The Cannabinoid CB1 Receptor Inverse Agonist/Antagonist SR141716A Activates the Adenylate Cyclase/PKA Signaling Pathway Among Other Intracellular Emetic Signals to Evoke Vomiting in Least Shrews (Cryptotis parva)
Abstract
1. Introduction
2. Results
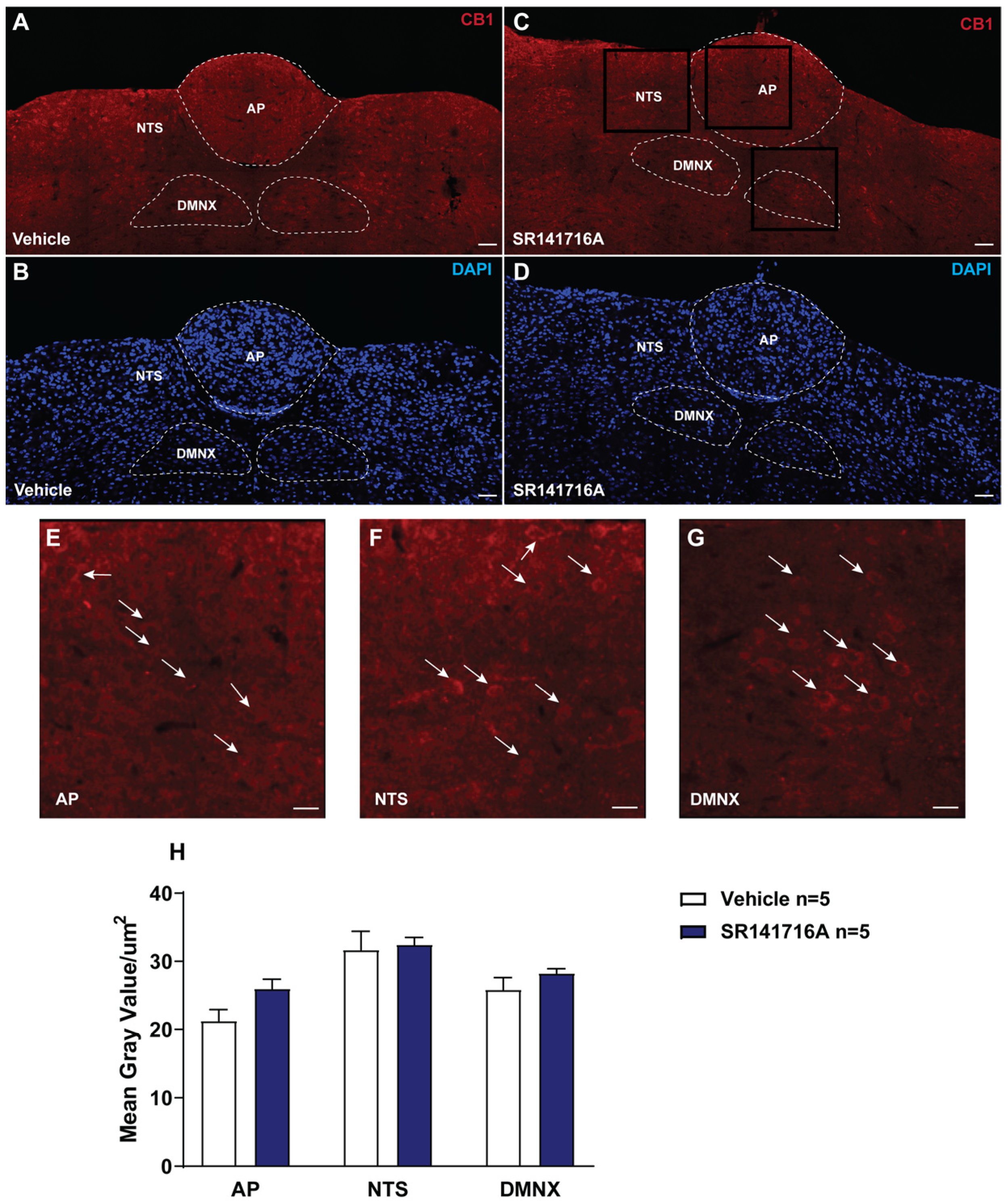
2.1. Acute Exposure to SR141716A Did Not Affect the Expression of CB1 Receptors in the Least Shrew Brainstem DVC
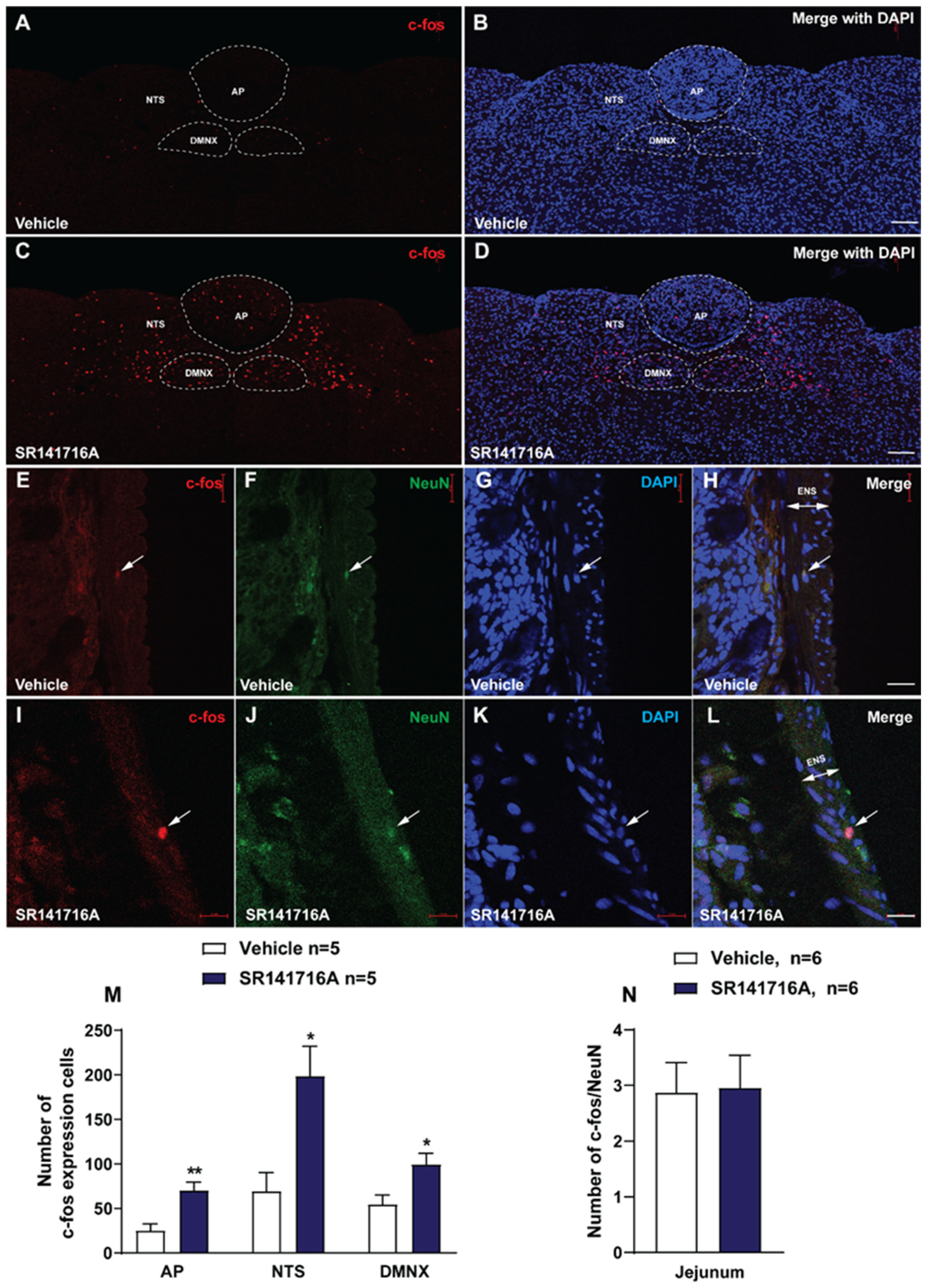
2.2. Administration of SR141716A (20 mg/kg, i.p.) Activates the Brainstem Emetic DVC
2.2.1. SR141716A Administration Significantly Increased c-fos Expression in the Brainstem DVC Emetic Nuclei
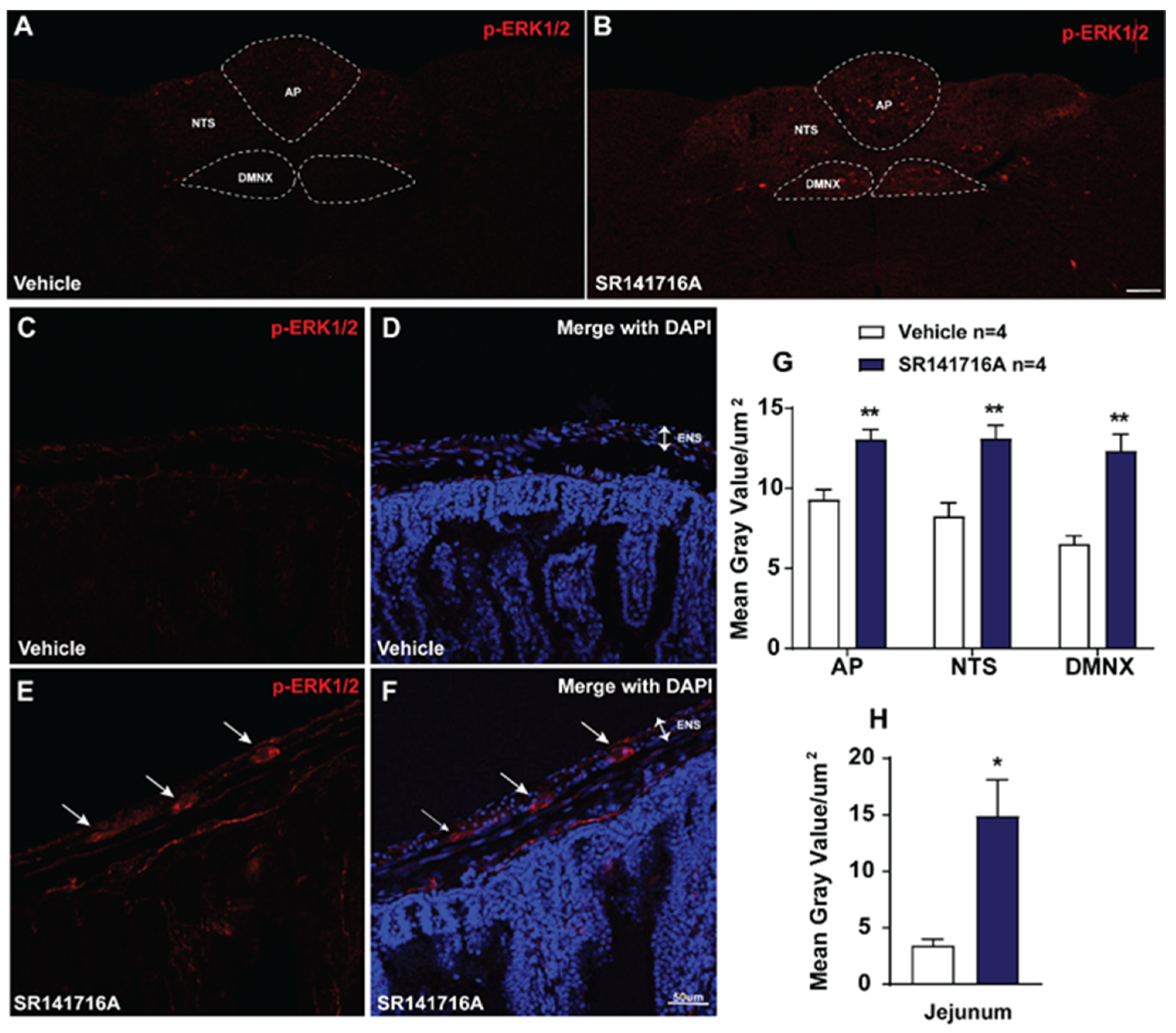
2.2.2. SR141716A Administration Evokes ERK1/2 Phosphorylation in Brainstem DVC and the Jejunum
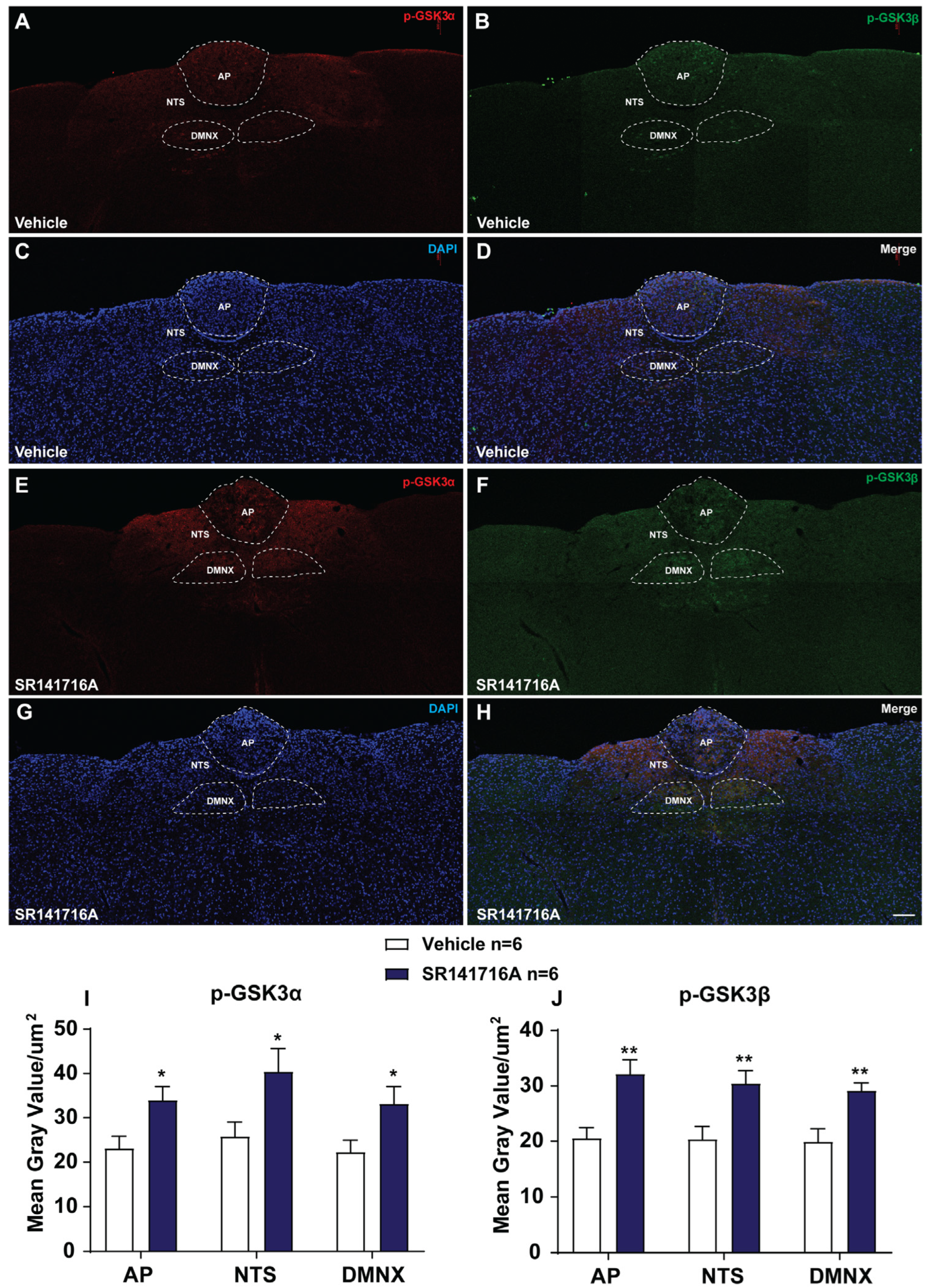
2.2.3. SR141716A Administration Induces GSK-3α/β Phosphorylation in Brainstem DVC
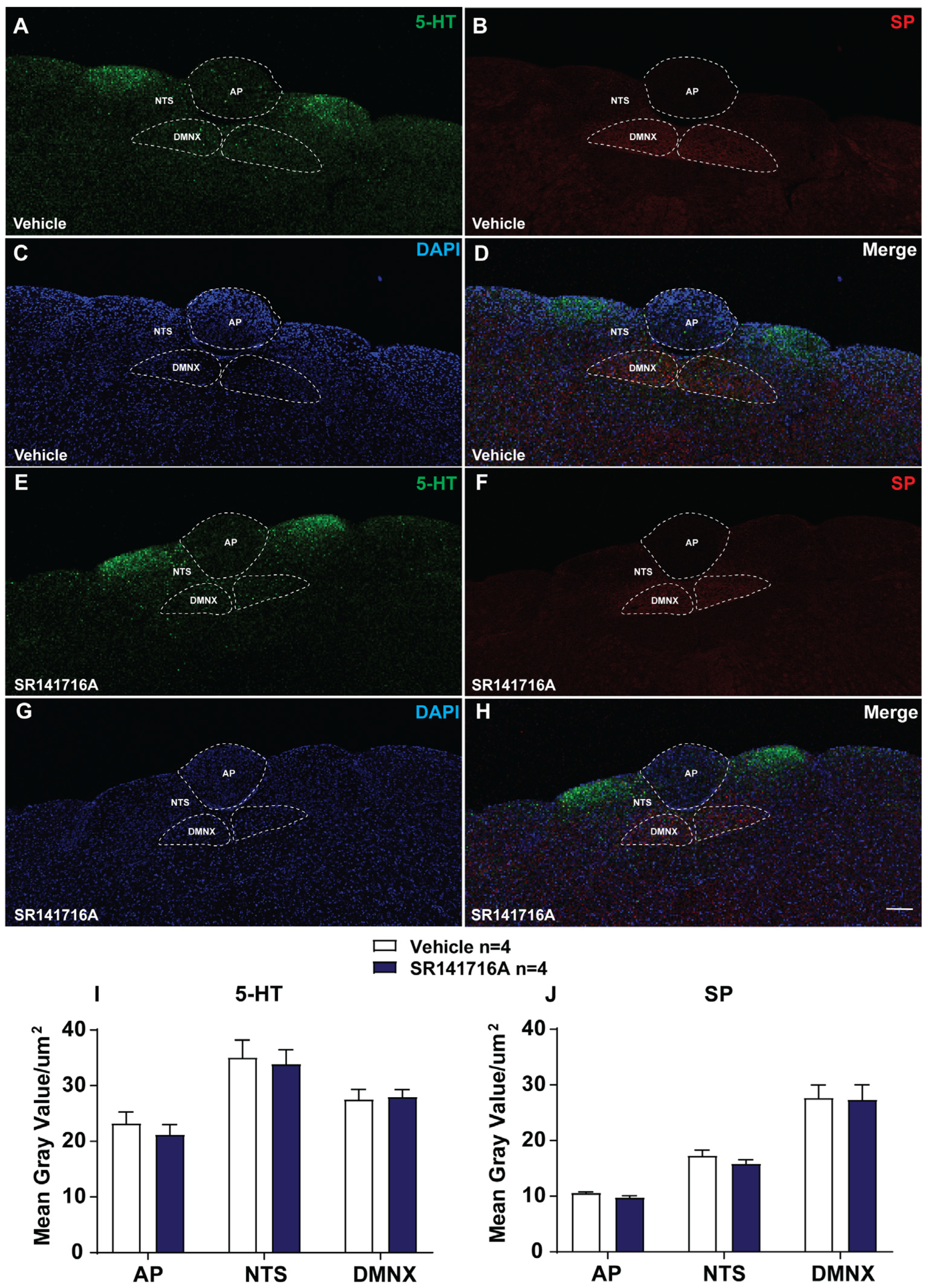
2.2.4. SR141716A Lacks Significant Effects on the Release of 5-HT and SP in Brainstem DVC
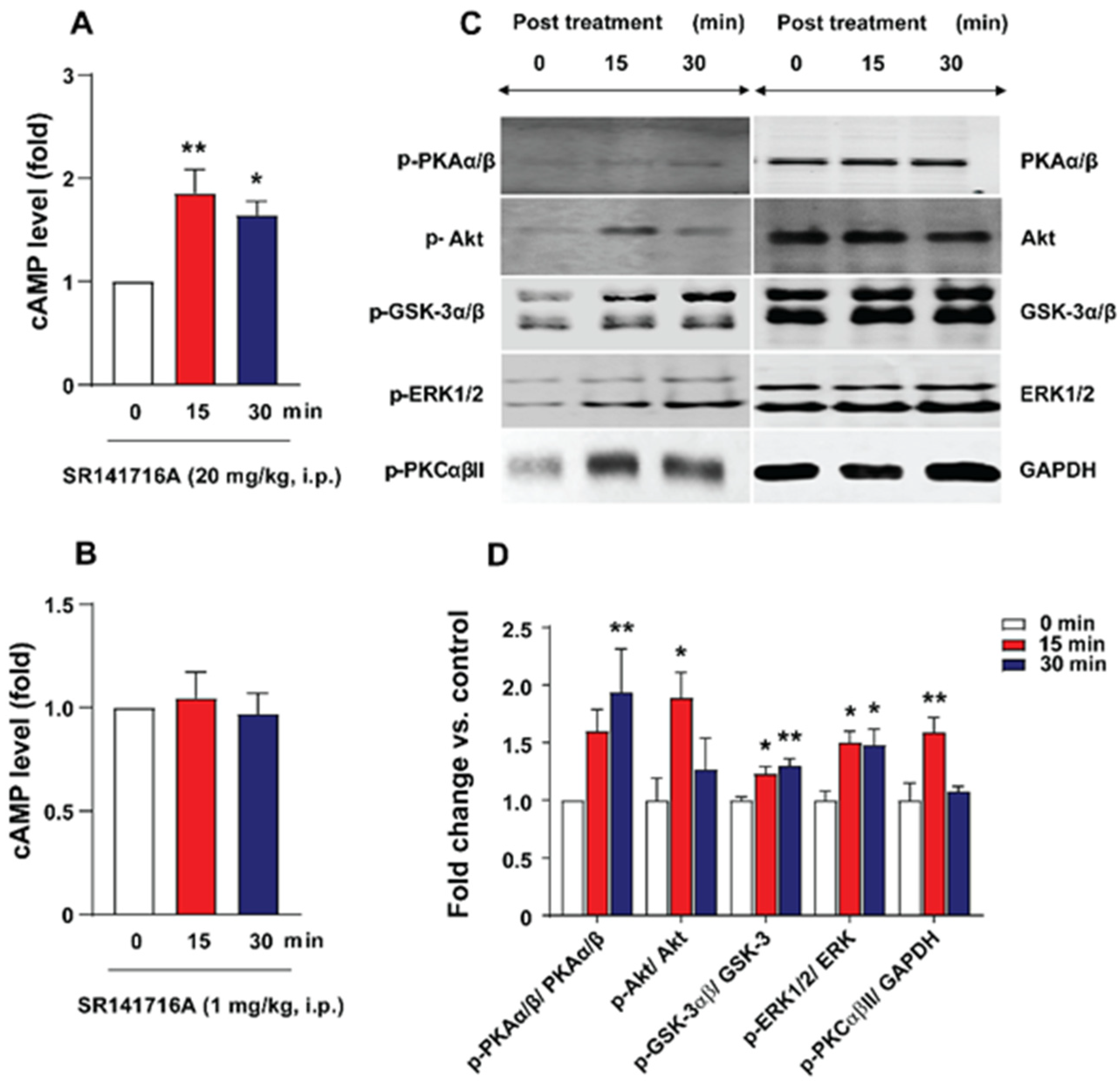
2.3. SR141716A Administration Increased Brainstem cAMP Levels
2.4. SR141716A Administration Increased Phosphorylation Levels of Emesis-Associated Proteins in Least Shrew Brainstems
2.5. Behavioral Studies
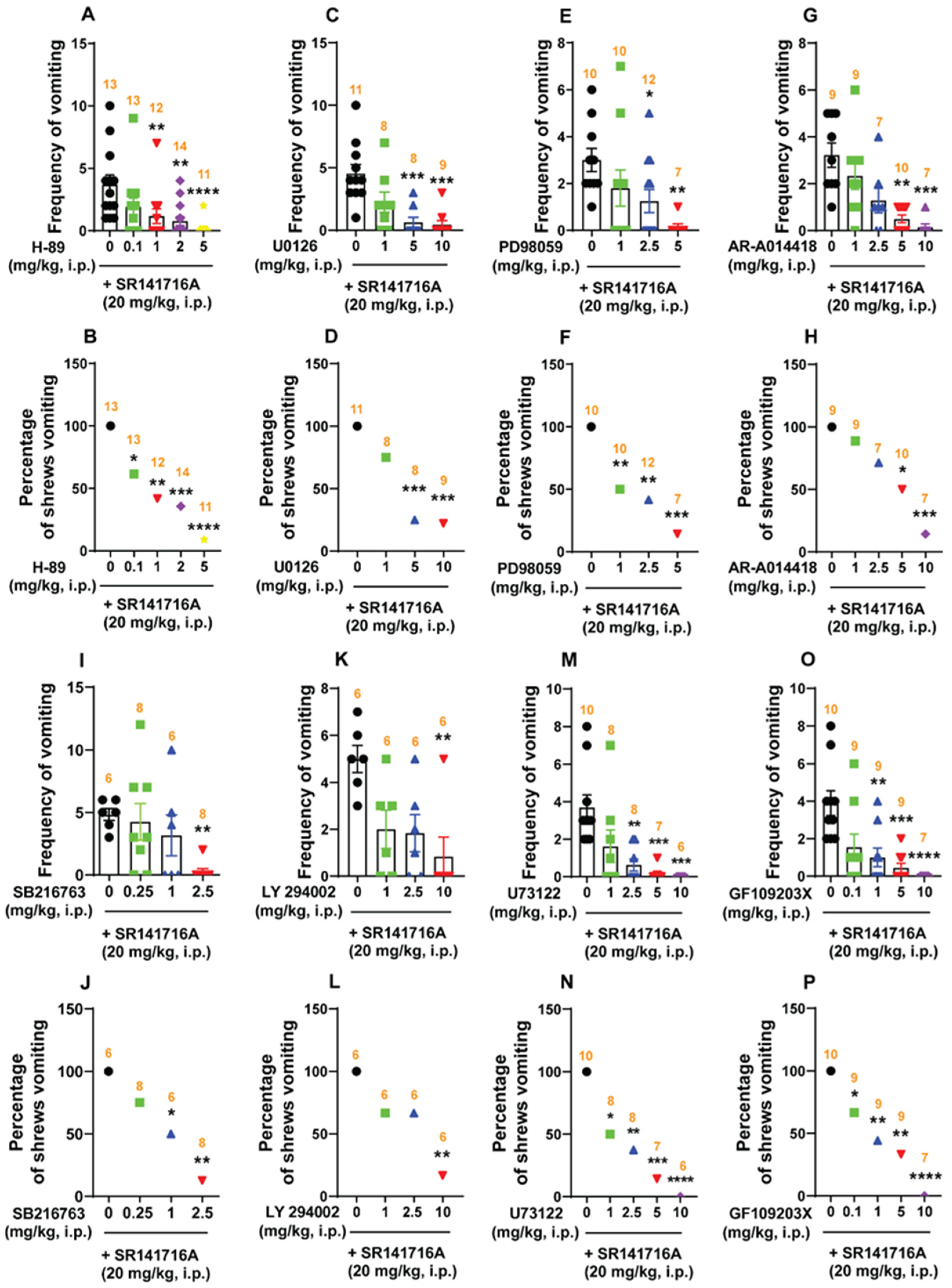
2.5.1. SR141716A (20 mg/kg, i.p.)-Induced Vomiting Involves Emesis-Associated PKA, ERK1/2, GSK-3α/β, PI3K, PLC, and PKCαβII Signals
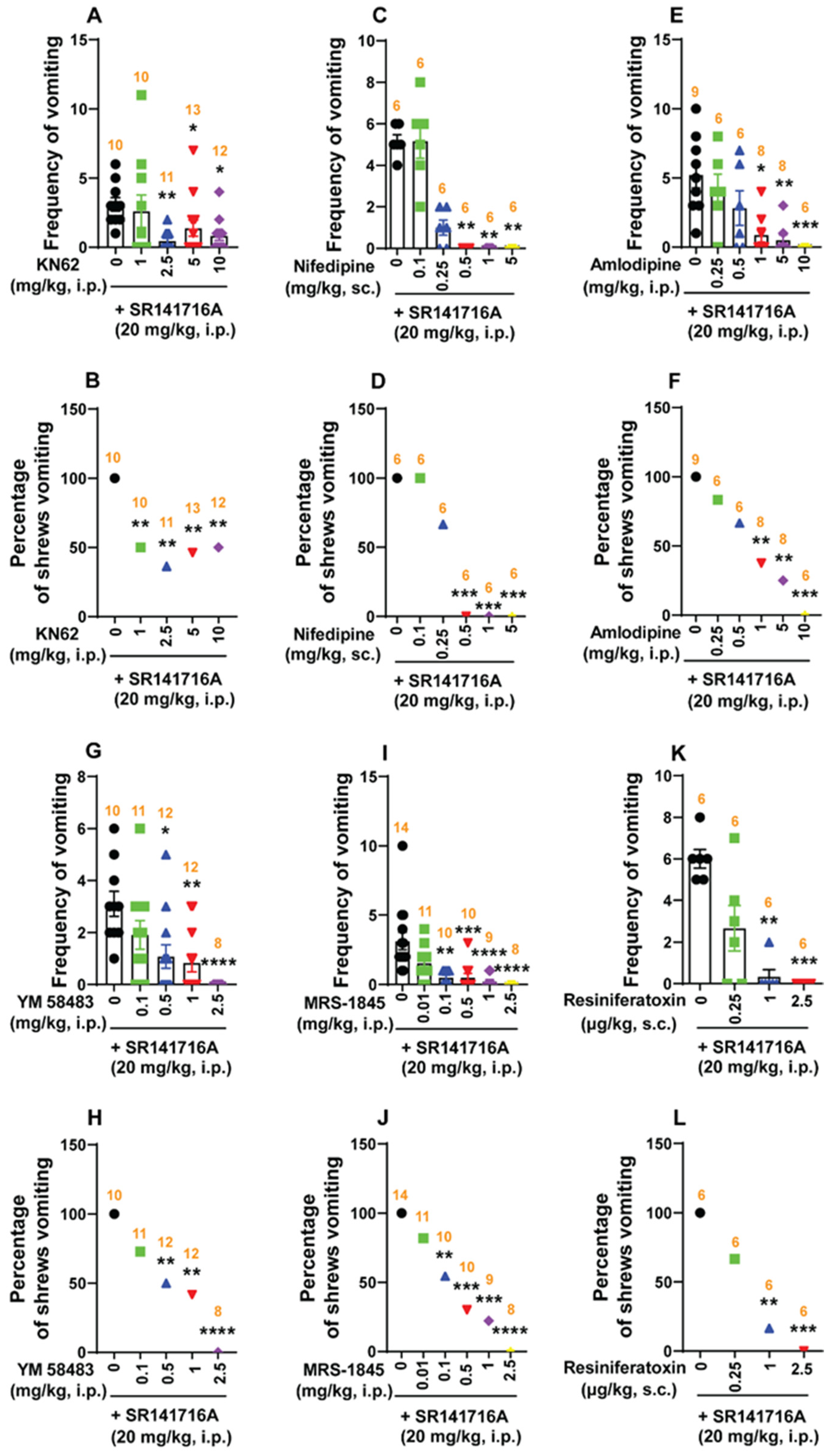
2.5.2. Ca2+ Channel Modulators Reduce SR141716A-Induced Emesis
Ca2+/Calmodulin-Dependent Kinase II (CaMKII)
Ca2+ Channel Blockers at the Cell Membrane Reduce SR141716A-Induced Emesis
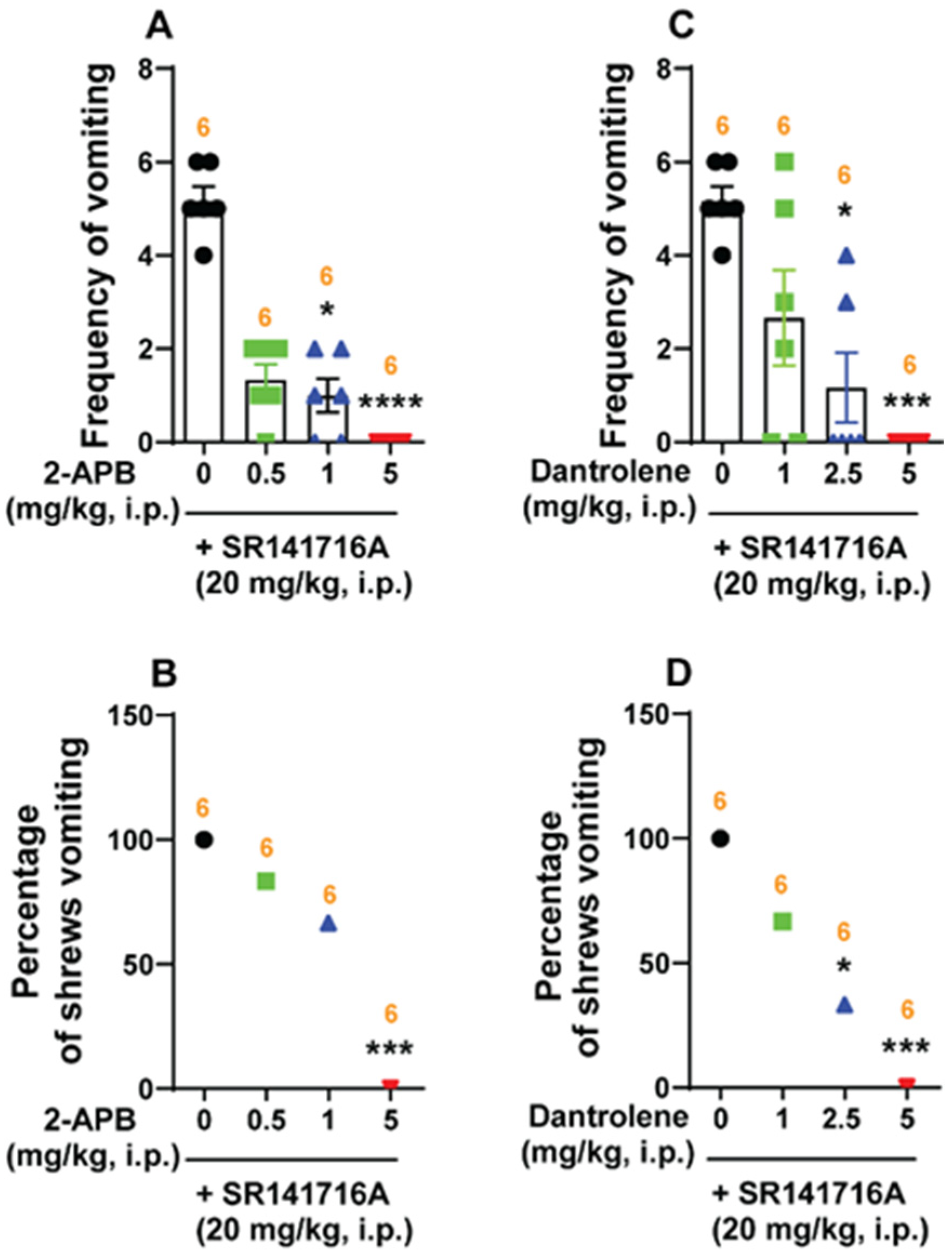
Intracellular Ca2+ Channel Blockers Reduce SR141716A-Induced Vomiting
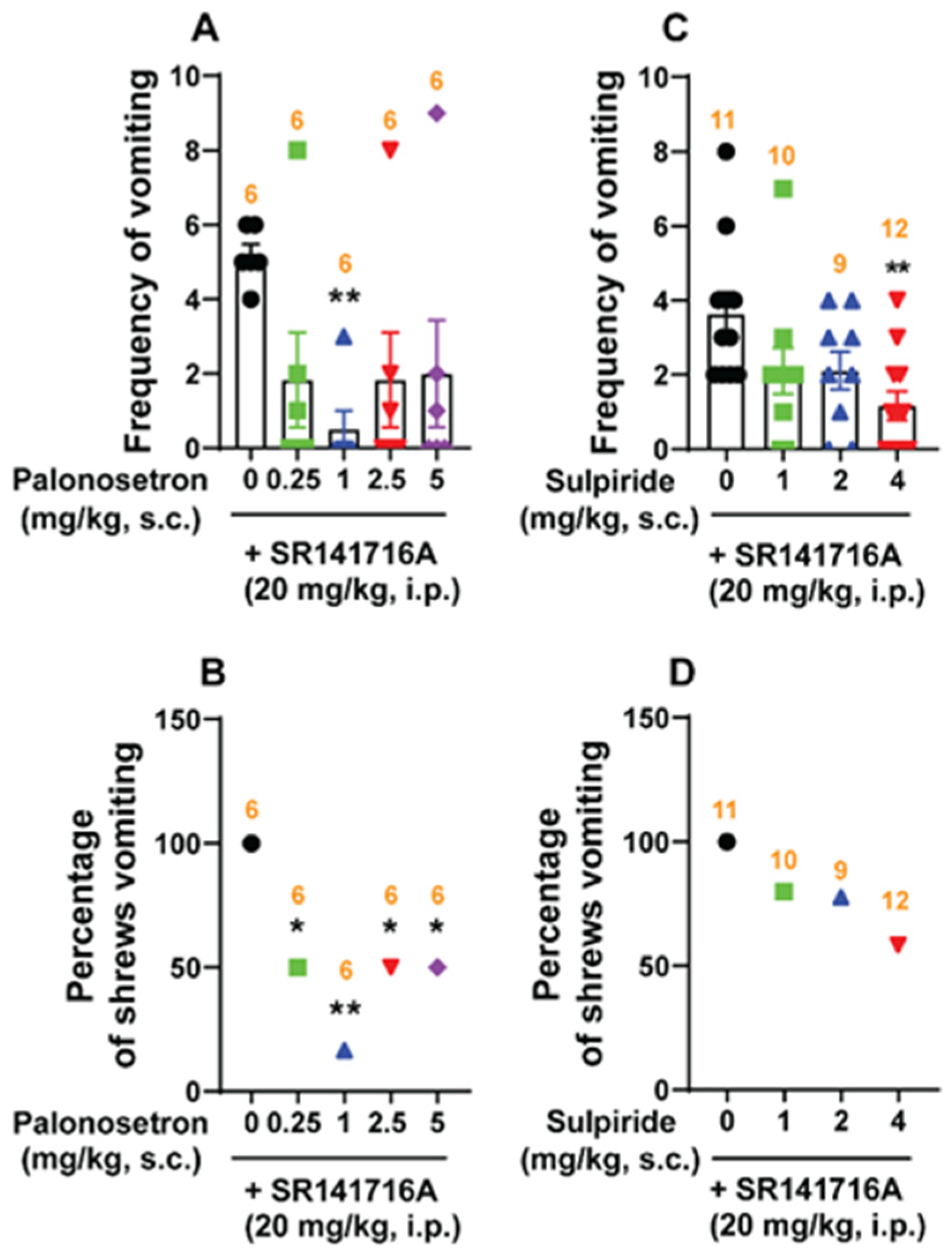
2.5.3. Classical Serotonin 5-HT3- and Dopamine D2/3-Receptor Antagonists Reduce SR141716A-Induced Emesis
3. Discussion
3.1. Acute Brief Exposure to SR141716A Did Not Alter the Cannabinoid CB1 Receptor Density in the Shrew Brainstem DVC Nuclei
3.2. SR141716A-Induced Vomiting Involves Multiple Intracellular Signaling Cascades
3.2.1. SR141716A (20 mg/kg) Induces Vomiting Through Both Central and Peripheral Mechanisms
3.2.2. SR141716A-Induced Emesis Involves the cAMP/PKA Signaling Pathway
3.2.3. SR141716A-Induced Vomiting Involves the PI3K/Akt Signaling Pathways
3.2.4. SR141716A-Induced Emesis Involves the PI3K/Akt/GSK-3 Signaling Pathway
3.2.5. SR141716A Promotes Vomiting via the PLC/PKCαβII Pathway
3.2.6. SR141716A Evokes Emesis Partly via Ca2+/Calmodulin (CaM)-Dependent Protein Kinase IIα (Ca2+/CaMKIIα)
3.3. SR141716A-Induced Vomiting Involves Ca2+-Ion Channel Modulators
3.4. Effects of Diverse Classical Emetic Receptor Antagonists on SR141716A-Induced Vomiting
3.5. SR141716A Produces its Emetic Signaling Effects via Inverse Agonism
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Behavioral Emesis Studies
4.4. Immunohistochemistry and Image Analysis
4.4.1. Immunohistochemical Distribution of CB1 Receptors in the Least Shrew DVC
4.4.2. c-fos Staining and Image Analysis
4.4.3. Phospho-ERK1/2 Immunohistochemistry
4.4.4. Phospho-GSK-3α/β Immunohistochemistry
4.4.5. 5-HT and SP Immunohistochemistry
4.5. Cyclic AMP Measurement
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darmani, N.A.; Ray, A.P. Evidence for a re-evaluation of the neurochemical and anatomical bases of chemotherapy-induced vomiting. Chem. Rev. 2009, 109, 3158–3199. [Google Scholar] [CrossRef]
- Travagli, R.A.; Anselmi, L. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 389–401. [Google Scholar] [CrossRef]
- Borison, H.L. Area postrema: Chemoreceptor circumventricular organ of the medulla oblongata. Prog. Neurobiol. 1989, 32, 351–390. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.Y.; Wu, C.; Yang, Z.Q.; Wang, K.X.; Shao, Z.H.; Yan, W. Advances in cannabinoid receptors pharmacology: From receptor structural insights to ligand discovery. Acta. Pharmacol. Sin. 2025, 46, 1495–1510. [Google Scholar] [CrossRef]
- Van Sickle, M.D.; Oland, L.D.; Ho, W.; Hillard, C.J.; Mackie, K.; Davison, J.S.; Sharkey, K.A. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology 2001, 121, 767–774. [Google Scholar] [CrossRef]
- Chiarlone, A.; Bellocchio, L.; Blazquez, C.; Resel, E.; Soria-Gomez, E.; Cannich, A.; Ferrero, J.J.; Sagredo, O.; Benito, C.; Romero, J.; et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc. Natl. Acad. Sci. USA 2014, 111, 8257–8262. [Google Scholar] [CrossRef]
- Izzo, A.A.; Fezza, F.; Capasso, R.; Bisogno, T.; Pinto, L.; Iuvone, T.; Esposito, G.; Mascolo, N.; Di Marzo, V.; Capasso, F. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br. J. Pharmacol. 2001, 134, 563–570. [Google Scholar] [CrossRef]
