Redox System Dysfunction as a Key Mechanism in Autism Spectrum Disorder Pathogenesis
Abstract
1. Introduction
2. Reconsidering the Concept of Oxidative Stress: Some Possible Shortcomings
3. Rethinking the Starting Point in ASD Pathophysiology: From Oxidative Stress to Redox System
4. Reactive Oxidant Species Levels Are Increased in ASD
4.1. Increased Production of ROS by Direct Mechanisms in ASD
4.2. Increased Production of ROS by Indirect/Catalytic Mechanisms in ASD
4.2.1. Increased Production of ROS by Inorganic Catalysts
4.2.2. Increased Production of ROS by Enzyme’s Dysregulation
Nicotinamide Adenine Dinucleotide Phosphate Oxidases
Myeloperoxidase
Nitric Oxide Synthases
The Unique Potential Role of Gamma-Glutamyl Transferase/Transpeptidase in ASD
Mitochondrial Pro-Oxidant Enzymes
Other Pro-Oxidant Enzymes
5. Biological Target Accessibility/Vulnerability Is Impaired in ASD
5.1. The Polyunsaturated Fatty Acid Target
5.2. The Thiol Proteins’ Molecular Target
5.3. Other Molecular Targets
5.4. Redox-Sensitive Molecular Pathways’ Targets
6. Decreased Bioavailability/Activity of Reducing/Antioxidant Systems in ASD
6.1. The Neglected Role of Glucose-6-P-Dehydrogenase
6.2. Glutathione Pathway
6.3. Other Relevant Antioxidant Enzymes
7. The Controversial Role of Superoxide Dismutases in ASD
8. Impairment of Neurons/Glia Subcellular Compartments in ASD
8.1. Mitochondrial Dysfunction and Altered Microbiota–Gut–Brain–Mitochondria Axis
8.2. Redox-Mediated Cell Membrane Dysfunction in ASD
8.2.1. Abnormalities in Neurons: Neurotransmission, Plasticity and Synaptic Functions, Morphogenesis
8.2.2. Abnormalities in Microglia: Neuro-Oxi-Inflammation
8.3. Redox-Mediated Lysosome Dysfunction in ASD
8.4. Redox-Mediated Peroxisomal Dysfunction in ASD
8.5. Redox-Mediated Endoplasmic Reticulum Dysfunction in ASD
8.6. Redox-Mediated Cytoskeleton Dysfunction in ASD
8.7. Redox-Mediated Nuclear Dysfunction in ASD
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-AG | 2-Arachidonoylglycerol |
| 4-HNE | 4-Hydroxynonenal |
| ACOX | Very-long-chain acyl-CoA oxidase |
| AOX | Reducing/Antioxidant (species) |
| AP-1 | Activator Protein 1 |
| ASD | Autism Spectrum Disorder |
| BDNF | Brain-Derived Neurotrophic Factor |
| BTBR | BTBR T+ Itpr3tf/J mouse strain (ASD model) |
| CAT | Catalase |
| CB | Cannabinoid (receptors) |
| CD200–CD200R | Cluster of Differentiation 200-Cluster of Differentiation 200 Receptor |
| cGMP | Cyclic Guanosine Monophosphate |
| CNS | Central Nervous System |
| CO | Carbon monoxide |
| COX | Cycloxygenase |
| CpG | Cytosine-phosphate-Guanine |
| CX3CL1–CX3CR1 | Fractalkine—chemokine receptor 1 |
| CYP | Cytochrome P450 |
| DUOX | Dual Oxidase (NADPH oxidase isoform) |
| ER | Endoplasmic Reticulum |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GABA | Gamma-aminobutyric acid |
| GAD (GAD65/67) | Glutamate Decarboxylase (65/67) |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| γ-GT | γ-glutamyltransferase/transpeptidase |
| GPx | Glutathione Peroxidase |
| GSH | Reduced glutathione/glutathione monomer |
| GSSG | Oxidized glutathione/glutathione dimer |
| GSTM | Glutathione S-Transferase Mu class |
| GSTP | Glutathione S-Transferase Pi class |
| GSTT | Glutathione S-Transferase Theta class |
| HIF-1α | Hypoxia-Inducible Factor 1-Alpha |
| HO-1 | Heme Oxygenase 1 |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| LOX | Lipoxygenase |
| LPS | Lipopolysaccharide |
| LTD | long-term depression |
| LTP | long-term potentiation |
| MAO | Monoamine Oxidase |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA | malondialdehyde |
| MIA | Maternal Immune Activation |
| mitNOS | Mitochondrial Nitric Oxide Synthase (putative) |
| MPO | myeloperoxidase |
| mtDNA | mitochondrial DNA |
| MTHFR | Methylenetetrahydrofolate Reductase |
| mTOR | Mechanistic Target of Rapamycin |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate (reduced form) |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NMDA | N-Methyl-D-Aspartate |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| nNOS/eNOS/iNOS | Neuronal/Endothelial/Inducible Nitric Oxide Synthase |
| NOX | NOX NADPH oxidase isoform |
| NRF2 | Nuclear factor erythroid 2–related factor 2 (gene) |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 (gene product) |
| OGG1 | 8-oxoguanine DNA glycosylase-1 |
| ONOO− | Peroxynitrite |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| PI3K-Akt | Phosphoinositide 3-Kinase—Protein Kinase B (Akt) (pathway) |
| poly I:C | Polyinosinic:polycytidylic acid |
| PON1 | Paraoxonase 1 |
| PUFA | Polyunsaturated Fatty Acids |
| RNS | Reactive Nitrogen Species |
| ROS | herein Reactive Oxidant Species (not Reactive Oxygen Species) |
| SAH | S-Adenosylhomocysteine |
| SAM | S-Adenosylmethionine |
| SHANK3 | SH3 and Multiple Ankyrin Repeat Domains 3 (gene) |
| Shank3 | SH3 and Multiple Ankyrin Repeat Domains 3 (gene product) |
| SNARE | Soluble N-ethylmaleimide-Sensitive Factor (NSF) Attachment Protein Receptor |
| SNPs | Single Nucleotide Polymorphisms |
| SOD | Superoxide Dismutase |
| TAS2Rs | Taste Receptor Type 2 family (bitter taste receptors) |
| TLR | Toll-Like Receptors |
| TNF-α | Tumor Necrosis Factor alpha |
| UPR | Unfolded protein response |
| VNTR | Variable Number Tandem Repeat |
| XO | Xanthine Oxidase |
References
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism Spectrum Disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR); American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Christensen, D.L.; Baio, J.; Van Naarden Braun, K.; Bilder, D.; Charles, J.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill. Summ. 2016, 65, 1–23. [Google Scholar] [CrossRef]
- Loke, Y.J.; Hannan, A.J.; Craig, J.M. The Role of Epigenetic Change in Autism Spectrum Disorders. Front. Neurol. 2015, 6, 107. [Google Scholar] [CrossRef]
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An Overview of the Main Genetic, Epigenetic and Environmental Factors Involved in Autism Spectrum Disorder Focusing on Synaptic Activity. Int. J. Mol. Sci. 2020, 21, 8290. [Google Scholar] [CrossRef]
- Frye, R.E.; James, S.J. Metabolic Pathology of Autism in Relation to Redox Metabolism. Biomark. Med. 2014, 8, 321–330. [Google Scholar] [CrossRef]
- Kuźniar-Pałka, A. The Role of Oxidative Stress in Autism Spectrum Disorder Pathophysiology, Diagnosis and Treatment. Biomedicines 2025, 13, 388. [Google Scholar] [CrossRef]
- Volk, H.E.; Park, B.; Hollingue, C.; Jones, K.L.; Ashwood, P.; Windham, G.C.; Lurman, F.; Alexeeff, S.E.; Kharrazi, M.; Pearl, M.; et al. Maternal Immune Response and Air Pollution Exposure during Pregnancy: Insights from the Early Markers for Autism (EMA) Study. J. Neurodev. Disord. 2020, 12, 42. [Google Scholar] [CrossRef]
- Zoghbi, H.Y.; Bear, M.F. Synaptic Dysfunction in Neurodevelopmental Disorders Associated with Autism and Intellectual Disabilities. Cold Spring Harb. Perspect. Biol. 2012, 4, a009886. [Google Scholar] [CrossRef]
- Liao, X.; Liu, Y.; Fu, X.; Li, Y. Postmortem Studies of Neuroinflammation in Autism Spectrum Disorder: A Systematic Review. Mol. Neurobiol. 2020, 57, 3424–3438. [Google Scholar] [CrossRef]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Evidence Linking Oxidative Stress, Mitochondrial Dysfunction, and Inflammation in the Brain of Individuals with Autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef]
- Bjørklund, G.; Doşa, M.D.; Maes, M.; Dadar, M.; Frye, R.E.; Peana, M.; Chirumbolo, S. The Impact of Glutathione Metabolism in Autism Spectrum Disorder. Pharmacol. Res. 2021, 166, 105437. [Google Scholar] [CrossRef]
- Renaldi, R.; Persico, A.M.; Wiguna, T.; Tanra, A.J. Breaking the Cycle of Oxidative Stress for Better Behavioral Health in Autism Spectrum Disorder: A Scoping Review. Asian J. Psychiatry 2025, 110, 104575. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Mormone, E.; Iorio, E.L. Editorial: Regenerative Medicine in Neurodegenerative Diseases and Aging: Challenging the Redox Homeostasis. Front. Neurosci. 2023, 17, 1238781. [Google Scholar] [CrossRef]
- Travagli, V.; Iorio, E.L. The Biological and Molecular Action of Ozone and Its Derivatives: State-of-the-Art, Enhanced Scenarios, and Quality Insights. Int. J. Mol. Sci. 2023, 24, 8465. [Google Scholar] [CrossRef]
- Macedo Signorelli, N.S.; Rende, S.G.S.; Iorio, E.L.; Ferraz, D.C.; Paranhos, L.R.; Moura, C.C.G. Identification of Oxidative Stress Biomarkers in Apical Periodontitis: A Scoping Review with Bibliometric Analysis. Aust. Endod. J. 2024, 50, 742–760. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Oxidative Stress in Autism. Pathophysiology 2006, 13, 171–181. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 3293. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stresses and their classifications. Ukr. Biochem. J. 2015, 87, 11–18. [Google Scholar] [CrossRef]
- Forman, H.J. Redox Signaling: An Evolution from Free Radicals to Aging. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Eustress: On Constant Alert for Redox Homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; ISBN 9780198717485. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Bienertova-Vasku, J.; Lenart, P.; Scheringer, M. Eustress and Distress: Neither Good Nor Bad, but Rather the Same? BioEssays 2020, 42, e1900238. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; W H Freeman & Co.: New York, NY, USA, 2017. [Google Scholar]
- WEISS, J. One-Electron versus Two-Electron Transfer Processes in the Mechanism of Oxidation-Reduction Reactions in Solution. Nature 1958, 181, 825–826. [Google Scholar] [CrossRef]
- Liochev, S.I.; Fridovich, I. The Role of O2·− in the Production of HO·: In Vitro and in Vivo. Free Radic. Biol. Med. 1994, 16, 29–33. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Santilli, G.; Lamorte, G.; Carlessi, L.; Ferrari, D.; Rota Nodari, L.; Binda, E.; Delia, D.; Vescovi, A.L.; De Filippis, L. Mild Hypoxia Enhances Proliferation and Multipotency of Human Neural Stem Cells. PLoS ONE 2010, 5, e8575. [Google Scholar] [CrossRef]
