Exploring the Role of Heat Shock Proteins in Neuroimmune Modulation in Rheumatoid Arthritis: Insights from a Rat Model
Abstract
1. Introduction
2. Results
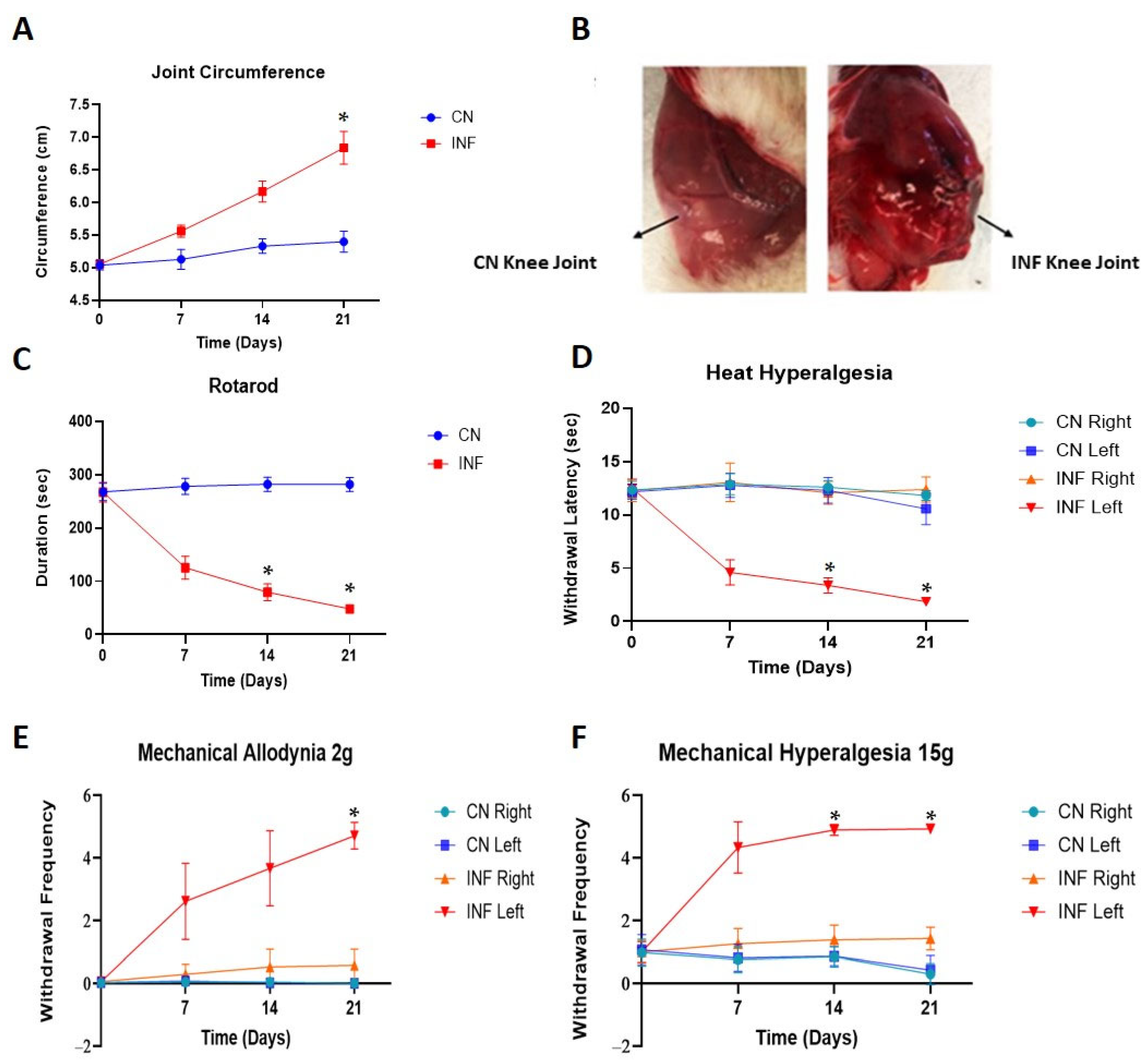
2.1. The Effect of Complete Freund’s Adjuvants (CFA) Injection on Joint Inflammation
2.2. The Effect of CFA-Induced Inflammation on Motor Coordination
2.3. Nociceptive Behavior Changes
2.3.1. The Effect of CFA-Induced Inflammation on Heat Hyperalgesia
2.3.2. The Effect of CFA-Induced Inflammation on Mechanical Allodynia and Mechanical Hyperalgesia
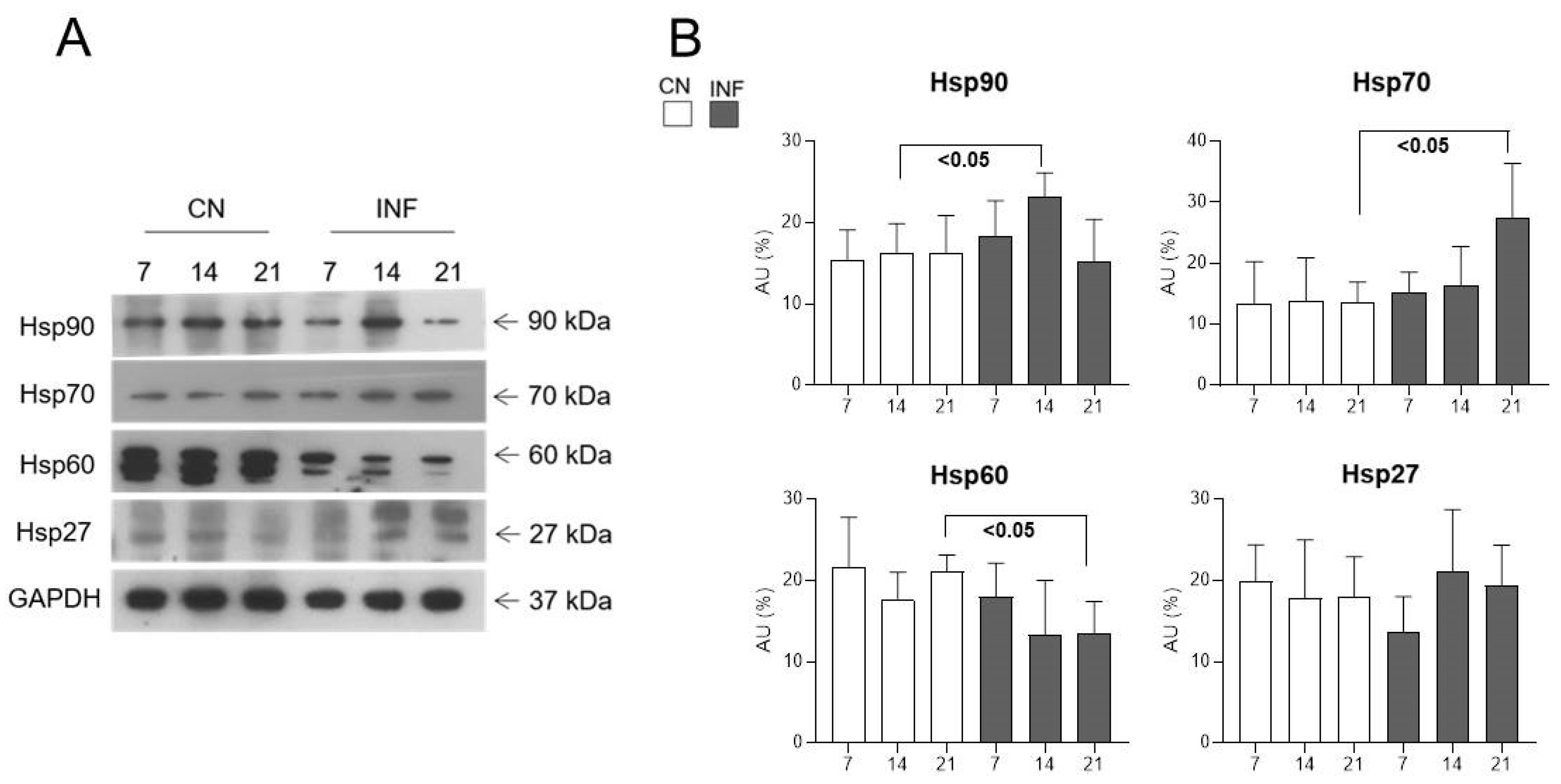
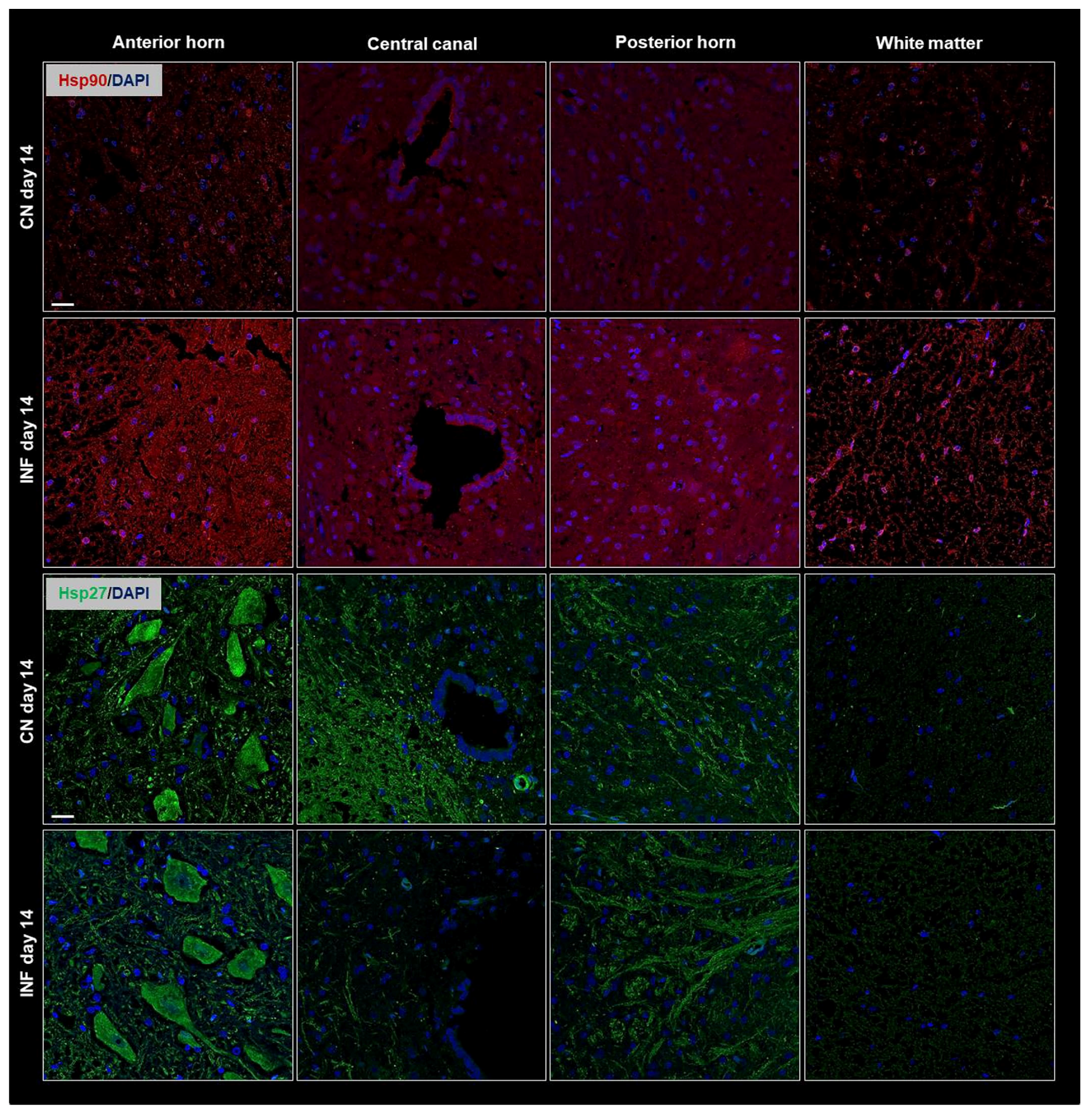
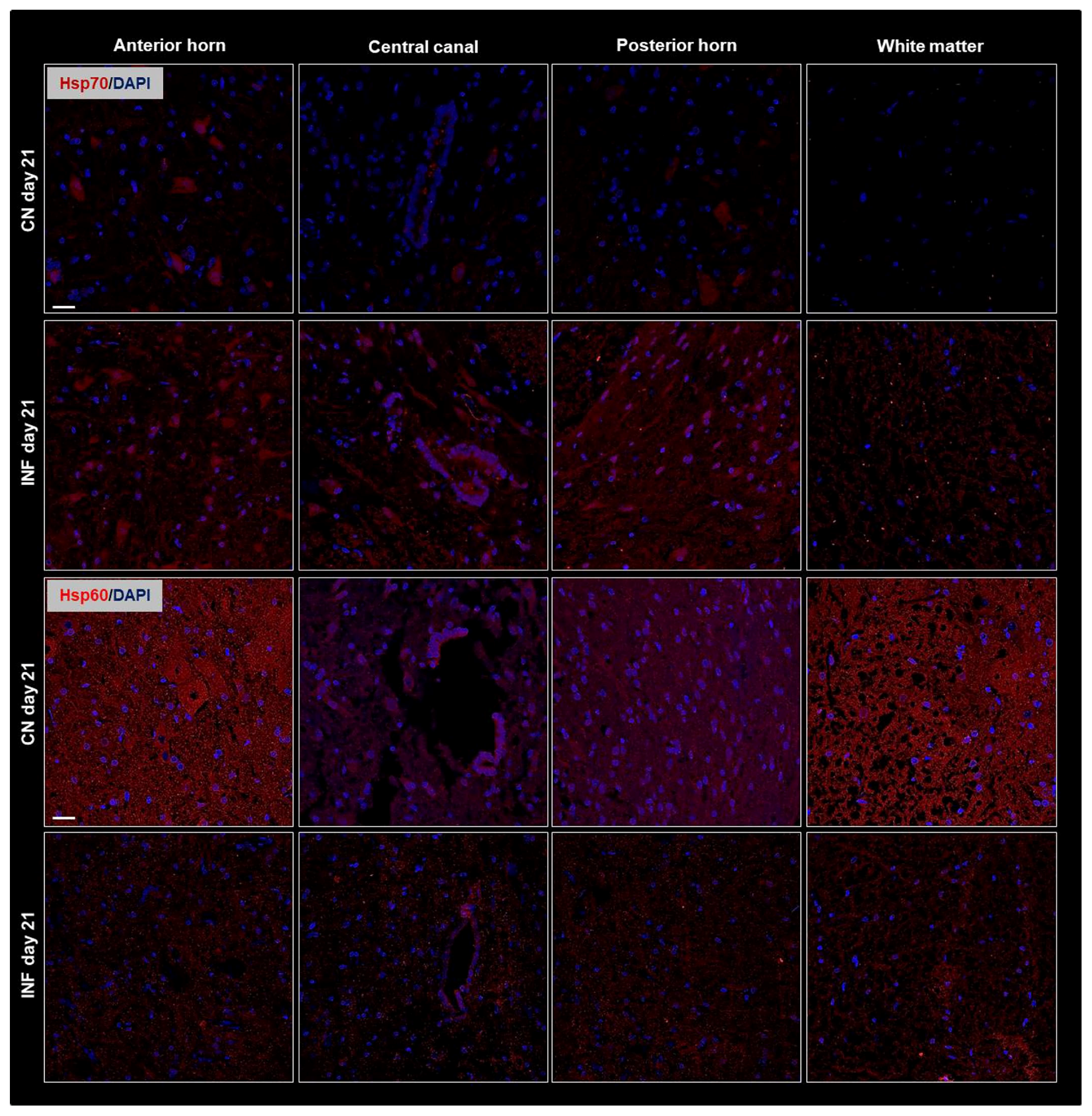
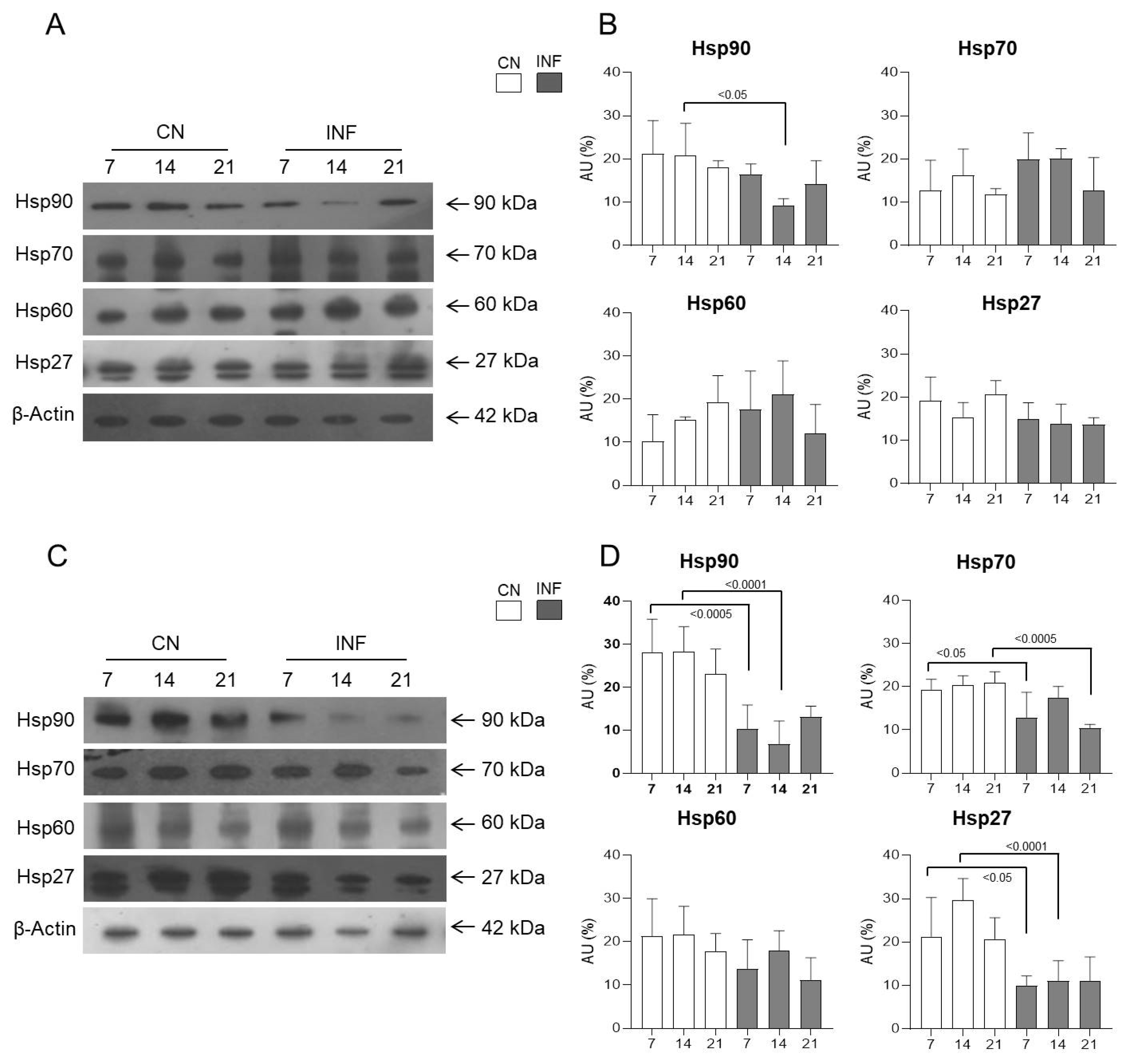
2.4. The Effect of CFA-Induced Inflammation on the Expression of HSPs in the Spinal Cord
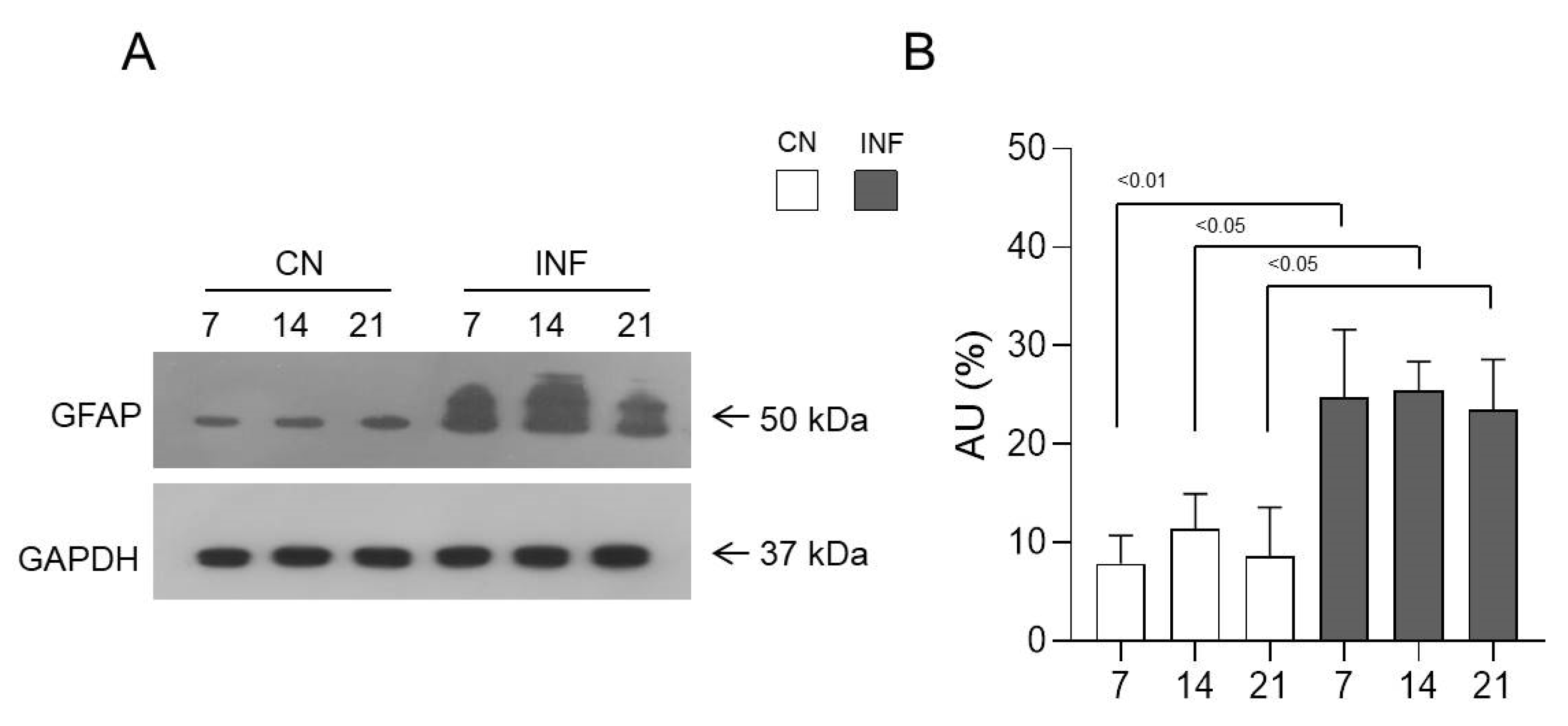
2.5. The Effect of CFA-Induced Inflammation on the Expression of GFAP in the Spinal Cord
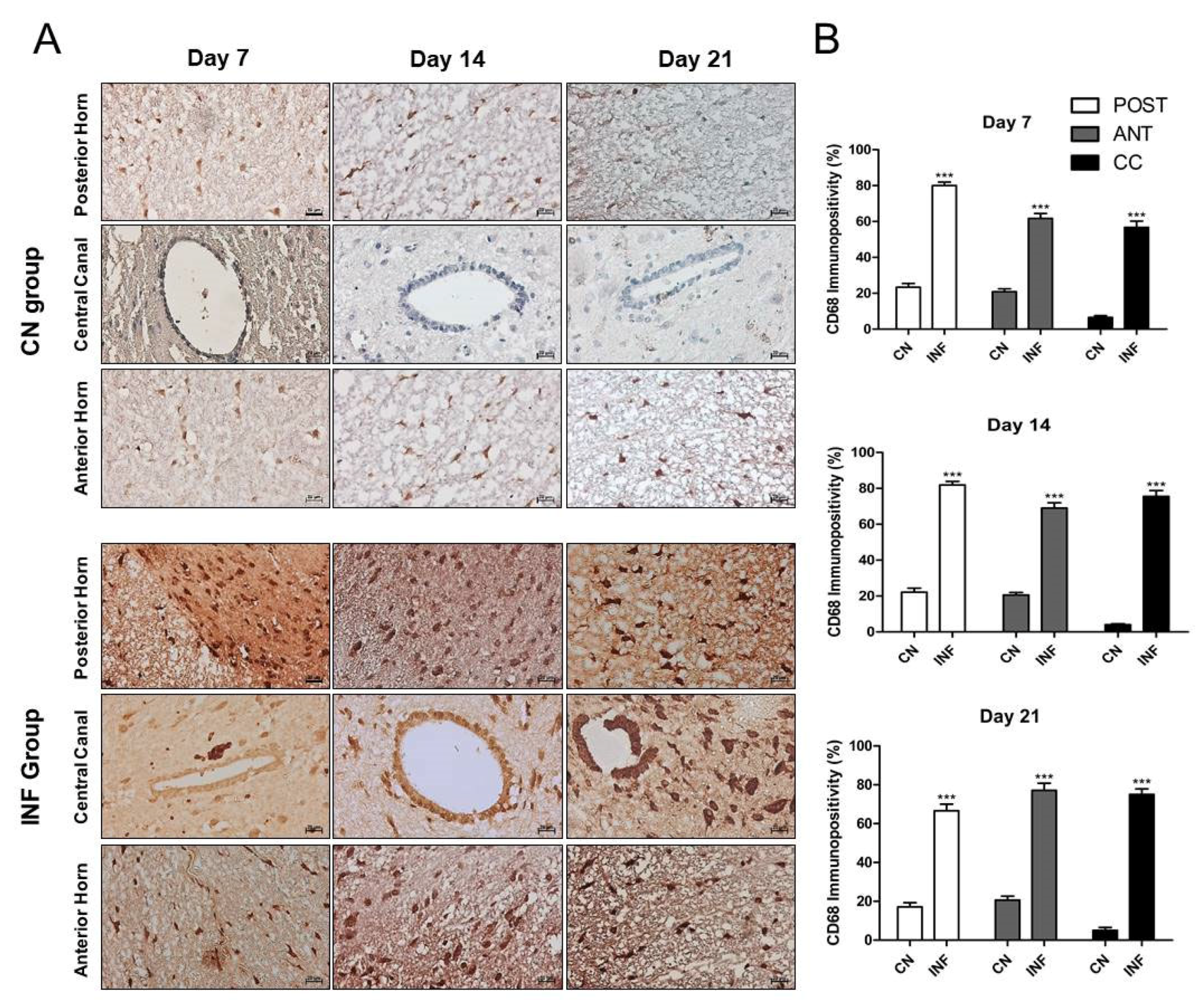
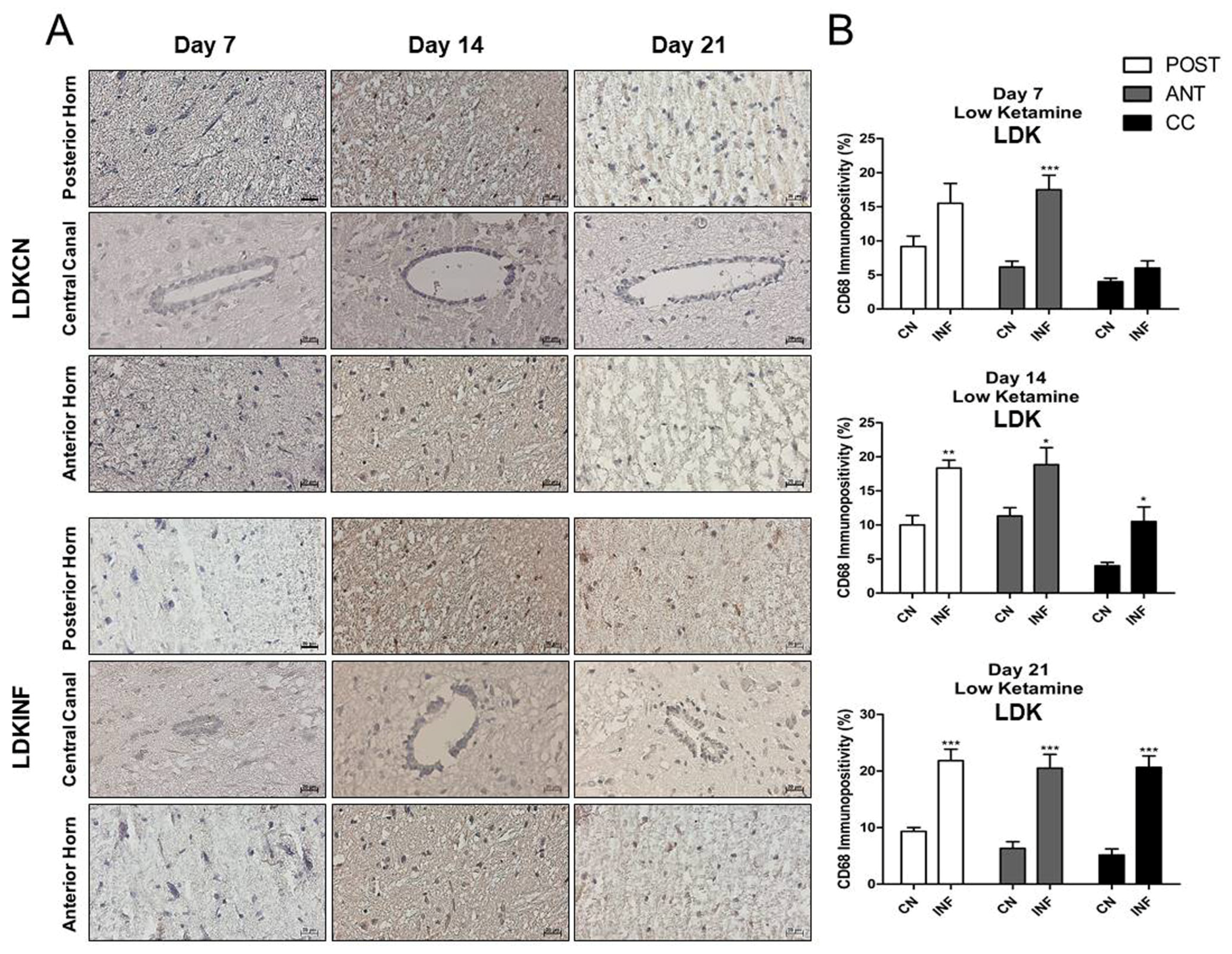
2.6. The Effect of CFA-Induced Inflammation on CD68-Immunopositivity in the Different Regions of the Spinal Cord
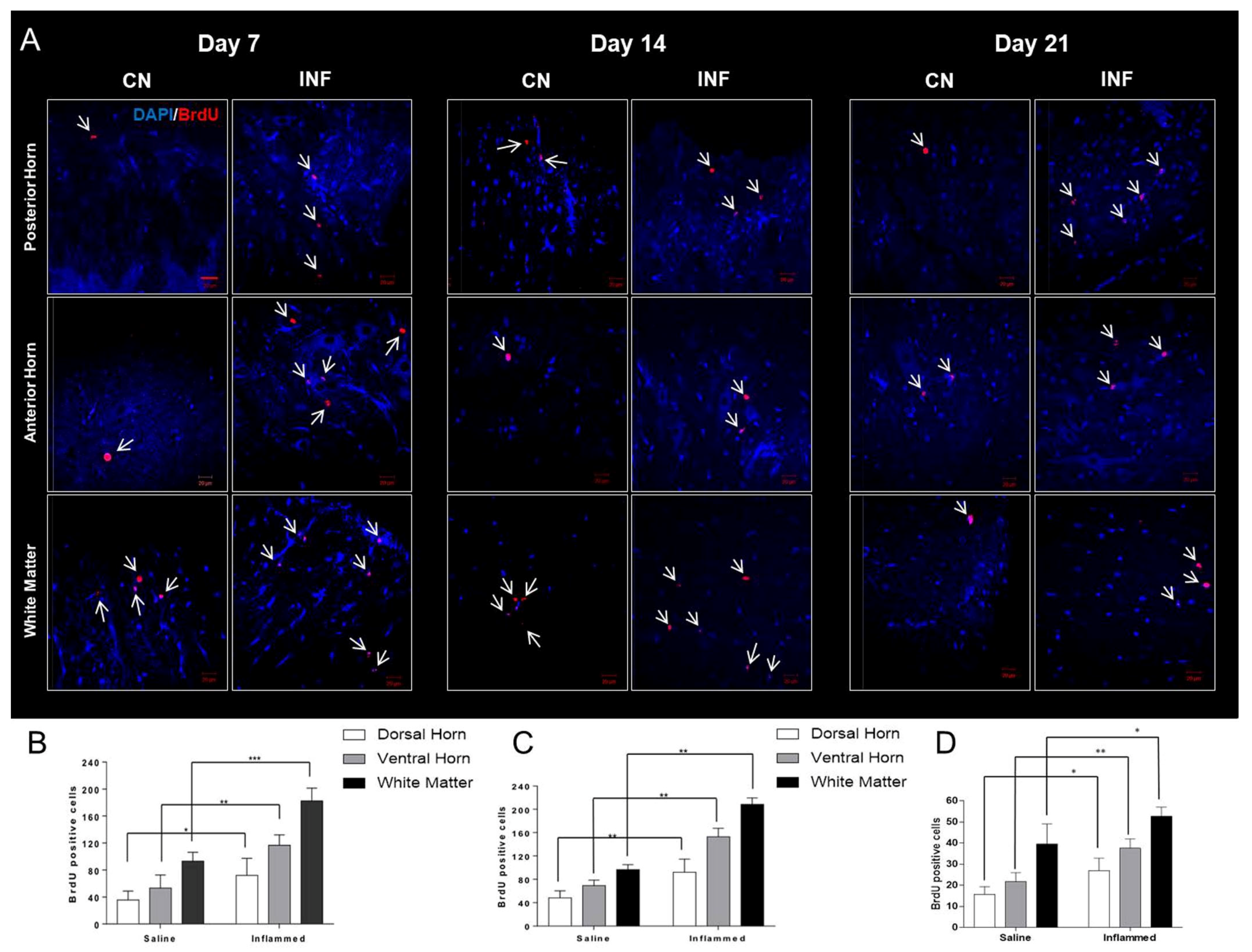
2.7. The Effect of CFA-Induced Inflammation on Cellular Proliferation Detected by BrdU-Positive Cells in the Spinal Cord
2.8. The Effect of the NMDA Receptor Antagonist Ketamine on the Expression of HSPs in the Spinal Cord
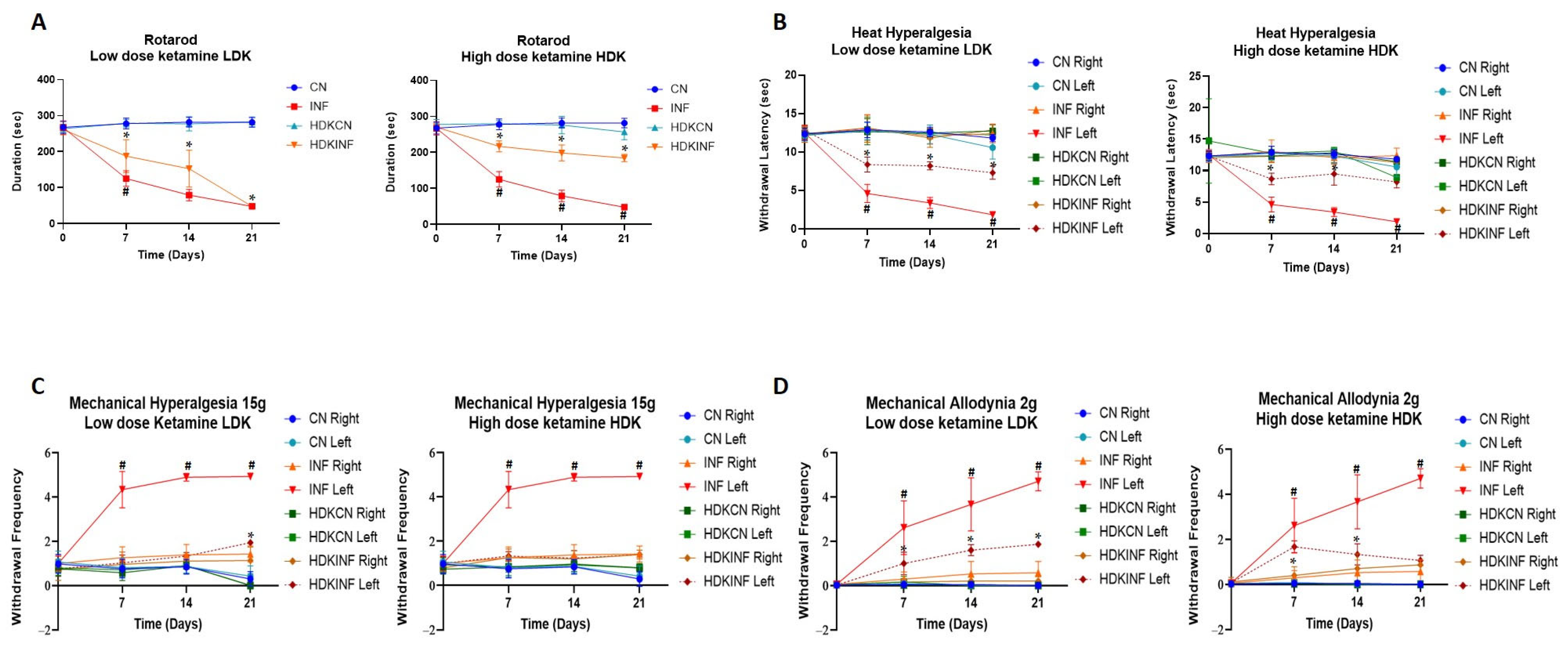
2.8.1. Motor Behavioral Testing
2.8.2. Heat Hyperalgesia
2.8.3. Mechanical Hyperalgesia
2.8.4. Mechanical Allodynia
2.8.5. Expression of HSPs in the Spinal Cord of Control and Inflamed Rats Treated with Low and High Doses of Ketamine
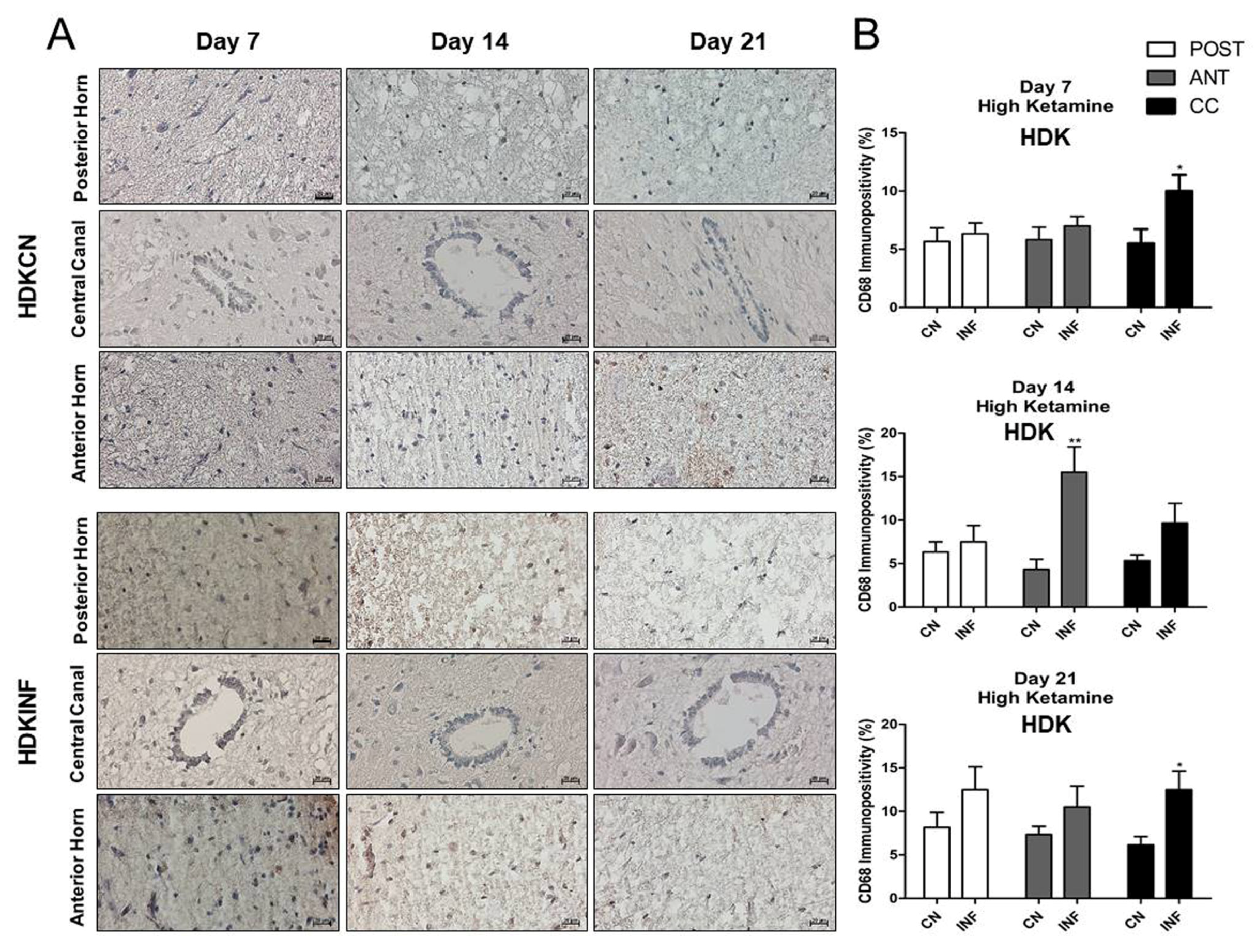
2.8.6. CD68-Immunopositivity in the Different Regions of the Spinal Cord of Rats Treated with Ketamine
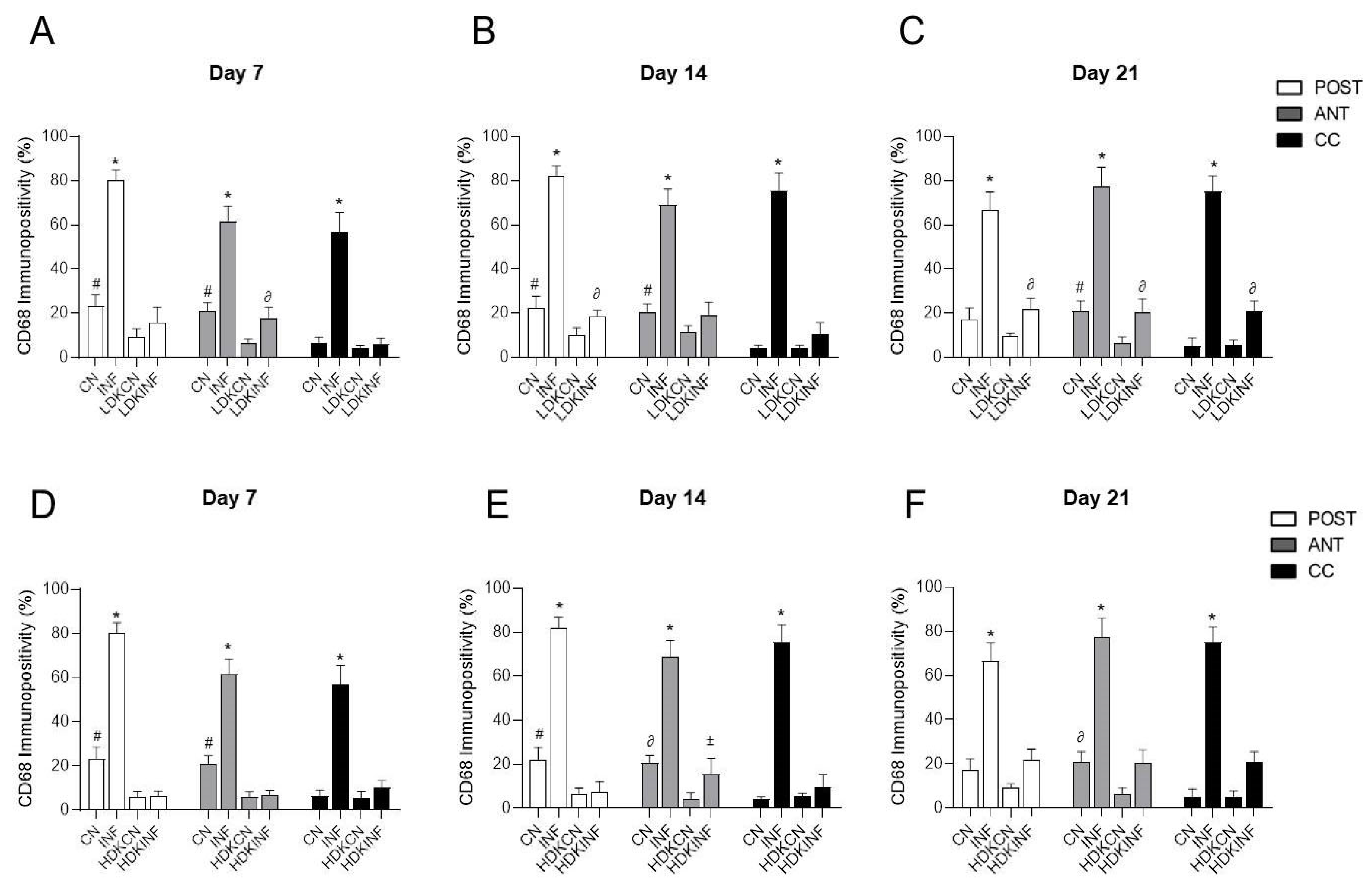
2.8.7. Comparisons of CD68-Immunopositivity in the Different Regions of the Spinal Cord of All Animal Groups
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Blocking of NMDA Receptors
4.3. BrdU Preparation and Administration
4.4. Joint Circumference Measurement
4.5. Nociceptive Behavioral Testing
4.5.1. Motor Behavioral Test
4.5.2. Heat Hyperalgesia Test
4.5.3. Mechanical Allodynia Test
4.5.4. Mechanical Hyperalgesia Test
4.6. Cervical Dislocation and Sample Collection
4.7. Animal Perfusion and Sample Collection
4.8. Tissue Processing
