The Role of Cadherin 17 (CDH17) in Cancer Progression via Wnt/β-Catenin Signalling Pathway: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Information Source and Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction and Analysis
2.6. Sensitivity Analysis
2.7. Quality Assessment
2.8. Effect Measures
2.9. Certainty of Evidence
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Quality and Risk of Bias Assessment
3.4. Certainity of Evidence
3.5. Narrative Synthesis
3.6. Meta-Analysis of Outcomes
3.6.1. CDH17 as a Pro-Tumorigenic Factor Driving Malignant Phenotypic Change
3.6.2. CDH17 as an Upstream Activator of the Wnt/β-Catenin Pathway
3.6.3. Sub-Group Analysis of Clinical Data Indicates Sex Dependent Role of CDH17 in Cancer Progression
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Adenomatous polyposis coli |
| CK1α | Casein kinase 1α |
| CRC | Colorectal cancer |
| DVL | Dishevelled |
| EMT | Epithelial–mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| FAK | Focal adhesion kinase |
| FZD | Wnt ligands bind frizzled |
| GC | Gastric cancer |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| GSK-3β | Glycogen synthase kinase 3β |
| HCC | Hepatocellular carcinoma |
| IHC | Immunohistochemistry |
| IV | Inverse variance |
| KD | Knockdown |
| KO | Knockout |
| LEF | Lymphoid enhancer-binding factor |
| LGR | Leucine-rich repeat-containing G-protein-coupled receptor |
| LRP5/6 | Low-density lipoprotein receptor-related proteins 5 and 6 |
| MA | Meta-analysis |
| MD | Mean difference |
| NEC | Neuroendocrine tumours |
| NOS | Newcastle–Ottawa Scale |
| OHAT | Office of Health Assessment and Translation |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | Prospective Register of Systematic Reviews |
| Rb | Retinoblastoma |
| RCTs | Non-randomised controlled trials |
| RoB | Risk of bias |
| SD | Standard deviation |
| SEM | Standard error of mean |
| shRNA | Short hairpin RNA |
| siRNA | Small interfering RNA |
| SMD | Standardised mean difference |
| SR | Systematic review |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation |
| TAZ | Transcriptional co-activator with PDZ-binding motif |
| TCF | T-cell factor |
| TNM | Tumour-Node-Metastasis |
| WB | Western blotting |
| YAP | Yes-associated protein |
References
- Berndorff, D.; Gessner, R.; Kreft, B.; Schnoy, N.; Lajous-Petter, A.M.; Loch, N.; Reutter, W.; Hortsch, M.; Tauber, R. Liver-intestine cadherin: Molecular cloning and characterization of a novel Ca2+-dependent cell adhesion molecule expressed in liver and intestine. J. Cell Biol. 1994, 125, 1353–1369. [Google Scholar] [CrossRef]
- Gessner, R.; Tauber, R. Intestinal cell adhesion molecules. Liver-intestine cadherin. Ann. N. Y. Acad. Sci. 2000, 915, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Altree-Tacha, D.; Tyrrell, J.; Haas, T. CDH17 Is a More Sensitive Marker for Gastric Adenocarcinoma Than CK20 and CDX2. Arch. Pathol. Lab. Med. 2017, 141, 144–150, Erratum in Arch. Pathol. Lab. Med. 2017, 141, 484. [Google Scholar] [CrossRef] [PubMed]
