Targeting the Aryl Hydrocarbon Receptor: The Potential of Indole Compounds in the Treatment of Cystic Fibrosis
Abstract
1. Introduction
2. CFTR
2.1. CFTR Function and Dysfunction
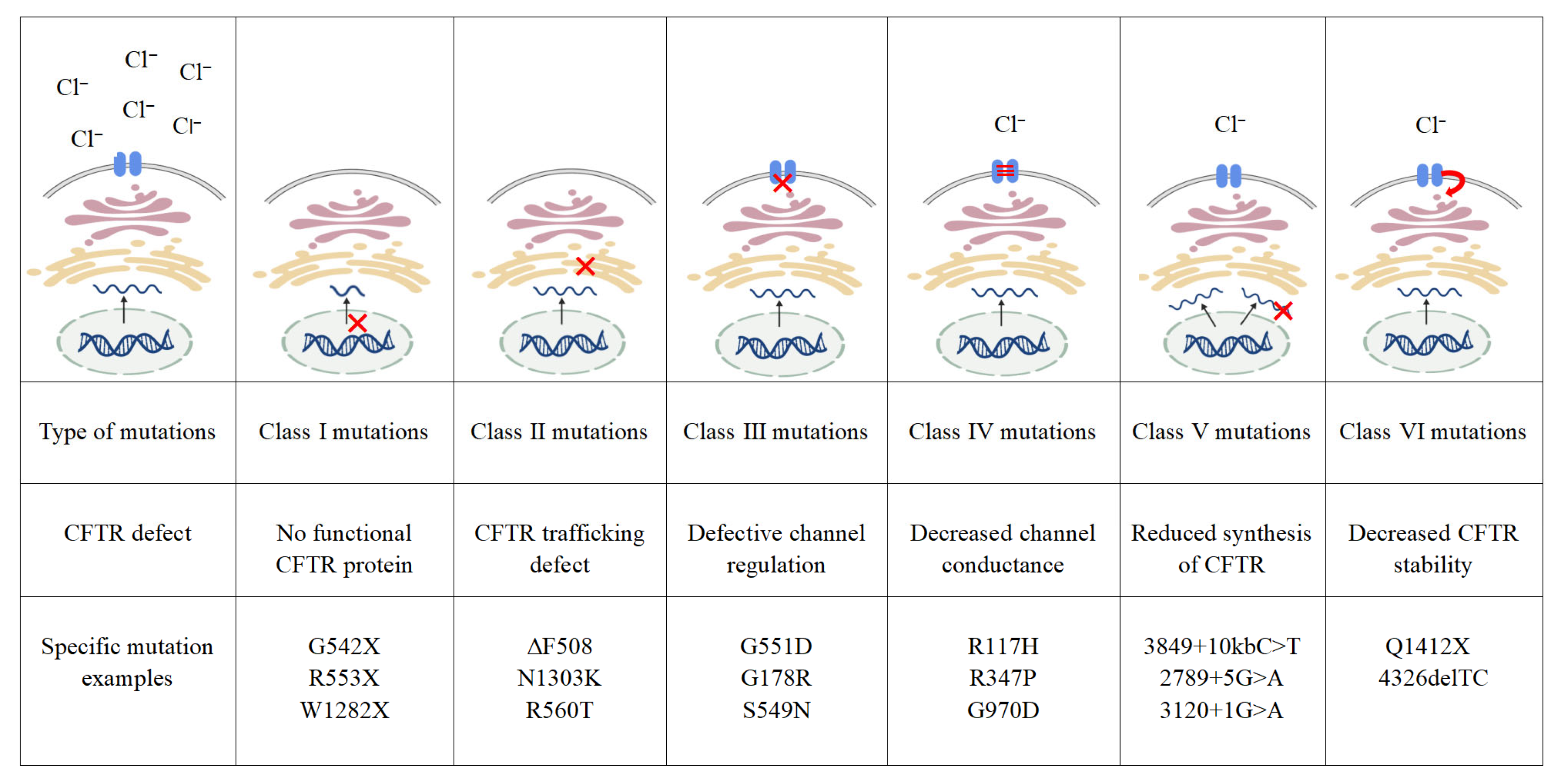
2.2. CFTR Mutation Classes
2.3. Therapeutic Advances in CF
3. AHR
3.1. AHR Signaling Pathway
3.2. Activation of AHR by Indole Compounds
4. AHR with CF
4.1. Pulmonary Infection and Inflammation
4.2. Intestinal Barrier and Microbiota
4.3. Immune Homeostasis
5. Indole Compounds and Derivatives with CF
5.1. Anti-Inflammatory and Antibacterial Mechanisms of Indole Compounds
| Ligand | Structure | Source | Model | Dose | Effect | Stage |
|---|---|---|---|---|---|---|
| DIM | 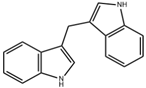 | Cruciferous vegetables | Liver-damaged mice [88] | 2.5–10 mg/kg (Subcutaneous injection) | Reduce inflammatory factors, anti-inflammatory effects | Preclinical trials |
| FICZ | 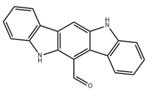 | Photo-oxidation | DSS-induced colitis in mice [57] | 1 μg/day (Intraperitoneal injection) | Reduce intestinal inflammation, enhance intestinal barrier | Preclinical trials |
| LPS-induced acute lung injury in mice [68] | 1 μg (Intranasal administration) | Reduce inflammatory factors, enhance pulmonary barrier function | ||||
| IPA | 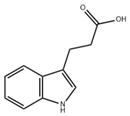 | Microbiota metabolism | LPS-induced inflammation in Caco-2/HT29 co-cultured cells [86] | 0.05–0.5 mM (In vitro processing) | Anti-inflammatory effect | Preclinical trials |
| DSS-induced colitis in mice [89] | 50 mg/kg/day (Oral gavage) | Enhance intestinal barrier | ||||
| I3C |  | Cruciferous vegetables | Alcohol-induced alcoholic liver injury in mice [90] | 40 mg/kg/day (Oral gavage) | Antioxidant, anti-inflammatory, anti-apoptotic effects on the gut-liver-adipose tissue axis | Preclinical trials |
| Influenza virus infection in ECΔAHR mice [67] | 1000 ppm I3C (Added to the diet) | Maintain lung barrier function, reduce lung injury | ||||
| ICA | 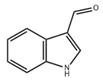 | Microbiota metabolism | RSV-induced inflammation in RAW264.7 cells [83] | 10–50 μM (In vitro processing) | Anti-inflammatory effects, reduce the production of pro-inflammatory cytokines | Preclinical trials |
| Mice infected with Aspergillus fumigatus spores via intranasal instillation [91] | 18 mg/kg (Intranasal delivery, oral administration) 4.5 mg/kg (Blowing into the lungs) | Possess antibacterial activity, prevent lung inflammation, alleviate lung infections | ||||
| IAA |  | Microbiota metabolism | HFD-induced obese mice [92] | 50 mg/kg/day (Oral gavage) | Reduce systemic inflammation, regulate gut microbiota composition, increase the abundance of beneficial bacteria | Preclinical trials |
| IA | 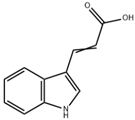 | Microbiota metabolism | DSS-induced colitis mice [93] | 1 × 109 CFU/day (Lactobacillus reuteri oral gavage) | Strengthen intestinal epithelial barrier, reduce inflammation | Preclinical trials |
5.2. Indole Compounds Related to CF
5.3. Indole Compounds and Their Derivatives as Candidate Therapeutics for CF
5.3.1. Preclinical Experimental Evidence
5.3.2. Clinical Translational Basis
5.3.3. Indole-Based CFTR Modulators
5.3.4. Future Development Prospects
6. Risks and Challenges
6.1. AHR Activation and Tumor Immune Escape Risk
6.2. The Pro-Fibrotic Effects of AHR Ligands
6.3. AHR-Mediated Multiorgan Toxicity
6.4. Challenges in AHR-Targeted Therapy for CF
7. Summary and Prospect
8. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ABCC4 | ATP-binding cassette subfamily C member 4 |
| AECs | Alveolar epithelial cells |
| AFB1 | Aflatoxin B1 |
| AHR | Aryl hydrocarbon receptor |
| AHRR | Aryl hydrocarbon receptor repressor |
| AKI | Acute kidney injury |
| AKT | Protein Kinase B |
| ARNT | Aryl hydrocarbon receptor nuclear translocator |
| ASL | Airway surface liquid |
| BAL | Bronchoalveolar lavage |
| BaP | Benzo[a]pyrene |
| bHLH | Basic helix-loop-helix |
| CAFs | Cancer-associated fibroblasts |
| CBAVD | Congenital bilateral absence of the vas deferens |
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CKD | Chronic kidney disease |
| CLL | Chronic lymphocytic leukemia |
| CUAVD | Congenital unilateral absence of the vas deferens |
| CYP1A1 | Cytochrome P450 family 1 subfamily A member 1 |
| CYP1B1 | Cytochrome P450 family 1 subfamily B member 1 |
| CYP2E1 | Cytochrome P450 family 2 subfamily E member 1 |
| DCs | Dendritic cells |
| DIM | 3,3′-diindolylmethane |
| DSS | Dextran sulfate sodium salt |
| ENaC | Epithelial sodium channel |
| ER | Endoplasmic reticulum |
| ERK | Extracellular regulated protein kinase |
| ERQC | Endoplasmic reticulum quality control |
| FICZ | 6-formylindole [3,2b]carbazole |
| FXR | Farnesoid X receptor |
| HSP | Heat shock protein |
| I3C | Indole-3-carbinol |
