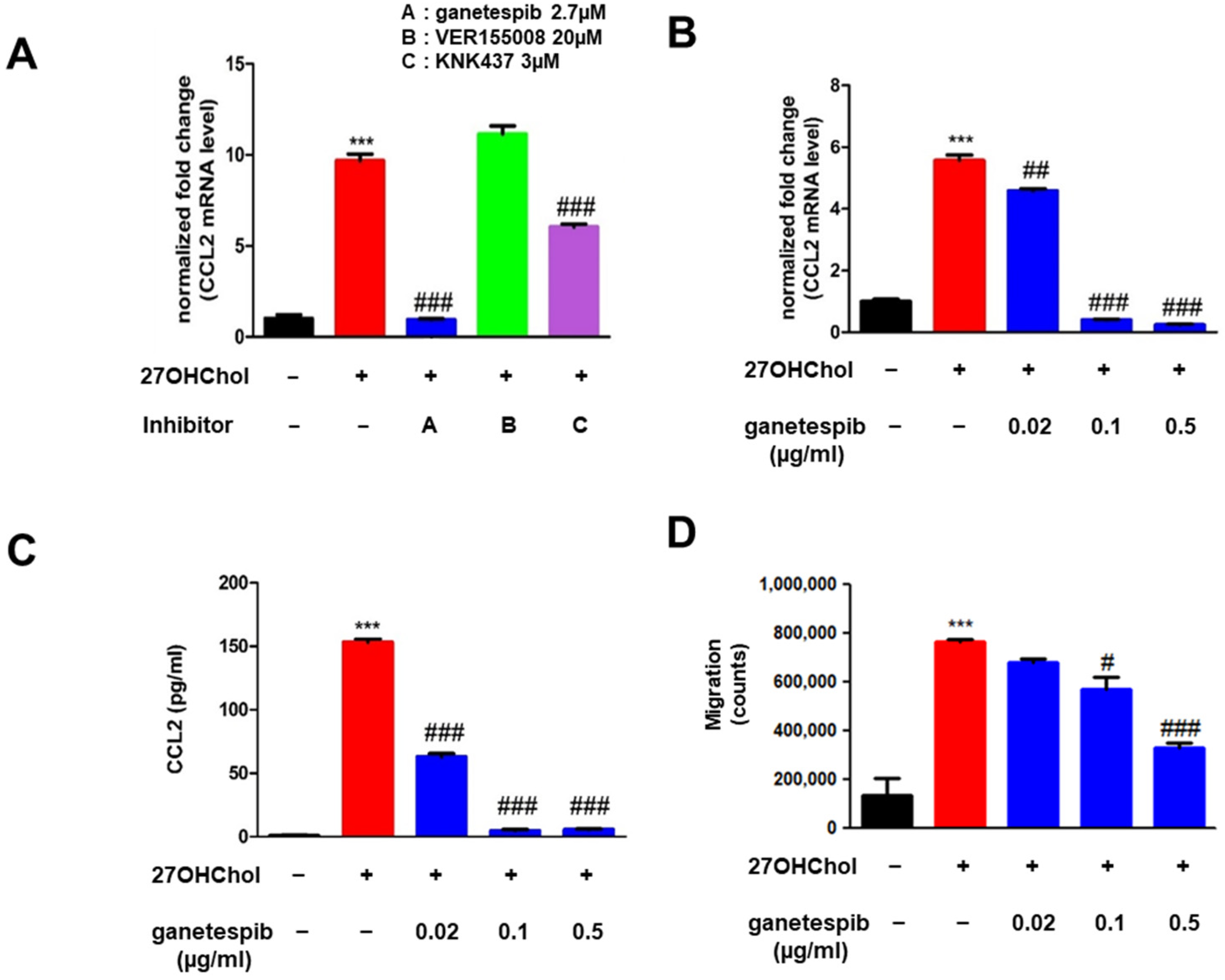
2.1. Ganetespib Decreases 27OHChol-Induced CCL2 Production and Monocytic Cell Migration
2.2. Ganetespib Suppresses the Superinduction of CCL2
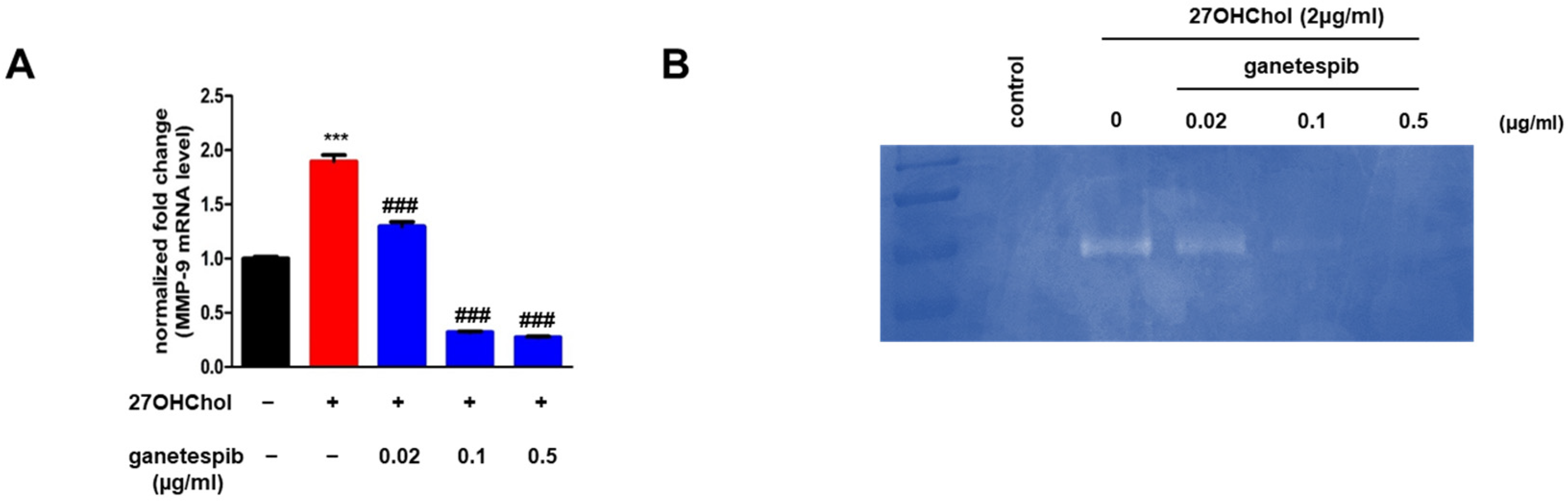
2.3. Ganetespib Suppresses the Expression of MMP-9 Induced by 27OHChol
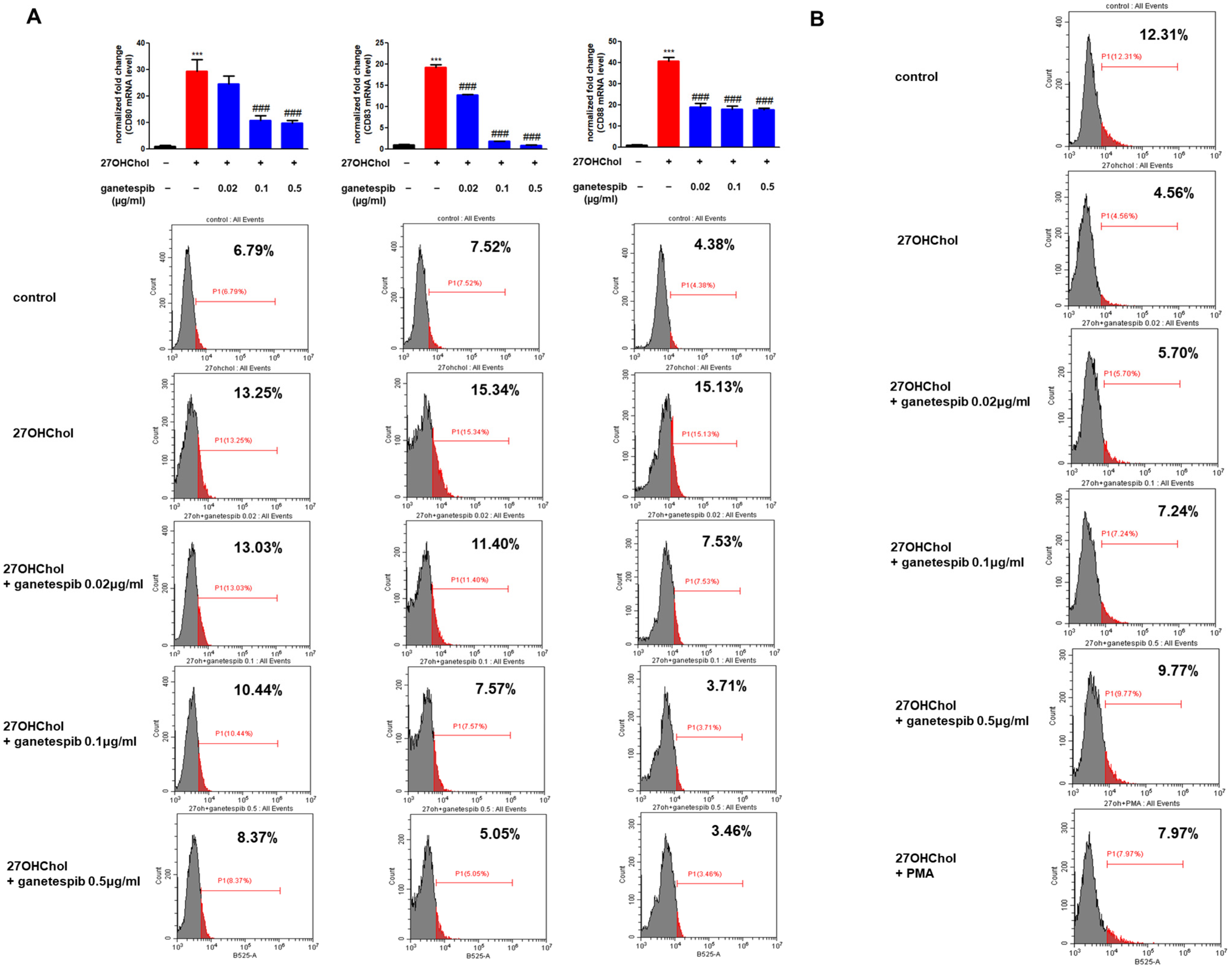
2.4. Ganetespib Influences 27OHChol-Induced Maturation and Functional Changes in Monocytes
2.5. Ganetespib Affects Phosphorylation of Downstream Protein of mTORC1 Signaling Pathway Increased in the Presence of 27OHChol
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. Reverse Transcription PCR (RT-PCR)
4.4. Western Blot Analysis
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Cell Migration Assay
4.7. MMP-9 Gelatin Zymography
4.8. Flow Cytometry
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El Hadri, K.; Smith, R.; Duplus, E.; El Amri, C. Inflammation, oxidative stress, senescence in atherosclerosis: Thioredoxine-1 as an emerging therapeutic target. Int. J. Mol. Sci. 2021, 23, 77. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef]
- Brown, A.J.; Jessup, W. Oxysterols and atherosclerosis. Atherosclerosis 1999, 142, 1–28. [Google Scholar] [CrossRef]
- Son, Y.; Choi, E.; Hwang, Y.; Kim, K. The role of 27-hydroxycholesterol in meta-inflammation. Korean J. Physiol. Pharmacol. 2024, 28, 107–112. [Google Scholar] [CrossRef]
- Baila-Rueda, L.; Cenarro, A.; Lamiquiz-Moneo, I.; Marco-Benedi, V.; Gracia-Rubio, I.; Casamayor-Franco, M.C.; Arbones-Mainar, J.M.; Civeira, F.; Laclaustra, M. Association of Cholesterol and Oxysterols in Adipose Tissue with Obesity and Metabolic Syndrome Traits. J. Clin. Endocrinol. Metab. 2022, 107, e3929–e3936. [Google Scholar] [CrossRef]
- Kim, S.-M.; Jang, H.; Son, Y.; Lee, S.-A.; Bae, S.-S.; Park, Y.C.; Eo, S.-K.; Kim, K. 27-hydroxycholesterol induces production of tumor necrosis factor-alpha from macrophages. Biochem. Biophys. Res. Commun. 2013, 430, 454–459. [Google Scholar] [CrossRef]
