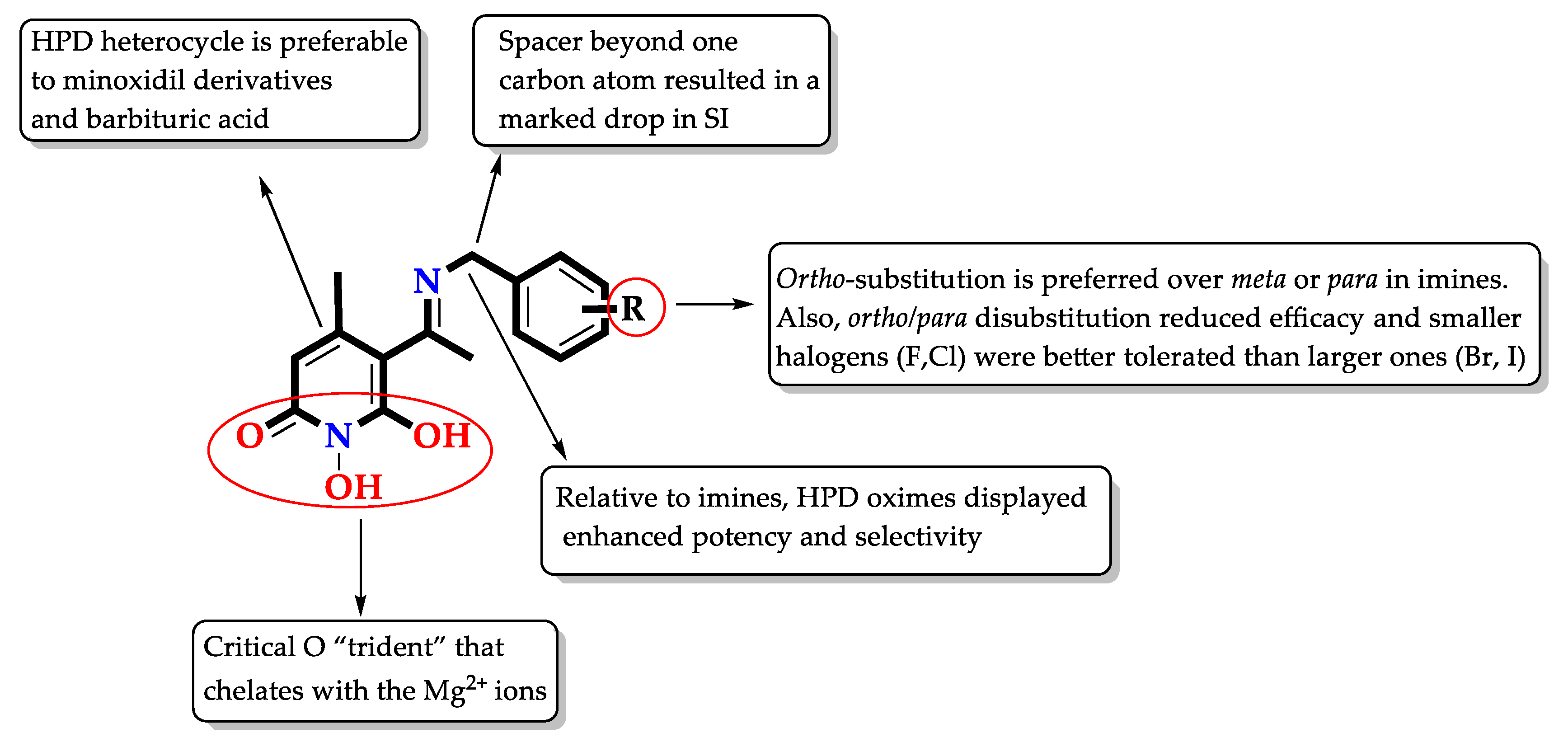
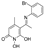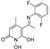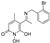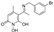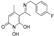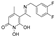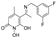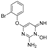3.2. Chemistry—Experimental Procedures and Compound Characterization
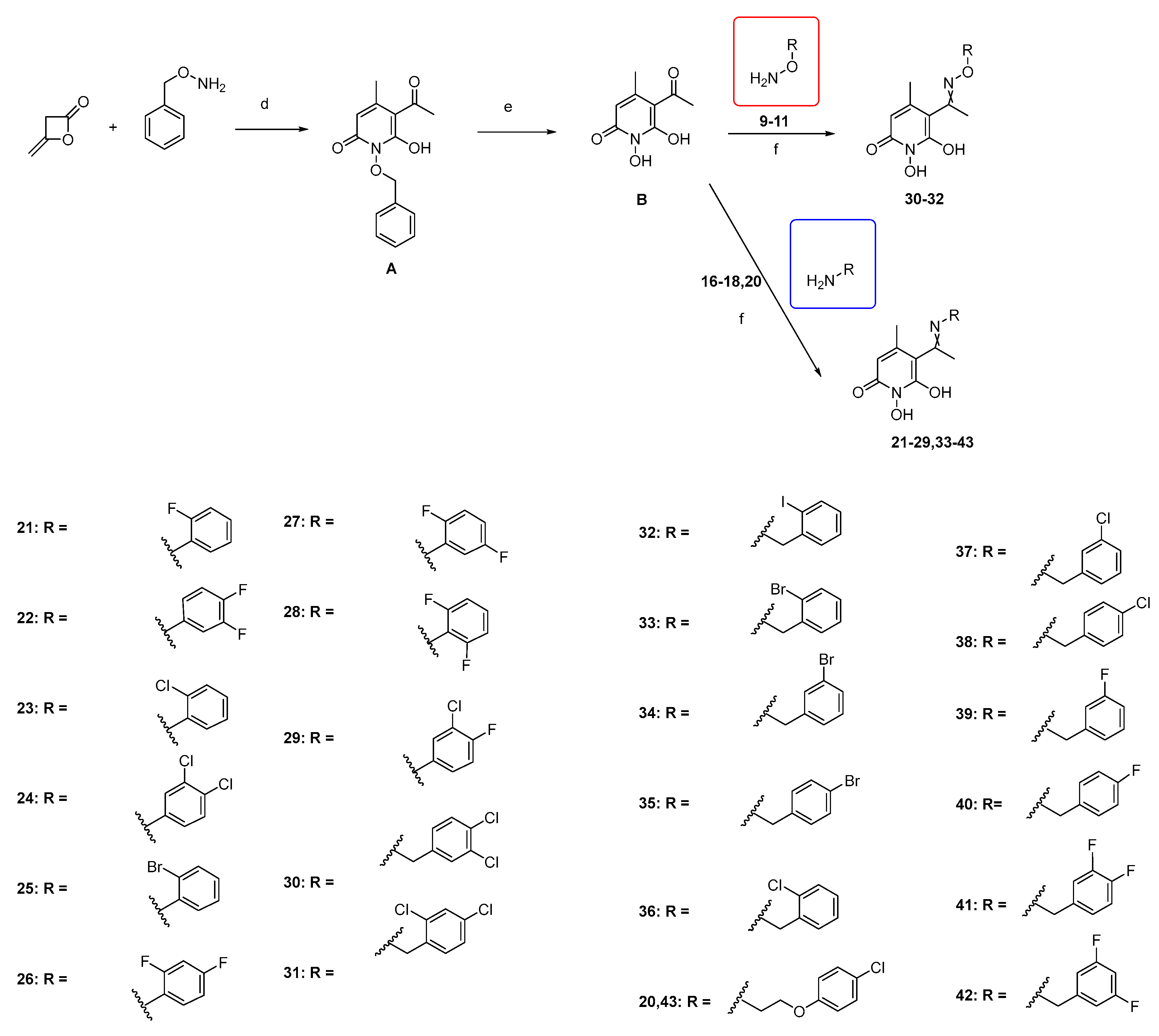
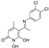
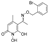
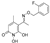
3.2.1. Synthesis of 5-Acetyl-1,6-dihydroxy-4-methylpyridin-2(1H)-one (Β)
5-Acetyl-1-(benzyloxy)-6-hydroxy-4-methylpyridin-2(1H)-one (A).
After cooling in an ice bath, diketene (4.07 g, 3.73 mL, 48.4 mmol, 2.0 eq) was added dropwise to a stirred solution of O-benzylhydroxylamine (2.98 g, 24.2 mmol, 1.0 equiv.) and triethylamine (2.45 g, 3.38 mL, 24.2 mmol, 1.0 equiv.) in 19 mL dry toluene.
After 4.5 h of stirring at 65 °C under Argon, the mixture was concentrated to dryness under reduced pressure, and 150 mL10% HCl was added. The residue was partitioned between the aqueous phase and AcOEt (300 mL), the organic phase was extracted once more with HCl 10% (150 mL), and the combined aqueous phases were extracted once more with 150 mL AcOEt. The combined organic phases were washed with brine (3 × 200 mL), dried over anhydrous Na
2SO
4, and the solvent was removed in vacuo. The residual brownish solid was triturated with Et
2O and AcOEt sequentially to afford the title compound
A as a beige crystalline solid (4.95 g, 75%); mp 144–146 °C (MeOH, AcOEt/n-pentane), R
f (NP-TLC) = 0.25 (AcOEt).
1H NMR (600.11 MHz, DMSO-
d6)
δ 2.34 (s, 3H, 4-CH
3), 2.60 (s, 3H, 7-CH
3), 5.05 (s, 2H, CH
2Ph) 5.83 (s, 1H, H
3), 7.37–7.44 (complex m, 3H, H
3’, H
4’, H
5’), 7.54 (dd, 2H,
J1 = 7.5 Hz,
J2 = 1.7 Hz, H
2’, H
6’).
13C NMR (50.32 MHz, DMSO-
d6)
δ 23.8 (4-CH
3), 29.0 (7-CH
3), 76.9 (CH
2Ph), 104.8 (C
5), 106.7 (C
3), 128.2 (C
3’, C
5’), 128.6 (C
4’), 129.3 (C
2’, C
6’), 134.9 (C
1’), 150.5 (C
4), 159.0 (C
2), 164.8 (C
6), 193.4 (C
7). Elemental analysis calcd. (%) for C
15H
15NO
4: C, 65.92; H, 5.53; N, 5.13; found: C, 66.00; H, 5.59; N, 5.08 [
20].
5-Acetyl-1,6-dihydroxy-4-methylpyridin-2(1H)-one (B).
A solution of A (2.0 g, 7.32 mmol) in 120 mL MeOH was hydrogenated for 20 min at rt and 40 psi pressure, in the presence of 200 mg Pd/C (10 wt.%) as a catalyst.
After filtering out the catalyst and washing it with hot MeOH (3 × 20 mL), the combined filtrates evaporated under reduced pressure. The beige crystalline product was treated with AcOEt to yield the
N-hydroxypyridinedione
B almost quantitatively (1.32 g, 98%); mp 178–179 °C (MeOH/
n-pentane, dry Et
2O), R
f (NP-TLC) = 0.06 (AcOEt), R
f (RP-TLC) = 0.85 (H
2O/ACN 7:3).
1H NMR (600.11 MHz, DMSO-
d6)
δ 2.32 (s, 3H, 4-CH
3), 2.56 (s, 3H, 7-CH
3), 5.80 (s, 1H, H
3).
13C NMR (100.61 MHz, DMSO-
d6)
δ 23.3 (4-CH
3), 28.0 (7-CH
3), 104.7 (C
5), 107.6 (C
3), 149.1 (C
4), 157.9 (C
2), 165.1 (C
6), 194.3 (C
7). Elemental analysis calcd. (%) for C
8H
9NO
4: C, 52.46; H, 4.95; N, 7.65; found: C, 52.51; H, 5.00; N, 7.58 [
20].
