Estrogenic Effect of Probiotics on Anxiety and Depression: A Narrative Review
Abstract
1. Introduction
2. Method
2.1. Design
2.2. Criteria
2.3. Article Research
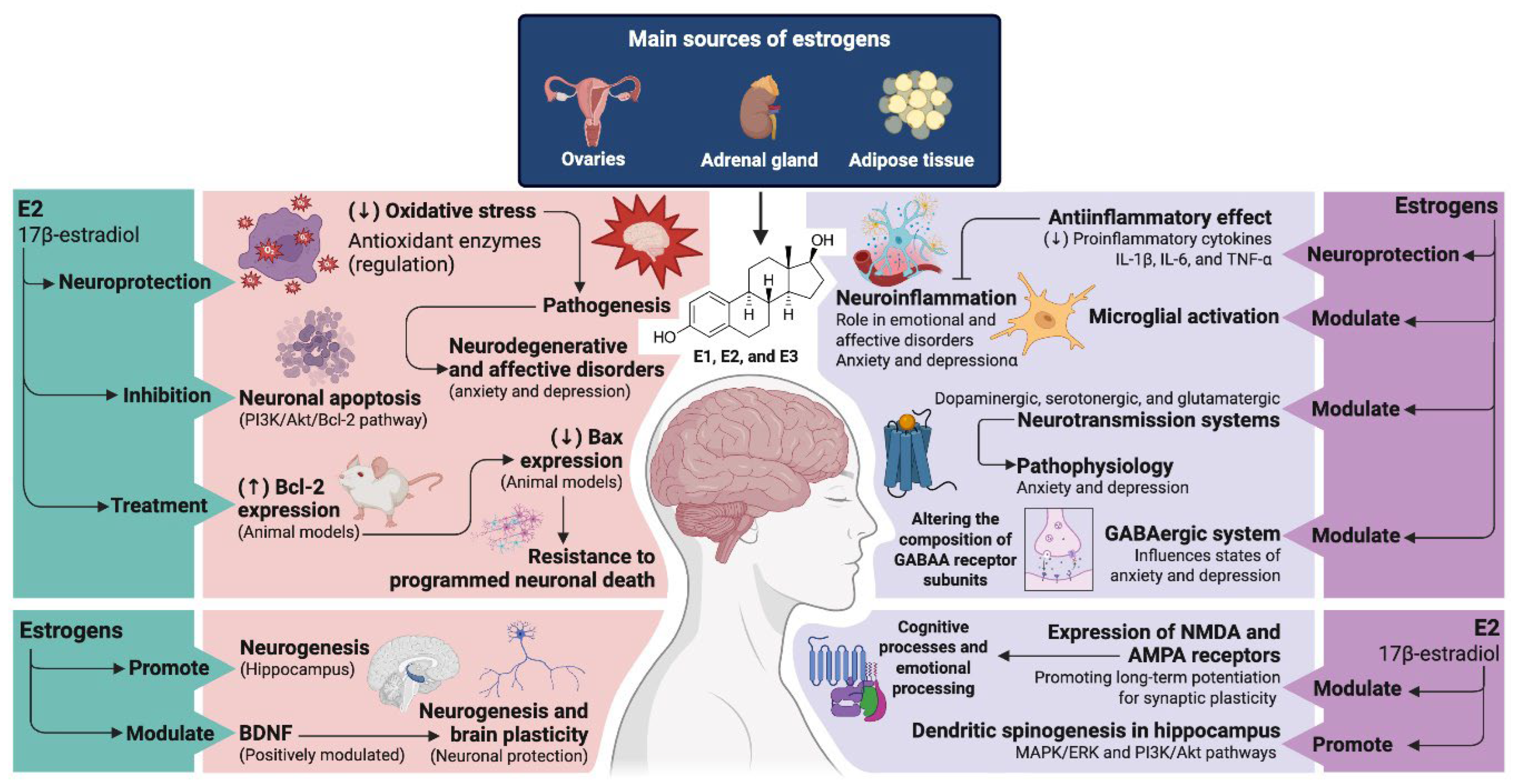
3. Physiological Basis: Estrogen, Brain and Mood
3.1. Function of Estrogen in the Brain
3.2. Relationship Between Fluctuating Hormone Levels and Mood Disorders
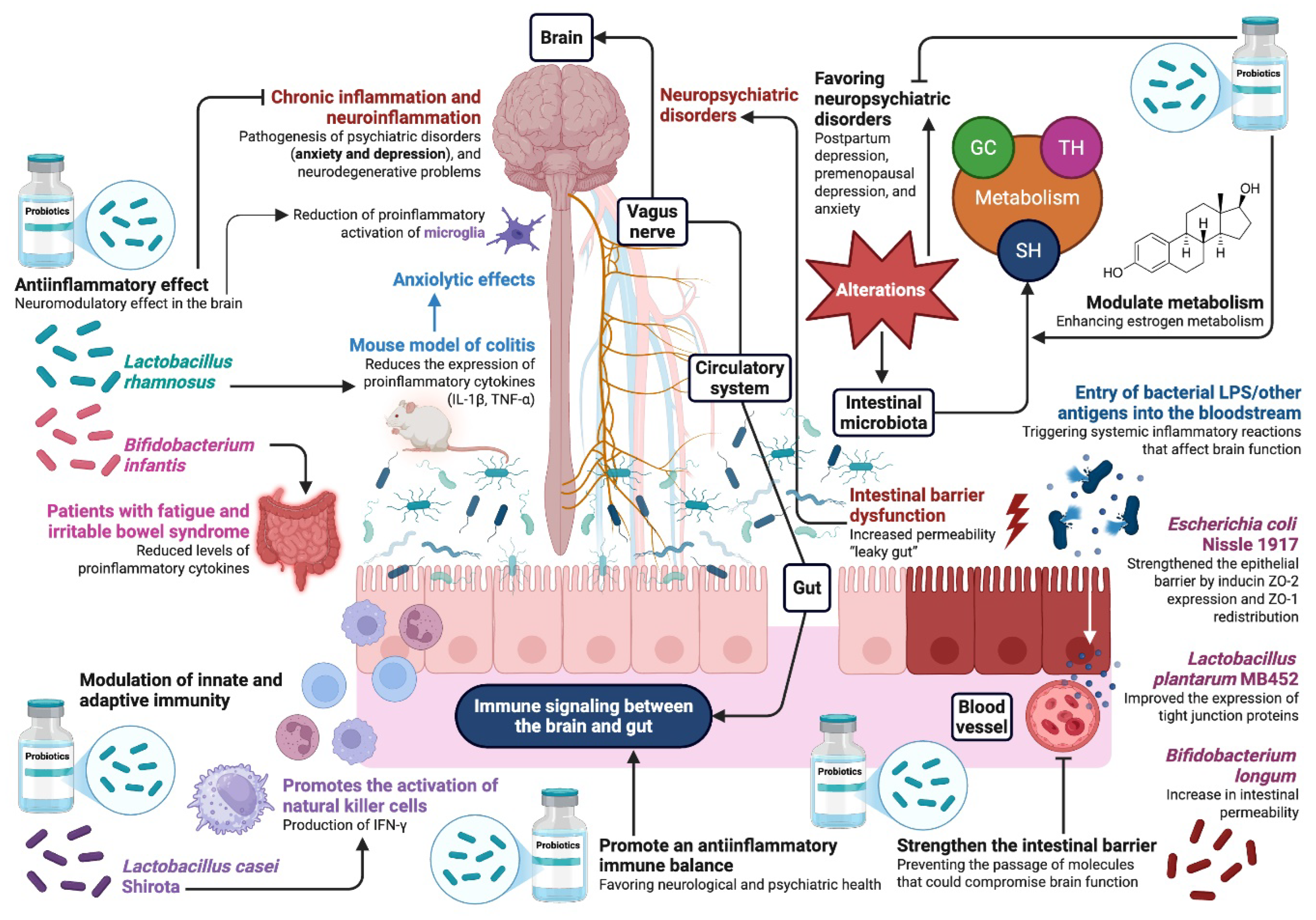
4. Role of Probiotics in Modulating the Gut–Brain Axis
4.1. Influence of the Gut Microbiota on the Production and Regulation of Neurotransmitters
4.2. Impact of Probiotics on Mental Health and Emotional Behavior
4.3. Potential Mechanisms of Action of Probiotics
5. Mechanisms of Action of Estrogenic Effect of Probiotics
Relation Between Estrogens and the Intestinal Microbiota in Mood Regulation
6. Preclinical Evidence of Use Probiotic
7. Clinical Evidence of the Effects of Probiotics on Depressive and Anxiety Symptoms in Women
8. Therapeutic and Preventive Applications
9. Challenges and Recommendations for Future Research
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MGB axis | Microbiota–gut–brain axis |
| 5-HT | Serotonin |
| GABA | Gamma-aminobutyric acid |
| DA | Dopamine |
| CNS | Central nervous system |
| HPA | Hypothalamic–pituitary–adrenal |
| SCFAs | Short-chain fatty acids |
| E1 | Estrone |
| E2 | 17β-estradiol |
| E3 | Estriol |
| PI3K | phosphatidylinositol 3-kinase |
| Akt | Protein kinase B |
| Bcl-2 | anti-apoptotic protein |
| BDNF | Brain-derived neurotrophic factor |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor alpha |
| SERT | 5-HT transporter |
| SSRIs | Selective serotonin reuptake inhibitors |
| NMDA | N-metil-D-aspartate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| LTP | Long-term potentiation |
| PSD-95 | Postsynaptic density protein 95 |
| MAPK | Mitogen-Activated Protein Kinase |
| ERK | Extracellular signal-regulated kinase |
| PMDD | Premenstrual dysphoric disorder |
| MDD | Major depressive disorder |
| NE | Norepinephrine |
| BBB | Blood–brain barrier |
| IFN-γ | Interferon gamma |
| LPS | Lipopolysaccharides |
| ZO-1 | Tight junction proteins |
| SHBG | Sex hormone-binding globulin |
| ERα | Estrogen receptor alpha |
| ERβ | Estrogen Receptor Beta |
| GH2 | Glycoside hydrolase 2 |
| gmGUS | Intestinal microbial β-glucuronidase |
| OVX | Ovariectomy |
| MAO | Enzyme monoamine oxidase |
| KP | Kynurenine pathway |
| IDO | Enzyme indoleamine 2,3-dioxygenase |
| KYNA | Kynurenic acid |
| QUIN | Quinolinic acid |
| TPH2 | Tryptophan hydroxylase-2 |
| DRN | Dorsal raphe nucleus |
| GPCR | G-protein-coupled membrane receptors |
| NF-KB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| TRP | Tryptophan |
| 3β-HSD | 3β-hydroxysteroid dehydrogenase |
| 3/17β-HSD | 3/17β-hydroxysteroid dehydrogenase |
| CUMS | Chronic unpredictable mild stress |
| CRP | C-reactive protein |
| CUS | Chronic unpredictable stress |
| NO | Nitric oxide |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| ACTH | Adrenocorticotropic hormone |
| CORT | Corticosterone |
| PCOS | Polycystic ovary syndrome |
| PMS | Premenstrual syndrome |
References
- World Health Organization. Depressive Disorder (Depression). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 31 July 2025).
