Mechanisms of Mitochondrial Impairment by SARS-CoV-2 Proteins: A Nexus of Pathogenesis with Significant Biochemical and Clinical Implications
Abstract
1. Introduction
1.1. SARS-CoV-2 Proteome and Mitochondria: Structural and Functional Relationships with Clinical Implications
- a.
- Structural Proteins
- Spike (S) protein: This trimeric protein is located on the virus’s surface and gives coronaviruses their distinctive “crown-like” appearance. It enables the virus to enter host cells by binding to the ACE2 receptor on the cell surface.
- Nucleocapsid (N) protein: This protein binds to the viral RNA genome to facilitate the formation of the helical ribonucleocapsid complex.
- Membrane (M) protein: This is the most abundant structural protein and is crucial for the virus’s assembly.
- Envelope (E) protein: This small protein helps assemble new virus particles and form the viral envelope.
- b.
- Non-Structural Proteins
- c.
- Accessory Proteins
1.2. The SARS-CoV-2-Human Protein Interactome: A Framework for Viral Pathogenesis
1.2.1. The Spike (S) Protein in Mitochondria
- -
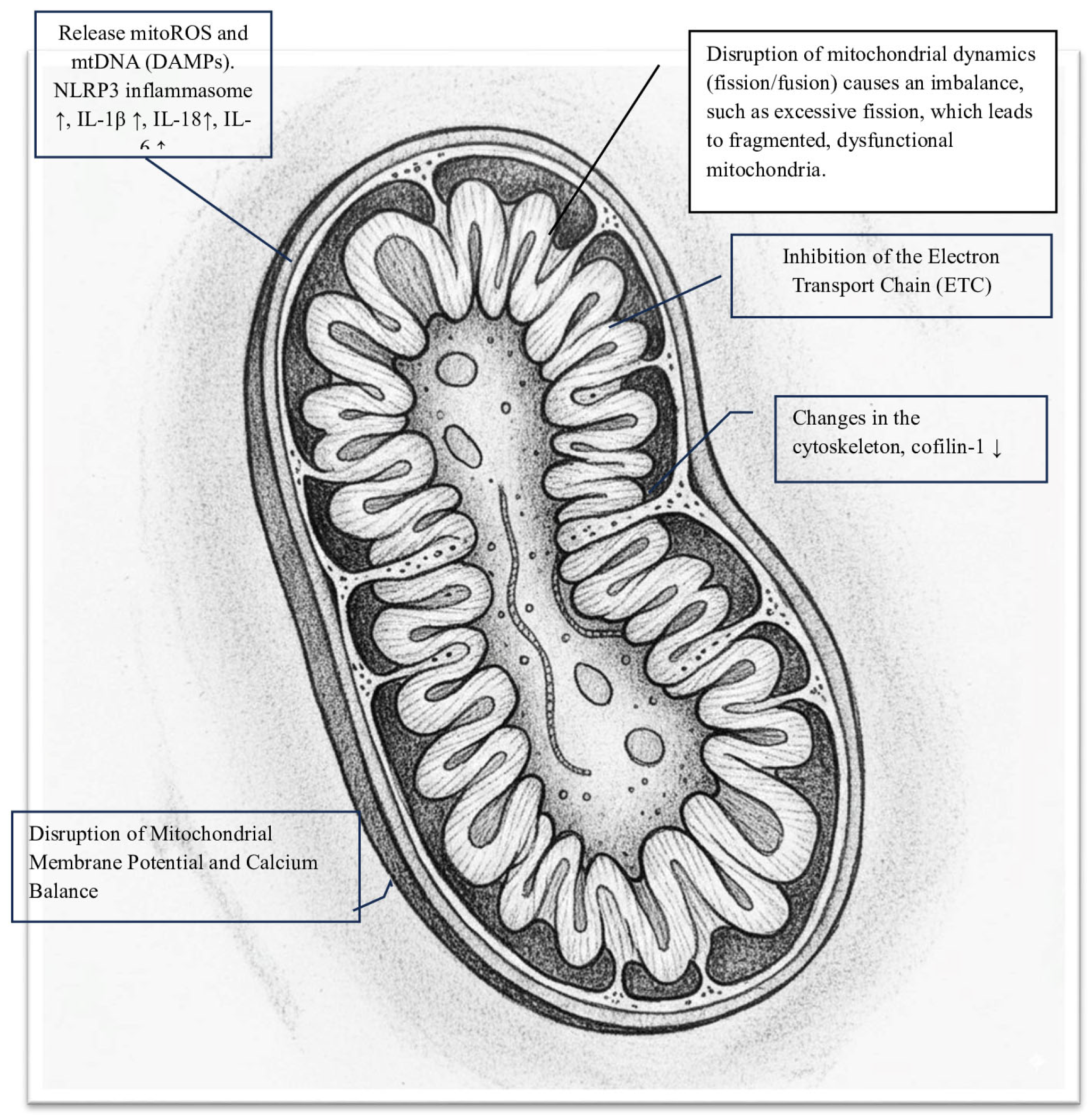
- Inhibition of the Electron Transport Chain (ETC): The S protein significantly disrupts oxidative phosphorylation by blocking enzyme activities within the ETC, leading to a notable decrease in mitochondrial oxygen consumption rate (OCR). Spectroscopic analysis reveals that the Spike protein diminishes the intensity of heme groups in Complex III and Complex IV, suggesting a disruption of their redox functions and creating a bottleneck in electron flow [12]. Specifically, in HLMVECs, the Spike RBD markedly lowers both the basal and maximal OCRs, as well as ATP-linked respiration. In respiratory epithelial cells, exposure to the S protein results in the downregulation of key mitochondrial proteins such as SIRT3 and TOMM22, and decreases the OCR, signaling a shift away from oxidative phosphorylation [13]. This impairment of the ETC causes significant oxidative stress, as the Spike protein increases superoxide production from Complex I and Complex III. This oxidative stress further damages mitochondrial structures and functions, creating a vicious cycle [7,14,15].