- Grötzinger, C.; Kneifel, J.; Patschan, D.; Schnoy, N.; Anagnostopoulos, I.; Faiss, S.; Tauber, R.; Wiedenmann, B.; Gessner, R. LI-cadherin: A marker of gastric metaplasia and neoplasia. Gut 2001, 49, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Bergsland, C.; Bruun, J.; Bjørnslett, M.; Vieira, A.F.; Mesquita, P.; Pinto, R.; Gomes, R.; Cavadas, B.; Bennett, E.; et al. A panel of intestinal differentiation markers (CDX2, GPA33, and LI-cadherin) identifies gastric cancer patients with favourable prognosis. Gastric Cancer 2020, 23, 811–823. [Google Scholar] [CrossRef]
- Liu, L.X.; Lee, N.P.; Chan, V.W.; Xue, W.; Zender, L.; Zhang, C.; Mao, M.; Dai, H.; Wang, X.L.; Xu, M.Z.; et al. Targeting cadherin-17 inactivates Wnt signaling and inhibits tumor growth in liver carcinoma. Hepatology 2009, 50, 1453–1463. [Google Scholar] [CrossRef]
- Wang, X.Q.; Luk, J.M.; Leung, P.P.; Wong, B.W.; Stanbridge, E.J.; Fan, S.T. Alternative mRNA splicing of liver intestine-cadherin in hepatocellular carcinoma. Clin. Cancer Res. 2005, 11, 483–489. [Google Scholar] [CrossRef]
- Wong, B.W.; Luk, J.M.; Ng, I.O.; Hu, M.Y.; Liu, K.D.; Fan, S.T. Identification of liver-intestine cadherin in hepatocellular carcinoma—A potential disease marker. Biochem. Biophys. Res. Commun. 2003, 311, 618–624. [Google Scholar] [CrossRef]
- Johnson, A.; Wright, J.P.; Zhao, Z.; Komaya, T.; Parikh, A.; Merchant, N.; Shi, C. Cadherin 17 is frequently expressed by ‘sclerosing variant’ pancreatic neuroendocrine tumour. Histopathology 2015, 66, 225–233. [Google Scholar] [CrossRef]
- Snow, A.N.; Mangray, S.; Lu, S.; Clubwala, R.; Li, J.; Resnick, M.B.; Yakirevich, E. Expression of cadherin 17 in well- differentiated neuroendocrine tumours. Histopathology 2015, 66, 1010–1021. [Google Scholar] [CrossRef]
- Huang, L.-P.; Yu, Y.-H.; Sheng, C.; Wang, S.-H. Up-regulation of cadherin 17 and down-regulation of homeodomain proteinDX2 correlate with tumor progression and unfavorable prognosis in epithelial ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Williamson, S.R.; Lopez-Beltran, A.; Montironi, R.; Huang, W.B.; Eble, J.N.; Grignon, D.J.; Koch, M.O.; Idrees, M.T.; Emerson, R.E.; et al. Distinguishing primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma: Extended immunohistochemical profiles emphasizing novel markers. Mod. Pathol. 2013, 26, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Su, M.C.; Yuan, R.H.; Lin, C.Y.; Jeng, Y.M. Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system. Mod. Pathol. 2008, 21, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Takamura, M.; Ichida, T.; Matsuda, Y.; Kobayashi, M.; Yamagiwa, S.; Genda, T.; Shioji, K.; Hashimoto, S.; Nomoto, M.; Hatakeyama, K.; et al. Reduced expression of liver-intestine cadherin is associated with progression and lymph node metastasis of human colorectal carcinoma. Cancer Lett. 2004, 212, 253–259. [Google Scholar] [CrossRef]