4.9. Immunofluorescence Detection and Quantification of BrdU
4.10. Immunohistochemistry
4.11. Immunofluorescence and Confocal Microscopy
4.12. Western Blot
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, H. What Is the Global Prevalence of Rheumatoid Arthritis (RA) Among Different Age Groups and Ethnicities. Medscape 2020, 1, 136. [Google Scholar]
- Fouani, M.; Basset, C.A.; Mangano, G.D.; Leone, L.G.; Lawand, N.B.; Leone, A.; Barone, R. Heat Shock Proteins Alterations in Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 2806. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Goldring, M.B. Cells of the Synovium in Rheumatoid Arthritis. Chondrocytes. Arthritis Res. Ther. 2007, 9, 220. [Google Scholar] [CrossRef]
- Choy, E. Understanding the Dynamics: Pathways Involved in the Pathogenesis of Rheumatoid Arthritis. Rheumatology 2012, 51, v3–v11. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.M.; McInnes, I.B. Evidence That Cytokines Play a Role in Rheumatoid Arthritis. J. Clin. Investig. 2008, 118, 3537–3545. [Google Scholar] [CrossRef]
- Min, H.K.; Kim, K.-W.; Lee, S.-H.; Kim, H.-R. Roles of Mast Cells in Rheumatoid Arthritis. Korean J. Intern. Med. 2020, 35, 12–24. [Google Scholar] [CrossRef]
- Yap, H.-Y.; Tee, S.; Wong, M.; Chow, S.-K.; Peh, S.-C.; Teow, S.-Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161. [Google Scholar] [CrossRef]
- Liu, P.-C.; Ssu, C.-T.; Tsao, Y.-P.; Liou, T.-L.; Tsai, C.-Y.; Chou, C.-T.; Chen, M.-H.; Leu, C.-M. Cytotoxic T Lymphocyte-Associated Antigen-4-Ig (CTLA-4-Ig) Suppresses Staphylococcus Aureus-Induced CD80, CD86, and pro-Inflammatory Cytokine Expression in Human B Cells. Arthritis Res. Ther. 2020, 22, 64. [Google Scholar] [CrossRef]
- Spierings, J.; van Eden, W. Heat Shock Proteins and Their Immunomodulatory Role in Inflammatory Arthritis. Rheumatology 2017, 56, 198–208. [Google Scholar] [CrossRef]
- Ngarmukos, S.; Scaramuzza, S.; Theerawattanapong, N.; Tanavalee, A.; Honsawek, S. Circulating and Synovial Fluid Heat Shock Protein 70 Are Correlated with Severity in Knee Osteoarthritis. CARTILAGE 2020, 11, 323–328. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Howe, F.A.; Allen, R.L.; Sofat, N. New Insights into the Impact of Neuro-Inflammation in Rheumatoid Arthritis. Front. Neurosci. 2014, 8, 357. [Google Scholar] [CrossRef]
