Synthesis, Characterization, and Evaluation of Folic Acid Release Ability of Acrylamide–Acrylic Acid Hydrogels and Acrylamide–Acrylic Acid/Functionalized Carbon Nanotube Nanocomposite Hydrogels
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis, Purification, Partial Oxidization, and Chemical Functionalization of CNTs
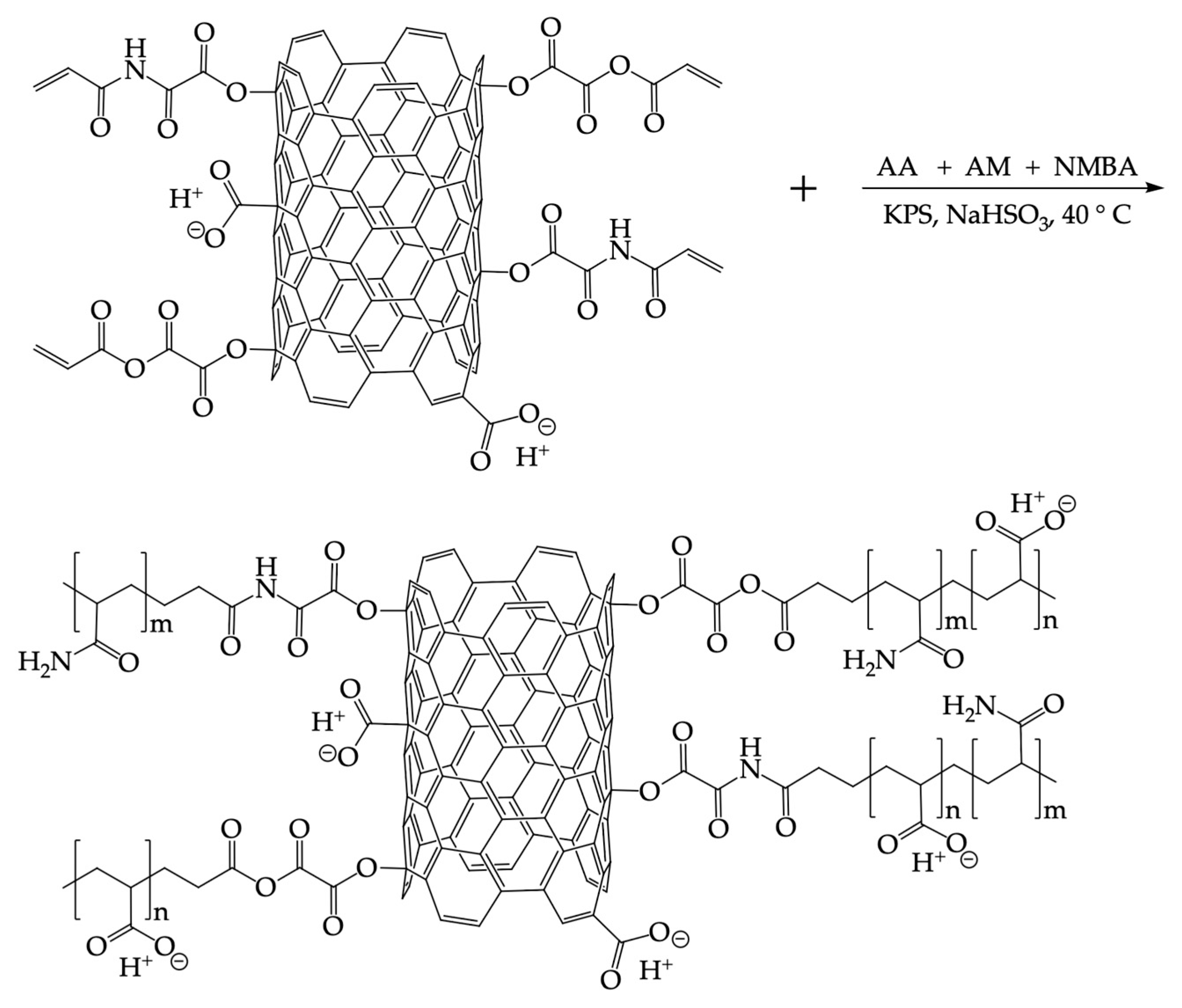
3.3. Synthesis and Purification of the AM–AA Hydrogels and AM–AA/CNTsOxCl Nanocomposite Hydrogels
3.4. Characterization of CNTs, AM–AA Hydrogels, and AM–AA/CNTsOxCl Nanocomposite Hydrogels
3.4.1. Transmission Electron Microscopy (TEM)
3.4.2. Raman Spectroscopy
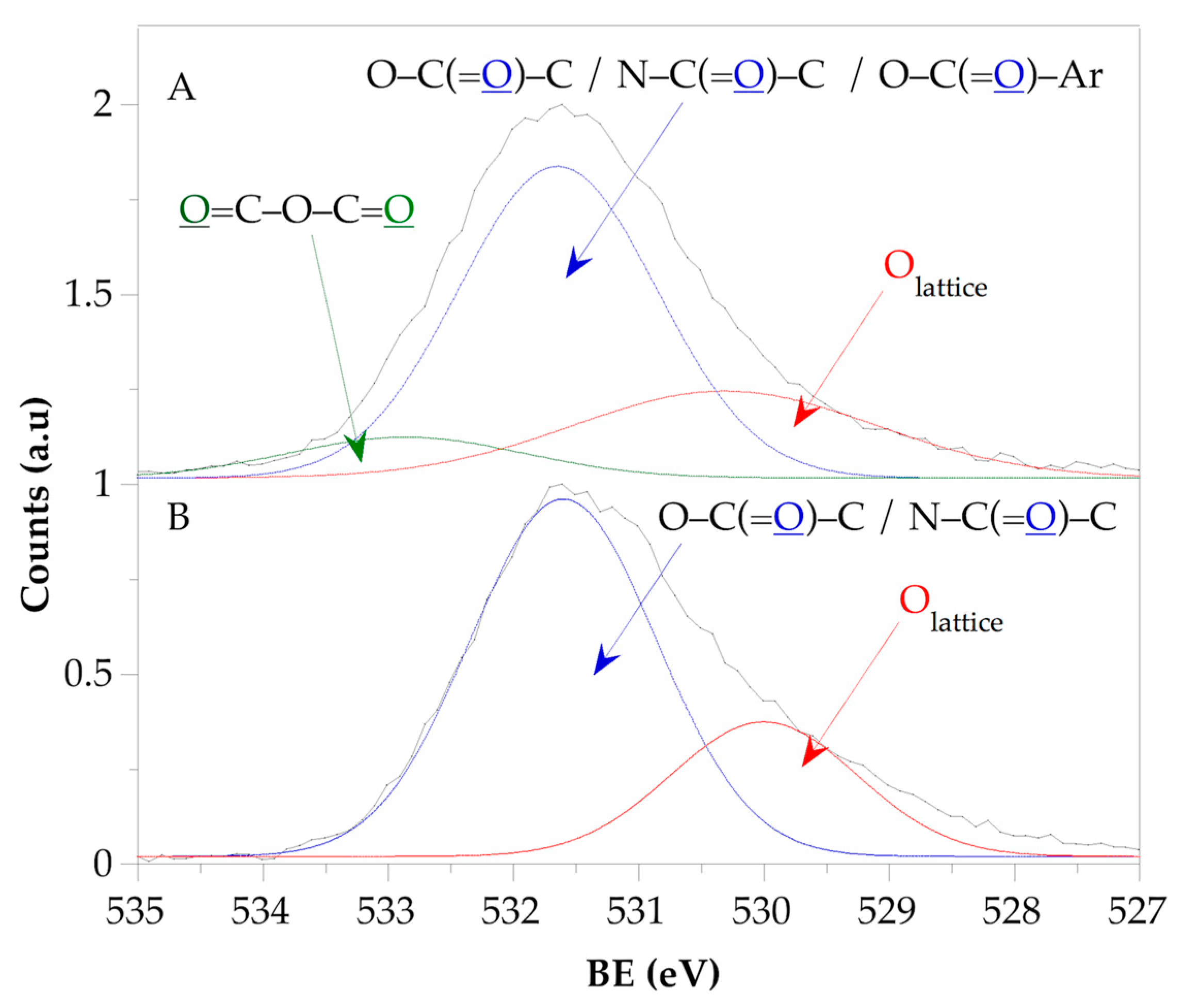
3.4.3. X-Ray Photoelectron Spectroscopy (XPS)
3.4.4. Field-Emission Scanning Electron Microscopy (FE–SEM)
3.4.5. Swelling Tests
3.4.6. Mechanical Tests
3.4.7. UV–Vis Spectroscopy
3.4.8. Differential Scanning Calorimetry (DSC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Acrylamide |
| AA | Acrylic acid |
| CNTs | Carbon nanotubes |
| CVD | Chemical vapor deposition |
| TEM | Transmission electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| FE-SEM | Field-emission scanning electron microscopy |
| SWCNTs | Single–walled carbon nanotubes |
| MWCNTs | Multi–walled carbon nanotubes |
| DDS | Drug delivery systems |
| UCST | Upper critical solution temperature |
| PPy | Polypyrrole |
| 5-FU | 5–fluorouracil |
| NIR | Near–infrared |
| PAAm | Polyacrylamide |
| PAAc | Polyacrylic acid |
| IPN | Interpenetrating network |
References
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive Lbl capsules and nanoshells for drug delivery. Adv. Drug Del. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Taylor, M.J.; Tomlins, P.; Sahota, T.S. Themoresposive Gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef]
- Alzari, V.; Ruiu, A.; Nuvoli, D.; Sanna, R.; Martinez, J.I.; Appelhans, D.; Voit, B.; Zschoche, S.; Mariani, A. Three component terpolymer and IPN hydrogels with response to stimuli. Polymer 2014, 55, 5305–5313. [Google Scholar] [CrossRef]
- Marcelo, G.; Areias, L.R.P.; Viciosa, M.T.; Martinho, J.M.G.; Farinha, J.P.S. PNIPAm-based microgels with a UCST response. Polymer 2017, 116, 261–267. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Zhou, X.; Weng, L.; Chen, Q.; Zhang, J.; Shen, D.; Li, Z.; Shao, M.; Xu, J. Investigation of pH sensitivity of poly(acrylic acid-co-acrylamide) hydrogel. Polym. Int. 2003, 52, 1153–1157. [Google Scholar] [CrossRef]
- Ding, C.; Guo, Z.; Xiong, J.; Wu, D.; Tao, Y.; Qin, Y.; Kong, Y. Rational design of a multi-responsive drug delivery platform based on SiO2@PPy@poly(acrylic acid-co-acrylamide). React. Funct. Polym. 2019, 137, 88–95. [Google Scholar] [CrossRef]
- Ray, D.; Mohapatra, D.K.; Mohapatra, R.K.; Mohanta, G.P.; Sahoo, P.K. Synthesis and colon-specific drug delivery of a poly(acrylic-co-acrylamide)/MBA nanosized Hydrogel. J. Biomater. Sci. Polym. Ed. 2008, 19, 1487–1502. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, M.; Puttagiddappa, M.G.; Thippaiah, D.; Satyanarayan, D.N. Poly(acrylamide-co-acrylic acid) synthesized, moxifloxacin drug-loaded hydrogel: Characterization and evaluation studies. J. Appl. Pharm. Sci. 2021, 11, 074–081. [Google Scholar] [CrossRef]
- Chavda, H.V.; Patel, C.N. Effect of crosslinker concentration on characteristics of superporous hydrogel. Int. J. Pharm. Investig. 2011, 1, 17–21. [Google Scholar] [CrossRef]
- Syduzzaman, M.; Islam Saad, M.S.; Piam, M.F.; Talukdar, T.A.; Shobdo, T.T.; Pritha, N.M. Carbon nanotubes: Structure, properties and applications in the aerospace industry. Results Mater. 2025, 25, 100654. [Google Scholar] [CrossRef]
- Yu, M.-F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef]
- Yu, M.-F.; Files, B.S.; Arepalli, S.; Ruoff, R.S. Tensile loading of ropes of single wall carbon nanotubes and their mechanical properties. Phys. Rev. Lett. 2000, 84, 5552. [Google Scholar] [CrossRef] [PubMed]
- Bellingeri, R.; Mulko, L.; Molina, M.; Picco, N.; Alustiza, F.; Grosso, C.; Vivas, A.; Acevedo, D.F.; Barbero, C.A. Nanocomposites based on pH-sensitive hydrogels and chitosan decorated carbon nanotubes with antibacterial properties. Mat. Sci. Eng. C Mater. 2018, 90, 461–467. [Google Scholar] [CrossRef]
