Role of Repeat Tract Structure and the rs7158733 SNP in Spinocerebellar Ataxia 3
Abstract
1. Introduction
2. Results
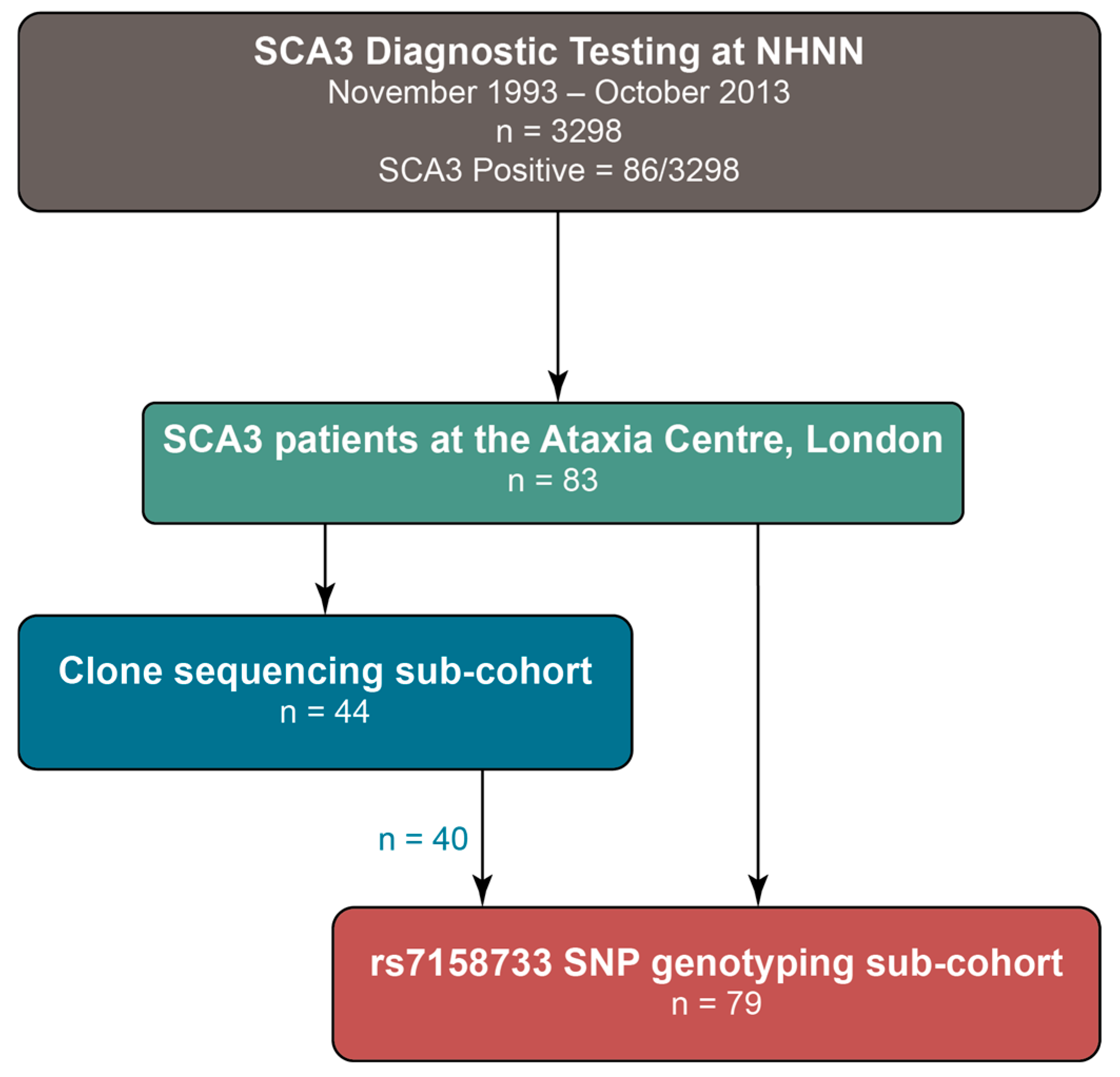
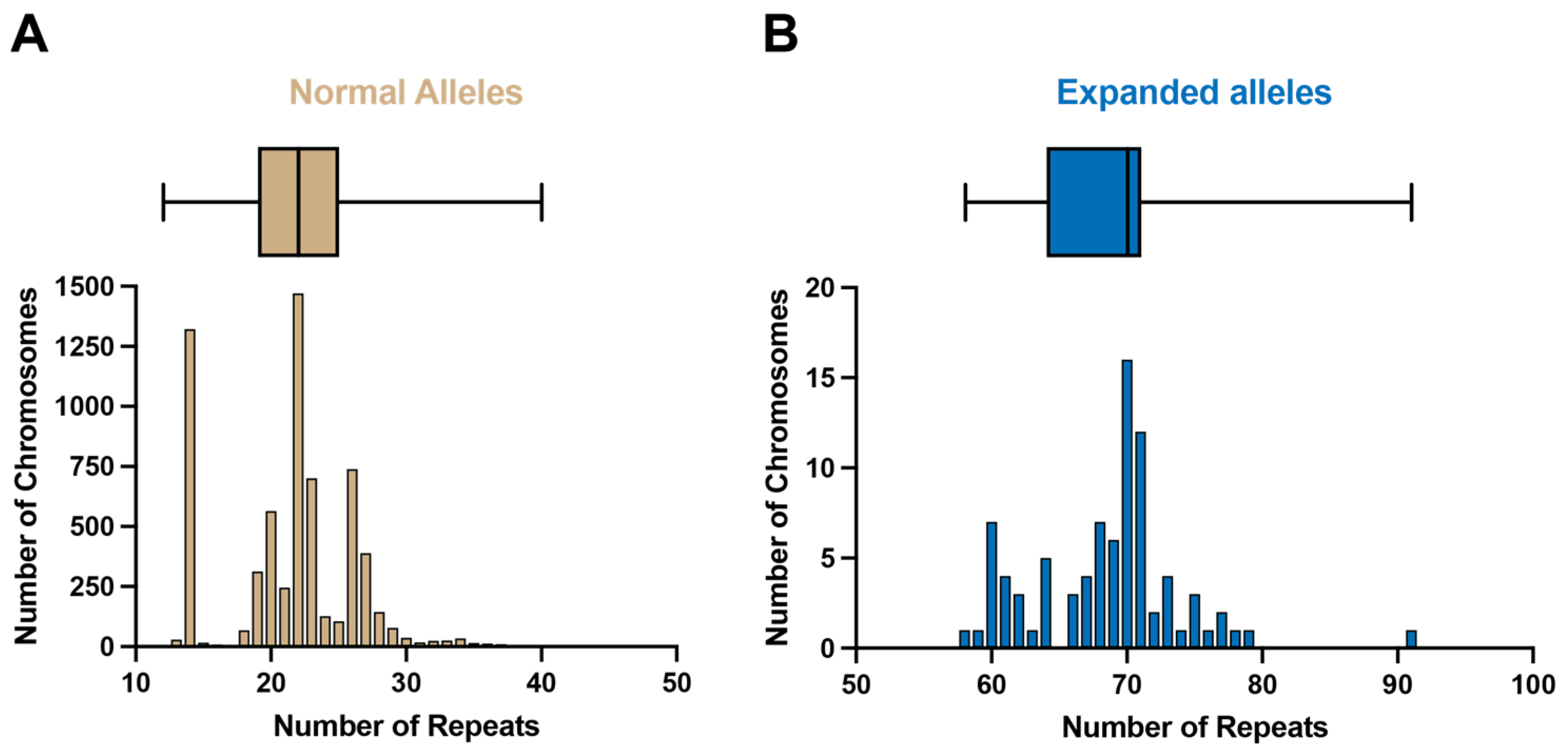
2.1. ATXN3 Allele Distribution in a Large UK Cohort
2.2. Clone Sequencing
2.2.1. Loss of Canonical Interruptions
2.2.2. Presence of Additional Interruptions
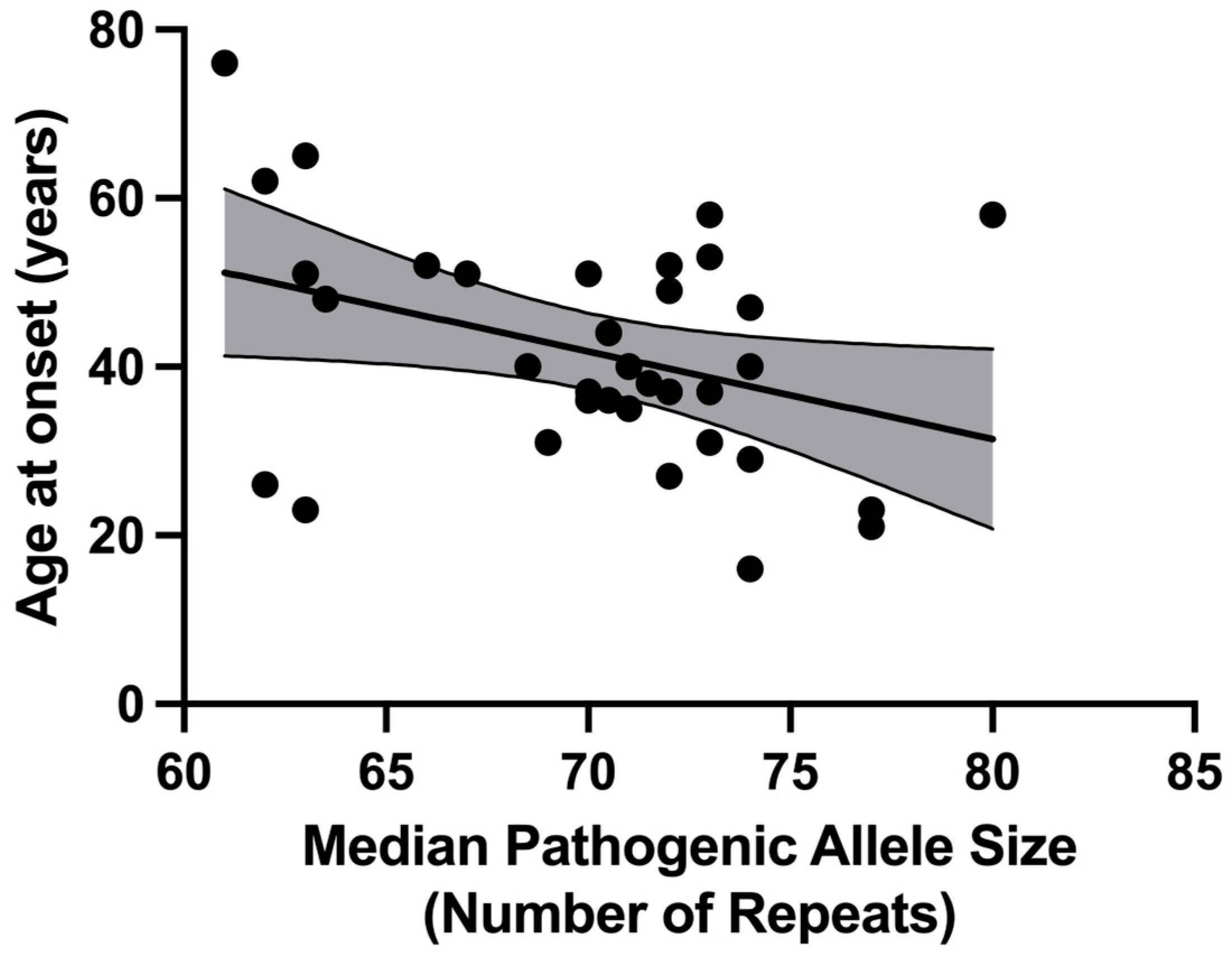
2.2.3. Correlation with Age at Disease Onset
2.2.4. Non-Expanded Alleles
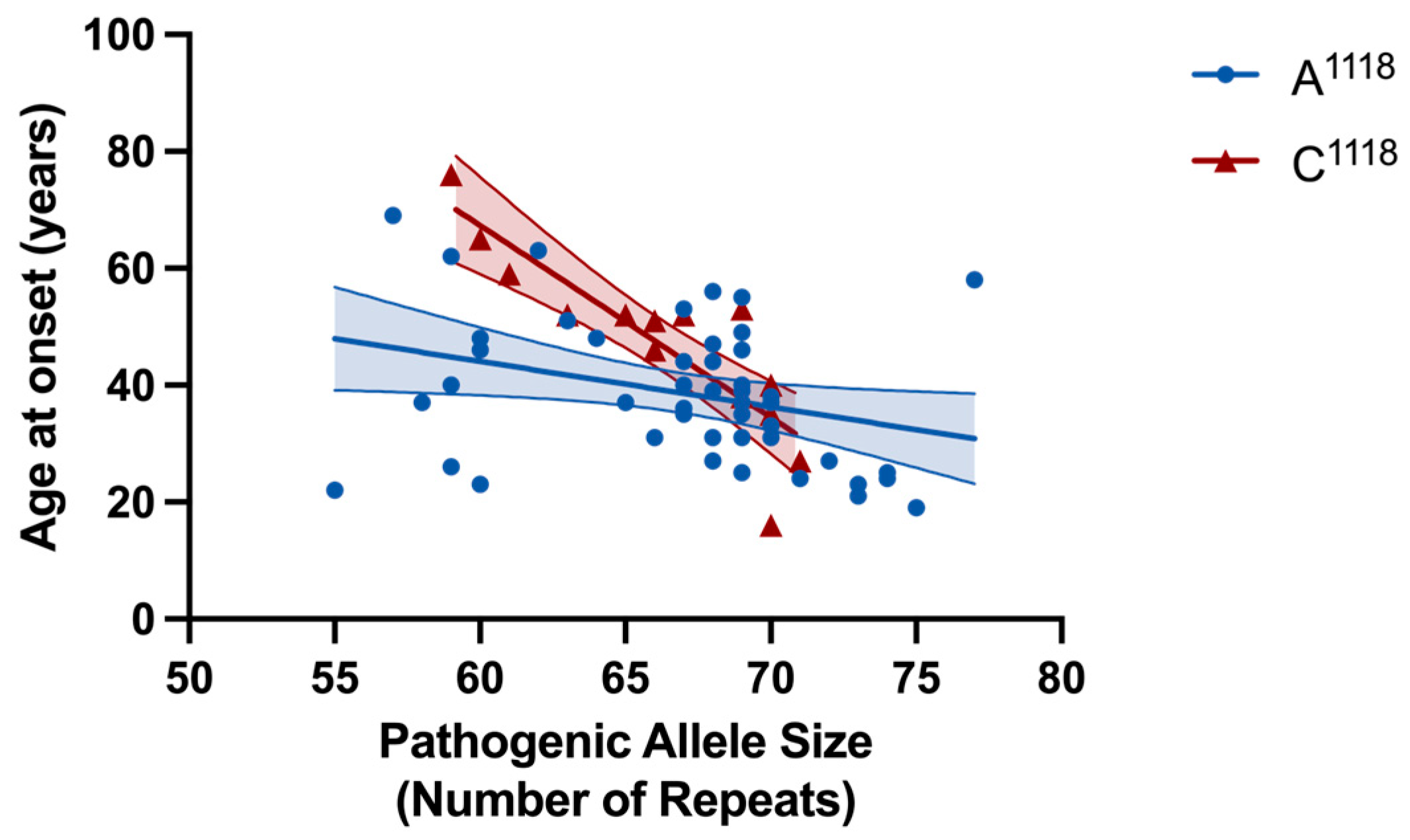
2.3. rs7158733 SNP (A1118/C1118) Allele Frequencies
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Patient Cohort
4.3. SCA3 Fragment Sizing and Cloning of SCA3 Allele CAG-Repeat Tracts
4.4. Characterisation of the rs7158733 SNP (A1118/C1118)
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | Activities of daily living |
| ESMI | European spinocerebellar ataxia type 3/Machado–Joseph disease initiative |
| EUROSCA | European integrated project on spinocerebellar ataxias |
| HD | Huntington’s Disease |
| INAS | Inventory of non-ataxia signs |
| MJD | Machado–Joseph disease |
| RBP | RNA-binding protein |
| SARA | Scale for the assessment and rating of ataxia |
| SCA | Spinocerebellar ataxia |
| SNP | Single-nucleotide polymorphism |
| TP-PCR | Triplet repeat primed PCR |
| WGS | Whole Genome Sequencing |
References
- do Carmo Costa, M.; Paulson, H.L. Toward understanding Machado-Joseph disease. Prog. Neurobiol. 2012, 97, 239–257. [Google Scholar]
- Kawaguchi, Y.; Okamoto, T.; Taniwaki, M.; Aizawa, M.; Inoue, M.; Katayama, S.; Kawakami, H.; Nakamura, S.; Nishimura, M.; Akiguchi, I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat. Genet. 1994, 8, 221–228. [Google Scholar] [CrossRef]
- Giunti, P.; Sweeney, M.G.; Harding, A.E. Detection of the Machado-Joseph disease/spinocerebellar ataxia three trinucleotide repeat expansion in families with autosomal dominant motor disorders, including the Drew family of Walworth. Brain A J. Neurol. 1995, 118 Pt 5, 1077–1085. [Google Scholar] [CrossRef]
