Integrated Absorption Spectroscopic Measurement of 2-Nitrophenol and Naphthalene
Abstract
1. Introduction
2. Results and Discussion
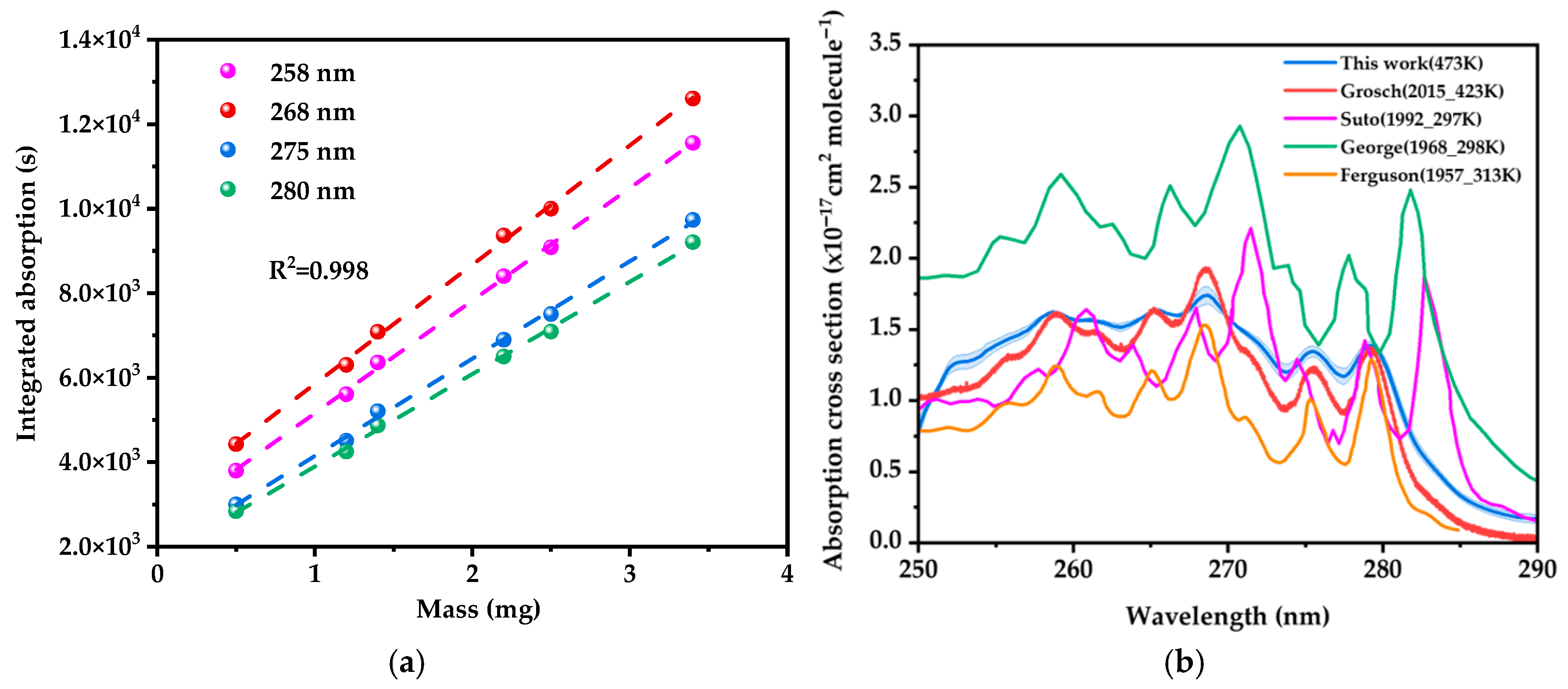
2.1. Absorption Cross Section of Naphthalene
2.2. Absorption Cross Section of 2-NP
3. Materials and Methods
3.1. Materials
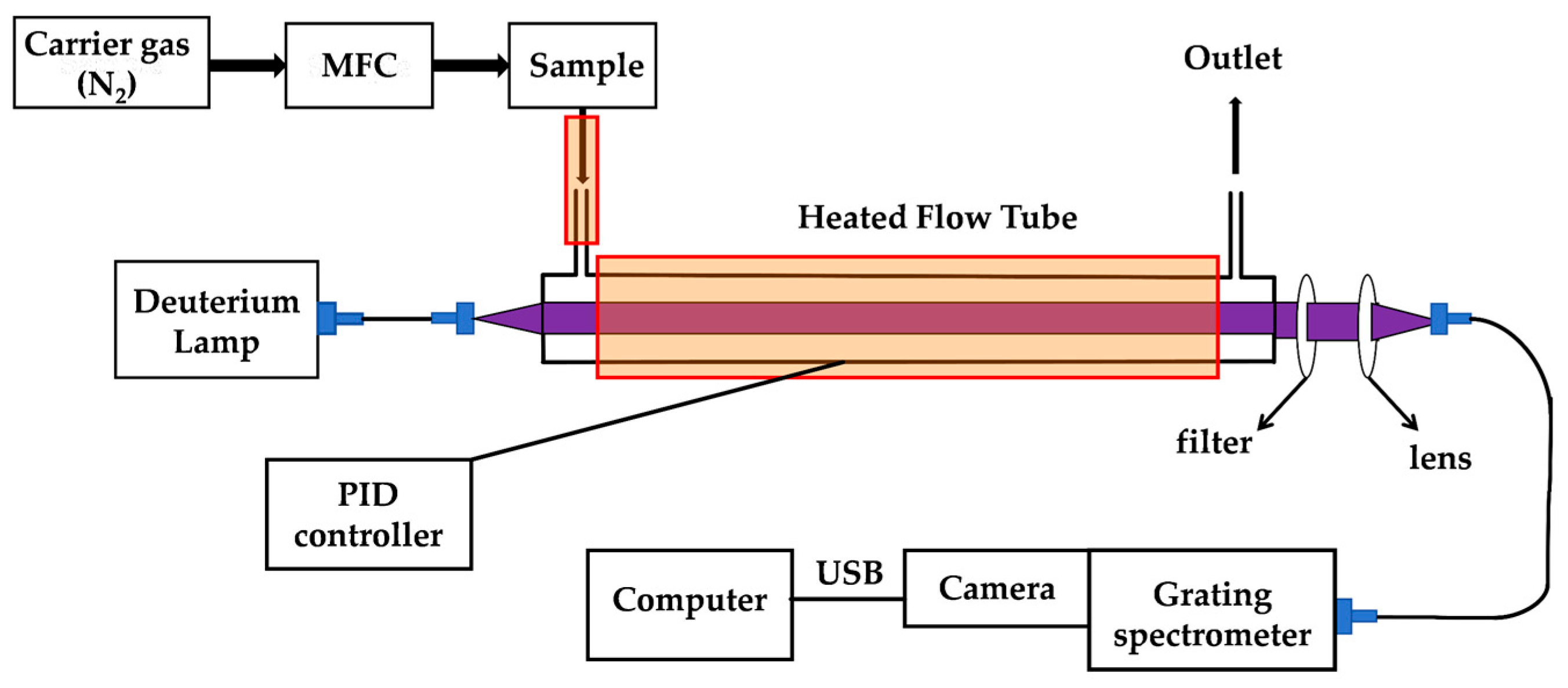
3.2. Experimental Setup
3.3. Integrated Absorption Measurement Principle
3.4. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, S.S.; Li, B.; Wei, N.N.; Hu, C.J.; Wang, Z.Y.; Zhang, W.J. Research progress on atmospheric oxidation reactions of low-volatility organic compounds and formation mechanisms of secondary organic aerosol. Environ. Chem. 2019, 38, 243–253. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Lu, C.; Li, R.; Zhang, J.; Dong, S.; Yang, L.; Xue, L.; Chen, J.; Wang, W. Nitrated phenols and the phenolic precursors in the atmosphere in urban Jinan, China. Sci. Total Environ. 2020, 714, 136760. [Google Scholar] [CrossRef]
- Wang, X.; Gu, R.; Wang, L.; Xu, W.; Zhang, Y.; Chen, B.; Li, W.; Xue, L.; Chen, J.; Wang, W. Emissions of fine particulate nitrated phenols from the burning of five common types of biomass. Environ. Pollut. 2017, 230, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, X.; Li, R.; Gu, R.; Zhang, Y.; Li, W.; Gao, R.; Chen, B.; Xue, L.; Wang, W. Emissions of fine particulate nitrated phenols from residential coal combustion in China. Atmos. Environ. 2019, 203, 10–17. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Gu, R.; Wang, H.; Wang, W. Observations of fine particulate nitrated phenols in four sites in northern China: Concentrations, source apportionment, and secondary formation. Atmos. Chem. Phys. 2018, 18, 4349–4359. [Google Scholar] [CrossRef]
- Xiang, J.; Xu, R.; Du, D.; Tang, B.; Yi, M.; Cai, F.; Yan, X.; Zheng, J.; Li, G.; An, T. The pollution characteristics, source identification and health risks of multiple classes atmospheric SVOCs with complex emission sources of the petrochemical plant and other industries. Atmos. Environ. 2023, 294, 119590. [Google Scholar] [CrossRef]
