Molecular and Histological Characterization of a Novel Hydrogel-Based Strategy for Inducing Experimental Glaucoma in Mice
Abstract
1. Introduction
2. Results
2.1. IOP Outcomes
2.2. RGC Survival
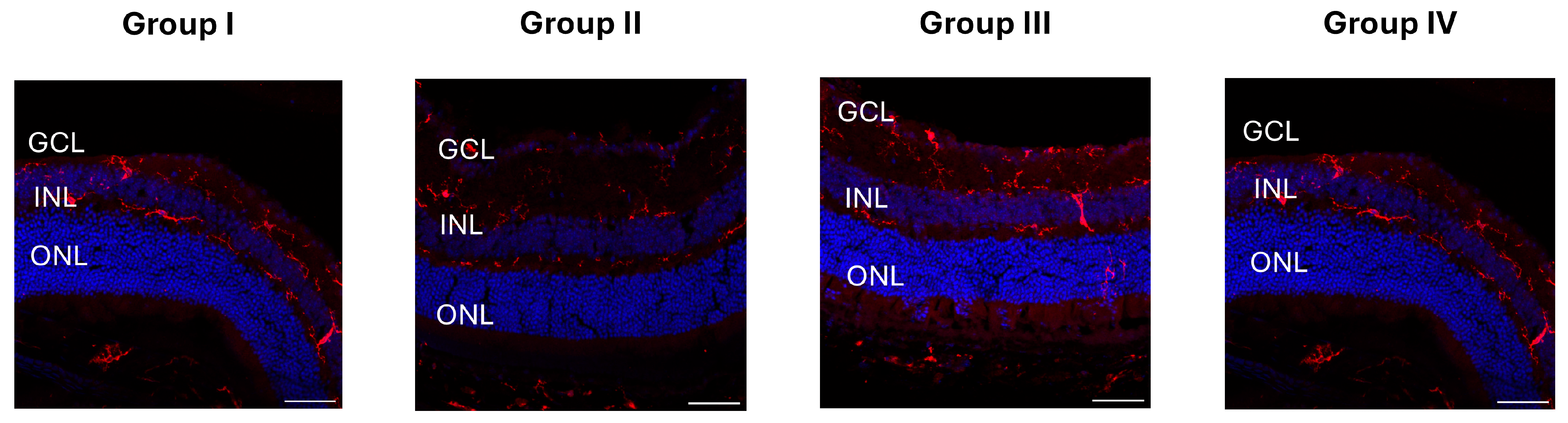
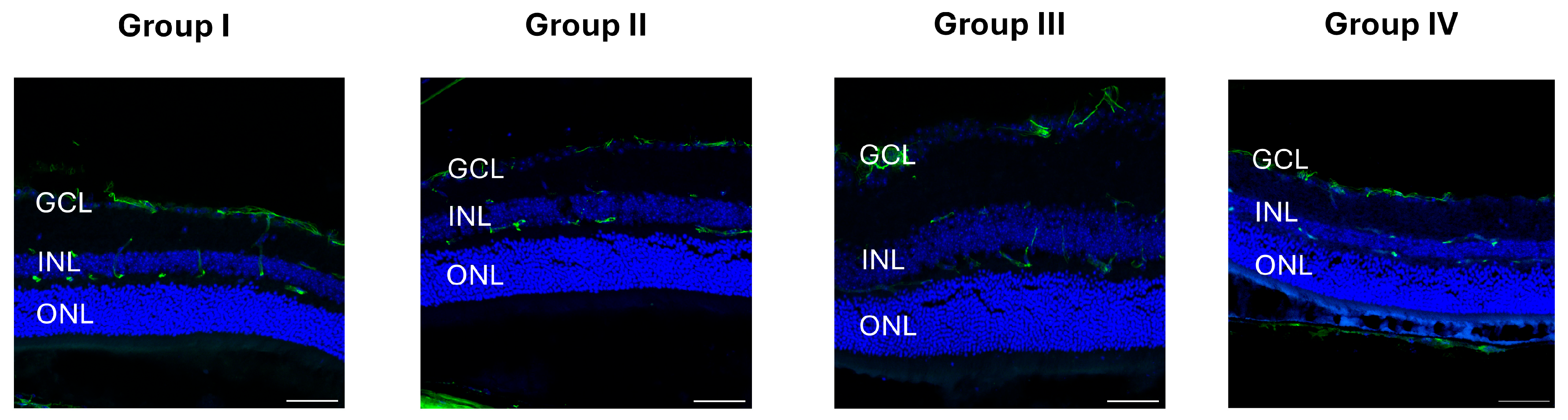
2.3. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Study Animals
4.2. Hydrogel-Induced Glaucoma Model
4.3. Measurement of IOP
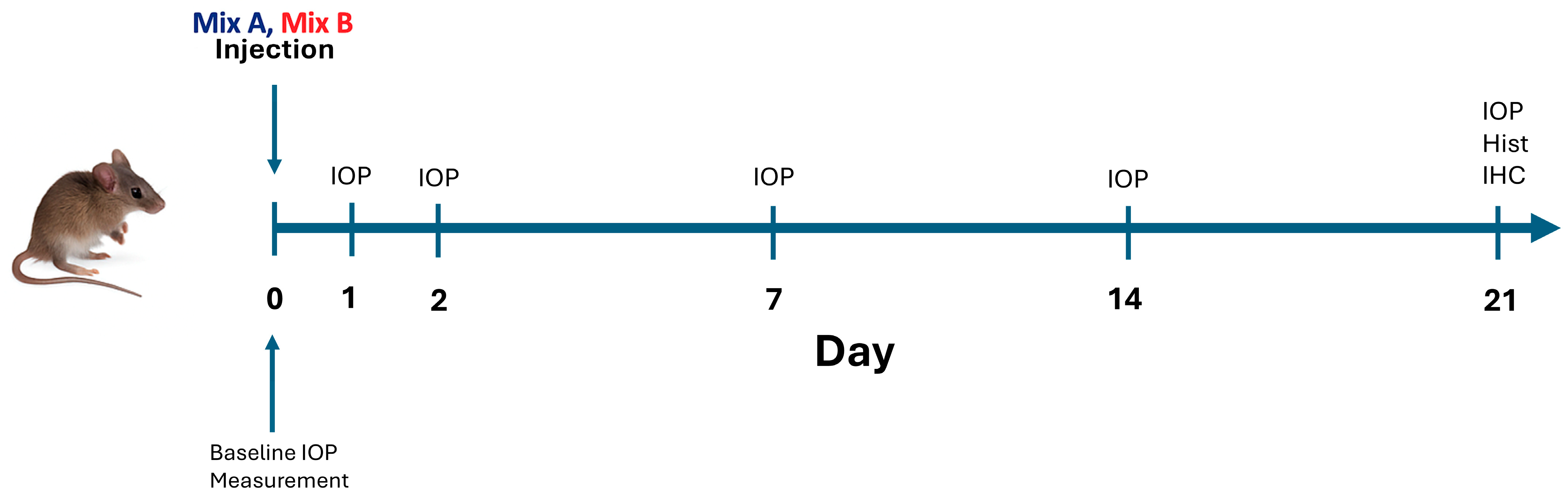
4.4. Experimental Groups and Study Design
4.5. Histological Analysis
4.6. Hematoxylin and Eosin
4.7. TUNEL Immunostaining
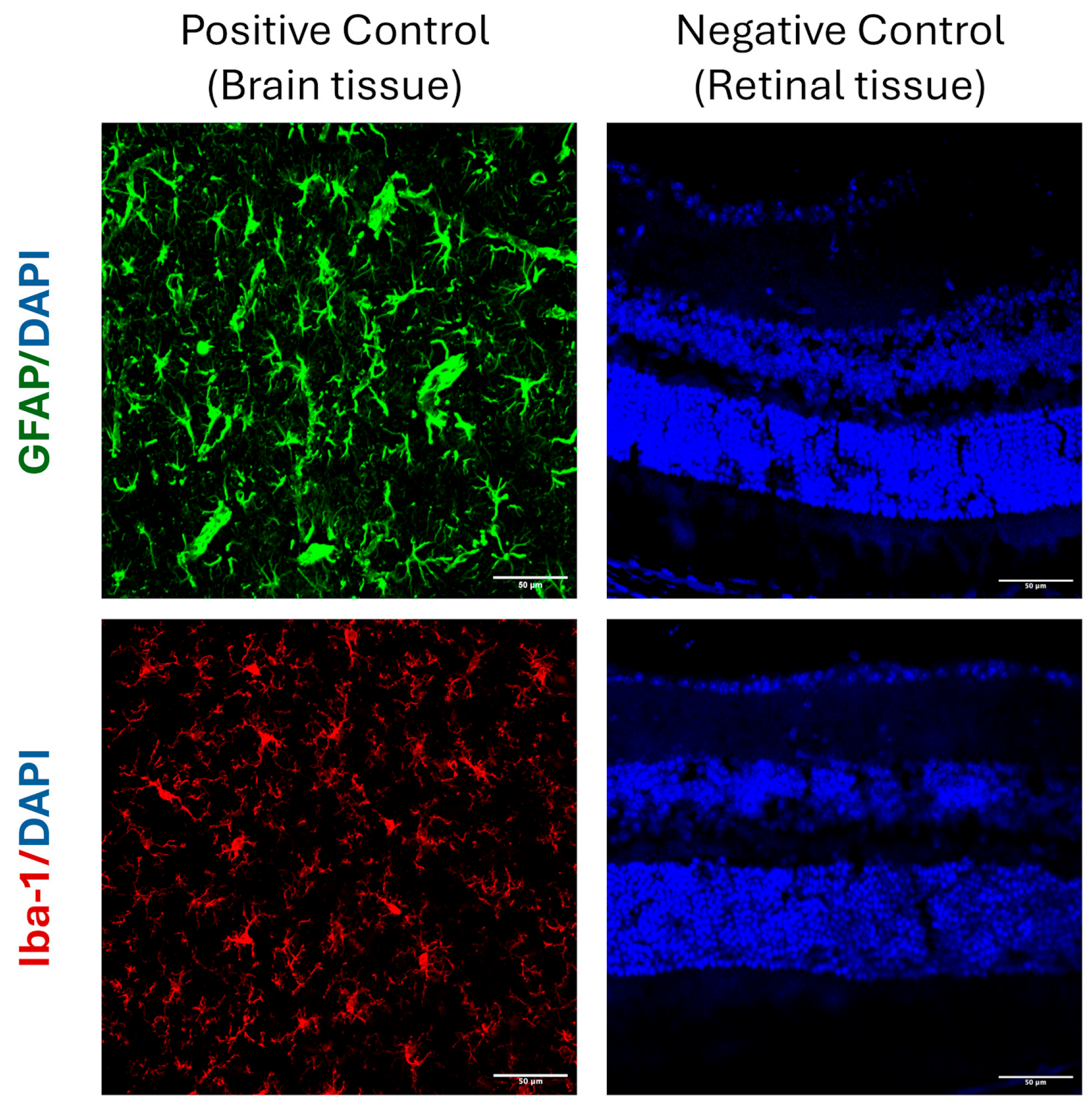
4.8. GFAP, NeuN, and Iba1 Immunostaining
4.9. RGC Counts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HA | Hyaluronic acid |
| FmocFF | Fluorenylmethoxycarbonyl-diphenylalanine |
| IOP | Intraocular pressure |
| RGC | Retinal ganglion cell |
| GFAP | Glial fibrillary acidic protein |
References
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.; Taylor, H.R.; Jonas, J.B.; Abdoli, A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.; et al. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet Glob. Health 2020, 9, e144, Correction in Lancet Glob. Health 2021, 2, 144–160. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Jonas, J.B.; Friedman, D.; Nangia, V.; Bron, A.; Tapply, I.; Fernandes, A.G.; Cicinelli, M.V.; Arrigo, A.; Leveziel, N.; et al. Global Estimates on the Number of People Blind or Visually Impaired by Glaucoma: A Meta-Analysis from 2000 to 2020. Eye 2024, 38, 2036–2046. [Google Scholar] [CrossRef]
- Qi, T.; Liu, H.; Frühn, L.; Löw, K.; Cursiefen, C.; Prokosch, V. Understanding Glaucoma: Why It Remains a Leading Cause of Blindness Worldwide. Klin. Monbl. Augenheilkd. 2025, 242, 712. [Google Scholar] [CrossRef]
