Nitrogen-Doped Carbon Dots Alleviate Pesticide Toxicity in Tomato by Regulating Antioxidant Systems
Abstract
1. Introduction
2. Results
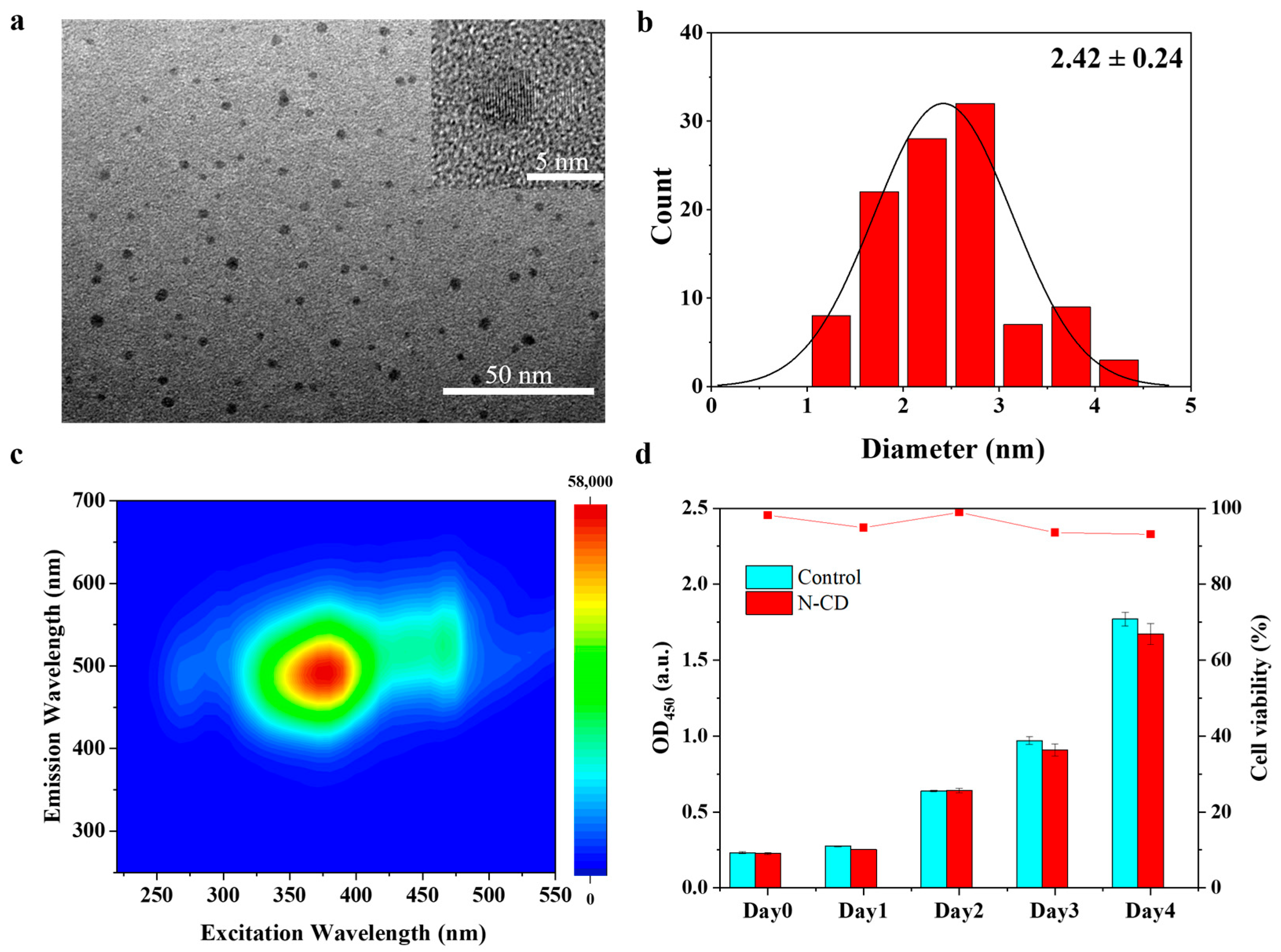
2.1. Synthesis of N-CDs with High Biological Safety
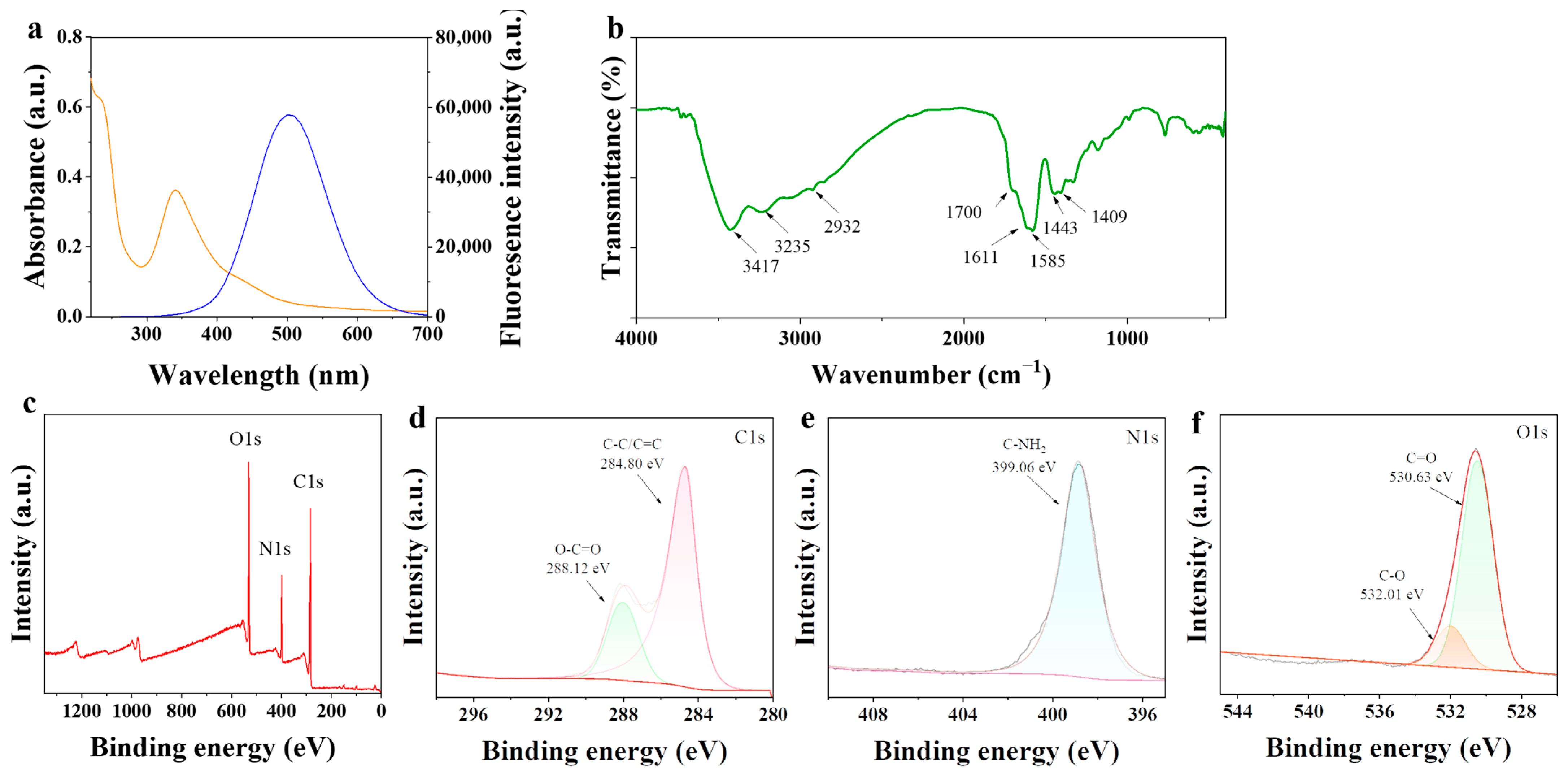
2.2. Analysis of Elemental Groups of N-CDs
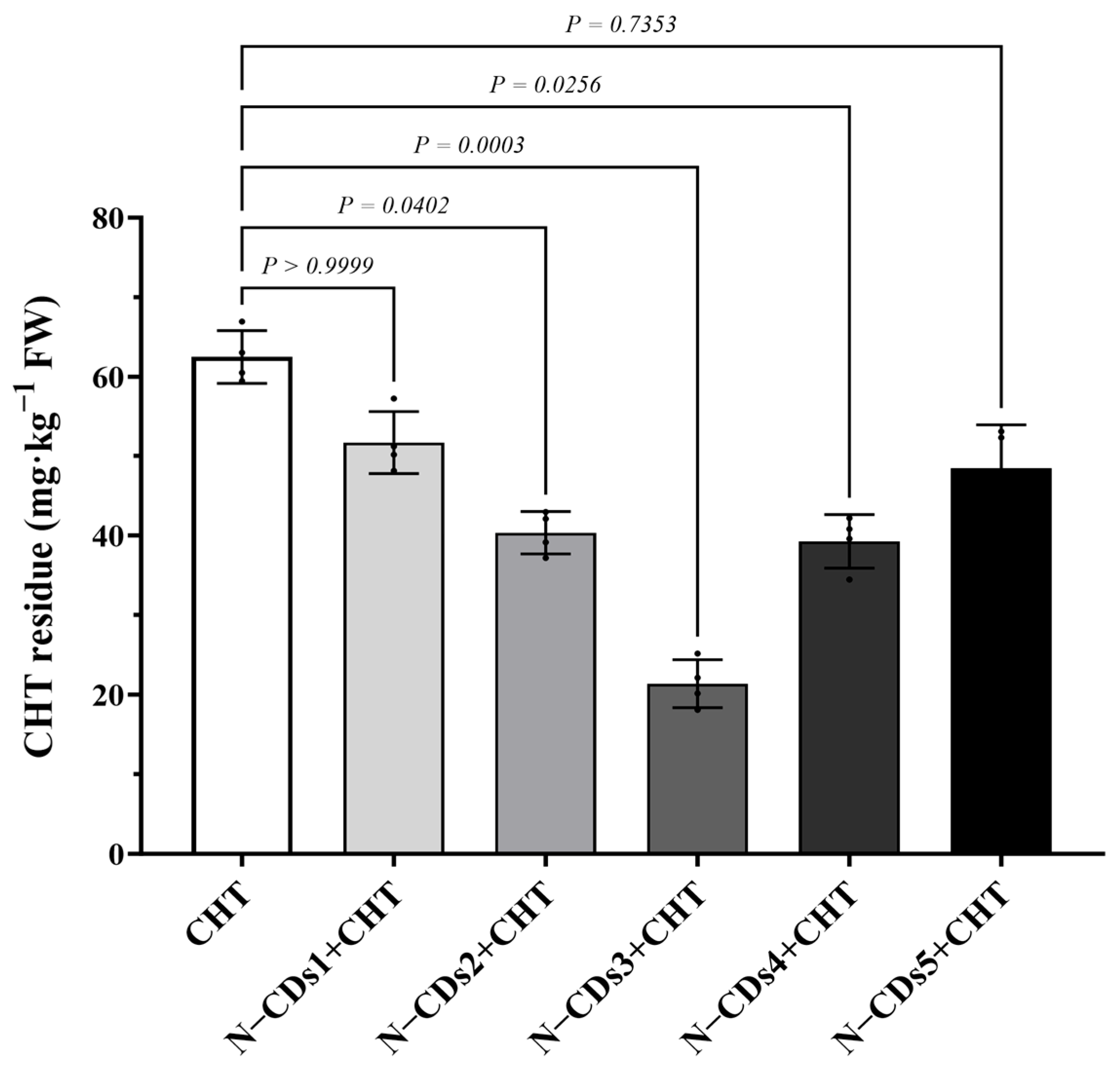
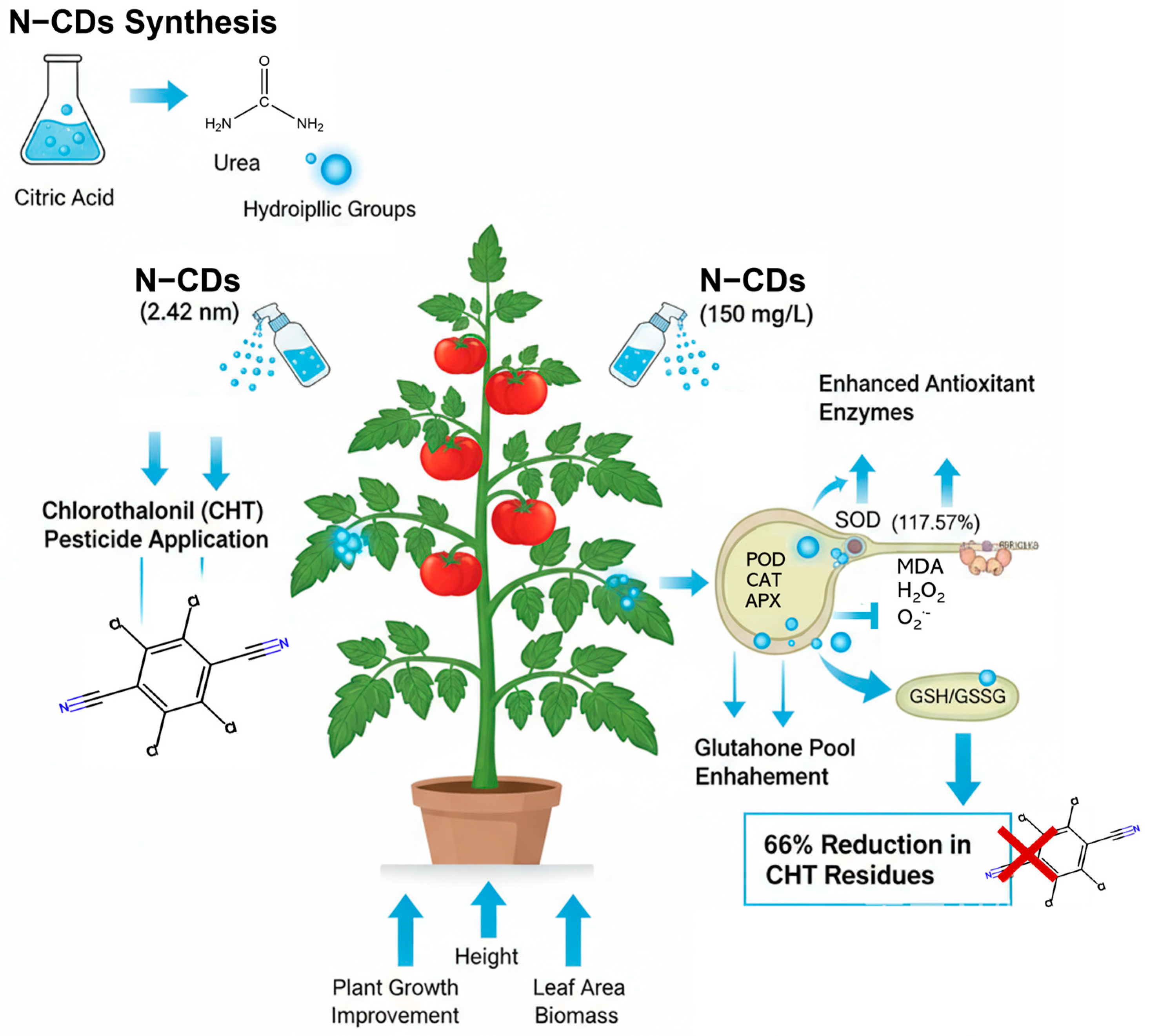
2.3. N-CDs Treatment Decrease CHT Residue in Tomato
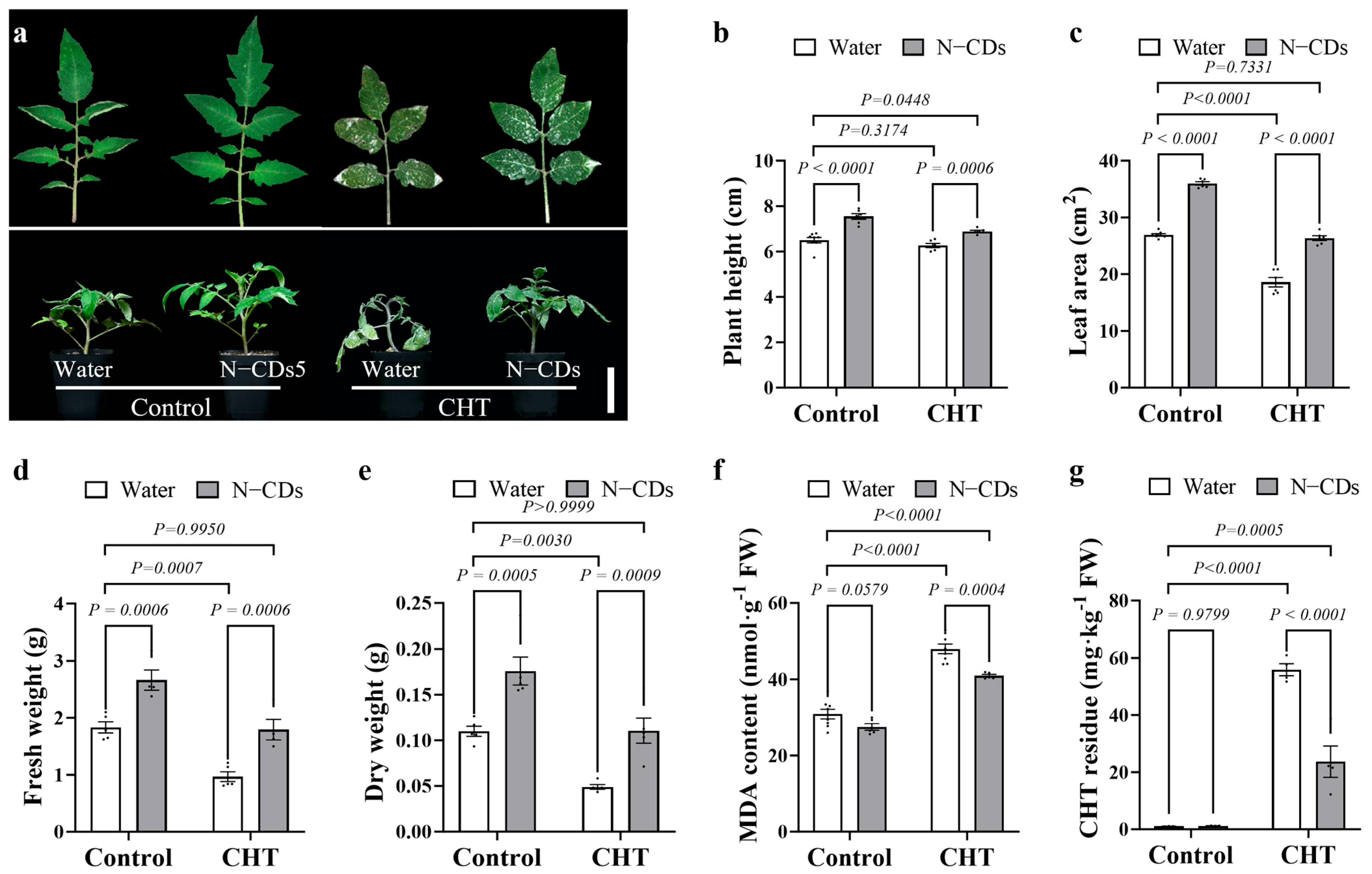
2.4. N-CDs Treatment Alleviated CHT-Induced Toxicity in Tomato
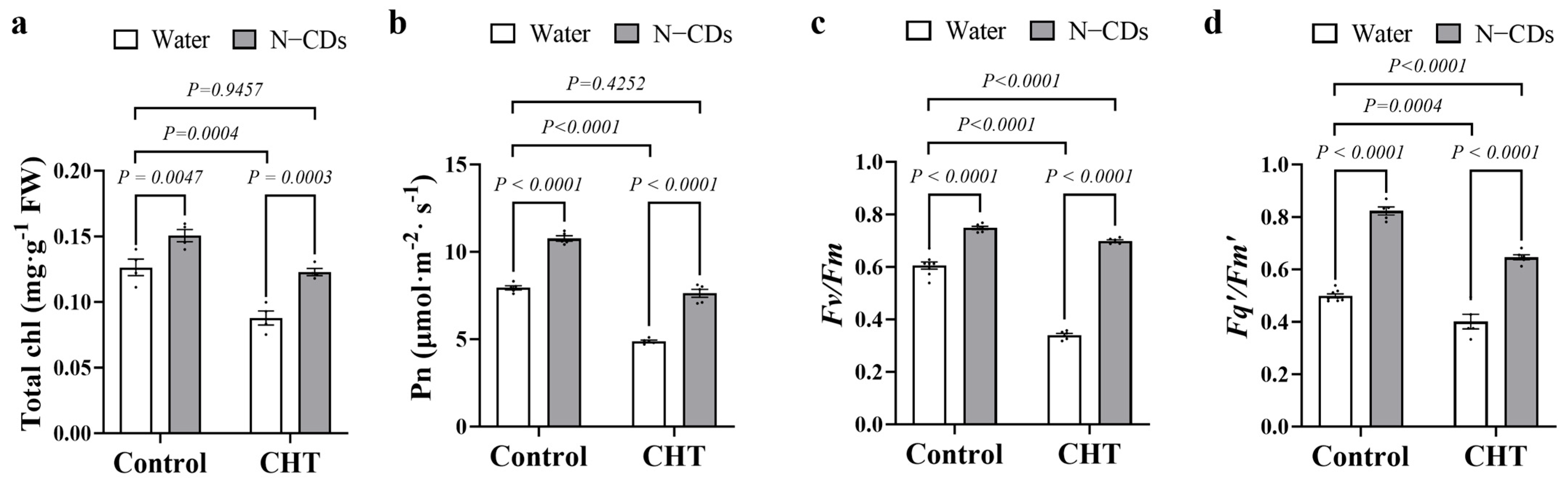
2.5. N-CDs Treatment Alleviated CHT-Induced Depression in Photosynthesis in Tomato
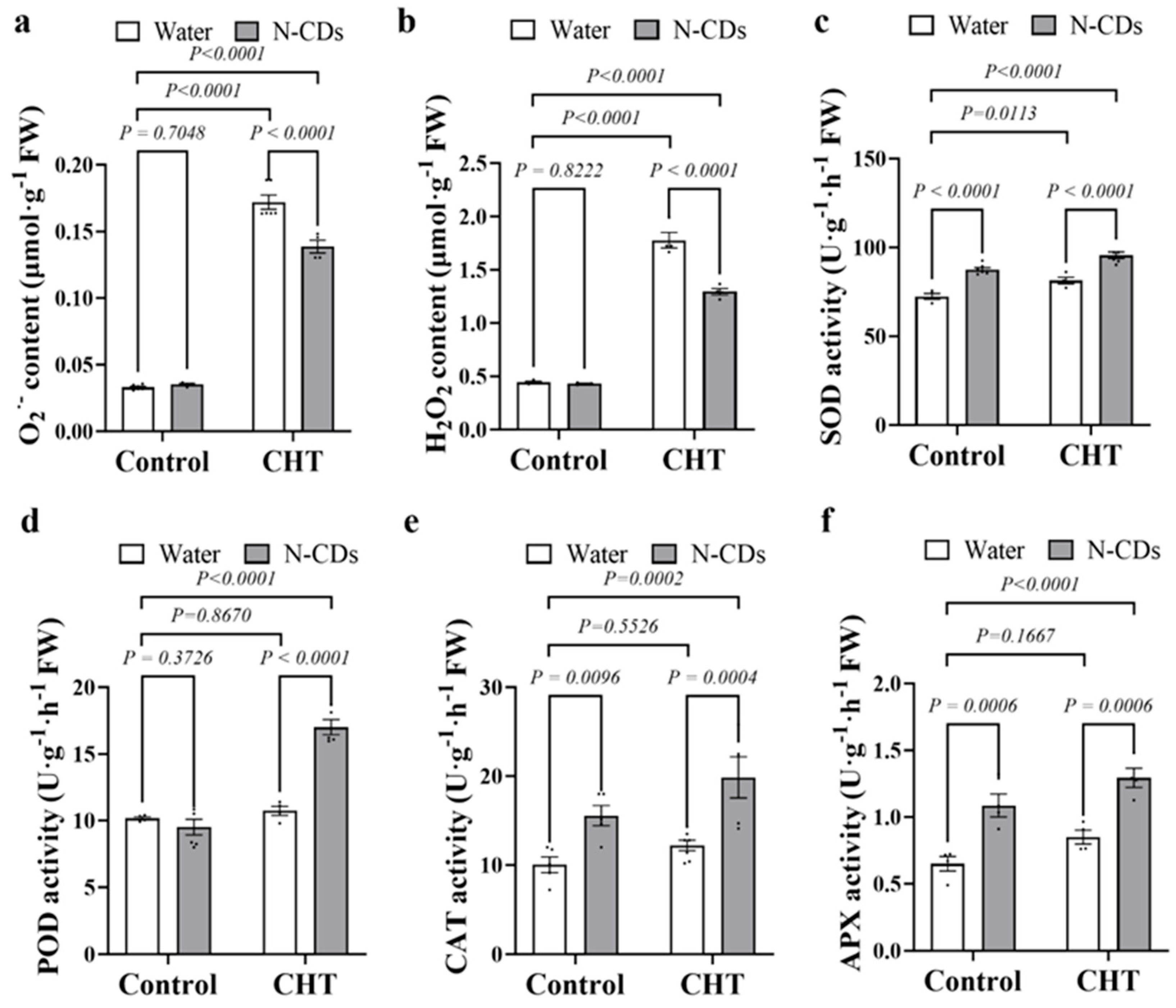
2.6. N-CDs Treatment Repressed ROS Accumulation and Activated the Antioxidant Enzyme Activity in Tomato Under CHT Condition
2.7. N-CDs Treatment Enhance the Detoxification Potential of Tomato
3. Discussion
4. Materials and Methods
4.1. The Synthesis, Characterization, and Cytotoxicity Test of N-CDs
4.2. Plant Materials and Pesticide Treatment
4.3. Growth Related Parameters Analysis
4.4. Photosynthetic Related Parameters Analysis
4.5. ROS Contents and Antioxidant Enzyme Activities Analysis
4.6. Analysis of CHT Content by HPLC
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.M.M.; Rocha, C.P.; Marques, J.C.; Gonçalves, F.J.M. Fatty acids as suitable biomarkers to assess pesticide impacts in freshwater biological scales—A review. Ecol. Indic. 2021, 122, 107299. [Google Scholar] [CrossRef]
