Gout Risk Allele Regulating IRF5 Expression Is Associated with Enhanced IL-1β Production in Response to Palmitate and Monosodium Urate Crystals
Abstract
1. Introduction
2. Results
2.1. Presence of rs4728141 Variant C Is Associated with Limited Changes in Transcription of Cytokine Genes in Unstimulated Primary Human PBMCs
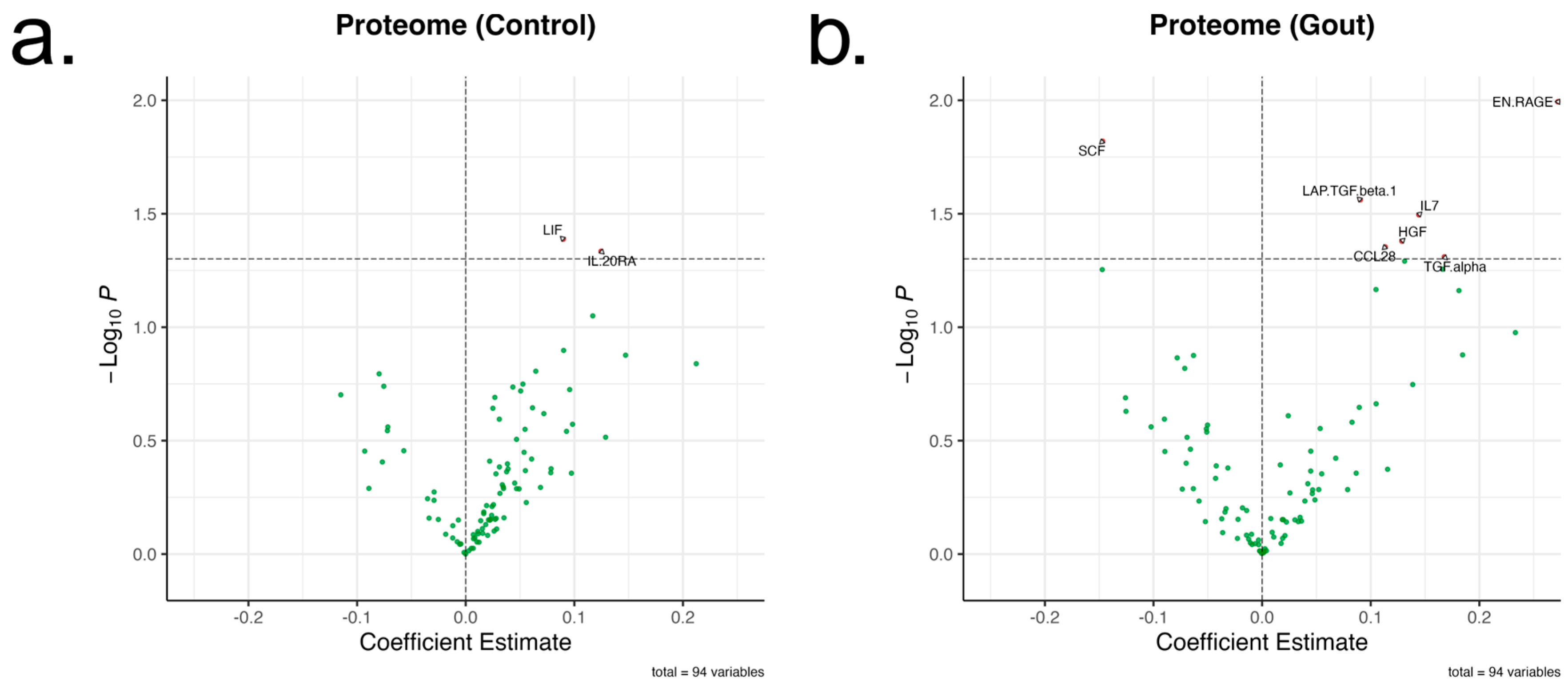
2.2. rs4728141 Is Not Associated with Significant Changes in Serum Cytokines and Chemokines
2.3. Transcriptional Analysis in Relation to rs4728141 in Stimulated PBMCs
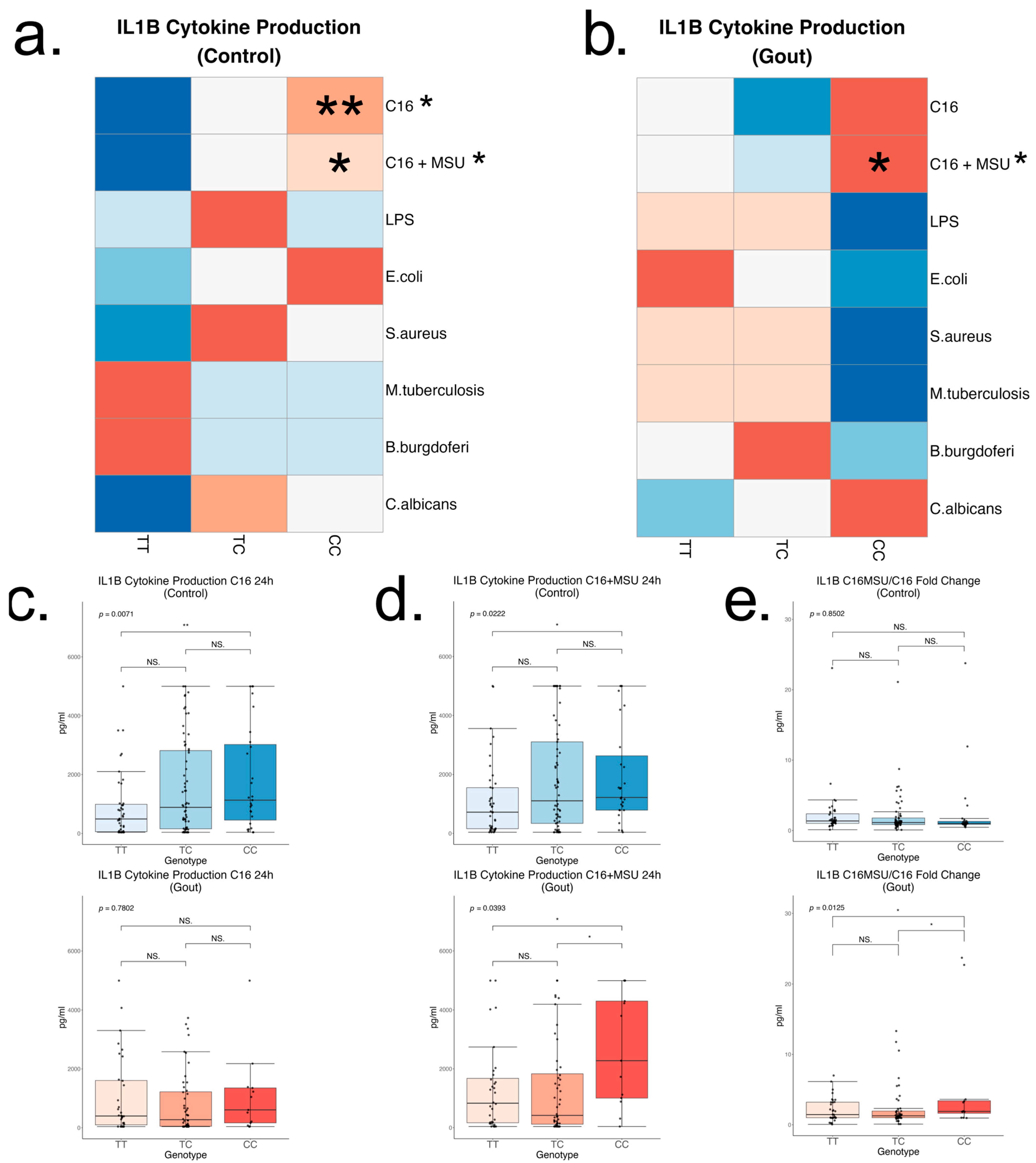
2.4. Rs4728141 Gout Risk Allele C Is Associated with Increased IL-1β Cytokine Release upon Stimulation with Palmitate in Presence or Absence of MSU Crystals
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. PBMC Isolation and Stimulation
4.3. Genotyping
4.4. Transcriptomics
4.5. Proteomics
4.6. Cytokine Measurements
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalbeth, N.; Choi, H.K.; Joosten, L.A.B.; Khanna, P.P.; Matsuo, H.; Perez-Ruiz, F.; Stamp, L.K. Gout. Nat. Rev. Dis. Primers 2019, 5, 69. [Google Scholar] [CrossRef]
- Dalbeth, N.; House, M.E.; Aati, O.; Tan, P.; Franklin, C.; Horne, A.; Gamble, G.D.; Stamp, L.K.; Doyle, A.J.; McQueen, F.M. Urate crystal deposition in asymptomatic hyperuricaemia and symptomatic gout: A dual