- Banik, S.; Ghosh, A.; Debi, H. The Prevalence Trend of Glaucoma by Age and Sex Difference in South Asia: A Systematic Review and Meta-Analysis of Population-Based Studies. Health Sci. Rep. 2025, 8, e70542. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; Matamoros, J.A.; Salobrar-García, E.; Elvira-Hurtado, L.; López-Cuenca, I.; Sánchez-Puebla, L.; Salazar, J.J.; Ramírez, J.M. Glaucoma: From Pathogenic Mechanisms to Retinal Glial Cell Response to Damage. Front. Cell. Neurosci. 2024, 18, 1354569. [Google Scholar] [CrossRef]
- Xue, J.; Zhu, Y.; Liu, Z.; Lin, J.; Li, Y.; Li, Y.; Zhuo, Y. Demyelination of the Optic Nerve: An Underlying Factor in Glaucoma? Front. Aging Neurosci. 2021, 13, 701322. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, A.; Vitiello, L.; Lixi, F.; Abbinante, G.; Coppola, A.; Gagliardi, V.; Pellegrino, A.; Giannaccare, G. Optic Nerve Neuroprotection in Glaucoma: A Narrative Review. J. Clin. Med. 2024, 13, 2214. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Park, D.Y.; Kee, C.; Han, J.C. Natural Course and Risk Factors of Glaucoma Development in the Untreated Fellow Eye in Unilateral Normal-Tension Glaucoma. Investig. Ophthalmol. Vis. Sci. 2025, 66, 16. [Google Scholar] [CrossRef] [PubMed]
- Naik, V.; Ohri, S.; Fernandez, E.; Mwanza, J.C.; Fleischman, D. Changes in Individuals’ Glaucoma Progression Velocity after IOP-Lowering Therapy: A Systematic Review. PLoS ONE 2025, 20, e0324806. [Google Scholar] [CrossRef]
- Hu, R.; Wu, K.; Shi, J.; Yu, J.; Yao, X. lei Glaucoma Animal Models in Rabbits: State of the Art and Perspectives—A Review. Anim. Models Exp. Med. 2025, 8, 429–440. [Google Scholar] [CrossRef]
- Agarwal, R.; Agarwal, P.; Iezhitsa, I. Exploring the Current Use of Animal Models in Glaucoma Drug Discovery: Where Are We in 2023? Expert Opin. Drug Discov. 2023, 18, 1287–1300. [Google Scholar] [CrossRef]
- Tsai, T.; Reinehr, S.; Deppe, L.; Strubbe, A.; Kluge, N.; Dick, H.B.; Joachim, S.C. Glaucoma Animal Models beyond Chronic IOP Increase. Int. J. Mol. Sci. 2024, 25, 906. [Google Scholar] [CrossRef]