- Chung, S.W.C. How effective are common household preparations on removing pesticide residues from fruit and vegetables? A review. J. Sci. Food Agric. 2018, 98, 2857–2870. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wang, Y.Q.; Mao, Q.; Wu, M.J.; Yan, Y.R.; Ren, J.J.; Wang, X.J.; Liu, A.R.; Chen, S.C. Dopamine alleviates bisphenol A-induced phytotoxicity by enhancing antioxidant and detoxification potential in cucumber. Environ. Pollut. 2020, 259, 113957. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, Z.L.; Gong, B.; Shi, Q.H. S-adenosylmethionine synthetase 1 confers drought and salt tolerance in transgenic tomato. Environ. Exp. Bot. 2020, 179, 104226. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Xie, D.L.; Yang, C.; Ahammed, G.J.; Qi, Z.Y.; Hasan, M.K.; Reiter, R.J.; Yu, J.Q.; Zhou, J. Melatonin promotes metabolism of bisphenol A by enhancing glutathione-dependent detoxification in Solanum lycopersicum L. J. Hazard. Mater. 2020, 388, 121727. [Google Scholar] [CrossRef]
- Lu, K.; Song, Y.Y.; Zeng, R.S. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef]
- Vaish, S.; Gupta, D.; Mehrotra, R.; Mehrotra, S.; Basantani, M.K. Glutathione S-transferase: A versatile protein family. 3 Biotech 2020, 10, 321. [Google Scholar] [CrossRef]
- Chen, S.C.; Yan, Y.R.; Wang, Y.Q.; Wu, M.J.; Mao, Q.; Chen, Y.F.; Ren, J.J.; Liu, A.R.; Lin, X.M.; Ahammed, G.J. Trichoderma asperellum reduces phoxim residue in roots by promoting plant detoxification potential in Solanum lycopersicum L. Environ. Pollut. 