- Biddle, G.; Beck, R.T.; Raslan, O.; Ebinu, J.; Jenner, Z.; Hamer, J.; Hacein-Bey, L.; Apperson, M.; Ivanovic, V. Autoimmune Diseases of the Spine and Spinal Cord. Neuroradiol. J. 2024, 37, 285–303. [Google Scholar] [CrossRef]
- Luo, X.; Zuo, X.; Zhou, Y.; Zhang, B.; Shi, Y.; Liu, M.; Wang, K.; McMillian, D.R.; Xiao, X. Extracellular Heat Shock Protein 70 Inhibits Tumour Necrosis Factor-α Induced Proinflammatory Mediator Production in Fibroblast-like Synoviocytes. Arthritis Res. Ther. 2008, 10, R41. [Google Scholar] [CrossRef]
- Quintana, F.J.; Carmi, P.; Mor, F.; Cohen, I.R. Inhibition of Adjuvant-Induced Arthritis by DNA Vaccination with the 70-Kd or the 90-Kd Human Heat-Shock Protein: Immune Cross-Regulation with the 60-Kd Heat-Shock Protein. Arthritis Rheum. 2004, 50, 3712–3720. [Google Scholar] [CrossRef]
- Swaroop, S.; Sengupta, N.; Suryawanshi, A.R.; Adlakha, Y.K.; Basu, A. HSP60 Plays a Regulatory Role in IL-1β-Induced Microglial Inflammation via TLR4-P38 MAPK Axis. J. Neuroinflamm. 2016, 13, 27. [Google Scholar] [CrossRef]
- Vercoulen, Y.; van Teijlingen, N.H.; de Kleer, I.M.; Kamphuis, S.; Albani, S.; Prakken, B.J. Heat Shock Protein 60 Reactive T Cells in Juvenile Idiopathic Arthritis: What Is New? Arthritis Res. Ther. 2009, 11, 231. [Google Scholar] [CrossRef]
- Miyakoshi, L.M.; Marques-Coelho, D.; De Souza, L.E.R.; Lima, F.R.S.; Martins, V.R.; Zanata, S.M.; Hedin-Pereira, C. Evidence of a Cell Surface Role for Hsp90 Complex Proteins Mediating Neuroblast Migration in the Subventricular Zone. Front. Cell. Neurosci. 2017, 11, 138. [Google Scholar] [CrossRef]
- Bobkova, N.V.; Evgen’ev, M.; Garbuz, D.G.; Kulikov, A.M.; Morozov, A.; Samokhin, A.; Velmeshev, D.; Medvinskaya, N.; Nesterova, I.; Pollock, A.; et al. Exogenous Hsp70 Delays Senescence and Improves Cognitive Function in Aging Mice. Proc. Natl. Acad. Sci. USA 2015, 112, 16006–16011. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Willis, W.D.; Westlund, K.N. Central Control of Peripheral Joint Inflammation and Heat Hyperalgesia. Prog. Pain Res. Manag. 1994, 2, 359–371. [Google Scholar] [PubMed]
- Zhang, X.; Nicholas, A.P.; Hökfelt, T. Ultrastructural Studies on Peptides in the Dorsal Horn of the Spinal Cord—I. Co-Existence of Galanin with Other Peptides in Primary Afferents in Normal Rats. Neuroscience 1993, 57, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Menniti, F.S.; Traynelis, S.F. NMDA Receptors in the Central Nervous System. Methods Mol. Biol. 2017, 1677, 1–80. [Google Scholar] [CrossRef] [PubMed]