- Heo, W.; Kim, S.-M.; Eo, S.-K.; Rhim, B.-Y.; Kim, K. FSL-1, a Toll-like Receptor 2/6 Agonist, Induces Expression of Interleukin-1alpha in the Presence of 27-hydroxycholesterol. Korean J. Physiol. Pharmacol. 2014, 18, 475–480. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, B.-Y.; Eo, S.-K.; Kim, C.-D.; Kim, K. 27-Hydroxycholesterol up-regulates CD14 and predisposes monocytic cells to superproduction of CCL2 in response to lipopolysaccharide. Biochim. Biophys. Acta 2015, 1852, 442–450. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.-M.; Lee, S.-A.; Eo, S.-K.; Kim, K. Oxysterols induce transition of monocytic cells to phenotypically mature dendritic cell-like cells. Biochem. Biophys. Res. Commun. 2013, 438, 161–168. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Son, Y.; Choi, J.; Eo, S.-K.; Park, Y.C.; Kim, K. 27-Hydroxycholesterol upregulates the production of heat shock protein 60 of monocytic cells. J. Steroid Biochem. Mol. Biol. 2017, 172, 29–35. [Google Scholar] [CrossRef]
- Kim, K.; Cho, H.-R.; Kim, B.-Y.; Kim, J.; Park, D.; Kwon, R.J.; Son, Y. Oxysterol Induces Expression of 60 kDa Chaperone Protein on Cell Surface of Microglia. Int. J. Mol. Sci. 2024, 25, 9073. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Shukla, H.D.; Pitha, P.M. Role of hsp90 in systemic lupus erythematosus and its clinical relevance. Autoimmune Dis. 2012, 2012, 728605. [Google Scholar] [CrossRef] [PubMed]
- Fung, P.C.W.; Kong, R.K.C. The Heat Shock Protein Story—From Taking mTORC1, 2 and Heat Shock Protein Inhibitors as Therapeutic Measures for Treating Cancers to Development of Cancer Vaccines. J. Cancer Ther. 2017, 8, 962–1029. [Google Scholar] [CrossRef][Green Version]
- Choi, J.; Kim, B.-Y.; Son, Y.; Lee, D.; Hong, Y.-S.; Kim, M.S.; Kim, K. Reblastatins Inhibit Phenotypic Changes of Monocytes/Macrophages in a Milieu Rich in 27-Hydroxycholesterol. Immune Netw. 2020, 20, e17. [Google Scholar] [CrossRef]