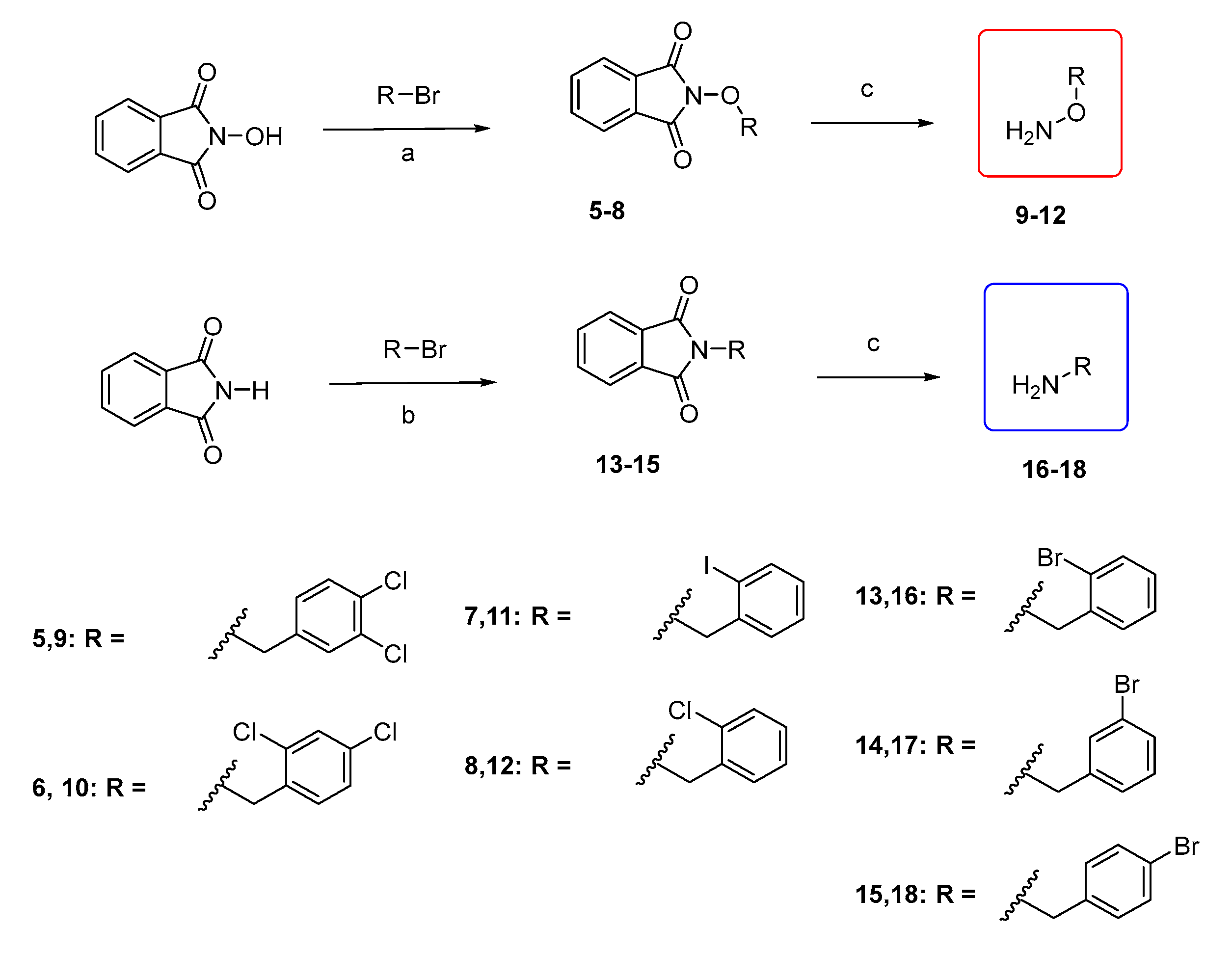
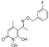
3.2.2. Synthesis of O-substituted N-hydroxyphthalimides (5–8)
General procedure:
To a solution of N-hydroxyphthalimide (2.45 mmol, 1 equiv.) in anhydrous DMF (2.4 mL), NaH (60% w/w, 3.07 mmol, 1.25 equiv.) is added at 0 °C. The mixture is stirred at RT for 30 min. Thereafter, the appropriate benzylalkylhalogenide (3.68 mmol, 1.5 equiv.) is added and the reaction is stirred at RT overnight. Then, water is added, and a solid precipitate is formed. The precipitate is filtered under vacuum and washed with water and a 7:3 solution of n-pentane/Et2O. The solid is then dried over P2O5 to afford the desired product.
2-((3,4-dichlorobenzyl)oxy)isoindoline-1,3-dione (5)
To a solution of
N-hydroxyphthalimide (2.0 g, 12.26 mmol, 1.0 equiv.) in dry DMF (12 mL), K
2CO
3 (3.4 g, 24.52 mmol, 2.0 eq.) was added, and the mixture obtained a red colour. Then, 3,4-dichlorobenzyl chloride was added (3.59 g, 24.52 mmol, 2.0 eq.) and the mixture was stirred at 80 °C for 18 h, under Argon. After the completion, white solid was precipitated. The precipitate was filtered under vacuum and washed with water and
n-pentane to afford
5 as a white solid (1.82 g, 46%) 1H NMR (400 MHz, CDCl
3) d: 7.82 (dt, J ¼ 8.4, 3.2 Hz, 2H, Ar-H), 7.75 (dt, J ¼ 8.4, 3.2 Hz, 2H, Ar-H), 7.64 (d, J ¼ 1.6 Hz, 1H, Ar-H), 7.46 (d, J ¼ 8.4 Hz, 1H, Ar-H), 7.39 (dt, J ¼ 8.4, 1.6 Hz, 1H, Ar-H), 5.15 (s, 2H, Ar-CH
2O-) [
28].
2-((2,4-dichlorobenzyl)oxy)isoindoline-1,3-dione (6), was synthesized from 1-(bromomethyl)-2,4-dichlorobenzene according to the general procedure. White solid (745.4 mg, 94%). Rf = 0.40 (CH2Cl2), mp: 154–156 °C, 1H NMR (400 MHz, CDCl3) δ: 7.82 (dt, J = 8.4, 3.2 Hz, 2H, Ar-Pthalimide), 7.75 (dt, J = 8.4, 3.2 Hz, 2H, Ar-Pthalimide), 7.59 (d, J = 8.4 Hz, 1H, Ar), 7.41 (d, J = 1.6 Hz, 1H, Ar), 7.27 (dt, J = 8.4, 1.6 Hz, 1H, Ar), 5.32 (s, 2H, CH2O). 13C NMR (100 MHz, CDCl3) δ: 163.3 (C1, C3), 135.8 (C1’), 135.3 (C4’), 134.5 (C2’), 132.4 (C7a, C3a), 130.5 (C6, C5), 129.5 (C3’), 128.7 (C6’), 127.4 (C5’), 123.6 (C7, C4), 75.7 (1’-CH2). Anal. Calcd for C15H9Cl2NO3: C, 55.93; H, 2.82; N, 4.35. Found: C, 55.97; H, 2.86; N, 4.39.
2-((2-iodobenzyl)oxy)isoindoline-1,3-dione (7)
To a solution of
N-hydroxyphthalimide (357.1 mg, 2.19 mmol, 1.3 equiv.) in anhydrous THF (6 mL), ET
3N (2.53 mmol, 1.5 eq.) was added, and the mixture was stirred at r.t. for 15 min. Then, 1-(bromomethyl)-2-iodobenzene (1.68 mmol, 1 eq.) was added and the mixture was stirred for 18 h at r.t. (overnight). The solvent was concentrated under reduced pressure, and 40 mL of H
2O was added to the residue. The product was extracted with CH
2Cl
2. The combined organic layers were washed with H
2O (3 × 20 mL) and with brine solution (20 mL). Then, they were dried with anh. Na
2SO
4 and the solvents were concentrated under reduced pressure. The white solid formed was purified by flash column chromatography (1:1 n-hexane/EtOAc and then EtOAc) to obtain a yellow solid (2710 mg, 43%).
1H NMR (400 MHz, DMSO) δ 7.90 (dd,
J = 7.9, 1.2 Hz, 1H), 7.86 (s, 4H), 7.61 (dd,
J = 7.7, 1.7 Hz, 1H), 7.44 (td,
J = 7.5, 1.3 Hz, 1H), 7.15 (td,
J = 7.7, 1.7 Hz, 1H), 5.25 (s, 2H) [
29].
2-((2-chlorobenzyl)oxy)isoindoline-1,3-dione (
8) was synthesized from 1-chloro-2-(chloromethyl)benzene (465 μL, 3.68 mmol) according to the general procedure. Pink solid (652.6 mg, 93%).
1H NMR (600 MHz, CDCl
3) δ 7.81 (dd,
J = 5.4, 3.1 Hz, 2H), 7.74 (dd,
J = 5.5, 3.1 Hz, 2H), 7.65–7.62 (m, 1H), 7.41–7.38 (m, 1H), 7.33–7.28 (m, 2H), 5.37 (s, 2H) [
28].
3.2.3. Synthesis of O-substituted Hydroxylamines (9–12)
General procedure:
To a solution of the appropriate N-hydroxyphthalimide (0.78 mmol, 1 equiv.) in CH2Cl2 (3 mL), H2NNH2 64% w/w (2 equiv.) is added, and the reaction is stirred at RT for 1–24 h. After the reaction is complete, the formed white precipitate is filtered, washed with CH2Cl2, and the filtrate is concentrated to afford the corresponding hydroxylamine.
O-(3,4-dichlorobenzyl)hydroxylamine (9)
To a solution of the compound (
5) (1.72 g, 5.34 mmol, 1 equiv.) in CH
2Cl
2 (18 mL), hydrazine monohydrate 64%
w/
w (534 mg, 10.68 mmol, 2 equiv.) is added, and the reaction is stirred at RT for 4 h. The formed white precipitate is filtered, washed with CH
2Cl
2, and the filtrate is concentrated to afford an off-yellow oil, which was further purified by flash column chromatography (CH
2Cl
2). (720 mg, 70%).
1H NMR (400 MHz, CDCl
3) d: 7.40 (s, 1H, Ar-H), 7.36 (d, J ¼ 8.0 Hz, 1H, Ar-H), 7.13 (d, J ¼ 8.0 Hz, 1H, Ar-H), 5.45 (brs, 2H, –NH
2), 4.57 (s, 2H, Ar-CH
2O-) [
28].
O-(2,4-dichlorobenzyl)hydroxylamine (10) was synthesized from compound (6) according to the general procedure, to afford an off-yellow oil (140 mg, 94%), Rf = 0.20 (CH2Cl2), 1H NMR (400 MHz, CDCl3) δ: 7.34 (s, 1H, Ar), 7.32 (d, J = 8.0 Hz, 1H, Ar), 7.20 (d, J = 8.0 Hz, 1H, Ar), 5.50 (broad s, 2H, –NH2), 4.72 (s, 2H, CH2O). 13C NMR (100 MHz, CDCl3) δ: 134.2 (C1), 134.0 (C2, C4), 130.6 (C3), 129.2 (C6), 126.9 (C5), 74.2 (OCH2).
