RNA Interference and Its Key Targets for Spinal Cord Injury Therapy: What Is Known So Far?
Abstract
1. Introduction
2. SCI: Sequence of Events
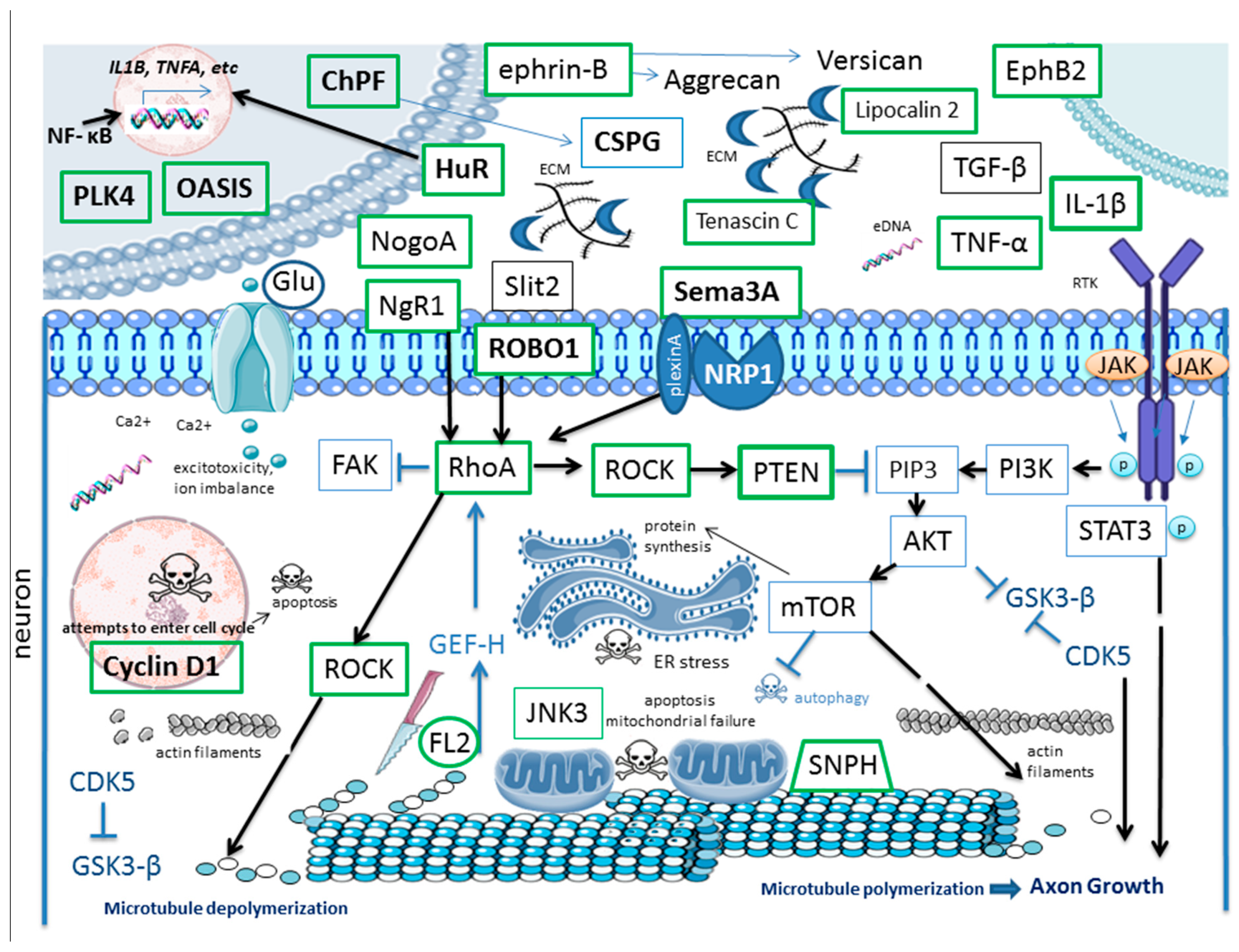
3. Targets for RNAi in SCI
3.1. ECM Remodeling and Scar Formation
3.2. Inflammation and Immune Response
3.3. Cell Death and Senescence
3.4. Axonal Growth, Guidance and Axonal Transport
3.5. Cytoskeletal Dynamics
3.6. Mitochondrial Dysfunction
3.7. Increased Blood–Spinal Cord Barrier (BSCB) Permeability
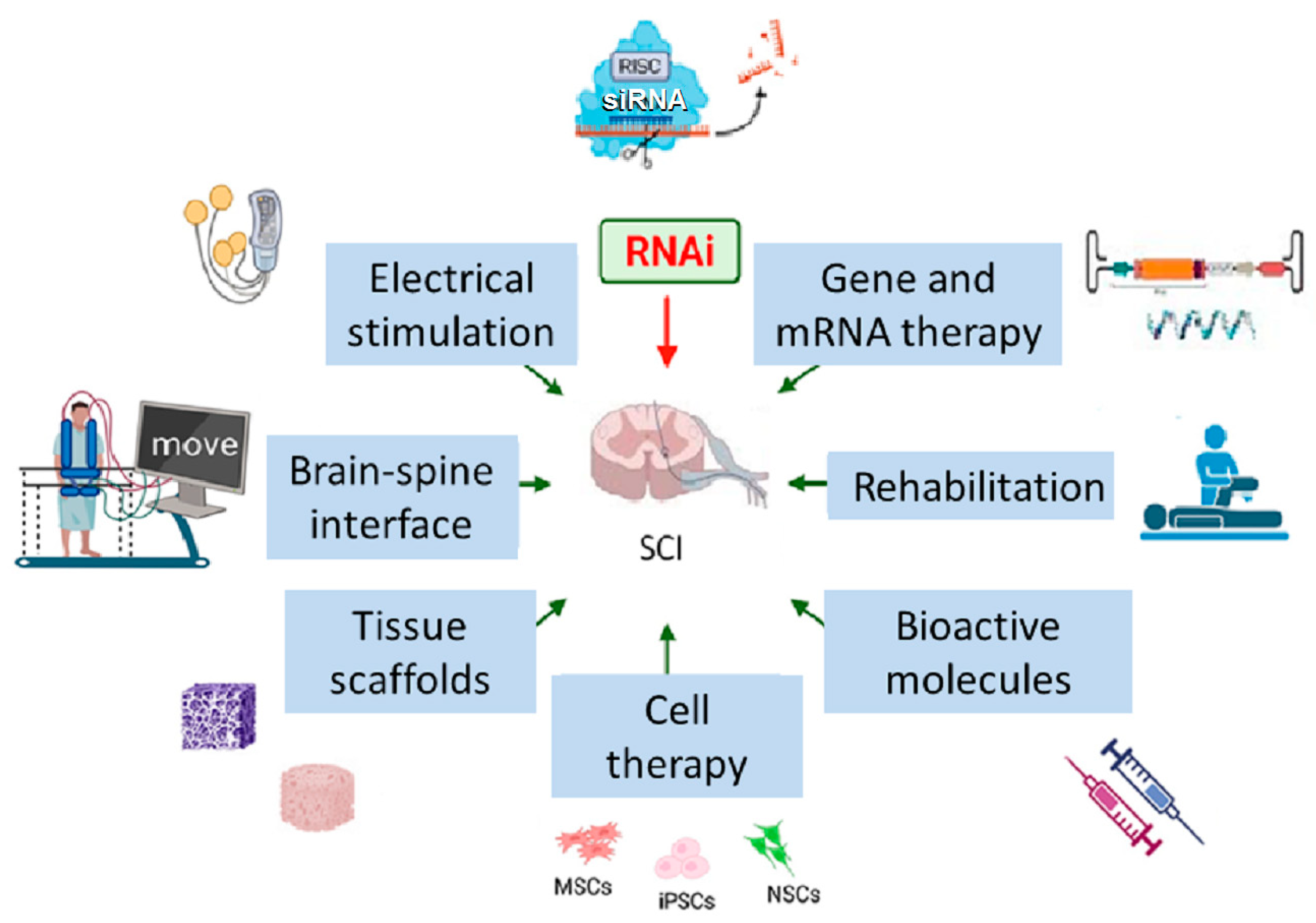
4. Combination of RNAi and Other SCI Therapies
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3WJ | Three-Way Junction (nanoparticles) |
| AAV | Adeno-Associated Virus |
| Ad4 LNPs | Adenosine-functionalized Lipid Nanoparticles |
| ADSCs | Adipose-Derived Stem Cells |
| Ago2 | Argonaute-2 Protein |
| anti-RGMa Ab | Antibody to RGMa |
| ASO | Single-stranded antisense RNA oligonucleotides |
| BBB | Blood–Brain Barrier |
| BDNF | Brain-Derived Neurotrophic Factor |
| BMSCs | Bone Marrow-Derived Mesenchymal Stem Cells |
| BSCB | Blood–Spinal Cord Barrier |
| CCL2 | Chemokine (C-C Motif) Ligand 2 |
| CD206 | Cluster of Differentiation 206 (Macrophage Mannose receptor) |
| CD86 | Cluster of Differentiation 86 |
| CDK5 | Cyclin dependent kinase 5 |
| ChABC | Chondroitinase ABC |
| ChPF | Chondroitin Polymerizing Factor |
| CNS | Central Nervous System |
| Col1a1 | Collagen Type I Alpha 1 Chain |
| CREB3L1 | cAMP-Responsive Element-Binding Protein 3-Like 1 (OASIS) |
| CSPGs | Chondroitin Sulfate Proteoglycans |
| CTGF | Connective Tissue Growth Factor |
| CX3CR1 | Chemokine Receptor 1 |
| DAMPs | Damage-Associated Molecular Patterns |
| ECM | Extracellular Matrix |
| EphB2 | Ephrin Receptor B2 |
| ESCs | Embryonic stem cells |
| EVs | Extracellular Vesicles |
| FDX1 | Ferredoxin 1 |
| FL2 | Fidgetin-Like 2 Protein |
| GAP-43 | Growth Associated Protein 43 |
| GelMA | Gelatin Methacrylate |
| GFAP | Glial Fibrillary Acidic Protein |
| GDNF | Glial Cell-Derived Neurotrophic Factor |
| GiPCRs | G Protein-Coupled Receptors |
| GSK-3β | Glycogen Synthase Kinase-3β |
| hATTR | Hereditary Transthyretin-mediated amyloidosis |
| HucMSCs | Human umbilical cord Mesenchymal Stem Cells |
| HuR | Hu Antigen R |
| IBA1 | Ionized Calcium Binding Adaptor Molecule 1 |
| ICH | Intracerebral Hemorrhage |
| IKKβ | Inhibitor of κB kinase subunit beta |
| IL-1β | Interleukin 1 Beta |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| iGluRs | Ionotropic Glutamate Receptors |
| iNSCs | Induced Neural Stem Cells |
| iNOS | Inducible Nitric Oxide Synthase |
| iPSCs | Induced pluripotent stem cells |
| IRF5 | Interferon Regulatory Factor 5 |
| JAK | Janus Kinase |
| Lcn2 | Lipocalin 2 |
| LNPs | Lipid Nanoparticles |
| LV | Lentiviral Vector |
| MAG | Myelin-Associated Glycoprotein |
| MAI | Myelin-Associated Inhibitor |
| MCAO | Middle Cerebral Artery Occlusion |
| METs | Macrophage Extracellular traps |
| MIF | Macrophage Migration Inhibitory Factor |
| miRNA | MicroRNA |
| mRNA | Messenger RNA |
| mTOR | Mechanistic Target of Rapamycin |
| MSCs | Mesenchymal Stem Cells |
| NETs | Neutrophil Extracellular traps |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of activated B cells |
| NeuN | Neuronal Nuclei Marker |
| NgR | Nogo-66 Receptor |
| Nogo-A | Neurite Outgrowth Inhibitor A |
| NSCs | Neural Stem Cells |
| OASIS | Old Astrocyte Specifically Induced Substance |
| OMgp | Oligodendrocyte Myelin Glycoprotein |
| P2X7R | P2X purinoceptor 7 receptor |
| PARP-1 | Poly(ADP-Ribose) Polymerase 1 |
| PBS | Phosphate-buffered Saline |
| PI3K | Phosphoinositide 3-Kinase |
| PLK4 | Polo-Like Kinase 4 |
| PLNG | Photocurable Lipid Nanoparticle GelMA |
| PMCID / PMID | PubMed Central Identification Number/ PubMed Identifier |
| PNNs | Perineuronal Nets |
| PTEN | Phosphatase and Tensin Homolog |
| RAGE | Receptor for Advanced Glycation End Products |
| RGMA | Repulsive Guidance Molecule A |
| RhoA | Ras Homolog Family Member A |
| RNAi | RNA Interference |
| Robo | transmembrane Roundabout receptors |
| ROCK | Rho-Associated Protein Kinase |