- Darmani, N.A.; Sim-Selley, L.J.; Martin, B.R.; Janoyan, J.J.; Crim, J.L.; Parekh, B.; Breivogel, C.S. Antiemetic and motor-depressive actions of CP55,940: Cannabinoid CB1 receptor characterization, distribution, and G-protein activation. Eur. J. Pharmacol. 2003, 459, 83–95. [Google Scholar] [CrossRef]
- Van Sickle, M.D.; Oland, L.D.; Mackie, K.; Davison, J.S.; Sharkey, K.A. Delta 9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G566–G576. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J. Cannabinoid signalling in the enteric nervous system. Neurogastroenterol. Motil. 2009, 21, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N.A. Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB(1) receptors in the least shrew. Pharmacol. Biochem. Behav. 2001, 69, 239–249. [Google Scholar] [CrossRef]
- Darmani, N.A. The cannabinoid CB1 receptor antagonist SR141716A reverses the antiemetic and motor depressant actions of WIN 55,212-2. Eur. J. Pharmacol. 2001, 430, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, M.; Parker, L.A.; Burton, P.; Mechoulam, R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology (Berl) 2004, 174, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N.A. Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB(1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology 2001, 24, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N.A.; Janoyan, J.J.; Kumar, N.; Crim, J.L. Behaviorally active doses of the CB1 receptor antagonist SR 141716A increase brain serotonin and dopamine levels and turnover. Pharmacol. Biochem. Behav. 2003, 75, 777–787. [Google Scholar] [CrossRef]
- Zou, P.; Yu, Y.; Zheng, N.; Yang, Y.; Paholak, H.J.; Yu, L.X.; Sun, D. Applications of human pharmacokinetic prediction in first-in-human dose estimation. AAPS J. 2012, 14, 262–281. [Google Scholar] [CrossRef]
- Robinson, J.D.; Cinciripini, P.M.; Karam-Hage, M.; Aubin, H.J.; Dale, L.C.; Niaura, R.; Anthenelli, R.M.; Group, S. Pooled analysis of three randomized, double-blind, placebo controlled trials with rimonabant for smoking cessation. Addict. Biol. 2018, 23, 291–303. [Google Scholar] [CrossRef]
- Gorelick, D.A.; Heishman, S.J.; Preston, K.L.; Nelson, R.A.; Moolchan, E.T.; Huestis, M.A. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. Am. Heart J. 2006, 151, 754.e1–754.e5. [Google Scholar] [CrossRef]
- Belkacemi, L.; Sun, Y.; Darmani, N.A. Evidence for Bell-Shaped Dose-Response Emetic Effects of Temsirolimus and Analogs: The Broad-Spectrum Antiemetic Efficacy of a Large Dose of Temsirolimus Against Diverse Emetogens in the Least Shrew (Cryptotis parva). Front. Pharmacol. 2022, 13, 848673. [Google Scholar] [CrossRef]
- Darmani, N.A.; Belkacemi, L.; Zhong, W. Delta(9)-THC and related cannabinoids suppress substance P- induced neurokinin NK(1)-receptor-mediated vomiting via activation of cannabinoid CB(1) receptor. Eur. J. Pharmacol. 2019, 865, 172806. [Google Scholar] [CrossRef]
- Sun, Y.; Darmani, N.A. A Comparative Study of the Antiemetic Effects of alpha(2)-Adrenergic Receptor Agonists Clonidine and Dexmedetomidine against Diverse Emetogens in the Least Shrew (Cryptotis parva) Model of Emesis. Int. J. Mol. Sci. 2024, 25, 4603. [Google Scholar] [CrossRef]
- Boczek, T.; Zylinska, L. Receptor-Dependent and Independent Regulation of Voltage-Gated Ca2+ Channels and Ca2+-Permeable Channels by Endocannabinoids in the Brain. Int. J. Mol. Sci. 2021, 22, 8168. [Google Scholar] [CrossRef] [PubMed]
- Console-Bram, L.; Marcu, J.; Abood, M.E. Cannabinoid receptors: Nomenclature and pharmacological principles. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 4–15. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.J.; Yin, N.; Gao, F.; Miao, Y.; Li, Q.; Li, F.; Sun, X.H.; Yang, X.L.; Wang, Z. Cannabinoid CB1 and CB2 receptors differentially modulate L- and T-type Ca2+ channels in rat retinal ganglion cells. Neuropharmacology 2017, 124, 143–156. [Google Scholar] [CrossRef]
- Turu, G.; Hunyady, L. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 2010, 44, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Rubino, T.; Vigano, D.; Zagato, E.; Sala, M.; Parolaro, D. In vivo characterization of the specific cannabinoid receptor antagonist, SR141716A: Behavioral and cellular responses after acute and chronic treatments. Synapse 2000, 35, 8–14. [Google Scholar] [CrossRef]
- Chen, W.; Xu, C.; Liu, H.Y.; Long, L.; Zhang, W.; Zheng, Z.B.; Xie, Y.D.; Wang, L.L.; Li, S. Novel selective cannabinoid CB(1) receptor antagonist MJ08 with potent in vivo bioactivity and inverse agonistic effects. Acta. Pharmacol. Sin. 2011, 32, 1148–1158. [Google Scholar] [CrossRef]
- Mato, S.; Pazos, A.; Valdizan, E.M. Cannabinoid receptor antagonism and inverse agonism in response to SR141716A on cAMP production in human and rat brain. Eur. J. Pharmacol. 2002, 443, 43–46. [Google Scholar] [CrossRef]