- De Filippis, L.; Delia, D. Hypoxia in the Regulation of Neural Stem Cells. Cell. Mol. Life Sci. 2011, 68, 2831–2844. [Google Scholar] [CrossRef]
- Mormone, E.; Iorio, E.L.; Abate, L.; Rodolfo, C. Sirtuins and Redox Signaling Interplay in Neurogenesis, Neurodegenerative Diseases, and Neural Cell Reprogramming. Front. Neurosci. 2023, 17, 1073689. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible to Oxidative Stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective Neuronal Vulnerability to Oxidative Stress in the Brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and in Vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P.; Jaquet, V.; Marcucci, F.; Schmidt, H.H.H.W. The Oxidative Stress Theory of Disease: Levels of Evidence and Epistemological Aspects. Br. J. Pharmacol. 2017, 174, 1784–1796. [Google Scholar] [CrossRef] [PubMed]
- Whayne, T.F.; Saha, S.P.; Mukherjee, D. Antioxidants in the Practice of Medicine; What Should the Clinician Know? Cardiovasc. Hematol. Disord. Drug Targets 2016, 16, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Liang, Z.; Ma, G.; Qureshi, A.; Ran, X.; Feng, C.; Liu, X.; Yan, X.; Shen, L. Autism Spectrum Disorder: Pathogenesis, Biomarker, and Intervention Therapy. MedComm 2024, 5, e497. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical Basis and Metabolic Interplay of Redox Regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef]
- Buettner, G.R. The Pecking Order of Free Radicals and Antioxidants: Lipid Peroxidation, Alpha-Tocopherol, and Ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Kumagai, Y.; Ihara, H.; Fujii, S.; Motohashi, H.; Akaike, T. Redox Signaling Regulated by Electrophiles and Reactive Sulfur Species. J. Clin. Biochem. Nutr. 2016, 58, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Medoro, A.; Siracusano, M.; Savino, R.; Saso, L.; Scapagnini, G.; Mazzone, L. Oxidative Stress Response and NRF2 Signaling Pathway in Autism Spectrum Disorder. Redox Biol. 2025, 83, 103661. [Google Scholar] [CrossRef]
- Cadenas, E.; Packer, L.; Traber, M.G. Antioxidants, Oxidants, and Redox Impacts on Cell Function—A Tribute to Helmut Sies. Arch. Biochem. Biophys. 2016, 595, 94–99. [Google Scholar] [CrossRef]
- Sevanian, A.; Muakkassah-Kelly, S.F.; Montestruque, S. The Influence of Phospholipase A2 and Glutathione Peroxidase on the Elimination of Membrane Lipid Peroxides. Arch. Biochem. Biophys. 1983, 223, 441–452. [Google Scholar] [CrossRef]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X.; Cao, X.; Shen, L. Oxidative Stress in Autism Spectrum Disorder-Current Progress of Mechanisms and Biomarkers. Front. Psychiatry 2022, 13, 813304. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Fang, J.; Sheng, R.; Qin, Z.-H. NADPH Oxidases in the Central Nervous System: Regional and Cellular Localization and the Possible Link to Brain Diseases. Antioxid. Redox Signal. 2021, 35, 951–973. [Google Scholar] [CrossRef]
- Zhang, X.; Ibi, M.; Haga, R.; Iwata, K.; Matsumoto, M.; Asaoka, N.; Liu, J.; Katsuyama, M.; Yabe-Nishimura, C. NOX1/NADPH Oxidase Affects the Development of Autism-like Behaviors in a Maternal Immune Activation Model. Biochem. Biophys. Res. Commun. 2021, 534, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Solanki, K.; Rajpoot, S.; Bezsonov, E.E.; Orekhov, A.N.; Saluja, R.; Wary, A.; Axen, C.; Wary, K.; Baig, M.S. The Expanding Roles of Neuronal Nitric Oxide Synthase (NOS1). PeerJ 2022, 10, e13651. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.N.; Raghanti, M.A. The Role of Monoamine Oxidase Enzymes in the Pathophysiology of Neurological Disorders. J. Chem. Neuroanat. 2021, 114, 101957. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Chauhan, V.; Chauhan, A. Monoamine Oxidase-A and B Activities in the Cerebellum and Frontal Cortex of Children and Young Adults with Autism. J. Neurosci. Res. 2017, 95, 1965–1972. [Google Scholar] [CrossRef]
- Yui, K.; Imataka, G.; Yoshihara, S. Lipid-Based Molecules on Signaling Pathways in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 9803. [Google Scholar] [CrossRef]
- El-Ansary, A.; Al-Ayadhi, L. Lipid Mediators in Plasma of Autism Spectrum Disorders. Lipids Health Dis. 2012, 11, 160. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Chen, A.; Chen, J.; Zhu, T.; Wang, S.; Qiao, W.; Zhou, D.; Zhang, X.; Chen, S.; et al. Involvement of Disulfidptosis in the Pathophysiology of Autism Spectrum Disorder. Life Sci. 2025, 369, 123531. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, J.; Luo, Z.; Li, Y.; Huang, Y. Emerging Mechanisms of Lipid Peroxidation in Regulated Cell Death and Its Physiological Implications. Cell Death Dis. 2024, 15, 859. [Google Scholar] [CrossRef]
- Ayoub, G. Neurodevelopment of Autism: Critical Periods, Stress and Nutrition. Cells 2024, 13, 1968. [Google Scholar] [CrossRef]
- Buss, C. Maternal Oxidative Stress during Pregnancy and Offspring Neurodevelopment. Brain Behav. Immun. 2021, 93, 6–7. [Google Scholar] [CrossRef]
- Carpita, B.; Muti, D.; Dell’Osso, L. Oxidative Stress, Maternal Diabetes, and Autism Spectrum Disorders. Oxidative Med. Cell. Longev. 2018, 2018, 3717215. [Google Scholar] [CrossRef] [PubMed]
- Marzola, P.; Melzer, T.; Pavesi, E.; Gil-Mohapel, J.; Brocardo, P.S. Exploring the Role of Neuroplasticity in Development, Aging, and Neurodegeneration. Brain Sci. 2023, 13, 1610. [Google Scholar] [CrossRef]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental Risk Factors for Autism: An Evidence-Based Review of Systematic Reviews and Meta-Analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Panisi, C.; Guerini, F.R.; Abruzzo, P.M.; Balzola, F.; Biava, P.M.; Bolotta, A.; Brunero, M.; Burgio, E.; Chiara, A.; Clerici, M.; et al. Autism Spectrum Disorder from the Womb to Adulthood: Suggestions for a Paradigm Shift. J. Pers. Med. 2021, 11, 70. [Google Scholar] [CrossRef]
- Singh, S.; Goel, I.; Tripathi, S.; Ahirwar, A.; Kumar, M.; Rana, A.; Dhar, R.; Karmakar, S. Effect of Environmental Air Pollutants on Placental Function and Pregnancy Outcomes: A Molecular Insight. Environ. Sci. Pollut. Res. Int. 2024, 31, 59819–59851. [Google Scholar] [CrossRef]
- Toscano, C.V.A.; Barros, L.; Lima, A.B.; Nunes, T.; Carvalho, H.M.; Gaspar, J.M. Neuroinflammation in Autism Spectrum Disorders: Exercise as a “Pharmacological” Tool. Neurosci. Biobehav. Rev. 2021, 129, 63–74. [Google Scholar] [CrossRef]
- She, K.; Yuan, N.; Huang, M.; Zhu, W.; Tang, M.; Ma, Q.; Chen, J. Emerging Role of Microglia in the Developing Dopaminergic System: Perturbation by Early Life Stress. Neural Regen. Res. 2024, 21, 126–140. [Google Scholar] [CrossRef]
- Meguid, N.A.; Dardir, A.A.; Abdel-Raouf, E.R.; Hashish, A. Evaluation of Oxidative Stress in Autism: Defective Antioxidant Enzymes and Increased Lipid Peroxidation. Biol. Trace Elem. Res. 2011, 143, 58–65. [Google Scholar] [CrossRef]
- Frye, R.E.; Rose, S.; Voinsky, I.; Gurwitz, D. Nitrosative Stress in Autism: Supportive Evidence and Implications for Mitochondrial Dysfunction. Adv. Sci. 2024, 11, e2304439. [Google Scholar] [CrossRef]
- Burke, S.L.; Cobb, J.; Agarwal, R.; Maddux, M.; Cooke, M.S. How Robust Is the Evidence for a Role of Oxidative Stress in Autism Spectrum Disorders and Intellectual Disabilities? J. Autism Dev. Disord. 2021, 51, 1428–1445. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox Regulation: Mechanisms, Biology and Therapeutic Targets in Diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Pal, A.; Goel, F.; Sharma, A.; Garg, V.K. Oxidative Stress and Antioxidant Therapeutics in Autism Spectrum Disorder: A Biochemical and Structure-Activity Relationship Perspective. Mol. Divers. 2025. [Google Scholar] [CrossRef]
- Yui, K.; Kawasaki, Y.; Yamada, H.; Ogawa, S. Oxidative Stress and Nitric Oxide in Autism Spectrum Disorder and Other Neuropsychiatric Disorders. CNS Neurol. Disord. Drug Targets 2016, 15, 587–596. [Google Scholar] [CrossRef]
- Delattre, J. [Introduction: From molecular oxygen to oxidative stress and radical biochemistry]. Ann. Pharm. Fr. 2006, 64, 363. [Google Scholar] [CrossRef] [PubMed]
- Mink, J.W.; Blumenschine, R.J.; Adams, D.B. Ratio of Central Nervous System to Body Metabolism in Vertebrates: Its Constancy and Functional Basis. Am. J. Physiol. 1981, 241, R203–R212. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Allaman, I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Fornstedt Wallin, B. Oxidation of Dopamine and Related Catechols in Dopaminergic Brain Regions in Parkinson’s Disease and during Ageing in Non-Parkinsonian Subjects. J. Neural Transm. 2024, 131, 213–228. [Google Scholar] [CrossRef]
- Umek, N.; Geršak, B.; Vintar, N.; Šoštarič, M.; Mavri, J. Dopamine Autoxidation Is Controlled by Acidic PH. Front. Mol. Neurosci. 2018, 11, 467. [Google Scholar] [CrossRef]
- Badillo-Ramírez, I.; Saniger, J.M.; Rivas-Arancibia, S. 5-S-Cysteinyl-Dopamine, a Neurotoxic Endogenous Metabolite of Dopamine: Implications for Parkinson’s Disease. Neurochem. Int. 2019, 129, 104514. [Google Scholar] [CrossRef]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef]
- Pavăl, D. The Dopamine Hypothesis of Autism Spectrum Disorder: A Comprehensive Analysis of the Evidence. Int. Rev. Neurobiol. 2023, 173, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Godar, S.C.; Alzghoul, L.; Zhang, J.; Darling, R.D.; Simpson, K.L.; Bini, V.; Chen, K.; Wellman, C.L.; Lin, R.C.S.; et al. Monoamine Oxidase A and A/B Knockout Mice Display Autistic-like Features. Int. J. Neuropsychopharmacol. 2013, 16, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H. The Centennial of the Fenton Reaction. Free Radic. Biol. Med. 1993, 15, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Catalano, A.; Sinicropi, M.S.; Genchi, G. Oxidative Stress and Neurodegeneration: The Involvement of Iron. Biometals 2018, 31, 715–735. [Google Scholar] [CrossRef]