| ROS | Reactive Oxygen Species |
| SASP | Senescence-Associated Secretory Phenotype |
| SC | Spinal Cord |
| SCI | Spinal Cord Injury |
| sem3A | Semaphorin 3A |
| shRNA | Short Hairpin RNA |
| siRNA | Small Interfering RNA |
| SNPH | Syntaphilin |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STING | Stimulator of Interferon Genes |
| TBI | Traumatic Brain Injury |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TLR4 | Toll-Like Receptor 4 |
| TLRs | Toll-Like Receptors |
| TMEM119 | Transmembrane Protein 119 |
| TMEM173 | Transmembrane Protein 173 |
| TNF-α | Tumor Necrosis Factor Alpha |
| TNC | Tenascin-C |
| TRPM7 | Transient Receptor Potential Melastatin 7 |
| TRITC | Tetramethylrhodamine |
| VIM | Vimentin |
References
- Kang, Y.; Ding, H.; Zhou, H.; Wei, Z.; Liu, L.; Pan, D.; Feng, S. Epidemiology of Worldwide Spinal Cord Injury: A Literature Review. J. Neurorestoratology 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal Cord Injury: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The Current State and Future Directions of RNAi-Based Therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Jo, S.B.; Kim, T.H.; Kim, H.W.; Chew, S.Y. RNA Interference in Glial Cells for Nerve Injury Treatment. J. Tissue Eng. 2020, 11, 2041731420939224. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sun, P. Promoting Functions of MicroRNA-29a/199B in Neurological Recovery in Rats with Spinal Cord Injury through Inhibition of the RGMA/STAT3 Axis. J. Orthop. Surg. Res. 2020, 15, 427. [Google Scholar] [CrossRef]
- Zhou, H.J.; Wang, L.Q.; Xu, Q.S.; Fan, Z.X.; Zhu, Y.; Jiang, H.; Zheng, X.J.; Ma, Y.H.; Zhan, R.Y. Downregulation of MiR-199b Promotes the Acute Spinal Cord Injury through IKKβ-NF-ΚB Signaling Pathway Activating Microglial Cells. Exp. Cell Res. 2016, 349, 60–67. [Google Scholar] [CrossRef]
- Michael, F.M.; Chandran, P.; Chandramohan, K.; Iyer, K.; Jayaraj, K.; Sundaramoorthy, R.; Venkatachalam, S. Prospects of SiRNA Cocktails as Tools for Modifying Multiple Gene Targets in the Injured Spinal Cord. Exp. Biol. Med. 2019, 244, 1096–1110. [Google Scholar] [CrossRef]
- Lennox, K.A.; Behlke, M.A. Cellular Localization of Long Non-Coding RNAs Affects Silencing by RNAi More than by Antisense Oligonucleotides. Nucleic Acids Res. 2016, 44, 863–877. [Google Scholar] [CrossRef]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell Biology of Spinal Cord Injury and Repair. J. Clin. Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Grin, A.A.; Kordonskiy, A.Y.; Abdukhalikov, B.A.; Arakelyan, S.L.; Lvov, I.S.; Kaikov, A.K.; Talypov, A.E.; Sytnik, A.V. Classification of Injuries of the Thoracic and Lumbar Spine. Russ. J. Neurosurg. 2021, 23, 112–127. [Google Scholar] [CrossRef]
- Sánchez-Ventura, J.; Lane, M.A.; Udina, E. The Role and Modulation of Spinal Perineuronal Nets in the Healthy and Injured Spinal Cord. Front. Cell Neurosci. 2022, 16, 893857. [Google Scholar] [CrossRef] [PubMed]
- Alastra, G.; Quadalti, C.; Baldassarro, V.A.; Giuliani, A.; Giardino, L.; Calzà, L. The Influence of Pathological Extracellular Matrix on the Biological Properties of Stem Cells: Possible Hints for Cell Transplantation Therapies in Spinal Cord Injury. Int. J. Mol. Sci. 2025, 26, 3969. [Google Scholar] [CrossRef]
- Burnside, E.R.; Bradbury, E.J. Review: Manipulating the Extracellular Matrix and Its Role in Brain and Spinal Cord Plasticity and Repair. Neuropathol. Appl. Neurobiol. 2014, 40, 26–59. [Google Scholar] [CrossRef]
- Rolls, A.; Shechter, R.; London, A.; Segev, Y.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Schwartz, M. Two Faces of Chondroitin Sulfate Proteoglycan in Spinal Cord Repair: A Role in Microglia/Macrophage Activation. PLoS Med. 2008, 5, e171. [Google Scholar] [CrossRef]
- Yang, D.; Han, Z.; Oppenheim, J.J. Alarmins and Immunity. Immunol. Rev. 2017, 280, 41–56. [Google Scholar] [CrossRef]
- Francos-Quijorna, I.; Sánchez-Petidier, M.; Burnside, E.R.; Badea, S.R.; Torres-Espin, A.; Marshall, L.; de Winter, F.; Verhaagen, J.; Moreno-Manzano, V.; Bradbury, E.J. Chondroitin Sulfate Proteoglycans Prevent Immune Cell Phenotypic Conversion and Inflammation Resolution via TLR4 in Rodent Models of Spinal Cord Injury. Nat. Commun. 2022, 13, 2933. [Google Scholar] [CrossRef] [PubMed]