- Soares, C.N.; Zitek, B. Reproductive hormone sensitivity and risk for depression across the female life cycle: A continuum of vulnerability? J. Psychiatry Neurosci. 2008, 33, 331–343. [Google Scholar] [CrossRef]
- Altemus, M.; Sarvaiya, N.; Neill-Epperson, C. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014, 35, 320–330. [Google Scholar] [CrossRef]
- Halbreich, U.; Kahn, L.S. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs 2001, 15, 797–817. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Akama, K.T.; Spencer-Segal, J.L.; Milner, T.A.; Waters, E.M. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 2012, 126, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.S.; Soares, C.N.; Vitonis, A.F.; Otto, M.W.; Harlow, B.L. Risk for new onset of depression during the menopausal transition: The Harvard study of moods and cycles. Arch. Gen. Psychiatry 2006, 63, 385–390. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Cryan, J.F.; O’RIordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Huang, R.; Wang, K.; Hu, J. Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef]
- Chao, L.; Liu, C.; Sutthawongwadee, S.; Li, Y.; Lv, W.; Chen, W.; Yu, L.; Zhou, J.; Guo, A.; Li, Z.; et al. Effects of probiotics on depressive or anxiety variables in healthy participants under stress conditions or with a depressive or anxiety diagnosis: A meta-analysis of randomized controlled trials. Front. Neurol. 2020, 11, 421. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Li, W.; Liang, H.; He, W.; Gao, X.; Wu, Z.; Hu, T.; Lin, X.; Wang, M.; Zhong, Y.; Zhang, H.; et al. Genomic and functional diversity of cultivated Bifidobacterium from human gut microbiota. Heliyon 2024, 10, e27270. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, X.; Wang, Z.; Kong, L.; Jiang, Z.; Shang, R.; Zhong, X.; Lv, S.; Zhang, G.; Gao, H.; et al. Microbiota–gut–brain axis: Natural antidepressants molecular mechanism. Phytomedicine 2024, 134, 156012. [Google Scholar] [CrossRef]
- Thakur, P.; Baraskar, K.; Shrivastava, V.K.; Medhi, B. Cross-talk between adipose tissue and microbiota-gut-brain-axis in brain development and neurological disorder. Brain Res. 2024, 1844, 149176. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocrinol. 2014, 35, 8–30. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.; Raz, L.; Wang, R.; Vadlamudi, R.; Zhang, Q. Oestrogen signalling and neuroprotection in cerebral ischaemia. J. Neuroendocrinol. 2012, 24, 34–47. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 2014, 12, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jover-Mengual, T.; Miyawaki, T.; Latuszek, A.; Alborch, E.; Zukin, R.S.; Etgen, A.M. Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway. Brain Res. 2010, 1321, 1–12. [Google Scholar] [CrossRef]
- Zaldivar, V.; Magri, M.L.; Zárate, S.; Jaita, G.; Eijo, G.; Radl, D.; Ferraris, J.; Pisera, D.; Seilicovich, A. Estradiol increases the Bax/Bcl-2 ratio and induces apoptosis in the anterior pituitary gland. Neuroendocrinology 2009, 90, 292–300. [Google Scholar] [CrossRef]
- Duarte-Guterman, P.; Lieblich, S.E.; Chow, C.; Galea, L.A. Estradiol and GPER activation differentially affect cell proliferation but not GPER expression in the hippocampus of adult female rats. PLoS ONE 2015, 10, e0129880. [Google Scholar] [CrossRef]
- Been, L.E.; Halliday, A.R.; Blossom, S.M.; Bien, E.M.; Bernhard, A.G.; Roth, G.E.; Kelly, M.E. Long-term oral tamoxifen administration decreases brain-derived neurotrophic factor in the hippocampus of female Long-Evans rats. Cancers 2024, 16, 1373. [Google Scholar] [CrossRef]
- Baker, E.E.; Brautigam, V.M.; Watters, J.J. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor β. Endocrinology 2004, 145, 5021–5032. [Google Scholar] [CrossRef]
- Saeed, K.; Jo, M.H.; Park, J.S.; Alam, S.I.; Khan, I.; Ahmad, R.; Khan, A.; Ullah, R.; Kim, M.O. 17β-estradiol abrogates oxidative stress and neuroinflammation after cortical stab wound injury. Antioxidants 2021, 10, 1682. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Unadkat, P.; Rebeiz, T.; Ajmal, E.; De Souza, V.; Xia, A.; Jinu, J.; Powell, K.; Li, C. Neurobiological mechanisms underlying psychological dysfunction after brain injuries. Cells 2025, 14, 74. [Google Scholar] [CrossRef]
- Hiroi, R.; McDevitt, R.A.; Neumaier, J.F. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: Association between gene expression and anxiety behavior in the open field. Biol. Psychiatry 2006, 60, 288–295. [Google Scholar] [CrossRef]
- Bendis, P.C.; Zimmerman, S.; Onisiforou, A.; Zanos, P.; Georgiou, P. The impact of estradiol on serotonin, glutamate, and dopamine systems. Front. Neurosci. 2024, 18, 1348551. [Google Scholar] [CrossRef] [PubMed]
- Zachry, J.E.; Nolan, S.O.; Brady, L.J.; Kelly, S.J.; Siciliano, C.A.; Calipari, E.S. Sex differences in dopamine release regulation in the striatum. Neuropsychopharmacology 2021, 46, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Clements, L.; Alexander, A.; Hamilton, K.; Irving, A.; Harvey, J. G-protein coupled estrogen receptor (GPER1) activation promotes synaptic insertion of AMPA receptors and induction of chemical LTP at hippocampal temporoammonic-CA1 synapses. Mol. Brain 2023, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Spurny-Dworak, B.; Handschuh, P.; Spies, M.; Kaufmann, U.; Seiger, R.; Klöbl, M.; Konadu, M.; Reed, M.; Ritter, V.; Baldinger-Melich, P.; et al. Effects of sex hormones on brain GABA and glutamate levels in a cis- and transgender cohort. Psychoneuroendocrinology 2022, 138, 105683. [Google Scholar] [CrossRef]
- Frye, C.A.; Cleveland, D.M.; Sadarangani, A.; Torgersen, J.K. Progesterone promotes anti-anxiety/depressant-like behavior and trophic actions of BDNF in the hippocampus of female nuclear progesterone receptor, but not 5α-reductase, knockout mice. Int. J. Mol. Sci. 2025, 26, 1173. [Google Scholar] [CrossRef]
- Appelbaum, L.G.; Shenasa, M.A.; Stolz, L.; Daskalakis, Z. Synaptic plasticity and mental health: Methods, challenges and opportunities. Neuropsychopharmacology 2023, 48, 113–120. [Google Scholar] [CrossRef]
- Bustamante-Barrientos, F.A.; Méndez-Ruette, M.; Ortloff, A.; Luz-Crawford, P.; Rivera, F.J.; Figueroa, C.D.; Bátiz, L.F. The impact of estrogen and estrogen-like molecules in neurogenesis and neurodegeneration: Beneficial or harmful? Front. Cell. Neurosci. 2021, 15, 636176. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Lange, C.A.; Levin, E.R. Membrane-initiated estrogen, androgen, and progesterone receptor signaling in health and disease. Endocr. Rev. 2022, 43, 720–742. [Google Scholar] [CrossRef]
- Jiang, H.H.; Wu, T.H.; Lee, L.J.; Lee, J.C.; Chung, B.C.; Yang, F.M.; Hu, M.C. Dendritic morphology of developing hippocampal neurons in Cyp11a1 null mice. Dev. Neurosci. 2025, 47, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Y.; Li, X.; Wang, D.; Zhang, A.; Pang, J.; He, J.; Chen, X.; Tang, N.-J. Perfluoroalkyl substances promote breast cancer progression via ERα and GPER mediated PI3K/Akt and MAPK/Erk signaling pathways. Ecotoxicol. Environ. Saf. 2023, 258, 114980. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.N. Menopause and mood: The role of estrogen in midlife depression and beyond. Psychiatr. Clin. N. Am. 2023, 46, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Targonskaya, A.; Maslowski, K. Reproductive hormones and female mental wellbeing. Women 2023, 3, 432–444. [Google Scholar] [CrossRef]
- Itriyeva, K. Premenstrual syndrome and premenstrual dysphoric disorder in adolescents. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101187. [Google Scholar] [CrossRef]
- Kundakovic, M.; Rocks, D. Sex hormone fluctuation and increased female risk for depression and anxiety disorders: From clinical evidence to molecular mechanisms. Front. Neuroendocrinol. 2022, 66, 101010. [Google Scholar] [CrossRef]
- Tak, S.; Lee, S.; Park, C.A.; Cheong, E.N.; Seok, J.W.; Sohn, J.H.; Cheong, C. Altered effective connectivity within the fronto-limbic circuitry in response to negative emotional task in female patients with major depressive disorder. Brain Connect. 2021, 11, 264–277. [Google Scholar] [CrossRef]