- Parada-Turska, J.; Rzeski, W.; Majdan, M.; Kandefer-Szerszeń, M.; Turski, W.A. Effect of Glutamate Receptor Antagonists and Antirheumatic Drugs on Proliferation of Synoviocytes In Vitro. Eur. J. Pharmacol. 2006, 535, 95–97. [Google Scholar] [CrossRef]
- Lam, F.F.; Ng, E.S. Substance P and Glutamate Receptor Antagonists Improve the Anti-Arthritic Actions of Dexamethasone in Rats: Combined Therapy on Experimental Arthritis. Br. J. Pharmacol. 2010, 159, 958–969. [Google Scholar] [CrossRef]
- Porto, R.R.; Dutra, F.D.; Crestani, A.P.; Holsinger, R.M.D.; Quillfeldt, J.A.; Homem De Bittencourt, P.I.; De Oliveira Alvares, L. HSP70 Facilitates Memory Consolidation of Fear Conditioning through MAPK Pathway in the Hippocampus. Neuroscience 2018, 375, 108–118. [Google Scholar] [CrossRef]
- Zhao, Z.; Faden, A.I.; Loane, D.J.; Lipinski, M.M.; Sabirzhanov, B.; Stoica, B.A. Neuroprotective Effects of Geranylgeranylacetone in Experimental Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 2013, 33, 1897–1908. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, R. Outpatient Ketamine Infusions for the Treatment of Fibromyalgia and Chronic Pain Syndrome: A Case Report. Cureus 2023, 15, e44909. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Measuring Motor Coordination in Mice. J. Vis. Exp. 2013, 29, e2609. [Google Scholar] [CrossRef] [PubMed]
- Dukay, B.; Csoboz, B.; Tóth, M.E. Heat-Shock Proteins in Neuroinflammation. Front. Pharmacol. 2019, 10, 920. [Google Scholar] [CrossRef]
- Raoof, R.; Willemen, H.L.D.M.; Eijkelkamp, N. Divergent Roles of Immune Cells and Their Mediators in Pain. Rheumatology 2018, 57, 429–440. [Google Scholar] [CrossRef]
- Hashmi, J.A.; Yashpal, K.; Holdsworth, D.W.; Henry, J.L. Sensory and Vascular Changes in a Rat Monoarthritis Model: Prophylactic and Therapeutic Effects of Meloxicam. Inflamm. Res. 2010, 59, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Redlich, K.; Xu, Q.; Bizan, P.; Gröger, M.; Tohidast-Akrad, M.; Kiener, H.; Smolen, J.; Steiner, G. Enhanced Expression of Heat Shock Protein 70 (Hsp70) and Heat Shock Factor 1 (HSF1) Activation in Rheumatoid Arthritis Synovial Tissue. Differential Regulation of Hsp70 Expression and Hsf1 Activation in Synovial Fibroblasts by Proinflammatory Cytokines, Shear Stress, and Antiinflammatory Drugs. J. Clin. Investig. 1998, 102, 302–311. [Google Scholar] [CrossRef]