2020, 259, 113893. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.B.; Zhang, Y.; Ahammed, G.J.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Glutathione biosynthesis and regeneration play an important role in the metabolism of chlorothalonil in tomato. Chemosphere 2013, 90, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Kunert, K. The ascorbate-glutathione cycle coming of age. J. Exp. Bot. 2024, 75, 2682–2699. [Google Scholar] [CrossRef] [PubMed]
- Flohe, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radical Bio. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef]
- Lin, Z.H.; Xu, D.H.; Zhao, Y.M.; Sheng, B.; Wu, Z.J.; Wen, X.B.; Zhou, J.; Chen, G.; Lv, J.; Wang, J.; et al. Micro/Nanoplastics in plantation agricultural products: Behavior process, phytotoxicity under biotic and abiotic stresses, and controlling strategies. J. Nanobiotechnol. 2025, 23, 231. [Google Scholar] [CrossRef]
- Li, Y.D.; Xu, X.K.; Wu, Y.; Zhuang, J.L.; Zhang, X.J.; Zhang, H.R.; Lei, B.F.; Hu, C.F.; Liu, Y.L. A review on the effects of carbon dots in plant systems. Mater. Chem. Front. 2020, 4, 437–448. [Google Scholar] [CrossRef]
- Kou, E.F.; Yao, Y.Y.; Yang, X.; Song, S.W.; Li, W.; Kang, Y.Y.; Qu, S.N.; Dong, R.Y.; Pan, X.Q.; Li, D.N.; et al. Regulation Mechanisms of Carbon Dots in the Development of Lettuce and Tomato. Acs Sustain. Chem. Eng. 2021, 9, 944–953. [Google Scholar] [CrossRef]
