Molecular Mechanisms and Therapeutic Perspectives of Gut Microbiota, Autophagy, and Apoptosis in Cholangiocarcinoma Pathophysiology
Abstract
1. Introduction
2. Literature Search and Selection
3. Cholangiocarcinoma: Pathophysiology and Molecular Features
Genetic and Epigenetic Alterations
4. Critical Signaling Pathway Disruptions
4.1. FGF/FGFR Pathway
4.2. PI3K/ERK/Akt/mTOR Pathway
4.3. Notch Pathway
4.4. Inflammatory and Cytokine Networks
4.5. Gut-Liver Axis and the Microbiota in Hepatobiliary Disease
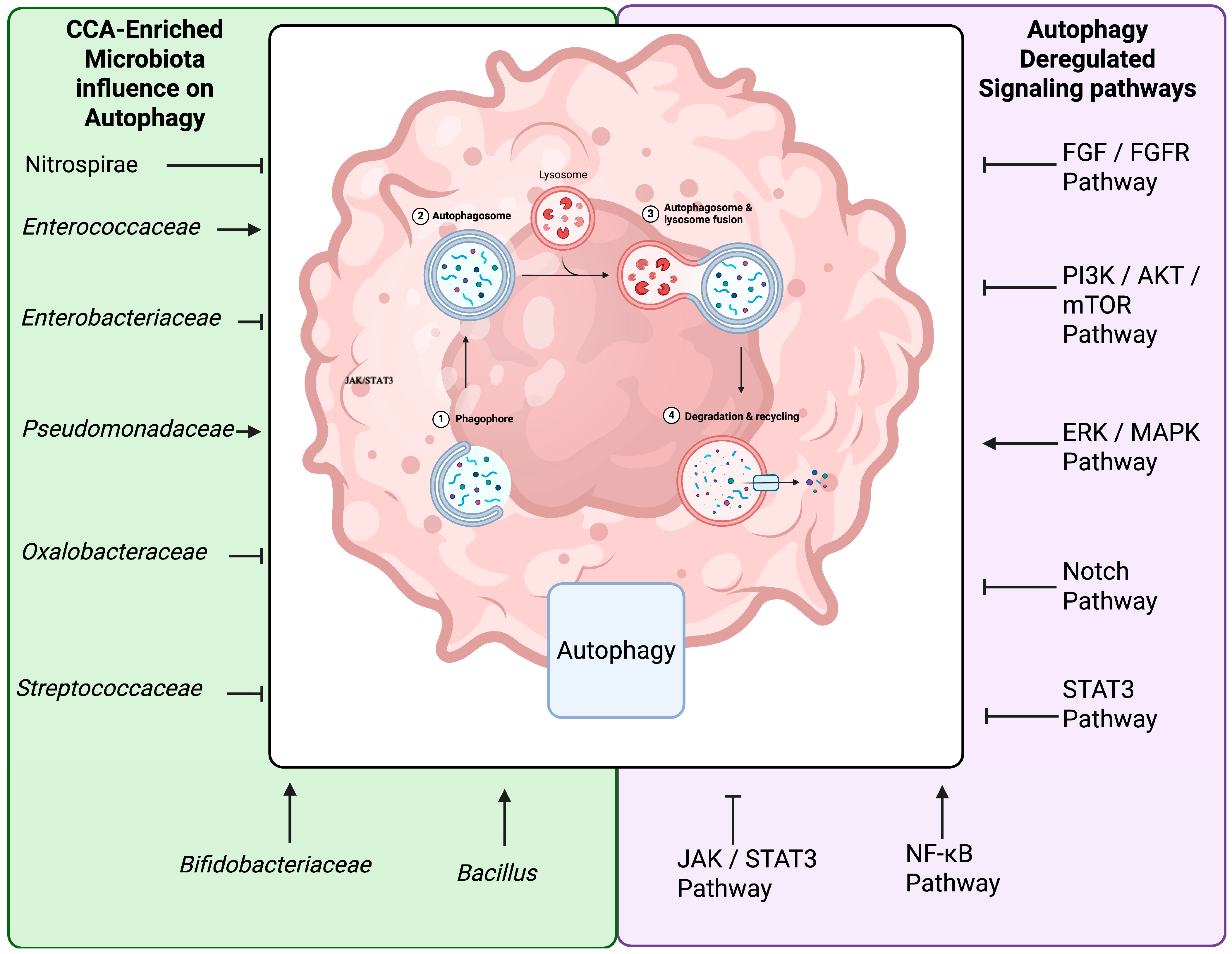
5. Modulation of Autophagy and Apoptosis by Gut Microbiota
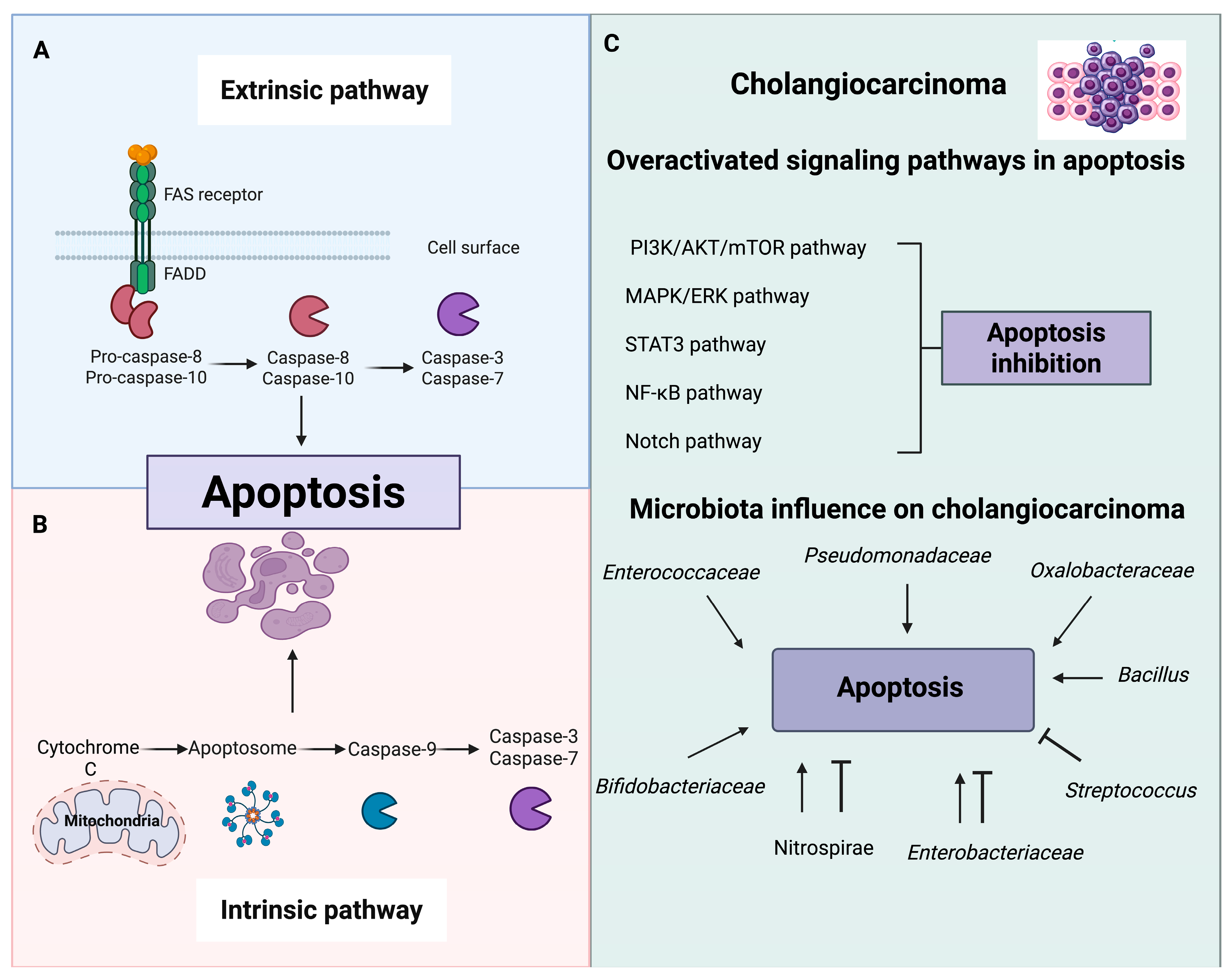
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilyas, S.I.; Affo, S.; Goyal, L.; Lamarca, A.; Sapisochin, G.; Yang, J.D.; Gores, G.J. Cholangiocarcinoma—Novel biological insights and therapeutic strategies. Nat. Rev. Clin. Oncol. 2023, 20, 470. [Google Scholar] [CrossRef] [PubMed]
- Elvevi, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristoferi, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann. Hepatol. 2022, 27, 100737. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2017, 15, 95. [Google Scholar]
- Zanuso, V.; Tesini, G.; Valenzi, E.; Rimassa, L. New systemic treatment options for advanced cholangiocarcinoma. J. Liver Cancer 2024, 24, 155–170. [Google Scholar] [CrossRef]
- Sato, K.; Glaser, S.; Alvaro, D.; Meng, F.; Francis, H.; Alpini, G. Cholangiocarcinoma: Novel therapeutic targets HHS Public Access. Expert. Opin. Ther. Targets 2020, 24, 345–357. [Google Scholar] [CrossRef]
- Qurashi, M.; Vithayathil, M.; Khan, S.A. Epidemiology of cholangiocarcinoma. Eur. J. Surg. Oncol. 2025, 51, 107064. [Google Scholar] [CrossRef]
- Prueksapanich, P.; Piyachaturawat, P.; Aumpansub, P.; Ridtitid, W.; Chaiteerakij, R.; Rerknimitr, R. Liver Fluke-Associated Biliary Tract Cancer. Gut Liver 2017, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.; Garikipati, S.C.; Roy, P. Cholangiocarcinoma. StatPearls. 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560708/ (accessed on 18 August 2025).
- Kirstein, M.M.; Vogel, A. Epidemiology and Risk Factors of Cholangiocarcinoma. Visc. Med. 2016, 32, 395. [Google Scholar] [CrossRef]
- Guevara-Ramirez, P.; Cadena-Ullauri, S.; Paz-Cruz, E.; Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Cabrera-Andrade, A.; Zambrano, A.K. Gut Microbiota Disruption in Hematologic Cancer Therapy: Molecular Insights and Implications for Treatment Efficacy. Int. J. Mol. Sci. 2024, 25, 10255. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Ramírez, P.; Cadena-Ullauri, S.; Paz-Cruz, E.; Tamayo-Trujillo, R.; Ruiz-Pozo, V.A.; Zambrano, A.K. Role of the gut microbiota in hematologic cancer. Front. Microbiol. 2023, 14, 1185787. [Google Scholar] [CrossRef]
- Jyoti, D.P. Mechanisms and implications of the gut microbial modulation of intestinal metabolic processes. NPJ Metab. Health Dis. 2025, 3, 24. [Google Scholar] [CrossRef]
- Gok Yavuz, B.; Datar, S.; Chamseddine, S.; Mohamed, Y.I.; LaPelusa, M.; Lee, S.S.; Hu, Z.I.; Koay, E.J.; Tran Cao, H.S.; Jalal, P.K.; et al. The Gut Microbiome as a Biomarker and Therapeutic Target in The Gut Microbiome. Cancers 2023, 15, 4875. [Google Scholar] [CrossRef]
- Ohtani, N.; Kawada, N. Role of the Gut–Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatol. Commun. 2019, 3, 456. [Google Scholar] [CrossRef]
- Artemev, A.; Naik, S.; Pougno, A.; Honnavar, P.; Shanbhag, N.M. The Association of Microbiome Dysbiosis With Colorectal Cancer. Cureus 2022, 14, e22156. [Google Scholar] [CrossRef] [PubMed]
- Fava, G. Molecular mechanisms of cholangiocarcinoma. World J. Gastrointest. Pathophysiol. 2010, 1, 12. [Google Scholar] [CrossRef]
- Glushko, T.; Costello, J.; Chima, R.; McGettigan, M.; Kim, R.; Jeong, D.; Qayyum, A. Molecular signatures of intrahepatic cholangiocarcinoma: Role in targeted therapy selection. Eur. J. Radiol. 2025, 187, 112056. [Google Scholar] [CrossRef]
- Tanaka, M.; Kunita, A.; Yamagishi, M.; Katoh, H.; Ishikawa, S.; Yamamoto, H.; Abe, J.; Arita, J.; Hasegawa, K.; Shibata, T.; et al. KRAS mutation in intrahepatic cholangiocarcinoma: Linkage with metastasis-free survival and reduced E-cadherin expression. Liver Int. 2022, 42, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Thongyoo, P.; Chindaprasirt, J.; Aphivatanasiri, C.; Intarawichian, P.; Kunprom, W.; Kongpetch, S.; Techasen, A.; Loilome, W.; Namwat, N.; Titapun, A.; et al. KRAS Mutations in Cholangiocarcinoma: Prevalence, Prognostic Value, and KRAS G12/G13 Detection in Cell-Free DNA. Cancer Genom. Proteom. 2025, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, Z.; Yu, Y.; Chen, Y.; Liu, H.; Guo, Y.; Peng, Z.; Cai, G.; Hua, Z.; Han, X.; et al. TP53 /KRAS Co-Mutations Create Divergent Prognosis Signatures in Intrahepatic Cholangiocarcinoma. Front. Genet. 2022, 13, 844800. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, W.; Gao, F.; Wu, Y.; Zhou, L.; Xu, H.; Yu, J.; Zhu, X.; Wang, L.; Li, L.; et al. Characteristic Analysis of Featured Genes Associated with Cholangiocarcinoma Progression. Biomedicines 2023, 11, 847. [Google Scholar] [CrossRef]