- Carvalho, D.R.; La Rocque-Ferreira, A.; Rizzo, I.M.; Imamura, E.U.; Speck-Martins, C.E. Homozygosity enhances severity in spinocerebellar ataxia type 3. Pediatr. Neurol. 2008, 38, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Sequeiros, J.; Coutinho, P. Epidemiology and clinical aspects of Machado-Joseph disease. Adv. Neurol. 1993, 61, 139–153. [Google Scholar]
- Jardim, L.B.; Pereira, M.L.; Silveira, I.; Ferro, A.; Sequeiros, J.; Giugliani, R. Neurologic findings in Machado-Joseph disease: Relation with disease duration, subtypes, and (CAG)n. Arch. Neurol. 2001, 58, 899–904. [Google Scholar] [CrossRef]
- Schols, L.; Amoiridis, G.; Epplen, J.T.; Langkafel, M.; Przuntek, H.; Riess, O. Relations between genotype and phenotype in German patients with the Machado-Joseph disease mutation. J. Neurol. Neurosurg. Psychiatry 1996, 61, 466–470. [Google Scholar] [CrossRef]
- Maciel, P.; Gaspar, C.; DeStefano, A.L.; Silveira, I.; Coutinho, P.; Radvany, J.; Dawson, D.M.; Sudarsky, L.; Guimaraes, J.; Loureiro, J.E.; et al. Correlation between CAG repeat length and clinical features in Machado-Joseph disease. Am. J. Hum. Genet. 1995, 57, 54–61. [Google Scholar]
- Menon, R.P.; Nethisinghe, S.; Faggiano, S.; Vannocci, T.; Rezaei, H.; Pemble, S.; Sweeney, M.G.; Wood, N.W.; Davis, M.B.; Pastore, A.; et al. The role of interruptions in polyQ in the pathology of SCA1. PLoS Genet. 2013, 9, e1003648. [Google Scholar]
- Wiethoff, S.; O’Connor, E.; Haridy, N.A.; Nethisinghe, S.; Wood, N.; Giunti, P.; Bettencourt, C.; Houlden, H. Sequencing analysis of the SCA6 CAG expansion excludes an influence of repeat interruptions on disease onset. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1226–1227. [Google Scholar] [CrossRef] [PubMed]
- Nethisinghe, S.; Lim, W.N.; Ging, H.; Zeitlberger, A.; Abeti, R.; Pemble, S.; Sweeney, M.G.; Labrum, R.; Cervera, C.; Houlden, H.; et al. Complexity of the Genetics and Clinical Presentation of Spinocerebellar Ataxia 17. Front. Cell. Neurosci. 2018, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Calafell, F.; Gaspar, C.; Wong, V.C.N.; Silveira, I.; Nicholson, G.A.; Brunt, E.R.; Tranebjaerg, L.; Stevanin, G.; Hsieh, M.; et al. Asian origin for the worldwide-spread mutational event in Machado-Joseph disease. Arch. Neurol. 