- Bartolomé, R.A.; Barderas, R.; Torres, S.; Fernandez-Aceñero, M.J.; Mendes, M.; García-Foncillas, J.; Lopez-Lucendo, M.; Casal, J.I. Cadherin-17 interacts with α2β1 integrin to regulate cell proliferation and adhesion in colorectal cancer cells causing liver metastasis. Oncogene 2014, 33, 1658–1669. [Google Scholar] [CrossRef]
- Takamura, M.; Sakamoto, M.; Ino, Y.; Shimamura, T.; Ichida, T.; Asakura, H.; Hirohashi, S. Expression of liver-intestine cadherin and its possible interaction with galectin-3 in ductal adenocarcinoma of the pancreas. Cancer Sci. 2003, 94, 425–430. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Yuan, H.; Qi, X.; Manjunath, Y.; Avella, D.; Kaifi, J.T.; Miao, Y.; Li, M.; Jiang, K.; et al. Disruption of oncogenic liver-intestine cadherin (CDH17) drives apoptotic pancreatic cancer death. Cancer Lett. 2019, 454, 204–214. [Google Scholar] [CrossRef]
- Li, X.; Ortiz, M.A.; Kotula, L. The physiological role of Wnt pathway in normal development and cancer. Exp. Biol. Med. 2020, 245, 411–426. [Google Scholar] [CrossRef]
- Song, P.; Gao, Z.; Bao, Y.; Chen, L.; Huang, Y.; Liu, Y.; Dong, Q.; Wei, X. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J. Hematol. Oncol. 2024, 17, 46. [Google Scholar] [CrossRef]
- Qiu, H.-B.; Zhang, L.-Y.; Ren, C.; Zeng, Z.-L.; Wu, W.-J.; Luo, H.-Y.; Zhou, Z.-W.; Xu, R.-H. Correction: Targeting CDH17 Suppresses Tumor Progression in Gastric Cancer by Downregulating Wnt/β-Catenin Signaling. PLoS ONE 2019, 14, e0217124. [Google Scholar] [CrossRef]
- Bartolomé, R.A.; Pintado-Berninches, L.; Robles, J.; Calvo-López, T.; Boukich, I.; Otero-Núñez, P.; Gonzalez-Sancho, J.M.; Casal, J.I. Loss of cadherin 17 downregulates LGR5 expression, stem cell properties and drug resistance in metastatic colorectal cancer cells. Cell Death Dis. 2025, 16, 475. [Google Scholar] [CrossRef]
- Qu, L.P.; Zhong, Y.M.; Zheng, Z.; Zhao, R.X. CDH17 is a downstream effector of HOXA13 in modulating the Wnt/β-catenin signaling pathway in gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1234–1241. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Mowbray, F.I.; Manlongat, D.; Shukla, M. Sensitivity Analysis: A Method to Promote Certainty and Transparency in Nursing and Health Research. Can. J. Nurs. Res. 2022, 54, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Chen, D.G.; Nishimura, K.; Miyamoto, Y. Comparative study of four methods in missing value imputations under missing completely at random mechanism. Open J. Stat. 2014, 4, 27–37. [Google Scholar] [CrossRef]