energy CT study. Ann. Rheum. Dis. 2015, 74, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.L. Genetics of hyperuricemia and gout: Insights from recent genome-wide association studies and Mendelian randomization studies. Tzu Chi Med. J. Wolters Kluwer Medknow Publ. 2022, 34, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) Pathway. Sci. Signal. 2010, 3, cm1. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Londe, A.C.; Fernandez-Ruiz, R.; Julio, P.R.; Appenzeller, S.; Niewold, T.B. Type I Interferons in Autoimmunity: Implications in Clinical Phenotypes and Treatment Response. Journal of Rheumatology. J. Rheumatol. 2023, 50, 1103–1113. [Google Scholar] [CrossRef]
- Crow, M.K.; Olferiev, M.; Kirou, K.A. Type I Interferons in Autoimmune Disease. Annu Rev Pathol Mech Dis 2019, 14, 369–393. [Google Scholar] [CrossRef]
- Eames, H.L.; Corbin, A.L.; Udalova, I.A. Interferon regulatory factor 5 in human autoimmunity and murine models of autoimmune disease. Transl. Res. 2016, 167, 167–182. [Google Scholar] [CrossRef]
- Weiss, M.; Byrne, A.J.; Blazek, K.; Saliba, D.G.; Pease, J.E.; Perocheau, D.; Feldmann, M.; Udalova, I.A. IRF5 controls both acute and chronic inflammation. Proc. Natl. Acad. Sci. USA 2015, 112, 11001–11006. [Google Scholar] [CrossRef]