- Pang, I.H.; Clark, A.F. Inducible Rodent Models of Glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.M.; Salobrar-García, E.; de Hoz, R.; Salazar, J.J.; Matamoros, J.A.; Sánchez-Puebla, L.; López-Cuenca, I.; Fernández-Albarral, J.A.; Ramírez, A.I. Laser-Induced Ocular Hypertension in a Mouse Model of Glaucoma. Methods Mol. Biol. 2023, 2708, 49–56. [Google Scholar] [CrossRef]
- Gossman, C.A.; Linn, D.M.; Linn, C. Glaucoma-inducing Procedure in an In Vivo Rat Model and Whole-mount Retina Preparation. J. Vis. Exp. 2016, 12, 53831. [Google Scholar] [CrossRef]
- Arabpour, Z.; Salehi, M.; An, S.; Moghtader, A.; Anwar, K.N.; Baharnoori, S.M.; Shah, R.J.; Abedi, F.; Djalilian, A.R. Exploring Hydrogel Nanoparticle Systems for Enhanced Ocular Drug Delivery. Gels 2024, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Hameed, H.; Faheem, S.; Paiva-Santos, A.C.; Sarwar, H.S.; Jamshaid, M. A Comprehensive Review of Hydrogel-Based Drug Delivery Systems: Classification, Properties, Recent Trends, and Applications. AAPS PharmSciTech 2024, 25, 64. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yi, P.; Liu, Z.; Zhang, W.; Mei, L.; Feng, C.; Tu, C.; Li, Z. Stem Cell-Laden Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 865770. [Google Scholar] [CrossRef]
- Hsiung, N.; Ju, Y.; Yang, K.; Yang, P.; Zeng, W.; Zhao, H.; Zou, P.; Ye, J.; Yi, K.; Wang, X. Organoid-Based Tissue Engineering for Advanced Tissue Repair and Reconstruction. Mater. Today Bio 2025, 33, 102093. [Google Scholar] [CrossRef]
- Fang, W.; Yang, M.; Wang, L.; Li, W.; Liu, M.; Jin, Y.; Wang, Y.; Yang, R.; Wang, Y.; Zhang, K.; et al. Hydrogels for 3D Bioprinting in Tissue Engineering and Regenerative Medicine: Current Progress and Challenges. Int. J. Bioprint 2023, 9, 759. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Dong, L.; Cheng, Y.; Huang, X.; Xue, B.; Jiang, C.; Cao, Y.; Yang, J. Hydrogel-Based Strategies for Liver Tissue Engineering. Chem. Bio Eng. 2024, 1, 887–915. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- City, C.M.; Nam, V.; Duc Huynh, P. Hydrogel Dressings: Revolutionizing Burn Care with Innovative Wound Healing Technology. Biomed. Res. Ther. 2025, 12, 7207–7223. [Google Scholar] [CrossRef]