- Chambel, S.S.; Cruz, C.D. Axonal Growth Inhibitors and Their Receptors in Spinal Cord Injury: From Biology to Clinical Translation. Neural Regen. Res. 2023, 18, 2573–2581. [Google Scholar] [CrossRef]
- Laabs, T.L.; Wang, H.; Katagiri, Y.; McCann, T.; Fawcett, J.W.; Geller, H.M. Inhibiting Glycosaminoglycan Chain Polymerization Decreases the Inhibitory Activity of Astrocyte-Derived Chondroitin Sulfate Proteoglycans. J. Neurosci. 2007, 27, 14494–14501. [Google Scholar] [CrossRef]
- Didangelos, A.; Puglia, M.; Iberl, M.; Sanchez-Bellot, C.; Roschitzki, B.; Bradbury, E.J. High-Throughput Proteomics Reveal Alarmins as Amplifiers of Tissue Pathology and Inflammation after Spinal Cord Injury. Sci. Rep. 2016, 6, 21607. [Google Scholar] [CrossRef]
- Chelluboina, B.; Chokkalla, A.K.; Mehta, S.L.; Morris-Blanco, K.C.; Bathula, S.; Sankar, S.; Park, J.S.; Vemuganti, R. Tenascin-C Induction Exacerbates Post-Stroke Brain Damage. J. Cereb. Blood Flow Metab. 2022, 42, 253–263. [Google Scholar] [CrossRef]
- Stepankova, K.; Smejkalova, B.; Machova Urdzikova, L.; Haveliková, K.; de Winter, F.; Suchankova, S.; Verhaagen, J.; Herynek, V.; Turecek, R.; Kwok, J.; et al. Activated Alpha 9 Integrin Expression Enables Sensory Pathway Reconstruction after Spinal Cord Injury. Acta Neuropathol. Commun. 2025, 13, 89. [Google Scholar] [CrossRef]
- Hirt, J.; Khanteymoori, A.; Hohenhaus, M.; Kopp, M.A.; Howells, D.W.; Schwab, J.M.; Watzlawick, R. Inhibition of the Nogo-Pathway in Experimental Spinal Cord Injury: A Meta-Analysis of 76 Experimental Treatments. Sci. Rep. 2023, 13, 22898. [Google Scholar] [CrossRef]
- Kucher, K.; Johns, D.; Maier, D.; Abel, R.; Badke, A.; Baron, H.; Thietje, R.; Casha, S.; Meindl, R.; Gomez-Mancilla, B.; et al. First-in-Man Intrathecal Application of Neurite Growth-Promoting Anti-Nogo-A Antibodies in Acute Spinal Cord Injury. Neurorehabilit. Neural Repair 2018, 32, 578–589. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Ma, Y.; Hua, Z.; Guo, Y.; Gu, X.; Zhang, Y. Nogo-A Expression Dynamically Varies after Spinal Cord Injury. Neural Regen. Res. 2015, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Fujitani, M.; Yasuda, Y.; Doya, H.; Saito, T.; Yamagishi, S.; Mueller, B.K.; Yamashita, T. RGMa Inhibition Promotes Axonal Growth and Recovery after Spinal Cord Injury. J. Cell Biol. 2006, 173, 47–58. [Google Scholar] [CrossRef]
- Qu, Y.; Zhao, J.; Wang, Y.; Gao, Z. Silencing EphrinB3 Improves Functional Recovery Following Spinal Cord Injury. Mol. Med. Rep. 2014, 9, 1761–1766. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, W.K.; Han, G.H.; Lee, D.; Cho, M.J.; Sheen, S.H.; Sohn, S. Axon Guidance Gene-Targeted SiRNA Delivery System Improves Neural Stem Cell Transplantation Therapy after Spinal Cord Injury. Biomater. Res. 2023, 27, 101. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Sun, Y.; Guo, H.; Lv, S.; Guo, W.; Ren, J.; Wang, Y.; Zu, J.; Yan, J.; et al. Targeting Astrocytes Polarization after Spinal Cord Injury: A Promising Direction. Front. Cell Neurosci. 2024, 18, 1478741. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Qu, M.; Li, L.; Liu, T.; Lin, M.; Yu, X. SiRNA in MSC-Derived Exosomes Silences CTGF Gene for Locomotor Recovery in Spinal Cord Injury Rats. Stem Cell Res. Ther. 2021, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, R.; Jiang, B.; Xu, X.; Guan, J.J.; Jiang, X.J.; Zhou, Y.; Zhou, Y.L.; Chen, X. Repair of Spinal Cord Injury by Inhibition of PLK4 Expression Through Local Delivery of SiRNA-Loaded Nanoparticles. J. Mol. Neurosci. 2022, 72, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Braga, A.; Verheyen, J.; Basilico, S.; Bandiera, S.; Alfaro-Cervello, C.; Peruzzotti-Jametti, L.; Shu, D.; Haque, F.; Guo, P.; et al. RNA Nanotherapeutics for the Amelioration of Astroglial Reactivity. Mol. Ther. Nucleic Acids 2018, 10, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.C.; Huang, Y.T.; Chiang, I.C.; Tsai, P.J.; Su, Y.W.; Chou, W.H. Lipocalin-2 Mediates the Rejection of Neural Transplants. FASEB J. 2021, 35, e21317. [Google Scholar] [CrossRef]