- Swift, T.A.; Fagan, D.; Benito-Alifonso, D.; Hill, S.A.; Yallop, M.L.; Oliver, T.A.A.; Lawson, T.; Galan, M.C.; Whitney, H.M. Photosynthesis and crop productivity are enhanced by glucose-functionalised carbon dots. New Phytol. 2021, 229, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Zhong, X.; Pan, Q.; Zhang, Y.; Deng, W.T.; Zou, G.Q.; Hou, H.S.; Ji, X.B. A review of carbon dots in synthesis strategy. Coordin. Chem. Rev. 2024, 498, 215468. [Google Scholar] [CrossRef]
- Li, Y.J.; Tang, Z.H.; Pan, Z.Y.; Wang, R.G.; Wang, X.; Zhao, P.; Liu, M.; Zhu, Y.X.; Liu, C.; Wang, W.C.; et al. Calcium-Mobilizing Properties of-Derived Carbon Dots Confer Enhanced Environmental Adaptability in Plants. Acs Nano 2022, 16, 4357–4370. [Google Scholar] [CrossRef]
- Peng, X.H.; Wang, N.N.; Sun, S.S.; Geng, L.J.H.; Guo, N.; Liu, A.R.; Chen, S.C.; Ahammed, G.J. Reactive oxygen species signaling is involved in melatonin-induced reduction of chlorothalonil residue in tomato leaves. J. Hazard. Mater. 2023, 443, 130212. [Google Scholar] [CrossRef]
- Ghirardello, M.; Ramos-Soriano, J.; Galan, M.C. Carbon Dots as an Emergent Class of Sustainable Antifungal Agents. Acs Nano 2025, 19, 24377–24403. [Google Scholar] [CrossRef]
- Delgado-Martín, J.; Delgado-Olidén, A.; Velasco, L. Carbon Dots Boost dsRNA Delivery in Plants and Increase Local and Systemic siRNA Production. Int. J. Mol. Sci. 2022, 23, 5338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Shen, D.K.; Liu, Q.; Luo, K.H.; Li, C. Mild Acidolysis-Assisted Hydrothermal Carbonization of Lignin for Simultaneous Preparation of Green and Blue Fluorescent Carbon Quantum Dots. Acs Sustain. Chem. Eng. 2022, 10, 9888–9898. [Google Scholar] [CrossRef]