O-(2-iodobenzyl)hydroxylamine (
11) was synthesized from compound (
7) (0.71 mmol) according to the general procedure (24 h), to afford an off-yellow oil (176 mg, 99%),
1H NMR (400 MHz, CDCl
3) δ 7.85 (d,
J = 7.9 Hz, 1H), 7.43–7.31 (m, 2H), 7.00 (t,
J = 7.5 Hz, 1H), 5.51 (br, s, 2H), 4.73 (s, 2H) [
29].
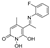
O-(2-chlorobenzyl)hydroxylamine (
12) was synthesized from compound (
8) (0.87 mmol) according to the general procedure (3 h), to afford a green oil (87.9 mg, 64%)
1H NMR (400 MHz, CDCl
3) δ 7.38–7.40 (m, 1H), 7.31–7.33 (m, 1H), 7.17–7.21 (m, 2H), 5.26 (brs, 2H), 4.76 (s, 2H) [
28].
3.2.4. Synthesis of N-substituted N-hydroxyphthalimides (13–15)
General procedure:
To a solution of phthalimide (500 mg, 3.40 mmol, 1 equiv.) in anhydrous DMF (3.4 mL), K2CO3 (1.5 eq.) was added, and the appropriate benzyl bromide (1.2 eq.). The mixture is stirred at 80 °C for 16–18 h, under Argon. After the completion of the reaction, H2O is added, and the product is extracted with EtOAc (3 × 30 mL). The combined organic layers were washed with brine solution (3 × 30 mL). Then, they were dried with anh. Na2SO4 and the solvents were concentrated under reduced pressure. The residue formed is purified by flash column chromatography or by recrystallization with EtOH.
2-(2-bromobenzyl)isoindoline-1,3-dione (
13) was synthesized according to the general procedure from 1-(bromomethyl)-2-bromobenzene (558.6 μL, 4.08 mmol). The solid was purified by recrystallization with EtOH, to obtain the desired product as a white solid (919.9 mg, 91%),
1H NMR (400 MHz, CDCl
3) δ 7.92–7.87 (m, 2H), 7.78–7.73 (m, 2H), 7.60–7.55 (m, 1H), 7.26–7.20 (m, 1H), 7.17–7.10 (m, 2H), 4.98 (s, 2H) [
30].
2-(3-bromobenzyl)isoindoline-1,3-dione (14)
To a solution of phthalimide (500 mg, 3.40 mmol, 1.1 equiv.) in anhydrous DMF (7.7 mL), K
2CO
3 (3 eq.) and 1-(bromomethyl-)3-bromobenzene (1 eq.) were added. The mixture is stirred at 40 °C overnight, under Argon. After the completion of the reaction, 40 mL of cold H
2O is added, and a white solid is precipitated. The solid formed was filtered off in vacuo and washed with 100 mL H
2O and 80 mL of a solution of PE/EtOAc 10/1. The desired product is obtained as a white solid (505,1 mg, 52%),
1H NMR (400 MHz, DMSO) δ 7.92–7.84 (m, 4H), 7.54 (s, 1H), 7.47 (dt,
J = 6.6, 2.2 Hz, 1H), 7.32–7.27 (m, 2H), 4.77 (s, 2H) [
31].
2-(4-bromobenzyl)isoindoline-1,3-dione (15)
To a solution of phthalimide (500 mg, 3.40 mmol, 1.1 equiv.) in anhydrous DMF (3.9 mL), Cs
2CO
3 (2 eq.) and 1-(bromomethyl-)4-bromobenzene (1 eq.) were added. The mixture is stirred at r.t. for 22 h, under Argon. After the completion of the reaction, 70 mL of H
2O is added, and the product is extracted from CH
2Cl
2 (2 × 70 mL). The combined organic layers were washed with brine solution (2 × 50 mL). Then, they were dried with anh. Na
2SO
4 and the solvents were concentrated under reduced pressure. The residue formed is purified by column chromatography (gravity, PE/EtOAc 3/1). The desired product is obtained as a white solid (607.9 mg, 56%),
1H NMR (400 MHz, CDCl
3) δ 7.87–7.81 (m, 2H), 7.74–7.68 (m, 2H), 7.44 (d,
J = 8.4 Hz, 2H), 7.31 (d,
J = 8.5 Hz, 2H), 4.79 (s, 2H) [
31].
3.2.5. Synthesis of Substituted Benzylamines (16–18)
General procedure:
To a solution of the appropriate phthalimide (0.98 mmol, 1 equiv.) in absolute EtOH (3 mL), hydrazine monohydrate 64% w/w (2 equiv.) is added, and the reaction is refluxed for 1 h. The appropriate amine is obtained with a different procedure each time.
(2-bromophenyl)methanamine (
16) was synthesized from compound (
13) (200 mg, 0.63 mmol). After the completion of the reaction, the solvent is concentrated under reduced pressure. Then, 5 mL of conc. HCl is added, and the mixture is stirred at 80 °C for 1 h. Next, solution of NaOH 2 N is added to the mixture, until it becomes basic (pH = 8). The product is extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with 50 mL H
2O and then with 50 mL brine solution. Then, they were dried with anh. Na
2SO
4 and the solvents were concentrated under reduced pressure. The desired product is obtained as a yellow oil (108.7 mg, 62%),
1H NMR (400 MHz, CDCl
3) δ 7.55 (d,
J = 8.0 Hz, 1H), 7.38 (d,
J = 6.9 Hz, 1H), 7.30 (t,
J = 7.4 Hz, 1H), 7.13 (dt,
J = 8.9, 4.5 Hz, 1H), 3.93 (s, 2H) [
32].
(3-bromophenyl)methanamine (
17) was synthesized from compound (
14) (300 mg, 0.95 mmol). After the completion of the reaction, the solvent is concentrated under reduced pressure. Then, 5 mL of conc. HCl is added, and the mixture is stirred at 80 °C for 1 h. Next, solution of aq. NaOH 2 N is added to the mixture, until it becomes basic (pH = 8). The product is extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with 50 mL H
2O and then with 50 mL brine solution. Then, they were dried with anhydrous. Na
2SO
4 and the solvents were concentrated under reduced pressure. The desired product is obtained as a yellow oil (100.0 mg, 57%),
1H NMR (400 MHz, CDCl
3) δ 7.48 (t,
J = 1.9 Hz, 1H), 7.38 (dt,
J = 7.5, 1.8 Hz, 1H), 7.25–7.17 (m, 2H), 3.86 (s, 2H) [
33].
(4-bromophenyl)methanamine (
18) was synthesized from compound (
15) (200 mg, 0.63 mmol). After the completion of the reaction, the solvent is concentrated under reduced pressure. Then, 5 mL of conc. HCl is added, and the mixture is stirred at 80 °C for 1 h. Next, it is added to the mixture solution of aq. NaOH 2 N, until it becomes basic (pH = 8). The product is extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with 50 mL H
2O and then with 50 mL brine solution. Then, they were dried with anh. Na
2SO
4 and the solvents were concentrated under reduced pressure. The desired product is obtained as a yellow oil (103.8 mg, 59%),
1H NMR (400 MHz, CDCl
3) δ 7.46 (d,
J = 8.4 Hz, 2H), 7.20 (d,
J = 8.4 Hz, 2H), 3.85 (s, 2H), 3.12 (s, 4H) [
32].
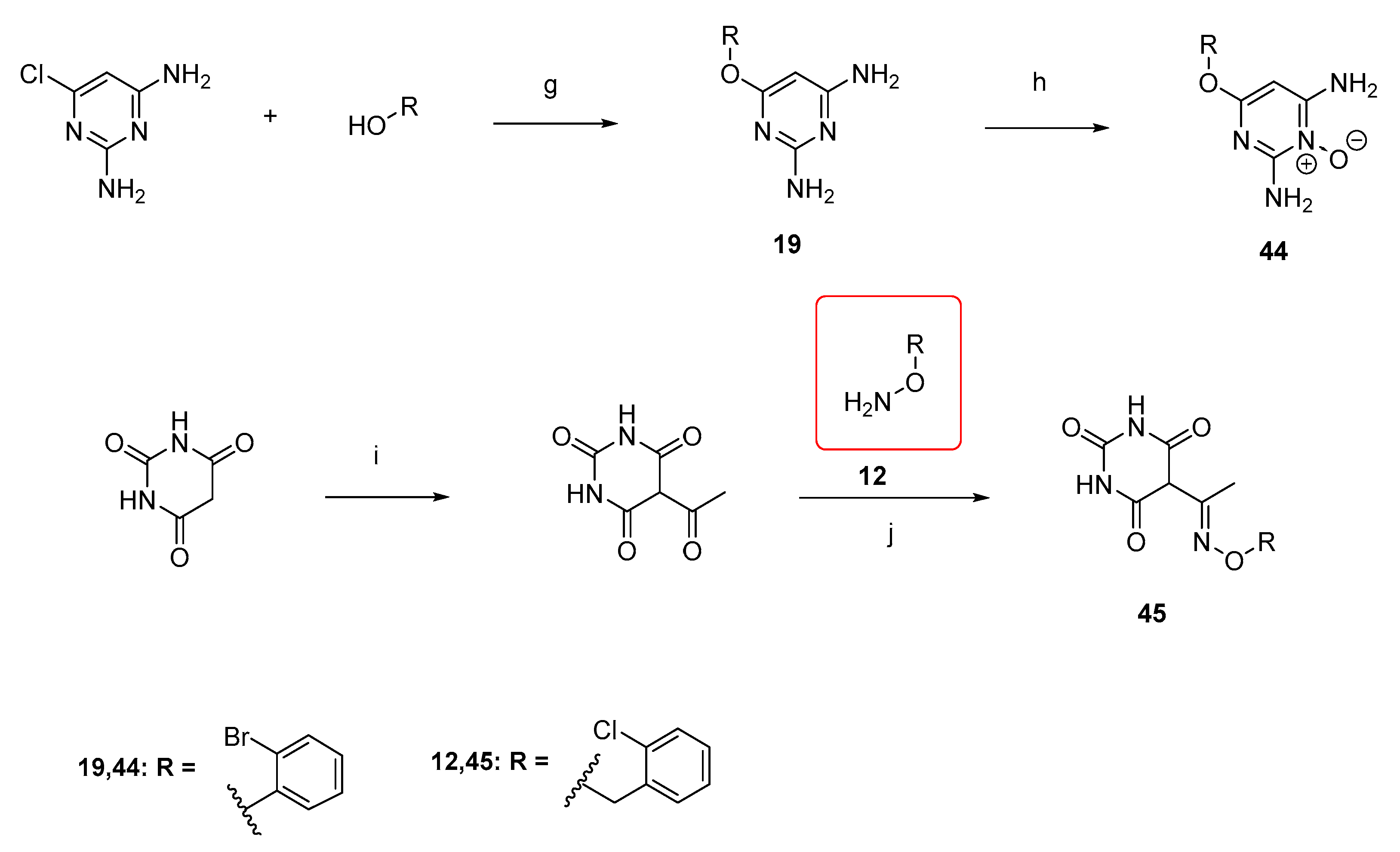
3.2.6. Synthesis of 6-Substituted 2,4-diaminopyrimidines (19)
General procedure:
To a flask containing the stirring corresponding alcohol (7.4 equiv.), NaH (1.2 equiv.) is added slowly, and the mixture is stirred at 150 °C for 1.5 h. Then, 6-chloropyrimidine-2,4-diamine is added (1 equiv.) and the resulting mixture is stirred at 140–150 °C overnight, under Argon. The brown residue is extracted with EtOAc (3 × 50 mL). The combined organic phases are washed with brine (3 × 40 mL), dried over anh. Na2SO4, filtered, and concentrated. The resulting crude mixture is purified by gravity column chromatography (1:1 CH2Cl2: AcOEt, AcOEt, 3:1 AcOEt: MeOH as eluent or recrystallized with EtOAc or triturated with Et2O).