- Santos, G.; Borges, J.M.P.; Avila-Rodriguez, M.; Gaíno, S.B.; Barreto, G.E.; Rúbio, É.P.; Aguiar, R.M.; Galembeck, E.; Bromochenkel, C.B.; de Oliveira, D.M. Copper and Neurotoxicity in Autism Spectrum Disorder. Curr. Pharm. Des. 2019, 25, 4747–4754. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Xu, K.; Wang, G.; Zhang, F. Copper Homeostasis and Neurodegenerative Diseases. Neural Regen. Res. 2025, 20, 3124–3143. [Google Scholar] [CrossRef]
- Zecca, L.; Stroppolo, A.; Gatti, A.; Tampellini, D.; Toscani, M.; Gallorini, M.; Giaveri, G.; Arosio, P.; Santambrogio, P.; Fariello, R.G.; et al. The Role of Iron and Copper Molecules in the Neuronal Vulnerability of Locus Coeruleus and Substantia Nigra during Aging. Proc. Natl. Acad. Sci. USA 2004, 101, 9843–9848. [Google Scholar] [CrossRef]
- Gangania, M.K.; Batra, J.; Kushwaha, S.; Agarwal, R. Role of Iron and Copper in the Pathogenesis of Parkinson’s Disease. Indian J. Clin. Biochem. 2017, 32, 353–356. [Google Scholar] [CrossRef]
- Deibel, M.A.; Ehmann, W.D.; Markesbery, W.R. Copper, Iron, and Zinc Imbalances in Severely Degenerated Brain Regions in Alzheimer’s Disease: Possible Relation to Oxidative Stress. J. Neurol. Sci. 1996, 143, 137–142. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, J. Why Not Try to Predict Autism Spectrum Disorder with Crucial Biomarkers in Cuproptosis Signaling Pathway? Front. Psychiatry 2022, 13, 1037503. [Google Scholar] [CrossRef]
- Oshodi, Y.; Ojewunmi, O.; Oshodi, T.A.; Ijarogbe, G.T.; Ogun, O.C.; Aina, O.F.; Lesi, F. Oxidative Stress Markers and Genetic Polymorphisms of Glutathione S-Transferase T1, M1, and P1 in a Subset of Children with Autism Spectrum Disorder in Lagos, Nigeria. Niger. J. Clin. Pract. 2017, 20, 1161–1167. [Google Scholar] [CrossRef]
- Katsuyama, M.; Matsuno, K.; Yabe-Nishimura, C. Physiological Roles of NOX/NADPH Oxidase, the Superoxide-Generating Enzyme. J. Clin. Biochem. Nutr. 2012, 50, 9–22. [Google Scholar] [CrossRef]
- Nisimoto, Y.; Jackson, H.M.; Ogawa, H.; Kawahara, T.; Lambeth, J.D. Constitutive NADPH-Dependent Electron Transferase Activity of the Nox4 Dehydrogenase Domain. Biochemistry 2010, 49, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Braughler, J.M.; Duncan, L.A.; Chase, R.L. The Involvement of Iron in Lipid Peroxidation. Importance of Ferric to Ferrous Ratios in Initiation. J. Biol. Chem. 1986, 261, 10282–10289. [Google Scholar] [CrossRef] [PubMed]
- Yui, K.; Imataka, G.; Shiohama, T. Lipid NOSation of the Docosahexaenoic Acid/Arachidonic Acid Ratio Relating to the Social Behaviors of Individuals with Autism Spectrum Disorder: The Relationship with Ferroptosis. Int. J. Mol. Sci. 2023, 24, 14796. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of Oxidative Damage and Inflammation Associated with Low Glutathione Redox Status in the Autism Brain. Transl. Psychiatry 2012, 2, e134. [Google Scholar] [CrossRef]
- Frustaci, A.; Neri, M.; Cesario, A.; Adams, J.B.; Domenici, E.; Dalla Bernardina, B.; Bonassi, S. Oxidative Stress-Related Biomarkers in Autism: Systematic Review and Meta-Analyses. Free Radic. Biol. Med. 2012, 52, 2128–2141. [Google Scholar] [CrossRef]
- Liu, L.; Lai, Y.; Zhan, Z.; Fu, Q.; Jiang, Y. Identification of Ferroptosis-Related Molecular Clusters and Immune Characterization in Autism Spectrum Disorder. Front. Genet. 2022, 13, 911119. [Google Scholar] [CrossRef]
- Rezzani, R.; Gianò, M.; Pinto, D.; Rinaldi, F.; van Noorden, C.J.F.; Favero, G. Hepatic Alterations in a BTBR T + Itpr3tf/J Mouse Model of Autism and Improvement Using Melatonin via Mitigation Oxidative Stress, Inflammation and Ferroptosis. Int. J. Mol. Sci. 2024, 25, 1086. [Google Scholar] [CrossRef]
- Wu, H.; Luan, Y.; Wang, H.; Zhang, P.; Liu, S.; Wang, P.; Cao, Y.; Sun, H.; Wu, L. Selenium Inhibits Ferroptosis and Ameliorates Autistic-like Behaviors of BTBR Mice by Regulating the Nrf2/GPx4 Pathway. Brain Res. Bull. 2022, 183, 38–48. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.S.; Katyal, A. Myeloperoxidase: Bridging the Gap in Neurodegeneration. Neurosci. Biobehav. Rev. 2016, 68, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pan, J.; Gong, Z.; Wu, M.; Zhang, X.; Chen, H.; Yang, D.; Qi, S.; Peng, Y.; Shen, J. Hypochlorous Acid Derived from Microglial Myeloperoxidase Could Mediate High-Mobility Group Box 1 Release from Neurons to Amplify Brain Damage in Cerebral Ischemia-Reperfusion Injury. J. Neuroinflamm. 2024, 21, 70. [Google Scholar] [CrossRef]
- Ceylan, M.F.; Tural Hesapcioglu, S.; Yavas, C.P.; Senat, A.; Erel, O. Serum Ischemia-Modified Albumin Levels, Myeloperoxidase Activity and Peripheral Blood Mononuclear Cells in Autism Spectrum Disorder (ASD). J. Autism Dev. Disord. 2021, 51, 2511–2517, Erratum in J. Autism Dev. Disord. 2021, 51, 2518. https://doi.org/10.1007/s10803-020-04834-4. [Google Scholar] [CrossRef]
- Haslacher, H.; Perkmann, T.; Lukas, I.; Barth, A.; Ponocny-Seliger, E.; Michlmayr, M.; Scheichenberger, V.; Wagner, O.; Winker, R. Myeloperoxidase Levels Predict Executive Function. Int. J. Sports Med. 2012, 33, 1034–1038. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.S.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively Activated Macrophages Produce Catecholamines to Sustain Adaptive Thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef]
- Mor, G.; Yue, W.; Santen, R.J.; Gutierrez, L.; Eliza, M.; Berstein, L.M.; Harada, N.; Wang, J.; Lysiak, J.; Diano, S.; et al. Macrophages, Estrogen and the Microenvironment of Breast Cancer. J. Steroid Biochem. Mol. Biol. 1998, 67, 403–411. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric Oxide Synthases: Structure, Function and Inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Filho, C.C.; Melfior, L.; Ramos, S.L.; Pizi, M.S.O.; Taruhn, L.F.; Muller, M.E.; Nunes, T.K.; Schmitt, L.d.O.; Gaspar, J.M.; de Oliveira, M.d.A.; et al. Tetrahydrobiopterin and Autism Spectrum Disorder: A Systematic Review of a Promising Therapeutic Pathway. Brain Sci. 2025, 15, 151. [Google Scholar] [CrossRef]
- Haynes, V.; Elfering, S.; Traaseth, N.; Giulivi, C. Mitochondrial Nitric-Oxide Synthase: Enzyme Expression, Characterization, and Regulation. J. Bioenerg. Biomembr. 2004, 36, 341–346. [Google Scholar] [CrossRef]
- Brown, G.C. Nitric Oxide and Mitochondrial Respiration. Biochim. Biophys. Acta 1999, 1411, 351–369. [Google Scholar] [CrossRef]
- Marks, J.D.; Boriboun, C.; Wang, J. Mitochondrial Nitric Oxide Mediates Decreased Vulnerability of Hippocampal Neurons from Immature Animals to NMDA. J. Neurosci. 2005, 25, 6561–6575. [Google Scholar] [CrossRef]
- Okamoto, S.; Lipton, S.A. S-Nitrosylation in Neurogenesis and Neuronal Development. Biochim. Biophys. Acta 2015, 1850, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharmacol. Rev. 2020, 72, 692–766. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine Oxidoreductase: One Enzyme for Multiple Physiological Tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Kartawy, M.; Amal, H. The Role of n itric Oxide in Brain Disorders: Autism Spectrum Disorder and Other Psychiatric, Neurological, and Neurodegenerative Disorders. Redox Biol. 2020, 34, 101567. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Dewald, H.D. Nitric Oxide and Peroxynitrite as New Biomarkers for Early Diagnosis of Autism. Brain Res. 2025, 1850, 149438. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M.; Lipinski, B.; Windom, H.; Audhya, T.; McGinnis, W. Oxidative Stress in Autism: Elevated Cerebellar 3-Nitrotyrosine Levels. Am. J. Biochem. Biotechnol. 2008, 4, 73–84. [Google Scholar] [CrossRef]
- Al-Garni, A.M.; Hosny, S.A.; Almasabi, F.; Shati, A.A.; Alzamil, N.M.; ShamsEldeen, A.M.; El-Shafei, A.A.; Al-Hashem, F.; Zafrah, H.; Maarouf, A.; et al. Identifying INOS and Glycogen as Biomarkers for Degenerated Cerebellar Purkinje Cells in Autism Spectrum Disorder: Protective Effects of Erythropoietin and Zinc Sulfate. PLoS ONE 2025, 20, e0317695. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Ojha, S.K.; Kartawy, M.; Hamoudi, W.; Choudhary, A.; Stern, S.; Aran, A.; Amal, H. The NO Answer for Autism Spectrum Disorder. Adv. Sci. 2023, 10, e2205783. [Google Scholar] [CrossRef] [PubMed]
- Steinert, J.R.; Amal, H. The Contribution of an Imbalanced Redox Signalling to Neurological and Neurodegenerative Conditions. Free Radic. Biol. Med. 2023, 194, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Khudhur, Z.O.; Abdullah, S.R.; Hussen, B.M.; Murad, N.A.; Sayad, A.; Ghafouri-Fard, S. Gasotransmitters and Their Influence on Autism Spectrum Disorders—A Systematic Review. Mol. Biol. Rep. 2025, 52, 595. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.B. Gamma Glutamyl Transferase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef]
- Hanigan, M.H.; Frierson, H.F.J. Immunohistochemical Detection of Gamma-Glutamyl Transpeptidase in Normal Human Tissue. J. Histochem. Cytochem. 1996, 44, 1101–1108. [Google Scholar] [CrossRef]
- Tzeng, Y.-Z.; Hu, C.-H. Radical-Induced Cis-Trans Isomerization of Fatty Acids: A Theoretical Study. J. Phys. Chem. A 2014, 118, 4554–4564. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Zambonin, L.; Altieri, A.; Ferreri, C.; Mulazzani, Q.G.; Landi, L. Geometrical Isomerism of Monounsaturated Fatty Acids: Thiyl Radical Catalysis and Influence of Antioxidant Vitamins. Free Radic. Biol. Med. 2002, 33, 1681–1692. [Google Scholar] [CrossRef]
- Accaoui, M.J.; Enoiu, M.; Mergny, M.; Masson, C.; Dominici, S.; Wellman, M.; Visvikis, A. Gamma-Glutamyltranspeptidase-Dependent Glutathione Catabolism Results in Activation of NF-KB. Biochem. Biophys. Res. Commun. 2000, 276, 1062–1067. [Google Scholar] [CrossRef]
- Paolicchi, A.; Minotti, G.; Tonarelli, P.; Tongiani, R.; De Cesare, D.; Mezzetti, A.; Dominici, S.; Comporti, M.; Pompella, A. Gamma-Glutamyl Transpeptidase-Dependent Iron Reduction and LDL Oxidation—A Potential Mechanism in Atherosclerosis. J. Investig. Med. 1999, 47, 151–160. [Google Scholar]