- Feng, H.; Zheng, T.; Wang, X.; Wang, H. Poly(acrylamide)-MWNTs hybrid hydrogel with extremely high mechanical strength. Open Chem. 2016, 14, 150–157. [Google Scholar] [CrossRef]
- Takada, T.; Morikawa, Y.; Kikuchi, Y.; Miyamoto, D.; Hayasaka, Y.; Abe, S. Mechanical and swelling properties of polyacrylamide/polyacrylic acid composite hydrogels: The effects of network structure and carbon nanotube reinforcement. Carbon Rep. 2024, 3, 29–36. [Google Scholar] [CrossRef]
- Kanca, Y.; Özdemir, R.; Dini, D.; Özkahraman, B. Multi-walled carbon nanotube reinforced polyacrylamide-acrylic acid nanocomposite hydrogels with superior mechanical and tribological performance as focal cartilage substitute. Polym. Eng. Sci. 2025, 65, 3988–4005. [Google Scholar] [CrossRef]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Rafahi, S.; Goushbolagh, N.A. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef]
- Fuster, M.G.; Wang, J.; Fandiño, O.; Víllora, G.; Paredes, A.J. Folic acid-decorated nanocrystals as highly loaded trojan horses to target cancer cells. Mol. Pharm. 2024, 21, 2781–2794. [Google Scholar] [CrossRef]
- Salvetat, J.-P.; Bonard, J.-M.; Thomson, N.H.; Kulik, A.J.; Forró, L.; Benoit, W.; Zuppiroli, L. Mechanical properties of carbon nanotubes. Appl. Phys. A 1999, 69, 255–260. [Google Scholar] [CrossRef]
- Torres-Ávalos, J.A.; Cajero-Zul, L.R.; Vázquez-Lepe, M.; López-Dellmary, F.A.; Martínez-Richa, A.; Barrera-Rivera, K.A.; López-Serrano, F.; Nuño-Donlucas, S.M. Synthesis of poly(methacrylic acid-co-butyl acrylate) grafted onto functionalized carbon nanotube nanocomposites for drug delivery. Polymers 2021, 13, 533. [Google Scholar] [CrossRef]
- Gómez-Vázquez, D.; Cajero-Zul, L.R.; Torres-Ávalos, J.A.; Sandoval-García, K.; Cortés-Ortega, J.A.; López-Dellamary, F.A.; Soltero-Martínez, J.F.A.; Martínez-Richa, A.; Nuño-Donlucas, S.M. Homogeneous hydrogels made with acrylic acid, acrylamide amd chemically functionalized carbon nanotubes. J. Macromol. Sci. Part A 2019, 56, 417–428. [Google Scholar] [CrossRef]
- Li, C.; Chou, T.-W. Elastic moduli of multi-walled carbon nanotubes and the effect of van der Waals forces. Compos. Sci. Technol. 2003, 63, 1517–1524. [Google Scholar] [CrossRef]
- Sivaganga, K.C.; Varughese, T. Physical properties of carbon nanotubes. In Handbook of Carbon Nanotubes, 1st ed.; Abraham, J., Thomas, S., Kalarikkal, N., Eds.; Springer: Cham, Switzerland, 2022; pp. 283–297. [Google Scholar]
- Xia, R.; Li, M.; Zhang, Y.; Qian, J.; Yuan, X. Surface modification of MWCNTs with BA-MMA-GMA terpolymer by single-step grafting technique. J. Appl. Polym. Sci. 2011, 119, 282–289. [Google Scholar] [CrossRef]
- Silva-Jara, J.M.; Manríquez-González, R.; López-Dellamary, F.A.; Puig, J.E.; Nuño-Donlucas, S.M. Semi-continuous heterophase polymerization to synthesize nanocomposites of poly(acrylic acid)-functionalized carbon nanotubes. J. Macromol. Sci. Part A 2015, 52, 732–744. [Google Scholar] [CrossRef]
- Thomsen, C.; Reich, S. Double resonant Raman scattering in graphite. Phys. Rev. Lett. 2000, 85, 5214. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.M.; Jorio, A.; Dresselhaus, M.S.; Dresselhaus, G. Observationsof the D-band feature in the Raman spectra of carbon nanotubes. Phys. Rev. B 2001, 64, 073403. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-Palen, E.; Kruszyńska, M.; Bachmatiuk, A.; Kaleńczuk, R.J. Characterization of carbon nanotubes by Raman spectroscopy. Mater. Sci.-Pol. 2008, 26, 433–441. [Google Scholar]
- Heise, H.M.; Kuckuk, R.; Ojha, A.K.; Srivastava, A.; Srivastava, V.; Asthana, B.P. Characterisation of carbonaceous materials using Raman spectroscopy: A comparison of carbon nanotube filters, single- and multi-walled nanotubes, graphitised porous carbon and graphite. J. Raman Spectrosc. 2009, 40, 344–353. [Google Scholar] [CrossRef]
- Fantini, C.; Usrey, M.L.; Strano, M.S. Investigation of electronic and vibrational properties of single-walled carbon nanotubes functionalized with diazonium salts. J. Phys. Chem. C 2007, 111, 17941–17946. [Google Scholar] [CrossRef]
- Cajero-Zul, L.R.; López-Dellmary, F.A.; Gómez-Salazar, S.; Vázquez-Lepe, M.; Vera-Graziano, R.; Torres-Vitela, M.R.; Olea-Rodríguez, M.A.; Nuño-Donlucas, S.M. Evaluation of the resistance to bacterial growth of star-shaped poly(e-caprolactone)-co-poly(ethylene glycol) grafted onto functionalized carbon nanotubes nanocomposites. J. Biomater. Sci. Polym. Ed. 2019, 30, 163–189. [Google Scholar] [CrossRef]
