Thermal Cycling Stimulation via Nasal Inhalation Attenuates Aβ25–35-Induced Cognitive Deficits in C57BL/6 Mice
Abstract
1. Introduction
2. Results
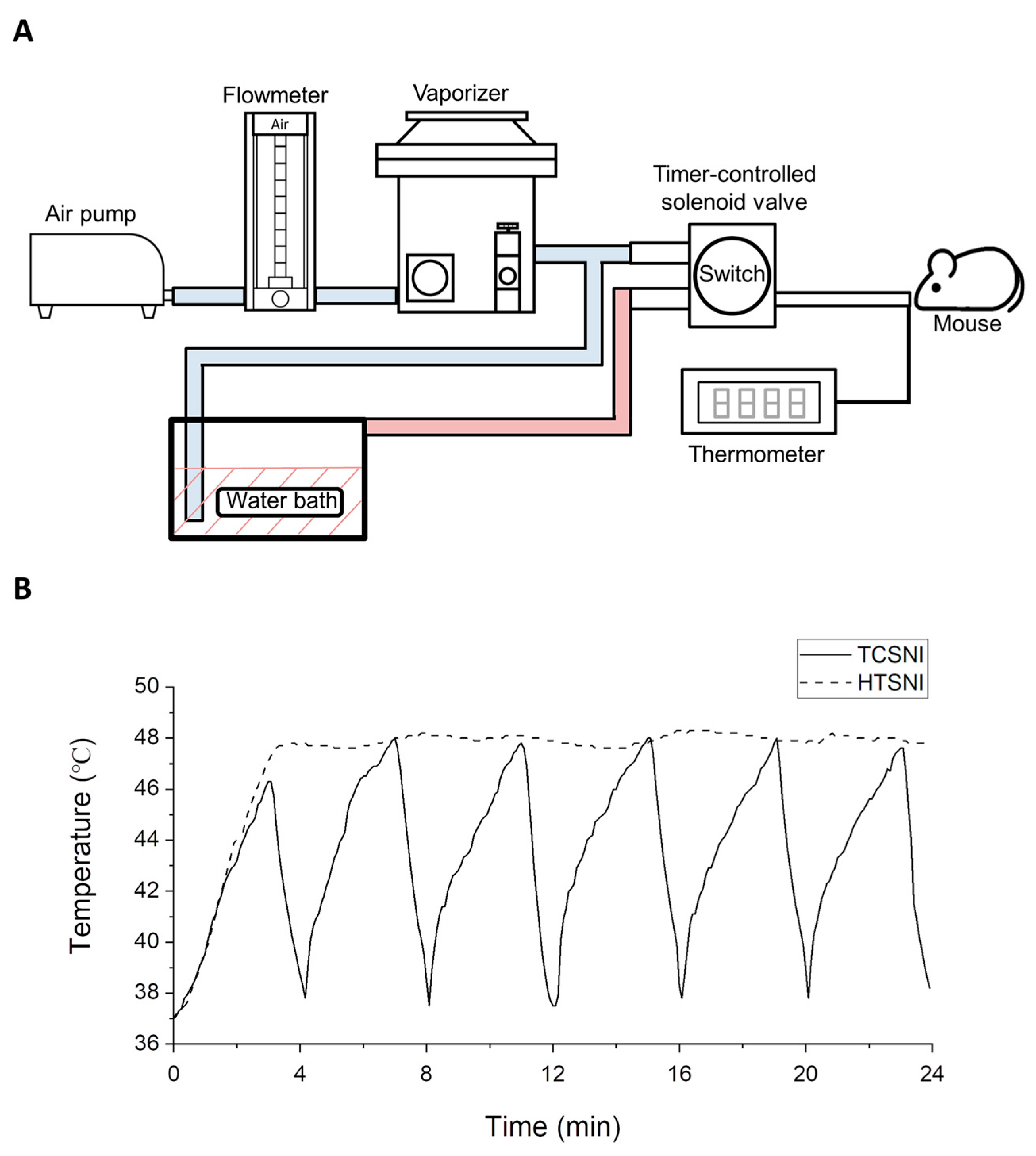
2.1. Effects of HTSNI and TCSNI on Olfactory Function in Mice
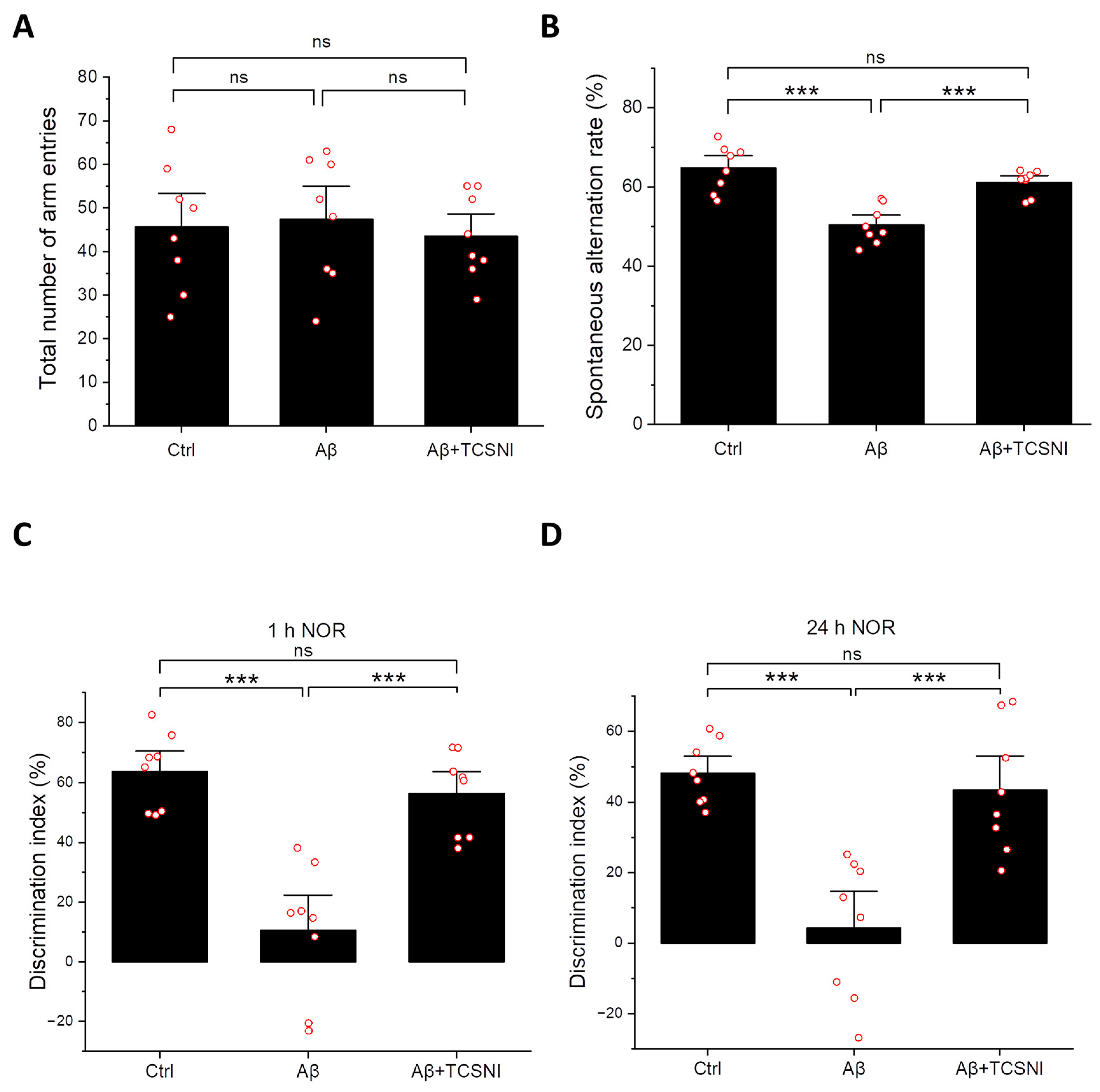
2.2. TCSNI Attenuates Aβ-Induced Cognitive Impairments in Mice
2.3. TCSNI Down-Regulates Aβ Accumulation and Elevates HSP70, IDE, and p-Akt Levels in the Mouse Hippocampus
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Housing
4.2. β-Amyloid Administration
4.3. HTSNI and TCSNI Applications
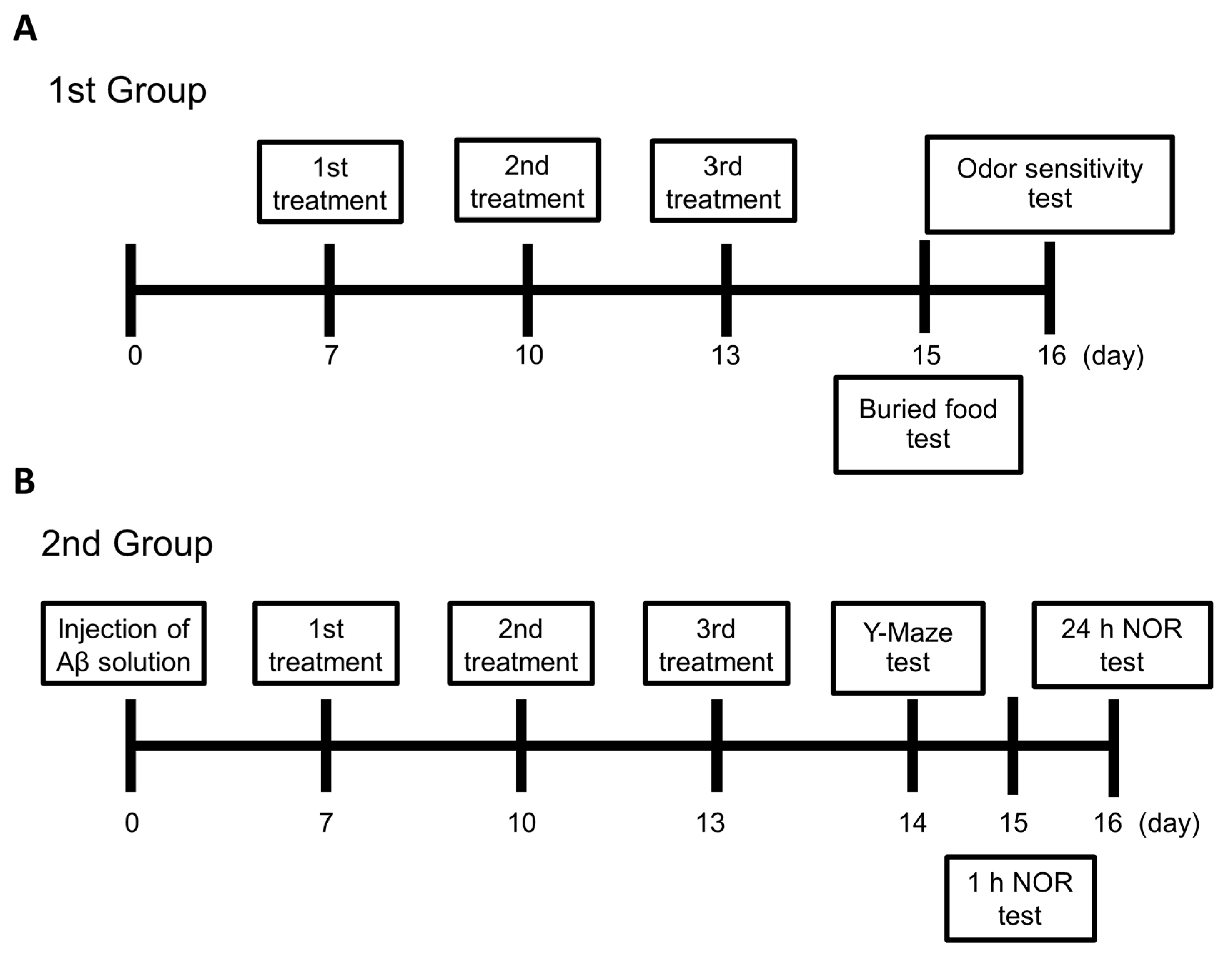
4.4. Experimental Design
4.5. Buried Food Test
4.6. Odor Sensitivity Test
4.7. Y-Maze Test
4.8. Novel Object Recognition Test
4.9. Collection of Brain Tissue and Preparation of Samples for Western Blot Analysis
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | β-amyloid |
| N-2-B | Nose-to-brain |
| BBB | Blood–brain barrier |
| HT | Hyperthermia |
| TC-HT | Thermal cycling hyperthermia |
| TCSNI | Thermal cycling stimulation via nasal inhalation |
| HTSNI | Hyperthermia stimulation via nasal inhalation |
| i.c.v. | Intracerebroventricular |
| NOR | Novel object recognition |
| HSP70 | Heat-shock protein 70 |
| IDE | Insulin-degrading enzyme |
| p-Akt | Phosphorylated Akt |
| ROS | Reactive oxygen species |
| ANOVA | Analysis of variance |