- -
- Cell surface receptor engagement: The interaction of the Spike protein with cell surface receptors activates signaling pathways that, along with oxidative stress, cause significant structural and functional damage to the mitochondrial network. Specifically, the engagement of the S protein’s S1 subunit with the ACE2 receptor leads to notable changes in mitochondrial shape. In human lung microvascular endothelial cells (HLMVECs), exposure to the Spike receptor-binding domain (RBD) results in mitochondrial fragmentation, swelling, and abnormal cristae remodeling, along with a decrease in cristae density [13]. Similarly, in human cardiomyocytes, prolonged exposure to the S1 subunit causes extensive mitochondrial fragmentation, confirming the role of induced mitochondrial stress. These structural damages are linked to reduced expression of TOM20, a key component of the translocase complex responsible for importing nuclear-encoded proteins into the mitochondria, indicating a disruption in mitochondrial shape and function [12].
- -
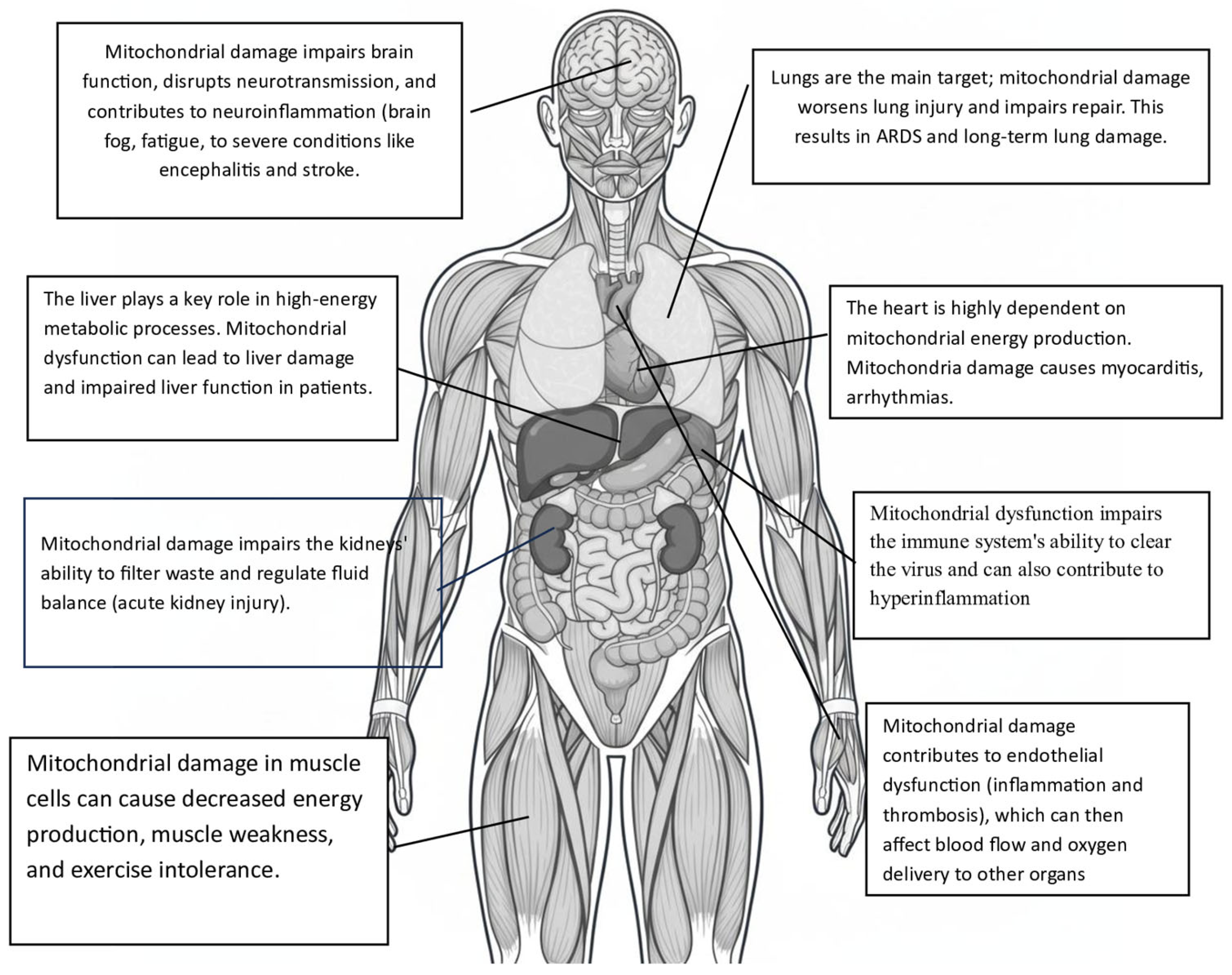
- Disruption of mitochondrial membrane potential and calcium balance: In cardiomyocytes, mitochondrial fragmentation is associated with a loss of mitochondrial membrane potential (Δψm), increased mitochondrial calcium (mCa2+) levels, and heightened production of reactive oxygen species (ROS). This damage to membrane integrity and calcium imbalance triggers cell death pathways. Notably, mitochondrial dysfunction caused by the Spike protein significantly contributes to the hyperinflammation seen in severe COVID-19 cases. Damaged mitochondria release mitoROS and mitochondrial DNA (mtDNA) into the cytosol, acting as powerful danger signals known as DAMPs. These signals activate the NLRP3 inflammasome, leading to the maturation and release of pro-inflammatory cytokines IL-1β and IL-18 [9,16]. Evidence suggests that the Spike protein primes and activates the NLRP3 inflammasome in immune cells, relying on mitochondrial reactive oxygen species (mitoROS) production [17,18]. This creates a harmful cycle in which mitochondrial damage, driven by the Spike protein, promotes inflammation and systemic cellular stress. Importantly, the toxic effects of the Spike protein provide a molecular explanation for several key clinical features of COVID-19, including significant