- Braga, A.; Bandiera, S.; Verheyen, J.; Hamel, R.; Rutigliani, C.; Edenhofer, F.; Smith, J.A.; Pluchino, S. Combination of In Situ Lcn2 PRNA-RNAi Nanotherapeutics and INSC Transplantation Ameliorates Experimental SCI in Mice. Mol. Ther. 2020, 28, 2677–2690. [Google Scholar] [CrossRef] [PubMed]
- Takazawa, A.; Kamei, N.; Adachi, N.; Ochi, M. Endoplasmic Reticulum Stress Transducer Old Astrocyte Specifically Induced Substance Contributes to Astrogliosis after Spinal Cord Injury. Neural Regen. Res. 2018, 13, 536–540. [Google Scholar] [CrossRef]
- Liu, S.; Lin, G.; Yang, Q.; Wang, P.; Ma, C.; Qian, X.; He, X.; Dong, Z.; Liu, Y.; Liu, M.; et al. Depletion of SASH1, an Astrocyte Differentiation-related Gene, Contributes to Functional Recovery in Spinal Cord Injury. CNS Neurosci. Ther. 2023, 29, 228–238. [Google Scholar] [CrossRef]
- Bundesen, L.Q.; Scheel, T.A.; Bregman, B.S.; Kromer, L.F. Ephrin-B2 and EphB2 Regulation of Astrocyte-Meningeal Fibroblast Interactions in Response to Spinal Cord Lesions in Adult Rats. J. Neurosci. 2003, 23, 7789–7800. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Tan, L.; Pan, J.Y.; Lin, W.W.; Wu, J.; Hu, W.; Chen, X.; Wang, X.D. RNAi-Mediated Ephrin-B2 Silencing Attenuates Astroglial-Fibrotic Scar Formation and Improves Spinal Cord Axon Growth. CNS Neurosci. Ther. 2017, 23, 779–789. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.; Ran, R.; Liao, H.; Lyu, J.; Ren, Y.; Lei, Z.; Zhang, H. TGF-β Signaling Pathway in Spinal Cord Injury: Mechanisms and Therapeutic Potential. J. Neurosci. Res. 2024, 102, e25255. [Google Scholar] [CrossRef]
- Park, S.M.; Jung, J.S.; Jang, M.S.; Kang, K.S.; Kang, S.K. Transforming Growth Factor-β1 Regulates the Fate of Cultured Spinal Cord-derived Neural Progenitor Cells. Cell Prolif. 2008, 41, 248–264. [Google Scholar] [CrossRef]
- Wang, X.; Cao, K.; Sun, X.; Chen, Y.; Duan, Z.; Sun, L.; Guo, L.; Bai, P.; Sun, D.; Fan, J.; et al. Macrophages in Spinal Cord Injury: Phenotypic and Functional Change from Exposure to Myelin Debris. Glia 2015, 63, 635–651. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Xu, H.; Fu, Q. Nanoparticle-Delivered IRF5 SiRNA Facilitates M1 to M2 Transition, Reduces Demyelination and Neurofilament Loss, and Promotes Functional Recovery After Spinal Cord Injury in Mice. Inflammation 2016, 39, 1704–1717. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-F.; Hu, X.; Xiong, L.; Wu, M.; Yang, X.; Wang, C.; Chen, S.; Xu, H.; Chen, H.; Ma, X.; et al. Interference of Interleukin-1 β Mediated by Lentivirus Promotes Functional Recovery of Spinal Cord Contusion Injury in Rats via the PI3K/AKT1 Signaling Pathway. Mediators Inflamm. 2022, 2022, 6285099. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.; Floyd, C.L.; Kim, S.; King, P.H. RNA Binding Protein Human Antigen R Is Translocated in Astrocytes Following Spinal Cord Injury and Promotes the Inflammatory Response. J. Neurotrauma 2017, 34, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, K.; Wu, Y.; Wu, S.; Ma, P.; Zhang, J.; Li, J.; Shen, G.; Men, K. Controlled Release of MIF SiRNA and GDNF Protein from a Photocurable Scaffold Efficiently Repairs Spinal Cord Injury. MedComm 2025, 6, e70099. [Google Scholar] [CrossRef]
- Krebs, D.L.; Hilton, D.J. SOCS Proteins: Negative Regulators of Cytokine Signaling. Stem Cells 2001, 19, 378–387. [Google Scholar] [CrossRef]
- Chen, X.-M.; Yu, Y.-H.; Wang, L.; Zhao, X.-Y.; Li, J.-R. Effect of the JAK2/STAT3 Signaling Pathway on Nerve Cell Apoptosis in Rats with White Matter Injury. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 321–327. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, C.; Chen, Z.; Wang, X.; Hong, F.; Hao, D. Regulation of the JAK/STAT Signaling Pathway in Spinal Cord Injury: An Updated Review. Front. Immunol. 2023, 14, 1276445. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, D.; Qiao, H.; Li, J.; Li, J.; Yang, Y.; Chang, S.; Li, F.; Wang, D.; Li, H.; et al. Macrophage Extracellular Traps Exacerbate Secondary Spinal Cord Injury by Modulating Macrophage/Microglia Polarization via LL37/P2X7R/NF- κ B Signaling Pathway. Oxid. Med. Cell Longev. 2022, 2022, 9197940. [Google Scholar] [CrossRef]