| IA | Indole-3-acrylic acid |
| IAA | Indole-3-acetic acid |
| IBD | Inflammatory bowel disease |
| ICA | Indole-3-carbaldehyde |
| IFN-α | Interferon-α |
| IL | Interleukin |
| ILA | Indole-3-lactic acid |
| ILC3s | Type 3 innate lymphoid cells |
| IPA | Indole-3-propionic acid |
| IS | Indoxyl sulfate |
| ITE | 2-(1′-H-indole-3′-carbonyl) -thiazole-4-carboxylic acid methyl ester |
| JAK2 | Janus kinase 2 |
| Kyn | Kynurenine |
| LPS | Lipopolysaccharide |
| MMP-1 | Matrix metalloproteinase-1 |
| MSD | Membrane-spanning domain |
| MUC | Mucin |
| NBD | Nucleotide-binding domain |
| NF-κB | Nuclear factor kappa-B |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| PAS | Per-ARNT-Sim |
| PGC1α | PPARG coactivator 1 α |
| R domain | Regulatory domain |
| RELMβ | Resistin-like molecule β |
| ROS | Reactive oxygen species |
| RSV | Respiratory syncytial virus |
| SOCS | Suppressor of cytokine signaling |
| SR1 | Stemregenin 1 |
| STAT | Signal transducer and activator of transcription |
| SULT1A1 | Sulfotransferase family 1A member 1 |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| TEER | Transepithelial electrical resistance |
| TFF3 | Trefoil factor 3 |
| TGF-β | Transforming growth factor-β |
| Th | T helper cell |
| TJs | Tight junctions |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| Tregs | Regulatory cells |
| Trp | Tryptophan |
| XAP2 | X-associated protein 2 |
References
- Elias, A.E.; McBain, A.J.; Aldehalan, F.A.; Taylor, G.; O’Neill, C.A. Activation of the aryl hydrocarbon receptor via indole derivatives is a common feature in skin bacterial isolates. J. Appl. Microbiol. 2024, 135, lxae273. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Al-Hussain, S.A. Design, Synthesis of New 1,2,4-Triazole/1,3,4-Thiadiazole with Spiroindoline, Imidazo[4,5-b]quinoxaline and Thieno[2,3-d]pyrimidine from Isatin Derivatives as Anticancer Agents. Molecules 2022, 27, 835. [Google Scholar] [CrossRef] [PubMed]
- Levring, J.; Terry, D.S.; Kilic, Z.; Fitzgerald, G.; Blanchard, S.C.; Chen, J. CFTR function, pathology and pharmacology at single-molecule resolution. Nature 2023, 616, 606–614, Erratum in Nature 2023, 617, E11. [Google Scholar] [CrossRef] [PubMed]
- Kleinfelder, K.; Somenza, E.; Farinazzo, A.; Conti, J.; Lotti, V.; Latorre, R.V.; Rodella, L.; Massella, A.; Tomba, F.; Bertini, M.; et al. CFTR Modulators Rescue the Activity of CFTR in Colonoids Expressing the Complex Allele p.[R74W;V201M;D1270N]/dele22_24. Int. J. Mol. Sci. 2023, 24, 5199. [Google Scholar] [CrossRef]
- Angyal, D.; Kleinfelder, K.; Ciciriello, F.; Groeneweg, T.A.; De Marchi, G.; de Pretis, N.; Bernardoni, L.; Rodella, L.; Tomba, F.; De Angelis, P.; et al. CFTR function is impaired in a subset of patients with pancreatitis carrying rare CFTR variants. Pancreatology 2024, 24, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Ébert, A.; Gál, E.; Tóth, E.; Szögi, T.; Hegyi, P.; Venglovecz, V. Role of CFTR in diabetes-induced pancreatic ductal fluid and HCO3− secretion. J. Physiol. 2024, 602, 1065–1083. [Google Scholar] [CrossRef]
- Luan, X.; Henao Romero, N.; Campanucci, V.A.; Le, Y.; Mustofa, J.; Tam, J.S.; Ianowski, J.P. Pulmonary Ionocytes Regulate Airway Surface Liquid pH in Primary Human Bronchial Epithelial Cells. Am. J. Respir. Crit. Care Med. 2024, 210, 788–800. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, L.; Tang, X.X.; Moninger, T.O.; Huang, T.J.; Stoltz, D.A.; Welsh, M.J. Acidic Submucosal Gland pH and Elevated Protein Concentration Produce Abnormal Cystic Fibrosis Mucus. Dev. Cell. 2020, 54, 488–500.E5. [Google Scholar] [CrossRef]
- Hanssens, L.S.; Duchateau, J.; Casimir, G.J. CFTR Protein: Not Just a Chloride Channel? Cells 2021, 10, 2844. [Google Scholar] [CrossRef]
- Anderson, K.J.; Cormier, R.T.; Scott, P.M. Role of ion channels in gastrointestinal cancer. World J. Gastroenterol. 2019, 25, 5732–5772. [Google Scholar] [CrossRef]
- Myerburg, M.M.; Butterworth, M.B.; McKenna, E.E.; Peters, K.W.; Frizzell, R.A.; Kleyman, T.R.; Pilewski, J.M. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: A mechanism for sodium hyperabsorption in cystic fibrosis. J. Biol. Chem. 2006, 281, 27942–27949. [Google Scholar] [CrossRef]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, Y.; Yang, Y.; Sun, Y.; Cheng, C.; Dai, J.; Meng, S.; Chen, K.; Zhao, Y.; Liu, X.; et al. Progression and mortality of patients with cystic fibrosis in China. Orphanet J. Rare Dis. 2025, 20, 6. [Google Scholar] [CrossRef]
- Grünewaldt, A.; Rohde, G. Retrospective cohort study of adult patients with cystic fibrosis supported with venovenous extracorporeal membrane oxygenation (VV ECMO) at a large German cystic fibrosis center. BMC Pulm. Med. 2025, 25, 276. [Google Scholar] [CrossRef] [PubMed]