- Henstridge, C.M.; Balenga, N.A.; Schroder, R.; Kargl, J.K.; Platzer, W.; Martini, L.; Arthur, S.; Penman, J.; Whistler, J.L.; Kostenis, E.; et al. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br. J. Pharmacol. 2010, 160, 604–614. [Google Scholar] [CrossRef]
- Jelsing, J.; Galzin, A.M.; Guillot, E.; Pruniaux, M.P.; Larsen, P.J.; Vrang, N. Localization and phenotypic characterization of brainstem neurons activated by rimonabant and WIN55,212-2. Brain Res. Bull. 2009, 78, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Mukhtarov, M.; Ragozzino, D.; Bregestovski, P. Dual Ca2+ modulation of glycinergic synaptic currents in rodent hypoglossal motoneurones. J. Physiol. 2005, 569, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Muthian, S.; Kearn, C.S. Effects of CB(1) cannabinoid receptor activation on cerebellar granule cell nitric oxide synthase activity. FEBS. Lett. 1999, 459, 277–281. [Google Scholar] [CrossRef]
- Hoddah, H.; Marcantoni, A.; Comunanza, V.; Carabelli, V.; Carbone, E. L-type channel inhibition by CB1 cannabinoid receptors is mediated by PTX-sensitive G proteins and cAMP/PKA in GT1-7 hypothalamic neurons. Cell Calcium 2009, 46, 303–312. [Google Scholar] [CrossRef]
- Le Bacquer, O.; Lanchais, K.; Combe, K.; Van Den Berghe, L.; Walrand, S. Acute rimonabant treatment promotes protein synthesis in C2C12 myotubes through a CB1-independent mechanism. J. Cell Physiol. 2021, 236, 2669–2683. [Google Scholar] [CrossRef]
- Lever, I.J.; Malcangio, M. CB(1) receptor antagonist SR141716A increases capsaicin-evoked release of Substance P from the adult mouse spinal cord. Br. J. Pharmacol. 2002, 135, 21–24. [Google Scholar] [CrossRef]
- Carpenter, D.O.; Briggs, D.B.; Knox, A.P.; Strominger, N. Excitation of area postrema neurons by transmitters, peptides, and cyclic nucleotides. J. Neurophysiol. 1988, 59, 358–369. [Google Scholar] [CrossRef]
- Robichaud, A.; Savoie, C.; Stamatiou, P.B.; Lachance, N.; Jolicoeur, P.; Rasori, R.; Chan, C.C. Assessing the emetic potential of PDE4 inhibitors in rats. Br. J. Pharmacol. 2002, 135, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Shahbaz, O.; Teskey, G.; Beever, A.; Kachour, N.; Venketaraman, V.; Darmani, N.A. Mechanisms of Nausea and Vomiting: Current Knowledge and Recent Advances in Intracellular Emetic Signaling Systems. Int. J. Mol. Sci. 2021, 22, 5797. [Google Scholar] [CrossRef]
- Hotokezaka, H.; Sakai, E.; Kanaoka, K.; Saito, K.; Matsuo, K.; Kitaura, H.; Yoshida, N.; Nakayama, K. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J. Biol. Chem. 2002, 277, 47366–47372. [Google Scholar] [CrossRef]
- Zhong, W.; Darmani, N.A. The pivotal role of glycogen synthase kinase 3 (GSK-3) in vomiting evoked by specific emetogens in the least shrew (Cryptotis parva). Neurochem. Int. 2020, 132, 104603. [Google Scholar] [CrossRef]
- Zhong, W.; Chebolu, S.; Darmani, N.A. Intracellular emetic signaling cascades by which the selective neurokinin type 1 receptor (NK(1)R) agonist GR73632 evokes vomiting in the least shrew (Cryptotis parva). Neurochem. Int. 2019, 122, 106–119. [Google Scholar] [CrossRef]
- Zhong, W.; Darmani, N.A. The Contribution of Phospholipase C in Vomiting in the Least Shrew (Cryptotis parva) Model of Emesis. Front. Pharmacol. 2021, 12, 736842. [Google Scholar] [CrossRef]
- Chaipunko, S.; Sookkua, T.; Nopparat, C.; Chutabhakdikul, N. Oxytocin Protects Against Corticosterone-Induced DA Dysfunction: An Involvement of the PKA/CREB Pathway. Neurochem. Res. 2024, 50, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Hutchinson, T.E.; Chebolu, S.; Darmani, N.A. Serotonin 5-HT3 receptor-mediated vomiting occurs via the activation of Ca2+/CaMKII-dependent ERK1/2 signaling in the least shrew (Cryptotis parva). PLoS ONE 2014, 9, e104718. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.E.; Zhong, W.; Chebolu, S.; Wilson, S.M.; Darmani, N.A. L-type calcium channels contribute to 5-HT3-receptor-evoked CaMKIIalpha and ERK activation and induction of emesis in the least shrew (Cryptotis parva). Eur. J. Pharmacol. 2015, 755, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, L.; Zhong, W.; Darmani, N.A. Signal transduction pathways involved in dopamine D(2) receptor-evoked emesis in the least shrew (Cryptotis parva). Auton. Neurosci. 2021, 233, 102807. [Google Scholar] [CrossRef]
- Darmani, N.A.; Henry, D.A.; Zhong, W.; Chebolu, S. Ultra-low doses of the transient receptor potential vanilloid 1 agonist, resiniferatoxin, prevents vomiting evoked by diverse emetogens in the least shrew (Cryptotis parva). Behav. Pharmacol. 2020, 31, 3–14. [Google Scholar] [CrossRef]
- Bullitt, E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J. Comp. Neurol. 1990, 296, 517–530. [Google Scholar] [CrossRef]