2007, 64, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Goto, J.; Watanabe, M.; Ichikawa, Y.; Yee, S.B.; Ihara, N.; Endo, K.; Igarashi, S.; Takiyama, Y.; Gaspar, C.; Maciel, P.; et al. Machado-Joseph disease gene products carrying different carboxyl termini. Neurosci. Res. 1997, 28, 373–377. [Google Scholar]
- Stevanin, G.; Lebre, A.S.; Mathieux, C.; Cancel, G.; Abbas, N.; Didierjean, O.; Durr, A.; Trottier, Y.; Agid, Y.; Brice, A. Linkage disequilibrium between the spinocerebellar ataxia 3/Machado-Joseph disease mutation and two intragenic polymorphisms, one of which, X359Y, affects the stop codon. Am. J. Hum. Genet. 1997, 60, 1548–1552. [Google Scholar] [PubMed]
- Gaspar, C.; Lopes-Cendes, I.; Hayes, S.; Goto, J.; Arvidsson, K.; Dias, A.; Silveira, I.; Maciel, P.; Coutinho, P.; Lima, M.; et al. Ancestral origins of the Machado-Joseph disease mutation: A worldwide haplotype study. Am. J. Hum. Genet. 2001, 68, 523–528. [Google Scholar] [CrossRef]
- Prudencio, M.; Garcia-Moreno, H.; Jansen-West, K.R.; Al-Shaikh, R.H.; Gendron, T.F.; Heckman, M.G.; Spiegel, M.R.; Carlomagno, Y.; Daughrity, L.M.; Song, Y.; et al. Toward allele-specific targeting therapy and pharmacodynamic marker for spinocerebellar ataxia type 3. Sci. Transl. Med. 2020, 12, eabb7086. [Google Scholar] [CrossRef]
- Melo, A.R.V.; Raposo, M.; Ventura, M.; Martins, S.; Pavao, S.; Alonso, I.; Bettencourt, C.; Lima, M. Genetic Variation in ATXN3 (Ataxin-3) 3’UTR: Insights into the Downstream Regulatory Elements of the Causative Gene of Machado-Joseph Disease/Spinocerebellar Ataxia Type 3. Cerebellum 2023, 22, 37–45. [Google Scholar]
- Weishäupl, D.; Schneider, J.; Peixoto Pinheiro, B.; Ruess, C.; Dold, S.M.; von Zweydorf, F.; Gloeckner, C.J.; Schmidt, J.; Riess, O.; Schmidt, T. Physiological and pathophysiological characteristics of ataxin-3 isoforms. J. Biol. Chem. 2019, 294, 644–661. [Google Scholar] [CrossRef]
- Tuite, P.J.; Rogaeva, E.A.; St George-Hyslop, P.H.; Lang, A.E. Dopa-responsive parkinsonism pheotype of Machado-Joseph disease: Confirmation of 14q CAG expansion. Ann. Neurol. 1995, 38, 684–687. [Google Scholar]