- Stefaniak, M.; Dmoch-Gajzlerska, E.; Jankowska, K.; Rogowski, A.; Kajdy, A.; Maksym, R.B. Progesterone and its metabolites play a beneficial role in affect regulation in the female brain. Pharmaceuticals 2023, 16, 520. [Google Scholar] [CrossRef]
- Kale, M.B.; Wankhede, N.L.; Goyanka, B.K.; Gupta, R.; Bishoyi, A.K.; Nathiya, D.; Kaur, P.; Shanno, K.; Taksande, B.G.; Khalid, M.; et al. Unveiling the neurotransmitter symphony: Dynamic shifts in neurotransmitter levels during menstruation. Reprod. Sci. 2025, 32, 26–40. [Google Scholar] [CrossRef]
- Sacher, J.; Zsido, R.G.; Barth, C.; Zientek, F.; Rullmann, M.; Luthardt, J.; Patt, M.; Becker, G.A.; Rusjan, P.; Witte, A.V.; et al. Increase in serotonin transporter binding in patients with premenstrual dysphoric disorder across the menstrual cycle: A case-control longitudinal neuroreceptor ligand positron emission tomography imaging study. Biol. Psychiatry 2023, 93, 1081–1088. [Google Scholar] [CrossRef]
- Alblooshi, S.; Taylor, M.; Gill, N. Does menopause elevate the risk for developing depression and anxiety? Results from a systematic review. Australas. Psychiatry 2023, 31, 165–173. [Google Scholar] [CrossRef]
- Badawy, Y.; Spector, A.; Li, Z.; Desai, R. The risk of depression in the menopausal stages: A systematic review and meta-analysis. J. Affect. Disord. 2024, 357, 126–133. [Google Scholar] [CrossRef]
- Sun, Q.; Li, G.; Zhao, F.; Dong, M.; Xie, W.; Liu, Q.; Yang, W.; Cui, R. Role of estrogen in treatment of female depression. Aging 2024, 16, 3021. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.Z.; Yahaya, M.F.; Mohd Fahami, N.A.; Abdul Manan, H.; Singh, M.; Damanhuri, H.A. Brain volumetric changes in menopausal women and its association with cognitive function: A structured review. Front. Aging Neurosci. 2023, 15, 1158001. [Google Scholar] [CrossRef] [PubMed]
- Rupanagunta, G.P.; Nandave, M.; Rawat, D.; Upadhyay, J.; Rashid, S.; Ansari, M.N. Postpartum depression: Aetiology, pathogenesis and the role of nutrients and dietary supplements in prevention and management. Saudi Pharm. J. 2023, 31, 1274. [Google Scholar] [CrossRef] [PubMed]
- Dimcea, D.A.M.; Petca, R.C.; Dumitrașcu, M.C.; Șandru, F.; Mehedințu, C.; Petca, A. Postpartum depression: Etiology, treatment, and consequences for maternal care. Diagnostics 2024, 14, 865. [Google Scholar] [CrossRef]
- Zhang, K.; He, L.; Li, Z.; Ding, R.; Han, X.; Chen, B.; Cao, G.; Ye, J.-H.; Li, T.; Fu, R. Bridging neurobiological insights and clinical biomarkers in postpartum depression: A narrative review. Int. J. Mol. Sci. 2024, 25, 8835. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Jiang, M.; Ni, X.; Du, M.; Jiang, H.; Bi, M.; Wang, Y.; Liu, C.; Liu, S. Limosilactobacillus reuteri alleviates anxiety-like behavior and intestinal symptoms in two stressed mouse models. Nutrients 2024, 16, 3209. [Google Scholar] [CrossRef]
- Landsman, A. Gene expression in depression: Molecular aspects of postpartum depression. In The Neuroscience of Depression; Academic Press: Cambridge, MA, USA, 2021; pp. 25–35. [Google Scholar] [CrossRef]
- Ferrari, S.; Mulè, S.; Parini, F.; Galla, R.; Ruga, S.; Rosso, G.; Brovero, A.; Molinari, C.; Uberti, F. The influence of the gut-brain axis on anxiety and depression: A review of the literature on the use of probiotics. J. Tradit. Complement. Med. 2024, 14, 237–255. [Google Scholar] [CrossRef]
- Rosas-Sánchez, G.U.; Germán-Ponciano, L.J.; Puga-Olguín, A.; Flores-Soto, M.E.; Nápoles-Medina, A.Y.; Muñoz-Carillo, J.L.; Rodríguez-Landa, J.F.; Soria-Fregozo, C. Gut–Brain Axis in Mood Disorders: A Narrative Review of Neurobiological Insights and Probiotic Interventions. Biomedicines 2025, 13, 1831. [Google Scholar] [CrossRef]
- Caldarelli, M.; Rio, P.; Marrone, A.; Ocarino, F.; Chiantore, M.; Candelli, M.; Cianci, R. Gut–brain axis: Focus on sex differences in neuroinflammation. Int. J. Mol. Sci. 2024, 25, 5377. [Google Scholar] [CrossRef]
- Kumari, N.; Kumari, R.; Dua, A.; Singh, M.; Kumar, R.; Singh, P.; Duyar-Ayerdi, S.; Pradeep, S.; Ojesina, A.I. From gut to hormones: Unraveling the role of gut microbiota in (phyto)estrogen modulation in health and disease. Mol. Nutr. Food Res. 2024, 68, 2300688. [Google Scholar] [CrossRef]
- Pires, L.; Gonzalez-Paramás, A.M.; Heleno, S.A.; Calhelha, R.C. Gut microbiota as an endocrine organ: Unveiling its role in human physiology and health. Appl. Sci. 2024, 14, 9383. [Google Scholar] [CrossRef]
- Kanova, M.; Kohout, P. Serotonin—Its synthesis and roles in the healthy and the critically ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef]
- Liu, N.; Sun, S.; Wang, P.; Sun, Y.; Hu, Q.; Wang, X. The mechanism of secretion and metabolism of gut-derived 5-hydroxytryptamine. Int. J. Mol. Sci. 2021, 22, 7931. [Google Scholar] [CrossRef]
- Cocean, A.M.; Vodnar, D.C. Exploring the gut-brain axis: Potential therapeutic impact of psychobiotics on mental health. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 134, 111073. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Y.; Cai, J.; Li, Y.; Su, Q.; Meng, X. Synbiotic potential of Bifidobacterium longum subsp. infantis H11 in combination with human milk oligosaccharides in cellular barrier protection. Food Biosci. 2025, 63, 105628. [Google Scholar] [CrossRef]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of oral gamma-aminobutyric acid (GABA) administration on stress and sleep in humans: A systematic review. Front. Neurosci. 2020, 14, 559962. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gutiérrez, L.; San Vicente, L.; Barrón, L.J.R.; del Carmen-Villarán, M.; Chávarri, M. Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 2020, 64, 103669. [Google Scholar] [CrossRef]
- Dalziel, J.E.; Zobel, G.; Dewhurst, H.; Hurst, C.; Olson, T.; Rodriguez-Sanchez, R.; Mace, L.; Parkar, N.; Thum, C.; Hannaford, R.; et al. A diet enriched with Lacticaseibacillus rhamnosus HN001 and milk fat globule membrane alters the gut microbiota and decreases amygdala GABA a receptor expression in stress-sensitive rats. Int. J. Mol. Sci. 2023, 24, 10433. [Google Scholar] [CrossRef]
- Gasmi, A.; Nasreen, A.; Menzel, A.; Gasmi-Benahmed, A.; Pivina, L.; Noor, S.; Peana, M.; Chirumbolo, S.; Bjørklund, G. Neurotransmitters regulation and food intake: The role of dietary sources in neurotransmission. Molecules 2022, 28, 210. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The critical modulators regulating gut–brain axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Ghazanfar, S.; Ul Haq, R.; Ullah, S.; Khan, S.; Wu, J.; Ahmad, W.; Tipu, M.K. Probiotics (Bacillus clausii and Lactobacillus fermentum NMCC-14) ameliorate stress behavior in mice by increasing monoamine levels and mRNA expression of dopamine receptors (D1 and D2) and synaptophysin. Front. Pharmacol. 2022, 13, 915595. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Li, M.M.; Zhou, M.F.; Xu, H.S.; Huan, F.; Liu, N.; Gao, R.; Wang, J.; Zhang, N.; Jiang, L. Links between gut dysbiosis and neurotransmitter disturbance in chronic restraint stress-induced depressive behaviours: The role of inflammation. Inflammation 2021, 44, 2448–2462. [Google Scholar] [CrossRef]
- Leite, J.A.; Orellana, A.M.M.; Kinoshita, P.F.; de Mello, N.P. Neuroinflammation and Neurotransmission Mechanisms Involved in Neuropsychiatric Disorders. In Mechanisms of Neuroinflammation; Gonçalves, F.L.T., Ed.; IntechOpen: London, UK, 2017; ISBN 978-953-51-5305-9. [Google Scholar] [CrossRef]
- Reis, D.J.; Ilardi, S.S.; Punt, S.E. The anxiolytic effect of probiotics: A systematic review and meta-analysis of the clinical and preclinical literature. PLoS ONE 2018, 13, e0199041. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Verdu, E.F. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Asad, A.; Kirk, M.; Zhu, S.; Dong, X.; Gao, M. Effects of prebiotics and probiotics on symptoms of depression and anxiety in clinically diagnosed samples: Systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2024, 83, e1504–e1520. [Google Scholar] [CrossRef]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Théodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J. Neurogastroenterol. Motil. 2018, 24, 138. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Suda, K.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes 2016, 7, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, N.; Chen, R.; Lee, T.; Gao, Y.; Yuan, Z.; Nie, Y.; Sun, T. Prenatal stress leads to deficits in brain development, mood related behaviors and gut microbiota in offspring. Neurobiol. Stress 2021, 15, 100333. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, W.; Jiang, Y.; Xiao, X.; Zou, Q.; Liang, J.; Zhao, Y.; Wang, Q.; Yuan, T.; Guo, R.; et al. A synbiotic formulation of Lactobacillus reuteri and inulin alleviates ASD-like behaviors in a mouse model: The mediating role of the gut–brain axis. Food Funct. 2024, 15, 387–400. [Google Scholar] [CrossRef]