- Wang, X.; Huang, K.; Zeng, L.; Zhang, X.; Cheng, D.; Li, R.; Zhou, Y.; Jing, T. Integration of 103 semivolatile organic compounds into one multianalyte method for human serum analysis: An innovative approach within exposure assessment. Environ. Sci. Technol. Lett. 2021, 8, 419–424. [Google Scholar] [CrossRef]
- Robinson, A.L.; Donahue, N.M.; Shrivastava, M.K.; Weitkamp, E.A.; Sage, A.M.; Grieshop, A.P.; Lane, T.E.; Pierce, J.R.; Pandis, S.N. Rethinking organic aerosols: Semivolatile emissions and photochemical aging. Science 2007, 315, 1259–1262. [Google Scholar] [CrossRef]
- Zaveri, R.A.; Shilling, J.E.; Zelenyuk, A.; Zawadowicz, M.A.; Suski, K.; China, S.; Bell, D.M.; Veghte, D.; Laskin, A. Particle-phase diffusion modulates partitioning of semivolatile organic compounds to aged secondary organic aerosol. Environ. Sci. Technol. 2020, 54, 2595–2605. [Google Scholar] [CrossRef]
- Huang, G.; Wang, S.; He, X.; Chang, X.; Li, Z.; Xie, J.; Zheng, H.; Zheng, X.; Wu, Y.; Hao, J. Speciated emissions of gas-phase intermediate volatility and semivolatile organic compounds (I/SVOCs) during petrochemical production and their secondary organic aerosol formation potential (SOAFP). Environ. Sci. Technol. 2025, 59, 3111–3120. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, S.; Wang, H.; Shen, R.; Zhu, W.; Tan, R.; Song, K.; Zhang, Z.; Li, S.; Chen, Y.; et al. Importance of semivolatile/intermediate volatility organic compounds to secondary organic aerosol formation from Chinese domestic cooking emissions. Environ. Sci. Technol. Lett. 2022, 9, 508–514. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, F.; Li, C.; Yang, X.; Li, N.; Liu, S.; Wei, J.; Qiu, X.; He, Q. A portable sensor for in-situ measurement of ammonia based on near-infrared laser absorption spectroscopy. Opt. Laser Eng. 2019, 115, 243–248. [Google Scholar] [CrossRef]
- Shao, L.; Fang, B.; Zheng, F.; Qiu, X.; He, Q.; Wei, J.; Li, C.; Zhao, W. Simultaneous detection of atmospheric CO and CH4 based on TDLAS using a single 2.3 μm DFB laser. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 117118. [Google Scholar] [CrossRef]