- Sojdeh, S.; Panjipour, A.; Bejandi, Z.B.; Salehi, M.; Yaghmour, A.; Arabpour, Z.; Djalilian, A.R.; Chan, R.V.P. Hydrogel-Based Vitreous Substitutes. Int. J. Mol. Sci. 2025, 26, 8406. [Google Scholar] [CrossRef]
- Fang, G.; Yang, X.; Wang, Q.; Zhang, A.; Tang, B. Hydrogels-Based Ophthalmic Drug Delivery Systems for Treatment of Ocular Diseases. Mater. Sci. Eng. C 2021, 127, 112212. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Tang, Y.; Ning, Z.; Zhou, Y.; Wu, H. Desired Properties of Polymeric Hydrogel Vitreous Substitute. Biomed. Pharmacother. 2024, 172, 116154. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef] [PubMed]
- Cie’slik, C.; Marczak, W.; Segneanu, C.; Bejenaru, A.-E.; Bejenaru, L.E.; Blendea, C.; Mogo¸sanu, A.; Biţăbiţă, G.D.; Boia, A.; Segneanu, A.-E.; et al. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef]
- Kudryavtseva, V.; Otero, M.; Zhang, J.; Bukatin, A.; Gould, D.; Sukhorukov, G.B. Drug-Eluting Sandwich Hydrogel Lenses Based on Microchamber Film Drug Encapsulation. ACS Nanosci. Au 2023, 3, 256–265. [Google Scholar] [CrossRef]
- Chan, K.C.; Yu, Y.; Ng, S.H.; Mak, H.K.; Yip, Y.W.Y.; van der Merwe, Y.; Ren, T.; Yung, J.S.Y.; Biswas, S.; Cao, X.; et al. Intracameral Injection of a Chemically Cross-Linked Hydrogel to Study Chronic Neurodegeneration in Glaucoma. Acta Biomater. 2019, 94, 219–231. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, Y.; Lv, K.; Wu, W.; Chen, C. Nanomedicine-Based Ophthalmic Drug Delivery Systems for the Treatment of Ocular Diseases. Int. J. Nanomed. 2025, 20, 9221–9249. [Google Scholar] [CrossRef]
- Lee, K.M.; Song, D.Y.; Kim, S.H. Effect of Strain on Rodent Glaucoma Models: Magnetic Bead Injection Versus Hydrogel Injection Versus Circumlimbal Suture. Transl. Vis. Sci. Technol. 2022, 11, 31. [Google Scholar] [CrossRef]