- Gupta, M.K.; Bansil, R. Laser Raman spectroscopy of polyacrylamide. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 353–360. [Google Scholar] [CrossRef]
- Amorim da Costa, A.M.; Amado, A.M. Cationic hydration in hydrogelic polyacrylamide-phosphoric acid network: A study by Raman spectroscopy. Solid State Ion. 2001, 145, 79–84. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, J.; Nakashima, K. Infrared, Raman, and near-infrared spectroscopic evidence for the coexistence of various hydrogen-bond forms in poly(acrylic acid). Macromolecules 1997, 30, 1111–1117. [Google Scholar] [CrossRef]
- Todica, M.; Stefan, R.; Pop, C.V.; Olar, L. IR and Raman investigation of some poly(acrylic) acid gels in aqueous and neutralized state. Acta Phys. Pol. A 2015, 128, 128–135. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Huang, B.; Wu, N.; Ouyang, S. Raman spectroscopy for the competition of hydrogen bonds in ternary (H2O-THF-DMSO) aqueous solutions. Molecules 2019, 24, 3666. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Gomez, A.; Bravo-Sanchez, M.; Ceballos-Sanchez, O.; Vazquez-Lepe, M.O. Practical methods for background substraction in photoemission spectra. Surf. Interface Anal. 2014, 46, 897–905. [Google Scholar] [CrossRef]
- Dietrich, P.M.; Henning, A.; Holzweber, M.; Thiele, T.; Borcherding, H.; Lippitz, A.; Schedler, U.; Resch-Genger, U.; Unger, W.E.S. Surface analytical study of poly(acrylic acid)-grafted microparticles (beads): Characterization, chemical derivatization, and quantification of surface carboxyl groups. J. Phys. Chem. C 2014, 118, 20393–20404. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database, 1st ed.; Wiley: Chichester, UK, 1992; pp. 110–111. [Google Scholar]
- Uchida, E.; Uyama, Y.; Iwata, H.; Ikada, Y. XPS analysis of the poly(ethylene terephthalate) film grafted with acrylamide. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 2837–2844. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mondal, M.H.; Mukherjee, M.; Doyle, B.P.; Nannarone, S. Onset kinetics of thermal degradation of ultrathin polyacrylamide films. Macromolecules 2009, 42, 7889–7896. [Google Scholar] [CrossRef]
- González-Iñiguez, K.J.; Figueroa-Ochoa, E.B.; Martínez-Richa, A.; Cajero-Zul, L.R.; Nuño-Donlucas, S.M. Synthesis of poly(L-lactide)-poly(ε-caprolactone)-poly(ethylene glycol) terpolymer grafted onto partially oxidixed carbon nanotube nanocomposites for drug delivery. Polymers 2024, 16, 2580. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, Y.; Nie, L.; Huang, Y. Crosslinked polymer coatings of poly(acrylic acid-co-acrylamide)/polyethyleneimine (P(AA-co-Aam)/PEI) on titanium alloy with excellent lubrication performance for artificial joints. Coatings 2024, 14, 28. [Google Scholar] [CrossRef]
- Khokhra, R.; Bharti, B.; Lee, H.-N.; Kumar, R. Visible and UV photo-detection in ZnO nanostructured thin films via simple tuning of solution method. Sci. Rep. 2017, 7, 15032. [Google Scholar] [CrossRef]
- Briggs, D.; Beamson, G. XPS studies of the oxygen 1s and 2s levels in a wide range of functional polymers. Anal. Chem. 1993, 65, 1517–1523. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, Y. Lattice oxygen activation for enhanced electrochemical oxygen evolution. J. Phys. Chem. C 2023, 127, 2147–2159. [Google Scholar] [CrossRef]
- Hepworth, S.J.; Leach, M.O.; Doran, S.J. Dynamics of polymerization in polyacrylamide gel (PAG) dosimeters: (II) modeling oxygen diffusion. Phys. Med. Biol. 1999, 44, 1875–1884. [Google Scholar] [CrossRef]
- Linert, W.; Lukovits, I. Aromaticity of carbon nanotubes. J. Chem. Inf. Model. 2007, 47, 887–890. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, W.; Jin, C. Protocol efficiently measuring the swelling rate of hydrogels. MethodsX 2020, 7, 100779. [Google Scholar] [CrossRef]
- Aydınoğlu, D. Investigation of pH-dependent swelling behavior and kinetic parameters of novel poly(acrylamide-co-acrylic acid) hydrogels with spirulina. e-Polymers 2015, 15, 81–93. [Google Scholar] [CrossRef]
- ImageJ, version 1.53; National Institutes of Health: Bethesda, MD, USA, 2024. Available online: https://imagej.net/ij/ (accessed on 12 July 2024).