- Lee, K.; Song, Y.S.; Shin, Y.; Wen, X.; Kim, Y.; Cho, N.Y.; Bae, J.M.; Kang, G.H. Intrahepatic cholangiocarcinomas with IDH1/2 mutation-associated hypermethylation at selective genes and their clinicopathological features. Sci. Rep. 2020, 10, 15820. [Google Scholar] [CrossRef]
- Furth, N.; Cohen, N.; Spitzer, A.; Salame, T.M.; Dassa, B.; Mehlman, T.; Brandis, A.; Moussaieff, A.; Friedmann-Morvinski, D.; Castro, M.G.; et al. Oncogenic IDH1mut drives robust loss of histone acetylation and increases chromatin heterogeneity. Proc. Natl. Acad. Sci. USA 2025, 122, e2403862122. [Google Scholar] [CrossRef]
- Angerilli, V.; Fornaro, L.; Pepe, F.; Rossi, S.M.; Perrone, G.; Malapelle, U.; Fassan, M. FGFR2 testing in cholangiocarcinoma: Translating molecular studies into clinical practice. Pathologica 2023, 115, 71. [Google Scholar] [CrossRef]
- De Santis, A.; Zhu, L.; Tao, J.; Reißfelder, C.; Schölch, S. Molecular subtypes of intrahepatic cholangiocarcinoma. Trends Mol. Med. 2025, 31, 755–769. [Google Scholar] [CrossRef]
- Stutes, M.; Tran, S.; DeMorrow, S. Genetic and epigenetic changes associated with cholangiocarcinoma: From DNA methylation to microRNAs. World J. Gastroenterol. WJG 2007, 13, 6465. [Google Scholar] [CrossRef] [PubMed]
- Armartmuntree, N.; Jusakul, A.; Sakonsinsiri, C.; Loilome, W.; Pinlaor, S.; Ungarreevittaya, P.; Yong, C.H.; Techasen, A.; Imtawil, K.; Kraiklang, R.; et al. Promoter hypermethylation of early B cell factor 1 (EBF1) is associated with cholangiocarcinoma progression. J. Cancer 2021, 12, 2673. [Google Scholar] [CrossRef]
- Shi, T.; Morishita, A.; Kobara, H.; Masaki, T. The Role of microRNAs in Cholangiocarcinoma. Int. J. Mol. Sci. 2021, 22, 7627. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, K.; Khalafizadeh, A.; Sheikhbahaei, M.; Soltaninejad, H.; Babashah, S. TET1: The epigenetic architect of clinical disease progression. Genes Dis. 2025, 12, 101513. [Google Scholar] [CrossRef]
- Ursu, S.; Majid, S.; Garger, C.; de Semir, D.; Bezrookove, V.; Desprez, P.Y.; McAllister, S.; Soroceanu, L.; Nosrati, M.; Yimam, K.; et al. Novel tumor suppressor role of miRNA-876 in cholangiocarcinoma. Oncogenesis 2019, 8, 42. [Google Scholar] [CrossRef]
- Barbato, A.; Piscopo, F.; Salati, M.; Reggiani-Bonetti, L.; Franco, B.; Carotenuto, P. Micro-RNA in Cholangiocarcinoma: Implications for Diagnosis, Prognosis, and Therapy. J. Mol. Pathol. 2022, 3, 88–103. [Google Scholar] [CrossRef]
- Idris, R.; Chaijaroenkul, W.; Na-Bangchang, K. Molecular Targets and Signaling Pathways in Cholangiocarcinoma: A Systematic Review. Asian Pac. J. Cancer Prev. 2023, 24, 741. [Google Scholar] [CrossRef]
- Wang, J.; Xing, X.; Li, Q.; Zhang, G.; Wang, T.; Pan, H.; Li, D. Targeting the FGFR signaling pathway in cholangiocarcinoma: Promise or delusion? Ther. Adv. Med. Oncol. 2020, 12, 1758835920940948. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215. [Google Scholar] [CrossRef]
- Narong, S.; Leelawat, K. Basic fibroblast growth factor induces cholangiocarcinoma cell migration via activation of the MEK1/2 pathway. Oncol. Lett. 2011, 2, 821. [Google Scholar] [CrossRef] [PubMed]
- Corti, F.; Nichetti, F.; Raimondi, A.; Niger, M.; Prinzi, N.; Torchio, M.; Tamborini, E.; Perrone, F.; Pruneri, G.; Di Bartolomeo, M.; et al. Targeting the PI3K/Akt/mTOR pathway in biliary tract cancers: A review of current evidences and future perspectives. Cancer Treat. Rev. 2019, 72, 45–55. [Google Scholar] [CrossRef]
- Ocana, A.; Vera-Badillo, F.; Al-Mubarak, M.; Templeton, A.J.; Corrales-Sanchez, V.; Diez-Gonzalez, L.; Cuenca-Lopez, M.D.; Seruga, B.; Pandiella, A.; Amir, E. Activation of the PI3K/mTOR/Akt Pathway and Survival in Solid Tumors: Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e95219. [Google Scholar] [CrossRef] [PubMed]
- Yothaisong, S.; Dokduang, H.; Techasen, A.; Namwat, N.; Yongvanit, P.; Bhudhisawasdi, V.; Puapairoj, A.; Riggins, G.J.; Loilome, W. Increased activation of PI3K/Akt signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumor Biol. 2013, 34, 3637–3648. [Google Scholar] [CrossRef]
- Heumann, P.; Albert, A.; Gülow, K.; Tümen, D.; Müller, M.; Kandulski, A. Current and Future Therapeutic Targets for Directed Molecular Therapies in Cholangiocarcinoma. Cancers 2024, 16, 1690. [Google Scholar] [CrossRef]