- Ferrini, F.; Russo, A.; Salio, C. Fos and pERK immunoreactivity in spinal cord slices: Comparative analysis of in vitro models for testing putative antinociceptive molecules. Ann. Anat. 2014, 196, 217–223. [Google Scholar] [CrossRef]
- Andrews, P.L.; Naylor, R.J.; Joss, R.A. Neuropharmacology of emesis and its relevance to anti-emetic therapy. Consensus and controversies. Support Care Cancer 1998, 6, 197–203. [Google Scholar] [PubMed]
- Zhong, W.; Chebolu, S.; Darmani, N.A. Intracellular emetic signaling evoked by the L-type Ca2+ channel agonist FPL64176 in the least shrew (Cryptotis parva). Eur. J. Pharmacol. 2018, 834, 157–168. [Google Scholar] [CrossRef]
- Zhong, W.; Chebolu, S.; Darmani, N.A. Thapsigargin-induced activation of Ca2+-CaMKII-ERK in brainstem contributes to substance P release and induction of emesis in the least shrew. Neuropharmacology 2016, 103, 195–210. [Google Scholar] [CrossRef]
- Robichaud, A.; Tattersall, F.D.; Choudhury, I.; Rodger, I.W. Emesis induced by inhibitors of type IV cyclic nucleotide phosphodiesterase (PDE IV) in the ferret. Neuropharmacology 1999, 38, 289–297, Erratum in Neuropharmacology 2001, 40, 465. [Google Scholar] [CrossRef] [PubMed]
- Alkam, T.; Chebolu, S.; Darmani, N.A. Cyclophosphamide causes activation of protein kinase A (PKA) in the brainstem of vomiting least shrews (Cryptotis parva). Eur. J. Pharmacol. 2014, 722, 156–164. [Google Scholar] [CrossRef]
- Means, C.K.; Lygren, B.; Langeberg, L.K.; Jain, A.; Dixon, R.E.; Vega, A.L.; Gold, M.G.; Petrosyan, S.; Taylor, S.S.; Murphy, A.N.; et al. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, E1227–E1235. [Google Scholar] [CrossRef]
- Akabane, S.; Oka, T. Insights into the regulation of mitochondrial functions by protein kinase A-mediated phosphorylation. J. Biochem. 2023, 175, 1–7. [Google Scholar] [CrossRef]
- Hudmon, A.; Schulman, H. Neuronal Ca2+/calmodulin-dependent protein kinase II: The role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 2002, 71, 473–510. [Google Scholar] [CrossRef]
- Mizuta, K.; Gallos, G.; Zhu, D.; Mizuta, F.; Goubaeva, F.; Xu, D.; Panettieri, R.A., Jr.; Yang, J.; Emala, C.W., Sr. Expression and coupling of neurokinin receptor subtypes to inositol phosphate and calcium signaling pathways in human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L523–L534. [Google Scholar] [CrossRef]
- Ray, A.P.; Griggs, L.; Darmani, N.A. Delta 9-tetrahydrocannabinol suppresses vomiting behavior and Fos expression in both acute and delayed phases of cisplatin-induced emesis in the least shrew. Behav. Brain Res. 2009, 196, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Storr, M.A.; Sharkey, K.A. The endocannabinoid system and gut-brain signalling. Curr. Opin. Pharmacol. 2007, 7, 575–582. [Google Scholar] [CrossRef]
- Rubino, T.; Vigano, D.; Massi, P.; Parolaro, D. Changes in the cannabinoid receptor binding, G protein coupling, and cyclic AMP cascade in the CNS of rats tolerant to and dependent on the synthetic cannabinoid compound CP55,940. J. Neurochem. 2000, 75, 2080–2086. [Google Scholar] [CrossRef]
- Ray, A.P.; Chebolu, S.; Darmani, N.A. Receptor-selective agonists induce emesis and Fos expression in the brain and enteric nervous system of the least shrew (Cryptotis parva). Pharmacol. Biochem. Behav. 2009, 94, 211–218. [Google Scholar] [CrossRef]
- Zhong, W.; Darmani, N.A. The HCN Channel Blocker ZD7288 Induces Emesis in the Least Shrew (Cryptotis parva). Front. Pharmacol. 2021, 12, 647021. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Chebolu, S.; Darmani, N.A. Central and peripheral emetic loci contribute to vomiting evoked by the Akt inhibitor MK-2206 in the least shrew model of emesis. Eur. J. Pharmacol. 2021, 900, 174065. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N.A.; Zhong, W.; Chebolu, S.; Mercadante, F. Differential and additive suppressive effects of 5-HT3 (palonosetron)- and NK1 (netupitant)-receptor antagonists on cisplatin-induced vomiting and ERK1/2, PKA and PKC activation. Pharmacol. Biochem. Behav. 2015, 131, 104–111. [Google Scholar] [CrossRef]
- Besnard, A.; Laroche, S.; Caboche, J. Comparative dynamics of MAPK/ERK signalling components and immediate early genes in the hippocampus and amygdala following contextual fear conditioning and retrieval. Brain Struct. Funct. 2014, 219, 415–430, Erratum in Brain Struct Funct. 2014, 219, 431. [Google Scholar] [CrossRef]
- Iqbal, J.; Zaidi, M. Molecular regulation of mechanotransduction. Biochem. Biophy.s Res. Commun. 2005, 328, 751–755. [Google Scholar] [CrossRef]