- Hsieh, M.; Tsai, H.F.; Lu, T.M.; Yang, C.Y.; Wu, H.M.; Li, S.Y. Studies of the CAG repeat in the Machado-Joseph disease gene in Taiwan. Hum. Genet. 1997, 100, 155–162. [Google Scholar] [CrossRef]
- Van Shaik, I.N.; Jöbsis, G.J.; Vermeulen, M.; Keizers, H.; Bolhuis, P.A.; de Visser, M. Machado-Joseph disease presenting as severe asymmetric proximal neuropathy. J. Neurol. Neurosurg. Psychiatry 1997, 63, 534–536. [Google Scholar] [CrossRef]
- Cagnoli, C.; Brussino, A.; Mancini, C.; Ferrone, M.; Orsi, L.; Salmin, P.; Pappi, P.; Giorgio, E.; Pozzi, E.; Cavalieri, S.; et al. Spinocerebellar Ataxia Tethering PCR: A Rapid Genetic Test for the Diagnosis of Spinocerebellar Ataxia Types 1, 2, 3, 6, and 7 by PCR and Capillary Electrophoresis. J. Mol. Diagn. 2018, 20, 289–297. [Google Scholar] [CrossRef]
- Mittal, U.; Sharma, S.; Chopra, R.; Dheeraj, K.; Pal, P.K.; Srivastava, A.K.; Mukerji, M. Insights into the mutational history and prevalence of SCA1 in the Indian population through anchored polymorphisms. Hum. Genet. 2005, 118, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.S.; Furtado, G.V.; Pedroso, J.L.; Barsottini, O.; Cornejo-Olivas, M.; Nobrega, P.R.; Braga Neto, P.; Soares, D.M.B.; Vargas, F.R.; Godeiro, C.; et al. Spinocerebellar ataxia type 2 has multiple ancestral origins. Park. Relat. Disord. 2024, 120, 105985. [Google Scholar] [CrossRef]
- Craig, K.; Takiyama, Y.; Soong, B.W.; Jardim, L.B.; Saraiva-Pereira, M.L.; Lythgow, K.; Morino, H.; Maruyama, H.; Kawakami, H.; Chinnery, P.F. Pathogenic expansions of the SCA6 locus are associated with a common CACNA1A haplotype across the globe: Founder effect or predisposing chromosome? Eur. J. Hum. Genet. 2008, 16, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, J.; Juvonen, V.; Sistonen, P.; Ignatius, J.; Johansson, D.; Bjorck, E.J.; Wahlstrom, J.; Melberg, A.; Holmgren, G.; Forsgren, L.; et al. Evidence for a common Spinocerebellar ataxia type 7 (SCA7) founder mutation in Scandinavia. Eur. J. Hum. Genet. 2000, 8, 918–922. [Google Scholar] [CrossRef]