- Rauff, B.; Malik, A.; Bhatti, Y.A.; Chudhary, S.A.; Qadri, I.; Rafiq, S. Notch signalling pathway in development of cholangiocarcinoma. World J. Gastrointest. Oncol. 2020, 12, 957. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.; Saucedo-Correa, G.; Oviedo-Boyso, J.; Valdez-Alarcón, J.J.; Baizabal-Aguirre, V.M.; Cajero-Juárez, M.; Bravo-Patiño, A. The CSL proteins, versatile transcription factors and context dependent corepressors of the notch signaling pathway. Cell Div. 2016, 11, 12. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Anusewicz, D.; Orzechowska, M.; Bednarek, A.K. Notch Signaling Pathway in Cancer—Review with Bioinformatic Analysis. Cancers 2021, 13, 768. [Google Scholar] [CrossRef]
- Guest, R.V.; Boulter, L.; Dwyer, B.J.; Kendall, T.J.; Man, T.Y.; Minnis-Lyons, S.E.; Lu, W.Y.; Robson, A.J.; Gonzalez, S.F.; Raven, A.; et al. Notch3 drives development and progression of cholangiocarcinoma. Proc. Natl. Acad. Sci. USA 2016, 113, 12250–12255. [Google Scholar] [CrossRef] [PubMed]
- Nwabo Kamdje, A.H.; Tagne Simo, R.; Fogang Dongmo, H.P.; Bidias, A.R.; Masumbe Netongo, P. Role of signaling pathways in the interaction between microbial, inflammation and cancer. Holist. Integr. Oncol. 2023, 2, 42. [Google Scholar] [CrossRef]
- Xu, Y.; Le, J.; Qin, J.; Zhang, Y.; Yang, J.; Chen, Z.; Li, C.; Qian, X.; Zhang, A. Decoding the microbiota metabolome in hepatobiliary and pancreatic cancers: Pathways to precision diagnostics and targeted therapeutics. Pharmacol. Res. 2024, 208, 107364. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, A.H.; Casolaro, V.; Bermúdez-Humarán, L.G.; Keyvani, H.; Taghinezhad, S.S. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut Microbes 2021, 13, 1886844. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef]
- Samad, M.A.; Ahmad, I.; Hasan, A.; Alhashmi, M.H.; Ayub, A.; Al-Abbasi, F.A.; Kumer, A.; Tabrez, S. STAT3 Signaling Pathway in Health and Disease. MedComm 2025, 6, e70152. [Google Scholar] [CrossRef]
- Yang, R.; Song, Y.; Shakoor, K.; Yi, W.; Peng, C.; Liu, S. Insights into the role of STAT3 in intrahepatic cholangiocarcinoma. Mol. Med. Rep. 2022, 25, 171. [Google Scholar] [CrossRef]
- Gu, Y.; Mohammad, I.S.; Liu, Z. Overview of the STAT-3 signaling pathway in cancer and the development of specific inhibitors. Oncol. Lett. 2020, 19, 2585. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef]
- Ke, J.; Zhang, C.; Wang, L.; Xie, F.S.; Wu, H.Y.; Li, T.; Bian, C.W.; Wu, R.L. Lipopolysaccharide promotes cancer cell migration and invasion through METTL3/PI3K/Akt signaling in human cholangiocarcinoma. Heliyon 2024, 10, e29683. [Google Scholar] [CrossRef]
- Hsu, C.L.; Schnabl, B. The gut–liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 2023, 21, 719. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Okada, H.; Nakamoto, N.; Taniki, N.; Chu, P.S.; Kanai, T. The gut-liver axis in hepatobiliary diseases. Inflamm. Regen. 2024, 44, 2. [Google Scholar] [CrossRef]
- Mpountouridis, A.; Tsigalou, C.; Bezirtzoglou, I.; Bezirtzoglou, E.; Stavropoulou, E. Gut microbiome in non-alcoholic fatty liver disease. Front. Gastroenterol. 2024, 3, 1534431. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Efremova, I.; Alieva, A.; Kashuh, E.; Tsvetaeva, E.; Poluektova, E.; Shirokova, E.; Ivashkin, K. Gut dysbiosis is associated with poorer long-term prognosis in cirrhosis. World J. Hepatol. 2021, 13, 557. [Google Scholar] [CrossRef]
- Caussy, C.; Tripathi, A.; Humphrey, G.; Bassirian, S.; Singh, S.; Faulkner, C.; Bettencourt, R.; Rizo, E.; Richards, L.; Xu, Z.Z.; et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat. Commun. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.-X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Tse, C.H.; Lam, T.T.Y.; Wong, G.L.H.; Chim, A.M.L.; Chu, W.C.W.; Yeung, D.K.W.; Law, P.T.W.; Kwan, H.S.; Yu, J.; et al. Molecular Characterization of the Fecal Microbiota in Patients with Nonalcoholic Steatohepatitis—A Longitudinal Study. PLoS ONE 2013, 8, e62885. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef]
- Pomyen, Y.; Chaisaingmongkol, J.; Rabibhadana, S.; Pupacdi, B.; Sripan, D.; Chornkrathok, C.; Budhu, A.; Budhisawasdi, V.; Lertprasertsuke, N.; Chotirosniramit, A.; et al. Gut dysbiosis in Thai intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Sci. Rep. 2023, 13, 11406. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.C.; Zhang, G.Z.; Zou, Y.W.; Ren, T.; Ren, H.Y.; Liu, C.; Yu, Z.J.; Ren, Z.G. Alterations in the human oral microbiome in cholangiocarcinoma. Mil. Med. Res. 2022, 9, 62. [Google Scholar] [CrossRef]
- Li, Z.; Chu, J.; Su, F.; Ding, X.; Zhang, Y.; Dou, L.; Liu, Y.; Ke, Y.; Liu, X.; Liu, Y.; et al. Characteristics of bile microbiota in cholelithiasis, perihilar cholangiocarcinoma, distal cholangiocarcinoma, and pancreatic cancer. Am. J. Transl. Res. 2022, 14, 2962. [Google Scholar] [PubMed]