- Sharma, A.; Gadly, T.; Neogy, S.; Ghosh, S.K.; Kumbhakar, M. Molecular Origin and Self-Assembly of Fluorescent Carbon Nanodots in Polar Solvents. J. Phys. Chem. Lett. 2017, 8, 5861–5864, Erratum in J. Phys. Chem. Lett. 2017, 8, 1044–1052. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Swiergosz, T.; Bednarz, S.; Walas, K.; Bashmakova, N.V.; Bogdal, D. Luminescence phenomena of carbon dots derived from citric acid and urea—A molecular insight. Nanoscale 2018, 10, 13889–13894. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.N.; Li, H.L.; Zhang, T.R.; Song, B.K.; Wang, X.H.; Gu, Z.J. Recent Advances in ZnO Nanomaterial-Mediated Biological Applications and Action Mechanisms. Nanomaterials 2023, 13, 1500. [Google Scholar] [CrossRef]
- Leskinen, J.; Tissari, J.; Uski, O.; Viren, A.; Torvela, T.; Kaivosoja, T.; Lamberg, H.; Nuutinen, I.; Kettunen, T.; Joutsensaari, J.; et al. Fine Particle Emissions in three Different Combustion Conditions of a Wood Chip-fired Appliance-Particulate Physico-chemical Properties and Induced Cell Death. Atmos. Environ. 2014, 86, 129–139. [Google Scholar] [CrossRef]
- Mu, L.; Gao, Y.; Hu, X.G. L-Cysteine: A Biocompatible, Breathable and Beneficial Voating for Graphene Oxide. Biomaterials 2015, 52, 301–311. [Google Scholar] [CrossRef]