- Zühlke, C.; Dalski, A.; Schwinger, E.; Finckh, U. Spinocerebellar ataxia type 17: Report of a family with reduced penetrance of an unstable Gln49 TBP allele, haplotype analysis supporting a founder effect for unstable alleles and comparative analysis of SCA17 genotypes. BMC Med. Genet. 2005, 6, 27. [Google Scholar] [CrossRef]
- Lund, A.; Udd, B.; Juvonen, V.; Andersen, P.M.; Cederquist, K.; Davis, M.; Gellera, C.; Kolmel, C.; Ronnevi, L.O.; Sperfeld, A.D.; et al. Multiple founder effects in spinal and bulbar muscular atrophy (SBMA, Kennedy disease) around the world. Eur. J. Hum. Genet. 2001, 9, 431–436. [Google Scholar] [CrossRef]
- Martins, S.; Matama, T.; Guimaraes, L.; Vale, J.; Guimaraes, J.; Ramos, L.; Coutinho, P.; Sequeiros, J.; Silveira, I. Portuguese families with dentatorubropallidoluysian atrophy (DRPLA) share a common haplotype of Asian origin. Eur. J. Hum. Genet. 2003, 11, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Veneziano, L.; Mantuano, E.; Catalli, C.; Gellera, C.; Durr, A.; Romano, S.; Spadaro, M.; Frontali, M.; Novelletto, A. A shared haplotype for dentatorubropallidoluysian atrophy (DRPLA) in Italian families testifies of the recent introduction of the mutation. J. Hum. Genet. 2014, 59, 153–157. [Google Scholar] [CrossRef]
- Burke, J.R.; Wingfield, M.S.; Lewis, K.E.; Roses, A.D.; Lee, J.E.; Hulette, C.; Pericak-Vance, M.A.; Vance, J.M. The Haw River syndrome: Dentatorubropallidoluysian atrophy (DRPLA) in an African-American family. Nat. Genet. 1994, 7, 521–524. [Google Scholar] [CrossRef]
- Warner, T.T.; Williams, L.; Harding, A.E. DRPLA in Europe. Nat. Genet. 1994, 6, 225. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Fujii, K.; Nagafuchi, S.; Nakahori, Y.; Nakagome, Y.; Akane, A.; Nakamura, M.; Sano, A.; Komure, O.; Kondo, I.; et al. A unique origin and multistep process for the generation of expanded DRPLA triplet repeats. Hum. Mol. Genet. 1996, 5, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Leggo, J.; Coetzee, G.A.; Irvine, R.A.; Buckley, M.; Ferguson-Smith, M.A. Sequence variation and size ranges of CAG repeats in the Machado-Joseph disease, spinocerebellar ataxia type 1 and androgen receptor genes. Hum. Mol. Genet. 1995, 4, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Genetic Modifiers of Huntington’s Disease Consortium. CAG Repeat Not Polyglutamine Length Determines Timing of Huntington’s Disease Onset. Cell 2019, 178, 887–900.e14. [Google Scholar] [CrossRef]