- Darmani, N.A.; Johnson, J.C. Central and peripheral mechanisms contribute to the antiemetic actions of delta-9-tetrahydrocannabinol against 5-hydroxytryptophan-induced emesis. Eur. J. Pharmacol. 2004, 488, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Goncalves de Moraes, V.L.; Singer, M.; Vargaftig, B.B.; Chignard, M. Effects of rolipram on cyclic AMP levels in alveolar macrophages and lipopolysaccharide-induced inflammation in mouse lung. Br. J. Pharmacol. 1998, 123, 631–636. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Huang, N.L.; Juang, J.M.; Wang, Y.H.; Hsueh, C.H.; Liang, Y.J.; Lin, J.L.; Tsai, C.T.; Lai, L.P. Rimonabant inhibits TNF-alpha-induced endothelial IL-6 secretion via CB1 receptor and cAMP-dependent protein kinase pathway. Acta. Pharmacol. Sin. 2010, 31, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, T.; Nishioka, C.; Bandobashi, K.; Yang, Y.; Kuwayama, Y.; Adachi, Y.; Takeuchi, T.; Koeffler, H.P.; Taguchi, H. Longitudinal inhibition of PI3K/Akt/mTOR signaling by LY294002 and rapamycin induces growth arrest of adult T-cell leukemia cells. Leuk. Res. 2007, 31, 673–682. [Google Scholar] [CrossRef]
- Tang, L.; Mo, Y.; Li, Y.; Zhong, Y.; He, S.; Zhang, Y.; Tang, Y.; Fu, S.; Wang, X.; Chen, A. Urolithin A alleviates myocardial ischemia/reperfusion injury via PI3K/Akt pathway. Biochem. Biophys. Res. Commun. 2017, 486, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Tadijan, A.; Vlasic, I.; Vlainic, J.; Dikic, D.; Orsolic, N.; Jazvinscak Jembrek, M. Intracellular Molecular Targets and Signaling Pathways Involved in Antioxidative and Neuroprotective Effects of Cannabinoids in Neurodegenerative Conditions. Antioxidants 2022, 11, 2049. [Google Scholar] [CrossRef]
- Jiang, H.; Fan, D.; Zhou, G.; Li, X.; Deng, H. Phosphatidylinositol 3-kinase inhibitor (LY294002) induces apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J. Exp. Clin. Cancer Res. 2010, 29, 34. [Google Scholar] [CrossRef]
- Howlett, A.C. Cannabinoid receptor signaling. Handb. Exp. Pharmacol. 2005, 168, 53–79. [Google Scholar]
- Galve-Roperh, I.; Rueda, D.; Gomez del Pulgar, T.; Velasco, G.; Guzman, M. Mechanism of extracellular signal-regulated kinase activation by the CB(1) cannabinoid receptor. Mol. Pharmacol. 2002, 62, 1385–1392. [Google Scholar] [CrossRef]
- Davis, M.I.; Ronesi, J.; Lovinger, D.M. A predominant role for inhibition of the adenylate cyclase/protein kinase A pathway in ERK activation by cannabinoid receptor 1 in N1E-115 neuroblastoma cells. J. Biol. Chem. 2003, 278, 48973–48980. [Google Scholar] [CrossRef] [PubMed]
- Olianas, M.C.; Dedoni, S.; Onali, P. The GABA(B) positive allosteric modulators CGP7930 and GS39783 stimulate ERK1/2 signalling in cells lacking functional GABA(B) receptors. Eur. J. Pharmacol. 2017, 794, 135–146. [Google Scholar] [CrossRef]
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Ding, J.; Liu, N.; Peng, S.; Wang, J.; Wang, F.; Zhang, Y. Targeting Akt by SC66 triggers GSK-3beta mediated apoptosis in colon cancer therapy. Cancer Cell Int. 2019, 19, 124. [Google Scholar] [CrossRef]
- Mazzardo-Martins, L.; Martins, D.F.; Stramosk, J.; Cidral-Filho, F.J.; Santos, A.R. Glycogen synthase kinase 3-specific inhibitor AR-A014418 decreases neuropathic pain in mice: Evidence for the mechanisms of action. Neuroscience 2012, 226, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Ozaita, A.; Puighermanal, E.; Maldonado, R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J. Neurochem. 2007, 102, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Shum, C.; Dutan, L.; Annuario, E.; Warre-Cornish, K.; Taylor, S.E.; Taylor, R.D.; Andreae, L.C.; Buckley, N.J.; Price, J.; Bhattacharyya, S.; et al. Delta(9)-tetrahydrocannabinol and 2-AG decreases neurite outgrowth and differentially affects ERK1/2 and Akt signaling in hiPSC-derived cortical neurons. Mol. Cell. Neurosci. 2020, 103, 103463. [Google Scholar] [CrossRef] [PubMed]
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar] [CrossRef]
- Goldsmith, Z.G.; Dhanasekaran, D.N. G protein regulation of MAPK networks. Oncogene 2007, 26, 3122–3142. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Jiang, Z.; Qing, H. G Protein-Coupled Receptors (GPCRs) in Alzheimer’s Disease: A Focus on BACE1 Related GPCRs. Front. Aging. Neurosci. 2016, 8, 58. [Google Scholar] [CrossRef]
- Sugiura, T.; Kodaka, T.; Kondo, S.; Nakane, S.; Kondo, H.; Waku, K.; Ishima, Y.; Watanabe, K.; Yamamoto, I. Is the cannabinoid CB1 receptor a 2-arachidonoylglycerol receptor? Structural requirements for triggering a Ca2+ transient in NG108-15 cells. J. Biochem. 1997, 122, 890–895. [Google Scholar] [CrossRef]
- Lauckner, J.E.; Hille, B.; Mackie, K. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 19144–19149. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Marini, P.; Matias, I.; Moriello, A.S.; Starowicz, K.; Cristino, L.; Nigam, S.; Di Marzo, V. Mechanisms for the coupling of cannabinoid receptors to intracellular calcium mobilization in rat insulinoma beta-cells. Exp. Cell Res. 2007, 313, 2993–3004. [Google Scholar] [CrossRef]