- Bejan, I.; Barnes, I.; Olariu, R.; Zhou, S.; Wiesen, P.; Benter, T. Investigations on the gas-phase photolysis and OH radical kinetics of methyl-2-nitrophenols. Phys. Chem. Chem. Phys. 2007, 9, 5686–5692. [Google Scholar] [CrossRef]
- Harrison, M.A.J.; Barra, S.; Borghesi, D.; Vione, D.; Arsene, C.; Olariu, R.I. Nitrated phenols in the atmosphere: A review. Atmos. Environ. 2005, 39, 231–248. [Google Scholar] [CrossRef]
- Bejan, I.G.; Olariu, R.I.; Wiesen, P. Secondary organic aerosol formation from nitrophenols photolysis under atmospheric conditions. Atmosphere 2020, 11, 1346. [Google Scholar] [CrossRef]
- Wang, M.; Connolly, S.C.; Venables, D.S. Deep-ultraviolet absorption cross sections of strongly absorbing atmospheric species. J. Quant. Spectrosc. Radiat. Transf. 2024, 323, 109050. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Miao, F. A method for measuring the absorption cross section of NO gas. J. Nanjing Univ. Inf. Sci. Technol. Nat. Sci. Ed. 2011, 3, 129–132. [Google Scholar] [CrossRef]
- Wang, M.; Liu, W.; Ding, X.; Liu, T.; Zhou, W.; Lou, S.; Venables, D.S.; Varma, R.; Huang, C.; Chen, J. Rapid and high-precision cavity-enhanced spectroscopic measurement of HONO and NO2: Application to emissions from heavy-duty diesel vehicles in chassis dynamometer tests and in mobile monitoring. Talanta 2025, 285, 127386. [Google Scholar] [CrossRef]
- Sangwan, M.; Zhu, L. Absorption cross sections of 2-nitrophenol in the 295–400 nm region and photolysis of 2-nitrophenol at 308 and 351 nm. J. Phys. Chem. A 2016, 120, 9958–9965. [Google Scholar] [CrossRef]
- Bardini, P. Atmospheric Chemistry of Dimethylphenols and Nitrophenols. Ph.D. Thesis, University College Cork, Cork, Ireland, 2006. [Google Scholar]
- Wang, M.; Varma, R.; Venables, D.S.; Zhou, W.; Chen, J. A demonstration of broadband cavity-enhanced absorption spectroscopy at deep-ultraviolet wavelengths: Application to sensitive real-time detection of the aromatic pollutants benzene, toluene, and xylene. Anal. Chem. 2022, 94, 4286–4293. [Google Scholar] [CrossRef]