- de Macedo, J.R.F.F.; Aubriot, A.S.; Reychler, G.; Penelle, M.; Gohy, S.; Poncin, W. The intermittent intrapulmonary deflation technique for airway clearance in patients with cystic fibrosis: A randomized trial. Respir. Med. Res. 2024, 86, 101094. [Google Scholar] [CrossRef] [PubMed]
- Long, A.M.; Jones, I.H.; Knight, M.; McNally, J. BAPS-CASS Early management of meconium ileus in infants with cystic fibrosis: A prospective population cohort study. J. Pediatr. Surg. 2021, 56, 1287–1292. [Google Scholar] [CrossRef]
- Gao, X.; Yeh, H.I.; Yang, Z.; Fan, C.; Jiang, F.; Howard, R.J.; Lindahl, E.; Kappes, J.C.; Hwang, T.C. Allosteric inhibition of CFTR gating by CFTRinh-172 binding in the pore. Nat. Commun. 2024, 15, 6668. [Google Scholar] [CrossRef]
- Infield, D.T.; Strickland, K.M.; Gaggar, A.; McCarty, N.A. The molecular evolution of function in the CFTR chloride channel. J. Gen. Physiol. 2021, 153, e202012625. [Google Scholar] [CrossRef]
- Cannarella, R.; Leitner, D.V.; Weiss, M.; Vij, S.C. Testicular function and fertility outcomes in males with CF: A multi center retrospective study of men with congenital bilateral absence of the vas deferens based on CFTR mutation status. J. Clin. Transl. Endocrinol. 2025, 40, 100388. [Google Scholar] [CrossRef]
- Jathal, I.; Stransky, O.M.; Wright, C.E.; Prangley, A.; Tangpricha, V.; Jain, R.; Taylor-Cousar, J.L.; Hughan, K.S.; Ladores-Barrett, S.; West, N.E.; et al. Fertility and family-building experiences and perspectives of males with cystic fibrosis. Reprod. Biol. Endocrinol. 2025, 23, 85. [Google Scholar] [CrossRef]
- Borgo, C.; D’Amore, C.; Capurro, V.; Tomati, V.; Pedemonte, N.; Bosello Travain, V.; Salvi, M. SUMOylation Inhibition Enhances Protein Transcription under CMV Promoter: A Lesson from a Study with the F508del-CFTR Mutant. Int. J. Mol. Sci. 2024, 25, 2302. [Google Scholar] [CrossRef]
- Guhr Lee, T.N.; Cholon, D.M.; Quinney, N.L.; Gentzsch, M.; Esther, C.R. Accumulation and persistence of ivacaftor in airway epithelia with prolonged treatment. J. Cyst. Fibros. 2020, 19, 746–751. [Google Scholar] [CrossRef]
- Amaral, M.D.; Farinha, C.M.; Matos, P.; Botelho, H.M. Investigating Alternative Transport of Integral Plasma Membrane Proteins from the ER to the Golgi: Lessons from the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). Methods Mol. Biol. 2016, 1459, 105–126. [Google Scholar]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- McDonald, E.F.; Woods, H.; Smith, S.T.; Kim, M.; Schoeder, C.T.; Plate, L.; Meiler, J. Structural Comparative Modeling of Multi-Domain F508del CFTR. Biomolecules 2022, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Thavamani, A.; Salem, I.; Sferra, T.J.; Sankararaman, S. Impact of Altered Gut Microbiota and Its Metabolites in Cystic Fibrosis. Metabolites 2021, 11, 123. [Google Scholar] [CrossRef]
- He, R.; Lin, F.; Deng, Z.; Yu, B. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with Phe508del mutation: Evidence from randomized controlled trials. SAGE Open Med. 2024, 12, 20503121231225874. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ford, R.C. Investigation of F508del CFTR unfolding and a search for stabilizing small molecules. Arch. Biochem. Biophys. 2024, 758, 110050. [Google Scholar] [CrossRef]
- Merlo, C.; Thorat, T.; McGarry, L.J.; Scirica, C.V.; DerSarkissian, M.; Nguyen, C.; Gu, Y.M.; Muthukumar, A.; Healy, J.; Rubin, J.L.; et al. A Retrospective, Longitudinal Registry Study on the Long-Term Durability of Ivacaftor Treatment in People with Cystic Fibrosis. Pulm. Ther. 2024, 10, 483–494. [Google Scholar] [CrossRef]
- Drummond, D.; Dana, J.; Berteloot, L.; Schneider-Futschik, E.K.; Chedevergne, F.; Bailly-Botuha, C.; Nguyen-Khoa, T.; Cornet, M.; Le Bourgeois, M.; Debray, D.; et al. Lumacaftor-ivacaftor effects on cystic fibrosis-related liver involvement in adolescents with homozygous F508 del-CFTR. J. Cyst. Fibros. 2022, 21, 212–219. [Google Scholar] [CrossRef]
- Bacalhau, M.; Camargo, M.; Magalhães-Ghiotto, G.A.V.; Drumond, S.; Castelletti, C.H.M.; Lopes-Pacheco, M. Elexacaftor-Tezacaftor-Ivacaftor: A Life-Changing Triple Combination of CFTR Modulator Drugs for Cystic Fibrosis. Pharmaceuticals 2023, 16, 410. [Google Scholar] [CrossRef]
- Dai, S.; Qu, L.; Li, J.; Zhang, Y.; Jiang, L.; Wei, H.; Guo, M.; Chen, X.; Chen, Y. Structural insight into the ligand binding mechanism of aryl hydrocarbon receptor. Nat. Commun. 2022, 13, 6234. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, Y.; Zhang, B.; Hang, Y.; Xu, L.; Chen, Y.; Xie, Q.; Zhao, Q.; Zhang, L.; Li, G.; et al. Cryo-EM structure of the cytosolic AhR complex. Structure 2023, 31, 295–308.E4. [Google Scholar] [CrossRef] [PubMed]
- Polonio, C.M.; McHale, K.A.; Sherr, D.H.; Rubenstein, D.; Quintana, F.J. The aryl hydrocarbon receptor: A rehabilitated target for therapeutic immune modulation. Nat. Rev. Drug Discov. 2025, 24, 610–630. [Google Scholar] [CrossRef]