- Wilson, C.H.; Ali, E.S.; Scrimgeour, N.; Martin, A.M.; Hua, J.; Tallis, G.A.; Rychkov, G.Y.; Barritt, G.J. Steatosis inhibits liver cell store-operated Ca2+ entry and reduces ER Ca2+ through a protein kinase C-dependent mechanism. Biochem. J. 2015, 466, 379–390. [Google Scholar] [CrossRef]
- Gustin, R.M.; Shonesy, B.C.; Robinson, S.L.; Rentz, T.J.; Baucum, A.J., 2nd; Jalan-Sakrikar, N.; Winder, D.G.; Stanwood, G.D.; Colbran, R.J. Loss of Thr286 phosphorylation disrupts synaptic CaMKIIalpha targeting, NMDAR activity and behavior in pre-adolescent mice. Mol. Cell Neurosci. 2011, 47, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Kitamura, Y.; Ogawa, H.; Oka, K. Serotonin induces the increase in intracellular Ca2+ that enhances neurite outgrowth in PC12 cells via activation of 5-HT3 receptors and voltage-gated calcium channels. J. Neurosci. Res. 2006, 84, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Ziviani, E.; Lippi, G.; Bano, D.; Munarriz, E.; Guiducci, S.; Zoli, M.; Young, K.W.; Nicotera, P. Ryanodine receptor-2 upregulation and nicotine-mediated plasticity. EMBO. J. 2011, 30, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Schlotthauer, K.; Bers, D.M. Transmission of information from cardiac dihydropyridine receptor to ryanodine receptor: Evidence from BayK 8644 effects on resting Ca2+ sparks. Circ. Res. 2000, 87, 106–111. [Google Scholar] [CrossRef]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef]
- Zurgil, N.; Zisapel, N. Calcium-dependent protein phosphorylation and dephosphorylation in intact brain neurons in culture. FEBS. Lett. 1983, 156, 257–261. [Google Scholar] [CrossRef]
- Zhong, W.; Picca, A.J.; Lee, A.S.; Darmani, N.A. Ca2+ signaling and emesis: Recent progress and new perspectives. Auton. Neurosci. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Szabo, B.; Schlicker, E. Effects of cannabinoids on neurotransmission. Handb. Exp. Pharmacol. 2005, 168, 327–365. [Google Scholar]
- Mackie, K.; Hille, B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc. Natl. Acad. Sci. USA 1992, 89, 3825–3829. [Google Scholar] [CrossRef]
- Mackie, K.; Lai, Y.; Westenbroek, R.; Mitchell, R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J. Neurosci. 1995, 15, 6552–6561. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Ikeda, S.R.; Lewis, D.L. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol. Pharmacol. 1996, 49, 707–714. [Google Scholar]
- Twitchell, W.; Brown, S.; Mackie, K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J. Neurophysiol. 1997, 78, 43–50. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshimaru, T.; Inoue, T.; Ra, C. Ca v 1.2 L-type Ca2+ channel protects mast cells against activation-induced cell death by preventing mitochondrial integrity disruption. Mol. Immunol. 2009, 46, 2370–2380. [Google Scholar] [CrossRef]
- Yoshimaru, T.; Suzuki, Y.; Inoue, T.; Ra, C. L-type Ca2+ channels in mast cells: Activation by membrane depolarization and distinct roles in regulating mediator release from store-operated Ca2+ channels. Mol. Immunol. 2009, 46, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Berrout, J.; Isokawa, M. Homeostatic and stimulus-induced coupling of the L-type Ca2+ channel to the ryanodine receptor in the hippocampal neuron in slices. Cell Calcium 2009, 46, 30–38. [Google Scholar] [CrossRef]
- Resende, R.R.; da Costa, J.L.; Kihara, A.H.; Adhikari, A.; Lorencon, E. Intracellular Ca2+ regulation during neuronal differentiation of murine embryonal carcinoma and mesenchymal stem cells. Stem Cells Dev. 2010, 19, 379–394. [Google Scholar] [CrossRef]
- Gebremedhin, D.; Lange, A.R.; Campbell, W.B.; Hillard, C.J.; Harder, D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999, 276, H2085–H2093. [Google Scholar] [CrossRef]
- Endoh, T. Pharmacological characterization of inhibitory effects of postsynaptic opioid and cannabinoid receptors on calcium currents in neonatal rat nucleus tractus solitarius. Br. J. Pharmacol. 2006, 147, 391–401. [Google Scholar] [CrossRef]
- Darmani, N.A.; Zhong, W.; Chebolu, S.; Vaezi, M.; Alkam, T. Broad-spectrum antiemetic potential of the L-type calcium channel antagonist nifedipine and evidence for its additive antiemetic interaction with the 5-HT(3) receptor antagonist palonosetron in the least shrew (Cryptotis parva). Eur. J. Pharmacol. 2014, 722, 2–12. [Google Scholar] [CrossRef]
- Moccia, F.; Zuccolo, E.; Soda, T.; Tanzi, F.; Guerra, G.; Mapelli, L.; Lodola, F.; D’Angelo, E. Stim and Orai proteins in neuronal Ca(2+) signaling and excitability. Front. Cell Neurosci. 2015, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.T.; Ong, H.L.; Liu, X.; Ambudkar, I.S. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr. Top Membr. 2013, 71, 149–179. [Google Scholar] [PubMed]