- Saab, M.; Mestivier, D.; Sohrabi, M.; Rodriguez, C.; Khonsari, M.R.; Faraji, A.; Sobhani, I. Characterization of biliary microbiota dysbiosis in extrahepatic cholangiocarcinoma. PLoS ONE 2021, 16, e0247798. [Google Scholar] [CrossRef]
- Chen, B.; Fu, S.W.; Lu, L.; Zhao, H. A Preliminary Study of Biliary Microbiota in Patients with Bile Duct Stones or Distal Cholangiocarcinoma. BioMed Res. Int. 2019, 2019, 1092563. [Google Scholar] [CrossRef]
- Chng, K.R.; Chan, S.H.; Ng, A.H.Q.; Li, C.; Jusakul, A.; Bertrand, D.; Wilm, A.; Choo, S.P.; Tan, D.M.Y.; Lim, K.H.; et al. Tissue Microbiome Profiling Identifies an Enrichment of Specific Enteric Bacteria in Opisthorchis viverrini Associated Cholangiocarcinoma. EBioMedicine 2016, 8, 195–202. [Google Scholar] [CrossRef]
- Wang, Y.; Du, J.; Wu, X.; Abdelrehem, A.; Ren, Y.; Liu, C.; Zhou, X.; Wang, S. Crosstalk between autophagy and microbiota in cancer progression. Mol. Cancer 2021, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Lapaquette, P.; Bizeau, J.B.; Acar, N.; Bringer, M.A. Reciprocal interactions between gut microbiota and autophagy. World J. Gastroenterol. 2021, 27, 8283. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Lessing, D.J.; Guo, M.; Chu, W. Fusobacterium nucleatum and its metabolite hydrogen sulfide alter gut microbiota composition and autophagy process and promote colorectal cancer progression. Microbiol. Spectr. 2023, 11, e02292-23. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Chu, L.T.; Lin, X.; Chen, H.; Chen, L.; Tang, J.; Zeng, T. Autophagy in cholangiocarcinoma: A comprehensive review about roles and regulatory mechanisms. Clin. Transl. Oncol. 2025, 27, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wei, J.; Zheng, W.; Li, Z. The dual role of autophagy in cancer stem cells: Implications for tumor progression and therapy resistance. J. Transl. Med. 2025, 23, 583. [Google Scholar] [CrossRef]
- Lendvai, G.; Szekerczés, T.; Illyés, I.; Csengeri, M.; Schlachter, K.; Szabó, E.; Lotz, G.; Kiss, A.; Borka, K.; Schaff, Z. Autophagy activity in cholangiocarcinoma is associated with anatomical localization of the tumor. PLoS ONE 2021, 16, e0253065. [Google Scholar] [CrossRef]
- Koustas, E.; Trifylli, E.M.; Sarantis, P.; Papavassiliou, A.G.; Karamouzis, M.V. Role of autophagy in cholangiocarcinoma: An autophagy-based treatment strategy. World J. Gastrointest. Oncol. 2021, 13, 1229. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Zhu, D.; Pan, W.; Li, H.; Hua, J.; Zhang, C.; Zhao, K. Innovative Applications of Bacteria and Their Derivatives in Targeted Tumor Therapy. ACS Nano 2025, 19, 5077–5109. [Google Scholar] [CrossRef]
- Duong, M.T.Q.; Qin, Y.; You, S.H.; Min, J.J. Bacteria-cancer interactions: Bacteria-based cancer therapy. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Du, M.; Chen, Z.; Yuan, Z. Recent Advances in Bacteria-Based Cancer Treatment. Cancers 2022, 14, 4945. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Ren, T.; Wang, X.; Wang, H.; Zou, Y.; Sun, Y.; Liu, S.; Ren, Z.; Yu, Z. Dysbiosis in the Human Microbiome of Cholangiocarcinoma. Front. Physiol. 2021, 12, 715536. [Google Scholar] [CrossRef]
- Elvevi, A.; Laffusa, A.; Gallo, C.; Invernizzi, P.; Massironi, S. Any Role for Microbiota in Cholangiocarcinoma? A Comprehensive Review. Cells 2023, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Nakanuma, Y. Innate Immunity in the Pathogenesis of Cholangiopathy: A Recent Update. Inflamm. Allergy-Drug Targets (Discontin.) 2012, 11, 478–483. [Google Scholar] [CrossRef]
- Mizutani, H.; Fukui, S.; Oosuka, K.; Ikeda, K.; Kobayashi, M.; Shimada, Y.; Nakazawa, Y.; Nishiura, Y.; Suga, D.; Moritani, I.; et al. Biliary microbiome profiling via 16 S rRNA amplicon sequencing in patients with cholangiocarcinoma, pancreatic carcinoma and choledocholithiasis. Sci. Rep. 2025, 15, 16966. [Google Scholar] [CrossRef]
- Vijayan, A.; Vattiringal Jayadradhan, R.K.; Pillai, D.; Pillai, D.; Prasannan Geetha, P.; Joseph, V.; Isaac Sarojini, B.S. Nitrospira as versatile nitrifiers: Taxonomy, ecophysiology, genome characteristics, growth, and metabolic diversity. J. Basic Microbiol. 2021, 61, 88–109. [Google Scholar] [CrossRef]
- Daims, H.; Wagner, M. Nitrospira. Trends Microbiol. 2018, 26, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Seyyedsalehi, M.S.; Mohebbi, E.; Tourang, F.; Sasanfar, B.; Boffetta, P.; Zendehdel, K. Association of Dietary Nitrate, Nitrite, and N-Nitroso Compounds Intake and Gastrointestinal Cancers: A Systematic Review and Meta-Analysis. Toxics 2023, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.O.; Son, Y.; Lee, J.H.; Choi, S.W.; Kim, S.H.; Cheong, Y.K.; Chung, H.T.; Pae, H.O. Both nitric oxide and nitrite prevent homocysteine-induced endoplasmic reticulum stress and subsequent apoptosis via cGMP-dependent pathway in neuronal cells. Biochem. Biophys. Res. Commun. 2017, 493, 164–169. [Google Scholar] [CrossRef]