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Langenbucher, F. Linearization of dissolution rate curves by the Weibull distribution. J. Pharm. Pharmacol. 1972, 24, 979–981. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modelling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Corsaro, C.; Neri, G.; Mezzasalma, A.M.; Fazio, E. Weibull modeling of controlled drug release from Ag-PMA nanosystems. Polymers 2021, 13, 2897. [Google Scholar] [CrossRef]
- Chattaraj, A.; Mishra, Y.; Aljabali, A.A.A.; Mishra, V. Development and evaluation of folic acid conjugated curcumin-loaded functionalized multiwalled carbon nanotubes for enhanced efficacy in ovarian cancer treatment. Carbon Trends 2025, 19, 100464. [Google Scholar] [CrossRef]
- Gangrade, A.; Mandal, B.B. Injectable carbon nanotube impregnated silk based multifunctional hydrogel for localized targeted and on-demand anticancer drug delivery. ACS Biomater. Sci. Eng. 2019, 5, 2365–2381. [Google Scholar] [CrossRef]
- Rúan-Esparza, L.; Soto, V.; Gómez-Salazar, S.; Rabelero, M.; Ávalos-Borja, M.; Luna-Bárcenas, G.; Prokhorov, E.; Nuño-Donlucas, S.M. Poly[ethylene-co-(acrylic acid)]-based nanocomposites: Thermal and mechanical properties and their structural characteristics studied by Raman spectroscopy. Polym. Compos. 2011, 32, 1181–1189. [Google Scholar] [CrossRef]
- Katime, I.; Velada, J.L.; Novoa, R.; Díaz de Apodaca, E.; Puig, J.; Mendizábal, E. Swelling kinetics of poly(acrylamide)/poly(mono-n-alkyl itaconates) hydrogels. Polym. Int. 1996, 40, 281–286. [Google Scholar] [CrossRef]
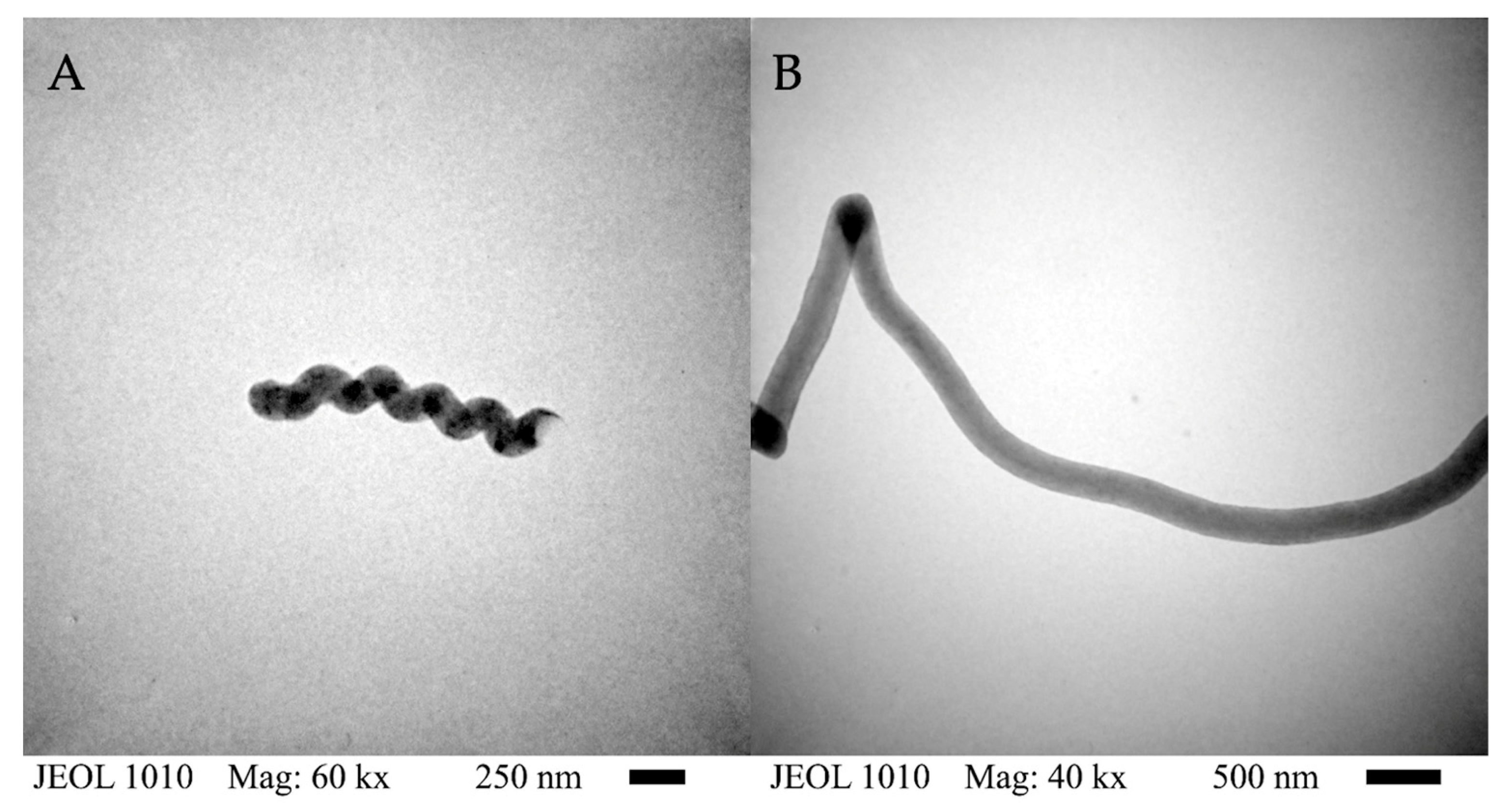

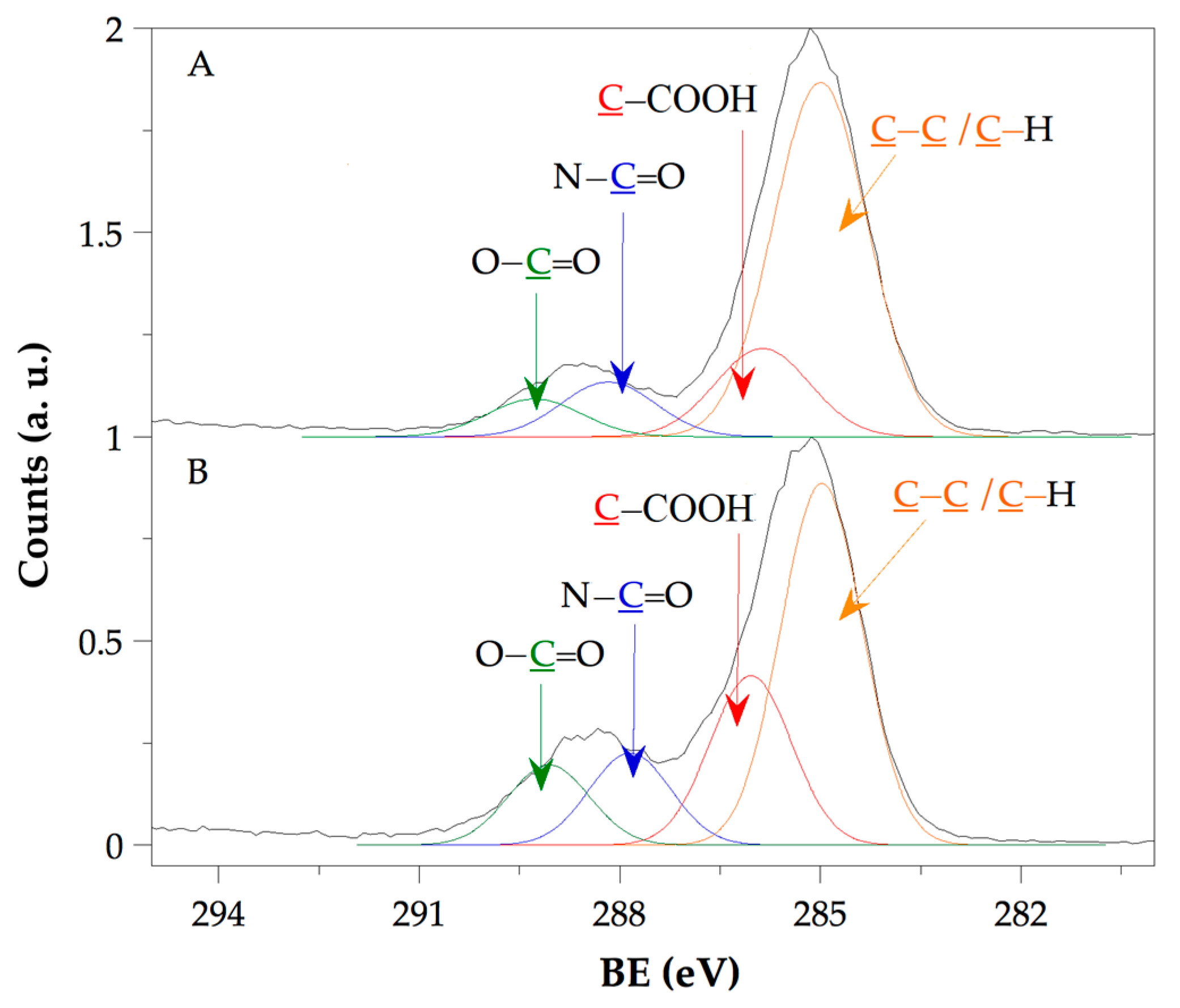
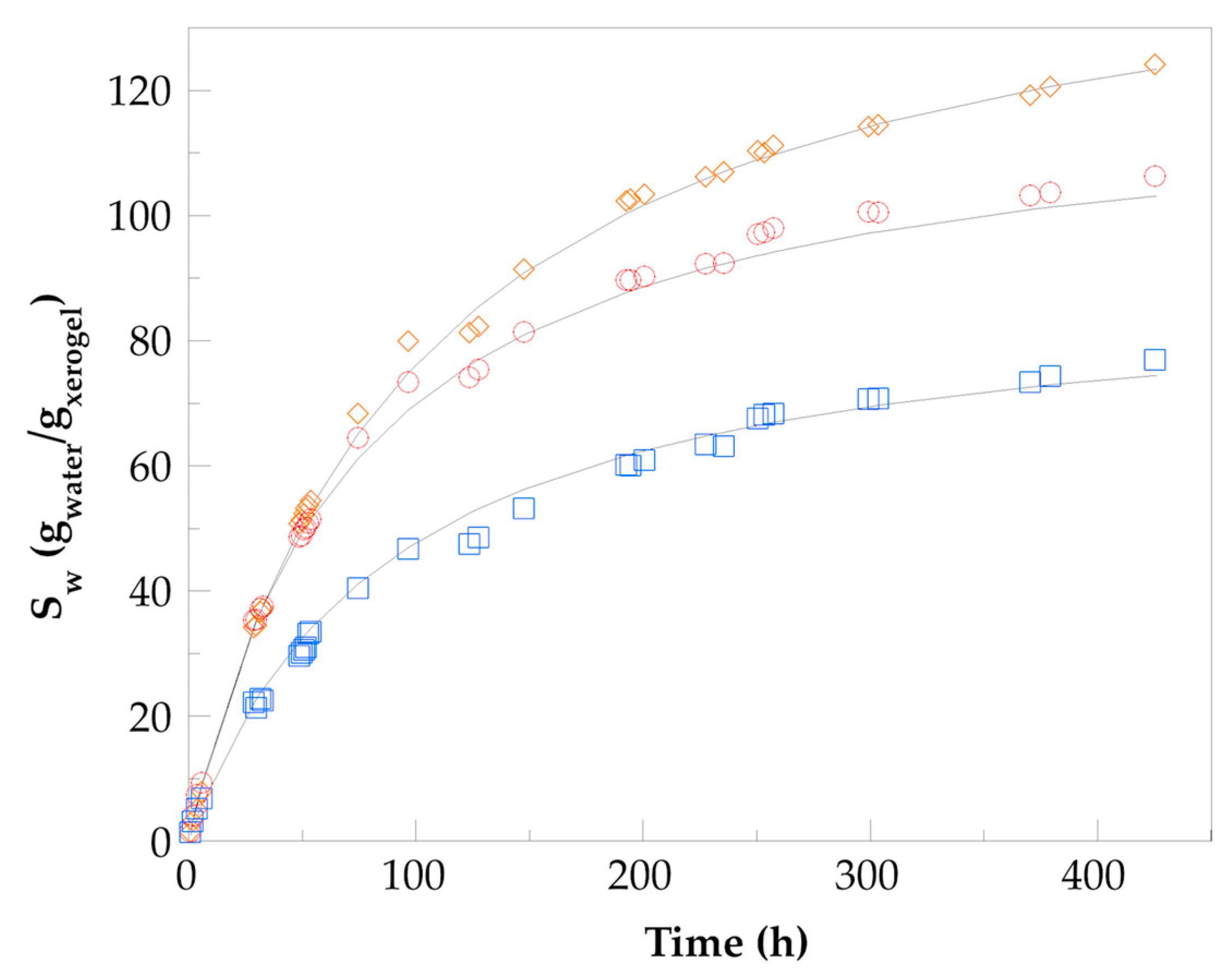
 ), Hydro 2 (
), Hydro 2 ( ), and Hydro 3 (
), and Hydro 3 ( ).
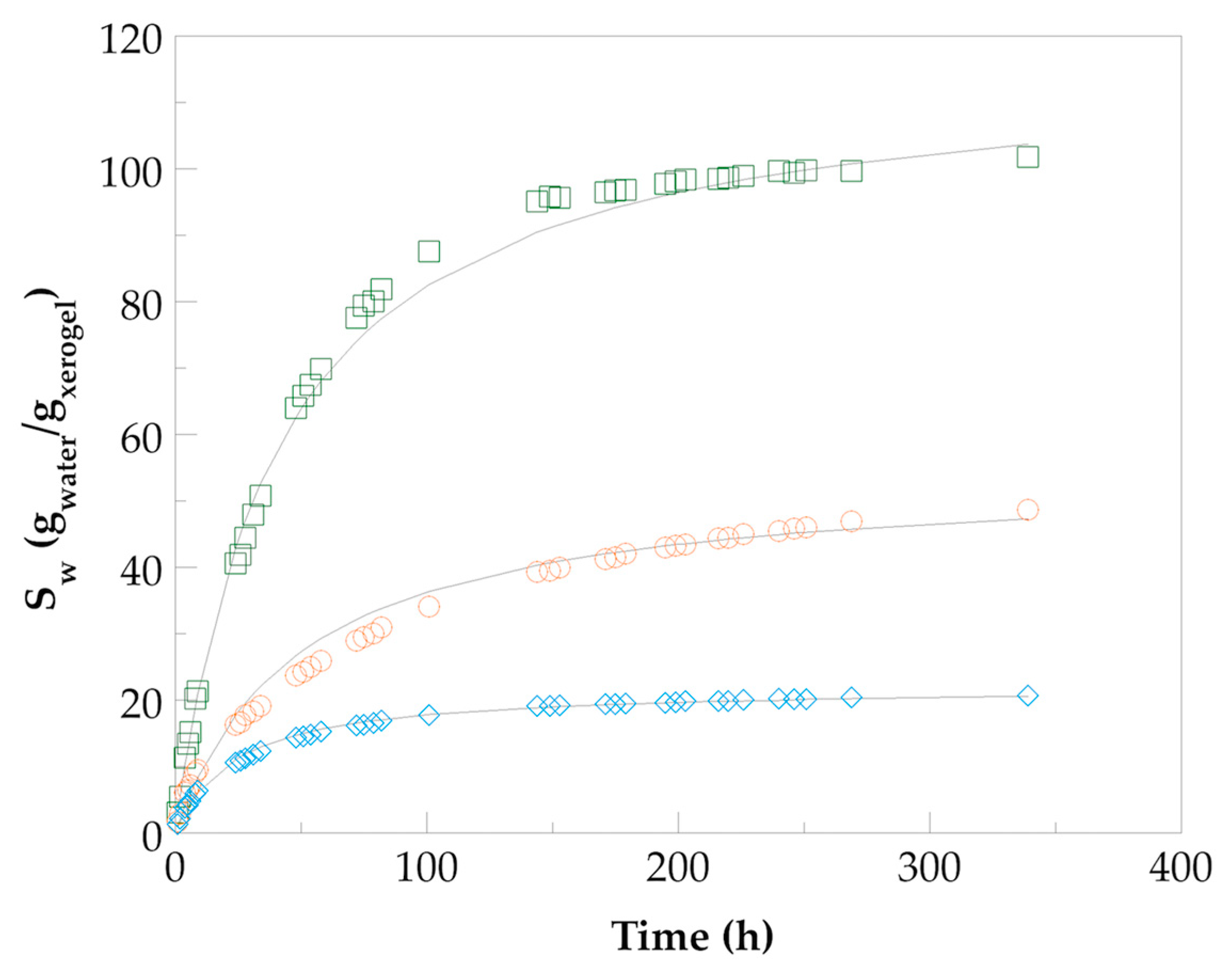
).