- Sondermann, N.C.; Faßbender, S.; Hartung, F.; Hätälä, A.M.; Rolfes, K.M.; Vogel, C.F.A.; Haarmann-Stemmann, T. Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway. Biochem. Pharmacol. 2023, 208, 115371. [Google Scholar] [CrossRef]
- Kobayashi, S.; Okamoto, H.; Iwamoto, T.; Toyama, Y.; Tomatsu, T.; Yamanaka, H.; Momohara, S. A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology 2008, 47, 1317–1322. [Google Scholar] [CrossRef]
- You, Y.; Cai, B.; Zhu, C.; Zhou, Z.; Xu, J.; Huang, L.; Jie, L.; Du, H. 3,3′-diindolylmethane, from cruciferous vegetables, ameliorates cigarette smoke-induced inflammatory amplification in CIA model mice by targeting the AhR/NF-κB crosstalk. J. Nutr. Biochem. 2025, 143, 109953. [Google Scholar] [CrossRef]
- Sawa-Wejksza, K.; Parada-Turska, J.; Turski, W. The Pharmacological Evidences for the Involvement of AhR and GPR35 Receptors in Kynurenic Acid-Mediated Cytokine and Chemokine Secretion by THP-1-Derived Macrophages. Molecules 2025, 30, 3133. [Google Scholar] [CrossRef]
- Ishihara, Y.; Kado, S.Y.; Bein, K.J.; He, Y.; Pouraryan, A.A.; Urban, A.; Haarmann-Stemmann, T.; Sweeney, C.; Vogel, C.F.A. Aryl Hydrocarbon Receptor Signaling Synergizes with TLR/NF-κB-Signaling for Induction of IL-22 Through Canonical and Non-Canonical AhR Pathways. Front. Toxicol. 2022, 3, 787360. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wu, X.; Shan, Q.Y.; Yang, Q.; Yu, X.Y.; Yang, J.H.; Miao, H.; Cao, G.; Zhao, Y.Y. Acteoside-containing caffeic acid is bioactive functional group of antifibrotic effect by suppressing inflammation via inhibiting AHR nuclear translocation in chronic kidney disease. Acta Pharmacol. Sin. 2025, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhang, X.; Zhang, Y.; Wu, B.; Fang, L.; Wang, N.; Yi, H.; Chang, N.; Chen, L.; Zhang, J. Aryl hydrocarbon receptor mediates Jak2/STAT3 signaling for non-small cell lung cancer stem cell maintenance. Exp. Cell Res. 2020, 396, 112288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, L.; Dong, J.; Yuan, Z.; Yao, W.; Ji, P.; Hua, Y.; Wei, Y. Shaoyao decoction improves damp-heat colitis by activating the AHR/IL-22/STAT3 pathway through tryptophan metabolism driven by gut microbiota. J. Ethnopharmacol. 2024, 326, 117874. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lee, Y.; Li, C.H.; Cheng, Y.W.; Kang, J.J. Down-regulation of aryl hydrocarbon receptor intensifies carcinogen-induced retinal lesion via SOCS3-STAT3 signaling. Cell Biol. Toxicol. 2020, 36, 223–242. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, H.; Wang, X.; Zhu, F.; Tian, N.; Xu, Z.; Yin, H.; Liang, M.; Yang, X.; Liu, X.; et al. Deubiquitination of aryl hydrocarbon receptor by USP21 negatively regulates T helper 17 cell differentiation. J. Leukoc. Biol. 2024, 117, qiae148. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, Z.; Mani, S.; Vondráček, J. Emerging approaches for antagonizing the aryl hydrocarbon receptor. Trends Pharmacol. Sci. 2025, 46, 629–637. [Google Scholar] [CrossRef]
- Veland, N.; Gleneadie, H.J.; Brown, K.E.; Sardini, A.; Pombo, J.; Dimond, A.; Burns, V.; Sarkisyan, K.; Schiering, C.; Webster, Z.; et al. Bioluminescence imaging of Cyp1a1-luciferase reporter mice demonstrates prolonged activation of the aryl hydrocarbon receptor in the lung. Commun. Biol. 2024, 7, 442. [Google Scholar] [CrossRef]
- Zhou, L.; Song, W.; Liu, T.; Yan, T.; He, Z.; He, W.; Lv, J.; Zhang, S.; Dai, X.; Yuan, L.; et al. Multi-omics insights into anti-colitis benefits of the synbiotic and postbiotic derived from wheat bran arabinoxylan and Limosilactobacillus reuteri. Int. J. Biol. Macromol. 2024, 278 Pt 3, 134860. [Google Scholar] [CrossRef]
- Dutta, B.; Tripathy, A.; Archana, P.R.; Kamath, S.U. Unraveling the complexities of diet induced obesity and glucolipid dysfunction in metabolic syndrome. Diabetol. Metab. Syndr. 2025, 17, 292. [Google Scholar] [CrossRef]
- Dvořák, Z.; Poulíková, K.; Mani, S. Indole scaffolds as a promising class of the aryl hydrocarbon receptor ligands. Eur. J. Med. Chem. 2021, 215, 113231. [Google Scholar] [CrossRef]