- Kim, M.; Jung, M.Y.; Lee, D.Y.; Ahn, S.M.; Lee, G.M.; Park, C.Y. How to Fabricate Hyaluronic Acid for Ocular Drug Delivery. Pharmaceutics 2024, 16, 1604. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Q.; Yin, Y.; Liang, G. Phe-Phe-Based Macroscopic Supramolecular Hydrogel Construction Strategies and Biomedical Applications. Chem. Bio Eng. 2024, 1, 664–677. [Google Scholar] [CrossRef]
- Tao, K.; Levin, A.; Adler-Abramovich, L.; Gazit, E. Fmoc-Modified Amino Acids and Short Peptides: Simple Bio-Inspired Building Blocks for the Fabrication of Functional Materials. Chem. Soc. Rev. 2016, 45, 3935–3953. [Google Scholar] [CrossRef]
- Arakawa, H.; Takeda, K.; Higashi, S.L.; Shibata, A.; Kitamura, Y.; Ikeda, M. Self-Assembly and Hydrogel Formation Ability of Fmoc-Dipeptides Comprising α-Methyl-L-Phenylalanine. Polym. J. 2020, 52, 923–930. [Google Scholar] [CrossRef]
- Aviv, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Buzhansky, L.; Mironi-Harpaz, I.; Seliktar, D.; Einav, S.; Nevo, Z.; Adler-Abramovich, L. Improving the Mechanical Rigidity of Hyaluronic Acid by Integration of a Supramolecular Peptide Matrix. ACS Appl. Mater. Interfaces 2018, 10, 41883–41891. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Rosa, E.; Morelli, G.; Accardo, A. Fmoc-Diphenylalanine Hydrogels: Optimization of Preparation Methods and Structural Insights. Pharmaceuticals 2022, 15, 1048. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, Q.; Zhang, Y.; Adler-Abramovich, L.; Fan, X.; Mei, D.; Gazit, E.; Tao, K. Fmoc-Diphenylalanine Gelating Nanoarchitectonics: A Simplistic Peptide Self-Assembly to Meet Complex Applications. J. Colloid. Interface Sci. 2023, 636, 113–133. [Google Scholar] [CrossRef]
- Halperin-Sternfeld, M.; Pokhojaev, A.; Ghosh, M.; Rachmiel, D.; Kannan, R.; Grinberg, I.; Asher, M.; Aviv, M.; Ma, P.X.; Binderman, I.; et al. Immunomodulatory Fibrous Hyaluronic Acid-Fmoc-Diphenylalanine-Based Hydrogel Induces Bone Regeneration. J. Clin. Periodontol. 2023, 50, 200–219. [Google Scholar] [CrossRef]