- Lazaro, I.; Oguiza, A.; Recio, C.; Mallavia, B.; Madrigal-Matute, J.; Blanco, J.; Egido, J.; Martin-Ventura, J.-L.; Gomez-Guerrero, C. Targeting HSP90 ameliorates nephropathy and atherosclerosis through suppression of NF-κB and STAT signaling pathways in diabetic mice. Diabetes 2015, 64, 3600–3613. [Google Scholar] [CrossRef]
- Son, H.; Choi, H.-S.; Baek, S.E.; Kim, Y.-H.; Hur, J.; Han, J.-H.; Moon, J.H.; Lee, G.S.; Park, S.G.; Woo, C.-H.; et al. Shear stress induces monocyte/macrophage-mediated inflammation by upregulating cell-surface expression of heat shock proteins. Biomed. Pharmacother. 2023, 161, 114566. [Google Scholar] [CrossRef]
- Tukaj, S.; Węgrzyn, G. Anti-Hsp90 therapy in autoimmune and inflammatory diseases: A review of preclinical studies. Cell Stress Chaperones 2016, 21, 213–218. [Google Scholar] [CrossRef]
- Kim, B.Y.; Son, Y.; Kim, M.S.; Kim, K. Prednisolone suppresses the immunostimulatory effects of 27-hydroxycholesterol. Exp. Ther. Med. 2020, 19, 2335–2342. [Google Scholar] [CrossRef]
- Lee, J.; Kim, B.Y.; Son, Y.; Giang, D.H.; Lee, D.; Eo, S.; Kim, K. 4′-O-Methylalpinumisoflavone inhibits the activation of monocytes/macrophages to an immunostimulatory phenotype induced by 27-hydroxycholesterol. Int. J. Mol. Med. 2019, 43, 2177–2186. [Google Scholar] [CrossRef]
- Son, Y.; Choi, J.; Kim, B.; Park, Y.C.; Eo, S.-K.; Cho, H.-R.; Bae, S.S.; Kim, C.D.; Kim, K. Cyclosporin A inhibits differentiation and activation of monocytic cells induced by 27-hydroxycholesterol. Int. Immunopharmacol. 2019, 69, 358–367. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Bear, M.; Du, Z.; Foley, K.P.; Ying, W.; Barsoum, J.; London, C. The novel HSP90 inhibitor STA-9090 exhibits activity against Kit-dependent and-independent malignant mast cell tumors. Exp. Hematol. 2008, 36, 1266–1277. [Google Scholar] [CrossRef]