- Parekh, A.B.; Putney, J.W., Jr. Store-operated calcium channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef] [PubMed]
- Kopach, O.; Vats, J.; Netsyk, O.; Voitenko, N.; Irving, A.; Fedirko, N. Cannabinoid receptors in submandibular acinar cells: Functional coupling between saliva fluid and electrolytes secretion and Ca2+ signalling. J. Cell Sci. 2012, 125, 1884–1895. [Google Scholar] [CrossRef]
- Bouron, A. Phyto and endocannabinoids exert complex actions on calcium and zinc signaling in mouse cortical neurons. Biochem. Pharmacol. 2018, 152, 244–251. [Google Scholar] [CrossRef]
- Szolcsanyi, J.; Szallasi, A.; Szallasi, Z.; Joo, F.; Blumberg, P.M. Resiniferatoxin: An ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J. Pharmacol. Exp. Ther. 1990, 255, 923–928. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef]
- Imler, E.; Zinsmaier, K.E. TRPV1 channels: Not so inactive on the ER. Neuron 2014, 84, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Tsang, S.Y. Versatile Roles of Intracellularly Located TRPV1 Channel. J. Cell Physiol. 2017, 232, 1957–1965. [Google Scholar] [CrossRef]
- Darmani, N.A.; Chebolu, S.; Zhong, W.; Trinh, C.; McClanahan, B.; Brar, R.S. Additive antiemetic efficacy of low-doses of the cannabinoid CB(1/2) receptor agonist Delta(9)-THC with ultralow-doses of the vanilloid TRPV1 receptor agonist resiniferatoxin in the least shrew (Cryptotis parva). Eur. J. Pharmacol. 2014, 722, 147–155. [Google Scholar] [CrossRef]
- Andrews, P.L.; Okada, F.; Woods, A.J.; Hagiwara, H.; Kakaimoto, S.; Toyoda, M.; Matsuki, N. The emetic and anti-emetic effects of the capsaicin analogue resiniferatoxin in Suncus murinus, the house musk shrew. Br. J. Pharmacol. 2000, 130, 1247–1254. [Google Scholar] [CrossRef]
- Yamakuni, H.; Sawai-Nakayama, H.; Imazumi, K.; Maeda, Y.; Matsuo, M.; Manda, T.; Mutoh, S. Resiniferatoxin antagonizes cisplatin-induced emesis in dogs and ferrets. Eur. J. Pharmacol. 2002, 442, 273–278. [Google Scholar] [CrossRef]
- Rudd, J.A.; Nalivaiko, E.; Matsuki, N.; Wan, C.; Andrews, P.L. The involvement of TRPV1 in emesis and anti-emesis. Temperature (Austin) 2015, 2, 258–276. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Baraldi, S.; Gessi, S.; Merighi, S.; Borea, P.A. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med. Res. Rev. 2017, 37, 936–983. [Google Scholar] [CrossRef] [PubMed]
- Hermann, H.; De Petrocellis, L.; Bisogno, T.; Schiano Moriello, A.; Lutz, B.; Di Marzo, V. Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell Mol. Life Sci. 2003, 60, 607–616. [Google Scholar] [CrossRef]
- Shirakawa, H.; Yamaoka, T.; Sanpei, K.; Sasaoka, H.; Nakagawa, T.; Kaneko, S. TRPV1 stimulation triggers apoptotic cell death of rat cortical neurons. Biochem. Biophys. Res. Commun. 2008, 377, 1211–1215. [Google Scholar] [CrossRef]
- Dezieck, L.; Hafez, Z.; Conicella, A.; Blohm, E.; O’Connor, M.J.; Schwarz, E.S.; Mullins, M.E. Resolution of cannabis hyperemesis syndrome with topical capsaicin in the emergency department: A case series. Clin. Toxicol. (Phila) 2017, 55, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; de Petrocellis, L.; Pryce, G.; Baker, D.; Guglielmotti, V.; Di Marzo, V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 2006, 139, 1405–1415. [Google Scholar] [CrossRef]
- Garaschuk, O.; Yaari, Y.; Konnerth, A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J. Physiol. 1997, 502 Pt 1, 13–30. [Google Scholar] [CrossRef]
- Gomez-Viquez, N.L.; Guerrero-Serna, G.; Arvizu, F.; Garcia, U.; Guerrero-Hernandez, A. Inhibition of SERCA pumps induces desynchronized RyR activation in overloaded internal Ca2+ stores in smooth muscle cells. Am. J. Physiol. Cell Physiol. 2010, 298, C1038–C1046. [Google Scholar] [CrossRef]
- Michelangeli, F.; East, J.M. A diversity of SERCA Ca2+ pump inhibitors. Biochem. Soc. Trans. 2011, 39, 789–797. [Google Scholar] [CrossRef]
- Beltran-Parrazal, L.; Fernandez-Ruiz, J.; Toledo, R.; Manzo, J.; Morgado-Valle, C. Inhibition of endoplasmic reticulum Ca2+ ATPase in preBotzinger complex of neonatal rat does not affect respiratory rhythm generation. Neuroscience 2012, 224, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Mombouli, J.V.; Schaeffer, G.; Holzmann, S.; Kostner, G.M.; Graier, W.F. Anandamide-induced mobilization of cytosolic Ca2+ in endothelial cells. Br. J. Pharmacol. 1999, 126, 1593–1600. [Google Scholar] [CrossRef]
- Pan, X.; Ikeda, S.R.; Lewis, D.L. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol. Pharmacol. 1998, 54, 1064–1072. [Google Scholar] [CrossRef]