- Sedlackova, L.; Nguyen, T.T.H.; Zlacka, D.; Sosna, A.; Hromadnikova, I. Cell Surface and Relative mRNA Expression of Heat Shock Protein 70 in Human Synovial Cells. Autoimmunity 2009, 42, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mantej, J.; Polasik, K.; Piotrowska, E.; Tukaj, S. Autoantibodies to Heat Shock Proteins 60, 70, and 90 in Patients with Rheumatoid Arthritis. Cell Stress Chaperones 2019, 24, 283–287. [Google Scholar] [CrossRef]
- Tukaj, S.; Mantej, J.; Sobala, M.; Potrykus, K.; Sitko, K. Autologous Extracellular Hsp70 Exerts a Dual Role in Rheumatoid Arthritis. Cell Stress Chaperones 2020, 25, 1105–1110. [Google Scholar] [CrossRef]
- Malange, K.F.; Navia-Pelaez, J.M.; Dias, E.V.; Lemes, J.B.P.; Choi, S.-H.; Dos Santos, G.G.; Yaksh, T.L.; Corr, M. Macrophages and Glial Cells: Innate Immune Drivers of Inflammatory Arthritic Pain Perception from Peripheral Joints to the Central Nervous System. Front. Pain Res. 2022, 3, 1018800. [Google Scholar] [CrossRef]
- Grundtman, C.; Kreutmayer, S.B.; Almanzar, G.; Wick, M.C.; Wick, G. Heat Shock Protein 60 and Immune Inflammatory Responses in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 960–968. [Google Scholar] [CrossRef]
- Uhelski, M.L.; Li, Y.; Fonseca, M.M.; Romero-Snadoval, E.A.; Dougherty, P.M. Role of Innate Immunity in Chemotherapy-Induced Peripheral Neuropathy. Neurosci. Lett. 2021, 755, 135941. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and Its Role in Neuroprotection, Axonal Regeneration and Functional Recovery after Spinal Cord Injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Giacobazzi, G.; Di Carlo, M. Chronic Pain in Inflammatory Arthritis: Mechanisms, Metrology, and Emerging Targets—A Focus on the JAK-STAT Pathway. Pain Res. Manag. 2018, 2018, 8564215. [Google Scholar] [CrossRef] [PubMed]
- Eminaga, S.; Teekakirikul, P.; Seidman, C.E.; Seidman, J.G. Detection of Cell Proliferation Markers by Immunofluorescence Staining and Microscopy Imaging in Paraffin-Embedded Tissue Sections. Curr. Protoc. Mol. Biol. 2016, 115, 14–25. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Chen, S.-R.; Pan, H.-L. Targeting N-Methyl-D-Aspartate Receptors for Treatment of Neuropathic Pain. Expert Rev. Clin. Pharmacol. 2011, 4, 379–388. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Tomitaka, S.; Hashimoto, K.; Narita, N.; Sakamoto, A.; Minabe, Y.; Tamura, A. Memantine Induces Heat Shock Protein HSP70 in the Posterior Cingulate Cortex, Retrosplenial Cortex and Dentate Gyrus of Rat Brain. Brain Res. 1996, 740, 1–5. [Google Scholar] [CrossRef]