- El-Nabarawy, N.A.; Gouda, A.S.; Khattab, M.A.; Rashed, L.A. Effects of nitrite graded doses on hepatotoxicity and nephrotoxicity, histopathological alterations, and activation of apoptosis in adult rats. Environ. Sci. Pollut. Res. 2020, 27, 14019–14032. [Google Scholar] [CrossRef]
- Yang, S.; Luo, J.; Huang, Y.; Yuan, Y.; Cai, S. Effect of sub-lethal ammonia and nitrite stress on autophagy and apoptosis in hepatopancreas of Pacific whiteleg shrimp Litopenaeus vannamei. Fish. Shellfish. Immunol. 2022, 130, 72–78. [Google Scholar] [CrossRef]
- Lin, D.; Gao, Y.; Zhao, L.; Chen, Y.; An, S.; Peng, Z. Enterococcus faecalis lipoteichoic acid regulates macrophages autophagy via PI3K/Akt/mTOR pathway. Biochem. Biophys. Res. Commun. 2018, 498, 1028–1036. [Google Scholar] [CrossRef]
- Daca, A.; Jarzembowski, T. From the Friend to the Foe—Enterococcus faecalis Diverse Impact on the Human Immune System. Int. J. Mol. Sci. 2024, 25, 2422. [Google Scholar] [CrossRef]
- Zou, J.; Shankar, N. The opportunistic pathogen Enterococcus faecalis resists phagosome acidification and autophagy to promote intracellular survival in macrophages. Cell Microbiol. 2016, 18, 831–843. [Google Scholar] [CrossRef]
- Molujin, A.M.; Abbasiliasi, S.; Nurdin, A.; Lee, P.C.; Gansau, J.A.; Jawan, R. Bacteriocins as Potential Therapeutic Approaches in the Treatment of Various Cancers: A Review of In Vitro Studies. Cancers 2022, 14, 4758. [Google Scholar] [CrossRef]
- Pourmollaei, S.; Farshbaf-Khalili, A.; Barzegari, A.; Bastani, S.; Babaie, S.; Fattahi, A.; Shahnazi, M. Anticancer Effect of Enterococcus faecium, Isolated from Vaginal Fluid, on Ovarian Cancer Cells. Iran. Biomed. J. 2023, 27, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Salek, F.; Mirzaei, H.; Khandaghi, J.; Javadi, A.; Nami, Y. Apoptosis induction in cancer cell lines and anti-inflammatory and anti-pathogenic properties of proteinaceous metabolites secreted from potential probiotic Enterococcus faecalis KUMS-T48. Sci. Rep. 2023, 13, 7813. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Sharma, A.; Kumar, S.; Malik, F. Purification and Characterization of Glutaminase Free Asparaginase from Enterobacter cloacae: In-Vitro Evaluation of Cytotoxic Potential against Human Myeloid Leukemia HL-60 Cells. PLoS ONE 2016, 11, e0148877. [Google Scholar] [CrossRef]
- Krzymińska, S.; Koczura, R.; Mokracka, J.; Puton, T.; Kaznowski, A. Isolates of the Enterobacter cloacae complex induce apoptosis of human intestinal epithelial cells. Microb. Pathog. 2010, 49, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Wang, X.; Guo, Z.; Xu, Z.; Zhao, Y.; Cai, J.; Liu, J. A polysaccharide from Enterobacter cloacae induces apoptosis of human osteosarcoma cells through the activation of p53 and mitochondrial intrinsic pathway. Int. J. Biol. Macromol. 2019, 122, 58–63. [Google Scholar] [CrossRef]
- Zhao, K.; Jin, M.; Chen, Q.; Zheng, P.S. Polysaccharides produced by Enterobacter cloacae induce apoptosis in cervical cancer cells. Int. J. Biol. Macromol. 2015, 72, 960–964, Erratum in Int. J. Biol. Macromol. 2015, 73, 279. [Google Scholar] [CrossRef]
- David, L.; Taieb, F.; Pénary, M.; Bordignon, P.J.; Planès, R.; Bagayoko, S.; Duplan-Eche, V.; Meunier, E.; Oswald, E. Outer membrane vesicles produced by pathogenic strains of Escherichia coli block autophagic flux and exacerbate inflammasome activation. Autophagy 2022, 18, 2913–2925. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Raouf, R.; Ouf, S.A.; Gabr, M.M.; Zakaria, M.M.; El-Yasergy, K.F.; Ali-El-Dein, B. Escherichia coli foster bladder cancer cell line progression via epithelial mesenchymal transition, stemness and metabolic reprogramming. Sci. Rep. 2020, 10, 18024. [Google Scholar] [CrossRef]
- Salesse, L.; Lucas, C.; Hoang, M.H.T.; Sauvanet, P.; Rezard, A.; Rosenstiel, P.; Damon-Soubeyrand, C.; Barnich, N.; Godfraind, C.; Dalmasso, G.; et al. Colibactin-producing escherichia coli induce the formation of invasive carcinomas in a chronic inflammation-associated mouse model. Cancers 2021, 13, 2060. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zheng, X.; Ren, L.; Yang, Y.; Li, W.; Fu, W.; Wang, J.; Du, G. Tumorigenic bacteria in colorectal cancer: Mechanisms and treatments. Cancer Biol. Med. 2022, 19, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Marzoog, T.R.; Jabir, M.S.; Ibraheem, S.; Jawad, S.F.; Hamzah, S.S.; Sulaiman, G.M.; Mohammed, H.A.; Khan, R.A. Bacterial extracellular vesicles induced oxidative stress and mitophagy through mTOR pathways in colon cancer cells, HT-29: Implications for bioactivity. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2023, 1870, 119486. [Google Scholar] [CrossRef] [PubMed]