- Office of Health Assessment and Translation (OHAT). Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. National Institute of Environmental Health Sciences. 2019. Available online: https://ntp.niehs.nih.gov/research/assessments/noncancer/handbook (accessed on 21 July 2025).
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Carra, M.C.; Romandini, P.; Romandini, M. Risk of Bias Evaluation of Cross-Sectional Studies: Adaptation of the Newcastle-Ottawa Scale. J. Periodontal Res. 2025. [Google Scholar] [CrossRef]
- Ryan, R.; Hill, S. How to GRADE the Quality of the Evidence. Cochrane Consumers and Communication Group. 2016. Version 3.0. Available online: http://cccrg.cochrane.org/author-resources (accessed on 22 July 2025).
- Wang, Y.; Shek, F.H.; Wong, K.F.; Liu, L.X.; Zhang, X.Q.; Yuan, Y.; Khin, E.; Hu, M.-Y.; Wang, J.H.; Poon, R.T.P.; et al. Anti-cadherin-17 antibody modulates beta-catenin signaling and tumorigenicity of hepatocellular carcinoma. PLoS ONE 2013, 8, e72386. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Bordonaro, M.; Lazarova, D.L.; Sartorelli, A.C. The activation of beta-catenin by Wnt signaling mediates the effects of histone deacetylase inhibitors. Exp. Cell Res. 2007, 313, 1652–1666. [Google Scholar] [CrossRef]
- Connell-Crowley, L.; Harper, J.W.; Goodrich, D.W. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol. Biol. Cell 1997, 8, 287–301. [Google Scholar] [CrossRef]
- El-Deiry, W.S.; Tokino, T.; Velculescu, V.E.; Levy, D.B.; Parsons, R.; Trent, J.M.; Lin, D.; Mercer, W.E.; Kinzler, K.W.; Vogelstein, B. WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75, 817–825. [Google Scholar] [CrossRef]
- Davidson, G.; Shen, J.; Huang, Y.L.; Su, Y.; Karaulanov, E.; Bartscherer, K.; Hassler, C.; Stannek, P.; Boutros, M.; Niehrs, C. Cell cycle control of wnt receptor activation. Dev. Cell 2009, 17, 788–799. [Google Scholar] [CrossRef]
- Bartolomé, R.A.; Pintado-Berninches, L.; Martín-Regalado, Á.; Robles, J.; Calvo-López, T.; Ortega-Zapero, M.; Llorente-Sáez, C.; Boukich, I.; Fernandez-Aceñero, M.J.; Casal, J.I. A complex of cadherin 17 with desmocollin 1 and p120-catenin regulates colorectal cancer migration and invasion according to the cell phenotype. J. Exp. Clin. Cancer Res. 2024, 43, 31. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, R.A.; Peláez-García, A.; Gomez, I.; Torres, S.; Fernandez-Aceñero, M.J.; Escudero-Paniagua, B.; Imbaud, J.I.; Casal, J.I. An RGD motif present in cadherin 17 induces integrin activation and tumor growth. J. Biol. Chem. 2014, 289, 34801–34814. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.I.; Bartolome, R.A. RGD cadherins and α2β1 integrin in cancer metastasis: A dangerous liaison. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 321–332. [Google Scholar] [CrossRef]