- Wright, G.E.B.; Collins, J.A.; Kay, C.; McDonald, C.; Dolzhenko, E.; Xia, Q.; Bečanović, K.; Drögemöller, B.I.; Semaka, A.; Nguyen, C.M.; et al. Length of Uninterrupted CAG, Independent of Polyglutamine Size, Results in Increased Somatic Instability, Hastening Onset of Huntington Disease. Am. J. Hum. Genet. 2019, 104, 1116–1126. [Google Scholar] [CrossRef]
- Coutinho, P.; Silva, C.C.; Gonçalves, A.F.; Graça, R.I.; Lourenço, E.; Sequeiros, J.; Loureiro, J.L.; Guimarães, J.; Ribeiro, P. Epidemiologia da doença Machado-Joseph em Portugal. Rev. Port. Neurol. 1994, 3, 69–76. [Google Scholar]
- Schöls, L.; Bauer, P.; Schmidt, T.; Schulte, T.; Riess, O. Autosomal dominant cerebellar ataxias: Clinical features, genetics, and pathogenesis. Lancet Neurol. 2004, 3, 291–304. [Google Scholar] [CrossRef]
- Vale, J.; Bugalho, P.; Silveira, I.; Sequeiros, J.; Guimaraes, J.; Coutinho, P. Autosomal dominant cerebellar ataxia: Frequency analysis and clinical characterization of 45 families from Portugal. Eur. J. Neurol. 2010, 17, 124–128. [Google Scholar] [CrossRef]
- Elter, T.L.; Sturm, D.; Santana, M.M.; Schaprian, T.; Raposo, M.; Melo, A.R.V.; Lima, M.; Koyak, B.; Oender, D.; Grobe-Einsler, M.; et al. Regional distribution of polymorphisms associated to the disease-causing gene of spinocerebellar ataxia type 3. J. Neurol. 2024, 272, 54. [Google Scholar] [CrossRef] [PubMed]
- Dolzhenko, E.; van Vugt, J.J.F.A.; Shaw, R.J.; Bekritsky, M.A.; van Blitterswijk, M.; Narzisi, G.; Ajay, S.S.; Rajan, V.; Lajoie, B.R.; Johnson, N.H.; et al. Detection of long repeat expansions from PCR-free whole-genome sequence data. Genome Res. 2017, 27, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Sequeiros, J.; Seneca, S.; Martindale, J. Consensus and controversies in best practices for molecular genetic testing of spinocerebellar ataxias. Eur. J. Hum. Genet. 2010, 18, 1188–1195. [Google Scholar] [CrossRef]