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, W.D.; Wang, Y.D. β-Amyloid: The key peptide in the pathogenesis of Alzheimer’s disease. Front. Pharmacol. 2015, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Leuner, K.; Schütt, T.; Kurz, C.; Eckert, S.H.; Schiller, C.; Occhipinti, A.; Mai, S.; Jendrach, M.; Eckert, G.P.; Kruse, S.E.; et al. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid. Redox Signal. 2012, 16, 1421–1433. [Google Scholar] [CrossRef]
- Miranda, S.; Opazo, C.; Larrondo, L.F.; Muñoz, F.J.; Ruiz, F.; Leighton, F.; Inestrosa, N.C. The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer’s disease. Prog. Neurobiol. 2000, 62, 633–648. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef]
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Rahman, A.; Hossen, M.A.; Chowdhury, M.F.I.; Bari, S.; Tamanna, N.; Sultana, S.S.; Haque, S.N.; Al Masud, A.; Saif-Ur-Rahman, K.M. Aducanumab for the treatment of Alzheimer’s disease: A systematic review. Psychogeriatrics 2023, 23, 512–522. [Google Scholar] [CrossRef]
- Hossain, M.F.; Husna, A.U.; Kharel, M. Use of lecanemab for the treatment of Alzheimer’s disease: A systematic review. Brain Behav. 2024, 14, e3592. [Google Scholar] [CrossRef]
- Cummings, J.; Apostolova, L.; Rabinovici, G.D.; Atri, A.; Aisen, P.; Greenberg, S.; Hendrix, S.; Selkoe, D.; Weiner, M.; Petersen, R.C.; et al. Lecanemab: Appropriate Use Recommendations. J. Prev. Alzheimer’s Dis. 2023, 10, 362–377. [Google Scholar] [CrossRef]