- Wang, B.; Sun, S.; Liu, M.; Chen, H.; Liu, N.; Wu, Z.; Wu, G.; Dai, Z. Dietary L-Tryptophan Regulates Colonic Serotonin Homeostasis in Mice with Dextran Sodium Sulfate-Induced Colitis. J. Nutr. 2020, 150, 1966–1976. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Nandi, B.; Paul, S.; Manna, S.; Maity, A.; Bandyopadhyay, K.; Panda, S.; Khan, S.; Nath, R.; Akhtar, M. Novel indole-based synthetic molecules in cancer treatment: Synthetic strategies and structure-activity relationship. Med. Drug Discov. 2025, 27, 100208. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Yan, J.; Sun, J.; Wang, Y.; Huang, G.; Zhang, F.; Cao, H.; Li, D. Biotherapeutic potential of gut microbiota-derived indole-3-acetic acid. Crit. Rev. Microbiol. 2025, 1–21. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Hou, Q.; Ye, L.; Liu, H.; Huang, L.; Yang, Q.; Turner, J.R.; Yu, Q. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018, 25, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Q.; Yu, K.; Fan, X.; Xiao, W.; Cai, Y.; Xu, P.; Yu, M.; Yang, H. 6-Formylindolo(3,2-b)carbazole induced aryl hydrocarbon receptor activation prevents intestinal barrier dysfunction through regulation of claudin-2 expression. Chem. Biol. Interact. 2018, 288, 83–90. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Jonić, N.; Koprivica, I.; Chatzigiannis, C.M.; Tsiailanis, A.D.; Kyrkou, S.G.; Tzakos, E.P.; Pavić, A.; Dimitrijević, M.; Jovanović, A.; Jovanović, M.B.; et al. Development of FluoAHRL: A Novel Synthetic Fluorescent Compound That Activates AHR and Potentiates Anti-Inflammatory T Regulatory Cells. Molecules 2024, 29, 2988. [Google Scholar] [CrossRef]
- Wellems, D.; Hu, Y.; Jennings, S.; Wang, G. Loss of CFTR function in macrophages alters the cell transcriptional program and delays lung resolution of inflammation. Front. Immunol. 2023, 14, 1242381. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Greenwald, M.A.; Wolfgang, M.C. The changing landscape of the cystic fibrosis lung environment: From the perspective of Pseudomonas aeruginosa. Curr. Opin. Pharmacol. 2022, 65, 102262. [Google Scholar] [CrossRef]
- Vohra, M.; Kamath, N.; Dubey, R.; Sharma, S.; Sharma, S. Complex Interplay between Biofilm Formation, Antibiotic Resistance, and Virulence in Pseudomonas aeruginosa: A Phenotypic and Genotypic Study. Mol. Genet. Microbiol. Virol. 2025, 40, 61–74. [Google Scholar] [CrossRef]
- Moura-Alves, P.; Puyskens, A.; Stinn, A.; Klemm, M.; Guhlich-Bornhof, U.; Dorhoi, A.; Furkert, J.; Kreuchwig, A.; Protze, J.; Lozza, L.; et al. Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science 2019, 366, eaaw1629. [Google Scholar] [CrossRef]
- Puccetti, M.; Gomes Dos Reis, L.; Pariano, M.; Costantini, C.; Renga, G.; Ricci, M.; Traini, D.; Giovagnoli, S. Development and in vitro-in vivo performances of an inhalable indole-3-carboxaldehyde dry powder to target pulmonary inflammation and infection. Int. J. Pharm. 2021, 607, 121004. [Google Scholar] [CrossRef]
- Britto, C.J.; Brady, V.; Lee, S.; Dela Cruz, C.S. Respiratory Viral Infections in Chronic Lung Diseases. Clin. Chest Med. 2017, 38, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Major, J.; Crotta, S.; Finsterbusch, K.; Chakravarty, P.; Shah, K.; Frederico, B.; D’Antuono, R.; Green, M.; Meader, L.; Suarez-Bonnet, A.; et al. Endothelial AHR activity prevents lung barrier disruption in viral infection. Nature 2023, 621, 813–820. [Google Scholar] [CrossRef]
- Zimmerman, E.; Sturrock, A.; Reilly, C.A.; Burrell-Gerbers, K.L.; Warren, K.; Mir-Kasimov, M.; Zhang, M.A.; Pierce, M.S.; Helms, M.N.; Paine, R. Aryl Hydrocarbon Receptor Activation in Pulmonary Alveolar Epithelial Cells Limits Inflammation and Preserves Lung Epithelial Cell Integrity. J. Immunol. 2024, 213, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Aljameeli, A.M.; Alsuwayt, B.; Bharati, D.; Gohri, V.; Mohite, P.; Singh, S.; Chidrawar, V. Chloride channels and mast cell function: Pioneering new frontiers in IBD therapy. Mol. Cell Biochem. 2025, 480, 3951–3969. [Google Scholar] [CrossRef]
- Duong, J.T.; Hayden, H.S.; Verster, A.J.; Pope, C.E.; Miller, C.; Penewit, K.; Salipante, S.J.; Rowe, S.M.; Solomon, G.M.; Nichols, D.; et al. Fecal microbiota changes in people with cystic fibrosis after 6 months of elexacaftor/tezacaftor/ivacaftor: Findings from the promise study. J. Cyst. Fibros. 2025, 24, 792–800. [Google Scholar] [CrossRef]
- Wang, G.; Fan, Y.; Zhang, G.; Cai, S.; Ma, Y.; Yang, L.; Wang, Y.; Yu, H.; Qiao, S.; Zeng, X. Microbiota-derived indoles alleviate intestinal inflammation and modulate microbiome by microbial cross-feeding. Microbiome 2024, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Justiniano, R.; Williams, J.D.; Cabello, C.M.; Qiao, S.; Wondrak, G.T. The Tryptophan-Derived Endogenous Aryl Hydrocarbon Receptor Ligand 6-Formylindolo[3,2-b]Carbazole Is a Nanomolar UVA Photosensitizer in Epidermal Keratinocytes. J. Investig. Dermatol. 2015, 135, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, S.; Li, S.; Zhang, Q.; Cai, Y.; Li, P.; Li, H.; Shen, B.; Liao, Q.; Hong, Y.; et al. Indoleacrylic acid produced by Parabacteroides distasonis alleviates type 2 diabetes via activation of AhR to repair intestinal barrier. BMC Biol. 2023, 21, 90. [Google Scholar] [CrossRef]
- de Vrankrijker, A.M.; Wolfs, T.F.; van der Ent, C.K. Challenging and emerging pathogens in cystic fibrosis. Paediatr. Respir. Rev. 2010, 11, 246–254. [Google Scholar] [CrossRef]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary Sodium Butyrate Decreases Postweaning Diarrhea by Modulating Intestinal Permeability and Changing the Bacterial Communities in Weaned Piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