- Varsamos, I.; Patilas, C.; Galanis, A.; Zachariou, D.; Tsalimas, G.; Sakellariou, E.; Spyrou, I.; Rozis, M.; Kaspiris, A.; Karampinas, P.K.; et al. The Impact of Nuclear Factor Kappa B on the Response of Microglia in Spinal Cord Injuries. Cureus 2025, 17, 79367. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Shen, D.; Zhao, L.J.; Zeng, N.; Hu, T.H. Sting Is a Critical Regulator of Spinal Cord Injury by Regulating Microglial Inflammation via Interacting with TBK1 in Mice. Biochem. Biophys. Res. Commun. 2019, 517, 741–748. [Google Scholar] [CrossRef]
- Bollaerts, I.; Van Houcke, J.; Andries, L.; De Groef, L.; Moons, L. Neuroinflammation as Fuel for Axonal Regeneration in the Injured Vertebrate Central Nervous System. Mediators Inflamm. 2017, 2017, 9478542. [Google Scholar] [CrossRef]
- Ribas, V.T.; Lingor, P. Autophagy in Degenerating Axons Following Spinal Cord Injury: Evidence for Autophagosome Biogenesis in Retraction Bulbs. Neural Regen. Res. 2015, 10, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Y.; Zhang, H.; Li, Y.; Wang, X.; Xu, C.; Ni, W.; Zhou, K. Role of Necroptosis in Traumatic Brain and Spinal Cord Injuries. J. Adv. Res. 2022, 40, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.Y.; Liu, X.L.; Deng, Z.Z.; Wei, D.M.; Zhang, D.; Xi, H.L.; Wang, Q.Y.; He, M.Z.; Yang, Y.L. Ferroptosis Is a New Therapeutic Target for Spinal Cord Injury. Front. Neurosci. 2023, 17, 1136143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, X.; Wang, Z.; Ng, L.; He, R.; Liu, C.; Liu, G.; Fan, X.; Mu, X.; Zhou, Y. Machine Learning-Driven Prediction Model for Cuproptosis-Related Genes in Spinal Cord Injury: Construction and Experimental Validation. Front. Neurol. 2025, 16, 1525416. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Jiang, R.; Zhang, C.; Qi, E.; Wu, M.; Zhang, M.; Zhao, H.; Zhao, F.; Zhou, H. Dynamic Changes in Pyroptosis Following Spinal Cord Injury and the Identification of Crucial Molecular Signatures through Machine Learning and Single-Cell Sequencing. J. Pharm. Biomed. Anal. 2024, 251, 116449. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, X.; Ge, M.; Hu, H.; Xu, C.; Wen, S.; Deng, H.; Mei, X. Zinc Defends against Parthanatos and Promotes Functional Recovery after Spinal Cord Injury through SIRT3-Mediated Anti-Oxidative Stress and Mitophagy. CNS Neurosci. Ther. 2023, 29, 2857–2872. [Google Scholar] [CrossRef]
- Guha, L.; Singh, N.; Kumar, H. Different Ways to Die: Cell Death Pathways and Their Association With Spinal Cord Injury. Neurospine 2023, 20, 430–448. [Google Scholar] [CrossRef]
- Becker, T.; Wullimann, M.F.; Becker, C.G.; Bernhardt, R.R.; Schachner, M. Axonal Regrowth after Spinal Cord Transection in Adult Zebrafish. J. Comp. Neurol. 1997, 377, 577–595. [Google Scholar] [CrossRef]
- Crowe, M.J.; Bresnahan, J.C.; Shuman, S.L.; Masters, J.N.; Beattie, M.S. Apoptosis and Delayed Degeneration after Spinal Cord Injury in Rats and Monkeys. Nat. Med. 1997, 3, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.S.; Farooqui, A.A.; Bresnahan, J.C. Review of Current Evidence for Apoptosis After Spinal Cord Injury. J. Neurotrauma 2009, 17, 915–925. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xu, Y.; Sun, J.; Li, L.; Zhang, J.H.; Wang, Y. Autophagy and Apoptosis in Acute Brain Injuries: From Mechanism to Treatment. Antioxid. Redox Signal 2023, 38, 234–257. [Google Scholar] [CrossRef]
- Feng, H.; Wang, H.; Li, J.; Ren, J.; Li, Y.; Li, C.; Chen, J.; Song, X.; Ning, G.; Feng, S. Exacerbation of Neuronal Senescence after Spinal Cord Injury: Role of the Macrophage-Derived Transforming Growth Factor-Β1-SMAD2 Signaling Axis. Neural Regen. Res. 2025. [Google Scholar] [CrossRef]
- Van Niekerk, E.A.; Tuszynski, M.H.; Lu, P.; Dulin, J.N. Molecular and Cellular Mechanisms of Axonal Regeneration After Spinal Cord Injury. Mol. Cell Proteom. 2015, 15, 394. [Google Scholar] [CrossRef]