endothelial dysfunction, cardiac injury, and systemic inflammation in severe cases. Additionally, ongoing mitochondrial stress and associated metabolic changes may be key factors in the development of chronic fatigue and multi-organ symptoms in the post-acute sequelae of SARS-CoV-2 infection (PASC), underscoring the lasting impact of the Spike protein on host cell function [19,20].
1.2.2. The Membrane (M) Protein in Mitochondria
- Pulmonary edema and ARDS: M protein damages the alveolar-capillary barrier, causing pulmonary edema, hypoxemia, and ARDS (Acute Respiratory Distress Syndrome) [28].
The SARS-CoV-2 Nucleocapsid (N) Protein in Mitochondria
The SARS-CoV-2 Envelope (E) Protein in Mitochondria
- -
- Mitochondrial calcium overload: Excess Ca2⁺ intake by mitochondria causes the opening of the mitochondrial permeability transition pore (mPTP), which can result in cell death.
- -
- Increased oxidative stress: This dysfunction boosts the production of mitochondrial reactive oxygen species (mtROS), which are highly damaging molecules that harm cells and impair mitochondrial function.
- -
- Loss of mitochondrial integrity: Calcium overload and oxidative stress harm the mitochondrial membrane, leading to depolarization and triggering apoptosis (programmed cell death).
- -
2. SARS-CoV-2 Accessory Proteins (ORFs) and Mitochondria: Structural and Functional Relationships with Clinical Implications
2.1. ORF3a
2.2. ORF3c
- i.
- Manipulation of mitochondrial fission and fusion (alteration of normal mitochondrial dynamics).
- ii.
- MAVS degradation (suppressing the host’s innate antiviral response).
- iii.
- Disruption of mitochondrial bioenergetics: interfering with respiration, ATP production, or other metabolic pathways to enhance viral replication.
- iv.
- Dysregulation of calcium homeostasis.
- v.
- Activation of cell death pathways, like apoptosis or necroptosis.
2.3. ORF6
2.4. ORF7a
2.5. ORF7b
2.6. ORF8
2.7. ORF9b
2.8. ORF9c
2.9. ORF10
- -
- Binding to mitochondrial proteins: Interacting with proteins like NIX and LC3B to promote mitophagy—the selective removal of damaged mitochondria.
- -
- Immune suppression: breaking down MAVS to prevent interferon responses.
- -
2.10. Potential Therapeutic Strategies
3. Conclusions
Induction of Mitochondrial Apoptosis
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 15 January 2023).