- Mirzarazi, M.; Bashiri, S.; Hashemi, A.; Vahidi, M.; Kazemi, B.; Bandehpour, M. The OmpA of commensal Escherichia coli of CRC patients affects apoptosis of the HCT116 colon cancer cell line. BMC Microbiol. 2022, 22, 139. [Google Scholar] [CrossRef]
- Zulpa, A.K.; Barathan, M.; Iyadorai, T.; Mariappan, V.; Vadivelu, J.; Teh, C.S.J.; Vellasamy, K.M. Selective pks+ Escherichia coli strains induce cell cycle arrest and apoptosis in colon cancer cell line. World J. Microbiol. Biotechnol. 2023, 39, 333. [Google Scholar] [CrossRef]
- Kaminski, A.; Gupta, K.H.; Goldufsky, J.W.; Lee, H.W.; Gupta, V.; Shafikhani, S.H. Pseudomonas aeruginosa ExoS Induces Intrinsic Apoptosis in Target Host Cells in a Manner That is Dependent on its GAP Domain Activity. Sci. Rep. 2018, 8, 14047. [Google Scholar] [CrossRef]
- Han, L.; Ma, Q.; Yu, J.; Gong, Z.; Ma, C.; Xu, Y.; Deng, G.; Wu, X. Autophagy plays a protective role during Pseudomonas aeruginosa-induced apoptosis via ROS–MAPK pathway. Innate Immun. 2020, 26, 580. [Google Scholar] [CrossRef]
- Bozic, D.; Živanović, J.; Živančević, K.; Baralić, K.; Đukić-Ćosić, D. Trends in Anti-Tumor Effects of Pseudomonas aeruginosa Mannose-Sensitive-Hemagglutinin (PA-MSHA): An Overview of Positive and Negative Effects. Cancers 2024, 16, 524. [Google Scholar] [CrossRef]
- Neroni, B.; Zingaropoli, M.A.; Radocchia, G.; Ciardi, M.R.; Mosca, L.; Pantanella, F.; Schippa, S. Evaluation of the anti-proliferative activity of violacein, a natural pigment of bacterial origin, in urinary bladder cancer cell lines. Oncol. Lett. 2022, 23, 132. [Google Scholar] [CrossRef]
- Aires-Lopes, B.; Zenker Justo, G.; Guimarães Cordeiro, H.; Durán, N.; Maria Azevedo-Martins, J.; Veríssima Ferreira Halder, C. Violacein improves vemurafenib response in melanoma spheroids. Nat. Prod. Res. 2024, 38, 3417–3420. [Google Scholar] [CrossRef]
- Gonçalves, P.R.; Rocha-Brito, K.J.P.; Fernandes, M.R.N.; Abrantes, J.L.; Durán, N.; Ferreira-Halder, C.V. Violacein induces death of RAS-mutated metastatic melanoma by impairing autophagy process. Tumor Biol. 2016, 37, 14049–14058. [Google Scholar] [CrossRef]
- Asghar, M.T.; Khurshid, M.; Nazir, J.; Shakoori, A.R. Induction of Apoptosis in Human Lung Epithelial Cell by Sphingomonas sp. Shah, a Recently Identified CeCulture Contaminant. Crit. Rev. Eukaryot. Gene Expr. 2021, 31, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, R.; Gulotta, G.; Andreoni, F.; Sumitomo, T.; Kawabata, S.; Zinkernagel, A.S.; Chhatwal, G.S.; Nizet, V.; Rohde, M.; Uchiyama, S. The Group A Streptococcus Interleukin-8 Protease SpyCEP Promotes Bacterial Intracellular Survival by Evasion of Autophagy. Infect. Microbes Dis. 2022, 4, 116–123. [Google Scholar] [CrossRef]
- Williams, J.G.; Ly, D.; Geraghty, N.J.; McArthur, J.D.; Vyas, H.K.N.; Gorman, J.; Tsatsaronis, J.A.; Sluyter, R.; Sanderson-Smith, M.L. Streptococcus pyogenes M1T1 Variants Induce an Inflammatory Neutrophil Phenotype Including Activation of Inflammatory Caspases. Front. Cell Infect. Microbiol. 2021, 10, 596023. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, Y.; Chen, L.; Gao, J.; Yang, D.Q. Effect of Streptococcus anginosus on biological response of tongue squamous cell carcinoma cells. BMC Oral Health 2021, 21, 141. [Google Scholar] [CrossRef]
- Huo, R.; Xu, Q.G.; You, Y.Q.; Chen, Y.L.; Su, G.J.; Yang, K.R.; Xiao, Y.P.; Xue, Z.; Li, Y.J.; Sun, P.; et al. Bifidobacterium boosts anti-PD-1 effectiveness through JAK pathway in hepatocellular carcinoma. NPJ Precis. Oncol. 2025, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Soraya, M.; Moazamian, E.; Shamsdin, S.A.; Dehghani, M. Bifidobacterium apoptosis induction by measuring bax and caspases on SW948 human colon cancer cell line. Int. J. Biochem. Cell Biol. 2025, 186, 106813. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Jiang, Y.; Zhao, X.Y.; Guan, Y.; Qian, W.; Fu, X.C.; Ren, H.Y.; Hou, X.H. Four types of Bifidobacteria trigger autophagy response in intestinal epithelial cells. J. Dig. Dis. 2014, 15, 597–605. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Xu, H.; Tang, L.; Li, Y.; Gong, L.; Wang, Y.; Li, W. Probiotic bacillus attenuates oxidative stress-induced intestinal injury via p38-mediated autophagy. Front. Microbiol. 2019, 10, 457971. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Zou, H.; Wang, B.; Sun, Q.; Fu, A.; Wang, Y.; Wang, Y.; Xu, X.; Li, W. Probiotic Bacillus amyloliquefaciens SC06 induces autophagy to protect against pathogens in macrophages. Front. Microbiol. 