- Hussain, Z.; Al-Shadidi, J.R.M.H.; Jagal, J.; Rawas-Qalaji, M. Overcoming stealthing effect of PEGylation on chitosan nanoparticles: Optimization, characterization, and assessment of cytotoxicity, apoptosis, cellular internalization, and antimetastatic efficacy. Int. J. Biol. Macromol. 2025, 321, 146514. [Google Scholar] [CrossRef]
- Hu, X.G.; Li, D.D.; Gao, Y.; Mu, L.; Zhou, Q.X. Knowledge gaps between nanotoxicological research and nanomaterial safety. Environ. Int. 2016, 94, 8–23. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.C.; Shi, Q.H.; Ren, Z.H. SIMYB102, an R2R3-type MYB gene, confers salt tolerance in transgenic tomato. Plant Sci. 2020, 291, 110356. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Willems, P.; Mhamdi, A.; Stael, S.; Storme, V.; Kerchev, P.; Noctor, G.; Gevaert, K.; Van Breusegem, F. The ROS Wheel: Refining ROS Transcriptional Footprints. Plant Physiol. 2016, 171, 1720–1733. [Google Scholar] [CrossRef]
- Guirguis, A.; Yang, W.; Conlan, X.A.; Cahill, D.M.; Wang, Y. Boosting Plant Photosynthesis with Carbon Dots: A Critical Review of Performance and Prospects. Small 2023, 19, 202300671. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, X.F.; Li, Y.Y.; Sun, Q.; Dai, L.N.; Li, J.W.; Guo, Z.J.; Zhang, L.; Ci, L.J. Functional carbon nanodots improve soil quality and tomato tolerance in saline-alkali soils. Sci. Total Environ. 2022, 830, 154817. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Liu, M.H.; Li, R.; Shi, Q.H.; Li, Y.; Gong, B. γ-Aminobutyric acid (GABA) improves pesticide detoxification in plants. Sci. Total Environ. 2022, 835, 155404. [Google Scholar] [CrossRef]