- Wu, X.; Xu, X.M. RhoA/Rho Kinase in Spinal Cord Injury. Neural Regen. Res. 2016, 11, 23–27. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, G.; Li, Y.; Guo, Z.; Zhang, X. The Molecular Genetics of PI3K/PTEN/AKT/MTOR Pathway in the Malformations of Cortical Development. Genes Dis. 2023, 11, 101021. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, Y.; Hayat, U.; Li, S. PTEN Inhibition and Axon Regeneration and Neural Repair. Neural Regen. Res. 2015, 10, 1363–1368. [Google Scholar] [CrossRef]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog SiRNA Repairs Complete Spinal Cord Injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef]
- Mirzaa, G.M.; Parry, D.A.; Fry, A.E.; Giamanco, K.A.; Schwartzentruber, J.; Vanstone, M.; Logan, C.V.; Roberts, N.; Johnson, C.A.; Singh, S.; et al. De Novo CCND2 Mutations Leading to Stabilization of Cyclin D2 Cause Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome. Nat. Genet. 2014, 46, 510–515. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Deng, B.; Lin, A.; Zhang, G.; Ma, M.; Wang, Y.; Yang, Y.; Kang, X. The PI3K/AKT Signalling Pathway in Inflammation, Cell Death and Glial Scar Formation after Traumatic Spinal Cord Injury: Mechanisms and Therapeutic Opportunities. Cell Prolif. 2022, 55, e13275. [Google Scholar] [CrossRef]
- Kimura, T.; Horikoshi, Y.; Kuriyagawa, C.; Niiyama, Y. Rho/ROCK Pathway and Noncoding RNAs: Implications in Ischemic Stroke and Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 11573. [Google Scholar] [CrossRef]
- Hu, J.; Selzer, M.E. RhoA as a Target to Promote Neuronal Survival and Axon Regeneration. Neural Regen. Res. 2017, 12, 525–528. [Google Scholar] [CrossRef]
- Danilov, C.A.; Thein, T.Z.; Tahara, S.M.; Schönthal, A.H.; Chen, T.C. Intranasal Delivery of MiR133b in a NEO100-Based Formulation Induces a Healing Response in Spinal Cord-Injured Mice. Cells 2023, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Gwak, S.J.; Macks, C.; Jeong, D.U.; Kindy, M.; Lynn, M.; Webb, K.; Lee, J.S. RhoA Knockdown by Cationic Amphiphilic Copolymer/SiRhoA Polyplexes Enhances Axonal Regeneration in Rat Spinal Cord Injury Model. Biomaterials 2017, 121, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Xu, X.; Shi, R.; Liu, J.; Yao, W.; Ke, C. Slit2/Robo1 Promotes Synaptogenesis and Functional Recovery of Spinal Cord Injury. Neuroreport 2017, 28, 75–81. [Google Scholar] [CrossRef]
- Matamoros, A.J.; Tom, V.J.; Wu, D.; Rao, Y.; Sharp, D.J.; Baas, P.W. Knockdown of Fidgetin Improves Regeneration of Injured Axons by a Microtubule-Based Mechanism. J. Neurosci. 2019, 39, 2011–2024. [Google Scholar] [CrossRef]
- Baker, L.; Tar, M.; Kramer, A.H.; Villegas, G.A.; Charafeddine, R.A.; Vafaeva, O.; Nacharaju, P.; Friedman, J.; Davies, K.P.; Sharp, D.J. Fidgetin-like 2 Negatively Regulates Axonal Growth and Can Be Targeted to Promote Functional Nerve Regeneration. JCI Insight 2021, 6, e138484. [Google Scholar] [CrossRef]
- Smith, A.N.; Nagrabski, S.; Baker, L.; Kramer, A.H.; Sharp, D.J.; Byrnes, K.R. Fidgetin-like 2 Knockdown Increases Acute Neuroinflammation and Improves Recovery in a Rat Model of Spinal Cord Injury. J. Neuroinflamm. 2025, 22, 73. [Google Scholar] [CrossRef]
- Shupp, A.; Casimiro, M.C.; Pestell, R.G. Biological Functions of CDK5 and Potential CDK5 Targeted Clinical Treatments. Oncotarget 2017, 8, 17373–17382. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.; Li, C.; Chen, J.; Tan, J.; Zeng, L. The Role of Cdk5 in Neurological Disorders. Front. Cell Neurosci. 2022, 16, 951202. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Zhou, J.; Dedhar, S.; Wu, Y.H.; Snider, W.D. NGF-Induced Axon Growth is Mediated by Localized Inactivation of GSK-3β and Functions of the Microtubule plus End Binding Protein APC. Neuron 2004, 42, 897–912. [Google Scholar] [CrossRef]