- Ying, W.; Du, Z.; Sun, L.; Foley, K.P.; Proia, D.A.; Blackman, R.K.; Zhou, D.; Inoue, T.; Tatsuta, N.; Sang, J.; et al. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol. Cancer Ther. 2012, 11, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, K.; Chen, F.; Liu, Q.; Ni, J.; Cao, W.; Hua, Y.; He, F.; Liu, Z.; Li, L.; et al. Role of the CCL2-CCR2 axis in cardiovascular disease: Pathogenesis and clinical implications. Front. Immunol. 2022, 13, 975367. [Google Scholar] [CrossRef]
- Brünnert, D.; Langer, C.; Zimmermann, L.; Bargou, R.C.; Burchardt, M.; Chatterjee, M.; Stope, M.B. The heat shock protein 70 inhibitor VER155008 suppresses the expression of HSP27, HOP and HSP90beta and the androgen receptor, induces apoptosis, and attenuates prostate cancer cell growth. J. Cell. Biochem. 2020, 121, 407–417. [Google Scholar] [CrossRef]
- Massey, A.J.; Williamson, D.S.; Browne, H.; Murray, J.B.; Dokurno, P.; Shaw, T.; Macias, A.T.; Daniels, Z.; Geoffroy, S.; Dopson, M.; et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother. Pharmacol. 2010, 66, 535–545. [Google Scholar] [CrossRef]
- Koishi, M.; Yokota, S.; Mae, T.; Nishimura, Y.; Kanamori, S.; Horii, N.; Shibuya, K.; Sasai, K.; Hiraoka, M. The effects of KNK437, a novel inhibitor of heat shock protein synthesis, on the acquisition of thermotolerance in a murine transplantable tumor in vivo. Clin. Cancer Res. 2001, 7, 215–219. [Google Scholar]
- Yokota, S.; Kitahara, M.; Nagata, K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000, 60, 2942–2948. [Google Scholar]
- Gong, Y.; Hart, E.; Shchurin, A.; Hoover-Plow, J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J. Clin. Investig. 2008, 118, 3012–3024. [Google Scholar] [CrossRef]
- Meschiari, C.A.; Jung, M.; Iyer, R.P.; Yabluchanskiy, A.; Toba, H.; Garrett, M.R.; Lindsey, M.L. Extracellular Matrix in Cardiovascular Pathophysiology: Macrophage overexpression of matrix metalloproteinase-9 in aged mice improves diastolic physiology and cardiac wound healing after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H224–H235. [Google Scholar] [CrossRef]
- Mellman, I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996, 12, 575–625. [Google Scholar] [CrossRef]