- Tang, R.Z.; Wang, H.; Liu, Y.; Guo, S. Composition of atmospheric semi-/intermediate-volatility organic compounds and their contribution to organic aerosol. Prog. Chem. 2019, 31, 180–190. [Google Scholar]
- Fullam, D.P.; Shoji, K.; Venables, D.S. Using integrated absorption to calibrate optical cavity spectrometers. Anal. Methods 2015, 7, 3298–3301. [Google Scholar] [CrossRef]
- Ray, S.S.; Das, T.R.; Kumar, A.; Paul, P.M.; Trivedi, R. Impact of transition metal decoration on the electronic and optical properties of polycyclic aromatic hydrocarbon “naphthalene”—A DFT investigation. Phys. B Condens. Matter 2025, 699, 416782. [Google Scholar] [CrossRef]
- Suto, M.; Wang, X.; Shan, J.; Lee, L.C. Quantitative photoabsorption and fluorescence spectroscopy of benzene, naphthalene, and some derivatives at 106–295 nm. J. Quant. Spectrosc. Radiat. Transfer 1992, 48, 79–89. [Google Scholar] [CrossRef]
- Grosch, H.; Sárossy, Z.; Egsgaard, H.; Fateev, A. UV absorption cross sections of phenol and naphthalene at temperatures up to 500 °C. J. Quant. Spectrosc. Radiat. Transfer 2015, 156, 17–23. [Google Scholar] [CrossRef]
- Fréreux, J.N.; Godard, M.; Dartois, E.; Pino, T. Ultraviolet electronic spectroscopy of heavily substituted naphthalene derivatives: Insights on the potential double aromatic ring substructures of interstellar bump carriers. Astron. Astrophys. 2023, 677, A98. [Google Scholar] [CrossRef]
- George, G.A.; Morris, G.C. The intensity of absorption of naphthalene from 30000 cm−1 to 53000 cm−1. J. Mol. Spectrosc. 1968, 26, 67–71. [Google Scholar] [CrossRef]
- Ferguson, J.; Reeves, L.W.; Schneider, W.G. Vapor absorption spectra and oscillator strengths of naphthalene, anthracene, and pyrene. Can. J. Chem. 1957, 35, 1117–1136. [Google Scholar] [CrossRef]
- Ernst, H.A.; Wolf, T.J.A.; Schalk, O.; González-García, N.; Boguslavskiy, A.E.; Stolow, A.; Oizmann, M.; Unterreiner, A.N. Ultrafast dynamics of o-nitrophenol: An experimental and theoretical study. J. Phys. Chem. A 2015, 119, 9225–9235. [Google Scholar] [CrossRef]
- Ciavardini, A.; Coreno, M.; Callegari, C.; Spezzani, C.; De Ninno, G.; Ressel, B.; Grazioli, C.; de Simone, M.; Kivimaki, A.; Miotti, P.; et al. Ultra-fast-VUV photoemission study of UV excited 2-nitrophenol. J. Phys. Chem. A 2019, 123, 1253–1262. [Google Scholar] [CrossRef]