6-(2-bromophenoxy)pyrimidine-2,4-diamine (19) was synthesized according to the general procedure, and it was purified by column chromatography and triturated as it has been described in the general procedure to afford (19) as an off-white solid. (591.6 mg, 61%). Rf = 0.38 (AcOEt), 1H NMR (500 MHz, DMSO) δ 7.68 (d, J = 8.0 Hz, 1H, Ph), 7.41 (t, J = 7.9 Hz, 1H, Ph), 7.26–7.13 (m, 2H, Ph), 6.27 (s, 2H, NH2), 5.99 (s, 2H, NH2), 5.05 (s, 1H, CHCNH2). 13C NMR (126 MHz, DMSO) δ 169.50 (C6), 166.50 (C4), 163.21 (C2), 150.21 (C1’), 133.23 (C3’), 129.00 (C5’), 126.70 (C4’), 124.32 (C6’), 115.96 (C2’), 76.77 (C5). Anal. Calcd for C10H9BrN4O: C, 42.73; H, 3.23; N, 19.93. Found: C, 42.76; H, 3.26; N, 19.96.
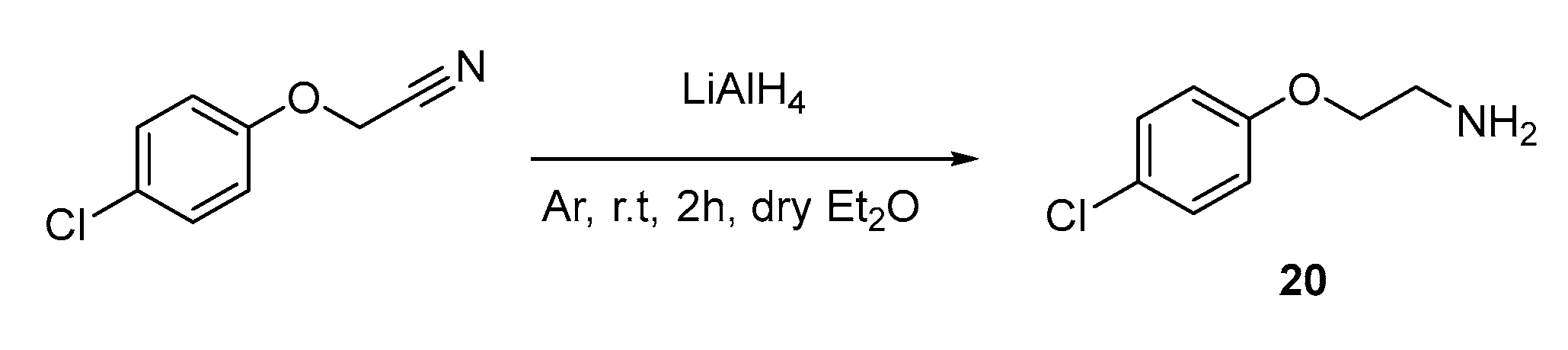
3.2.7. Synthesis of 2-(4-Chlorophenoxy)ethan-1-amine (20)
In a solution of (4-chlorophenoxy)acetonitrile (200 mg, 1,19 mmol, 1 eq) in anhydrous Et2O in ambient temperature, LiAlH4 (135.8 mg, 3.58 mmol, 3 eq) is added, and the mixture is stirred for 2 h. After the completion of the reactions, the mixture is cooled to 0 °C and EtOH, H2O and aq. NaOH 15% w/v is added sequentially and dropwise, until LiAlH4 discoloration to white. The solid is filtered with hot EtOAc using a folded filtered paper. The filtrate is concentrated under reduced pressure to obtain colourless oil (130 mg, 64%).
1H NMR (300 MHz, CDCl
3, δ): 7.28–7.21 (m, 2H), 6.89–6.81 (m, 2H), 4.26 (t,
J = 6.4 Hz, 2H), 3.62 (t,
J = 6.4 Hz, 2H) [
34].
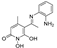
3.2.8. Synthesis of N-hydroxypyridinedione Imines (21–29)
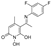
5-(1-((2-fluorophenyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (21)
To a stirred solution of B (200 mg, 1.09 mmol, 1.0 eq) in 4 mL of absolute EtOH at 75 °C activated 4Å molecular sieves, 2-fluoroaniline (133 mg, 116 μL, 1.2 mmol, 1.1 eq), and a drop of sulfuric acid were added successively. The mixture was refluxed for 4.5 h at 60 °C under Argon and for 2.5 h at 30 °C. During the reaction, a yellow solid was formed. The precipitate was filtered off in vacuo and washed with small portions of EtOH and EtOAc to afford (21) as a yellow crystalline solid (180 mg, 60%); mp 190–193 °C (AcOEt/n-pentane), Rf (alox) = 0.05 (MeOH), Rf (RP-TLC) = 0.07 (H2O/ACN 7:3); 1H NMR (600.11 MHz, DMSO-d6) δ (ppm) 2.38 (s, 3H, 4-CH3), 2.46 (s, 3H, 7-CH3), 5.73 (s, 1H, H3), 7.30-7.37 (m, 1H, H4’), 7.41–7.47 (complex m, 2H, H3’, H6’), 7.49 (t, 1H, J = 8.0 Hz, H5’), 10.11 (s, 1H, 1-OH), 14.78 (s, 1H, 6-OH); 13C NMR (150.9 MHz, DMSO-d6) δ (ppm) 20.4 (7-CH3), 25.1 (4-CH3), 99.7 (C5), 110.8 (C3), 116.4, 116.5 (d, JC-F = 19.5 Hz, C3’), 124.6, 124.7 (d, JC-F = 12.2 Hz, C1’), 125.11, 125.12 (d, JC-F = 2.8 Hz, C4’), 128.2 (C5’), 129.38, 129.43 (d, JC-F = 7.9 Hz, C6’), 148.3 (C4), 155.0, 156.6 (d, JC-F = 247.1 Hz, C2’), 158.8 (C2), 164.6 (C6), 169.1 (C7). Anal. Calcd for C14H13FN2O3: C, 60.87; H, 4.74; N, 10.14. Found: C, 60.91; H, 4.79; N, 10.18.
5-(1-((3,4-difluorophenyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (22)
To a stirred solution of B (200 mg, 1.09 mmol, 1.0 eq) in 4 mL of absolute EtOH activated 4Å molecular sieves, 3,4-difluoroaniline (155 mg, 119 μL, 1.2 mmol, 1.1 eq), and a drop of sulfuric acid were added successively. The mixture was refluxed for 4.5 h at 60 °C under Argon. The precipitate formed was filtered off in vacuo and washed with EtOH to afford (22) as a beige crystalline solid (240 mg, 75%); mp 217–219 °C (EtOH), Rf (alox) = 0.05 (MeOH), Rf (RP-TLC) = 0.05 (H2O/ACN 7:3); 1H NMR (600.11 MHz, DMSO-d6) δ (ppm) 2.36 (s, 3H, 4-CH3), 2.45 (s, 3H, 7-CH3), 5.71 (s, 1H, H3), 7.22 (ddd, 1H, J1 = 9.2 Hz, J2 = 4.3 Hz, J3 = 2.1 Hz, H6’), 7.54–7.60 (complex m, 2H, H2’, H5’), 10.09 (s, 1H, 1-OH), 14.77 (s, 1H, 6-OH); 13C NMR (150.9 MHz, DMSO-d6) δ (ppm) 20.7 (7-CH3), 25.1 (4-CH3), 99.5 (C5), 110.6 (C3), 115.6, 115.8 (d, JC-F = 19.1 Hz, C5’), 118.0, 118.2 (d, JC-F = 18.2 Hz, C2’), 123.0 (C6’), 133.7 (C1’), 147.6, 147.7, 148.4, 148.5 (d, J1(C-F) = 121.2 Hz, J2(C-F) = 13.2 Hz, C4’), 148.3 (C4), 149.2, 149.3, 150.1, 150.1 (d, J1(C-F) = 122.5 Hz, J2(C-F) = 13.0 Hz, C3’), 158.8 (C2), 164.4 (C6), 168.8 (C7). Anal. Calcd for C14H12F2N2O3: C, 57.15; H, 4.11; N, 9.52. Found: C, 57.19; H, 4.05; N, 9.58.
5-(1-((2-chlorophenyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (23)
To a stirred solution of B (200 mg, 1.09 mmol, 1.0 eq) in 4 mL of absolute EtOH at 75 °C activated 4Å molecular sieves, 2-chloroaniline (153 mg, 126 μL, 1.2 mmol, 1.1 eq), and a drop of sulfuric acid were added successively. The mixture was refluxed for 24 h at 60 °C. After the competition of the reaction the solvent was evaporated in half of its volume, and the solid formed was filtered off in vacuo and washed with small portions of EtOH to afford (23) as a yellow crystalline solid (80 mg, 25%); mp 184186 °C (AcOEt/n-pentane), Rf (alox) = 0.01 (MeOH), Rf (RP-TLC) = 0.05 (H2O/ACN 7:3); 1H NMR (600.11 MHz, DMSO-d6) δ (ppm) 2.39 (s, 3H, 4-CH3), 2.42 (s, 3H, 7-CH3), 5.73 (s, 1H, H3), 7.43 (td, 1H, J1 = 7.7 Hz, J2 = 1.7 Hz, H5’), 7.49 (td, 1H, J1 = 7.6 Hz, J2 = 1.5 Hz, H4’), 7.54 (dd, 1H, J1 = 7.9 Hz, J2 = 1.7 Hz, H3’), 7.68 (dd, 1H, J1 = 8.0 Hz, J2 = 1.5 Hz, H6’), 10.12 (s, 1H, 1-OH), 14.94 (s, 1H, 6-OH); 13C NMR (150.9 MHz, DMSO-d6) δ (ppm) 20.6 (7-CH3), 25.2 (4-CH3), 99.5 (C5), 110.8 (C3), 28.1 (C4’), 128.6 (C3’), 129.1 (C2’), 129.2 (C5’), 130.1 (C6’), 134.4 (C1’), 148.4 (C4), 158.8 (C2), 164.7 (C6), 168.9 (C7). Anal. Calcd for C14H13ClN2O3: C, 57.45; H, 4.48; N, 9.57. Found: C, 57.53; H, 4.50; N, 9.47.
5-(1-((3,4-dichlorophenyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (24)
To a stirred solution of B (200 mg, 1.09 mmol, 1.0 eq) in 4 mL of absolute EtOH activated 4Å molecular sieves, 3,4-dichloroaniline (194 mg, 1.2 mmol, 1.1 eq), and a drop of sulfuric acid were added successively. The mixture was refluxed for 4 h at 60 °C under Argon. The precipitate formed was filtered off in vacuo and washed with EtOH to afford (24) as a yellow crystalline solid (250 mg, 70%); mp 217–219 °C (EtOH), Rf (alox) = 0.01 (MeOH), Rf (RP-TLC) = 0.01 (H2O/ACN 7:3); 1H NMR (600.11 MHz, DMSO-d6) δ (ppm) 2.37 (s, 3H, 4-CH3), 2.48 (s, 3H, 7-CH3), 5.73 (s, 1H, H3), 7.35 (dd, 1H, J1 = 8.6 Hz, J2 = 2.5 Hz, H6’), 7.71 (d, 1H, J = 2.5 Hz, H2’), 7.75 (d, 1H, J = 8.5 Hz, H5’), 10.10 (s, 1H, 1-OH), 14.79 (s, 1H, 6-OH); 13C NMR (150.9 MHz, DMSO-d6) δ (ppm) 20.8 (7-CH3), 25.0 (4-CH3), 99.9 (C5), 110.8 (C3), 126.1 (C6’), 127.7 (C2’), 129.8 (C4’), 131.2 (C5’), 131.7 (C3’), 137.0 (C1’), 148.3 (C4), 158.8 (C2), 164.4 (C6), 168.4 (C7). Anal. Calcd for C14H12Cl2N2O3: C, 51.40; H, 3.70; N, 8.56. Found: C, 51.44; H, 3.65; N, 8.60.