- Rogers, R.C.; Nasse, J.S.; Hermann, G.E. Live-cell imaging methods for the study of vagal afferents within the nucleus of the solitary tract. J. Neurosci. Methods 2006, 150, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N.A.; Crim, J.L.; Janoyan, J.J.; Abad, J.; Ramirez, J. A re-evaluation of the neurotransmitter basis of chemotherapy-induced immediate and delayed vomiting: Evidence from the least shrew. Brain Res. 2009, 1248, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Cadogan, A.K.; Alexander, S.P.; Boyd, E.A.; Kendall, D.A. Influence of cannabinoids on electrically evoked dopamine release and cyclic AMP generation in the rat striatum. J. Neurochem. 1997, 69, 1131–1137. [Google Scholar] [CrossRef]
- Alonso, R.; Voutsinos, B.; Fournier, M.; Labie, C.; Steinberg, R.; Souilhac, J.; Le Fur, G.; Soubrie, P. Blockade of cannabinoid receptors by SR141716 selectively increases Fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function. Neuroscience 1999, 91, 607–620. [Google Scholar] [CrossRef]
- Tzavara, E.T.; Perry, K.W.; Rodriguez, D.E.; Bymaster, F.P.; Nomikos, G.G. The cannabinoid CB(1) receptor antagonist SR141716A increases norepinephrine outflow in the rat anterior hypothalamus. Eur. J. Pharmacol. 2001, 426, R3–R4. [Google Scholar] [CrossRef]
- Roche, M.; O’Connor, E.; Diskin, C.; Finn, D.P. The effect of CB(1) receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. Eur. J. Neurosci. 2007, 26, 2643–2653. [Google Scholar] [CrossRef]
- Stocker, F.P.; Kinser, J.; Weber, J.W.; Rosler, H.; Noelpp, U. Isotopic investigations in pediatric cardiology (author’s transl). Schweiz. Rundsch. Med. Prax. 1975, 64, 1220–1231. [Google Scholar]
- Kenakin, T. Functional selectivity through protean and biased agonism: Who steers the ship? Mol. Pharmacol. 2007, 72, 1393–1401. [Google Scholar] [CrossRef]
- Deng, M.Y.; Cheng, J.; Gao, N.; Li, X.Y.; Liu, H.; Wang, Y.X. Dexamethasone attenuates neuropathic pain through spinal microglial expression of dynorphin A via the cAMP/PKA/p38 MAPK/CREB signaling pathway. Brain Behav. Immun. 2024, 119, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N.A.; Wang, Y.; Abad, J.; Ray, A.P.; Thrush, G.R.; Ramirez, J. Utilization of the least shrew as a rapid and selective screening model for the antiemetic potential and brain penetration of substance P and NK1 receptor antagonists. Brain Res. 2008, 1214, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Chebolu, S.; Wang, Y.; Ray, A.P.; Darmani, N.A. Pranlukast prevents cysteinyl leukotriene-induced emesis in the least shrew (Cryptotis parva). Eur. J. Pharmacol. 2010, 628, 195–201. [Google Scholar] [CrossRef] [PubMed]
| Antibody Name | Antibody Dilution | Manufacturer |
|---|---|---|
| Total PKAα/β | 1:5000 | Invitrogen (Carlsbad, CA, USA) |
| p-PKAα/β (Thr197) | 1:5000 | Invitrogen (Carlsbad, CA, USA) |
| Total Akt | 1:2000 | Cell Signaling Tech (Danvers, MA, USA) |
| p-Akt (Ser473) | 1:2000 | Cell Signaling Tech (Danvers, MA, USA) |
| Total ERK1/2 | 1:3000 | Cell Signaling Tech (Danvers, MA, USA) |
| p-ERK1/2 (Th202/204) | 1:1000 | Cell Signaling Tech (Danvers, MA, USA) |
| Total GSK-3α/β mouse | 1:2000 | Invitrogen Carlsbad, CA, USA) |
| p-GSK-3α/β rabbit | 1:1000 | Cell Signaling Tech (Danvers, MA, USA) |
| p-PKCαβII (Thr638/641) | 1:2000 | Cell Signaling Tech (Danvers, MA, USA) |
| GAPDH | 1:10,000 | Millipore Sigma (Burlington, MA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Belkacemi, L.; Zhong, W.; Daily, Z.; Darmani, N.A. The Cannabinoid CB1 Receptor Inverse Agonist/Antagonist SR141716A Activates the Adenylate Cyclase/PKA Signaling Pathway Among Other Intracellular Emetic Signals to Evoke Vomiting in Least Shrews (Cryptotis parva). Int. J. Mol. Sci. 2025, 26, 9884. https://doi.org/10.3390/ijms26209884
Sun Y, Belkacemi L, Zhong W, Daily Z, Darmani NA. The Cannabinoid CB1 Receptor Inverse Agonist/Antagonist SR141716A Activates the Adenylate Cyclase/PKA Signaling Pathway Among Other Intracellular Emetic Signals to Evoke Vomiting in Least Shrews (Cryptotis parva). International Journal of Molecular Sciences. 2025; 26(20):9884. https://doi.org/10.3390/ijms26209884
Chicago/Turabian StyleSun, Yina, Louiza Belkacemi, Weixia Zhong, Zollie Daily, and Nissar A. Darmani. 2025. "The Cannabinoid CB1 Receptor Inverse Agonist/Antagonist SR141716A Activates the Adenylate Cyclase/PKA Signaling Pathway Among Other Intracellular Emetic Signals to Evoke Vomiting in Least Shrews (Cryptotis parva)" International Journal of Molecular Sciences 26, no. 20: 9884. https://doi.org/10.3390/ijms26209884
APA StyleSun, Y., Belkacemi, L., Zhong, W., Daily, Z., & Darmani, N. A. (2025). The Cannabinoid CB1 Receptor Inverse Agonist/Antagonist SR141716A Activates the Adenylate Cyclase/PKA Signaling Pathway Among Other Intracellular Emetic Signals to Evoke Vomiting in Least Shrews (Cryptotis parva). International Journal of Molecular Sciences, 26(20), 9884. https://doi.org/10.3390/ijms26209884