- Guthmuller, J. Assessment of TD-DFT and CC2 methods for the calculation of resonance Raman intensities: Application to o-nitrophenol. J. Chem. Theory Comput. 2011, 7, 1082–1089. [Google Scholar] [CrossRef]
- Abeygunewardane, D.; Weinacht, T.; Matsika, S. How excitation wavelength affects excited state dynamics in o-nitrophenol: A theoretical perspective. J. Chem. Phys. 2025, 163, 024302. [Google Scholar] [CrossRef]
- Shama, S.A. Vacuum Ultraviolet Absorption Spectra of Organic Compounds in Gas-Phase and Liquid State. Ph.D. Thesis, Faculty of Science, Zagazig University, Zagazig, Egypt, 1991. [Google Scholar]
- Chen, J.; Wenger, J.C.; Venables, D.S. Near-ultraviolet absorption cross sections of nitrophenols and their potential influence on tropospheric oxidation capacity. J. Phys. Chem. A 2011, 115, 12235–12242. [Google Scholar] [CrossRef]
- Brion, J.; Chakir, A.; Daumont, D.; Malicet, J.; Parisse, C. High-resolution laboratory absorption cross section of O3. Temperature effect. Chem. Phys. Lett. 1993, 213, 610–612. [Google Scholar] [CrossRef]
- Osthoff, H.D.; Pilling, M.J.; Ravishankara, A.R.; Brown, S.S. Temperature dependence of the NO3 absorption cross section above 298 K and determination of the equilibrium constant for NO3 + NO2 ⇌ N2O5 at atmospherically relevant conditions. Phys. Chem. Chem. Phys. 2007, 9, 5785–5793. [Google Scholar] [CrossRef]
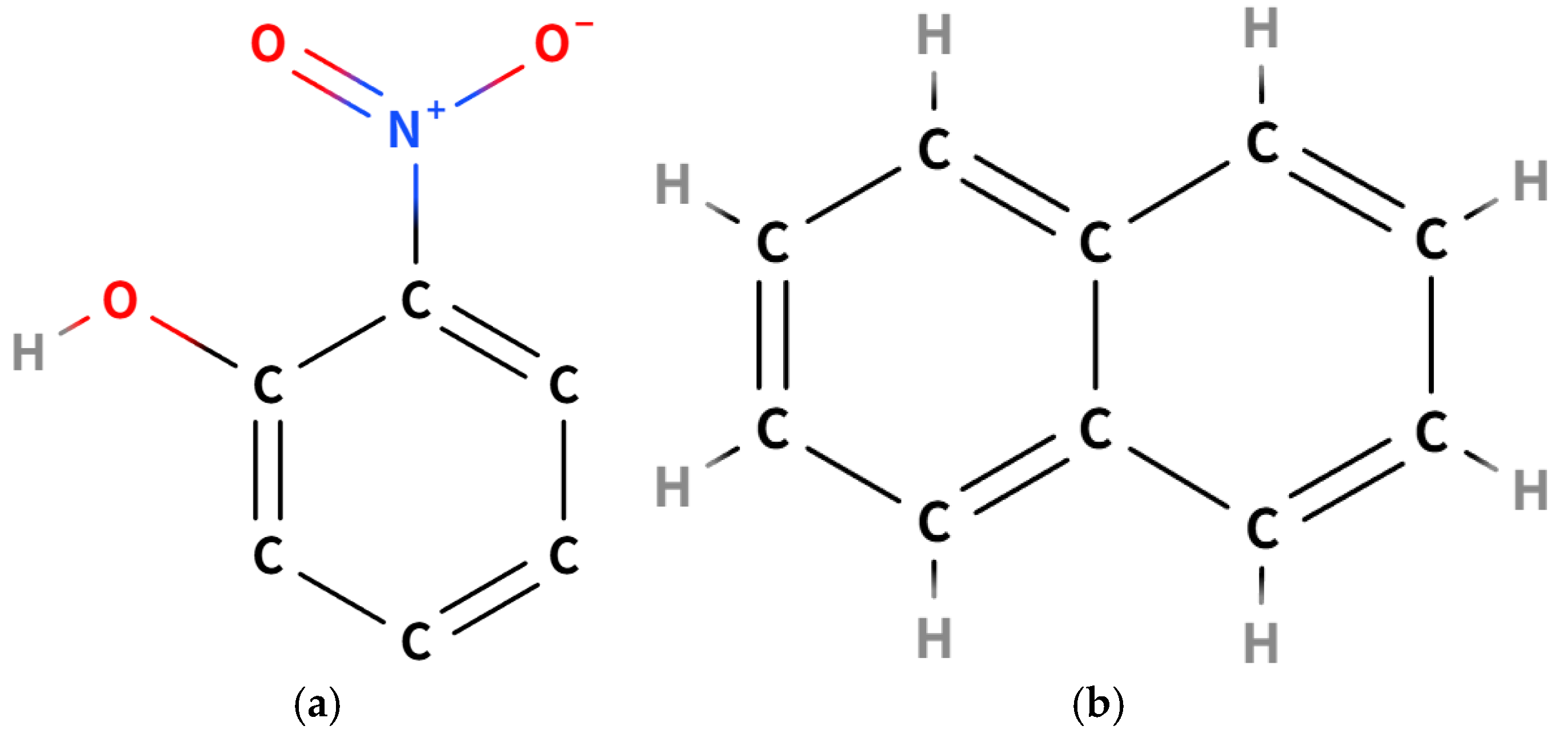


| Wavelength Range (nm) | Transition Energy Levels | Electronic Transition Type | Oscillator Strength (f) |
|---|---|---|---|
| 220–230 | S0 → S3 | π → π* | 0.059 |
| 230–290 | S0 → S2 (1B2u ← 1Ag) | π → π* | 0.089 |
| Wavelength Range (nm) | Transition Energy Levels | Electronic Transition Type | Oscillator Strength (f) |
|---|---|---|---|
| 255–275 | S0 → S4 | π → π* | 0.19 |
| 320–350 | S0 → S1 | π → π* | 0.06 |
| Module | Manufacturer | Model/Specifications | Key Features |
|---|---|---|---|
| Light Source System | |||