5-(1-((2-bromophenyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (25)
To a stirred solution of B (300 mg, 1.64 mmol, 1.0 eq) in 5 mL of absolute EtOH at 75 °C activated 4Å molecular sieves, 2-bromoaniline (310 mg, 1.8 mmol, 1.1 eq), and 2 drops of sulfuric acid were added successively. The mixture was refluxed for 48 h at 65 °C under Argon. The solvent was concentrated under reduced pressure, and the brown residue was triturated with 3 mL EtOAc. The precipitate formed was filtered off in vacuo and washed with MeOH. The filtrate was concentrated under reduced pressure and chromatographed on a Sephadex LH-20 column eluted with MeOH. The solid was triturated with dry Et2O and abs. EtOH to afford (25) as a yellow crystalline solid (140 mg, 25%); mp: 198–200 °C (MeOH/dry Et2O), Rf (alox) = 0.13 (MeOH), Rf (RP-TLC) = 0.09 (H2O/ACN 7:3). 1H NMR (600.11 MHz, DMSO-d6) δ (ppm): 2.38 (s, 3H, 4-CH3), 2.40 (s, 3H, 7-CH3), 5.73 (s, 1H, H3), 7.36 (ddd, 1H, J1 = 8.5 Hz, J2 = 5.8 Hz, J3 = 3.3 Hz, H4’), 7.50–7.55 (m, 2H, H5’, H6’), 7.83 (d, 1H, J = 7.6 Hz, H3’), 10.14 (s, 1H, 1-OH), 14.92 (s, 1H, 6-OH); 13C NMR (150.9 MHz, DMSO-d6) δ (ppm): 20.6 (7-CH3), 25.3 (4-CH3), 99.4 (C5), 110.7 (C3), 119.8 (C2’), 128.8 (C5’), 128.8 (C6’), 129.5 (C4’), 133.3 (C3’), 136.0 (C1’), 148.5 (C4), 158.8 (C2), 164.7 (C6), 168.9 (C7). Anal. Calcd for C14H13BrN2O3: C, 49.87; H, 3.89; N, 8.31. Found: C, 49.79; H, 3.86; N, 8.34.
5-(1-((2,4-difluorophenyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (26)
To a stirred solution of B (200 mg, 1.09 mmol, 1.0 eq) in 4 mL of absolute EtOH at 75 °C activated 4Å molecular sieves, 2,4-difluoroaniline (155 mg, 120 μL, 1.2 mmol, 1.1 eq), and a drop of sulfuric acid were added successively. The mixture was refluxed for 4 h at 60 °C under Argon. The precipitate formed was filtered off in vacuo and washed with EtOH to afford (26) as a yellow crystalline solid (270 mg, 84%); mp 230–233 °C (EtOH), Rf (alox) = 0.01 (MeOH), Rf (RP-TLC) = 0.02 (H2O/ACN 7:3); 1H NMR (600.11 MHz, DMSO-d6) δ (ppm) 2.38 (s, 3H, 4-CH3), 2.43 (s, 3H, 7-CH3), 5.73 (s, 1H, H3), 7.24 (td, 1H, J1 = 8.3 Hz, J2 = 2.8 Hz, H5’), 7.52 (ddd, 1H, J1 = 11.1 Hz, J2 = 8.9 Hz, J3 = 2.8 Hz, H3’), 7.56 (td, 1H, J1 = 9.0 Hz, J2 = 5.9 Hz, H6’), 10.11 (s, 1H, 1-OH), 14.64 (s, 1H, 6-OH); 13C NMR (150.9 MHz, DMSO-d6) δ (ppm) 20.3 (7-CH3), 25.1 (4-CH3), 99.7 (C5), 105.1 (d, JC-F = 26.5 Hz, C3’), 110.8 (C3), 112.18 (dd, J1(C-F) = 22.5 Hz, J2(C-F) = 2.8 Hz, C5’), 121.44 (d, JC-F = 13.4 Hz, C1’), 129.51 (d, JC-F = 13.4 Hz, C6’), 148.3 (C4), 156.09 (dd, J1(C-F) = 249.5 Hz, J2(C-F) = 13.2 Hz, C2’), 158.8 (C2), 160.97 (dd, J1(C-F) = 247.4 Hz, J2(C-F) = 11.7 Hz, C4’), 164.6 (C6), 169.3 (C7). Anal. Calcd for C14H12F2N2O3: C, 57.15; H, 4.11; N, 9.52. Found: C, 57.20; H, 4.15; N, 9.50.
5-(1-((2,5-difluorophenyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (27)
To a stirred solution of B (200 mg, 1.09 mmol, 1.0 eq) in 4 mL of absolute EtOH at 75 °C, 2,5-difluoroaniline (155 mg, 120 μL, 1.2 mmol, 1.1 eq) was added, and the mixture was refluxed for 4 h at 60 °C under Argon. The solvent was concentrated under reduced pressure, and the residue was triturated with ET2O. The precipitate formed was filtered off in vacuo and washed with EtOH. The solid was chromatographed on a Sephadex LH-20 column eluted with MeOH to afford (27) as a yellow crystalline solid (230 mg, 72%); mp 173–178 °C (EtOH), Rf (alox) = 0.01 (MeOH), Rf (RP-TLC) = 0.01 (H2O/ACN 7:3); 1H NMR (600.11 MHz, DMSO-d6) δ (ppm) 2.38 (s, 3H, 4-CH3), 2.49 (s, 3H, 7-CH3), 5.76 (s, 1H, H3), 7.30 (ddd, 1H, J1 = 8.9 Hz, J2 = 6.9 Hz, J3 = 3.6 Hz, H4’), 7.47–7.53 (m, 2H, H3’, H6’), 10.19 (s, 1H, 1-OH), 14.79 (s, 1H, 6-OH); 13C NMR (150.9 MHz, DMSO-d6) δ (ppm) 20.5 (7-CH3), 25.0 (4-CH3), 100.2 (C5), 111.2 (C3), 115.04 (d, JC-F = 26.3 Hz, C6’), 115.46 (dd, J1(C-F) = 23.9 Hz, J2(C-F) = 8.0 Hz, C4’), 117.50 (dd, J1(C-F) = 22.6 Hz, J2(C-F) = 9.6 Hz, C3’), 125.85 (dd, J1(C-F) = 14.2 Hz, J2(C-F) = 11.5 Hz, C1’), 148.2 (C4), 152.28 (d, JC-F = 243.2 Hz, C2’), 157.72 (d, JC-F = 242.1 Hz, C5’), 158.8 (C2), 164.7 (C6), 168.7 (C7). Anal. Calcd for C14H12F2N2O3: C, 57.15; H, 4.11; N, 9.52. Found: C, 57.11; H, 4.08; N, 9.55.
5-(1-((2,6-difluorophenyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (28)
To a stirred solution of B (200 mg, 1.09 mmol, 1.0 eq) in 4 mL of absolute EtOH at 75 °C, 2,6-difluoroaniline (155 mg, 120 μL, 1.2 mmol, 1.1 eq) was added and the mixture was refluxed for 16 h at 60 °C under Argon. The solvent was concentrated under reduced pressure and the orange residue was chromatographed on a Sephadex LH-20 column eluted with MeOH to afford (28) as a yellow crystalline solid (145 mg, 45%); mp 188–194 °C (EtOH/dry Et2O), Rf (alox) = 0.02 (MeOH), Rf (RP-TLC) = 0.06 (H2O/ACN 7:3); 1H NMR (600.11 MHz, DMSO-d6) δ (ppm) 2.38 (s, 3H, 4-CH3), 2.49 (s, 3H, 7-CH3), 5.76 (d, 1H, J = 1.1 Hz, H3), 7.30 (ddd, 1H, J1 = 8.9 Hz, J2 = 6.9 Hz, J3 = 3.6 Hz, H4’), 7.47–7.53 (m, 2H, H3’, H6’), 10.15 (s, 1H, 1-OH), 14.51 (s, 1H, 6-OH); 13C NMR (150.9 MHz, DMSO-d6) δ (ppm) 20.5 (7-CH3), 25.0 (4-CH3), 99.9 (C5), 111.3 (C3), 115.04 (d, JC-F = 26.3 Hz, C6’), 115.46 (dd, J1(C-F) = 23.9 Hz, J2(C-F) = 8.0 Hz, C4’), 117.50 (dd, J1(C-F) = 22.6 Hz, J2(C-F) = 9.6 Hz, C3’), 125.85 (dd, J1(C-F) = 14.2 Hz, J2(C-F) = 11.5 Hz, C1’), 148.3 (C4), 152.28 (d, JC-F = 243.2 Hz, C2’), 157.72 (d, JC-F = 242.1 Hz, C5’), 158.8 (C2), 164.9 (C6), 169.6 (C7). Anal. Calcd for C14H12F2N2O3: C, 57.15; H, 4.11; N, 9.52. Found: C, 57.20; H, 4.13; N, 9.52.