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef]
- Denaro, M.; Ferro, E.; Barrano, G.; Meli, S.; Busacca, M.; Corallo, D.; Capici, A.; Zisa, A.; Cucuzza, L.; Gradante, S.; et al. Monitoring of SARS-CoV-2 Infection in Ragusa Area: Next Generation Sequencing and Serological Analysis. Int. J. Mol. Sci. 2023, 24, 4742. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Gupta, S.; Paramo, M.I.; Hou, Y.; Mao, C.; Luo, Y.; Judd, J.; Wierbowski, S.; Bertolotti, M.; et al. A comprehensive SARS-CoV-2-human protein-protein interactome network identifies pathobiology and host-targeting therapies for COVID-19. Nat. Biotechnol. 2023, 41, 128–139. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Feng, X.; Nie, L.; Tang, M.; Zhang, H.; Xiong, Y.; Swisher, S.K.; Srivastava, M.; Chen, J. Interactomes of SARS-CoV-2 and human coronaviruses reveal host factors potentially affecting pathogenesis. EMBO J. 2021, 40, e107776. [Google Scholar] [CrossRef]
- Brant, A.C.; Tian, W.; Majerciak, V.; Yang, W.; Zheng, Z.-M. SARS-CoV-2: From its discovery to genome structure, transcription, and replication. Cell Biosci. 2021, 11, 136. [Google Scholar] [CrossRef]
- Mariano, G.; Farthing, R.J.; Lale-Farjat, S.L.M.; Bergeron, J.R.C. Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be. Front. Mol. Biosci. 2020, 7, 605236. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis, and therapies: Structural genomics approach. BBA Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Tamura, T.; Wang, L.; Mori, K.; Tsuda, M.; Suzuki, R.; Suzuki, S.; Yoshimatsu, K.; Tanaka, S.; Fukuhara, T. Involvement of SARS-CoV-2 accessory proteins in immunopathogenesis. Microbiol Immunol. 2024, 68, 237–247. [Google Scholar]
- Peng, R.; Wu, L.A.; Wang, Q.; Qi, J.; Gao, G.F. Cell entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef]
- Huynh, T.V.; Rethi, L.; Lee, T.W.; Higa, S.; Kao, Y.H.; Chen, Y.J. Spike Protein Impairs Mitochondrial Function in Human Cardiomyocytes: Mechanisms Underlying Cardiac Injury in COVID-19. Cells 2023, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Kulkovienė, G.; Narauskaitė, D.; Tunaitytė, A.; Volkevičiūtė, A.; Balion, Z.; Kutakh, O.; Gečys, D.; Kairytė, M.; Uldukytė, M.; Stankevičius, E.; et al. Differential Mitochondrial, Oxidative Stress and Inflammatory Responses to SARS-CoV-2 Spike Protein Receptor Binding Domain in Human Lung Microvascular, Coronary Artery Endothelial and Bronchial Epithelial Cells. Int. J. Mol. Sci. 2024, 25, 3188. [Google Scholar] [CrossRef]
- Keturakis, V.; Narauskaitė, D.; Balion, Z.; Gečys, D.; Kulkovienė, G.; Kairytė, M.; Žukauskaitė, I.; Benetis, R.; Stankevičius, E.; Jekabsone, A. The Effect of SARS-CoV-2 Spike Protein RBD-Epitope on Immunometabolic State and Functional Performance of Cultured Primary Cardiomyocytes Subjected to Hypoxia and Reoxygenation. Int. J. Mol. Sci. 2023, 24, 16554. [Google Scholar] [CrossRef]
- Yeung-Luk, B.H.; Narayanan, G.A.; Ghosh, B.; Wally, A.; Lee, E.; Mokaya, M.; Wankhade, E.; Zhang, R.; Lee, B.; Park, B.; et al. SARS-CoV-2 infection alters mitochondrial and cytoskeletal function in human respiratory epithelial cells mediated by expression of spike protein. mBio 2023, 14, e0082023. [Google Scholar] [CrossRef]
- Bhowal, C.; Ghosh, S.; Ghatak, D.; De, R. Pathophysiological involvement of host mitochondria in SARS-CoV-2 infection that causes COVID-19: A comprehensive evidential insight. Mol. Cell. Biochem. 2023, 478, 1325–1343. [Google Scholar] [CrossRef]