- Neto, E.D.S.; Pedro, P.P.D.A.; Cartágenes, M.D.S.D.S.; Neto, J.O.B.; Garcia, J.B.S. The Effect of Low Dose Intra-Articular S(+) Ketamine on Osteoarthritis in Rats: An Experimental Study. Braz. J. Anesthesiol. 2024, 74, 844502. [Google Scholar] [CrossRef]
- Lu, W.; Wang, L.; Wo, C.; Yao, J. Ketamine Attenuates Osteoarthritis of the Knee via Modulation of Inflammatory Responses in a Rabbit Model. Mol. Med. Rep. 2016, 13, 5013–5020. [Google Scholar] [CrossRef] [PubMed]
- Sevin, M.; Girodon, F.; Garrido, C.; de Thonel, A. HSP90 and HSP70: Implication in Inflammation Processes and Therapeutic Approaches for Myeloproliferative Neoplasms. Mediat. Inflamm. 2015, 2015, 970242. [Google Scholar] [CrossRef]
- Singh, M.K.; Shin, Y.; Han, S.; Ha, J.; Tiwari, P.K.; Kim, S.S.; Kang, I. Molecular Chaperonin HSP60: Current Understanding and Future Prospects. Int. J. Mol. Sci. 2024, 25, 5483. [Google Scholar] [CrossRef] [PubMed]
- Boettger, M.K.; Weber, K.; Gajda, M.; Bräuer, R.; Schaible, H.-G. Spinally Applied Ketamine or Morphine Attenuate Peripheral Inflammation and Hyperalgesia in Acute and Chronic Phases of Experimental Arthritis. Brain Behav. Immun. 2010, 24, 474–485. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Z.; Shen, J.; Xu, J.; Li, H. Intravenous Anesthetic Ketamine Attenuates Complete Freund’s Adjuvant-Induced Arthritis in Rats via Modulation of MAPKs/NF-κB. Inflamm. Res. 2019, 68, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, K.E.; Vanderah, T.W. Constitutive Activity at the Cannabinoid CB1 Receptor and Behavioral Responses. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 484, pp. 3–30. ISBN 978-0-12-381298-8. [Google Scholar]
- Noh, A.S.M.; Chuan, T.D.; Khir, N.A.M.; Zin, A.A.M.; Ghazali, A.K.; Long, I.; Ab Aziz, C.B.; Ismail, C.A.N. Effects of Different Doses of Complete Freund’s Adjuvant on Nociceptive Behaviour and Inflammatory Parameters in Polyarthritic Rat Model Mimicking Rheumatoid Arthritis. PLoS ONE 2021, 16, e0260423. [Google Scholar] [CrossRef]
- Cheah, M.; Fawcett, J.; Andrews, M. Assessment of Thermal Pain Sensation in Rats and Mice Using the Hargreaves Test. Bio-Protocol 2017, 7, e2506. [Google Scholar] [CrossRef]