5-(1-((3-chloro-4-fluorophenyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (29)
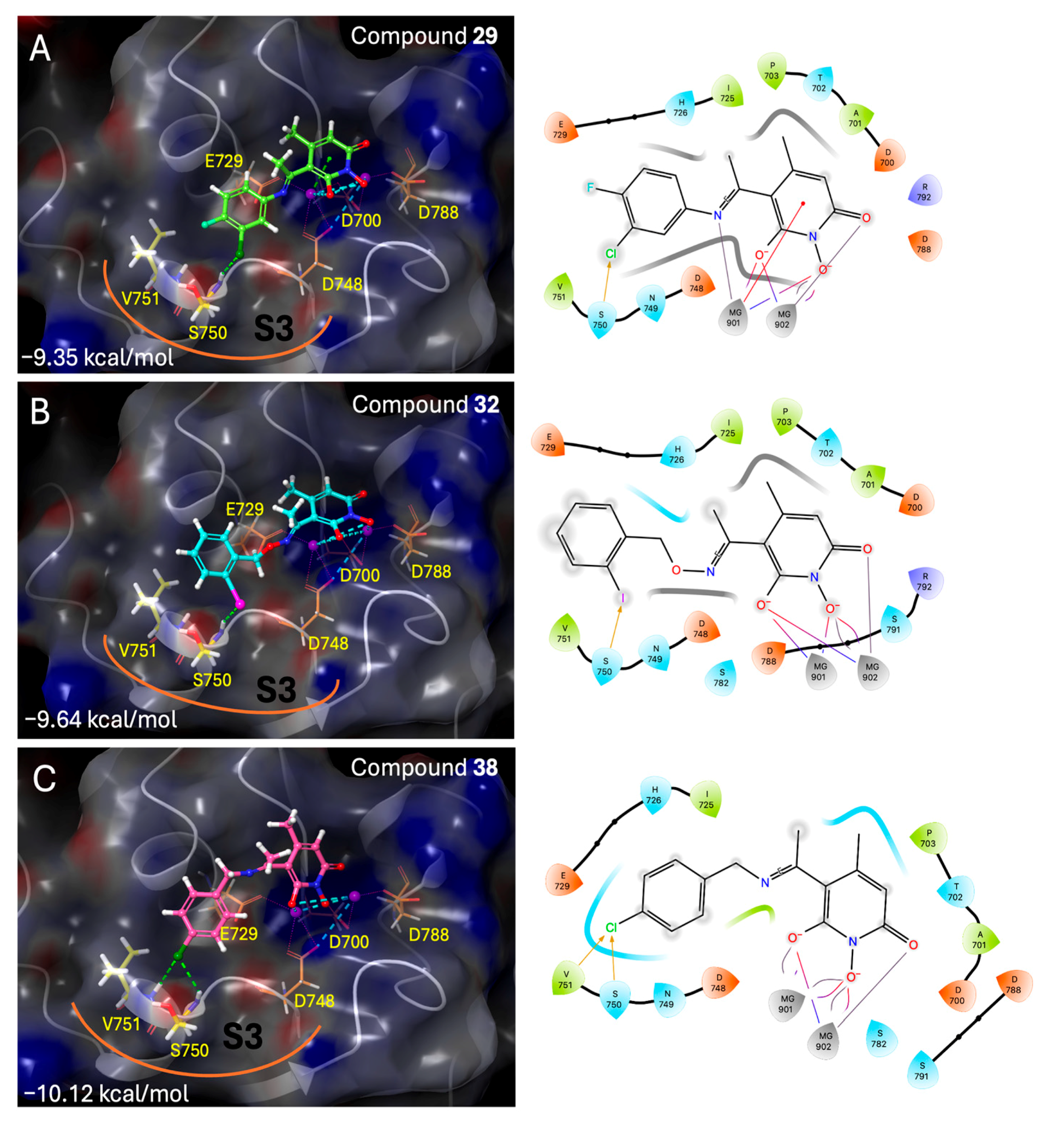
To a stirred solution of B (200 mg, 1.09 mmol, 1.0 eq) in 4 mL of absolute EtOH activated 4Å molecular sieves, 3-chloro-4-fluoroaniline (175 mg, 1.2 mmol, 1.1 eq) and a drop of sulfuric acid were added successively, and a yellow solid was formed. The mixture was refluxed for 20 h at 60 °C under Argon. The precipitate formed was filtered off in vacuo and washed with EtOH and MeOH to afford (29) as a pale yellow crystalline solid (195 mg, 57%). Mp: 189–191 °C (EtOH/dry Et2O), Rf (alox) = 0.10 (MeOH), Rf (RP-TLC) = 0.07 (H2O/ACN 7:3). 1H NMR (600.11 MHz, DMSO-d6) δ (ppm): 2.36 (s, 3H, 4-CH3), 2.45 (s, 3H, 7-CH3), 5.71 (s, 1H, H3), 7.34–7.40 (m, 1H, H6’), 7.55 (t, 1H, J = 8.9 Hz, H5’), 7.68 (dd, 1H, J1 = 6.7 Hz, J2 = 2.6 Hz, H2’), 10.08 (s, 1H, 1-OH), 14.74 (s, 1H, 6-OH); 13C NMR (150.9 MHz, DMSO-d6) δ (ppm): 20.7 (7-CH3), 25.0 (4-CH3), 99.5 (C5), 110.5 (C3), 117.5, 117.6 (d, JC-F = 22.3 Hz, C5’), 120.1, 120.2 (d, JC-F = 18.9 Hz, C3’), 126.7 (C6’), 128.1 (C2’), 134.1 (C1’), 148.3 (C4), 155.3, 156.9 (d, JC-F = 247.2 Hz, C4’), 158.8 (C2), 164.3 (C6), 168.8 (C7). Anal. Calcd for C14H12ClFN2O3: C, 54.12; H, 3.89; N, 9.02. Found: C, 54.16; H, 3.92; N, 9.08.
3.2.9. Synthesis of N-hydroxypyridinedione Oximes (30–32)
General procedure:
5-acetyl-1,6-dihydroxy-4-methylpyridin-2(1H)-one (B) (0.55 mmol, 1 equiv.) is added to a solution of the appropriate hydroxylamine (0.57 mmol, 1.1 equiv.) in abs. EtOH (2 mL), and the reaction mixture is stirred at RT, under Argon, for 1.5–22 h. Thereafter, the solvent is evaporated under vacuum. The solid residue is triturated with Et2O under ice to afford the desired compound.
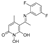
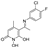
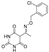
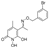
5-(1-(((3,4-dichlorobenzyl)oxy)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (30)
To a solution of the
O-(3,4-dichlorobenzyl)hydroxylamine (
9) (330 mg, 1.64 mmol, 1.0 equiv.) in abs. EtOH (6 mL), 5-acetyl-1,6-dihydroxy-4-methylpyridin-2(1
H)-one (
B) (300 mg, 1.72 mmol, 1.05 equiv.) is added, and the reaction mixture is stirred at RT, under Argon, for 24h. Thereafter, the solvent is evaporated under vacuum. The solid residue is triturated with 2 mL Et
2O under ice. The precipitate formed was filtered off in vacuo and chromatographed on a Sephadex LH-20 column eluted with MeOH. The green solid was triturated with dry Et
2O and drops of EtOAc to afford [
30] as a green crystalline solid (150 mg, 26%); mp 103–105 °C (AcOEt/dry Et
2O), R
f (NP-TLC) = 0.10 (AcOEt), R
f (RP-TLC) = 0.03 (H
2O/ACN 7:3);
1H NMR (600.11 MHz, DMSO-
d6)
δ (ppm) 1.76, 1.88 (s + s, 2.85H, 4-CH
3), 1.93, 1.96 (s + s, 0.8H, 7-CH
3), 1.99, 2.03 (s + s, 1.9H, 7-CH
3), 4.96, 5.01 (s + s, 0.55H, OCH
23,4-Cl
2C
6H
4), 5.09, 5.13 (s + s, 1.4H, OCH
23,4-Cl
2C
6H
4), 5.48, 5.52 (s + s, 1H, H
3), 7.28 (dd, 0.3H,
J1 = 8.2 Hz,
J2 = 2.0 Hz, H
6’), 7.35 (dd, 0.7H,
J1 = 8.2 Hz,
J2 = 2.0 Hz, H
6’), 7.54 (d, 0.3H,
J = 2.0 Hz, H
2’), 7.59 (d, 0.7H,
J = 1.9 Hz, H
2’), 7.59 (d, 0.3H,
J = 8.3 Hz, H
5’), 7.63 (d, 0.7H,
J = 8.3 Hz, H
5’), 10.18 (low), 11.60 (s + v br s, 1H, 1-OH, 6-OH);
13C NMR (150.9 MHz, DMSO-
d6)
δ (ppm) 15.9 (7-CH
3), 19.3, 19.4 (4-CH
3), 19.8 (7-CH
3), 72.8 (OCH
23,4-Cl
2C
6H
4), 91.0 (C
3), 112.0 (C
5), 127.8 (C
6’), 129.5 (C
2’), 129.9 (C
4’), 130.4 (C
5’), 130.8 (C
3’), 139.8, 140.1 (C
1’), 146.4, 149.5 (C
4), 153.7, 155.0 (C
7), 155.1 (C
6), 156.4 (C
2). Anal. Calcd for C
15H
14Cl
2N
2O
4: C, 50.44; H, 3.95; N, 7.84. Found: C, 50.48; H, 4.04; N, 7.89.
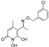
5-(1-(((2,4-dichlorobenzyl)oxy)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (31) was synthesized from compound (10) according to the general procedure. Green solid (93.6 mg, 50%), Rf 0.20 (AcOEt), mp: 145–150 °C, 1H NMR (400 MHz, DMSO) δ 7.65–7.39 (m, 3H, Ar), 5.48 (d, J = 19.7 Hz, 1H, CHC=ON), 5.09 (d, J = 50.9 Hz, 2H, OCH2), 2.00 (d, J = 28.0 Hz, 3H, CH3C=N), 1.88 (d, J = 6.2 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 132.50 (C3’), 129.22 (C5’), 128.28 (C6’), 92.19 (C3), 71.56 (OCH2), 20.42 (7-CH3), 16.42 (4-CH3). Anal. Calcd for C15H14Cl2N2O4: C, 50.44; H, 3.95; N, 7.84. Found: C, 50.48; H, 3.99; N, 7.88.
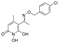
1,6-dihydroxy-5-(1-(((2-iodobenzyl)oxy)imino)ethyl)-4-methylpyridin-2(1H)-one (32), was synthesized from compound (11) (170 mg, 0.68 mmol) according to the general procedure. Green solid (99.5 mg, 39%), mp 131–133 °C (deco), Rf = 0.07 (EtOAc/MeOH 3:1). 1H NMR (600 MHz, DMSO) δ 7.86 (dd, J = 19.2, 8.0 Hz, 1H, Ar), 7.45–7.34 (m, 2H, Ar), 7.17–6.99 (m, 2H, Ar), 5.39 (s, 1H, H3), 5.06 (s, 2H, –CH2-), 2.06 (s, 3H, 7-CH3), 1.88 (s, 3H, 4-CH3). 13C NMR (151 MHz, DMSO) δ 154.38 (C2), 153.09 (C6), 150.81 (C7), 136.73 (C2‘), 133.67 (C3‘), 135.54 (C1‘), 131.17 (C4‘), 128.10 (C5‘), 127.09 (C6‘), 125.58 (C5), 122.25 (C3), 91.19 (C4), 73.67 (–CH2-), 22.10 (4-CH3), 19.78 (7-CH3). Anal. Calcd for C15H15IN2O4: C, 43.50; H, 3.65; N, 6.76. Found: C, 43.51; H, 3.64; N, 6.78.
3.2.10. Synthesis of N-hydroxypyridinedione Imines (33–43)
General procedure:
To a solution of A (1 eq) in absolute EtOH at 60 °C, the appropriate amine (1.1 eq) and activated 4Å molecular sieves were added. The mixture was refluxed overnight at 60 °C under Argon. After the completion of the reaction, the solvent is evaporated under vacuum. The solid residue is triturated with Et2O under ice to afford the desired compound as a solid.
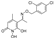
5-(1-((2-bromobenzyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (33) was synthesized from compound (16) (100 mg, 0.54 mmol) according to the general procedure. Green solid (130.2 mg, 76%)%). mp 140–142 °C, Rf = 0.06 (EtOAc/MeOH 3:1). 1H NMR (400 MHz, DMSO) δ 7.71 (d, J = 7.9 Hz, 2H, -OH), 7.45 (d, J = 4.5 Hz, 2H, Ar), 7.37–7.27 (m, 2H, Ar), 5.58 (s, 1H, H3), 4.82 (s, 2H, H10), 2.35 (s, 2H, H9), 2.32 (s, 3H, 4-CH3), 2.16 (s, 1H, H9). 13C NMR (101 MHz, DMSO) δ 170.67 (C2), 163.86 (C8), 158.71 (C6), 148.04 (C2’), 135.60 (C1’), 133.02 (C3’), 130.18 (C5’), 130.05 (C4’), 128.37 (C6’), 123.02 (C3), 109.13 (C5), 101.25 (C4), 98.35 (C10), 25.24 (C9), 23.86 (C4). Anal. Calcd for C15H15BrN2O3: C, 51.30; H, 4.31; N, 7.98. Found: C, 51.35; H, 4.34; N, 7.99.