- Li, S.; Liao, X.; Pan, Y.; Xiang, X.; Zhang, Y. Gamma-Glutamyl Transferase Levels Are Associated with the Occurrence of Post-Stroke Cognitive Impairment: A Multicenter Cohort Study. BMC Neurol. 2022, 22, 65. [Google Scholar] [CrossRef]
- Starkov, A.A.; Fiskum, G.; Chinopoulos, C.; Lorenzo, B.J.; Browne, S.E.; Patel, M.S.; Beal, M.F. Mitochondrial Alpha-Ketoglutarate Dehydrogenase Complex Generates Reactive Oxygen Species. J. Neurosci. 2004, 24, 7779–7788. [Google Scholar] [CrossRef]
- Jodeiri Farshbaf, M.; Kiani-Esfahani, A. Succinate Dehydrogenase: Prospect for Neurodegenerative Diseases. Mitochondrion 2018, 42, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mrácek, T.; Pecinová, A.; Vrbacký, M.; Drahota, Z.; Houstek, J. High Efficiency of ROS Production by Glycerophosphate Dehydrogenase in Mammalian Mitochondria. Arch. Biochem. Biophys. 2009, 481, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Vivar, J.; Kalyanaraman, B.; Kennedy, M.C. Mitochondrial Aconitase Is a Source of Hydroxyl Radical. An Electron Spin Resonance Investigation. J. Biol. Chem. 2000, 275, 14064–14069. [Google Scholar] [CrossRef] [PubMed]
- Hey-Mogensen, M.; Goncalves, R.L.S.; Orr, A.L.; Brand, M.D. Production of Superoxide/H2O2 by Dihydroorotate Dehydrogenase in Rat Skeletal Muscle Mitochondria. Free Radic. Biol. Med. 2014, 72, 149–155. [Google Scholar] [CrossRef]
- Fox, R.J.; Wiendl, H.; Wolf, C.; De Stefano, N.; Sellner, J.; Gryb, V.; Rejdak, K.; Bozhinov, P.S.; Tomakh, N.; Skrypchenko, I.; et al. A Double-Blind, Randomized, Placebo-Controlled Phase 2 Trial Evaluating the Selective Dihydroorotate Dehydrogenase Inhibitor Vidofludimus Calcium in Relapsing-Remitting Multiple Sclerosis. Ann. Clin. Transl. Neurol. 2022, 9, 977–987. [Google Scholar] [CrossRef]
- Sabol, S.Z.; Hu, S.; Hamer, D. A Functional Polymorphism in the Monoamine Oxidase A Gene Promoter. Hum. Genet. 1998, 103, 273–279. [Google Scholar] [CrossRef]
- Harneit, A.; Braun, U.; Geiger, L.S.; Zang, Z.; Hakobjan, M.; van Donkelaar, M.M.J.; Schweiger, J.I.; Schwarz, K.; Gan, G.; Erk, S.; et al. MAOA-VNTR Genotype Affects Structural and Functional Connectivity in Distributed Brain Networks. Hum. Brain Mapp. 2019, 40, 5202–5212. [Google Scholar] [CrossRef]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine Oxidase: From Genes to Behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef]
- Wang, C.C.; Billett, E.; Borchert, A.; Kuhn, H.; Ufer, C. Monoamine Oxidases in Development. Cell. Mol. Life Sci. 2013, 70, 599–630. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Baes, M.; Ribeiro, D.; Ferdinandusse, S.; Waterham, H.R. The Physiological Functions of Human Peroxisomes. Physiol. Rev. 2023, 103, 957–1024. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.A.; Waterham, H.R. Peroxisomal Disorders I: Biochemistry and Genetics of Peroxisome Biogenesis Disorders. Clin. Genet. 2005, 67, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Lipinski, M.M. Role and Function of Peroxisomes in Neuroinflammation. Cells 2024, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Alhakbany, M.; Aldbass, A.; Qasem, H.; Al-Mazidi, S.; Bhat, R.S.; Al-Ayadhi, L. Alpha-Synuclein, Cyclooxygenase-2 and Prostaglandins-EP2 Receptors as Neuroinflammatory Biomarkers of Autism Spectrum Disorders: Use of Combined ROC Curves to Increase Their Diagnostic Values. Lipids Health Dis. 2021, 20, 155, Erratum in Lipids Health Dis. 2021, 20, 172. https://doi.org/10.1186/s12944-021-01598-3. [Google Scholar] [CrossRef]
- Zhao, G.; Gao, J.; Liang, S.; Wang, X.; Sun, C.; Xia, W.; Hao, Y.; Li, X.; Cao, Y.; Wu, L. Study of the Serum Levels of Polyunsaturated Fatty Acids and the Expression of Related Liver Metabolic Enzymes in a Rat Valproate-Induced Autism Model. Int. J. Dev. Neurosci. 2015, 44, 14–21. [Google Scholar] [CrossRef]
- Sun, C.; Zou, M.; Wang, X.; Xia, W.; Ma, Y.; Liang, S.; Hao, Y.; Wu, L.; Fu, S. FADS1-FADS2 and ELOVL2 Gene Polymorphisms in Susceptibility to Autism Spectrum Disorders in Chinese Children. BMC Psychiatry 2018, 18, 283. [Google Scholar] [CrossRef]
- Yui, K.; Imataka, G.; Ichihashi, M. Prostaglandins: Biological Action, Therapeutic Aspects, and Pathophysiology of Autism Spectrum Disorders. Curr. Issues Mol. Biol. 2025, 47, 71. [Google Scholar] [CrossRef]
- Gasser, B.A.; Kurz, J.; Dick, B.; Mohaupt, M.G. How Is CYP17A1 Activity Altered in Autism? A Pilot Study to Identify Potential Pharmacological Targets. Life 2022, 12, 867. [Google Scholar] [CrossRef]
- Durairaj, P.; Liu, Z.L. Brain Cytochrome P450: Navigating Neurological Health and Metabolic Regulation. J. Xenobiot. 2025, 15, 44. [Google Scholar] [CrossRef]
- Ballester, P.; Espadas, C.; Almenara, S.; Barrachina, J.; Muriel, J.; Ramos, E.; Toral, N.; Belda, C.; Peiró, A.M. CYP2D6 Genotype and Pharmacovigilance Impact on Autism Spectrum Disorder: A Naturalistic Study with Extreme Phenotype Analysis. Pharmaceuticals 2023, 16, 954. [Google Scholar] [CrossRef]
- Dhulkifle, H.; Agouni, A.; Zeidan, A.; Al-Kuwari, M.S.; Parray, A.; Tolefat, M.; Korashy, H.M. Influence of the Aryl Hydrocarbon Receptor Activating Environmental Pollutants on Autism Spectrum Disorder. Int. J. Mol. Sci. 2021, 22, 9258. [Google Scholar] [CrossRef]
- Harrison, R. Structure and Function of Xanthine Oxidoreductase: Where Are We Now? Free Radic. Biol. Med. 2002, 33, 774–797. [Google Scholar] [CrossRef]
- Godber, B.L.; Doel, J.J.; Sapkota, G.P.; Blake, D.R.; Stevens, C.R.; Eisenthal, R.; Harrison, R. Reduction of Nitrite to Nitric Oxide Catalyzed by Xanthine Oxidoreductase. J. Biol. Chem. 2000, 275, 7757–7763. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, M.; Bilenberg, N.; Thorsen, L.; Michel, T.M. Oxidative Stress in Adults with Autism Spectrum Disorder: A Case Control Study. J. Autism Dev. Disord. 2022, 52, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Zoroglu, S.S.; Armutcu, F.; Ozen, S.; Gurel, A.; Sivasli, E.; Yetkin, O.; Meram, I. Increased Oxidative Stress and Altered Activities of Erythrocyte Free Radical Scavenging Enzymes in Autism. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zielke, H.R.; Yeh, D.; Yang, P. Cellular Stress and Apoptosis Contribute to the Pathogenesis of Autism Spectrum Disorder. Autism Res. 2018, 11, 1076–1090. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Kynurenine Pathway and Human Systems. Exp. Gerontol. 2020, 129, 110770. [Google Scholar] [CrossRef]
- Bryn, V.; Verkerk, R.; Skjeldal, O.H.; Saugstad, O.D.; Ormstad, H. Kynurenine Pathway in Autism Spectrum Disorders in Children. Neuropsychobiology 2017, 76, 82–88. [Google Scholar] [CrossRef]
- Notarangelo, F.M.; Pocivavsek, A. Elevated Kynurenine Pathway Metabolism during Neurodevelopment: Implications for Brain and Behavior. Neuropharmacology 2017, 112, 275–285. [Google Scholar] [CrossRef]
- Santana-Coelho, D. Does the Kynurenine Pathway Play a Pathogenic Role in Autism Spectrum Disorder? Brain Behav. Immun.-Health 2024, 40, 100839. [Google Scholar] [CrossRef]
- Le Belle, J.E.; Sperry, J.; Ngo, A.; Ghochani, Y.; Laks, D.R.; López-Aranda, M.; Silva, A.J.; Kornblum, H.I. Maternal Inflammation Contributes to Brain Overgrowth and Autism-Associated Behaviors through Altered Redox Signaling in Stem and Progenitor Cells. Stem Cell Rep. 2014, 3, 725–734. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Attia, S.M.; Alshammari, M.A.; Alzahrani, K.S.; Bakheet, S.A. Increased Oxidative Stress in the Cerebellum and Peripheral Immune Cells Leads to Exaggerated Autism-like Repetitive Behavior Due to Deficiency of Antioxidant Response in BTBR T + tf/J Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.C.; Selvakumar, B.; Weil, Z.M.; Snyder, S.H.; Nelson, R.J. Neuronal Nitric Oxide Synthase and NADPH Oxidase Interact to Affect Cognitive, Affective, and Social Behaviors in Mice. Behav. Brain Res. 2013, 256, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Stein, T.P.; Brimacombe, M.; Johnson, W.G.; Lambert, G.H.; Wagner, G.C. Increased Excretion of a Lipid Peroxidation Biomarker in Autism. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K. 4-Hydroxy-2-Nonenal: A Product and Mediator of Oxidative Stress. Prog. Lipid Res. 2003, 42, 318–343. [Google Scholar] [CrossRef]
- Wu, X.; Li, R.; Hong, Q.; Chi, X. Development and Validation of a Novel Diagnostic Model for Childhood Autism Spectrum Disorder Based on Ferroptosis-Related Genes. Front. Psychiatry 2022, 13, 886055. [Google Scholar] [CrossRef]
- Bjørklund, G.; Tinkov, A.A.; Hosnedlová, B.; Kizek, R.; Ajsuvakova, O.P.; Chirumbolo, S.; Skalnaya, M.G.; Peana, M.; Dadar, M.; El-Ansary, A.; et al. The Role of Glutathione Redox Imbalance in Autism Spectrum Disorder: A Review. Free Radic. Biol. Med. 2020, 160, 149–162. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, G.; Liu, S.; Zhang, Q.; Wang, P.; Cao, Y.; Wu, L. Supplementation with Selenium Attenuates Autism-like Behaviors and Improves Oxidative Stress, Inflammation and Related Gene Expression in an Autism Disease Model. J. Nutr. Biochem. 2022, 107, 109034. [Google Scholar] [CrossRef]
- Yui, K.; Imataka, G.; Shiohama, T. Lipid Peroxidation via Regulating the Metabolism of Docosahexaenoic Acid and Arachidonic Acid in Autistic Behavioral Symptoms. Curr. Issues Mol. Biol. 2023, 45, 9149–9164. [Google Scholar] [CrossRef]
- Zambonin, L.; Ferreri, C.; Cabrini, L.; Prata, C.; Chatgilialoglu, C.; Landi, L. Occurrence of Trans Fatty Acids in Rats Fed a Trans-Free Diet: A Free Radical-Mediated Formation? Free Radic. Biol. Med. 2006, 40, 1549–1556. [Google Scholar] [CrossRef]
- Neish, A.S.; Jones, R.M. Redox Signaling Mediates Symbiosis between the Gut Microbiota and the Intestine. Gut Microbes 2014, 5, 250–253. [Google Scholar] [CrossRef]