- Zahavi, A.; Friedman Gohas, M.; Sternfeld, A.; Daoud Zreiq, N.; Muhsinoglu, O.; Ofri, R.; BarKana, Y.; Goldenberg-Cohen, N. Histological and Molecular Characterization of Glaucoma Model Induced by One or Two Injections of Microbeads to the Anterior Chamber of Mice. Int. Ophthalmol. 2022, 42, 3763–3775. [Google Scholar] [CrossRef]
- Sela, T.C.; Zahavi, A.; Friedman-Gohas, M.; Weiss, S.; Sternfeld, A.; Ilguisonis, A.; Badash, D.; Geffen, N.; Ofri, R.; BarKana, Y.; et al. Azithromycin and Sildenafil May Have Protective Effects on Retinal Ganglion Cells via Different Pathways: Study in a Rodent Microbead Model. Pharmaceuticals 2023, 16, 486. [Google Scholar] [CrossRef]
- Wang, L.H.; Huang, C.H.; Lin, I.C. Advances in Neuroprotection in Glaucoma: Pharmacological Strategies and Emerging Technologies. Pharmaceuticals 2024, 17, 1261. [Google Scholar] [CrossRef]
- Cone, F.E.; Gelman, S.E.; Son, J.L.; Pease, M.E.; Quigley, H.A. Differential Susceptibility to Experimental Glaucoma among 3 Mouse Strains Using Bead and Viscoelastic Injection. Exp. Eye Res. 2010, 91, 415–424. [Google Scholar] [CrossRef]
- Cohen-Gerassi, D.; Messer, O.; Finkelstein-Zuta, G.; Aviv, M.; Favelukis, B.; Shacham-Diamand, Y.; Sokol, M.; Adler-Abramovich, L. Conductive Peptide-Based MXene Hydrogel as a Piezoresistive Sensor. Adv. Healthc. Mater. 2024, 13, 2303632. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xue, J.; Xu, Q.; Liu, Z.; Zhao, C.; Tang, J.; Han, J.; Sigen, A.; Wang, W.; Zhuo, Y.; et al. In Situ-Crosslinked Hydrogel-Induced Experimental Glaucoma Model with Persistent Ocular Hypertension and Neurodegeneration. Biomater. Sci. 2022, 10, 5006–5017. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhong, H.; Chen, J.; Sun, J.; Huang, P.; Xu, X.; Huang, S.; Zhong, Y. Efficacy, Drug Sensitivity, and Safety of a Chronic Ocular Hypertension Rat Model Established Using a Single Intracameral Injection of Hydrogel into the Anterior Chamber. Med. Sci. Monit. 2020, 26, e925852-1. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, Characterization, and Potential Application of Chitosan, Chitosan Derivatives, and Chitosan Metal Nanoparticles in Pharmaceutical Drug Delivery. Drug Des. Devel Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef]