5-(1-((3-bromobenzyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (34) was synthesized from compound (17) (100 mg, 0.54 mmol) according to the general procedure. Green solid (116.2 mg, 68%). mp 146-148 °C, Rf = 0.06 (EtOAc/MeOH 3:1). 1H NMR (500 MHz, DMSO) δ 7.60 (s, 1H, Ar), 7.55 (s, 2H, Ar), 7.38 (s, 1H, Ar), 5.57 (s, 1H, H3), 4.80 (s, 2H, H10), 2.48 (s, 3H, H9), 2.31 (s, 3H, H4). 13C NMR (126 MHz, DMSO) δ 170.72 (C2), 163.80 (C8), 158.72 (C6), 148.03 (C5’), 139.56 (C2’), 131.06 (C1’), 130.64 (C6’), 130.32 (C4’), 126.59 (C3’), 122.04 (C5), 109.01 (C4), 98.35 (C3), 46.52 (C10), 25.18 (C4), 19.09 (C9). Anal. Calcd for C15H15BrN2O3: C, 51.30; H, 4.31; N, 7.98. Found: C, 51.32; H, 4.35; N, 8.00.
5-(1-((4-bromobenzyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (35) was synthesized from compound (18) (103.8 mg, 0.56 mmol), according to the general procedure. Green solid (117.3 mg, 66%), mp 146–148 °C, Rf = 0.06 (EtOAc/MeOH 3:1). 1H NMR (400 MHz, DMSO) δ 7.61 (d, J = 8.1 Hz, 2H, Ar), 7.51 (br, s, 2H, –OH), 7.39–7.23 (m, 2H, Ar), 5.57 (s, 1H, H3), 4.77 (s, 2H, H10), 2.35 (s, 1H, H9), 2.31 (s, 3H, H7), 2.16 (s, 2H, H9). 13C NMR (101 MHz, DMSO) δ 170.74 (C2), 163.79 (C8), 158.74 (C6), 148.03 (C4’), 136.23 (C1’), 131.78 (C3’,5’), 131.22 (C2’,6’), 129.75 (C3), 120.89 (C5), 108.96 (C4), 46.57 (C10), 25.18 (C9), 19.10 (C7). Anal. Calcd for C15H15BrN2O3: C, 51.30; H, 4.31; N, 7.98. Found: C, 51.36; H, 4.40; N, 7.99.
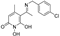
5-(1-((2-chlorobenzyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (36) was synthesized from 2-chlorobenzylamine, (42.5 mg, 36.2 μL, 0.3 mmol, 1.1 eq) according to the general procedure. The solid residue was purified by MPLC method Column: FP Si 12g, Mode: Flash; Solvent A: EtOAc, Solvent B: MeOH; Gradient (%2nd: 0–30%; Flow Rate: 30 mL/min). The solid was then triturated with Et2O and EtOAc and filtered off under vacuum to afford the title compound as a yellow solid. (53.9 mg, 64.4%); m.p 116–118 °C, Rf = 0.26 (EtOAc/MeOH 3:1), Rf (RP-TLC) = 0.66 (H2O/ACN 7:3). 1H NMR (400 MHz, DMSO-d6) δ 7.55 (dd, J = 5.7, 3.5 Hz, 1H,Ar), 7.44–7.39 (m, 1H,Ar), 7.36–7.24 (m, 2H, Ar), 5.58 (s, 1H, CHC=ON), 4.93 (d, J = 68.8 Hz, 2H,1’-CH2), 2.35 (s, 3H,4-CH3), 2.17 (s, 3H,7-CH3). 13C NMR (101 MHz, DMSO) δ 170.71 (C7), 161.18 (C6), 157.63 (C2), 148.82 (C4), 133.99 (C1’), 132.66 (C2’), 129.96 (Ar), 128.48 (Ar), 127.81 (Ar), 127.19 (Ar), 109.08 (C3), 98.35 (C5), 45.37 (1’-CH2), 25.22 (4-CH3), 23.82 (7-CH3). Anal. Calcd for C15H15ClN2O3: C, 58.73; H, 4.93; Cl, 11.56; N, 9.13; O, 15.65. Found: C, 60.00; H, 5.05; Cl, 11.58; N, 9.50; O, 15.75.
5-(1-((3-chlorobenzyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (37) was synthesized from 3-chlorobenzylamine, (102.1 mg, 87 μL, 0.7 mmol, 1.1 eq) according to the general procedure. The solid residue was triturated with Et2O and n-pentane. The solid was filtered off under vacuum to afford the title compound as a brown solid. (137.40 mg, 68.4%); m.p 102–120 °C (dec.), Rf = 0.27 (EtOAc/MeOH 3:1), Rf (RP-TLC) = 0.60 (H2O/ACN 7:3). 1H NMR (500 MHz, DMSO-d6) δ 7.48–7.25 (m, 4H,Ar), 5.57 (s, 1H,H3), 4.92 (d, J = 113.2 Hz, 2H,-1’-CH2), 2.48 (s, 1H,7-CH3), 2.33 (d, J = 22.1 Hz, 3H,4-CH3), 2.17 (s, 2H, 7-CH3). 13C NMR (126 MHz, DMSO-d6) δ 170.75 (C7),158.77 (C2), 148.92 (C6), 148.02 (C4), 133.45 (C1’), 130.79 (C3’), 127.75 (Ar), 127.43 (Ar), 127.27 (Ar), 126.22 (Ar), 109.00 (C3), 101.15 (1’-CH2), 98.39 (C5), 46.61 (1’-CH2), 32.50 (4-CH3), 25.18 (7-CH3), 23.81 (7-CH3), 19.10 (7-CH3). Anal. Calcd for C15H15ClN2O3: C, 58.73; H, 4.93; Cl, 11.56; N, 9.13; O, 15.65. Found: C, 60.00; H, 5.05; Cl,12.00; N, 9.50; O, 16.00.
5-(1-((4-chlorobenzyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (38) was synthesized from 4-chlorobenzylamine, (102.1 mg, 87.7 μL, 0.7 mmol, 1.1 eq) according to the general procedure. The solid residue was triturated with Et2O and drops of EtOH. The solid was filtered off under vacuum to afford the title compound as a brown solid. (165.80 mg, 82.5%); m.p 115–118 °C, Rf = 0.30 (EtOAc/MeOH 3:1), Rf (RP-TLC) = 0.66 (H2O/ACN 7:3). 1H NMR (400 MHz, DMSO-d6) δ 7.48 (d, J = 7.9 Hz, 2H, H2’,6’), 7.40 (d, J = 8.3 Hz, 2H, H3’,5’), 5.57 (s, 1H, H3), 4.79 (s, 2H, 1’-CH2), 2.48 (s, 2H, 7-CH3), 2.31 (s, 3H, 4-CH3), 2.16 (s, 1H, 7-CH3). 13C NMR (101 MHz, DMSO) δ 170.70 (C7), 158.71 (C2), 148.75 (C6) 147.99 (C4), 135.79 (C4’), 132.36 (C1’), 129.42 (C2’,6’), 128.84 (C3’,5’), 108.93 (C3), 98.32 (C5), 46.51 (1’-CH2), 25.15 (4-CH3), 19.08 (7-CH3). Anal. Calcd for C15H15ClN2O3: C, 58.73; H, 4.93; Cl, 11.56; N, 9.13; O, 15.65. Found: C, 59.00; H, 5.00; Cl,12.05; N, 9.50; O, 16.00.
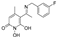
5-(1-((3-fluorobenzyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (39) was synthesized from 3-fluorobenzylamine, (52.6 mg, 47.9 μL, 0.42 mmol, 1.1 eq) according to the general procedure. The solid residue was triturated with Et2O and drops of EtOH. The solid was filtered off under vacuum to afford the title compound as a brown solid. (53.3 mg, 48.4%). Rf = 0.13(EtoAc/MeOH 3:1). mp. 138–140 °C. 1H NMR (500 MHz, DMSO) δ 7.47 (q, J = 7.3 Hz, 1H), 7.22 (d, J = 8.3 Hz, 2H), 7.17 (d, J = 8.8 Hz, 1H), 5.57 (s, 1H), 4.81 (s, 2H), 2.35 (s, 1H), 2.31 (s,2H), 2.16 (s, 3H). 13C NMR (126 MHz, DMSO) δ 170.75 (C8), 163.79 (C17), 161.35(C19), 158.71 (C2), 148.75 (C4), 139.65 (C15), 130.97 (C18), 123.52 (C20),115.42 (C16),108.97(C1), 101.22 (C5), 98.35 (C6), 46.68 (C14), 25.76 (8-CH3), 25.16 (6-CH3). Anal. Calcd (%) for C15H15FN2O3: C, 62.06; H, 5.21; F, 6.54; N,9.65; O, 16.53. Found: C, 61.97; H, 5.24; F, 6.40; N, 9.59; O, 16.63.
5-(1-((4-fluorobenzyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (40) was synthesized from 4-fluorobenzylamine, (74.53 mg, 68 μL, 0.3 mmol, 1.1 eq) according to the general procedure. The solid residue was triturated with Et2O and drops of EtOH. The solid was filtered off under vacuum to afford the title compound as a brown solid. (74.6 mg, 43%); m.p. 138–140 °C, Rf = 0.15 (EtOAc/MeOH 3:1). 1H NMR (400 MHz, DMSO) δ 7.28–7.20 (m, 4H), 5.56 (s, 1H), 4.76 (s,2H), 2.35 (s, 2H), 2.31 (s, 1H), 2.16 (s, 3H,). 13C NMR (101 MHz, DMSO) δ 170.87 (C11), 163.52 (C18), 157.79 (C2), 149.94 (C6), 148,91 (C4), 133.28 (C15), 129.75 (C16–20), 129.67 (C17–19), 115.52 (C15), 108.71 (C1), 101.09 (C5), 98.93 (C6), 48.50(C14), 25.05 (11-CH3), 23.68 (6-CH3)., Anal. Calcd (%) for C15H15FN2O3: C, 62.06; H, 5.21; F, 6.54; N, 9.65; O, 16.53. Found: C, 61.97; H, 5.29; F, 6.42; N, 9.61; O, 16.60.
5-(1-((3,4-difluorobenzyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (41) was synthesized from 3,4-difluorobenzylamine, (103.2 mg, 85.3 μL, 0.7 mmol, 1.1 eq) according to the general procedure. The solid residue was triturated with Et2O and drops of EtOH. The solid was filtered off under vacuum to afford the title compound as a brown solid. (101 mg, 50%); m.p 102–107 °C, Rf = 0.19 (EtOAc/MeOH 3:1), Rf (RP-TLC) = 0.28 (H2O/ACN 7:3). 1H NMR (400 MHz, DMSO-d6) δ 7.51–7.45 (m, 1H,H6’), 7.39–7.32 (m, 1H,H5’), 7.24 (s, 1H,H2’), 5.57 (s, 1H,H3), 4.78 (s, 2H,1’-CH2), 2.48 (s, 3H,7-CH3), 2.31 (s, 3H,4-CH3). 13C NMR (101 MHz, DMSO) δ 170.89 (C7), 163.96 (C6), 158.92 (C2), 148.19 (C4), 134.78 (C1’), 124.66 (C2’), 118.22 (C4’), 118.05 (C3’), 117.13 (C6’), 116.95 (C5’), 109.16 (C3), 98.62 (C5), 46.37 (1’-CH2), 25.33 (4-CH3), 19.28 (7-CH3). Anal. Calcd for C15H14F2N2O3: C, 58.44; H, 4.58; F, 12.33; N, 9.09; O, 15.57. Found: C, 59.00; H, 5.60; F,12.35; N, 9.15; O, 15.65
5-(1-((3,5-difluorobenzyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (42) was synthesized from 3,5-difluorobenzylamine, (41.3 mg, 34.1 μL, 0.3 mmol, 1.1 eq) according to the general procedure. The solid residue was triturated with Et2O and drops of EtOH. The solid was filtered off under vacuum to afford the title compound as a brown solid. (64.3mg, 80.2%); m.p 136–140 °C, Rf = 0.24 (EtOAc/MeOH 3:1), Rf (RP-TLC) = 0.60 (H2O/ACN 7:3). 1H NMR (400 MHz, DMSO-d6) δ 7.40–6.99 (m, 3H, Ar), 5.58 (s, 1H, H3), 4.92 (d, J = 75.5 Hz, 2H, 1’-CH2), 2.33 (d, J = 15.1 Hz, 3H, 4-CH3), 2.19–2.04 (m, 3H, 7-CH3). 13C NMR (101 MHz, DMSO) δ 171.43 (C7), 164.30 (C6), 158,66 (C2) 148.55 (C4), 131.88 (C1’), 111.05 (Ar), 109.61 (C3), 103.96 (Ar), 103.70 (Ar), 103.45 (Ar), 98.99 (C5), 46.81 (1’-CH2), 25.64 (7-CH3), 19.55 (4-CH3). Anal. Calcd for C15H14F2N2O3: C, 58.44; H, 4.58; F, 12.33; N, 9.09; O, 15.57. Found: C, 59.05; H, 5.65; F,12.40; N, 9.13; O, 15.70.