- Casal, J.I.; Bartolomé, R.A. Beyond N-cadherin, relevance of cadherins 5, 6 and 17 in cancer progression and metastasis. Int. J. Mol. Sci. 2019, 20, 3373. [Google Scholar] [CrossRef]
- Que, Z.; Qi, D.; Yang, Y.; Yao, W.; Liu, J.; Li, Y.; Yu, Y.; Wang, L.; Li, F.; Zhang, G.; et al. Regulating chemoresistance and cancer stemness: The CDH17-YAP pathway in distinct cellular states of lung cancer CTC clusters. Cell Mol. Biol. Lett. 2025, 30, 23. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

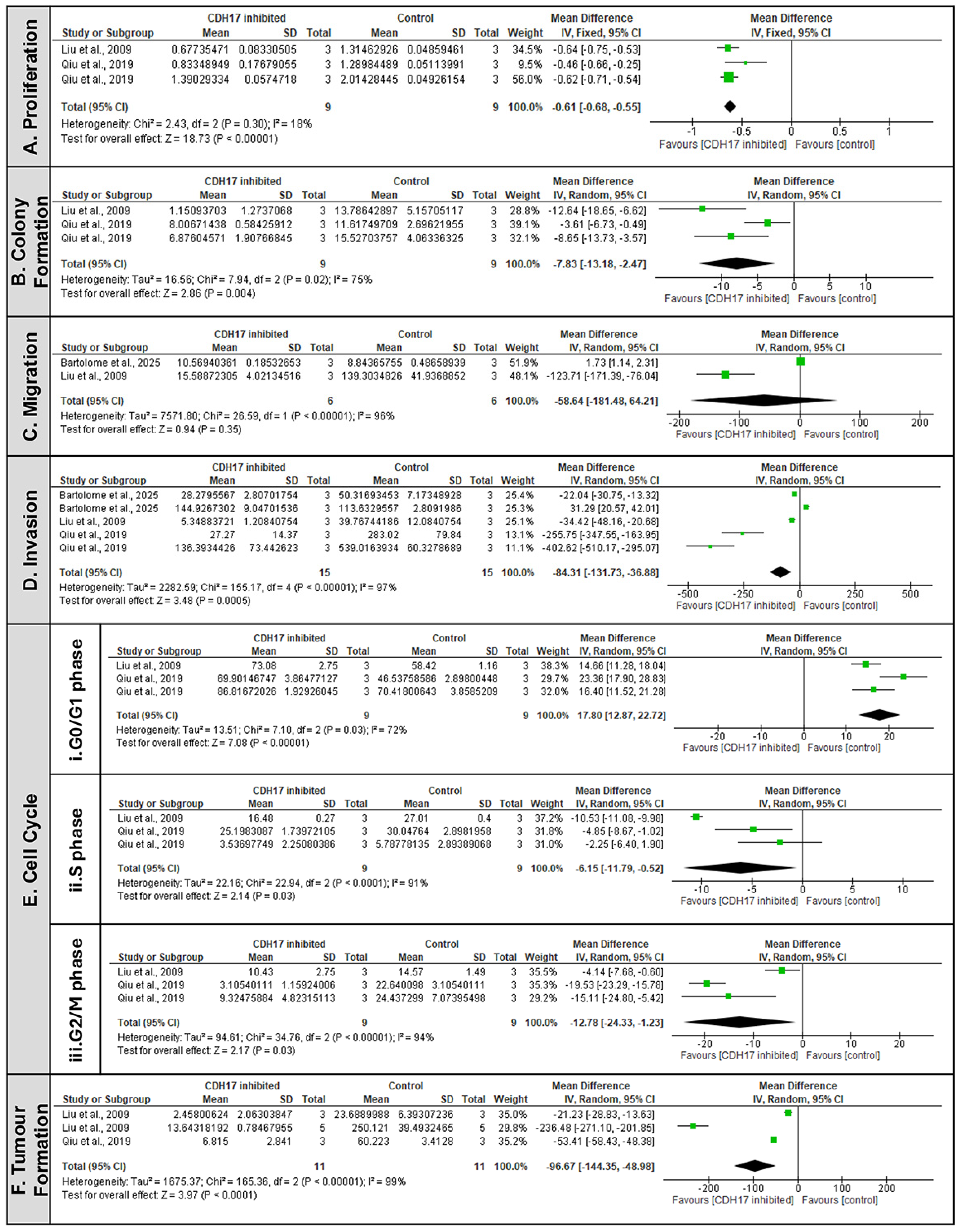
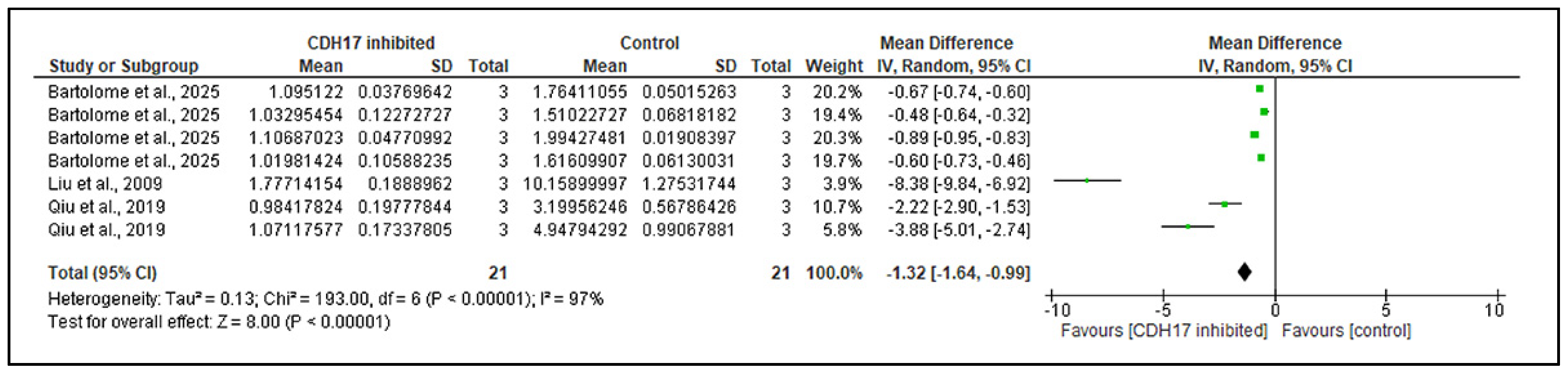

| Articles | Year | Cancer Type | Study Design | Models | Groups (Exposure vs. Control) | Outcome Measures |
|---|---|---|---|---|---|---|
| Bartolomé et al. [21] | 2025 | CRC | Preclinical study | In vitro (cell-line; KM12SM & SW620; n = 3) | CDH17-specific shRNA (sh60) vs. scrambled shRNA (control) | TOP/FOP reporter assay, gene expression |
| Liu et al. [6] | 2009 | HCC | Preclinical study; cross-sectional | In vitro (cell-line; MHCC97H, n = NS) In vivo (athymic BALB/c nu/nu mice; n = 3, n = 5), Clinical (n = 46) | CDH17-specific constructs and shRNA vs. empty vector | TOP/FOP assay, WB |
| Wang et al. [30] | 2013 | HCC | Preclinical study | In vitro (cell-line; MHCC97L, MHCC97H n = 3), in vivo (BALB/c ByJ mice; n = 6) | Lic5 antibody-treated (CDH17-suppressed) MHCC97L cells vs. PBS-treated MHCC97L cells | WB, qPCR, IHC |
| Qiu et al. [20] | 2019 | GC | Preclinical study; cross-sectional | In vitro (AGS & MKN-45, n = 3), In vivo (nude mice; n = 5), Clinical (n = 156) | CDH17 shRNA vector/CDH17 lentiviral shRNAmir vs. mock control) | TOP/FOP assay, WB |
| Qu et al. [22] | 2017 | GC | Preclinical study | In vitro (AGS & SGC-7901 cells, n = 3) | CDH17 lentiviral shRNA (sc-43014-V) vs. empty vector | WB |
| References | Title | Comparison Detail | Comparison Outcome |
|---|---|---|---|
| [21] | Loss of cadherin 17 downregulates LGR5 expression, stem cell properties and drug resistance in metastatic colorectal cancer cells | Global transcriptomic analysis was performed to compare shRNA (KD) of KM12SM and SW620 cells with the scramble control (SCR) to identify affected genes and signalling pathways. Wnt/β-catenin signalling activity was assessed using TOP/FOP reporter assays and Western blot. | CDH17 KD group > control group (GSK3B expression, AXIN expression, CSNK1A expression, DKK4 and DKK1) CDH17 KD group < control group (responsiveness to Wnt3a in TOP/FOP assays) |
| [6] | Targeting cadherin-17 inactivates Wnt signalling and inhibits tumour growth in liver carcinoma | A TOP/FOP Flash luciferase assay was performed in MHCC97H cells with or without CDH17 shRNA transfection (Vector and Mock controls). Western blotting was used to examine Wnt pathway proteins. In vivo, immunohistochemistry was carried out for tumour tissues from both treated and control groups. | CDH17 KD group < control group (total β-catenin levels, cyclin D1 levels, phospho-GSK-3β levels, TOP/FOP luciferase activity) CDH17 KD > Vector/Mock controls (Rb expression) |