2017, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yan, L.; Xu, X.; Jiang, C.; Shi, J.; Zhang, Y.; Liu, L.; Lei, S.; Shao, D.; Huang, Q. Potential of Bacillus subtilis lipopeptides in anti-cancer I: Induction of apoptosis and paraptosis and inhibition of autophagy in K562 cells. AMB Express 2018, 8, 78. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, X.; Lei, S.; Shao, D.; Jiang, C.; Shi, J.; Zhang, Y.; Liu, L.; Lei, S.; Sun, H.; et al. Iturin A-like lipopeptides from Bacillus subtilis trigger apoptosis, paraptosis, and autophagy in Caco-2 cells. J. Cell Physiol. 2019, 234, 6414–6427. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, X.; Yuan, W.; Xiang, Y.; Guo, X.; Wei, W.; Soberón, M.; Bravo, A.; Liu, K. Bacillus thuringiensis cry toxin triggers autophagy activity that may enhance cell death. Pestic. Biochem. Physiol. 2021, 171, 104728. [Google Scholar] [CrossRef]
- Tang, L.; Zeng, Z.; Zhou, Y.; Wang, B.; Zou, P.; Wang, Q.; Ying, J.; Wang, F.; Li, X.; Xu, S.; et al. Bacillus amyloliquefaciens SC06 Induced Akt–FOXO Signaling Pathway-Mediated Autophagy to Alleviate Oxidative Stress in IPEC-J2 Cells. Antioxidants 2021, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Oliero, M.; Hajjar, R.; Cuisiniere, T.; Fragoso, G.; Calvé, A.; Dagbert, F.; Loungnarath, R.; Sebajang, H.; Schwenter, F.; Wassef, R.; et al. Prevalence of pks + bacteria and enterotoxigenic Bacteroides fragilis in patients with colorectal cancer. Gut Pathog. 2022, 14, 51. [Google Scholar] [CrossRef]
- Purcell, R.V.; Permain, J.; Keenan, J.I. Enterotoxigenic Bacteroides fragilis activates IL-8 expression through Stat3 in colorectal cancer cells. Gut Pathog. 2022, 14, 16. [Google Scholar] [CrossRef]
- Jasemi, S.; Molicotti, P.; Fais, M.; Cossu, I.; Simula, E.R.; Sechi, L.A. Biological Mechanisms of Enterotoxigenic Bacteroides fragilis Toxin: Linking Inflammation, Colorectal Cancer, and Clinical Implications. Toxins 2025, 17, 305. [Google Scholar] [CrossRef]
- Peng, J.; Fang, S.; Li, M.; Liu, Y.; Liang, X.; Li, Z.; Chen, G.; Peng, L.; Chen, N.; Liu, L.; et al. Genetic alterations of KRAS and TP53 in intrahepatic cholangiocarcinoma associated with poor prognosis. Open Life Sci. 2023, 18, 20220652. [Google Scholar] [CrossRef]
- Ferreira, A.; Pereira, F.; Reis, C.; Oliveira, M.J.; Sousa, M.J.; Preto, A. Crucial Role of Oncogenic KRAS Mutations in Apoptosis and Autophagy Regulation: Therapeutic Implications. Cells 2022, 11, 2183. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.K.; Görrissen, N.; Nguyen, T.T.; Kreutz, C.; Rasel, H.; Bartsch, F.; Lang, H.; Endres, K. Exploring the effects of gut microbiota on cholangiocarcinoma progression by patient-derived organoids. J. Transl. Med. 2025, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Wellhöner, F.; Plumeier, I.; Kahl, S.; Chhatwal, P.; Vital, M.; Voigtländer, T.; Pieper, D.H.; Manns, M.P.; Lenzen, H.; et al. The biliary microbiome in ischaemic-type biliary lesions can be shaped by stenting but is resilient to antibiotic treatment. Liver Int. 2022, 42, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, R.C.; Kilgour, E.; Jacobs, T.; Lamarca, A.; Hubner, R.A.; Valle, J.W.; McNamara, M.G. Potential influence of the microbiome environment in patients with biliary tract cancer and implications for therapy. Br. J. Cancer 2021, 126, 693. [Google Scholar] [CrossRef]
- Lyu, Z.; Yu, T.; Zhang, L.; Xu, X.; Zhang, Y.; Li, J.; Li, Z.; Zhang, W.; Hou, S. Analysis of the relationship between bile duct and duodenal microbiota reveals that potential dysbacteriosis is the main cause of primary common bile duct stones. Synth. Syst. Biotechnol. 2021, 6, 414. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Park, S.Y.; Chung, J.O.; Choi, Y.D.; Lee, S.M.; Cho, S.H.; Kim, D.H.; Kim, H.S.; Jung, Y. Effect of bile reflux on gastric juice microbiota in patients with different histology phenotypes. Gut Pathog. 2024, 16, 26. [Google Scholar] [CrossRef]
- Kazantseva, J.; Malv, E.; Kaleda, A.; Kallastu, A.; Meikas, A. Optimisation of sample storage and DNA extraction for human gut microbiota studies. BMC Microbiol. 2021, 21, 158. [Google Scholar] [CrossRef]
- Sune, D.; Rydberg, H.; Augustinsson, Å.N.; Serrander, L.; Jungeström, M.B. Optimization of 16S rRNA gene analysis for use in the diagnostic clinical microbiology service. J. Microbiol. Methods 2020, 170, 105854. [Google Scholar] [CrossRef]
- Elie, C.; Perret, M.; Hage, H.; Sentausa, E.; Hesketh, A.; Louis, K.; Fritah-Lafont, A.; Leissner, P.; Vachon, C.; Rostaing, H.; et al. Comparison of DNA extraction methods for 16S rRNA gene sequencing in the analysis of the human gut microbiome. Sci. Rep. 2023, 13, 10279. [Google Scholar] [CrossRef]