5-(1-((2-(4-chlorophenoxy)ethyl)imino)ethyl)-1,6-dihydroxy-4-methylpyridin-2(1H)-one (43) was synthesized from 2-(4-chlorophenoxy)ethan-1-amine (20), (101 mg, 0.58 mmol, 1.2 eq) according to the general procedure. The solid residue was triturated with Et2O, EtOAc, and drops of EtOH. The solid was filtered off under vacuum to afford the title compound as a red solid. (30 mg, 19.6%) Rf = 0.27 (EtOAc/MeOH 3:1). mp. 138–140 °C. 1H NMR (400 MHz, DMSO) δ 7.35 (dd, J = 132.1, J = 8.6 Hz, 2H), 7.03 (dd, J = 132.1, 9.0 Hz, 2H), 5.55 (s, 1H), 4.24 (q, J = 5.0 Hz, 2H), 3.91 (q, J = 5.0 Hz,2H), 2.35 (s, 2H), 2.31 (s, 1H), 2.16 (s, 3H). 13C NMR (101 MHz, DMSO) δ 171.49 (C11), 159.7 (C2), 157.43 (C17),148.52 (C4),129.81 (C18–22), 125.31 (C20), 116.97 (C19–21), 108.95 (C1), 101.64 (C5), 98.59 (C6), 66.83 (C15), 43.63 (C14), 25.66 (11-CH3), 24.33 (6-CH3). Anal. Calcd (%) for C16H17ClN2O4: C, 57.06;H, 5.09; Cl, 10.53; N, 8.32; O, 19.00. Found: C, 57.01; H, 5.02; Cl, 10.48; N,8.31; O, 19.13.
3.2.11. Synthesis of Minoxidil Derivative (44)
General procedure:
To a solution of the corresponding 6-substituted 2,4-diaminopyrimidine intermediate (700 mg, 2.49 mmol, 1 equiv.) in MeOH (6.2 mL), a solution of mCPBA (1.1 g, 6.23 mmol, 2.5 equiv.) in MeOH (5 mL) is added dropwise over a time period of 1–1.5 h at 0 °C. The reaction mixture is stirred at 0 °C overnight. Then, aq. NaOH 6 M is added until basic pH = 8. The organic solvent is evaporated under vacuum, and the formed white solid precipitate is filtered under vacuum and washed with ice-cooled water (1 mL). The aqueous filtrate is extracted with EtOAc (4 × 100 mL). The combined organic layers are washed with brine (3 × 50 mL), then dried over anh. Na2SO4, filtered, and concentrated. The resulting crude oil is purified using flash column chromatography (EtOAc/MeOH, 0–30%). The oil product was then triturated with Et2O to afford the product as a white solid.
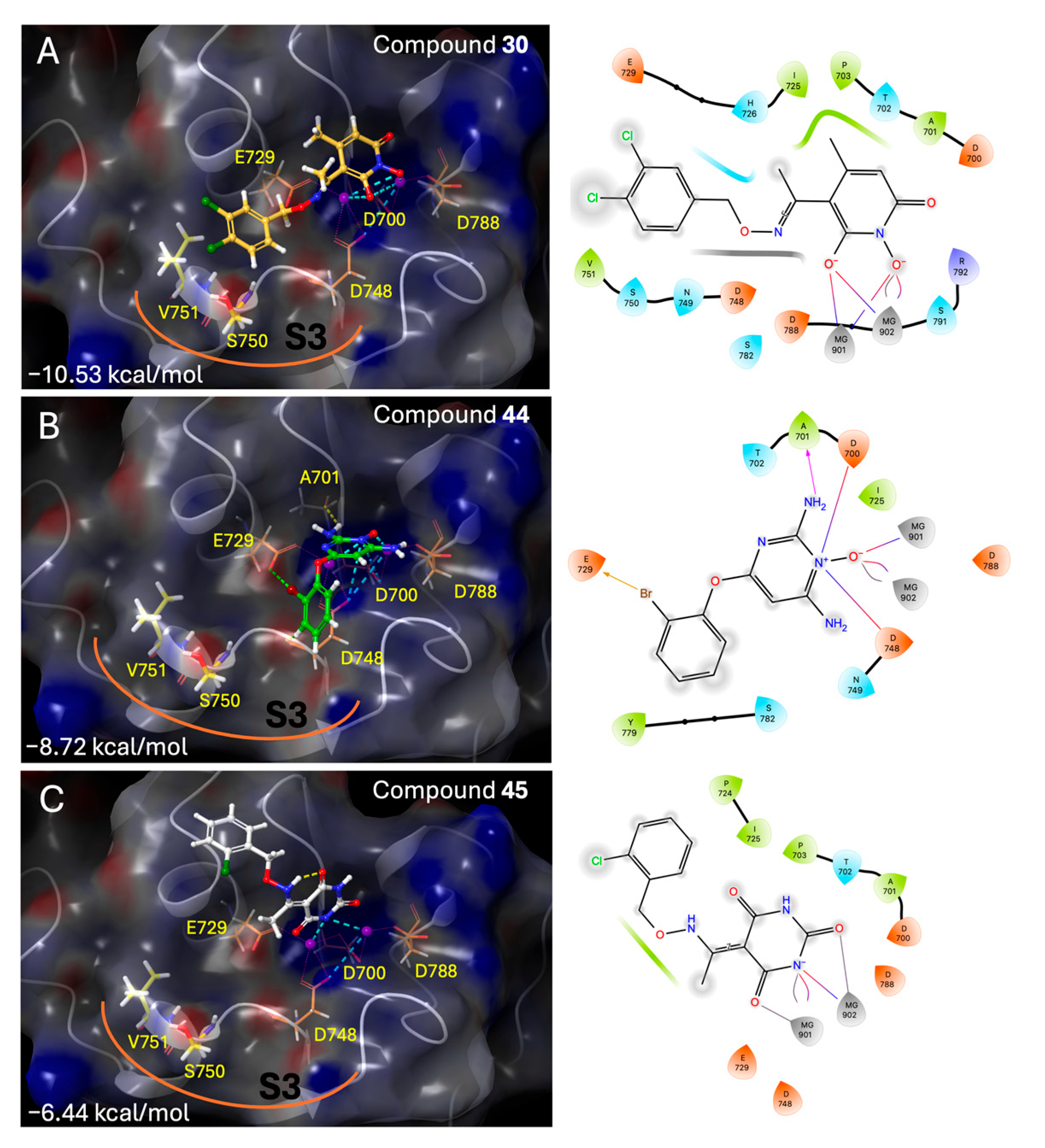
2-amino-4-(2-bromophenoxy)-6-iminopyrimidin-1(6H)-ol (44) was synthesized according to the general procedure from the 6-(2-bromophenoxy)pyrimidine-2,4-diamine (19), to afford a white solid (250.2 mg, 50%) Rf = 0.30 (3:1 AcOEt: MeOH), mp > 250 °C (dec.: 200 °C), 1H NMR (500 MHz, DMSO) δ 7.71 (d, J = 7.9 Hz, 1H, Ph), 7.44 (t, J = 7.8 Hz, 2H, Ph, OH), 7.30 (d, J = 8.1 Hz, 1H, Ph), 7.20 (t, J = 7.7 Hz, 1H, Ph), 5.50 (s, 1H, CHC=NH). 13C NMR (126 MHz, DMSO) δ 158.72 (C4), 154.31 (C6), 152.58 (C2), 150.04 (C1’), 133.45 (C3’), 129.27 (C5’), 127.14 (C4’), 124.17 (C6’), 115.82 (C2’), 76.92 (C5). HRMS/ESI+ (m/z): Calcd for C10H9BrN4O2: 295.9909; Found: 296.9979. Anal. Calcd for C10H9 BrN4O2: C, 40.43; H, 3.05; N, 18.86. Found: C, 40.46; H, 3.08; N, 18.89.
3.2.12. Synthesis of 5-Acetyl Barbituric Acid
Acetic anhydride (23.4 mL) was used to suspend barbituric acid (1.0 g, 7.81 mmol), then 7 drops of concentrated H
2SO
4 were added. The barbituric acid was dissolved completely after 10 min, resulting in a yellow–brown solution. The reaction mixture was stirred at 110 °C for 1.5 h. Thereafter, the mixture was concentrated to half its volume and cooled down to 0 °C. The formed precipitate was filtered off and washed with hot water and acetone to afford a beige crystalline solid (1.15 g, 89%).
1H NMR (400 MHz, DMSO)
δ 11.77 (s, 1H), 11.05 (s, 1H,), 2.58 (s, 3H) ppm [
35].
3.2.13. Synthesis of Barbituric Acid Analogue (45)
5-(1-(((2-chlorobenzyl)oxy)imino)ethyl)pyrimidine-2,4,6(1H,3H,5H)-trione (45)
5-Acetyl-barbituric acid (0.386 mmol, 1 equiv.) is suspended in abs. EtOH (2 mL) at ~90 °C, and molecular sieves and the appropriate hydroxylamine (12) (82.2 mg, 0.52 mmol, 1.1 eq) are added. The mixture is refluxed for 3 days at 70 °C under an Argon atmosphere. The suspended solid formed is filtered under vacuum and washed with Et2O and EtOH. The molecular sieves are removed to afford the desired product as a pink solid. (122 mg, 83%). mp >250 °C, Rf = 0.26 (EtOAc/MeOH 3:1). 1H NMR (400 MHz, DMSO) δ 10.58 (s, 1H, H5), 7.61–7.52 (m, 2H, Ar), 7.49–7.38 (m, 2H, Ar), 5.20 (s, 2H, –CH2-), 2.54 (s, 3H, H8). 13C NMR (125 MHz, DMSO) δ 167.28 (C4,6), 161.29 (C7), 151.41 (C2), 133.87 (C1’), 133.51 (C2’), 129.94 (C6’), 129.42 (C3’), 128.88 (C4’), 127.49 (C5’), 75.31 (C9), 54.39 (C5), 19.20 (C8). Anal. Calcd for C13H12ClN3O4: C, 50.42; H, 3.91; N, 13.57. Found: C, 50.44; H, 3.92; N, 13.55.