- Duffau, P.; Menn-Josephy, H.; Cuda, C.M.; Dominguez, S.; Aprahamian, T.R.; Watkins, A.A.; Yasuda, K.; Monach, P.; Lafyatis, R.; Rice, L.M.; et al. Promotion of Inflammatory Arthritis by Interferon Regulatory Factor 5 in a Mouse Model. Arthritis Rheumatol. 2015, 67, 3146–3157. [Google Scholar] [CrossRef]
- Ryzhakov, G.; Eames, H.L.; Udalova, I.A. Activation and Function of Interferon Regulatory Factor 5. J. Interferon Cytokine Res. 2015, 35, 71–78. [Google Scholar] [CrossRef]
- Eames, H.L.; Saliba, D.G.; Krausgruber, T.; Lanfrancotti, A.; Ryzhakov, G.; Udalova, I.A. KAP1/TRIM28: An inhibitor of IRF5 function in inflammatory macrophages. Immunobiology 2012, 217, 1315–1324. [Google Scholar] [CrossRef]
- Khoyratty, T.E.; Udalova, I.A. Diverse mechanisms of IRF5 action in inflammatory responses. Int. J. Biochem. Cell Biol. 2018, 99, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Major, T.J.; Takei, R.; Matsuo, H.; Leask, M.P.; Sumpter, N.A.; Topless, R.K.; Shirai, Y.; Wang, W.; Cadzow, M.J.; Phipps-Green, A.J.; et al. A genome-wide association analysis reveals new pathogenic pathways in gout. Nat. Genet. 2024, 56, 2392–2406, Erratum in Nat. Genet. 2024, 56, 2577. [Google Scholar] [CrossRef] [PubMed]
- GTEx Portal, dbGaP, Accession Number phs000424.vN.pN. Available online: https://www.gtexportal.org/home/snp/rs4728141 (accessed on 18 June 2025).