- Mishra, V.; Yadav, D.; Solanki, K.S.; Koul, B.; Song, M. 2023. A review on the protective effects of probiotics against Alzheimer’s disease. Biology 2023, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Bi, N.; Wang, T.; Huang, C.; Wang, R.; Xu, Y.; Wang, H.L. Probiotic Lactobacillus rhamnosus GR-1 supplementation attenuates Pb-induced learning and memory deficits by reshaping the gut microbiota. Front. Nutr. 2022, 9, 934118. [Google Scholar] [CrossRef]
- Yin, X.; Liu, W.; Feng, H.; Huang, J.; Wang, Q.; Zhang, Q.; He, J.; Wang, R. Bifidobacterium animalis subsp. lactis A6 attenuates hippocampal damage and memory impairments in an ADHD rat model. Food Funct. 2024, 15, 2668–2678. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kuhara, T.; Oki, M.; Xiao, J.Z. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial. Benef. Microbes 2019, 10, 511–520. [Google Scholar] [CrossRef]
- Rhie, S.J.; Jung, E.Y.; Shim, I. The role of neuroinflammation on pathogenesis of affective disorders. J. Exerc. Rehabil. 2020, 16, 2–9. [Google Scholar] [CrossRef]
- Rezaie, N.; Aghamohammad, S.; Haj Agha Gholizadeh Khiavi, E.; Khatami, S.; Sohrabi, A.; Rohani, M. The comparative anti-oxidant and anti-inflammatory efficacy of postbiotics and probiotics through Nrf-2 and NF-κB pathways in DSS-induced colitis model. Sci. Rep. 2024, 14, 11560. [Google Scholar] [CrossRef]
- Amdekar, S.; Singh, V. Studies on anti-inflammatory and analgesic properties of Lactobacillus rhamnosus in experimental animal models. J. Complement. Integr. Med. 2016, 13, 145–150. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Xie, A.; Sun, J.; Yang, H.; Li, J.; Li, Y.; Chen, F.; Mei, Y.; Liang, Y. Lactobacillus rhamnosus and L. plantarum combination treatment ameliorated colitis symptoms in a mouse model by altering intestinal microbial composition and suppressing inflammatory response. Mol. Nutr. Food Res. 2023, 67, 2200340. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Jin, H.; Liao, N.; Li, J.; Jiang, C.; Shao, D.; Shi, J. Lactobacillus rhamnosus from human breast milk ameliorates ulcerative colitis in mice via gut microbiota modulation. Food Funct. 2021, 12, 5171–5186. [Google Scholar] [CrossRef] [PubMed]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Quigley, E.M.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Campos Pérez, W.; Martínez López, E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158900. [Google Scholar] [CrossRef]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front. Immunol. 2018, 9, 1832. [Google Scholar] [CrossRef]
- Min, Y.W.; Rhee, P.L. The role of microbiota on the gut immunology. Clin. Ther. 2015, 37, 968–975. [Google Scholar] [CrossRef]
- Chornchoem, P.; Tandhavanant, S.; Saiprom, N.; Preechanukul, A.; Thongchompoo, N.; Sensorn, I.; Chantratita, W.; Chantratita, N. Metagenomic evaluation, antimicrobial activities, and immune stimulation of probiotics from dietary supplements and dairy products. Sci. Rep. 2025, 15, 11537. [Google Scholar] [CrossRef]
- Kasarello, K.; Cudnoch Jedrzejewska, A.; Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef]
- Wasiak, J.; Gawlik Kotelnicka, O. Intestinal permeability and its significance in psychiatric disorders: A narrative review and future perspectives. Behav. Brain Res. 2023, 448, 114459. [Google Scholar] [CrossRef]
- Ferrari, S.; Galla, R.; Mulè, S.; Rosso, G.; Brovero, A.; Macchi, V.; Ruga, S.; Uberti, F. The role of Bifidobacterium bifidum novaBBF7, B. longum novaBLG2 and Lactobacillus paracasei TJB8 to improve mechanisms linked to neuronal cell protection against oxidative condition in a gut-brain axis model. Int. J. Mol. Sci. 2023, 24, 12281. [Google Scholar] [CrossRef]
- Zyrek, A.A.; Cichon, C.; Helms, S.; Enders, C.; Sonnenborn, U.; Schmidt, M.A. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζ redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007, 9, 804–816. [Google Scholar] [CrossRef]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef]
- Tian, P.; Zou, R.; Song, L.; Zhang, X.; Jiang, B.; Wang, G.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W. Ingestion of Bifidobacterium longum subspecies infantis strain CCFM687 regulated emotional behavior and the central BDNF pathway in chronic stress-induced depressive mice through reshaping the gut microbiota. Food Funct. 2019, 10, 7588–7598. [Google Scholar] [CrossRef]
- Fenneman, A.C.; Bruinstroop, E.; Nieuwdorp, M.; van der Spek, A.H.; Boelen, A. A comprehensive review of thyroid hormone metabolism in the gut and its clinical implications. Thyroid 2023, 33, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Grau-Del Valle, C.; Fernández, J.; Solá, E.; Montoya-Castilla, I.; Morillas, C.; Bañuls, C. Association between gut microbiota and psychiatric disorders: A systematic review. Front. Psychol. 2023, 14, 1215674. [Google Scholar] [CrossRef] [PubMed]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan metabolites along the microbiota-gut-brain axis: An interkingdom communication system influencing the gut in health and disease. Int. J. Tryptophan Res. 2020, 13, 1178646920928984. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F. Roles of estrogens, estrogen-like compounds, and endocrine disruptors in adipocytes. Front. Endocrinol. 2022, 13, 921504. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes 2023, 15, 2236749. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Chen, K.L.; Madak-Erdogan, Z. Estrogen and microbiota crosstalk: Should we pay attention? Trends Endocrinol. Metab. 2016, 27, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota: Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 1, 227–238. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Kwon, H.; Lim, W.; Moon, B.I. Staphylococcus aureus-derived extracellular vesicles enhance the efficacy of endocrine therapy in breast cancer cells. J. Clin. Med. 2022, 11, 2030. [Google Scholar] [CrossRef]
- Pollet, R.M.; D’AGostino, E.H.; Walton, W.G.; Xu, Y.; Little, M.S.; Biernat, K.A.; Pellock, S.J.; Patterson, L.M.; Creekmore, B.C.; Isenberg, H.N.; et al. An atlas of β-glucuronidases in the human intestinal microbiome. Structure. 2017, 25, 967–977.e5. [Google Scholar] [CrossRef]
- Creekmore, B.C.; Gray, J.H.; Walton, W.G.; Biernat, K.A.; Little, M.S.; Xu, Y.; Liu, J.; Gharaibeh, R.Z.; Redinbo, M.R. Mouse gut microbiome-encoded β-glucuronidases identified using metagenome analysis guided by protein structure. MSystems 2019, 4, e00452-19. [Google Scholar] [CrossRef]
- Roy, D.; Ward, P. Rapid detection of Bifidobacterium dentium by enzymatic hydrolysis of beta-glucoronide substrates. J. Food Prot. 1992, 55, 291–295. [Google Scholar] [CrossRef]
- Beaud, D.; Tailliez, P.; Anba-Mondoloni, J. Genetic characterization of the beta-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 2005, 151, 2323–2330. [Google Scholar] [CrossRef]
- Dabek, M.; McCrae, S.I.; Stevens, V.J.; Duncan, S.H.; Louis, P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 2008, 66, 487–495. [Google Scholar] [CrossRef]