- Fleck, L.M. Alzheimer’s and Aducanumab: Unjust Profits and False Hopes. Hastings Cent. Rep. 2021, 51, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Alzheimer’s disease drug development and the problem of the blood-brain barrier. Alzheimer’s Dement. 2009, 5, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Rattan, S.I. Hormetic modulation of aging and longevity by mild heat stress. Dose Response 2006, 3, 533–546. [Google Scholar] [CrossRef]
- Schirrmacher, V. Less Can Be More: The Hormesis Theory of Stress Adaptation in the Global Biosphere and Its Implications. Biomedicines 2021, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Kouda, K.; Iki, M. Beneficial effects of mild stress (hormetic effects): Dietary restriction and health. J. Physiol. Anthropol. 2010, 29, 127–132. [Google Scholar] [CrossRef]
- Calabrese, V.; Giordano, J.; Signorile, A.; Laura Ontario, M.; Castorina, S.; De Pasquale, C.; Eckert, G.; Calabrese, E.J. Major pathogenic mechanisms in vascular dementia: Roles of cellular stress response and hormesis in neuroprotection. J. Neurosci. Res. 2016, 94, 1588–1603. [Google Scholar] [CrossRef]
- Rattan, S.I.; Demirovic, D. Hormesis can and does work in humans. Dose Response 2009, 8, 58–63. [Google Scholar] [CrossRef]
- Adlard, P.A.; Engesser-Cesar, C.; Cotman, C.W. Mild stress facilitates learning and exercise improves retention in aged mice. Exp. Gerontol. 2011, 46, 53–59, Correction in Exp Gerontol. 2011, 46, 841. [Google Scholar] [CrossRef]