- De Nicolo, B.; Cataldi-Stagetti, E.; Diquigiovanni, C.; Bonora, E. Calcium and Reactive Oxygen Species Signaling Interplays in Cardiac Physiology and Pathologies. Antioxidants 2023, 12, 353. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kucia, M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia 2020, 34, 1726–1729. [Google Scholar] [CrossRef]
- Guarnieri, J.W.; Angelin, A.; Murdock, D.G.; Schaefer, P.; Portluri, P.; Lie, T.; Huang, J.; Wallace, D.C. SARS-COV-2 viroporins activate the NLRP3-inflammasome by the mitochondrial permeability transition pore. Front. Immunol. 2023, 14, 1064293. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z. More than a key—The pathological roles of SARS-CoV-2 spike protein in COVID-19 related cardiac injury. Sports Med. Health Sci. 2023, 6, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Neuman, B.W.; Kiss, G.; Kunding, A.H.; Bhella, D.; Baksh, M.F.; Connelly, S.; Droese, B.; Klaus, J.P.; Makino, S.; Sawicki, S.G.; et al. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011, 174, 11–22. [Google Scholar] [CrossRef]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, A.; Fang, Y.; Shu, T.; Wu, D.; Wang, C.; Huang, M.; Min, J.; Jin, L.; Zhou, W.; et al. SARS-CoV-2 Membrane Glycoprotein M Triggers Apoptosis With the Assistance of Nucleocapsid Protein N in Cells. Front. Cell. Infect. Microbiol. 2021, 11, 706252. [Google Scholar] [CrossRef]
- Castillo-Galán, S.; Grünenwald, F.; Hidalgo, Y.; Cárdenas, J.C.; Cadiz, M.I.; Alcayaga-Miranda, F.; Khoury, M.; Cuenca, J. Mitochondrial Antiviral Signaling Protein Activation by Retinoic Acid-Inducible Gene I Agonist Triggers Potent Antiviral Defense in Umbilical Cord Mesenchymal Stromal Cells Without Compromising Mitochondrial Function. Int. J. Mol. Sci. 2025, 26, 4686. [Google Scholar] [CrossRef]
- Duan, X.; Liu, R.; Lan, W.; Liu, S. The Essential Role of Mitochondrial Dynamics in Viral Infections. Int. J. Mol. Sci. 2025, 26, 1955. [Google Scholar] [CrossRef]
- Chen, T.H.; Jeng, T.H.; Lee, M.Y.; Wang, H.C.; Tsai, K.F.; Chou, C.K. Viral mitochondriopathy in COVID-19. Redox Biol. 2025, 85, 103766. [Google Scholar] [CrossRef]
- Hsu, P.C.; Shahed-Al-Mahmud, M. SARS-CoV-2 mediated neurological disorders in COVID-19: Measuring the pathophysiology and immune response. Life Sci. 2022, 308, 120981. [Google Scholar] [CrossRef]
- Cui, X.; Chen, W.; Zhou, H.; Gong, Y.; Zhu, B.; Lv, X.; Guo, H.; Duan, J.; Zhou, J.; Marcon, E.; et al. Pulmonary Edema in COVID-19 Patients: Mechanisms and Treatment Potential. Front. Pharmacol. 2021, 12, 664349. [Google Scholar] [CrossRef]
- Wang, F.; Han, H.; Wang, C.; Wang, J.; Peng, Y.; Chen, Y.; He, Y.; Deng, Z.; Li, F.; Rong, Y.; et al. SARS-CoV-2 membrane protein induces neurodegeneration via affecting Golgi-mitochondria interaction. Transl. Neurodegener. 2024, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Meng, X.; Wang, Z.; Younis, M.; Liu, Y.; Wang, P.; Huang, X. SARS-CoV-2 membrane protein causes the mitochondrial apoptosis and pulmonary edema via targeting BOK. Cell Death Differ. 2022, 29, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, J.W.; Lie, T.; Albrecht, Y.E.S.; Hewin, P.; Jurado, K.A.; Widjaja, G.A.; Zhu, Y.; McManus, M.J.; Kilbaugh, T.J.; Keith, K.; et al. Mitochondrial antioxidants abate SARS-COV-2 pathology in mice. Proc. Natl. Acad. Sci. USA 2024, 121, e2321972121. [Google Scholar] [CrossRef] [PubMed]
- Detrimental Effects of Anti-Nucleocapsid Antibodies in COVID-19—Thailand Medical News. Available online: https://www.thailandmedical.news/news/detrimental-effects-of-anti-nucleocapsid-antibodies-in-covid-19 (accessed on 25 August 2025).