- Gloux, K.; Berteau, O.; El Oumami, H.; Béguet, F.; Leclerc, M.; Doré, J. A metagenomic β-glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proc. Natl. Acad. Sci. USA 2010, 108, 4539–4546. [Google Scholar] [CrossRef]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.E.; Herbert, W.G.; Song, S.D.; Raman, H.N.; Zhu, J.E.; Gonzalez, P.E.; Walther-António, M.R.S.; Tetel, M.J. Gut and vaginal microbiomes on steroids: Implications for women’s health. Trends Endocrinol. Metab. 2021, 32, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.G.; Hale, G.E.; Robertson, D.M.; Dennerstein, L. A review of hormonal changes during the menopausal transition: Focus on findings from the Melbourne Women’s Midlife Health Project. Hum. Reprod. Update 2007, 13, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Lin, J.; Qi, Q.; Usyk, M.; Isasi, C.R.; Mossavar-Rahmani, Y.; Derby, C.A.; Santoro, N.; Perreira, K.M.; Daviglus, M.L.; et al. Menopause is associated with an altered gut microbiome and estrobolome, with implications for adverse cardiometabolic risk in the Hispanic Community Health Study/Study of Latinos. MSystems 2022, 7, e0027322. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Mao, T.; Huang, Y.; Liang, J.; Zhu, M.; Yao, P.; Zong, Y.; Lang, J.; Zhang, Y.; et al. The relationship between menopausal syndrome and gut microbes. BMC Womens Health 2022, 22, 437. [Google Scholar] [CrossRef]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol. 2016, 39, 1–12. [Google Scholar]
- Meng, Q.; Ma, M.; Zhang, W.; Bi, Y.; Cheng, P.; Yu, X.; Fu, Y.; Chao, Y.; Ji, T.; Li, J.; et al. The gut microbiota during the progression of atherosclerosis in the perimenopausal period shows specific compositional changes and significant correlations with circulating lipid metabolites. Gut Microbes 2021, 13, 1–27. [Google Scholar] [CrossRef]
- Insenser, M.; Murri, M.; Del Campo, R.; Martínez-García, M.Á.; Fernández-Durán, E.; Escobar-Morreale, H.F. Gut microbiota and the polycystic ovary syndrome: Influence of sex, sex hormones, and obesity. J. Clin. Endocrinol. Metab. 2018, 103, 2552–2562. [Google Scholar] [CrossRef]
- Luqman, A.; He, M.; Hassan, A.; Ullah, M.; Zhang, L.; Rashid Khan, M.; Din, A.U.; Ullah, K.; Wang, W.; Wang, G. Mood and microbes: A comprehensive review of intestinal microbiota’s impact on depression. Front. Psychiatry 2024, 15, 1295766. [Google Scholar] [CrossRef]
- Sui, Y.; Wu, J.; Chen, J. The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.; Tramullas, M.; Kiely, B.; Dinan, T.; Cryan, J. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015, 287, 59–72. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Pierozan, P.; Biasibetti, H.; Schmitz, F.; Ávila, H.; Parisi, M.M.; Barbe-Tuana, F.; Wyse, A.T.; Pessoa-Pureur, R. Quinolinic acid neurotoxicity: Differential roles of astrocytes and microglia via FGF-2-mediated signaling in redox-linked cytoskeletal changes. Biochim. Biophys. Acta. 2016, 1863, 3001–3014. [Google Scholar] [CrossRef]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Moschen, A.R.; Kaser, A.; Obrist, P.; Fuchs, D.; Brandacher, G.; Winkler, C.; Geboes, K.; et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin. Immunol. 2004, 113, 47–55. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112 Pt B, 399–412. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1–16. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Qi, Q.; Li, J.; Yu, B.; Moon, J.Y.; Chai, J.C.; Merino, J.; Hu, J.; Ruiz-Canela, M.; Rebholz, C.; Wang, Z.; et al. Host and gut microbial tryptophan metabolism and type 2 diabetes: An integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut 2022, 71, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, Y.; Xu, L.; Yu, Z.; Wang, S.; Xu, H.; Zhang, J.; Zhang, L.; Liu, W.; Wu, L.; et al. Microbiome and tryptophan metabolomics analysis in adolescent depression: Roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 2023, 11, 145. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Yang, C.; Xu, J.; Xu, X.; Xu, W.; Tong, B.; Wang, S.; Ji, R.; Tan, Y.; Zhu, Y. Characteristics of gut microbiota in patients with metabolic associated fatty liver disease. Sci. Rep. 2023, 13, 9988. [Google Scholar] [CrossRef]
- Mitra, S.W.; Hoskin, E.; Yudkovitz, J.; Pear, L.; Wilkinson, H.A.; Hayashi, S.; Pfaff, D.W.; Ogawa, S.; Rohrer, S.P.; Schaeffer, J.M.; et al. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology 2003, 144, 2055–2067. [Google Scholar] [CrossRef]
- Imwalle, D.B.; Gustafsson, J.A.; Rissman, E.F. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol. Behav. 2005, 84, 157–163. [Google Scholar] [CrossRef]
- Sheng, Z.; Kawano, J.; Yanai, A.; Fujinaga, R.; Tanaka, M.; Watanabe, Y.; Shinoda, K. Expression of estrogen receptors (α, β) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci. Res. 2004, 49, 185–196. [Google Scholar] [CrossRef]
- Hildebrandt, T.; Alfano, L.; Tricamo, M.; Pfaff, D.W. Conceptualizing the role of estrogens and serotonin in the development and maintenance of bulimia nervosa. Clin. Psychol. Rev. 2010, 30, 655–668. [Google Scholar] [CrossRef]
- Hudon Thibeault, A.A.; Sanderson, J.T.; Vaillancourt, C. Serotonin-estrogen interactions: What can we learn from pregnancy? Biochimie 2019, 161, 88–108. [Google Scholar] [CrossRef]
- Nomura, M.; Akama, K.T.; Alves, S.E.; Korach, K.S.; Gustafsson, J.Å.; Pfaff, D.W.; Ogawa, S. Differential distribution of estrogen receptor (ER)-α and ER-β in the midbrain raphe nuclei and periaqueductal gray in male mouse: Predominant role of ER-β in midbrain serotonergic systems. Neuroscience 2005, 130, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Donner, N.; Handa, R.J. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience 2009, 163, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, R.; Handa, R.J. Estrogen receptor-β regulates human tryptophan hydroxylase-2 through an estrogen response element in the 5′ untranslated region. J. Neurochem. 2013, 127, 487–495. [Google Scholar] [CrossRef] [PubMed]
- McQueen, J.K.; Wilson, H.; Fink, G. Estradiol-17β increase serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Mol. Brain Res. 1997, 45, 13–23. [Google Scholar] [CrossRef]
- Hernández-Hernández, O.T.; Martínez-Mota, L.; Herrera-Pérez, J.J.; Jiménez-Rubio, G. Role of estradiol in the expression of genes involved in serotonin neurotransmission: Implications for female depression. Curr. Neuropharmacol. 2019, 17, 459–471. [Google Scholar] [CrossRef]
- Bethea, C.L.; Phu, K.; Belikova, Y.; Bethea, S.C. Localization and regulation of reproductive steroid receptors in the raphe serotonin system of male macaques. J. Chem. Neuroanat. 2015, 66, 19–27. [Google Scholar] [CrossRef]
- Bertrand, P.P.; Paranavitane, U.T.; Chavez, C.; Gogos, A.; Jones, M.; van den Buuse, M. The effect of low estrogen state on serotonin transporter function in mouse hippocampus: A behavioral and electrochemical study. Brain Res. 2005, 1064, 10–20. [Google Scholar] [CrossRef]
- Fatima-Shad, K. Serotonin-Neurotransmitter and Hormone of Brain, Bowels and Blood: Neurotransmitter and Hormone of Brain, Bowels and Blood. In BoD–Books on Demand; IntechOpen: London, UK, 2024. [Google Scholar]
- Tejeda-Martínez, A.R.; Ramos-Molina, A.R.; Brand-Rubalcava, P.A.; Flores-Soto, M.E. Involvement of serotonergic receptors in depressive processes and their modulation by β-arrestins: A review. Medicine 2024, 103, e38943. [Google Scholar] [CrossRef]
- Paredes, S.; Cantillo, S.; Candido, K.D.; Knezevic, N.N. An association of serotonin with pain disorders and its modulation by estrogens. Int. J. Mol. Sci. 2019, 20, 5729. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, H.; Tang, Z.; Lu, J.; Ni, X. Inflammation and increased IDO in hippocampus contribute to depression-like behavior induced by estrogen deficiency. Behav. Brain Res. 2015, 288, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, X.; Hu, S.; Dai, H.; Zhang, H.; Hang, Y.; Xie, X.; Yang, Y.; Wu, F. Effects of E2 on the IDO1-mediated metabolic KYN pathway in OVX female mice. J. Cell. Mol. Med. 2024, 28, e70179. [Google Scholar] [CrossRef] [PubMed]
- Hases, L.; Archer, A.; Williams, C. ERβ and inflammation. Adv. Exp. Med. Biol. 2022, 1390, 213–225. [Google Scholar] [CrossRef]
- Li, D.; Sun, T.; Tong, Y.; Le, J.; Yao, Q.; Tao, J.; Liu, H.; Jiao, W.; Mei, Y.; Chen, J.; et al. Gut-microbiome-expressed 3β-hydroxysteroid dehydrogenase degrades estradiol and is linked to depression in premenopausal females. Cell Metab. 2023, 35, 685–694. [Google Scholar] [CrossRef]
- Li, D.; Liu, R.; Wang, M.; Peng, R.; Fu, S.; Fu, A.; Le, J.; Yao, Q.; Yuan, T.; Chi, H.; et al. 3β-Hydroxysteroid dehydrogenase expressed by gut microbes degrades testosterone and is linked to depression in males. Cell Host Microbe 2022, 30, 329–339.e5. [Google Scholar] [CrossRef]