 ), Nano Hydro 2 (
), Nano Hydro 2 ( ), and Nano Hydro 3 (
), and Nano Hydro 3 ( ).
).
 ), Nano Hydro 2 (
), Nano Hydro 2 ( ), and Nano Hydro 3 (
), and Nano Hydro 3 ( ).
).

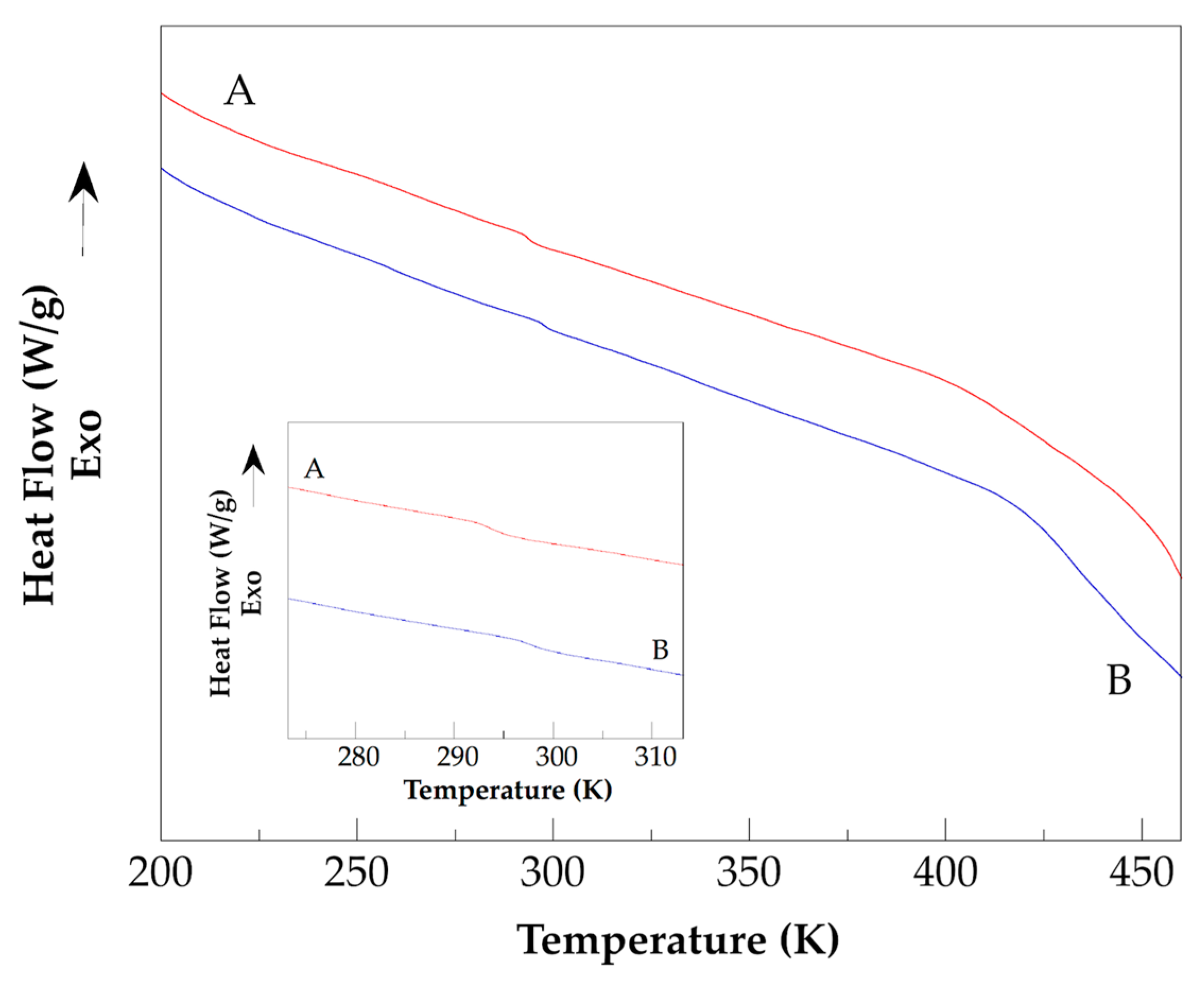
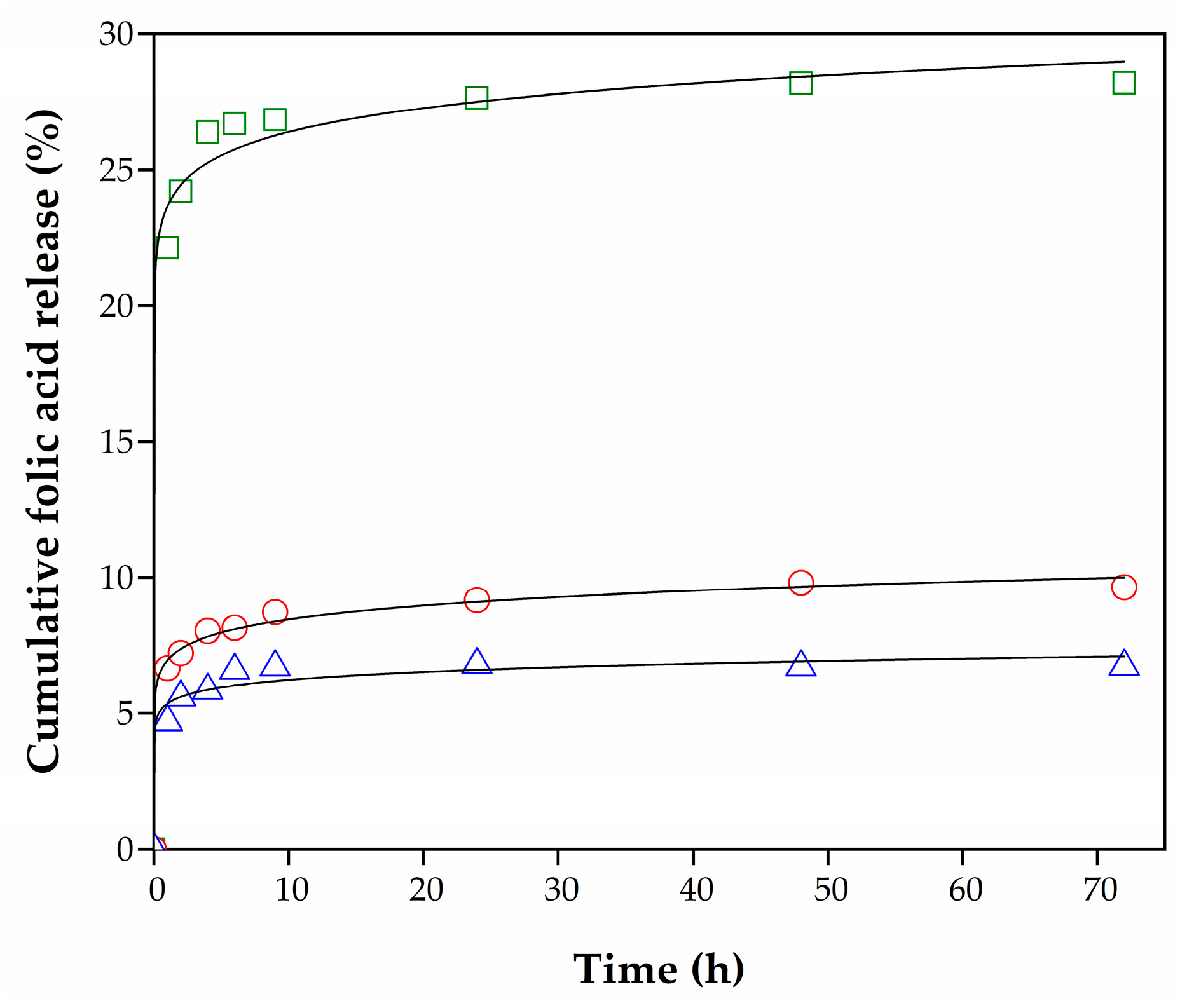
 ), Hydro 2 (
), Hydro 2 ( ), and Hydro 3 (
), and Hydro 3 ( ).