- Schupack, D.A.; Mars, R.A.T.; Voelker, D.H.; Abeykoon, J.P.; Kashyap, P.C. The promise of the gut microbiome as part of individualized treatment strategies. Nat. Rev. Gastroenterol. Hepatol. 2021, 19, 7. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Gulliver, E.L.; Young, R.B.; Chonwerawong, M.; D’Adamo, G.L.; Thomason, T.; Widdop, J.T.; Rutten, E.L.; Rossetto Marcelino, V.; Bryant, R.V.; Costello, S.P.; et al. Review article: The future of microbiome-based therapeutics. Aliment. Pharmacol. Ther. 2022, 56, 192. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.; Naderian, R.; Bahar, A.; Babaeizad, A.; Rezanavaz Gheshlagh, S.; Oksenych, V.; Tahmasebi, H. Microbiota as diagnostic biomarkers: Advancing early cancer detection and personalized therapeutic approaches through microbiome profiling. Front. Immunol. 2025, 16, 1559480. [Google Scholar] [CrossRef]

| Model | Liver Disease | Type of Microbiota and Sample | Dysbiosis at the Genus or Family Level Microbiome | Technique | Reference |
|---|---|---|---|---|---|
| Human | NAFLD | Gut microbiota Stool | NAFLD-cirrhosis: ↑ Streptococcus ↑ Gallibacterium NAFLD without advance fibrosis: ↑ Streptococcus, ↑ Bacillus ↑ Lactococcus | 16S rRNA sequencing | [59] |
| Animal | NAFLD | Gut microbiota Stool | ↓ Bifidobacterium ↓ Bacteroides ↑ Mucispirillum ↑ Desulfovibrio ↑ Anaerotruncus ↑ Desulfovibrionaceae | 16S rRNA sequencing | [60] |
| Human | NASH | Gut microbiota Fecal | ↑ Faecalibacterium ↑ Anaerosporobacter ↓ Parabacteroides ↓ Allisonella | 16S ribosomal RNA pyrosequencing | [61] |
| Human | Cirrhosis survival vs. death | Gut microbiota Stool | ↑ Enterobacteriaceae, ↑ Lactobacillaceae ↓ Ruminococcus ↓ Lachnospiraceae | 16S ribosomal RNA sequencing | [58] |
| Human | Cirrhosis survival vs. death | Gut microbiota Stool | ↑ Propionibacteriaceae ↑ Halomonadaceae ↓ Lachnospiraceae ↓ Veillonellaceae | Multi-tagged pyrosequencing techniques and ribosomal data (RDP10) taxa analysis | [62] |
| Human | Intrahepatic cholangiocarcinoma Hepatocellular carcinoma | Gut microbiota Stool | ↑ Veillonellaceae ↑ Lactobacillales ↑ Actinomycetaceae ↑ Streptococcaceae ↑ Neisseriaceae ↓ Lachnospiraceae, ↓ Eubacteriaceae ↑ Blautia | Whole-genome metagenomic shotgun | [63] |
| Human | Cholangiocarcinoma | Oral microbiota Saliva | ↑ Streptococcus ↑ Veillonella ↑ Haemophilus ↑ Leptotrichia ↑ Granulicatella ↑ Capnocytophaga ↑ Alloprevotella ↓ Actinomyces | 16S rRNA sequencing | [64] |
| Human | Perihilar cholangiocarcinoma | Bile microbiota Bile sample | ↑ Pseudomonas, ↑ Sphingomonas ↑ Halomonas | 16S rRNA gene analysis and next-generation sequencing | [65] |
| Human | Extrahepatic cholangiocarcinoma | Bile microbiota Biliary fluid | ↑ Bacteroides ↑ Geobacillus ↑ Meiothermus ↑ Anoxybacillus | 16S rRNA sequencing | [66] |
| Human | Distal cholangiocarcinoma | Bile microbiota Bile sample | ↑ Gemmatimonas ↑ Nitrospira ↑ Chloroflexus ↑ Planctomyces | 16S rRNA sequencing | [67] |
| Human | Cholangiocarcinoma | Bile microbiota Bile duct tissue Gastric microbiota Gastric mucosal tissue | ↑ Dietziaceae ↑ Pseudomonadaceae ↑ Oxalobacteraceae ↑ Moraxellaceae ↓ Burkholderiaceae | 16S rRNA sequencing | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Pozo, V.A.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Tamayo-Trujillo, R.; Paz-Cruz, E.; Cabrera-Andrade, A.; Zambrano, A.K. Molecular Mechanisms and Therapeutic Perspectives of Gut Microbiota, Autophagy, and Apoptosis in Cholangiocarcinoma Pathophysiology. Int. J. Mol. Sci. 2025, 26, 9949. https://doi.org/10.3390/ijms26209949
Ruiz-Pozo VA, Cadena-Ullauri S, Guevara-Ramírez P, Tamayo-Trujillo R, Paz-Cruz E, Cabrera-Andrade A, Zambrano AK. Molecular Mechanisms and Therapeutic Perspectives of Gut Microbiota, Autophagy, and Apoptosis in Cholangiocarcinoma Pathophysiology. International Journal of Molecular Sciences. 2025; 26(20):9949. https://doi.org/10.3390/ijms26209949
Chicago/Turabian StyleRuiz-Pozo, Viviana A., Santiago Cadena-Ullauri, Patricia Guevara-Ramírez, Rafael Tamayo-Trujillo, Elius Paz-Cruz, Alejandro Cabrera-Andrade, and Ana Karina Zambrano. 2025. "Molecular Mechanisms and Therapeutic Perspectives of Gut Microbiota, Autophagy, and Apoptosis in Cholangiocarcinoma Pathophysiology" International Journal of Molecular Sciences 26, no. 20: 9949. https://doi.org/10.3390/ijms26209949
APA StyleRuiz-Pozo, V. A., Cadena-Ullauri, S., Guevara-Ramírez, P., Tamayo-Trujillo, R., Paz-Cruz, E., Cabrera-Andrade, A., & Zambrano, A. K. (2025). Molecular Mechanisms and Therapeutic Perspectives of Gut Microbiota, Autophagy, and Apoptosis in Cholangiocarcinoma Pathophysiology. International Journal of Molecular Sciences, 26(20), 9949. https://doi.org/10.3390/ijms26209949