- Ru, Y.; Waterhouse, G.I.N.; Lu, S.Y. Aggregation in carbon dots. Aggregate 2022, 3, e296. [Google Scholar] [CrossRef]
- Du, H.; Zhang, X.; Yao, M.R.; Yang, Q.L.; Wu, W. Aptamer-guided luminous microsphere for anchoring and lightening. Sens. Actuators B Chem. 2022, 366, 131938. [Google Scholar] [CrossRef]
- Du, H.; Ping, T.; Wu, W.; Yang, Q.L. Sandwich Fluorescence Detection of Foodborne Pathogen with CD Fluorescence Signal Amplification in Food Samples. Foods 2022, 11, 945. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.X.; Chen, L.C.; Ren, Z.H. Comprehensive Analysis of Multiprotein Bridging Factor 1 Family Genes and Negatively Regulate the Resistance to in Tomato. BMC Plant Biol. 2019, 19, 437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, S.J.; Lv, J.L.; Liu, Z.B.; Wang, Y.; Ma, N.N.; Meng, Q.W. SUMO E3 Ligase SlSIZ1 Facilitates Heat Tolerance in Tomato. Plant Cell Physiol. 2018, 59, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Deng, Y.S.; Zhou, B.; Wang, G.D.; Wang, Y.; Meng, Q.W. A chloroplast-targeted DnaJ protein contributes to maintenance of photosystem II under chilling stress. J. Exp. Bot. 2014, 65, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Gong, B.; Jin, Z.Y.; Wang, X.F.; Wei, M.; Yang, F.J.; Li, Y.; Shi, Q.H. Sodic alkaline stress mitigation by exogenous melatonin in tomato needs nitric oxide as a downstream signal. J. Plant Physiol. 