- Durda-Masny, M.; Goździk-Spychalska, J.; Morańska, K.; Pawłowska, N.; Mazurkiewicz, M.; Skrzypczak, I.; Cofta, S.; Szwed, A. Gut microbiota in adults with cystic fibrosis: Implications for the severity of the CFTR gene mutation and nutritional status. J. Cyst. Fibros. 2024, 23, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Hayden, H.S.; Eng, A.; Pope, C.E.; Brittnacher, M.J.; Vo, A.T.; Weiss, E.J.; Hager, K.R.; Martin, B.D.; Leung, D.H.; Heltshe, S.L.; et al. Fecal dysbiosis in infants with cystic fibrosis is associated with early linear growth failure. Nat. Med. 2020, 26, 215–221. [Google Scholar] [CrossRef]
- Sun, M.; Ma, N.; He, T.; Johnston, L.J.; Ma, X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit. Rev. Food Sci. Nutr. 2020, 60, 1760–1768. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, G.; Wang, K.; Liu, K.; Gao, Y.; Chen, J.; Li, Y.; Yan, J. The Role of Lactiplantibacillus plantarum CGMCC9513 in Alleviating Colitis by Synergistic Enhancement of the Intestinal Barrier Through Modulating Gut Microbiota and Activating the Aryl Hydrocarbon Receptor. Probiotics Antimicrob Proteins 2025, 1–13. [Google Scholar] [CrossRef]
- Jaudas, F.; Bartenschlager, F.; Shashikadze, B.; Santamaria, G.; Reichart, D.; Schnell, A.; Stöckl, J.B.; Degroote, R.L.; Cambra, J.M.; Graeber, S.Y.; et al. Perinatal dysfunction of innate immunity in cystic fibrosis. Sci. Transl. Med. 2025, 17, eadk9145. [Google Scholar] [CrossRef]
- Koeppen, K.; Nymon, A.; Barnaby, R.; Li, Z.; Hampton, T.H.; Ashare, A.; Stanton, B.A. CF monocyte-derived macrophages have an attenuated response to extracellular vesicles secreted by airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L530–L544. [Google Scholar] [CrossRef]
- Goudot, C.; Coillard, A.; Villani, A.C.; Gueguen, P.; Cros, A.; Sarkizova, S.; Tang-Huau, T.L.; Bohec, M.; Baulande, S.; Hacohen, N.; et al. Aryl Hydrocarbon Receptor Controls Monocyte Differentiation into Dendritic Cells versus Macrophages. Immunity 2017, 47, 582–596.E6. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, X.; Bi, J.; Zhu, A.; He, L. Indole-3-carboxaldehyde regulates RSV-induced inflammatory response in RAW264.7 cells by moderate inhibition of the TLR7 signaling pathway. J. Nat. Med. 2021, 75, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Piñero-Fernandez, S.; Chimerel, C.; Keyser, U.F.; Summers, D.K. Indole transport across Escherichia coli membranes. J. Bacteriol. 2011, 193, 1793–1798. [Google Scholar] [CrossRef]
- Jing, W.; Dong, S.; Xu, Y.; Liu, J.; Ren, J.; Liu, X.; Zhu, M.; Zhang, M.; Shi, H.; Li, N.; et al. Gut microbiota-derived tryptophan metabolites regulated by Wuji Wan to attenuate colitis through AhR signaling activation. Acta Pharm. Sin. B 2025, 15, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef]
- Gao, H.; Sun, M.; Li, A.; Gu, Q.; Kang, D.; Feng, Z.; Li, X.; Wang, X.; Chen, L.; Yang, H.; et al. Microbiota-derived IPA alleviates intestinal mucosal inflammation through upregulating Th1/Th17 cell apoptosis in inflammatory bowel disease. Gut Microbes 2025, 17, 2467235. [Google Scholar] [CrossRef]
- Munakarmi, S.; Chand, L.; Shin, H.B.; Jang, K.Y.; Jeong, Y.J. Indole-3-Carbinol Derivative DIM Mitigates Carbon Tetrachloride-Induced Acute Liver Injury in Mice by Inhibiting Inflammatory Response, Apoptosis and Regulating Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 2048. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, H.; Hou, X.; Chen, Y.; Xu, K. Pretreatment with IPA ameliorates colitis in mice: Colon transcriptome and fecal 16S amplicon profiling. Front. Immunol. 2022, 13, 1014881. [Google Scholar] [CrossRef]
- Choi, Y.; Abdelmegeed, M.A.; Song, B.J. Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis. J. Nutr. Biochem. 2018, 55, 12–25. [Google Scholar] [CrossRef]
- Puccetti, M.; Pariano, M.; Renga, G.; Santarelli, I.; D’Onofrio, F.; Bellet, M.M.; Stincardini, C.; Bartoli, A.; Costantini, C.; Romani, L.; et al. Targeted Drug Delivery Technologies Potentiate the Overall Therapeutic Efficacy of an Indole Derivative in a Mouse Cystic Fibrosis Setting. Cells 2021, 10, 1601. [Google Scholar] [CrossRef]
- Ding, Y.; Yanagi, K.; Yang, F.; Callaway, E.; Cheng, C.; Hensel, M.E.; Menon, R.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Oral supplementation of gut microbial metabolite indole-3-acetate alleviates diet-induced steatosis and inflammation in mice. eLife 2024, 12, RP87458. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37.E6. [Google Scholar] [CrossRef]
- Genestet, C.; Le Gouellec, A.; Chaker, H.; Polack, B.; Guery, B.; Toussaint, B.; Stasia, M.J. Scavenging of reactive oxygen species by tryptophan metabolites helps Pseudomonas aeruginosa escape neutrophil killing. Free Radic. Biol. Med. 2014, 73, 400–410. [Google Scholar] [CrossRef]