- Trostchansky, A.; Mastrogiovanni, M.; Miquel, E.; Rodríguez-Bottero, S.; Martínez-Palma, L.; Cassina, P.; Rubbo, H. Profile of Arachidonic Acid-Derived Inflammatory Markers and Its Modulation by Nitro-Oleic Acid in an Inherited Model of Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2018, 11, 131. [Google Scholar] [CrossRef]
- Hung, W.-L.; Sun Hwang, L.; Shahidi, F.; Pan, M.-H.; Wang, Y.; Ho, C.-T. Endogenous Formation of Trans Fatty Acids: Health Implications and Potential Dietary Intervention. J. Funct. Foods 2016, 25, 14–24. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of Saturated and Trans Unsaturated Fatty Acids and Risk of All Cause Mortality, Cardiovascular Disease, and Type 2 Diabetes: Systematic Review and Meta-Analysis of Observational Studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Schilter, D. Thiol Oxidation: A Slippery Slope. Nat. Rev. Chem. 2017, 1, 13. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Hydrogen Peroxide Reactivity and Specificity in Thiol-Based Cell Signalling. Biochem. Soc. Trans. 2020, 48, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Schrier, M.S.; Zhang, Y.; Trivedi, M.S.; Deth, R.C. Decreased Cortical Nrf2 Gene Expression in Autism and Its Relationship to Thiol and Cobalamin Status. Biochimie 2022, 192, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rincon, N.; McCarty, P.J.; Brister, D.; Scheck, A.C.; Rossignol, D.A. Biomarkers of Mitochondrial Dysfunction in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Neurobiol. Dis. 2024, 197, 106520. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal Gut Bacteria Promote Neurodevelopmental Abnormalities in Mouse Offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef]
- Napoli, E.; Wong, S.; Hertz-Picciotto, I.; Giulivi, C. Deficits in Bioenergetics and Impaired Immune Response in Granulocytes from Children with Autism. Pediatrics 2014, 133, e1405–e1410. [Google Scholar] [CrossRef]
- Ayaydın, H.; Kılıçaslan, F.; Koyuncu, İ.; Çelik, H.; Çalık, M.; Güzelçiçek, A.; Kirmit, A. Impaired Thiol/Disulfide Homeostasis in Children Diagnosed with Autism: A Case-Control Study. J. Mol. Neurosci. 2021, 71, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Teke, H.; Balci, S.; Neselioglu, S.; Teke, S.; Erel, O.; Tamer, L.; Toros, F. Oxidative Stress and Dynamic Thiol/Disulfide Homeostasis in Autism: A Focus on Early Childhood. J. Mol. Neurosci. 2025, 75, 62. [Google Scholar] [CrossRef] [PubMed]
- Efe, A.; Neşelioğlu, S.; Soykan, A. An Investigation of the Dynamic Thiol/Disulfide Homeostasis, As a Novel Oxidative Stress Plasma Biomarker, in Children With Autism Spectrum Disorders. Autism Res. 2021, 14, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Cortés, E.; Aguilar, M.J.; Rizo, M.M.; Gil, V.; Hidalgo, M. Trans Fatty Acids in the Nutrition of Children with Neurological Disorders. Nutr. Hosp. 2013, 28, 1140–1144. [Google Scholar]
- Hamoudi, W.; Tripathi, M.K.; Ojha, S.K.; Amal, H. A Cross-Talk between Nitric Oxide and the Glutamatergic System in a Shank3 Mouse Model of Autism. Free Radic. Biol. Med. 2022, 188, 83–91. [Google Scholar] [CrossRef]
- Kang, K.W.; Choi, S.H.; Kim, S.G. Peroxynitrite Activates NF-E2-Related Factor 2/Antioxidant Response Element through the Pathway of Phosphatidylinositol 3-Kinase: The Role of Nitric Oxide Synthase in Rat Glutathione S-Transferase A2 Induction. Nitric Oxide Biol. Chem. 2002, 7, 244–253. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Ayadhi, L.Y.; Attia, S.M.; Al-Harbi, N.O.; Alzahrani, K.S.; Bakheet, S.A. Differential Regulation of Nrf2 Is Linked to Elevated Inflammation and Nitrative Stress in Monocytes of Children with Autism. Psychoneuroendocrinology 2020, 113, 104554. [Google Scholar] [CrossRef]
- Söğüt, S.; Zoroğlu, S.S.; Ozyurt, H.; Yilmaz, H.R.; Ozuğurlu, F.; Sivasli, E.; Yetkin, O.; Yanik, M.; Tutkun, H.; Savaş, H.A.; et al. Changes in Nitric Oxide Levels and Antioxidant Enzyme Activities May Have a Role in the Pathophysiological Mechanisms Involved in Autism. Clin. Chim. Acta 2003, 331, 111–117. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Y.; Pan, J.; Yang, A.; Niu, L.; Min, J.; Meng, X.; Liao, L.; Zhang, K.; Shen, L. Redox Proteomic Identification of Carbonylated Proteins in Autism Plasma: Insight into Oxidative Stress and Its Related Biomarkers in Autism. Clin. Proteom. 2017, 14, 2. [Google Scholar] [CrossRef]
- Hu, T.; Dong, Y.; He, C.; Zhao, M.; He, Q. The Gut Microbiota and Oxidative Stress in Autism Spectrum Disorders (ASD). Oxidative Med. Cell. Longev. 2020, 2020, 8396708. [Google Scholar] [CrossRef]
- Rose, S.; Frye, R.E.; Slattery, J.; Wynne, R.; Tippett, M.; Pavliv, O.; Melnyk, S.; James, S.J. Oxidative Stress Induces Mitochondrial Dysfunction in a Subset of Autism Lymphoblastoid Cell Lines in a Well-Matched Case Control Cohort. PLoS ONE 2014, 9, e85436. [Google Scholar] [CrossRef]
- Rose, S.; Bennuri, S.C.; Wynne, R.; Melnyk, S.; James, S.J.; Frye, R.E. Mitochondrial and Redox Abnormalities in Autism Lymphoblastoid Cells: A Sibling Control Study. FASEB J. 2017, 31, 904–909. [Google Scholar] [CrossRef]
- Osredkar, J.; Kumer, K.; Fabjan, T.; Jekovec Vrhovšek, M.; Maček, J.; Zupan, M.; Bobrowska-Korczak, B.; Gątarek, P.; Rosiak, A.; Giebułtowicz, J.; et al. Determination of Modified Nucleosides in the Urine of Children with Autism Spectrum Disorder. Acta Biochim. Pol. 2023, 70, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, S.; Fuchs, G.J.; Schulz, E.; Lopez, M.; Kahler, S.G.; Fussell, J.J.; Bellando, J.; Pavliv, O.; Rose, S.; Seidel, L.; et al. Metabolic Imbalance Associated with Methylation Dysregulation and Oxidative Damage in Children with Autism. J. Autism Dev. Disord. 2012, 42, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Liu, Q.; Xing, F.; Zeng, C.; Wang, W. Disulfidptosis: A New Form of Programmed Cell Death. J. Exp. Clin. Cancer Res. 2023, 42, 137. [Google Scholar] [CrossRef] [PubMed]
- Howsmon, D.P.; Kruger, U.; Melnyk, S.; James, S.J.; Hahn, J. Classification and Adaptive Behavior Prediction of Children with Autism Spectrum Disorder Based upon Multivariate Data Analysis of Markers of Oxidative Stress and DNA Methylation. PLoS Comput. Biol. 2017, 13, e1005385. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, S.; Shi, J.; Guo, Y.; Li, Z.; Cheng, Y.; Liu, Y. Association between MTHFR C677T/A1298C and Susceptibility to Autism Spectrum Disorders: A Meta-Analysis. BMC Pediatr. 2020, 20, 449. [Google Scholar] [CrossRef]
- Pu, D.; Shen, Y.; Wu, J. Association between MTHFR Gene Polymorphisms and the Risk of Autism Spectrum Disorders: A Meta-Analysis. Autism Res. 2013, 6, 384–392. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Tancredi, D.J.; Ozonoff, S.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tassone, F.; Hertz-Picciotto, I. Maternal Periconceptional Folic Acid Intake and Risk of Autism Spectrum Disorders and Developmental Delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) Case-Control Study. Am. J. Clin. Nutr. 2012, 96, 80–89. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016, 10, 321. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Fu, H.-G.; Cheng, H.; Zheng, R.-J.; Wang, G.; Li, S.; Li, E.-Y.; Li, L.-G. Electroacupuncture at Zusanli Ameliorates the Autistic-like Behaviors of Rats through Activating the Nrf2-Mediated Antioxidant Responses. Gene 2022, 828, 146440. [Google Scholar] [CrossRef]
- Morris, G.; Walder, K.; Berk, M.; Carvalho, A.F.; Marx, W.; Bortolasci, C.C.; Yung, A.R.; Puri, B.K.; Maes, M. Intertwined Associations between Oxidative and Nitrosative Stress and Endocannabinoid System Pathways: Relevance for Neuropsychiatric Disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 114, 110481. [Google Scholar] [CrossRef]
- Aran, A.; Eylon, M.; Harel, M.; Polianski, L.; Nemirovski, A.; Tepper, S.; Schnapp, A.; Cassuto, H.; Wattad, N.; Tam, J. Lower Circulating Endocannabinoid Levels in Children with Autism Spectrum Disorder. Mol. Autism 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, B.; Persico, A.; Battista, N.; Maccarrone, M. Endocannabinoid Signaling in Autism. Neurotherapeutics 2015, 12, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Chełchowska, M.; Gajewska, J.; Szczepanik, E.; Mazur, J.; Cychol, A.; Kuźniar-Pałka, A.; Ambroszkiewicz, J. Oxidative Stress Indicated by Nuclear Transcription Factor Nrf2 and Glutathione Status in the Blood of Young Children with Autism Spectrum Disorder: Pilot Study. Antioxidants 2025, 14, 320. [Google Scholar] [CrossRef] [PubMed]
- Ayaydin, H.; Akaltun, İ.; Koyuncu, İ.; Çelİk, H.; Kİrmİt, A.; Takatak, H. High KEAP1, NRF2 and Low HO-1 Serum Levels in Children with Autism. Noro Psikiyatr. Ars. 2020, 57, 274–279. [Google Scholar] [CrossRef]
- Subasi Turgut, F.; Karadag, M.; Taysi, S.; Hangül, Z.; Gokcen, C. NRF2, KEAP1 and GSK-3 Levels in Autism Spectrum Disorder: A Case Control Study. Int. J. Dev. Disabil. 2024, 70, 1441–1451. [Google Scholar] [CrossRef]
- Furnari, M.A.; Saw, C.L.-L.; Kong, A.-N.; Wagner, G.C. Altered Behavioral Development in Nrf2 Knockout Mice Following Early Postnatal Exposure to Valproic Acid. Brain Res. Bull. 2014, 109, 132–142. [Google Scholar] [CrossRef]
- Shah, A.; Varma, M.; Bhandari, R. Exploring Sulforaphane as Neurotherapeutic: Targeting Nrf2-Keap & Nf-Kb Pathway Crosstalk in ASD. Metab. Brain Dis. 2024, 39, 373–385. [Google Scholar] [CrossRef]
- Panes, J.D.; Wendt, A.; Ramirez-Molina, O.; Castro, P.A.; Fuentealba, J. Deciphering the Role of PGC-1α in Neurological Disorders: From Mitochondrial Dysfunction to Synaptic Failure. Neural Regen. Res. 2022, 17, 237–245. [Google Scholar] [CrossRef]
- Souder, D.C.; McGregor, E.R.; Rhoads, T.W.; Clark, J.P.; Porter, T.J.; Eliceiri, K.; Moore, D.L.; Puglielli, L.; Anderson, R.M. Mitochondrial Regulator PGC-1a in Neuronal Metabolism and Brain Aging. bioRxiv 2023. [Google Scholar] [CrossRef]