2015, 186, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, M.; Latif, S.; Ahmed, M.; Shehzadi, W.; Imran, M.; Ahmad, M.; Asari, A.; Jehangir, M.; Mahmud, Z. Modified matrix solid phase dispersion−HPLC method for determination of pesticide residue in vegetables and their impact on human health: A risk assessment. Front. Chem. 2022, 10, 1084350. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xin, Y.; Wang, H.; Dang, Y.; Wang, W.; Gao, Y.; Han, Y.; Kang, R.; Shi, Q.; Du, H. Nitrogen-Doped Carbon Dots Alleviate Pesticide Toxicity in Tomato by Regulating Antioxidant Systems. Int. J. Mol. Sci. 2025, 26, 9916. https://doi.org/10.3390/ijms26209916
Zhang X, Xin Y, Wang H, Dang Y, Wang W, Gao Y, Han Y, Kang R, Shi Q, Du H. Nitrogen-Doped Carbon Dots Alleviate Pesticide Toxicity in Tomato by Regulating Antioxidant Systems. International Journal of Molecular Sciences. 2025; 26(20):9916. https://doi.org/10.3390/ijms26209916
Chicago/Turabian StyleZhang, Xu, Yu Xin, Hao Wang, Yuting Dang, Wenhui Wang, Yi Gao, Yu Han, Rongrui Kang, Qinghua Shi, and Han Du. 2025. "Nitrogen-Doped Carbon Dots Alleviate Pesticide Toxicity in Tomato by Regulating Antioxidant Systems" International Journal of Molecular Sciences 26, no. 20: 9916. https://doi.org/10.3390/ijms26209916
APA StyleZhang, X., Xin, Y., Wang, H., Dang, Y., Wang, W., Gao, Y., Han, Y., Kang, R., Shi, Q., & Du, H. (2025). Nitrogen-Doped Carbon Dots Alleviate Pesticide Toxicity in Tomato by Regulating Antioxidant Systems. International Journal of Molecular Sciences, 26(20), 9916. https://doi.org/10.3390/ijms26209916