- Ahmed, Z.; Morgan-Warren, P.J.; Berry, M.; Scott, R.A.H.; Logan, A. Effects of SiRNA-Mediated Knockdown of GSK3β on Retinal Ganglion Cell Survival and Neurite/Axon Growth. Cells 2019, 8, 956. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Xie, Y.; Ordaz, J.D.; Huh, A.J.; Huang, N.; Wu, W.; Liu, N.; Chamberlain, K.A.; Sheng, Z.H.; Xu, X.M. Restoring Cellular Energetics Promotes Axonal Regeneration and Functional Recovery after Spinal Cord Injury. Cell Metab. 2020, 31, 623–641.e8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.T.; Huang, N.; Sheng, Z.H. Programming Axonal Mitochondrial Maintenance and Bioenergetics in Neurodegeneration and Regeneration. Neuron 2022, 110, 1899–1923. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Lu, S.; Shen, Y. Roles of Syntaphilin and Armadillo Repeat-Containing X-Linked Protein 1 in Brain Injury after Experimental Intracerebral Hemorrhage. Neurosci. Lett. 2023, 809, 137300. [Google Scholar] [CrossRef]
- Jin, L.-Y.; Li, J.; Wang, K.-F.; Xia, W.-W.; Zhu, Z.-Q.; Wang, C.-R.; Li, X.-F.; Liu, H.-Y. Blood-Spinal Cord Barrier in Spinal Cord Injury: A Review. J. Neurotrauma 2021, 38, 1203–1224. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Lee, J.Y.; Seo, K.J.; Kim, I.Y.; Ju, B.G.; Yune, T.Y. TRPM7 Mediates BSCB Disruption After Spinal Cord Injury by Regulating the MTOR/JMJD3 Axis in Rats. Mol. Neurobiol. 2024, 61, 662–677. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, J.Y.; Choi, H.Y.; Yune, T.Y. Suppression of Transient Receptor Potential Melastatin 7 by Carvacrol Protects against Injured Spinal Cord by Inhibiting Blood-Spinal Cord Barrier Disruption. J. Neurotrauma 2022, 39, 735–749. [Google Scholar] [CrossRef]
- Xiao, X.; Li, W.; Rong, D.; Xu, Z.; Zhang, Z.; Ye, H.; Xie, L.; Wu, Y.; Zhang, Y.; Wang, X. Human Umbilical Cord Mesenchymal Stem Cells-Derived Extracellular Vesicles Facilitate the Repair of Spinal Cord Injury via the MiR-29b-3p/PTEN/Akt/MTOR Axis. Cell Death Discov. 2021, 7, 212. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Zhao, L.; Li, H.; Wang, S.; Shen, Y. Bone Marrow Mesenchymal Stem Cells with Nogo-66 Receptor Gene Silencing for Repair of Spinal Cord Injury. Neural Regen. Res. 2014, 9, 806–814. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Z.; Zhu, X.; Renn, C.L.; Dorsey, S.G.; Faden, A.I. Cell Cycle Inhibition Limits Development and Maintenance of Neuropathic Pain Following Spinal Cord Injury. Pain 2016, 157, 488. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, Q.J.; Sun, J.C.; Xu, X.M.; Yang, Y.; Liu, N.; Shi, J.G. Protective Effect of Epigenetic Silencing of CyclinD1 against Spinal Cord Injury Using Bone Marrow-Derived Mesenchymal Stem Cells in Rats. J. Cell Physiol. 2018, 233, 5361–5369. [Google Scholar] [CrossRef]
- Baklaushev, V.P.; Durov, O.V.; Kalsin, V.A.; Gulaev, E.V.; Kim, S.V.; Gubskiy, I.L.; Revkova, V.A.; Samoilova, E.M.; Melnikov, P.A.; Karal-Ogly, D.D.; et al. Disease Modifying Treatment of Spinal Cord Injury with Directly Reprogrammed Neural Precursor Cells in Non-Human Primates. World J. Stem Cells 2021, 13, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Xiao, X.; Zhao, H.; Yang, W.; Gao, X.; Yang, K.; Zheng, K.; Ji, Y.; Xu, D.; Fu, R.; et al. The Key to Spinal Cord Recovery: Harnessing P21 Inhibition to Boost Neural Stem/Progenitor Cell Proliferation. ACS Nano 2025, 19, 27406–27423. [Google Scholar] [CrossRef]
- Zhu, S.; Diao, S.; Liu, X.; Zhang, Z.; Liu, F.; Chen, W.; Lu, X.; Luo, H.; Cheng, X.; Liao, Q.; et al. Biomaterial-Based Strategies: A New Era in Spinal Cord Injury Treatment. Neural Regen. Res. 2025, 20, 3476–3500. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, K.; Wu, S.; Wu, J.; Zhang, J.; Li, J.; Lei, S.; Duan, X.; Men, K. Injectable and Photocurable Gene Scaffold Facilitates Efficient Repair of Spinal Cord Injury. ACS Appl. Mater. Interfaces 2024, 16, 4375–4394. [Google Scholar] [CrossRef]
- Dong, X.; Lu, Y.; Hu, Q.; Zeng, C.; Zheng, J.; Huang, J.; Dong, H.; Zou, P.; Wang, T.; Wu, Y.; et al. Engineered Exosome-Loaded Silk Fibroin Composite Hydrogels Promote Tissue Repair in Spinal Cord Injury Via Immune Checkpoint Blockade. Small 2025, 21, 2412170. [Google Scholar] [CrossRef] [PubMed]
- Cheriyan, T.; Ryan, D.J.; Weinreb, J.H.; Cheriyan, J.; Paul, J.C.; Lafage, V.; Kirsch, T.; Errico, T.J. Spinal Cord Injury Models: A Review. Spinal Cord 2014, 52, 588–595. [Google Scholar] [CrossRef]