- Tao, J.; Dai, W.; Lyu, Y.; Liu, H.; Le, J.; Sun, T.; Yao, Q.; Zhao, Z.; Jiang, X.; Li, Y. Role of intestinal testosterone-degrading bacteria and 3/17β-HSD in the pathogenesis of testosterone deficiency-induced hyperlipidemia in males. NPJ Biofilms Microbiomes 2024, 10, 123. [Google Scholar] [CrossRef]
- Schmidt, N.P.; Molz, P.; Fraga, B.S.; Bondarczuk, N.H.; Silveira, P.D.; Ferri, M.H.; Crestani, T.B.; Breyer, G.M.; Guimarães, G.R.; Motta, A.d.S.d.; et al. Effect of probiotic Lacticaseibacillus rhamnosus LB1.5 on anxiety-like behavior, neuroprotection and neuroinflammation markers of male mice fed a high-fat diet. Nutrients 2024, 16, 879. [Google Scholar] [CrossRef]
- Pei, J.; Zhang, C.; Zhang, Q.; Yu, H.; Yuan, H.; Guo, Y.; Shen, H.; Liu, H.; Wang, C.; Meng, F.; et al. Probiotics Alleviate Chronic Ethanol Exposure-Induced Anxiety-like Behavior and Hippocampal Neuroinflammation in Male Mice through Gut Microbiota-Derived Extracellular Vesicles. J. Nanobiotechnol. 2024, 22, 730. [Google Scholar] [CrossRef]
- Satti, S.; Palepu, M.S.K.; Singh, A.A.; Jaiswal, Y.; Dash, S.P.; Gajula, S.N.R.; Chaganti, S.; Samanthula, G.; Sonti, R.; Dandekar, M.P. Anxiolytic- and Antidepressant-Like Effects of Bacillus Coagulans Unique IS-2 Mediate via Reshaping of Microbiome Gut-Brain Axis in Rats. Neurochem. Int. 2023, 163, 105483. [Google Scholar] [CrossRef]
- Ye, M.; Ji, F.; Huang, C.; Li, F.; Zhang, C.; Zhang, Y.; Wang, R.; Ma, K.; Lu, X.; Wang, H. A Novel Probiotic Formula, BLLL, Ameliorates Chronic Stress-Induced Depression-like Behaviors in Mice by Reducing Neuroinflammation and Increasing Neurotrophic Factors. Front. Pharmacol. 2024, 15, 1398292. [Google Scholar] [CrossRef]
- Hayley, S.; Scharf, J.; Anisman, H. Central Administration of Murine Interferon-α Induces Depressive-like Behavioral, Brain Cytokine and Neurochemical Alterations in Mice: A Mini-Review and Original Experiments. Brain Behav. Immun. 2013, 31, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.L.; Liu, Y.N.; Liu, L.; Wang, X.; Jiang, C.L.; Wang, Y.X. Inducible Nitric Oxide Synthase Is Involved in the Modulation of Depressive Behaviors Induced by Unpredictable Chronic Mild Stress. J. Neuroinflamm. 2012, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.N.S.; Medeiros, I.d.S.; Vasconcelos, G.S.; de Aquino, G.A.; Filho, F.M.S.C.; Cysne, J.C.d.A.; Macêdo, D.S.; Vasconcelos, S.M.M. Involvement of Oxidative Pathways and BDNF in the Antidepressant Effect of Carvedilol in a Depression Model Induced by Chronic Unpredictable Stress. Psychopharmacology 2022, 239, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Niu, X.; Tang, C.; Wang, H.; Gao, J.; Hu, J. Probiotics Alleviate Depressive Behavior in Chronic Unpredictable Mild Stress Rat Models by Remodeling Intestinal Flora. Neuroreport 2021, 32, 686–693. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of Regulating Gut Microbiota on the Serotonin Metabolism in the Chronic Unpredictable Mild Stress Rat Model. Neurogastroenterol. Motil. 2019, 31, e13677. [Google Scholar] [CrossRef]
- Kochalska, K.; Oakden, W.; Słowik, T.; Chudzik, A.; Pankowska, A.; Łazorczyk, A.; Kozioł, P.; Andres-Mach, M.; Pietura, R.; Rola, R.; et al. Dietary Supplementation with Lactobacillus Rhamnosus JB-1 Restores Brain Neurochemical Balance and Mitigates the Progression of Mood Disorder in a Rat Model of Chronic Unpredictable Mild Stress. Nutr. Res. 2020, 82, 44–57. [Google Scholar] [CrossRef]
- Gu, F.; Wu, Y.; Liu, Y.; Dou, M.; Jiang, Y.; Liang, H. Lactobacillus Casei Improves Depression-like Behavior in Chronic Unpredictable Mild Stress-Induced Rats by the BDNF-TrkB Signal Pathway and the Intestinal Microbiota. Food Funct. 2020, 11, 6148–6157. [Google Scholar] [CrossRef]
- Karen, C.; Shyu, D.J.H.; Rajan, K.E. Lactobacillus Paracasei Supplementation Prevents Early Life Stress-Induced Anxiety and Depressive-Like Behavior in Maternal Separation Model—Possible Involvement of Microbiota-Gut-Brain Axis in Differential Regulation of MicroRNA124a/132 and Glutamate Receptors. Front. Neurosci. 2021, 15, 719933. [Google Scholar] [CrossRef]
- Acharya, K.D.; Graham, M.; Raman, H.; Parakoyi, A.E.R.; Corcoran, A.; Belete, M.; Ramaswamy, B.; Koul, S.; Sachar, I.; Derendorf, K.; et al. Estradiol-Mediated Protection against High-Fat Diet Induced Anxiety and Obesity Is Associated with Changes in the Gut Microbiota in Female Mice. Sci. Rep. 2023, 13, 4776. [Google Scholar] [CrossRef]
- Huang, F.; Liu, X.; Xu, S.; Hu, S.; Wang, S.; Shi, D.; Wang, K.; Wang, Z.; Lin, Q.; Li, S.; et al. Prevotella Histicola Mitigated Estrogen Deficiency-Induced Depression via Gut Microbiota-Dependent Modulation of Inflammation in Ovariectomized Mice. Front. Nutr. 2022, 8, 805465. [Google Scholar] [CrossRef] [PubMed]
- Mesripour, A.; Meshkati, A.; Hajhashemi, V. A Synbiotic Mixture Augmented the Efficacy of Doxepin, Venlafaxine, and Fluvoxamine in a Mouse Model of Depression. Turk. J. Pharm. Sci. 2020, 17, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.F.Q.; Guimarães, I.C.; González, L.N.; Cuasnicu, P.S.; Cohen, D.J.; De Grava Kempinas, W. Could Probiotics Be Used as a Novel Therapeutic Approach to Alleviate the Reproductive and Neurobehavioral Side Effects of Sertraline? A Study in Male Mice. Reprod. Toxicol. 2025, 131, 108755. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, T.; Shen, H.; Jiang, Y.; Yang, Q.; Su, S.; Wu, L.; Fan, X.; Gao, M.; Wu, Y.; et al. Mixed Probiotics Modulated Gut Microbiota to Improve Spermatogenesis in Bisphenol A-Exposed Male Mice. Ecotoxicol. Environ. Saf. 2024, 270, 115922. [Google Scholar] [CrossRef]
- Di Benedetto, M.G.; Landi, P.; Mencacci, C.; Cattaneo, A. Depression in Women: Potential Biological and Sociocultural Factors Driving the Sex Effect. Neuropsychobiology 2024, 83, 2–16. [Google Scholar] [CrossRef]
- Hulubă, I.P.; Crecan-Suciu, B.D.; Păunescu, R.; Micluția, I.V. The link between sex hormones and depression over a woman’s lifespan. Biomed. Rep. 2025, 22, 71. [Google Scholar] [CrossRef]
- Oroojzadeh, P.; Bostanabad, S.Y.; Lotfi, H. Psychobiotics: The influence of gut microbiota on the gut-brain axis in neurological disorders. J. Mol. Neurosci. 2022, 72, 1952–1964. [Google Scholar] [CrossRef]
- Cagnacci, A.; Venier, M. The controversial history of hormone replacement therapy. Medicina 2019, 55, 602. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Chen, H.-Y.; Ho, C.-H.; Huang, W.-C. Impact of probiotic supplementation and high-intensity interval training on primary dysmenorrhea: A double-blind, randomized controlled trial investigating inflammation and hormonal modulation. Nutrients 2025, 17, 622. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Yasui, T.; Kuwano, Y.; Rokutan, K. Daily intake of Lactobacillus gasseri CP2305 ameliorates psychological premenstrual symptoms in young women: A randomized, double-blinded, placebo-controlled study. J. Funct. Foods 2021, 80, 104426. [Google Scholar] [CrossRef]
- Bjelica, A.; Cetkovic, N.; Trninic-Pjevic, A.; Mladenovic-Segedi, L. The phenomenon of pregnancy—A psychological view. Ginekol. Pol. 2018, 89, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, Z.; Pourasghar, M.; Khalilian, A.; Salehi, F. A review of the effects of anxiety during pregnancy on children’s health. Mater. Socio-Med. 2015, 27, 200–202. [Google Scholar] [CrossRef]
- Hakanen, H.; Flykt, M.; Sinervä, E.; Nolvi, S.; Kataja, E.L.; Pelto, J.; Korja, R. How maternal pre- and postnatal symptoms of depression and anxiety affect early mother–infant interaction? J. Affect. Disord. 2019, 257, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Caparros-Gonzalez, R.A.; Romero-Gonzalez, B.; Strivens-Vilchez, H.; Gonzalez-Perez, R.; Martinez-Augustin, O.; Peralta-Ramirez, M.I. Hair cortisol levels, psychological stress and psychopathological symptoms as predictors of postpartum depression. PLoS ONE 2017, 12, e0182817. [Google Scholar] [CrossRef] [PubMed]
- Dawe, J.P.; McCowan, L.M.E.; Wilson, J.; Okesene-Gafa, K.A.M.; Serlachius, A.S. Probiotics and maternal mental health: A randomised controlled trial among pregnant women with obesity. Sci. Rep. 2020, 10, 1291. [Google Scholar] [CrossRef]