- Yu, H.; Yang, L.; Han, Z.; Zhou, X.; Zhang, Z.; Sun, T.; Zheng, F.; Yang, J.; Guan, F.; Xie, J.; et al. SARS-CoV-2 nucleocapsid protein enhances the level of mitochondrial reactive oxygen species. J. Med. Virol. 2023, 95, e29270. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Huang, D.; Tan, Y.-J. The Role of SARS-CoV-2 Nucleocapsid Protein in Host Inflammation. Viruses 2025, 17, 1046. [Google Scholar] [CrossRef]
- Georgieva, E.; Ananiev, J.; Yovchev, Y.; Arabadzhiev, G.; Abrashev, H.; Abrasheva, D.; Atanasov, V.; Kostandieva, R.; Mitev, M.; Petkova-Parlapanska, K.; et al. COVID-19 Complications: Oxidative Stress, Inflammation, and Mitochondrial and Endothelial Dysfunction. Int. J. Mol. Sci. 2023, 24, 14876. [Google Scholar] [CrossRef]
- Gokhale, N.S.; Sam, R.K.; Somfleth, K.; Thompson, M.G.; Marciniak, D.M.; Smith, J.R.; Genoyer, E.; Eggenberger, J.; Chu, L.H.; Park, M.; et al. Cellular RNA interacts with MAVS to promote antiviral signaling. Science 2024, 386, eadl0429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Di, B.; Xu, L.L. The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021, 61, 2–15. [Google Scholar] [CrossRef]
- Molnar, T.; Lehoczki, A.; Fekete, M.; Varnai, R.; Zavori, L.; Erdo-Bonyar, S.; Simon, D.; Berki, T.; Csecsei, P.; Ezer, E. Mitochondrial dysfunction in long COVID: Mechanisms, consequences, and potential therapeutic approaches. Geroscience 2024, 46, 5267–5286. [Google Scholar] [CrossRef]
- Chen, T.H.; Chang, C.J.; Hung, P.H. Possible Pathogenesis and Prevention of Long COVID: SARS-CoV-2-Induced Mitochondrial Disorder. Int. J. Mol. Sci. 2023, 24, 8034. [Google Scholar] [CrossRef]
- Tang, N.; Kido, T.; Shi, J.; McCafferty, E.; Ford, J.M.; Dal Bon, K.; Pulliam, L. Blood Markers Show Neural Consequences of LongCOVID-19. Cells 2024, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lv, P.; Li, M.; Chen, Z.; Xin, H.; Reilly, S.; Zhang, X. SARS-CoV-2 E protein: Pathogenesis and potential therapeutic development. Biomed. Pharmacother. 2023, 159, 114242. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zheng, Y.; Zeng, X.; He, B.; Cheng, W. Structural biology of SARS-CoV-2: Open the door for novel therapies. Signal Transduct. Target. Ther. 2022, 7, 26. [Google Scholar] [CrossRef]
- Mehregan, A.; Pérez-Conesa, S.; Zhuang, Y.; Elbahnsi, A.; Pasini, D.; Lindahl, E.; Howard, R.J.; Ulens, C.; Delemotte, L. Probing effects of the SARS-CoV-2 E protein on membrane curvature and intracellular calcium. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183994. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.; de Melo, G.D.; Larrous, F.; Kergoat, L.; Boëda, B.; Michel, V.; Seilhean, D.; Tichit, M.; Hing, D.; Hardy, D.; et al. The SARS-CoV-2 envelope PDZ binding motif acts as a virulence factor disrupting host’s epithelial cell—Cell junctions. Cell. Mol. Biol. Lett. 2025, 30, 80. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Patra, S.; Bhol, C.S.; Panigrahi, D.P.; Praharaj, P.P.; Singh, A.; Patil, S.; Dhiman, R.; et al. Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics. Int. J. Biochem. Cell Biol. 2021, 136, 106013. [Google Scholar] [CrossRef]
- Kakavandi, S.; Zare, I.; VaezJalali, M.; Dadashi, M.; Azarian, M.; Akbari, A.; Ramezani Farani, M.; Zalpoor, H.; Hajikhani, B. Structural and non-structural proteins in SARS-CoV-2: Potential aspects to COVID-19 treatment or prevention of progression of related diseases. Cell Commun. Signal. 2023, 21, 110. [Google Scholar] [CrossRef]
- Low, Z.Y.; Zabidi, N.Z.; Yip, A.J.W.; Puniyamurti, A.; Chow, V.T.K.; Lal, S.K. SARS-CoV-2 Non-Structural Proteins and Their Roles in Host Immune Evasion. Viruses 2022, 14, 1991. [Google Scholar] [CrossRef]
- Zimmermann, L.; Zhao, X.; Makroczyova, J.; Wachsmuth-Melm, M.; Prasad, V.; Hensel, Z.; Bartenschlager, R.; Chlanda, P. SARS-CoV-2 nsp3 and nsp4 are minimal constituents of a pore spanning replication organelle. Nat. Commun. 2023, 14, 7894. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Deng, J.; Han, L.; Zhuang, M.-W.; Xu, Y.; Zhang, J.; Nan, M.-L.; Xiao, Y.; Zhan, P.; Liu, X.; et al. SARS-CoV-2 NSP5 and N protein counteract the RIG-I signaling pathway by suppressing the formation of stress granules. Signal Transduct. Target. Ther. 2022, 7, 22. [Google Scholar] [CrossRef]
- Peng, Q.; Peng, R.; Yuan, B.; Zhao, J.; Wang, M.; Wang, X.; Wang, Q.; Sun, Y.; Fan, Z.; Qi, J.; et al. Structural and Biochemical Characterization of the nsp12-nsp7-nsp8 Core Polymerase Complex from SARS-CoV-2. Cell Rep. 2020, 31, 107774. [Google Scholar] [CrossRef]