- Sobolev, V.E.; Sysoev, Y.I.; Vyunova, T.V.; Musienko, P.E. Animal Models of Spinal Cord Injury. Biomedicines 2025, 13, 1427. [Google Scholar] [CrossRef]
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA Interference-Based Therapy and Its Delivery Systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Kashaw, S.; Iyer, A. SiRNA Delivery Strategies: A Comprehensive Review of Recent Developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef]
- Tang, Q.; Khvorova, A. RNAi-Based Drug Design: Considerations and Future Directions. Nat. Rev. Drug Discov. 2024, 23, 341–364. [Google Scholar] [CrossRef]
- Kurreck, J. RNA Interference: Perspectives and Caveats. J. RNAi Gene Silenc. 2005, 1, 50–51. [Google Scholar]
- Meng, Z.; Lu, M. RNA Interference-Induced Innate Immunity, Off-Target Effect, or Immune Adjuvant? Front. Immunol. 2017, 8, 331. [Google Scholar] [CrossRef]
- Xiao, H.; Amarsaikhan, O.; Zhao, Y.; Yu, X.; Hu, X.; Han, S.; Chaolumen Baigude, H. Astrocyte-Targeted SiRNA Delivery by Adenosine-Functionalized LNP in Mouse TBI Model. Mol. Ther. Nucleic Acids 2023, 34, 102065. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, X.; Tian, X.; Mo, B.; Xu, H.; Zhang, L.; Huang, L.; Yao, S.; Huang, Z.; Wang, Y.; et al. Adipose Mesenchymal Stem Cell Transplantation Alleviates Spinal Cord Injury-Induced Neuroinflammation Partly by Suppressing the Jagged1/Notch Pathway. Stem Cell Res. Ther. 2020, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.E.; Lim, C.K.W.; Krishnan, R.; McCallister, T.X.; Saporito-Magriña, C.; Zeballos, M.A.; McPheron, G.D.; Gaj, T. Targeted Gene Silencing in the Nervous System with CRISPR-Cas13. Sci. Adv. 2022, 8, eabk2485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, D.; Zhang, Y.; Li, J.; Ma, S.; Zhang, J.; Xiong, Y.; Wang, W.; Li, N.; Xia, L. Knockdown of LncRNA BDNF-AS Suppresses Neuronal Cell Apoptosis via Downregulating MiR-130b-5p Target Gene PRDM5 in Acute Spinal Cord Injury. RNA Biol. 2018, 15, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Zeng, L.-N.; Yang, W.-Y.; Ding, L.; Chen, K.-Z.; Fu, W.-J.; Zeng, S.-Q.; Liang, Y.-R.; Chen, G.-H.; Wu, H.-F. Inhibition of LncRNA Vof-16 Expression Promotes Nerve Regeneration and Functional Recovery after Spinal Cord Injury. Neural Regen. Res. 2022, 17, 217–227. [Google Scholar] [CrossRef]
- Cui, S.-Y.; Zhang, W.; Cui, Z.-M.; Yi, H.; Xu, D.-W.; Liu, W.; Zhu, X.-H. Knockdown of Long Non-Coding RNA LEF1-AS1 Attenuates Apoptosis and Inflammatory Injury of Microglia Cells Following Spinal Cord Injury. J. Orthop. Surg. Res. 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huang, Z.; Liu, Y. LncRNA ZFAS1 Combined with SRSF1 Regulate CNPY2 Expression and Leads to Microglia Endoplasmic Reticulum Stress–Induced Spinal Cord Injury. Mol. Neurobiol. 2025, 62, 12924–12937. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudakova, D.; Kovalev, V.; Shkap, M.; Sigal, E.; Biktimirov, A.; Soboleva, A.; Baklaushev, V. RNA Interference and Its Key Targets for Spinal Cord Injury Therapy: What Is Known So Far? Int. J. Mol. Sci. 2025, 26, 9861. https://doi.org/10.3390/ijms26209861
Chudakova D, Kovalev V, Shkap M, Sigal E, Biktimirov A, Soboleva A, Baklaushev V. RNA Interference and Its Key Targets for Spinal Cord Injury Therapy: What Is Known So Far? International Journal of Molecular Sciences. 2025; 26(20):9861. https://doi.org/10.3390/ijms26209861
Chicago/Turabian StyleChudakova, Daria, Vladimir Kovalev, Matthew Shkap, Elizaveta Sigal, Arthur Biktimirov, Alesya Soboleva, and Vladimir Baklaushev. 2025. "RNA Interference and Its Key Targets for Spinal Cord Injury Therapy: What Is Known So Far?" International Journal of Molecular Sciences 26, no. 20: 9861. https://doi.org/10.3390/ijms26209861
APA StyleChudakova, D., Kovalev, V., Shkap, M., Sigal, E., Biktimirov, A., Soboleva, A., & Baklaushev, V. (2025). RNA Interference and Its Key Targets for Spinal Cord Injury Therapy: What Is Known So Far? International Journal of Molecular Sciences, 26(20), 9861. https://doi.org/10.3390/ijms26209861