- Cabău, G.; Gaal, O.; Badii, M.; Nica, V.; Mirea, A.M.; Hotea, I.; Pamfil, C.; Popp, R.A.; Netea, M.G.; Rednic, S.; et al. Hyperuricemia remodels the serum proteome toward a higher inflammatory state. iScience 2023, 26, 107909. [Google Scholar] [CrossRef]
- Bentham, J.; Morris, D.L.; Cunninghame Graham, D.S.; Pinder, C.L.; Tombleson, P.; Behrens, T.W.; Martín, J.; Fairfax, B.P.; Knight, J.C.; Chen, L.; et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 2015, 47, 1457–1464. [Google Scholar] [CrossRef]
- Saevarsdottir, S.; Stefansdottir, L.; Sulem, P.; Thorleifsson, G.; Ferkingstad, E.; Rutsdottir, G.; Glintborg, B.; Westerlind, H.; Grondal, G.; Loft, I.C.; et al. Multiomics analysis of rheumatoid arthritis yields sequence variants that have large effects on risk of the seropositive subset. Ann. Rheum. Dis. 2022, 81, 1085–1095. [Google Scholar] [CrossRef]
- Lessard, C.J.; Li, H.; Adrianto, I.; Ice, J.A.; Rasmussen, A.; Grundahl, K.M.; Kelly, J.A.; Dozmorov, M.G.; Miceli-Richard, C.; Bowman, S.; et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren’s syndrome. Nat. Genet. 2013, 45, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, A.; Yanai, H.; Kondo, S.; Duncan, G.; Negishi, H.; Mizutani, T.; Kano, S.-I.; Honda, K.; Ohba, Y.; Mak, T.W.; et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 2005, 434, 243–249. [Google Scholar] [CrossRef]
- Steinhagen, F.; McFarland, A.P.; Rodriguez, L.G.; Tewary, P.; Jarret, A.; Savan, R.; Klinman, D. IRF-5 and NF-κB p50 co-regulate IFN-β and IL-6 expression in TLR9-stimulated human plasmacytoid dendritic cells. Eur. J. Immunol. 2013, 43, 1896–1906. [Google Scholar] [CrossRef]
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S.; et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.D.; Kellis, M. HaploReg: A resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012, 40, D930–D934. [Google Scholar] [CrossRef] [PubMed]
- ENCSR000ATW. ENCODE Datasets. Available online: https://www.encodeproject.org/experiments/ENCSR000ATW/ (accessed on 18 June 2025).
- Uckelmann, M.; Levina, V.; Taveneau, C.; Ng, X.H.; Pandey, V.; Martinez, J.; Mendiratta, S.; Houx, J.; Boudes, M.; Venugopal, H.; et al. Dynamic PRC1–CBX8 stabilizes a porous structure of chromatin condensates. Nat. Struct. Mol. Biol. 2025, 32, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Zhen, C.Y.; Tatavosian, R.; Huynh, T.N.; Duc, H.N.; Das, R.; Kokotovic, M.; Grimm, J.B.; Lavis, L.D.; Lee, J.; Mejia, F.J.; et al. Live-cell single-molecule tracking reveals co-recognition of H3K27me3 and DNA targets polycomb Cbx7-PRC1 to chromatin. Elife 2016, 5, e17667. [Google Scholar] [CrossRef]
- Badii, M.; Nica, V.; Straton, A.R.; Kischkel, B.; Gaal, O.; Cabău, G.; Klück, V.; Hotea, I.; HINT Consortium; Novakovic, B.; et al. Downregulation of type I interferon signalling pathway by urate in primary human PBMCs. Immunology 2024, 174, 100–112. [Google Scholar] [CrossRef]
- He, Y.; Franchi, L.; Núñez, G. TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J. Immunol. 2013, 190, 334–339. [Google Scholar] [CrossRef]
- Cleophas, M.C.P.; Crişan, T.O.; Lemmers, H.; Toenhake-Dijkstra, H.; Fossati, G.; Jansen, T.L.; Dinarello, C.A.; Netea, M.G.; Joosten, L.A.B. Suppression of monosodium urate crystal-induced cytokine production by butyrate is mediated by the inhibition of class I histone deacetylases. Ann. Rheum. Dis. 2016, 75, 593–600. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Huang, S.; Choi, I.W.; Rutledge, J.C.; Hwang, D.H. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J. Immunol. 2013, 191, 4337–4347. [Google Scholar] [CrossRef]