- Newman, J.A.; Douangamath, A.; Yadzani, S.; Yosaatmadja, Y.; Aimon, A.; Brandão-Neto, J.; Dunnett, L.; Gorrie-Stone, T.; Skyner, R.; Fearon, D.; et al. Structure, mechanism and crystallographic fragment screening of the SARS-CoV-2 NSP13 helicase. Nat. Commun. 2021, 12, 4848. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.Y.; Yan, H.; Guo, Z.; Lian, Z.; Yao, H.; Yuan, S.; Ge, X.Y.; Qiu, Y. The P132H mutation of SARS-CoV-2 NSP5 relieves its inhibition on interferon-β activation via blocking MAVS degradation. Cell. Mol. Life Sci. 2025, 82, 293. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.D. The ‘cytokine storm’: Molecular mechanisms and therapeutic prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef]
- Mone, P.; Agyapong, E.D.; Morciano, G.; Jankauskas, S.S.; De Luca, A.; Varzideh, F.; Pinton, P.; Santulli, G. Dysfunctional mitochondria elicit bioenergetic decline in the aged heart. J. Cardiovasc. Aging 2024, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.V.W.; Guy, G.W.; Brysch, W.; Bell, J.D. Understanding Long COVID; Mitochondrial Health and Adaptation—Old Pathways, New Problems. Biomedicines 2022, 10, 3113. [Google Scholar] [CrossRef] [PubMed]
- Nazerian, Y.; Ghasemi, M.; Yassaghi, Y.; Nazerian, A.; Hashemi, S.M. Role of SARS-CoV-2-induced cytokine storm in multi-organ failure: Molecular pathways and potential therapeutic options. Int. Immunopharmacol. 2022, 113, 109428. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Belinchón-deMiguel, P.; Martinez-Guardado, I.; Dalamitros, A.A.; Yáñez-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488. [Google Scholar] [CrossRef]
- Redondo, N.; Zaldívar-López, S.; Garrido, J.J.; Montoya, M. SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns. Front. Immunol. 2021, 12, 708264. [Google Scholar] [CrossRef]
- Zandi, M.; Shafaati, M.; Kalantar-Neyestanaki, D.; Pourghadamyari, H.; Fani, M.; Soltani, S.; Kaleji, H.; Abbasi, S. The role of SARS-CoV-2 accessory proteins in immune evasion. Biomed. Pharmacother. 2022, 156, 113889. [Google Scholar] [CrossRef]
- Si, F.; Song, S.; Yu, R.; Li, Z.; Wei, W.; Wu, C. Coronavirus accessory protein ORF3 biology and its contribution to viral behavior and pathogenesis. iScience 2023, 26, 106280. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.; Lu, Y.; O’Keefe, S.; Valpadashi, A.; Cruz-Zaragoza, L.D.; Michel, H.A.; Nguyen, S.K.; Carnell, G.W.; Lukhovitskaya, N.; Milligan, R.; et al. The SARS-CoV-2 protein ORF3c is a mitochondrial modulator of innate immunity. iScience 2023, 26, 108080. [Google Scholar] [CrossRef]
- Mozzi, A.; Oldani, M.; Forcella, M.E.; Vantaggiato, C.; Cappelletti, G.; Pontremoli, C.; Valenti, F.; Forni, D.; Saresella, M.; Biasin, M.; et al. SARS-CoV-2 ORF3c impairs mitochondrial respiratory metabolism, oxidative stress, and autophagic flux. iScience 2023, 26, 107118. [Google Scholar] [CrossRef] [PubMed]
- Miorin, L.; Kehrer, T.; Sanchez-Aparicio, M.T.; Zhang, K.; Cohen, P.; Patel, R.S.; Cupic, A.; Makio, T.; Mei, M.; Moreno, E.; et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 28344–28354. [Google Scholar] [CrossRef]
- Wen, Y.; Li, C.; Tang, T.; Luo, C.; Lu, S.; Lyu, N.; Li, Y.; Wang, R. SARS-CoV-2 ORF7a Protein Impedes Type I Interferon-Activated JAK/STAT Signaling by Interacting with HNRNPA2B1. Int. J. Mol. Sci. 2025, 26, 5536. [Google Scholar] [CrossRef]
- Mansueto, G.; Fusco, G.; Colonna, G. A Tiny Viral Protein, SARS-CoV-2-ORF7b: Functional Molecular Mechanisms. Biomolecules 2024, 14, 541. [Google Scholar] [CrossRef]
- Flower, T.G.; Buffalo, C.Z.; Hooy, R.M.; Allaire, M.; Ren, X.; Hurley, J.H. Structure of SARS-CoV-2 ORF8, a rapidly evolving immune evasion protein. Proc. Natl. Acad. Sci. USA 2021, 118, e2021785118. [Google Scholar] [CrossRef]
- Vinjamuri, S.; Li, L.; Bouvier, M. SARS-CoV-2 ORF8: One protein, seemingly one structure, and many functions. Front. Immunol. 2022, 13, 1035559. [Google Scholar] [CrossRef]
- Lenhard, S.; Gerlich, S.; Khan, A.; Rödl, S.; Bökenkamp, J.E.; Peker, E.; Zarges, C.; Faust, J.; Storchova, Z.; Räschle, M.; et al. The Orf9b protein of SARS-CoV-2 modulates mitochondrial protein biogenesis. J. Cell Biol. 2023, 222, e202303002. [Google Scholar] [CrossRef] [PubMed]