- Mustafina, M.; Silantyev, A.; Krasovskiy, S.; Chernyak, A.; Naumenko, Z.; Suvorov, A.; Gognieva, D.; Abdullaev, M.; Bektimirova, A.; Bykova, A.; et al. Exhaled breath analysis in adult patients with cystic fibrosis by real-time proton mass spectrometry. Clin. Chim. Acta 2024, 560, 119733. [Google Scholar] [CrossRef] [PubMed]
- Mustafina, M.; Silantyev, A.; Krasovskiy, S.; Chernyak, A.; Naumenko, Z.; Suvorov, A.; Gognieva, D.; Abdullaev, M.; Suvorova, O.; Schmidt, A.; et al. Identification of Exhaled Metabolites Correlated with Respiratory Function and Clinical Features in Adult Patients with Cystic Fibrosis by Real-Time Proton Mass Spectrometry. Biomolecules 2024, 14, 1189. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, M.; Pariano, M.; Wojtylo, P.; Schoubben, A.; Giovagnoli, S.; Ricci, M. Turning Microbial AhR Agonists into Therapeutic Agents via Drug Delivery Systems. Pharmaceutics 2023, 15, 506. [Google Scholar] [CrossRef]
- Rueda, G.H.; Causada-Calo, N.; Borojevic, R.; Nardelli, A.; Pinto-Sanchez, M.I.; Constante, M.; Libertucci, J.; Mohan, V.; Langella, P.; Loonen, L.M.P.; et al. Oral tryptophan activates duodenal aryl hydrocarbon receptor in healthy subjects: A crossover randomized controlled trial. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 326, G687–G696. [Google Scholar] [CrossRef]
- Zeng, W.; Han, C.; Mohammed, S.; Li, S.; Song, Y.; Sun, F.; Du, Y. Indole-containing pharmaceuticals: Targets, pharmacological activities, and SAR studies. RSC Med. Chem. 2024, 15, 788–808. [Google Scholar] [CrossRef]
- Fiedorczuk, K.; Chen, J. Mechanism of CFTR correction by type I folding correctors. Cell 2022, 185, 158–168.E11. [Google Scholar] [CrossRef]
- Ruah, S.S.H.; Grootenhuis, P.D.; Van Goor, F.; Zhou, J.; Bear, B.; Miller, M.T.; Numa, M.M.D.; Yang, X. Indole Derivatives as CFTR Modulators. U.S. Patent No. 7,645,789, 10 June 2025. [Google Scholar]
- Son, J.H.; Phuan, P.W.; Zhu, J.S.; Lipman, E.; Cheung, A.; Tsui, K.Y.; Tantillo, D.J.; Verkman, A.S.; Haggie, P.M.; Kurth, M.J. 1-BENZYLSPIRO[PIPERIDINE-4,1′-PYRIDO[3,4-b]indole] ‘co-potentiators’ for minimal function CFTR mutants. Eur. J. Med. Chem. 2021, 209, 112888. [Google Scholar] [CrossRef]
- Brindani, N.; Gianotti, A.; Giovani, S.; Giacomina, F.; Di Fruscia, P.; Sorana, F.; Bertozzi, S.M.; Ottonello, G.; Goldoni, L.; Penna, I.; et al. Identification, Structure-Activity Relationship, and Biological Characterization of 2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indoles as a Novel Class of CFTR Potentiators. J. Med. Chem. 2020, 63, 11169–11194. [Google Scholar] [CrossRef]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270.E34. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.B.; Zhu, S.P.; Liu, T.P.; Zhao, H.; Chen, P.F.; Duan, Y.J.; Hu, R. Cancer Associated Fibroblasts Promote Renal Cancer Progression Through a TDO/Kyn/AhR Dependent Signaling Pathway. Front. Oncol. 2021, 11, 628821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, D.; Zhang, J.; Tang, H.; Li, F.; Peng, Y.; Duan, X.; Meng, E.; Zhang, C.; Zeng, T.; et al. Role of macrophage AHR/TLR4/STAT3 signaling axis in the colitis induced by non-canonical AHR ligand aflatoxin B1. J. Hazard. Mater. 2023, 452, 131262. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Deng, Y.; Yang, H.; Yu, M. The aryl hydrocarbon receptor: A promising target for intestinal fibrosis therapy. Pharmacol. Res. 2025, 219, 107909. [Google Scholar] [CrossRef]
- Li, M.D.; Chen, L.H.; Xiang, H.X.; Jiang, Y.L.; Lv, B.B.; Xu, D.X.; Zhao, H.; Fu, L. Benzo[a]pyrene evokes epithelial-mesenchymal transition and pulmonary fibrosis through AhR-mediated Nrf2-p62 signaling. J. Hazard. Mater. 2024, 473, 134560. [Google Scholar] [CrossRef]
- Luo, M.; Luo, D.; Liu, J.; Wang, H.; Liu, X.; Yang, M.; Tian, F.; Qin, S.; Li, Y. Ameliorative effect of the probiotic peptide against benzo(α)pyrene-induced inflammatory damages in enterocytes. Int. Immunopharmacol. 2022, 112, 109255. [Google Scholar] [CrossRef]
- Xie, H.; Yang, N.; Lu, L.; Sun, X.; Li, J.; Wang, X.; Guo, H.; Zhou, L.; Liu, J.; Wu, H.; et al. Uremic Toxin Receptor AhR Facilitates Renal Senescence and Fibrosis via Suppressing Mitochondrial Biogenesis. Adv. Sci. 2024, 11, e2402066. [Google Scholar] [CrossRef]
- Gao, X.; Xie, C.; Wang, Y.; Luo, Y.; Yagai, T.; Sun, D.; Qin, X.; Krausz, K.W.; Gonzalez, F.J. The antiandrogen flutamide is a novel aryl hydrocarbon receptor ligand that disrupts bile acid homeostasis in mice through induction of Abcc4. Biochem. Pharmacol. 2016, 119, 93–104. [Google Scholar] [CrossRef]
- Salminen, A. Activation of aryl hydrocarbon receptor (AhR) in Alzheimer’s disease: Role of tryptophan metabolites generated by gut host-microbiota. J. Mol. Med. 2023, 101, 201–222. [Google Scholar] [CrossRef]