- You, W.; Knoops, K.; Berendschot, T.T.J.M.; Benedikter, B.J.; Webers, C.A.B.; Reutelingsperger, C.P.M.; Gorgels, T.G.M.F. PGC-1a Mediated Mitochondrial Biogenesis Promotes Recovery and Survival of Neuronal Cells from Cellular Degeneration. Cell Death Discov. 2024, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Bam, S.; Buchanan, E.; Mahony, C.; O’Ryan, C. DNA Methylation of PGC-1α Is Associated With Elevated MtDNA Copy Number and Altered Urinary Metabolites in Autism Spectrum Disorder. Front. Cell Dev. Biol. 2021, 9, 696428. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Otterbein, L.E.; Morse, D.; Choi, A.M.K. Heme Oxygenase/Carbon Monoxide Signaling Pathways: Regulation and Functional Significance. Mol. Cell. Biochem. 2002, 234–235, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Augusto, O.; Truzzi, D.R. Carbon Dioxide Redox Metabolites in Oxidative Eustress and Oxidative Distress. Biophys. Rev. 2021, 13, 889–891. [Google Scholar] [CrossRef]
- Jo-Watanabe, A.; Inaba, T.; Osada, T.; Hashimoto, R.; Nishizawa, T.; Okuno, T.; Ihara, S.; Touhara, K.; Hattori, N.; Oh-Hora, M.; et al. Bicarbonate Signalling via G Protein-Coupled Receptor Regulates Ischaemia-Reperfusion Injury. Nat. Commun. 2024, 15, 1530. [Google Scholar] [CrossRef]
- Whiteman, M.; Cheung, N.S.; Zhu, Y.-Z.; Chu, S.H.; Siau, J.L.; Wong, B.S.; Armstrong, J.S.; Moore, P.K. Hydrogen Sulphide: A Novel Inhibitor of Hypochlorous Acid-Mediated Oxidative Damage in the Brain? Biochem. Biophys. Res. Commun. 2005, 326, 794–798. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen Sulfide as a Neuromodulator. Mol. Neurobiol. 2002, 26, 13–19. [Google Scholar] [CrossRef]
- Manivasagam, T.; Arunadevi, S.; Essa, M.M.; SaravanaBabu, C.; Borah, A.; Thenmozhi, A.J.; Qoronfleh, M.W. Role of Oxidative Stress and Antioxidants in Autism. Adv. Neurobiol. 2020, 24, 193–206. [Google Scholar] [CrossRef]
- Bonomini, F.; Siniscalco, D.; Schultz, S.; Carnovale, C.; Barthélémy, C.; Fazzi, E.M. Editorial: Antioxidants in Autism Spectrum Disorders. Front. Psychiatry 2022, 13, 889865. [Google Scholar] [CrossRef]
- Essa, M.M.; Braidy, N.; Waly, M.I.; Al-Farsi, Y.M.; Al-Sharbati, M.; Subash, S.; Amanat, A.; Al-Shaffaee, M.A.; Guillemin, G.J. Impaired Antioxidant Status and Reduced Energy Metabolism in Autistic Children. Res. Autism Spectr. Disord. 2013, 7, 557–565. [Google Scholar] [CrossRef]
- Mondal, A.; Mukherjee, S.; Dar, W.; Singh, S.; Pati, S. Role of Glucose 6-Phosphate Dehydrogenase (G6PD) Deficiency and Its Association to Autism Spectrum Disorders. Biochimica et biophysica acta. Mol. Basis Dis. 2021, 1867, 166185. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 6th ed.; Palgrave MacMillan: London, UK, 2013. [Google Scholar]
- Al-Salehi, S.M.; Al-Hifthy, E.H.; Ghaziuddin, M. Autism in Saudi Arabia: Presentation, Clinical Correlates and Comorbidity. Transcult. Psychiatry 2009, 46, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Jeng, W.; Loniewska, M.M.; Wells, P.G. Brain Glucose-6-Phosphate Dehydrogenase Protects against Endogenous Oxidative DNA Damage and Neurodegeneration in Aged Mice. ACS Chem. Neurosci. 2013, 4, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Loniewska, M.M.; Gupta, A.; Bhatia, S.; MacKay-Clackett, I.; Jia, Z.; Wells, P.G. DNA Damage and Synaptic and Behavioural Disorders in Glucose-6-Phosphate Dehydrogenase-Deficient Mice. Redox Biol. 2020, 28, 101332. [Google Scholar] [CrossRef] [PubMed]
- Al-Gadani, Y.; El-Ansary, A.; Attas, O.; Al-Ayadhi, L. Metabolic Biomarkers Related to Oxidative Stress and Antioxidant Status in Saudi Autistic Children. Clin. Biochem. 2009, 42, 1032–1040. [Google Scholar] [CrossRef]
- Chauhan, A.; Audhya, T.; Chauhan, V. Brain Region-Specific Glutathione Redox Imbalance in Autism. Neurochem. Res. 2012, 37, 1681–1689. [Google Scholar] [CrossRef]
- Gu, F.; Chauhan, V.; Chauhan, A. Glutathione Redox Imbalance in Brain Disorders. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 89–95. [Google Scholar] [CrossRef]
- Endres, D.; Tebartz van Elst, L.; Meyer, S.A.; Feige, B.; Nickel, K.; Bubl, A.; Riedel, A.; Ebert, D.; Lange, T.; Glauche, V.; et al. Glutathione Metabolism in the Prefrontal Brain of Adults with High-Functioning Autism Spectrum Disorder: An MRS Study. Mol. Autism 2017, 8, 10. [Google Scholar] [CrossRef]
- Bowers, K.; Li, Q.; Bressler, J.; Avramopoulos, D.; Newschaffer, C.; Fallin, M.D. Glutathione Pathway Gene Variation and Risk of Autism Spectrum Disorders. J. Neurodev. Disord. 2011, 3, 132–143. [Google Scholar] [CrossRef]
- Hodgson, N.W.; Waly, M.I.; Al-Farsi, Y.M.; Al-Sharbati, M.M.; Al-Farsi, O.; Ali, A.; Ouhtit, A.; Zang, T.; Zhou, Z.S.; Deth, R.C. Decreased Glutathione and Elevated Hair Mercury Levels Are Associated with Nutritional Deficiency-Based Autism in Oman. Exp. Biol. Med. 2014, 239, 697–706. [Google Scholar] [CrossRef]
- Scapagnini, G.; Vasto, S.; Abraham, N.G.; Caruso, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE Pathway by Food Polyphenols: A Nutritional Neuroprotective Strategy for Cognitive and Neurodegenerative Disorders. Mol. Neurobiol. 2011, 44, 192–201, Erratum in Mol. Neurobiol. 2011, 44, 202. [Google Scholar] [CrossRef] [PubMed]
- Porokhovnik, L.N.; Pisarev, V.M.; Chumachenko, A.G.; Chudakova, J.M.; Ershova, E.S.; Veiko, N.N.; Gorbachevskaya, N.L.; Mamokhina, U.A.; Sorokin, A.B.; Basova, A.Y.; et al. Association of NEF2L2 Rs35652124 Polymorphism with Nrf2 Induction and Genotoxic Stress Biomarkers in Autism. Genes 2023, 14, 718. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Johnson, W.G.; Stenroos, E.S.; Mars, A.; Lambert, G.H.; Buyske, S. Genetic Variant of Glutathione Peroxidase 1 in Autism. Brain Dev. 2010, 32, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, X.-J.; Liu, H.; Mao, X.; Gui, L.-N.; Wang, H.; Cheng, Y. Oxidative Stress Marker Aberrations in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis of 87 Studies (N = 9109). Transl. Psychiatry 2021, 11, 15. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- James, S.J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, D.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic Endophenotype and Related Genotypes Are Associated with Oxidative Stress in Children with Autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2006, 141B, 947–956. [Google Scholar] [CrossRef]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic Biomarkers of Increased Oxidative Stress and Impaired Methylation Capacity in Children with Autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The Thioredoxin Antioxidant System. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione Peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Savaskan, N.E.; Bräuer, A.U.; Kühbacher, M.; Eyüpoglu, I.Y.; Kyriakopoulos, A.; Ninnemann, O.; Behne, D.; Nitsch, R. Selenium Deficiency Increases Susceptibility to Glutamate-Induced Excitotoxicity. FASEB J. 2003, 17, 112–114. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Crosstalk between Neuron and Glial Cells in Oxidative Injury and Neuroprotection. Int. J. Mol. Sci. 2021, 22, 13315. [Google Scholar] [CrossRef] [PubMed]
- Dawi, J.; Mohan, A.S.; Misakyan, Y.; Affa, S.; Gonzalez, E.; Hajjar, K.; Nikoghosyan, D.; Fardeheb, S.; Tuohino, C.; Venketaraman, V. The Role of Oxidative Stress in TB Meningitis and Therapeutic Options. Diseases 2024, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P.B. Catalase, a Remarkable Enzyme: Targeting the Oldest Antioxidant Enzyme to Find a New Cancer Treatment Approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Mugnaini, E.; Cerù, M.P. Immunocytochemical Localization of Catalase in the Central Nervous System of the Rat. J. Histochem. Cytochem. 1995, 43, 1253–1267. [Google Scholar] [CrossRef]
- Schad, A.; Fahimi, H.D.; Völkl, A.; Baumgart, E. Expression of Catalase MRNA and Protein in Adult Rat Brain: Detection by Nonradioactive in Situ Hybridization with Signal Amplification by Catalyzed Reporter Deposition (ISH-CARD) and Immunohistochemistry (IHC)/Immunofluorescence (IF). J. Histochem. Cytochem. 2003, 51, 751–760. [Google Scholar] [CrossRef]
- Yenkoyan, K.; Harutyunyan, H.; Harutyunyan, A. A Certain Role of SOD/CAT Imbalance in Pathogenesis of Autism Spectrum Disorders. Free Radic. Biol. Med. 2018, 123, 85–95. [Google Scholar] [CrossRef]
- Wu, Y.H.; Hsieh, H.L. Roles of Heme Oxygenase-1 in Neuroinflammation and Brain Disorders. Antioxidants 2022, 11, 923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ba, X.; Boldogh, I. 8-Oxoguanine DNA Glycosylase 1: Beyond Repair of the Oxidatively Modified Base Lesions. Redox Biol. 2018, 14, 669–678. [Google Scholar] [CrossRef]
- Shpyleva, S.; Ivanovsky, S.; de Conti, A.; Melnyk, S.; Tryndyak, V.; Beland, F.A.; James, S.J.; Pogribny, I.P. Cerebellar Oxidative DNA Damage and Altered DNA Methylation in the BTBR T+tf/J Mouse Model of Autism and Similarities with Human Post Mortem Cerebellum. PLoS ONE 2014, 9, e113712. [Google Scholar] [CrossRef] [PubMed]
- Markkanen, E.; Meyer, U.; Dianov, G.L. DNA Damage and Repair in Schizophrenia and Autism: Implications for Cancer Comorbidity and Beyond. Int. J. Mol. Sci. 2016, 17, 856. [Google Scholar] [CrossRef] [PubMed]
- Gaita, L.; Manzi, B.; Sacco, R.; Lintas, C.; Altieri, L.; Lombardi, F.; Pawlowski, T.L.; Redman, M.; Craig, D.W.; Huentelman, M.J.; et al. Decreased Serum Arylesterase Activity in Autism Spectrum Disorders. Psychiatry Res. 2010, 180, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Paşca, S.P.; Nemeş, B.; Vlase, L.; Gagyi, C.E.; Dronca, E.; Miu, A.C.; Dronca, M. High Levels of Homocysteine and Low Serum Paraoxonase 1 Arylesterase Activity in Children with Autism. Life Sci. 2006, 78, 2244–2248. [Google Scholar] [CrossRef]
- D’Amelio, M.; Ricci, I.; Sacco, R.; Liu, X.; D’Agruma, L.; Muscarella, L.A.; Guarnieri, V.; Militerni, R.; Bravaccio, C.; Elia, M.; et al. Paraoxonase Gene Variants Are Associated with Autism in North America, but Not in Italy: Possible Regional Specificity in Gene-Environment Interactions. Mol. Psychiatry 2005, 10, 1006–1016. [Google Scholar] [CrossRef]