- Ibeas Moreno, E.; Alonso, M.J.; Abbadessa, A. Intraocular Injectable Hydrogels for the Delivery of Cells and Nanoparticles. Mater. Today Bio 2025, 32, 101767. [Google Scholar] [CrossRef]
- Rohiwal, S.S.; Ellederová, Z.; Ardan, T.; Klima, J. Advancement in Nanostructure-Based Tissue-Engineered Biomaterials for Retinal Degenerative Diseases. Biomedicines 2021, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In Situ Forming Injectable Hydrogels for Drug Delivery and Wound Repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Kim, D.; Fang, R.; Zhang, P.; Yan, Z.; Sun, C.; Li, G.; Montgomery, C.; John, S.W.M.; Stamer, W.D.; Zhang, H.F.; et al. In Vivo Quantification of Anterior and Posterior Chamber Volumes in Mice: Implications for Aqueous Humor Dynamics. Investig. Ophthalmol. Vis. Sci. 2025, 66, 18. [Google Scholar] [CrossRef]
- Johnson, T.V.; Tomarev, S.I. Rodent Models of Glaucoma. Brain Res. Bull. 2010, 81, 349–358. [Google Scholar] [CrossRef]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal Models of Glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Calkins, D.J. Critical Pathogenic Events Underlying Progression of Neurodegeneration in Glaucoma. Prog. Retin. Eye Res. 2012, 31, 702–719. [Google Scholar] [CrossRef]
- Burgoyne, C.F. A Biomechanical Paradigm for Axonal Insult within the Optic Nerve Head in Aging and Glaucoma. Exp. Eye Res. 2011, 93, 120–132. [Google Scholar] [CrossRef]

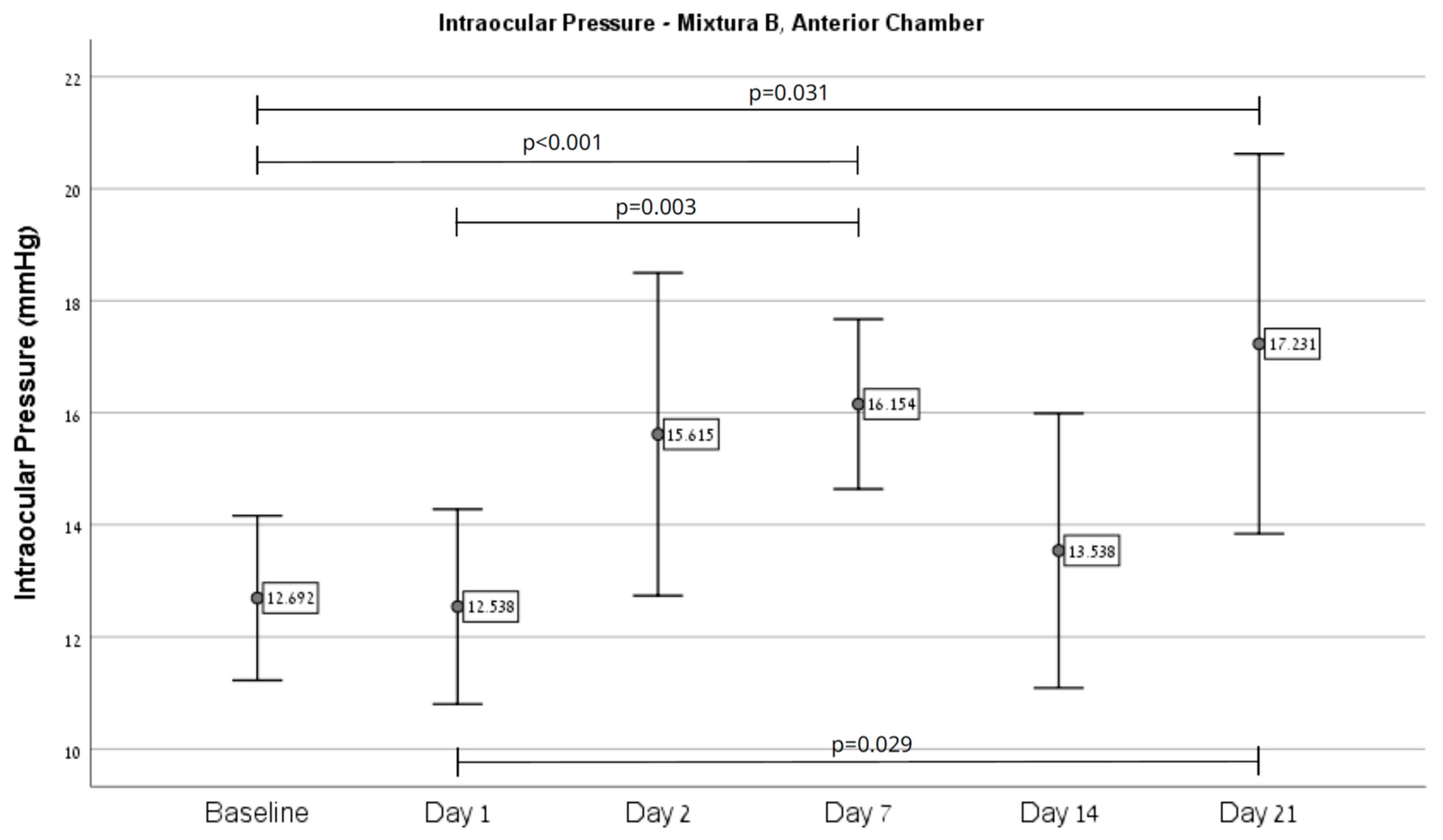


| IOP (mmHg) | Mixture A | Mixture B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior Chamber | Intravitreal | All Eyes | Anterior Chamber | Intravitreal | All Eyes | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Baseline | 10.96 | 1.94 | 9.25 | 0.50 | 10.70 | 1.90 | 12.69 | 2.43 | 8.50 | 0.53 | 11.10 | 2.83 |
| Day 1 | 8.09 | 0.95 | 13.00 | 10.68 | 8.81 | 4.13 | 12.54 | 2.88 | 9.25 | 1.04 | 11.29 | 2.83 |