- Notartomaso, S.; Scarselli, P.; Di Pietro, P.; Battaglia, G.; Llebaria, A.; Ciruela, F.; Nicoletti, F. Mechanical Allodynia Assessment in a Murine Neuropathic Pain Model. Bio-Protocol 2018, 8, e2671. [Google Scholar] [CrossRef]
- Woode, E.; Abotsi, W.K.M.; Adosraku, R.; Ameyaw, E.; Boakye-Gyasi, E.; Kyekyeku, J. Anti-Allodynic and Anti-Hyperalgesic Effects of an Ethanolic Extract and Xylopic Acid from the Fruits of Xylopia Aethiopica in Murine Models of Neuropathic Pain. Pharmacogn. Res. 2014, 6, 172. [Google Scholar] [CrossRef]
- Chamaa, F.; Sweidan, W.; Nahas, Z.; Saade, N.; Abou-Kheir, W. Thalamic Stimulation in Awake Rats Induces Neurogenesis in the Hippocampal Formation. Brain Stimul. 2016, 9, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Macaluso, F.; Sangiorgi, C.; Campanella, C.; Marino Gammazza, A.; Moresi, V.; Coletti, D.; Conway De Macario, E.; Macario, A.J.; Cappello, F.; et al. Skeletal Muscle Heat Shock Protein 60 Increases after Endurance Training and Induces Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1 A1 Expression. Sci. Rep. 2016, 6, 19781. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Sangiorgi, C.; Marino Gammazza, A.; D’Amico, D.; Salerno, M.; Cappello, F.; Pomara, C.; Zummo, G.; Farina, F.; Di Felice, V.; et al. Effects of Conjugated Linoleic Acid Associated With Endurance Exercise on Muscle Fibres and Peroxisome Proliferator-Activated Receptor γ Coactivator 1 α Isoforms. J. Cell. Physiol. 2017, 232, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Rappa, F.; Sciume, C.; Lo Bello, M.; Bavisotto, C.C.; Marino Gammazza, A.; Barone, R.; Campanella, C.; David, S.; Carini, F.; Zarcone, F.; et al. Comparative Analysis of Hsp10 and Hsp90 Expression in Healthy Mucosa and Adenocarcinoma of the Large Bowel. Anticancer Res. 2014, 34, 4153–4159. [Google Scholar]
- Rappa, F.; Pitruzzella, A.; Marino Gammazza, A.; Barone, R.; Mocciaro, E.; Tomasello, G.; Carini, F.; Farina, F.; Zummo, G.; De Macario, E.C.; et al. Quantitative Patterns of Hsps in Tubular Adenoma Compared with Normal and Tumor Tissues Reveal the Value of Hsp10 and Hsp60 in Early Diagnosis of Large Bowel Cancer. Cell Stress Chaperones 2016, 21, 927–933. [Google Scholar] [CrossRef]

| Primary Antibody | Animal Host | Distributor | WB | IF | IHC |
|---|---|---|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | Rabbit | LOT-3557958, Rabbit Polyclonal EMD Millipore Corp., Burlington, MA, USA | 1:1000 | - | - |