- Martindale, J.E. Diagnosis of Spinocerebellar Ataxias Caused by Trinucleotide Repeat Expansions. Curr. Protoc. Hum. Genet. 2017, 92, 1–22. [Google Scholar] [CrossRef]
- Costa, I.P.D.; Almeida, B.C.; Sequeiros, J.; Amorim, A.; Martins, S. A Pipeline to Assess Disease-Associated Haplotypes in Repeat Expansion Disorders: The Example of MJD/SCA3 Locus. Front. Genet. 2019, 10, 38. [Google Scholar] [CrossRef]
- Lopes, S.M.; Faro, R.; Lopes, M.M.; Onofre, I.; Mendonça, N.; Ribeiro, J.; Januário, C.; Nobre, R.J.; Pereira de Almeida, L. Protocol for the Characterization of the Cytosine-Adenine-Guanine Tract and Flanking Polymorphisms in Machado-Joseph Disease: Impact on Diagnosis and Development of Gene-Based Therapies. J. Mol. Diagn. 2020, 22, 782–793. [Google Scholar] [CrossRef]
- Ibañez, K.; Polke, J.; Hagelstrom, R.T.; Dolzhenko, E.; Pasko, D.; Thomas, E.R.A.; Daugherty, L.C.; Kasperaviciute, D.; Smith, K.R.; WGS for Neurological Diseases Group; et al. Whole genome sequencing for the diagnosis of neurological repeat expansion disorders in the UK: A retrospective diagnostic accuracy and prospective clinical validation study. Lancet Neurol. 2022, 21, 234–245. [Google Scholar] [CrossRef]
- Borghero, G.; Pugliatti, M.; Marrosu, F.; Marrosu, M.G.; Murru, M.R.; Floris, G.; Cannas, A.; Parish, L.D.; Cau, T.B.; Loi, D.; et al. ATXN2 is a modifier of phenotype in ALS patients of Sardinian ancestry. Neurobiol. Aging 2015, 36, 2906.e1–2906.e5. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.L.; Spataro, R.; Sproviero, W.; Mazzei, R.; Cavalcanti, F.; Condino, F.; Simone, I.L.; Logroscino, G.; Patitucci, A.; Magariello, A.; et al. Ataxin-1 and ataxin-2 intermediate-length PolyQ expansions in amyotrophic lateral sclerosis. Neurology 2012, 79, 2315–2320. [Google Scholar] [CrossRef] [PubMed]


| Criteria | Sequence and Information |
|---|---|
| Most frequent non-expanded allele | (CAG)2(CAA)(AAG)(CAG)(CAA)(CAG)17 |
| 23 repeats (7.5% of all clones; 10 participants) | |
| Most frequent expanded allele | (CAG)2(CAA)(AAG)(CAG)(CAA)(CAG)68 |