- Dufault, R.; Lukiw, W.J.; Crider, R.; Schnoll, R.; Wallinga, D.; Deth, R. A Macroepigenetic Approach to Identify Factors Responsible for the Autism Epidemic in the United States. Clin. Epigenet. 2012, 4, 6. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Gulesserian, T.; Seidl, R.; Hardmeier, R.; Cairns, N.; Lubec, G. Superoxide Dismutase SOD1, Encoded on Chromosome 21, but Not SOD2 Is Overexpressed in Brains of Patients with Down Syndrome. J. Investig. Med. 2001, 49, 41–46. [Google Scholar] [CrossRef]
- Shareef, A.A.; Kheder, A.H.; Albarzinji, N.; Karim, K.J.; Smail, S.W.; Mahmood, A.A.; Amin, K. Oxidative Markers and SOD Variant: Predictors of Autism Severity and Susceptibility. Future Sci. OA 2025, 11, 2483628. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, Y.; Wei, J.; Zhao, G.; Hao, W.; Lv, Z.; Chen, X.; Liu, Y.; Wei, F. Shorter Telomere Length in Children with Autism Spectrum Disorder Is Associated with Oxidative Stress. Front. Psychiatry 2023, 14, 1209638. [Google Scholar] [CrossRef]
- Vellingiri, B.; Venkatesan, D.; Iyer, M.; Mohan, G.; Krishnan, P.; Sai Krishna, K.; R, S.; Narayanasamy, A.; Gopalakrishnan, A.V.; Kumar, N.S.; et al. Concurrent Assessment of Oxidative Stress and MT-ATP6 Gene Profiling to Facilitate Diagnosis of Autism Spectrum Disorder (ASD) in Tamil Nadu Population. J. Mol. Neurosci. 2023, 73, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, J.; Zhang, J.; Li, K. Serum Levels of SOD and Risk of Autism Spectrum Disorder: A Case-Control Study. Int. J. Dev. Neurosci. 2016, 51, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Ahmad, S.F.; Attia, S.M.; Al-Ayadhi, L.Y.; Al-Harbi, N.O.; Bakheet, S.A. Dysregulated Enzymatic Antioxidant Network in Peripheral Neutrophils and Monocytes in Children with Autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 88, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Afrazeh, M.; Saedisar, S.; Khakzad, M.R.; Hojati, M. Measurement of Serum Superoxide Dismutase and Its Relevance to Disease Intensity Autistic Children. Maedica 2015, 10, 315–318. [Google Scholar]
- Yui, K.; Tanuma, N.; Yamada, H.; Kawasaki, Y. Reduced Endogenous Urinary Total Antioxidant Power and Its Relation of Plasma Antioxidant Activity of Superoxide Dismutase in Individuals with Autism Spectrum Disorder. Int. J. Dev. Neurosci. 2017, 60, 70–77. [Google Scholar] [CrossRef]
- Salari, Z.; Moslemizadeh, A.; Tezerji, S.S.; Sabet, N.; Parizi, A.S.; Khaksari, M.; Sheibani, V.; Jafari, E.; Shafieipour, S.; Bashiri, H. Sex-Dependent Alterations of Inflammatory Factors, Oxidative Stress, and Histopathology of the Brain-Gut Axis in a VPA-Induced Autistic-like Model of Rats. Birth Defects Res. 2024, 116, e2310. [Google Scholar] [CrossRef]
- Yorbik, O.; Sayal, A.; Akay, C.; Akbiyik, D.I.; Sohmen, T. Investigation of Antioxidant Enzymes in Children with Autistic Disorder. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 341–343. [Google Scholar] [CrossRef]
- El-Ansary, A.; Bjørklund, G.; Chirumbolo, S.; Alnakhli, O.M. Predictive Value of Selected Biomarkers Related to Metabolism and Oxidative Stress in Children with Autism Spectrum Disorder. Metab. Brain Dis. 2017, 32, 1209–1221. [Google Scholar] [CrossRef]
- Bjørklund, G.; Zou, L.; Peana, M.; Chasapis, C.T.; Hangan, T.; Lu, J.; Maes, M. The Role of the Thioredoxin System in Brain Diseases. Antioxidants 2022, 11, 2161. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef]
- Ornoy, A.; Reece, E.A.; Pavlinkova, G.; Kappen, C.; Miller, R.K. Effect of Maternal Diabetes on the Embryo, Fetus, and Children: Congenital Anomalies, Genetic and Epigenetic Changes and Developmental Outcomes. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Hollis, F.; Kanellopoulos, A.K.; Bagni, C. Mitochondrial Dysfunction in Autism Spectrum Disorder: Clinical Features and Perspectives. Curr. Opin. Neurobiol. 2017, 45, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Al-Kafaji, G.; Jahrami, H.A.; Alwehaidah, M.S.; Alshammari, Y.; Husni, M. Mitochondrial DNA Copy Number in Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2023, 14, 1196035. [Google Scholar] [CrossRef] [PubMed]
- Kovacheva, E.; Gevezova, M.; Mehterov, N.; Kazakova, M.; Sarafian, V. The Intersection of Mitophagy and Autism Spectrum Disorder: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2217. [Google Scholar] [CrossRef]
- Citrigno, L.; Muglia, M.; Qualtieri, A.; Spadafora, P.; Cavalcanti, F.; Pioggia, G.; Cerasa, A. The Mitochondrial Dysfunction Hypothesis in Autism Spectrum Disorders: Current Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 5785. [Google Scholar] [CrossRef]
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Frye, R.E.; Rossignol, D.A.; Scahill, L.; McDougle, C.J.; Huberman, H.; Quadros, E.V. Treatment of Folate Metabolism Abnormalities in Autism Spectrum Disorder. Semin. Pediatr. Neurol. 2020, 35, 100835. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The Epigenetic Effects of Butyrate: Potential Therapeutic Implications for Clinical Practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef]
- Thomas, R.H.; Meeking, M.M.; Mepham, J.R.; Tichenoff, L.; Possmayer, F.; Liu, S.; MacFabe, D.F. The Enteric Bacterial Metabolite Propionic Acid Alters Brain and Plasma Phospholipid Molecular Species: Further Development of a Rodent Model of Autism Spectrum Disorders. J. Neuroinflam. 2012, 9, 153. [Google Scholar] [CrossRef]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood-Brain Barrier and Intestinal Epithelial Barrier Alterations in Autism Spectrum Disorders. Mol. Autism 2016, 7, 49. [Google Scholar] [CrossRef]
- Richardson, L.A. Evolving as a Holobiont. PLoS Biol. 2017, 15, e2002168. [Google Scholar] [CrossRef] [PubMed]
- Modafferi, S.; Lupo, G.; Tomasello, M.; Rampulla, F.; Ontario, M.; Scuto, M.; Salinaro, A.T.; Arcidiacono, A.; Anfuso, C.D.; Legmouz, M.; et al. Antioxidants, Hormetic Nutrition, and Autism. Curr. Neuropharmacol. 2024, 22, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Sterling, K.G.; Dodd, G.K.; Alhamdi, S.; Asimenios, P.G.; Dagda, R.K.; De Meirleir, K.L.; Hudig, D.; Lombardi, V.C. Mucosal Immunity and the Gut-Microbiota-Brain-Axis in Neuroimmune Disease. Int. J. Mol. Sci. 2022, 23, 13328. [Google Scholar] [CrossRef] [PubMed]
- Pridmore, R.D.; Pittet, A.-C.; Praplan, F.; Cavadini, C. Hydrogen Peroxide Production by Lactobacillus Johnsonii NCC 533 and Its Role in Anti-Salmonella Activity. FEMS Microbiol. Lett. 2008, 283, 210–215. [Google Scholar] [CrossRef]
- Johnson, S.L.; Kirk, R.D.; DaSilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol Microbial Metabolites Exhibit Gut and Blood−Brain Barrier Permeability and Protect Murine Microglia against LPS-Induced Inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef]
- Vauzour, D. Dietary Polyphenols as Modulators of Brain Functions: Biological Actions and Molecular Mechanisms Underpinning Their Beneficial Effects. Oxidative Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The Neuropharmacology of Butyrate: The Bread and Butter of the Microbiota-Gut-Brain Axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Halt, A.R.; Stary, J.M.; Kanodia, R.; Schulz, S.C.; Realmuto, G.R. Glutamic Acid Decarboxylase 65 and 67 KDa Proteins Are Reduced in Autistic Parietal and Cerebellar Cortices. Biol. Psychiatry 2002, 52, 805–810. [Google Scholar] [CrossRef]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D.G. Glutamate and GABA in Autism Spectrum Disorder—A Translational Magnetic Resonance Spectroscopy Study in Man and Rodent Models. Transl. Psychiatry 2018, 8, 106. [Google Scholar] [CrossRef]
- Carpita, B.; Nardi, B.; Palego, L.; Cremone, I.M.; Massimetti, G.; Carmassi, C.; Betti, L.; Giannaccini, G.; Dell’Osso, L. Kynurenine Pathway and Autism Spectrum Phenotypes: An Investigation among Adults with Autism Spectrum Disorder and Their First-Degree Relatives. CNS Spectr. 2023, 28, 374–385. [Google Scholar] [CrossRef]
- Almulla, A.F.; Thipakorn, Y.; Zhou, B.; Vojdani, A.; Paunova, R.; Maes, M. The Tryptophan Catabolite or Kynurenine Pathway in Long COVID Disease: A Systematic Review and meta-Analysis. Neuroscience 2024, 563, 268–277. [Google Scholar] [CrossRef]
- Yip, J.; Soghomonian, J.-J.; Blatt, G.J. Decreased GAD67 MRNA Levels in Cerebellar Purkinje Cells in Autism: Pathophysiological Implications. Acta Neuropathol. 2007, 113, 559–568. [Google Scholar] [CrossRef]
- Jin, F.; Wang, Z. Mapping the Structure of Biomarkers in Autism Spectrum Disorder: A Review of the Most Influential Studies. Front. Neurosci. 2024, 18, 1514678. [Google Scholar] [CrossRef] [PubMed]
- Anashkina, A.A.; Erlykina, E.I. Molecular Mechanisms of Aberrant Neuroplasticity in Autism Spectrum Disorders (Review). Sovrem. Tekhnologii Meditsine 2021, 13, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; McAllister, A.K. Maternal Immune Activation: Implications for Neuropsychiatric Disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Nadeem, A.; Ahmad, S.F.; Al-Ayadhi, L.Y.; Al-Harbi, M.M.; As Sobeai, H.M.; Ibrahim, K.E.; Bakheet, S.A. Elevated Expression of Toll-like Receptor 4 Is Associated with NADPH Oxidase-Induced Oxidative Stress in B Cells of Children with Autism. Int. Immunopharmacol. 2020, 84, 106555. [Google Scholar] [CrossRef]
- Abdel-Haq, M.; Ojha, S.K.; Hamoudi, W.; Kumar, A.; Tripathi, M.K.; Khaliulin, I.; Domb, A.J.; Amal, H. Effects of Extended-Release 7-Nitroindazole Gel Formulation Treatment on the Behavior of Shank3 Mouse Model of Autism. Nitric Oxide 2023, 140–141, 41–49. [Google Scholar] [CrossRef]
- Hughes, H.K.; Moreno, R.J.; Ashwood, P. Innate Immune Dysfunction and Neuroinflammation in Autism Spectrum Disorder (ASD). Brain Behav. Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, J.; Chen, W.; Li, W.; Chen, Z. Vascular Macrophages in Atherosclerosis. J. Immunol. Res. 2019, 2019, 4354786. [Google Scholar] [CrossRef]