- Risé, P.; Eligini, S.; Ghezzi, S.; Colli, S.; Galli, C. Fatty acid composition of plasma, blood cells and whole blood: Relevance for the assessment of the fatty acid status in humans. Prostaglandins Leukot Essent Fat. Acids 2007, 76, 363–369. [Google Scholar] [CrossRef]
- Nicholas, D.A.; Zhang, K.; Hung, C.; Glasgow, S.; Aruni, A.W.; Unternaehrer, J.; Payne, K.J.; Langridge, W.H.R.; De Leon, M. Palmitic acid is a toll-like receptor 4 ligand that induces human dendritic cell secretion of IL-1β. PLoS ONE 2017, 12, e0176793. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Langley, K.G.; Berglund, N.A.; Kammoun, H.L.; Reibe, S.; Estevez, E.; Weir, J.; Mellett, N.A.; Pernes, G.; Conway, J.R.W.; et al. Evidence that TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab. 2018, 27, 1096–1110.e5. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, X.; Lu, Z.; Perry, D.M.; Li, Y.; Russo, S.B.; Cowart, L.A.; Hannun, Y.A.; Huang, Y. Acid sphingomyelinase plays a key role in palmitic acid-amplified inflammatory signaling triggered by lipopolysaccharide at low concentrations in macrophages. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E853–E867. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, X.; Deng, B.; Xiao, J.; Jin, J.; Huang, Z. Lipopolysaccharide and palmitic acid synergistically induced MCP-1 production via MAPK-meditated TLR4 signaling pathway in RAW264.7 cells. Lipids Health Dis. 2019, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Orliaguet, L.; Ejlalmanesh, T.; Humbert, A.; Ballaire, R.; Diedisheim, M.; Julla, J.B.; Chokr, D.; Cuenco, J.; Michieletto, J.; Charbit, J.; et al. Early macrophage response to obesity encompasses Interferon Regulatory Factor 5 regulated mitochondrial architecture remodelling. Nat. Commun. 2022, 13, 5089. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Chen, Y.J.; Wei, Y.H.; Lin, H.A.; Chen, C.C.; Liu, T.F.; Hsieh, Y.L.; Huang, K.Y.; Lin, K.H.; Wang, H.H.; et al. Lactic Acid Fermentation Is Required for NLRP3 Inflammasome Activation. Front. Immunol. 2021, 12, 630380. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nica, V.; Gaal, O.; Badii, M.; Cabău, G.; Mirea, A.-M.; Hotea, I.; Pamfil, C.; Rednic, S.; Popp, R.A.; Netea, M.G.; et al. Gout Risk Allele Regulating IRF5 Expression Is Associated with Enhanced IL-1β Production in Response to Palmitate and Monosodium Urate Crystals. Int. J. Mol. Sci. 2025, 26, 9930. https://doi.org/10.3390/ijms26209930
Nica V, Gaal O, Badii M, Cabău G, Mirea A-M, Hotea I, Pamfil C, Rednic S, Popp RA, Netea MG, et al. Gout Risk Allele Regulating IRF5 Expression Is Associated with Enhanced IL-1β Production in Response to Palmitate and Monosodium Urate Crystals. International Journal of Molecular Sciences. 2025; 26(20):9930. https://doi.org/10.3390/ijms26209930
Chicago/Turabian StyleNica, Valentin, Orsolya Gaal, Medeea Badii, Georgiana Cabău, Andreea-Manuela Mirea, Ioana Hotea, Cristina Pamfil, Simona Rednic, Radu A. Popp, Mihai G. Netea, and et al. 2025. "Gout Risk Allele Regulating IRF5 Expression Is Associated with Enhanced IL-1β Production in Response to Palmitate and Monosodium Urate Crystals" International Journal of Molecular Sciences 26, no. 20: 9930. https://doi.org/10.3390/ijms26209930
APA StyleNica, V., Gaal, O., Badii, M., Cabău, G., Mirea, A.-M., Hotea, I., Pamfil, C., Rednic, S., Popp, R. A., Netea, M. G., Crișan, T. O., Joosten, L. A. B., & HINT Consortium. (2025). Gout Risk Allele Regulating IRF5 Expression Is Associated with Enhanced IL-1β Production in Response to Palmitate and Monosodium Urate Crystals. International Journal of Molecular Sciences, 26(20), 9930. https://doi.org/10.3390/ijms26209930