- Dominguez Andres, A.; Feng, Y.; Campos, A.R.; Yin, J.; Yang, C.C.; James, B.; Murad, R.; Kim, H.; Deshpande, A.J.; Gordon, D.E.; et al. SARS-CoV-2 ORF9c Is a Membrane-Associated Protein that Suppresses Antiviral Responses in Cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Li, X.; Hou, P.; Ma, W.; Wang, X.; Wang, H.; Yu, Z.; Chang, H.; Wang, T.; Jin, S.; Wang, X.; et al. SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy. Cell. Mol. Immunol. 2022, 19, 67–78. [Google Scholar] [CrossRef]
| SARS-CoV-2 Protein | Type (Structural/Non-Structural/Accessory) | Primary Role in Virus | Mitochondrial Interaction/Impact |
|---|---|---|---|
| ORF9b | Accessory Protein | Unknown direct viral function; known host interaction | Targets outer mitochondrial membrane (OMM); induces mitochondrial fragmentation; inhibits mitochondrial protein import; impairs mitochondrial dynamics and function. |
| Nsp5 (3CLpro) | Non-Structural Protein | Main protease; cleaves viral polyprotein | Indirectly impacts mitochondria by increasing cellular stress due to essential role in viral replication. |
| Nsp12 (RdRp) | Non-Structural Protein | Core enzyme for viral genome replication and transcription | Places strain on mitochondrial ATP production due to high energy demands of viral replication. |
| Spike (S) protein | Structural Protein | Host cell entry | Induces inflammation and cellular stress; indirectly affects mitochondrial function in various tissues through systemic inflammation. |
| Envelope (E) protein | Structural Protein | Viral assembly and budding | Viroporin activity; alters ion homeostasis; potentially impacts mitochondrial calcium signaling and membrane potential. |
| Membrane (M) protein | Structural Protein | Viral assembly | Indirectly influences cellular processes relying on mitochondrial function through interactions with other viral proteins and host membranes. |
| Nucleocapsid (N) protein | Structural Protein | Binds viral RNA; replication and transcription | Contributes to cellular stress upon accumulation in the cytoplasm, which in turn affects mitochondria. |
| Therapeutic Strategy | Rationale/Goal | Examples/Mechanism (Based on Provided Text) |
|---|---|---|
| Mitochondria-Targeted Antioxidants | To combat oxidative stress induced by SARS-CoV-2 infection. | Focus on neutralizing Reactive Oxygen Species (ROS) within mitochondria. |
| Mitochondrial Biogenesis Enhancers | To promote the formation of new, healthy mitochondria. | Aim to increase the number and quality of mitochondria within cells. |
| Drugs Modulating Mitochondrial Dynamics | To restore proper balance between mitochondrial fission and fusion. | Address issues like excessive mitochondrial fragmentation or impaired fusion. |
| Inhibitors of Viral Proteins Targeting Mitochondria | To prevent mitochondrial damage caused by specific viral proteins. | Specifically target proteins like ORF9b to block their detrimental effects on mitochondria. |
| Metabolic Reprogramming Agents | To support overall mitochondrial function and cellular metabolism. | Aims to optimize metabolic pathways that rely on or are linked to mitochondria. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Refrigeri, M.; Tola, A.; Mogavero, R.; Pietracupa, M.M.; Gionta, G.; Scatena, R. Mechanisms of Mitochondrial Impairment by SARS-CoV-2 Proteins: A Nexus of Pathogenesis with Significant Biochemical and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 9885. https://doi.org/10.3390/ijms26209885
Refrigeri M, Tola A, Mogavero R, Pietracupa MM, Gionta G, Scatena R. Mechanisms of Mitochondrial Impairment by SARS-CoV-2 Proteins: A Nexus of Pathogenesis with Significant Biochemical and Clinical Implications. International Journal of Molecular Sciences. 2025; 26(20):9885. https://doi.org/10.3390/ijms26209885
Chicago/Turabian StyleRefrigeri, Marco, Alessandra Tola, Rosangela Mogavero, Maria Michela Pietracupa, Giulia Gionta, and Roberto Scatena. 2025. "Mechanisms of Mitochondrial Impairment by SARS-CoV-2 Proteins: A Nexus of Pathogenesis with Significant Biochemical and Clinical Implications" International Journal of Molecular Sciences 26, no. 20: 9885. https://doi.org/10.3390/ijms26209885
APA StyleRefrigeri, M., Tola, A., Mogavero, R., Pietracupa, M. M., Gionta, G., & Scatena, R. (2025). Mechanisms of Mitochondrial Impairment by SARS-CoV-2 Proteins: A Nexus of Pathogenesis with Significant Biochemical and Clinical Implications. International Journal of Molecular Sciences, 26(20), 9885. https://doi.org/10.3390/ijms26209885