- Walker, J.A.; Richards, S.; Belghasem, M.E.; Arinze, N.; Yoo, S.B.; Tashjian, J.Y.; Whelan, S.A.; Lee, N.; Kolachalama, V.B.; Francis, J.; et al. Temporal and tissue-specific activation of aryl hydrocarbon receptor in discrete mouse models of kidney disease. Kidney Int. 2020, 97, 538–550. [Google Scholar] [CrossRef]
- Tanaka, M.; Fujikawa, M.; Oguro, A.; Itoh, K.; Vogel, C.F.A.; Ishihara, Y. Involvement of the Microglial Aryl Hydrocarbon Receptor in Neuroinflammation and Vasogenic Edema after Ischemic Stroke. Cells 2021, 10, 718. [Google Scholar] [CrossRef]
- Moriguchi, T.; Motohashi, H.; Hosoya, T.; Nakajima, O.; Takahashi, S.; Ohsako, S.; Aoki, Y.; Nishimura, N.; Tohyama, C.; Fujii-Kuriyama, Y.; et al. Distinct response to dioxin in an arylhydrocarbon receptor (AHR)-humanized mouse. Proc. Natl. Acad. Sci. USA 2003, 100, 5652–5657. [Google Scholar] [CrossRef]
- Shen, Q.; Yu, H.; Liu, Y.; Li, G.; An, T. Combined exposure of MAHs and PAHs enhanced amino acid and lipid metabolism disruption in epithelium leading asthma risk. Environ. Pollut. 2024, 343, 123261. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Li, J.L. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019, 12, 5467–5484. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Guo, M.; Li, X.; Jia, F.; Li, C.; Yang, Y.; Cao, M.; Jiang, N.; Ma, E.; Zhai, X. Discovery of Novel Indole-Based Allosteric Highly Potent ATX Inhibitors with Great In Vivo Efficacy in a Mouse Lung Fibrosis Model. J. Med. Chem. 2020, 63, 7326–7346. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, B.; Ouyang, H.; Wang, X.; Li, C.; Li, L.; Zhang, J. Advanced nanotherapies for precision treatment of inflammatory lung diseases. Bioact. Mater. 2025, 53, 329–365. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Wang, Q.; Yuan, Y.; Hao, Q.; Bi, Y.; He, Y.; Zhao, J.; Hao, J. Fabrication and in vitro/in vivo characterization of Eudragit enteric nanoparticles loaded with indomethacin. Chem. Pap. 2022, 76, 1119–1133. [Google Scholar] [CrossRef]

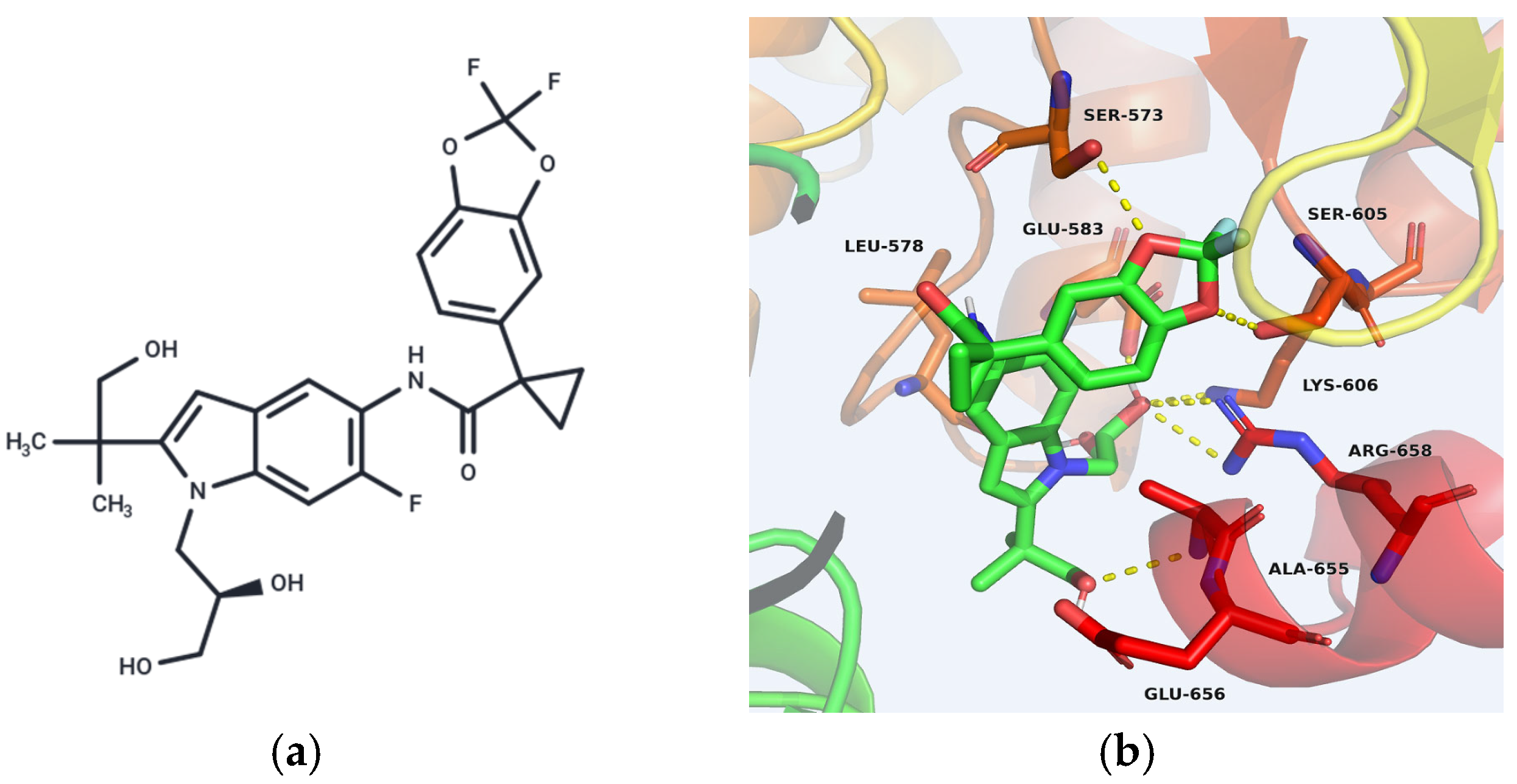
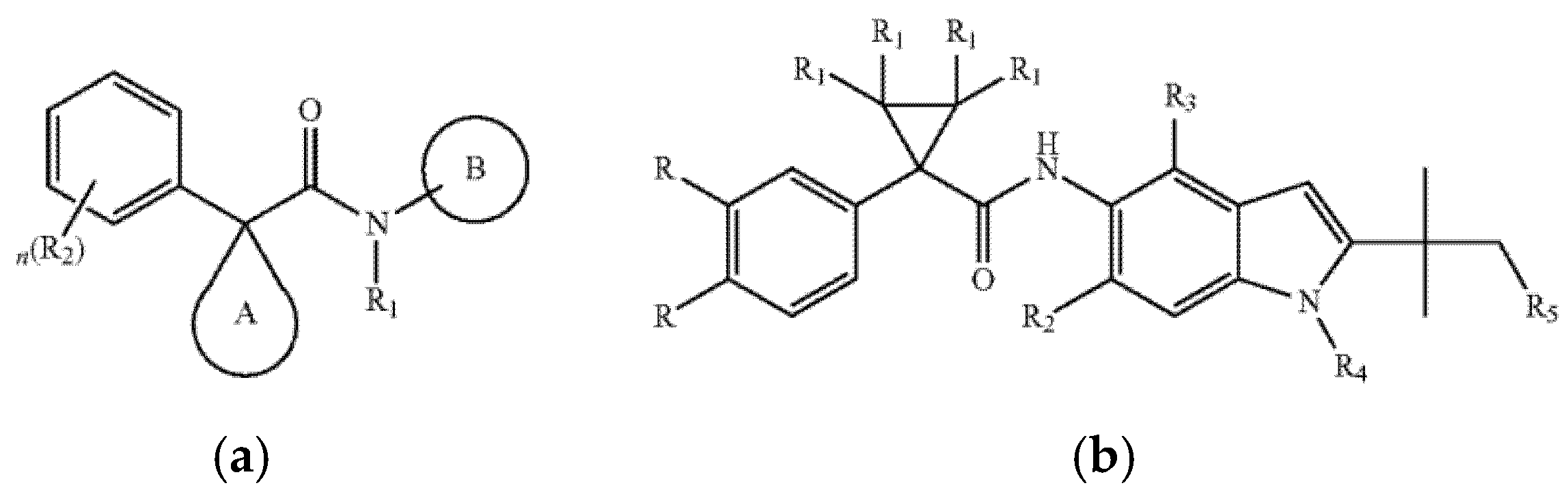
| Risk Category | Potential Risks | Research Gaps |
|---|---|---|
| Tissue specific risk | Pulmonary effects: Induce oxidative stress and exacerbate airway inflammation [116]; Liver damage: Inhibition of the FXR signaling pathway induces liver injury [111] | Lack of targeted toxicity assessment for specific organs such as the lungs, liver, and kidneys in CF patients |
| Exposure duration | Long-term risk: Animal studies indicate that prolonged use may induce CYP450, potentially affecting the metabolism of other drugs [117] | Lack of long-term pharmacokinetic studies in CF patients |
| Immunosuppression | Inhibition of the NF-κB signaling pathway in the tumor microenvironment weakens the tumor immune response and promotes tumor immune escape [104,105] | The equilibrium point between immunosuppressive effects and anti-inflammatory actions remains unclear |
| Drug–drug interactions | Metabolic interference: Indole compounds may affect the concentrations of other drugs by influencing the metabolic enzyme CYP450 [117] | Lack of interaction studies between indole compounds and CFTR modulators |
| Drug interactions: The risk of interactions with other CF treatments such as antibiotics, bronchodilators, and CFTR modulators is unknown | Lack of research on the metabolic patterns of compounds under the unique metabolic state of CF patients | |
| Clinical status | Existing research directions predominantly focus on cancer or inflammatory bowel disease, with insufficient preclinical data for CF patients | Lack of preclinical safety data for long-term administration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Yue, Q.; Hou, X.; Wu, Q. Targeting the Aryl Hydrocarbon Receptor: The Potential of Indole Compounds in the Treatment of Cystic Fibrosis. Int. J. Mol. Sci. 2025, 26, 9876. https://doi.org/10.3390/ijms26209876
Hou S, Yue Q, Hou X, Wu Q. Targeting the Aryl Hydrocarbon Receptor: The Potential of Indole Compounds in the Treatment of Cystic Fibrosis. International Journal of Molecular Sciences. 2025; 26(20):9876. https://doi.org/10.3390/ijms26209876
Chicago/Turabian StyleHou, Sen, Qingkun Yue, Xia Hou, and Qingtian Wu. 2025. "Targeting the Aryl Hydrocarbon Receptor: The Potential of Indole Compounds in the Treatment of Cystic Fibrosis" International Journal of Molecular Sciences 26, no. 20: 9876. https://doi.org/10.3390/ijms26209876
APA StyleHou, S., Yue, Q., Hou, X., & Wu, Q. (2025). Targeting the Aryl Hydrocarbon Receptor: The Potential of Indole Compounds in the Treatment of Cystic Fibrosis. International Journal of Molecular Sciences, 26(20), 9876. https://doi.org/10.3390/ijms26209876