).
 ), Nano Hydro 2 (
), Nano Hydro 2 ( ), and Nano Hydro 3 (
), and Nano Hydro 3 ( ).
).
| Samples | D-Band (cm−1) | G-Band (cm−1) | G′-Band (cm−1) | G-Band Area (u. a.) | ID/IG |
|---|---|---|---|---|---|
| CNTspoxi | 1233 | 1485 | 2436 | 19.49 | 0.81 |
| CNTsOxCl | 1235 | 1466 | 2461 | 41.43 | 1.56 |
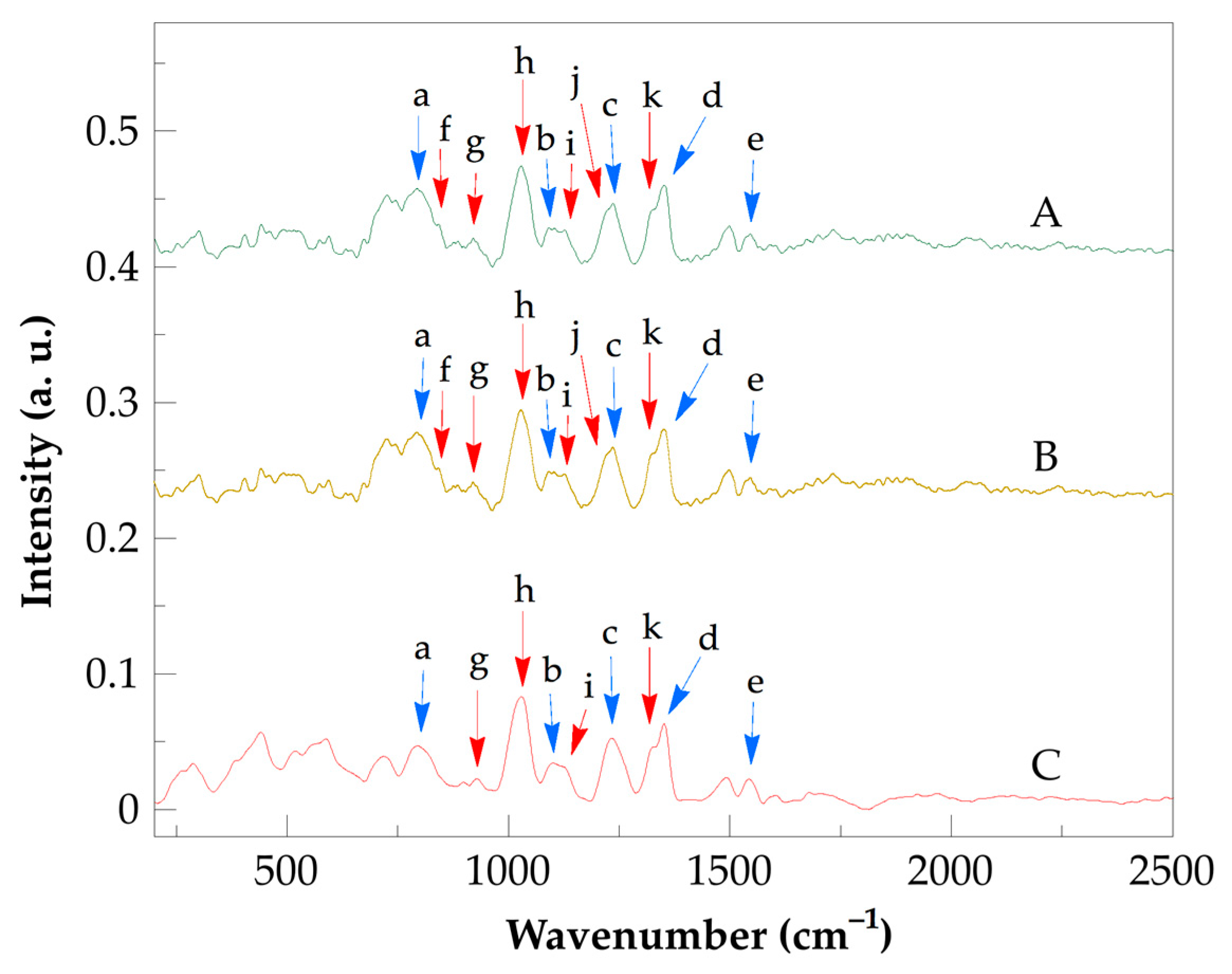
| Band | Assignment |
|---|---|
| a | CH2 rocking |
| b | C–C skeletal stretching |
| c | NH2 wagging |
| d | CH2 wagging |
| e | amide I, C=O stretching |
| f | C–COOH stretching |
| g | COOH dimer stretching |
| h | CH2 rocking |
| i | C-CH2 stretching |
| j | C–O stretching coupled with O–H in-plane bending |
| k | –CCO stretching |
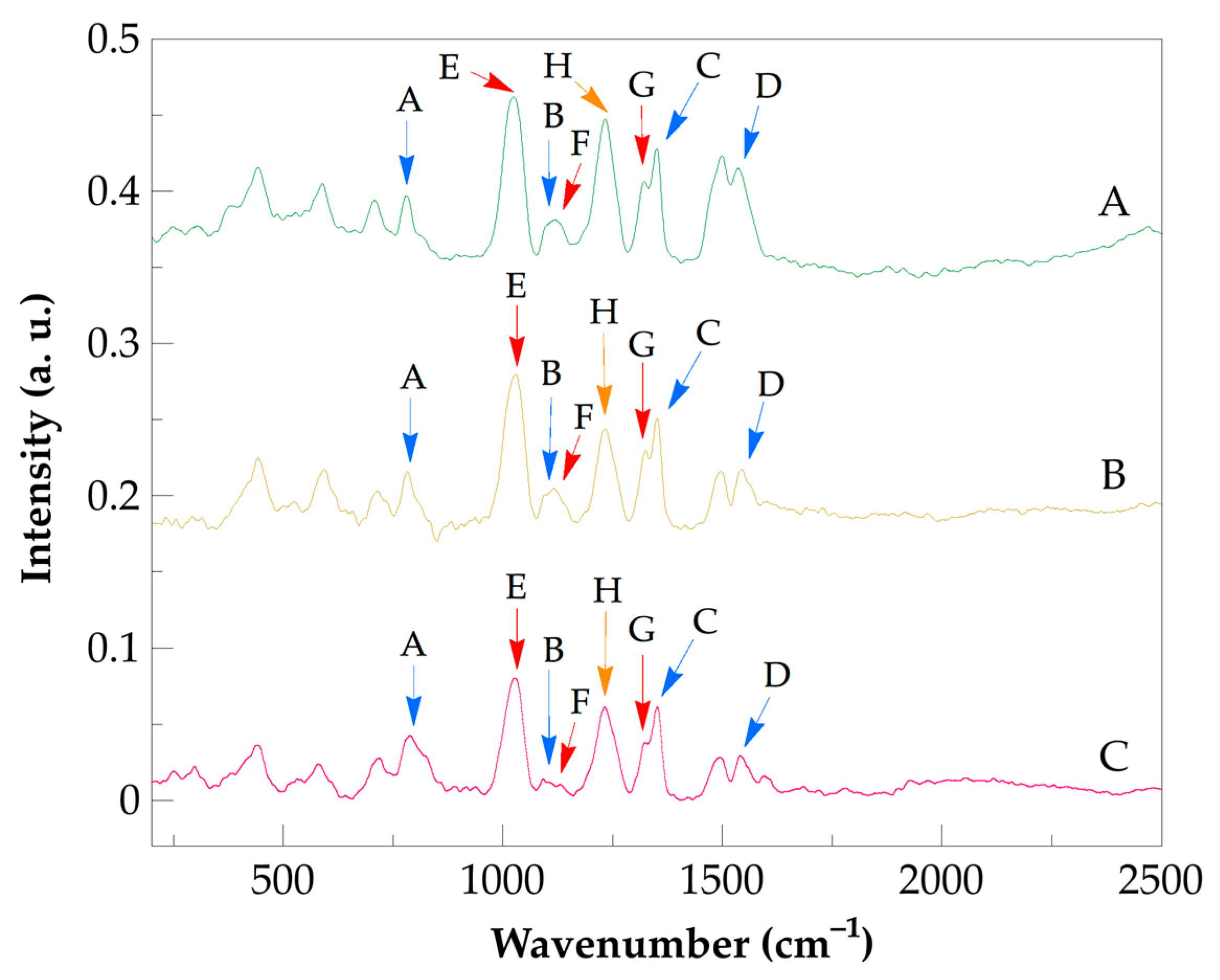
| Band | Assignment |
|---|---|
| A | CH2 rocking |
| B | C–C skeletal stretching |
| C | CH2 wagging |
| D | amide I, C=O stretching |
| E | CH2 rocking |
| F | C-CH2 stretching |
| G | –CCO stretching |
| H | NH2 wagging/D-band |
| Samples | |||
|---|---|---|---|
| Hydro 1 | 77.0 | 74.4 | 1.0 |
| Hydro 2 | 106.3 | 103.1 | 1.5 |
| Hydro 3 | 124.2 | 123.4 | 1.7 |
| Nano Hydro 1 | 20.6 | 20.5 | 1.0 |
| Nano Hydro 2 | 48.6 | 47.3 | 1.1 |
| Nano Hydro 3 | 101.3 | 103.6 | 2.9 |
| Samples | E (kPa) | Coefficient of Determination (R2) | (K) |
|---|---|---|---|
| Hydro 1 | 6.6 | 0.994 | 293.35 |
| Hydro 2 | 7.6 | 0.994 | 296.05 |
| Hydro 3 | 8.8 | 0.994 | 305.35 |
| Nano Hydro 1 | 9.2 | 0.996 | 297.55 |
| Nano Hydro 2 | 10.3 | 0.990 | 298.35 |
| Nano Hydro 3 | 10.6 | 0.983 | 306.75 |
| Samples | Parameters of the Weibull Model | Coefficient of Determination (R2) | ||
|---|---|---|---|---|
| Hydro 1 | 0.96 | 8.0 | 0.057 | 0.993 |
| Hydro 2 | 0.98 | 4.9 | 0.054 | 0.973 |
| Hydro 3 | 0.96 | 3.3 | 0.009 | 0.999 |
| Nano Hydro 1 | 0.99 | 16.6 | 0.042 | 0.987 |
| Nano Hydro 2 | 0.75 | 13.1 | 0.074 | 0.997 |
| Nano Hydro 3 | 0.97 | 3.4 | 0.037 | 0.998 |
| Samples | AM | AA | NMBA | Na2SO3 | KPS | CNTsOxCl | H2O |
|---|---|---|---|---|---|---|---|