- Fries, L.R.; Boehme, M.; Lavalle, L.; Sakwinska, O.; Chughlay, F.; Keddani, S.; Chan, S.Y. The impact of ingestion of Bifidobacterium longum NCC3001 on perinatal anxiety and depressive symptoms: A randomized controlled trial. Sci. Rep. 2025, 15, 11250. [Google Scholar] [CrossRef]
- Ertmann, R.K.; Lyngsøe, B.K.; Nicolaisdottir, D.R.; Kragstrup, J.; Siersma, V. Mental vulnerability before and depressive symptoms during pregnancy and postpartum: A prospective population-based cohort study from general practice. Nord. J. Psychiatry 2021, 76, 243–249. [Google Scholar] [CrossRef]
- Barbu, R.M.; Gavrilescu, C.M.; Cojocaru, E.; Popescu, R.I.; Ababei, D.; Bild, W. Hormonal effects of estrogen and progesterone in postpartum depression. Bull. Integr. Psychiatry 2020, 26, 87–95. [Google Scholar] [CrossRef]
- Vicariotto, F.; Malfa, P.; Torricelli, M.; Lungaro, L.; Caio, G.; De Leo, V. Beneficial effects of Limosilactobacillus reuteri PBS072 and Bifidobacterium breve BB077 on mood imbalance, self-confidence, and breastfeeding in women during the first trimester postpartum. Nutrients 2023, 15, 3513. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef]
- Kulkarni, J.; Gurvich, C.; Mu, E.; Molloy, G.; Lovell, S.; Mansberg, G.; Szoeke, C. Menopause depression: Under recognised and poorly treated. Aust. N. Z. J. Psychiatry 2024, 58, 636–640. [Google Scholar] [CrossRef]
- Sawada, D.; Sugawara, T.; Hirota, T.; Nakamura, Y. Effects of Lactobacillus gasseri CP2305 on mild menopausal symptoms in middle-aged women. Nutrients 2022, 14, 1695. [Google Scholar] [CrossRef]
- Shafie, M.; Rad, A.H.; Mohammad-Alizadeh-Charandabi, S.; Mirghafourvand, M. The effect of probiotics on mood and sleep quality in postmenopausal women: A triple-blind randomized controlled trial. Clin. Nutr. ESPEN 2022, 50, 15–23. [Google Scholar] [CrossRef]
- Hashemi-Mohammadabad, N.; Taghavi, S.A.; Lambert, N.; Moshtaghi, R.; Bazarganipour, F.; Sharifi, M. Adjuvant Administration of Probiotic Effects on Sexual Function in Depressant Women Undergoing SSRIs Treatment: A Double-Blinded Randomized Controlled Trial. BMC Psychiatry 2024, 24, 44. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Jamilian, M.; Bahmani, F.; Asemi, Z. Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J. Ovarian Res. 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Accettulli, A.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Campaniello, D.; Racioppo, A.; Altieri, C.; Bevilacqua, A. Psycho-Microbiology, a New Frontier for Probiotics: An Exploratory Overview. Microorganisms 2022, 10, 2141. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Front. Cell. Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a Translational Psychobiotic: Modulation of Stress, Electrophysiology and Neurocognition in Healthy Volunteers. Transl. Psychiatry 2016, 6, e93. [Google Scholar] [CrossRef]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W. Prebiotic Intake Reduces the Waking Cortisol Response and Alters Emotional Bias in Healthy Volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef]
- Yun, T.J.; Kim, Y.; Lee, J.J.; Park, J.Y.; Kim, J.H. Protective effects of probiotics against menopausal symptoms in ovariectomized mice. Food Biosci. 2024, 61, 104611. [Google Scholar] [CrossRef]
- Honda, S.; Tominaga, Y.; Espadaler-Mazo, J.; Huedo, P.; Aguiló, M.; Perez, M.; Ueda, T.; Sawashita, J. Supplementation with a probiotic formula having β-glucuronidase activity modulates serum estrogen levels in healthy peri- and postmenopausal women. J. Med. Food 2024, 27, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Basnet, J.; Eissa, M.A.; Yanes Cardozo, L.L.; Romero, D.G.; Rezq, S. Impact of probiotics and prebiotics on gut microbiome and hormonal regulation. Gidisord 2024, 6, 801–815. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health effects and sources of prebiotic dietary fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef]
- Schaub, A.-C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: A randomized controlled trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, L.; Cheng, Y.; Lei, W.; Wang, Y.; Liu, X.; Zheng, N.; Shao, L.; Chen, X.; Sun, Y.; et al. Probiotics for the treatment of depression and its comorbidities: A systemic review. Front. Cell. Infect. Microbiol. 2023, 13, 1167116. [Google Scholar] [CrossRef]
- Szydłowska, I.; Marciniak, A.; Brodowska, A.; Loj, B.; Ciećwież, S.; Skonieczna-Żydecka, K.; Palmas, J.; Loniewski, I.; Stachowska, E. Effects of probiotics supplementation on the hormone and body mass index in perimenopausal and postmenopausal women using the standardized diet: A 5-week double-blind, placebo-controlled, randomized clinical study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6022–6032. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, B.; Wang, S.; Qian, X.; Li, X.; Zhao, J.; Wang, G. Modulation of the gut microbiota structure with probiotics and isoflavone alleviates metabolic disorder in ovariectomized mice. Nutrients 2021, 13, 1793. [Google Scholar] [CrossRef]
- Simpson, E.R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef]
- Merlotti, D.; Gennari, L.; Stolakis, K.; Nuti, R. Aromatase activity and bone loss in men. J. Osteoporos. 2011, 2011, 230671. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, P.; Thaiss, C.A. The microbiome–adipose tissue axis in systemic metabolism. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G717–G724. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef]
- Ergang, P.; Vagnerová, K.; Hermanová, P.; Vodička, M.; Jágr, M.; Šrůtková, D.; Pácha, J. The gut microbiota affects corticosterone production in the murine small intestine. Int. J. Mol. Sci. 2021, 22, 4229. [Google Scholar] [CrossRef]
| Probiotic | Experimental Subject | Dosage and Treatment Time | Experimental Model | Effect on Anxiety and Depression | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Lacticaseibacillus rhamnosus LB1.5 | Male mice C57BL/6. | 3.1 × 108 CFU/mL three times a week for 13 weeks. | Mice on a high-fat diet Light–dark test. | ↓ Anxiety (↑ distance and time in illuminated compartment in light–dark test). | ↓ IL-6, neuroprotection, reduction in neuroinflammation. | [173]. |
| Limosilactobacillus reuteri | Male mice C57BL/6. | 2.0 × 109 CFU/0.1 mL daily for 9 weeks. | Mice with sleep deprivation and water restriction Elevated plus-maze Y maze test. | ↓ Anxiety (↑ distance and time in open arms in elevated plus-maze and Y maze test). | ↓ Cortisone and CRH; positive modulation of the HPA axis; ↓ visceral hypersensitivity. | [60]. |
| Mixture: Bifidobacterium, Lactobacillus acidophilus, L. rhamnosus, Bifodan A/S | Male mice C57BL/6N. | 1 × 109 CFU/mL, 200 µL/daily for 30 days. | Mice with chronic exposure to ethanol Elevated plus-maze Open field test. | ↓ Anxiety (reversal of effects on elevated plus-maze and open field test). | ↓ NLRP3, NF-κB, IL-1β in hippocampus; reduction in neuroinflammation via extracellular vesicles. | [174]. |
| Bacillus coagulans Unique IS-2 | Female and male Sprague Dawley rats. | 2 × 109 CFU daily for 6 weeks. | Rats with maternal separation + CUMS. Elevated plus-maze Forced swim test Sugar water consumption test. | ↓ Anxiety and depression (elevated plus-maze; forced swim test; sugar water consumption test). Effects independent of sex. | ↑ Hippocampal BDNF; ↓ TNF-α, CRP, IL-1β; ↑ L-tryptophan; sex-dependent effects on monoamines: ♂ ↑ DA reversal; ♀ ↑↑ DA and 5-HT (double effect). | [175]. |
| Mixture: Bifidobacterium breve (25%), Lactobacillus plantarum (25%), L. paracasei (25%), L. helveticus (25%) | Male mice C57BL/6J. | 2, 4 and 8 × 109 CFU daily for 10 days. | Mice with chronic unpredictable stress (CUS) Tail suspension test Forced swim test Open field test Sucrose preference test. | ↓ Depression (↑ sucrose preference; ↓ tail suspension immobility and forced swimming). | ↓ IL-1β, IL-6, TNF-α; ↑ IL-4, IL-10, Ym-1; ↓ nitroxidative stress (NO, MDA); ↑ GSH; ↑ BDNF in hippocampus and medial prefrontal cortex. | [176]. |
| Mixture of probiotics | Male Sprague Dawley rats. | Unspecified dose. | Rats subjected to CUMS Forced swim test Open field test. | ↓ Depression (reversal of effects in forced swimming and open field test). | ↑ Serotonin and norepinephrine in the hippocampus; ↓ ACTH and CORT in plasma; ↑ Lactobacillus and Bifidobacterium; ↓ E. coli and E. faecalis. | [180]. |