| Deuterium lamp | Avantes (Apeldoor, The Netherlands) | Avalight-D-S-DUV | Spectral range: 175–400 nm |
| Emission fiber | Avantes (Apeldoorn, The Netherlands) | FC-UVIR400-0.5-ME | Core diameter: 400 μm |
| Reflective collimator | Thorlabs (Newton, NJ, USA) | RC04SMA-P01 | 2D-adjustable design |
| Absorption Cell and Spectral Detection System | |||
| Quartz absorption cell | – | Custom-built | Length: 420 mm, Gas port ID: 8 mm |
| Precision balance | Ohaus (Parsippany, NJ, USA) | PWS224ZH | ±0.1 mg |
| Temperature control unit | YuDian (Shanghai, China) | AI-206/207 | Proportional–integral–derivative (PID)-controlled |
| Short-pass filters | Semrock (Rochester, NY, USA) | FF01-300/SP-25 (300 nm) | UV band cutoff |
| Asahi Spectra (Torrance, CA, USA) | XHS0450 (450 nm) | Visible band cutoff | |
| Focusing lens | – | f = 50 mm | Fiber optic coupling |
| Spectrograph | Andor Tech (Belfast, UK) | Kymera-328i-A | UV–Vis spectral analysis |
| CCD detector | Andor Tech (Belfast, UK) | DU420A-BU | High-sensitivity detection |
| Fiber spectrometer | Avantes (Apeldoorn, The Netherlands) | ULS2048XL-RS-EVO | Grating: 2400 lines/mm, slit50, 0.26–0.34, 200 nm–320 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Wang, M.; Venables, D.S.; Chen, J. Integrated Absorption Spectroscopic Measurement of 2-Nitrophenol and Naphthalene. Int. J. Mol. Sci. 2025, 26, 9904. https://doi.org/10.3390/ijms26209904
Yang Z, Wang M, Venables DS, Chen J. Integrated Absorption Spectroscopic Measurement of 2-Nitrophenol and Naphthalene. International Journal of Molecular Sciences. 2025; 26(20):9904. https://doi.org/10.3390/ijms26209904
Chicago/Turabian StyleYang, Zhongmei, Meng Wang, Dean S. Venables, and Jun Chen. 2025. "Integrated Absorption Spectroscopic Measurement of 2-Nitrophenol and Naphthalene" International Journal of Molecular Sciences 26, no. 20: 9904. https://doi.org/10.3390/ijms26209904
APA StyleYang, Z., Wang, M., Venables, D. S., & Chen, J. (2025). Integrated Absorption Spectroscopic Measurement of 2-Nitrophenol and Naphthalene. International Journal of Molecular Sciences, 26(20), 9904. https://doi.org/10.3390/ijms26209904