- Martínez de Toda, I.; Ceprián, N.; Díaz-Del Cerro, E.; De la Fuente, M. The Role of Immune Cells in Oxi-Inflamm-Aging. Cells 2021, 10, 2974. [Google Scholar] [CrossRef]
- Meyer, U. Prenatal Poly(I:C) Exposure and Other Developmental Immune Activation Models in Rodent Systems. Biol. Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The Maternal Interleukin-17a Pathway in Mice Promotes Autism-like Phenotypes in Offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tan, L.; Zhang, X. Prenatal Exposure to Valproic Acid may Alter CD200/CD200R Signaling Pathways in a Rat Model of Autism Spectrum Disorder. Alpha Psychiatry 2025, 26, 39444. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G. Myeloperoxidase—A Bridge Linking Inflammation and Oxidative Stress with Cardiovascular Disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF Biology, Pathogenic Mechanisms and Emerging Therapeutic Strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Pivtoraiko, V.N.; Stone, S.L.; Roth, K.A.; Shacka, J.J. Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death. Antioxid. Redox Signal. 2009, 11, 481–496. [Google Scholar] [CrossRef]
- Finkbeiner, S. The Autophagy Lysosomal Pathway and Neurodegeneration. Cold Spring Harb. Perspect. Biol. 2020, 12, a033993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Zhang, J.-X.; Zhang, Q.-L. PI3K/AKT/MTOR-Mediated Autophagy in the Development of Autism Spectrum Disorder. Brain Res. Bull. 2016, 125, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Chaudry, S.; Vasudevan, N. mTOR-Dependent Spine Dynamics in Autism. Front. Mol. Neurosci. 2022, 15, 877609. [Google Scholar] [CrossRef] [PubMed]
- Switon, K.; Kotulska, K.; Janusz-Kaminska, A.; Zmorzynska, J.; Jaworski, J. Molecular neurobiology of mTOR. Neuroscience 2017, 341, 112–153. [Google Scholar] [CrossRef]
- Tang, G.; Gudsnuk, K.; Kuo, S.H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef]
- Zapata-Muñoz, J.; Villarejo-Zori, B.; Largo-Barrientos, P.; Boya, P. Towards a better understanding of the neuro-developmental role of autophagy in sickness and in health. Cell Stress 2021, 5, 99–118. [Google Scholar] [CrossRef]
- Ganesan, H.; Balasubramanian, V.; Iyer, M.; Venugopal, A.; Subramaniam, M.D.; Cho, S.G.; Vellingiri, B. mTOR signalling pathway—A root cause for idiopathic autism? BMB Rep. 2019, 52, 424–433. [Google Scholar] [CrossRef]
- Berger, J.; Dorninger, F.; Forss-Petter, S.; Kunze, M. Peroxisomes in brain development and function. Biochim. Biophys. Acta 2016, 1863, 934–955. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Altun, H.; Şahin, N.; Kurutaş, E.B.; Karaaslan, U.; Sevgen, F.H.; Fındıklı, E. Assessment of malondialdehyde levels, superoxide dismutase, and catalase activity in children with autism spectrum disorders. Psychiatry Clin. Psychopharmacol. 2018, 28, 408–415. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, N.; Choubey, V.; Dhingra, N.; Kaur, T. Endoplasmic Reticulum Stress and Its Role in Various Neurodegenerative Diseases. Brain Res. 2024, 1826, 148742. [Google Scholar] [CrossRef]
- Wilson, C.; González-Billault, C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: Implications for neuronal development and trafficking. Front. Cell. Neurosci. 2015, 9, 381. [Google Scholar] [CrossRef]
- Livanos, P.; Galatis, B.; Apostolakos, P. The interplay between ROS and tubulin cytoskeleton in plants. Plant Signal. Behav. 2014, 9, e28069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, C.K.; Nakamura, T.; Zhang, X.; Lipton, S.A. Redox regulation, protein S-nitrosylation, and synapse loss in Alzheimer’s and related dementias. Neuron 2024, 112, 3823–3850. [Google Scholar] [CrossRef] [PubMed]
- Landino, L.M.; Hasan, R.; McGaw, A.; Cooley, S.; Smith, A.W.; Masselam, K.; Kim, G. Peroxynitrite oxidation of tubulin sulfhydryls inhibits microtubule polymerization. Arch. Biochem. Biophys. 2002, 398, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Man, H.Y. Fundamental Elements in Autism: From Neurogenesis and Neurite Growth to Synaptic Plasticity. Front. Cell. Neurosci. 2017, 11, 359. [Google Scholar] [CrossRef]
- Oswald, M.C.; Brooks, P.S.; Zwart, M.F.; Mukherjee, A.; West, R.J.; Giachello, C.N.; Morarach, K.; Baines, R.A.; Sweeney, S.T.; Landgraf, M. Reactive oxygen species regulate activity-dependent neuronal plasticity in Drosophila. eLife 2018, 7, e39393. [Google Scholar] [CrossRef]
- Sanders, S.J.; Ercan-Sencicek, A.G.; Hus, V.; Luo, R.; Murtha, M.T.; Moreno-De-Luca, D.; Chu, S.H.; Moreau, M.P.; Gupta, A.R.; Thomson, S.A.; et al. Multiple Recurrent de Novo CNVs, Including Duplications of the 7q11.23 Williams Syndrome Region, Are Strongly Associated with Autism. Neuron 2011, 70, 863–885. [Google Scholar] [CrossRef]
- Tang, G.; Gutierrez Rios, P.; Kuo, S.-H.; Akman, H.O.; Rosoklija, G.; Tanji, K.; Dwork, A.; Schon, E.A.; Dimauro, S.; Goldman, J.; et al. Mitochondrial Abnormalities in Temporal Lobe of Autistic Brain. Neurobiol. Dis. 2013, 54, 349–361. [Google Scholar] [CrossRef]
- Napoli, E.; Wong, S.; Giulivi, C. Evidence of Reactive Oxygen Species-Mediated Damage to Mitochondrial DNA in Children with Typical Autism. Mol. Autism 2013, 4, 2. [Google Scholar] [CrossRef]
- Landrigan, P.J. What Causes Autism? Exploring the Environmental Contribution. Curr. Opin. Pediatr. 2010, 22, 219–225. [Google Scholar] [CrossRef]
- Oudin, A.; Frondelius, K.; Haglund, N.; Källén, K.; Forsberg, B.; Gustafsson, P.; Malmqvist, E. Prenatal Exposure to Air Pollution as a Potential Risk Factor for Autism and ADHD. Environ. Int. 2019, 133, 105149. [Google Scholar] [CrossRef]
- Lyall, K.; Schmidt, R.J.; Hertz-Picciotto, I. Maternal Lifestyle and Environmental Risk Factors for Autism Spectrum Disorders. Int. J. Epidemiol. 2014, 43, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Cocchia, N.; Ciani, F.; Tafuri, S.; Iorio, E.L.; Landolfi, F. Redoxomics and Oxidative Stress: From the Basic Research to the Clinical Practice. In Free Radicals and Diseases; Ahmad, R., Ed.; IntechOpen: Rijeka, Croatia, 2016; ISBN 978-953-51-2747-5. [Google Scholar]
- Lei, X.; Xie, X.N.; Yang, J.X.; Li, Y.M. The emerging role of extracellular vesicles in the diagnosis and treatment of autism spectrum disorders. Psychiatry Res. 2024, 337, 115954. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.C.; Costa, A.R.; Gonçalves, I.; Quintela, T.; Preissner, R.; Santos, C.R.A. The druggability of bitter taste receptors for the treatment of neurodegenerative disorders. Biochem. Pharmacol. 2022, 197, 114915. [Google Scholar] [CrossRef] [PubMed]
- Dobrachinski, F.; Ribeiro, K.A.; Bezerra, I.C.; da Silva, A.J.; Pereira, C.M.M.; Vellasques, K.; Padilha, H.A.; Haas, S.E.; Ávila, D.S.; Gubert, P. Nutraceutical approaches for Autism Spectrum Disorder treatment. Behav. Brain Res. 2025, 492, 115653. [Google Scholar] [CrossRef]
- Hamilton, C.; Liebert, A.; Pang, V.; Magistretti, P.; Mitrofanis, J. Lights on for Autism: Exploring Photobiomodulation as an Effective Therapeutic Option. Neurol. Int. 2022, 14, 884–893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
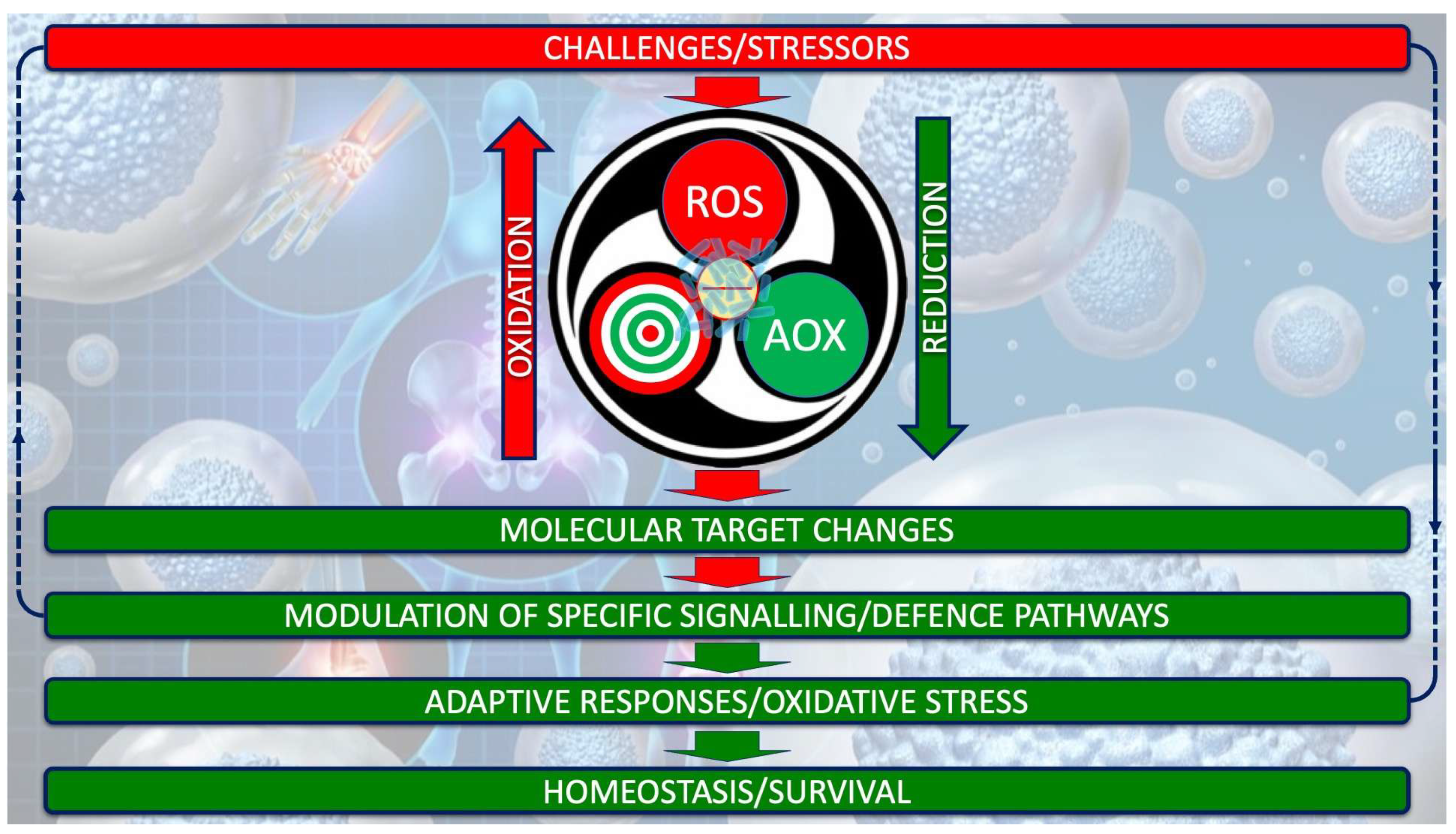
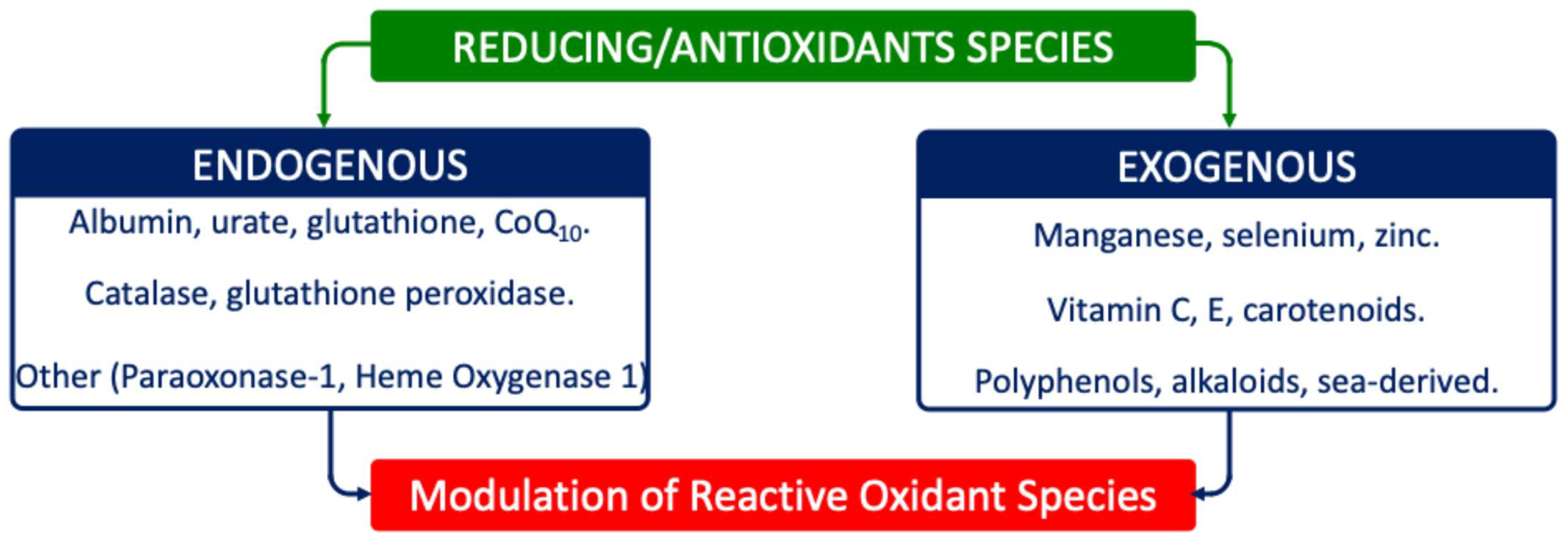
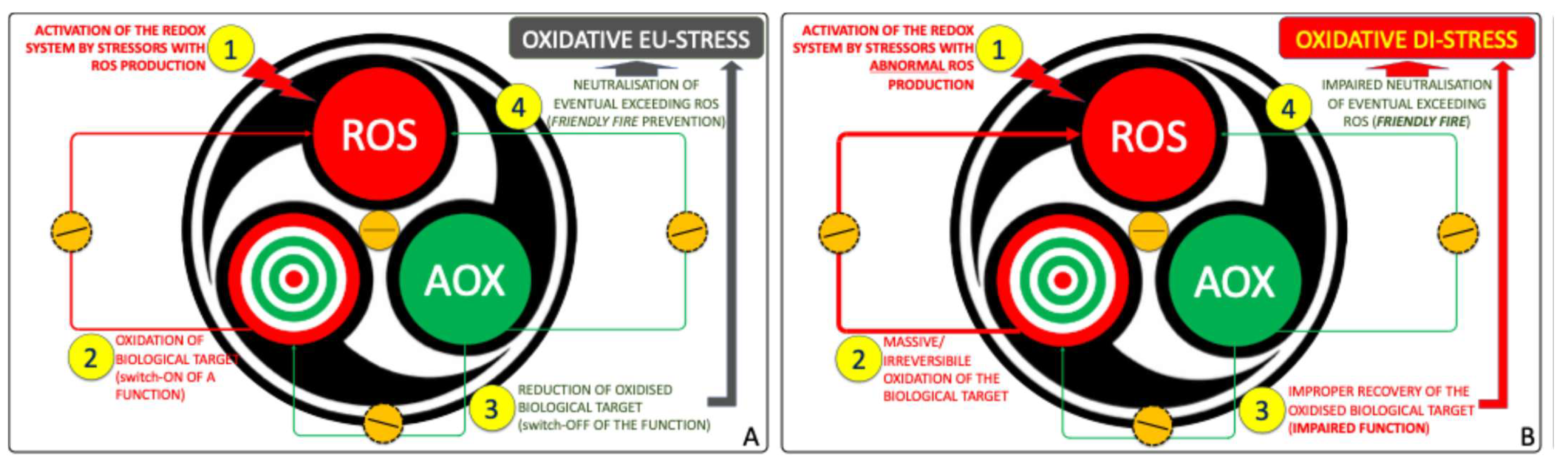

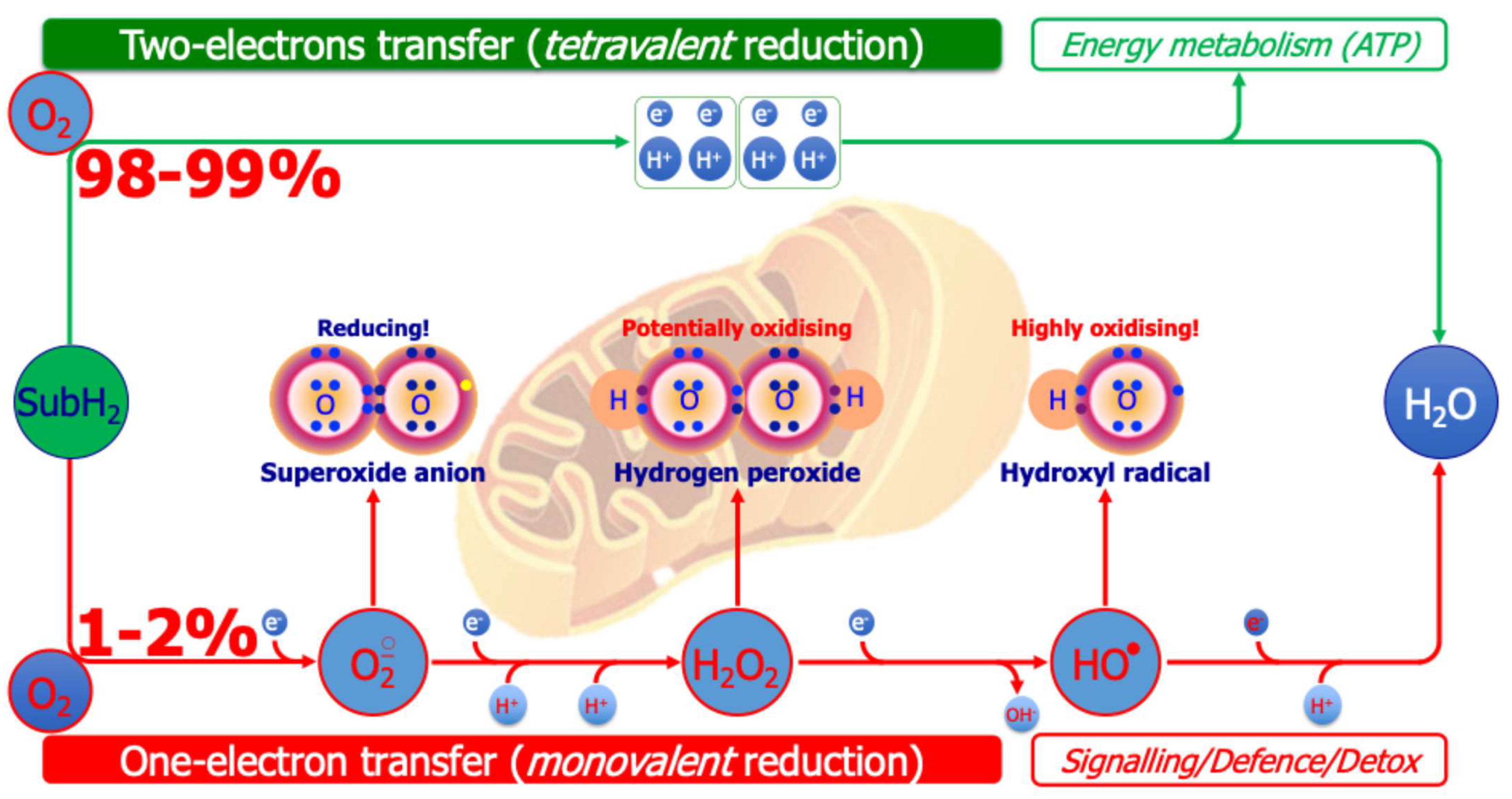
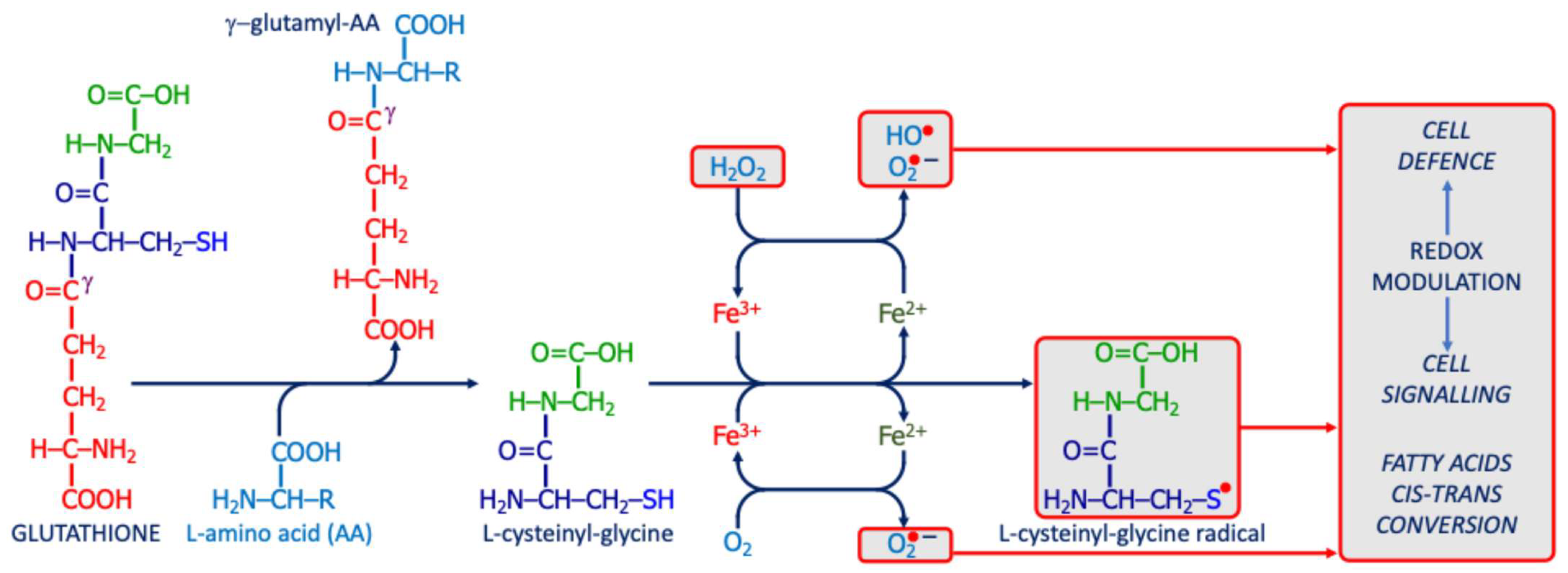

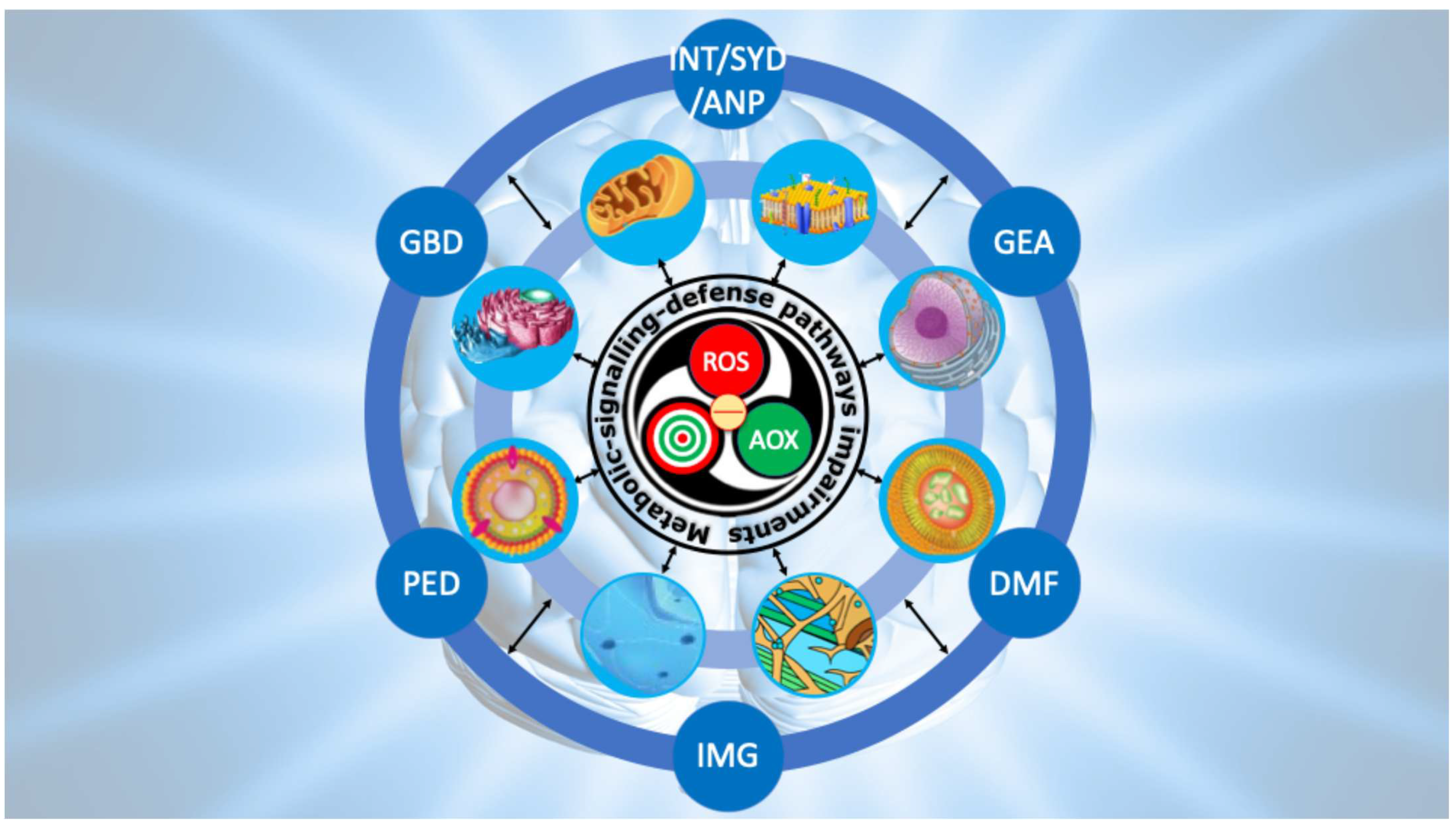
| Chemical Species | Formula | Class | Chemical Species | Formula | Class |
|---|---|---|---|---|---|
| Singlet oxygen | 1O2 | Non radical | Nitric oxide | NO• | Radical |
| Superoxide anion (Reducing species) | O2•− | Radical | Nitrous acid | HNO2 | Non radical |
| Ozone | O3 | Non radical | Nitric tetroxide | N2O4 | Non radical |
| Hydroxyl radical | HO• | Radical | Nitric trioxide | N2O3 | Non radical |
| Hydrogen peroxide | H2O2 | Non radical | Peroxynitrite | ONOO− | Non radical |
| Alkyl radical | R• | Radical | Peroxynitrous acid | ONOOH | Non radical |
| (Alkyl-)peroxyl radical | ROO• | Radical | Nitronium cation | NO2+ | Non radical |
| (Alkyl)hydroperoxide | ROOH | Non radical | (Alkyl)peroxynitrite | ROONO− | Non radical |
| Semiquinone (from CoQ10) | Q• | Radical | Hypochlorous acid | HClO | Non radical |
| Tocopheryl (from vit-E) | E-O• | Radical | Thiyl | -S• | Radical |
| Reactive Oxygen Species | Main Sources of Production | Mean Migration Distance | T1/2 | Main Molecular Targets | Scavenging Systems |
|---|---|---|---|---|---|
Singlet oxygen | Plasmamembrane Mitochondria | 30 nm | 1–4 μs | PUFA double bonds Protein coupled thiol | Carotenoids Tocopherols |
Superoxide anion | Plasmamembrane Mitochondria | 30 nm | 1–4 μs | Iron-Sulphur clusters | Superoxide dismutases |
Hydroxyl radical | Membranes Mitochondria Cytosol | 1 nm | 1 μs | Any organic molecule (rapidly reacting with DNA) | Flavonoids |
Hydrogen peroxide | Plasmamembrane Mitochondria Peroxisomes | 1 μm | 1 ms | Preferably cysteine’s moieties | Catalase Glutathione peroxidases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, C.A.; Iorio, E.L.; Espíndola, F.S. Redox System Dysfunction as a Key Mechanism in Autism Spectrum Disorder Pathogenesis. Int. J. Mol. Sci. 2025, 26, 9850. https://doi.org/10.3390/ijms26209850
de Oliveira CA, Iorio EL, Espíndola FS. Redox System Dysfunction as a Key Mechanism in Autism Spectrum Disorder Pathogenesis. International Journal of Molecular Sciences. 2025; 26(20):9850. https://doi.org/10.3390/ijms26209850
Chicago/Turabian Stylede Oliveira, Clarissa Aires, Eugenio Luigi Iorio, and Foued Salmen Espíndola. 2025. "Redox System Dysfunction as a Key Mechanism in Autism Spectrum Disorder Pathogenesis" International Journal of Molecular Sciences 26, no. 20: 9850. https://doi.org/10.3390/ijms26209850
APA Stylede Oliveira, C. A., Iorio, E. L., & Espíndola, F. S. (2025). Redox System Dysfunction as a Key Mechanism in Autism Spectrum Disorder Pathogenesis. International Journal of Molecular Sciences, 26(20), 9850. https://doi.org/10.3390/ijms26209850