| Bifidobacterium longum and L. rhamnosus | Male Wistar rats. | 1 × 109 CFU per 100 g weight for 4 weeks. | Rats subjected to CUMS Forced swim test Sucrose preference test. | ↓ Depression (better performance in forced swimming; ↑ sucrose preference). | ↓ 5-HT in the colon; ↑ 5-HT in the hippocampus and prefrontal cortex (redistribution associated with antidepressant effect). | [181]. |
| Lactobacillus rhamnosus JB-1 | Male mice BALB/c. | 5 × 109 per ml for 28 days. | Mice subjected to Open field test Stress-induced hyperthermia Elevated plus maze Fear conditioning Forced swim test. | ↓ Anxiety and depression (elevated plus-maze; forced swim test. | ↓ Corticosterone; modulation of central GABA receptors via the vagus nerve. | [15]. |
| Lactobacillus rhamnosus JB-1 | Male Wistar rats | 0.2 mL of bacteria suspension (~1.7 × 109 CFU) daily for 4 weeks. | Rats subjected to CUMS Elevated plus maze. | Restoration of neurochemical brain balance. | ↑ GSH, glutamate and norepinephrine transporters. | [182]. |
| Lactobacillus casei | Male Sprague Dawley rats | 8 × 108 CFU kg−1 day−1 for 3 weeks. | Rats were treated with CUMS once a day and lasted for 7 weeks Open field test Forced swim test Sucrose preference test. | ↓ Depression. | ↑ NE, 5-HT, DA, BDNF, and BDNF-TrkB signaling pathway. | [183]. |
| Lactobacillus paracasei HT6 | Pregnant Wistar rats. | 1 × 109 CFU daily for 14 days, from PND-2 to PND-16. | Maternal separation model (early stress) Open field test Tail suspension test. | ↓ Anxiety and depression. | Normalization of ACTH, CORT, GR, 5-HT, NE, DA; regulation of microRNA124a/132 and glutamate receptors. | [184]. |
| Prevotella histicola | Female C57BL/6 Mice. | 0.2 mL of a P. histicola suspension with a concentration of 1 × 109 CFU/mL for 4 weeks. | Ovariectomized mice (estradiol deficiency) Open field test Elevated plus-maze Forced swim test Tail suspension test. | ↓ Anxiety (open field, raised arms maze) and depression (tail suspension test and forced swim test). | Reversal of neuronal damage and inflammation in the CA3 region of the hippocampus; ↑↑ serotonergic, dopaminergic, and BDNF receptors (promoting neuronal proliferation); reversal of ovariectomy-induced dysbiosis. | [186]. |
| Mixture: L. casei, L. acidophilus, L. rhamnosus, L. bulgaricus, Bifidobacterium breve, B. infantis, Streptococcus thermophilus + FOS | Male Swiss mice | 6.25, 12.5, and 25 × 106 CFU for 7–14 days. | Swiss mice, depression model Marble-burying test Forced swim test. | Potentiation of antidepressant effects: Venlafaxine (SNRI): synergistic effect from day 7 in FST and MBT; Doxepin (TCA): synergistic effect from day 7 in FST; Fluvoxamine (SSRI): synergistic effect only after 14 days in FST | Modulation of serotonergic (Marble burying test) and noradrenergic (forced swim test) systems; restoration of monoamine levels in brain regions ↑ Tryptophan attenuation of proinflammatory response; mediated by vagus nerve; more potent synergistic effect with SNRI (venlafaxine) | [187]. |
| Lactobacillus rhamnosus | Male mice (C57BL/6 × BALB/c). | 1 × 109 CFU daily for 30 days. | Mice treated with sertraline (20 mg/kg) | Reversal of sertraline side effects: Prevention of ↓ body weight; Prevention of ↓ seminal vesicle weight; ↓ number of embryonic resorptions; improvement in exploratory profile; ↓ sertraline-induced anxiety | Protection against adverse effects: ↑ sperm quality; improved natural fertility; regulation of testosterone levels (spermatogenesis); modulation of intestinal microbiota; first demonstration of probiotic alleviating side effects of SSRIs | [188]. |
| Reference | Population (Age, Condition) | Intervention/Probiotic | Duration | Outcomes Evaluated | Main Findings | Biomarker Assessment | Study Status |
|---|---|---|---|---|---|---|---|
| [194] | 65 women (18–40 years | B. longum subsp. longum OLP-01, L. plantarum PL-02, L. lactis LY-66 | 10 weeks | Premenstrual symptoms: emotional (anger, depression, crying, anxiety), fatigue, social limitations | Significant improvement in overall premenstrual symptoms, particularly emotional, fatigue, and social domains vs. placebo | Not assessed | Completed |
| [195] | Young women, 20–35 years | L. gasseri CP2305 tablets, daily | 183 days | Anxiety, depression, fatigue | Reduction in anxiety, depression, and fatigue; improvement | Estrogen and progesterone increased during luteal phase | Completed |
| [200] | Pregnant women, 36 weeks gestation | L. rhamnosus GG + B. lactis BB12, capsules, daily | 36 weeks | Depression, anxiety, functional health, general well-being | No significant improvements observed, possible insufficient dose and floor effect | Not assessed | Completed |
| [201] | Pregnant women, third trimester (≥21 years) | Bifidobacterium longum NCC3001 | Third trimester of pregnancy | Anxiety, depression symptoms | No significant effects on anxiety or depression; evidence limited and inconclusive | Not assessed | Completed |
| [204] | Healthy pregnant women, 18–50 years | Limosilactobacillus reuteri PBS072 + Bifidobacterium breve BB077 | 90 days | Anxiety and depression symptoms | Reduction in anxiety and depression | Not assessed | Completed |
| [207] | Japanese premenopausal women, 40–60 years | Lactobacillus gasseri CP2305 tablets, once daily | Six menstrual cycles | Depressive symptoms, insomnia, dizziness, hot flushes | Alleviation of depressive symptoms, insomnia, dizziness, and hot flushes | Serum estrogen measured, no conclusive results | Completed |
| [208] | Postmenopausal women, 45–55 years | 100 g yogurt containing L. bulgaricus, S. thermophilus, B. lactis, L. acidophilus, daily | 6 weeks | Anxiety, stress, depressive symptoms, sleep quality | Reduction in anxiety and stress; no significant effects on depressive symptoms or sleep quality | Not assessed | Completed |
| [209] | Women with major depressive disorder, on antidepressant treatment | Oral capsules containing L. acidophilus, B. bifidum, L. reuteri, L. fermentum, daily | 2 months | Depression scores, sexual function, sexual satisfaction | Reduction in depression scores; improvement in sexual function and satisfaction | Not assessed | Completed |
| [211] | Women with PCOS, 18–40 years | Co-administration of vitamin D + probiotic supplements (L. acidophilus, B. bifidum, L. reuteri, L. fermentum), daily | 12 weeks | Anxiety and depression symptoms | Significant improvement in anxiety and depression | CRP levels reduced | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Sánchez, G.U.; Germán-Ponciano, L.J.; Rodríguez-Landa, J.F.; Bonilla-Jaime, H.; Limón-Morales, O.; García-Ríos, R.I.; Muñoz-Carrillo, J.L.; Gutiérrez-Coronado, O.; Villalobos-Gutiérrez, P.T.; Soria-Fregozo, C. Estrogenic Effect of Probiotics on Anxiety and Depression: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 9948. https://doi.org/10.3390/ijms26209948
Rosas-Sánchez GU, Germán-Ponciano LJ, Rodríguez-Landa JF, Bonilla-Jaime H, Limón-Morales O, García-Ríos RI, Muñoz-Carrillo JL, Gutiérrez-Coronado O, Villalobos-Gutiérrez PT, Soria-Fregozo C. Estrogenic Effect of Probiotics on Anxiety and Depression: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(20):9948. https://doi.org/10.3390/ijms26209948
Chicago/Turabian StyleRosas-Sánchez, Gilberto Uriel, León Jesús Germán-Ponciano, Juan Francisco Rodríguez-Landa, Herlinda Bonilla-Jaime, Ofelia Limón-Morales, Rosa Isela García-Ríos, José Luis Muñoz-Carrillo, Oscar Gutiérrez-Coronado, Paola Trinidad Villalobos-Gutiérrez, and César Soria-Fregozo. 2025. "Estrogenic Effect of Probiotics on Anxiety and Depression: A Narrative Review" International Journal of Molecular Sciences 26, no. 20: 9948. https://doi.org/10.3390/ijms26209948
APA StyleRosas-Sánchez, G. U., Germán-Ponciano, L. J., Rodríguez-Landa, J. F., Bonilla-Jaime, H., Limón-Morales, O., García-Ríos, R. I., Muñoz-Carrillo, J. L., Gutiérrez-Coronado, O., Villalobos-Gutiérrez, P. T., & Soria-Fregozo, C. (2025). Estrogenic Effect of Probiotics on Anxiety and Depression: A Narrative Review. International Journal of Molecular Sciences, 26(20), 9948. https://doi.org/10.3390/ijms26209948