| Heat Shock Protein 90 (Hsp90) | Rabbit | Ab-13495, Rabbit Monoclonal Abcam, Cambridge, UK | 1:1000 | 1:50 | - |
| Heat Shock Protein 70 (Hsp70) | Mouse | Ab-2787, Mouse Monoclonal Abcam, Cambridge, UK | 1:1000 | 1:50 | - |
| Heat Shock Protein 60 (Hsp60) | Mouse | Ab-13532, Mouse Monoclonal Abcam, Cambridge, UK | 1:1000 | 1:50 | - |
| Heat Shock Protein 27 (Hsp27) | Goat | Sc-1048, Goat Polyclonal Santa Cruz Biotechnology, Dallas, TX, USA | 1:1000 | 1:50 | - |
| Glial Fibrillary Acidic Protein (GFAP) | Mouse | Sc-33673, Mouse Monoclonal Santa Cruz Biotechnology, Dallas, TX, USA | 1:1000 | 1:50 | - |
| Bromodeoxyuridine (BrdU) | Mouse | Sc-32323, Mouse Monoclonal Santa Cruz Biotechnology, Dallas, TX, USA | - | 1:100 | - |
| Neuronal Nuclear Antigen (NeuN) | Rabbit | R-A22113, Rabbit Monoclonal Neuromics, Edina, MN, USA | - | 1:500 | - |
| Cluster of Differentiation 68 (CD68) | Mouse | Sc-20060, Mouse Monoclonal Santa Cruz Biotechnology, Dallas, TX, USA | - | - | 1:150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouani, M.; Scalia, F.; Mangano, G.D.; Rappa, F.; Abou-Kheir, W.; Leone, A.; Lawand, N.; Barone, R. Exploring the Role of Heat Shock Proteins in Neuroimmune Modulation in Rheumatoid Arthritis: Insights from a Rat Model. Int. J. Mol. Sci. 2025, 26, 9743. https://doi.org/10.3390/ijms26199743
Fouani M, Scalia F, Mangano GD, Rappa F, Abou-Kheir W, Leone A, Lawand N, Barone R. Exploring the Role of Heat Shock Proteins in Neuroimmune Modulation in Rheumatoid Arthritis: Insights from a Rat Model. International Journal of Molecular Sciences. 2025; 26(19):9743. https://doi.org/10.3390/ijms26199743
Chicago/Turabian StyleFouani, Malak, Federica Scalia, Giuseppe Donato Mangano, Francesca Rappa, Wassim Abou-Kheir, Angelo Leone, Nada Lawand, and Rosario Barone. 2025. "Exploring the Role of Heat Shock Proteins in Neuroimmune Modulation in Rheumatoid Arthritis: Insights from a Rat Model" International Journal of Molecular Sciences 26, no. 19: 9743. https://doi.org/10.3390/ijms26199743
APA StyleFouani, M., Scalia, F., Mangano, G. D., Rappa, F., Abou-Kheir, W., Leone, A., Lawand, N., & Barone, R. (2025). Exploring the Role of Heat Shock Proteins in Neuroimmune Modulation in Rheumatoid Arthritis: Insights from a Rat Model. International Journal of Molecular Sciences, 26(19), 9743. https://doi.org/10.3390/ijms26199743