| [30] | Anti-cadherin-17 antibody modulates beta-catenin signalling and tumorigenicity of hepatocellular carcinoma | AGS & SGC-7901 cells treated with anti-CDH17 antibody were analysed by immunofluorescence to assess total and phosphorylated β-catenin levels. Real-time qPCR was used to measure cyclin D1 gene expression. In vivo, Wnt/β-catenin pathway targets were examined using Western blot and immunohistochemistry. | CDH17 inhibited group < control group (total β-catenin, phospho-β-catenin [Thr41/Ser45], cyclin D1 expression) Lic5-treated group > control group (Rb expression) |
| [20] | Targeting CDH17 Suppresses Tumour Progression in Gastric Cancer by Downregulating Wnt/beta-Catenin Signalling | A TOP flash/FOP flash reporter assay was conducted in AGS and MKN-45 cells following CDH17 silencing. To further validate the modulation of the Wnt/β-catenin pathway, Western blot analysis was used to assess the expression of key pathway components. | CDH17 KD group < control group (total and nuclear β-catenin, Phospho-GSK-3β, Cyclin D1, TCF/LEF transactivation activity) CDH17 KD group > control group (Rb, p53, p21 expression) |
| [22] | CDH17 is a downstream effector of HOXA13 in modulating the Wnt/beta-catenin signalling pathway in gastric cancer | Western blot assay was used to assess β-catenin between the knock down group and the control group in SGC-7901 cells. | CDH17 KD group < control group (β-catenin expression) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tha Shrestha, B.; Feng, Y.; Lad, A.; Bates, A.; Chen, J.; Brown, K.; Zeng, F.; Wang, N. The Role of Cadherin 17 (CDH17) in Cancer Progression via Wnt/β-Catenin Signalling Pathway: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 9838. https://doi.org/10.3390/ijms26209838
Tha Shrestha B, Feng Y, Lad A, Bates A, Chen J, Brown K, Zeng F, Wang N. The Role of Cadherin 17 (CDH17) in Cancer Progression via Wnt/β-Catenin Signalling Pathway: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(20):9838. https://doi.org/10.3390/ijms26209838
Chicago/Turabian StyleTha Shrestha, Bipusha, Yahui Feng, Aaron Lad, Anthony Bates, Jing Chen, Karen Brown, Feier Zeng, and Ning Wang. 2025. "The Role of Cadherin 17 (CDH17) in Cancer Progression via Wnt/β-Catenin Signalling Pathway: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 20: 9838. https://doi.org/10.3390/ijms26209838
APA StyleTha Shrestha, B., Feng, Y., Lad, A., Bates, A., Chen, J., Brown, K., Zeng, F., & Wang, N. (2025). The Role of Cadherin 17 (CDH17) in Cancer Progression via Wnt/β-Catenin Signalling Pathway: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(20), 9838. https://doi.org/10.3390/ijms26209838