| 74 repeats (5.5% of all clones; 12 participants) | |
| Most frequent loss of canonical interruption allele | (CAG)2(CAA)(AAG)(CAG)58 |
| 62 repeats (0.7% of all clones; 1 participant, #17) |
| Non-Expanded Allele | Total | |||
|---|---|---|---|---|
| A1118 | C1118 | |||
| Expanded allele | A1118 | 13 | 46 | 59 (74.7%) |
| C1118 | 13 | 7 | 20 (25.3%) | |
| Total | 26 (32.9%) | 53 (67.1%) | 79 (100%) | |
| A1118 | C1118 | ||
|---|---|---|---|
| Female (n = 79; %) | 34 (57.6) | 12 (60.0) | = 0.03; df = 1; p = 0.852 a |
| Baseline age [n = 72; years, median (Q1, Q3)] | 51.0 (40.0, 58.5) | 57.0 (49.0, 65.0) | p = 0.029 b |
| CAG repeats, expanded allele [n = 71; median (Q1, Q3)] | 68.0 (63.0, 70.0) | 66.0 (61.0, 69.0) | p = 0.294 b |
| CAG repeats, non-expanded allele [n = 77; median (Q1, Q3)] | 22.0 (18.0, 26.0) | 22.0 (20.5, 29.5) | p = 0.339 b |
| Disease duration [n = 71; years, median (Q1, Q3)] | 11.0 (6.0, 16.75) | 9.0 (6.0, 14.0) | p = 0.583 b |
| Age at onset [n = 71; years, median (Q1, Q3)] | 37.0 (29.5, 46.0) | 51.0 (38.0, 53.0) | p = 0.017 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nethisinghe, S.; Garcia-Moreno, H.; Alwan, J.; Labrum, R.; Giunti, P. Role of Repeat Tract Structure and the rs7158733 SNP in Spinocerebellar Ataxia 3. Int. J. Mol. Sci. 2025, 26, 9836. https://doi.org/10.3390/ijms26209836
Nethisinghe S, Garcia-Moreno H, Alwan J, Labrum R, Giunti P. Role of Repeat Tract Structure and the rs7158733 SNP in Spinocerebellar Ataxia 3. International Journal of Molecular Sciences. 2025; 26(20):9836. https://doi.org/10.3390/ijms26209836
Chicago/Turabian StyleNethisinghe, Suran, Hector Garcia-Moreno, Jude Alwan, Robyn Labrum, and Paola Giunti. 2025. "Role of Repeat Tract Structure and the rs7158733 SNP in Spinocerebellar Ataxia 3" International Journal of Molecular Sciences 26, no. 20: 9836. https://doi.org/10.3390/ijms26209836
APA StyleNethisinghe, S., Garcia-Moreno, H., Alwan, J., Labrum, R., & Giunti, P. (2025). Role of Repeat Tract Structure and the rs7158733 SNP in Spinocerebellar Ataxia 3. International Journal of Molecular Sciences, 26(20), 9836. https://doi.org/10.3390/ijms26209836