| Day 2 | 11.57 | 3.80 | 11.50 | 5.07 | 11.56 | 3.90 | 15.62 | 4.77 | 9.00 | 0.76 | 13.10 | 4.97 |
| Day 7 | 12.35 | 2.77 | 21.00 | 16.75 | 13.63 | 6.98 | 16.15 | 2.51 | 11.50 | 1.41 | 14.38 | 3.14 |
| Day 14 | 11.83 | 3.47 | 11.25 | 5.25 | 11.74 | 3.66 | 13.54 | 4.05 | 9.25 | 0.71 | 11.90 | 3.82 |
| Day 21 | 11.61 | 3.60 | 11.75 | 4.19 | 11.63 | 3.61 | 17.23 | 5.61 | 9.50 | 0.53 | 14.29 | 5.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obied, B.; Richard, S.; Kramarz Dadon, J.; Sela, T.C.; Geffen, N.; Halperin-Sternfeld, M.; Adler-Abramovich, L.; Goldenberg-Cohen, N.; Zahavi, A. Molecular and Histological Characterization of a Novel Hydrogel-Based Strategy for Inducing Experimental Glaucoma in Mice. Int. J. Mol. Sci. 2025, 26, 9860. https://doi.org/10.3390/ijms26209860
Obied B, Richard S, Kramarz Dadon J, Sela TC, Geffen N, Halperin-Sternfeld M, Adler-Abramovich L, Goldenberg-Cohen N, Zahavi A. Molecular and Histological Characterization of a Novel Hydrogel-Based Strategy for Inducing Experimental Glaucoma in Mice. International Journal of Molecular Sciences. 2025; 26(20):9860. https://doi.org/10.3390/ijms26209860
Chicago/Turabian StyleObied, Basel, Stephen Richard, Judith Kramarz Dadon, Tal Corina Sela, Noa Geffen, Michal Halperin-Sternfeld, Lihi Adler-Abramovich, Nitza Goldenberg-Cohen, and Alon Zahavi. 2025. "Molecular and Histological Characterization of a Novel Hydrogel-Based Strategy for Inducing Experimental Glaucoma in Mice" International Journal of Molecular Sciences 26, no. 20: 9860. https://doi.org/10.3390/ijms26209860
APA StyleObied, B., Richard, S., Kramarz Dadon, J., Sela, T. C., Geffen, N., Halperin-Sternfeld, M., Adler-Abramovich, L., Goldenberg-Cohen, N., & Zahavi, A. (2025). Molecular and Histological Characterization of a Novel Hydrogel-Based Strategy for Inducing Experimental Glaucoma in Mice. International Journal of Molecular Sciences, 26(20), 9860. https://doi.org/10.3390/ijms26209860