| Hydro 1 | 9 | 1 | 0.1 | 0.05 | 0.05 | 0 | 89.8 |
| Hydro 2 | 8 | 2 | 0.1 | 0.05 | 0.05 | 0 | 89.8 |
| Hydro 3 | 7 | 3 | 0.1 | 0.05 | 0.05 | 0 | 89.8 |
| Nano Hydro 1 | 9 | 1 | 0.1 | 0.05 | 0.05 | 0.1 | 89.7 |
| Nano Hydro 2 | 8 | 2 | 0.1 | 0.05 | 0.05 | 0.1 | 89.7 |
| Nano Hydro 3 | 7 | 3 | 0.1 | 0.05 | 0.05 | 0.1 | 89.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval-García, K.; Cortés-Ortega, J.A.; Figueroa-Ochoa, E.B.; Antolín-Cerón, V.H.; Nuño-Donlucas, S.M. Synthesis, Characterization, and Evaluation of Folic Acid Release Ability of Acrylamide–Acrylic Acid Hydrogels and Acrylamide–Acrylic Acid/Functionalized Carbon Nanotube Nanocomposite Hydrogels. Int. J. Mol. Sci. 2025, 26, 9847. https://doi.org/10.3390/ijms26209847
Sandoval-García K, Cortés-Ortega JA, Figueroa-Ochoa EB, Antolín-Cerón VH, Nuño-Donlucas SM. Synthesis, Characterization, and Evaluation of Folic Acid Release Ability of Acrylamide–Acrylic Acid Hydrogels and Acrylamide–Acrylic Acid/Functionalized Carbon Nanotube Nanocomposite Hydrogels. International Journal of Molecular Sciences. 2025; 26(20):9847. https://doi.org/10.3390/ijms26209847
Chicago/Turabian StyleSandoval-García, Karina, Jorge A. Cortés-Ortega, Edgar B. Figueroa-Ochoa, Víctor H. Antolín-Cerón, and Sergio M. Nuño-Donlucas. 2025. "Synthesis, Characterization, and Evaluation of Folic Acid Release Ability of Acrylamide–Acrylic Acid Hydrogels and Acrylamide–Acrylic Acid/Functionalized Carbon Nanotube Nanocomposite Hydrogels" International Journal of Molecular Sciences 26, no. 20: 9847. https://doi.org/10.3390/ijms26209847
APA StyleSandoval-García, K., Cortés-Ortega, J. A., Figueroa-Ochoa, E. B., Antolín-Cerón, V. H., & Nuño-Donlucas, S. M. (2025). Synthesis, Characterization, and Evaluation of Folic Acid Release Ability of Acrylamide–Acrylic Acid Hydrogels and Acrylamide–Acrylic Acid/Functionalized Carbon Nanotube Nanocomposite Hydrogels. International Journal of Molecular Sciences, 26(20), 9847. https://doi.org/10.3390/ijms26209847