- Wayne, N.; Mishra, P.; Bolon, D.N. Hsp90 and client protein maturation. Methods Mol. Biol. 2011, 787, 33–44. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Kole, T.P.; Cotter, R.J.; Powell, J.D. Enhanced interaction between Hsp90 and raptor regulates mTOR signaling upon T cell activation. Mol. Immunol. 2009, 46, 2694–2698. [Google Scholar] [CrossRef]
- Mediani, L.; Antoniani, F.; Galli, V.; Vinet, J.; Carrà, A.D.; Bigi, I.; Tripathy, V.; Tiago, T.; Cimino, M.; Leo, G.; et al. Hsp90-mediated regulation of DYRK3 couples stress granule disassembly and growth via mTORC1 signaling. EMBO Rep. 2021, 22, e51740. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kwon, M.; Park, D.; Kang, N.; Son, Y.; Baryawno, N.; Kim, B.S.; Yoon, S.; Oh, S.-O.; Lee, D.; et al. HSP90 Inhibition Disrupts 27-Hydroxycholesterol-Induced Inflammatory Signaling in Monocytic Cells. Int. J. Mol. Sci. 2025, 26, 9963. https://doi.org/10.3390/ijms26209963
Kim J, Kwon M, Park D, Kang N, Son Y, Baryawno N, Kim BS, Yoon S, Oh S-O, Lee D, et al. HSP90 Inhibition Disrupts 27-Hydroxycholesterol-Induced Inflammatory Signaling in Monocytic Cells. International Journal of Molecular Sciences. 2025; 26(20):9963. https://doi.org/10.3390/ijms26209963
Chicago/Turabian StyleKim, Jaesung, Munju Kwon, Dongha Park, Nakyung Kang, Yonghae Son, Ninib Baryawno, Byoung Soo Kim, Sik Yoon, Sae-Ock Oh, Dongjun Lee, and et al. 2025. "HSP90 Inhibition Disrupts 27-Hydroxycholesterol-Induced Inflammatory Signaling in Monocytic Cells" International Journal of Molecular Sciences 26, no. 20: 9963. https://doi.org/10.3390/ijms26209963
APA StyleKim, J., Kwon, M., Park, D., Kang, N., Son, Y., Baryawno, N., Kim, B. S., Yoon, S., Oh, S.-O., Lee, D., & Kim, K. (2025). HSP90 Inhibition Disrupts 27-Hydroxycholesterol-Induced Inflammatory Signaling in Monocytic Cells. International Journal of Molecular Sciences, 26(20), 9963. https://doi.org/10.3390/ijms26209963