- Minois, N. Longevity and aging: Beneficial effects of exposure to mild stress. Biogerontology 2000, 1, 15–29. [Google Scholar] [CrossRef]
- Parihar, V.K.; Hattiangady, B.; Kuruba, R.; Shuai, B.; Shetty, A.K. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol. Psychiatry 2011, 16, 171–183. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Mao, L.; Franke, J. Hormesis in aging and neurodegeneration-a prodigy awaiting dissection. Int. J. Mol. Sci. 2013, 14, 13109–13128. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Hormesis, cell death, and regenerative medicine for neurode-generative diseases. Dose Response 2012, 11, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Leak, R.K. The hormesis principle of neuroplasticity and neuroprotection. Cell Metab. 2024, 36, 315–337. [Google Scholar] [CrossRef]
- Mane, N.R.; Gajare, K.A.; Deshmukh, A.A. Mild heat stress induces hormetic effects in protecting the primary culture of mouse prefrontal cerebrocortical neurons from neuropathological alterations. IBRO Rep. 2018, 5, 110–115. [Google Scholar] [CrossRef]
- Smith Sonneborn, J. Alternative strategy for Alzheimer’s disease: Stress response triggers. Int. J. Alzheimer’s Dis. 2012, 2012, 684283. [Google Scholar] [CrossRef]
- Patrick, R.P.; Johnson, T.L. Sauna use as a lifestyle practice to extend healthspan. Exp. Gerontol. 2021, 154, 111509. [Google Scholar] [CrossRef]
- Ji, L.L. Exercise-induced modulation of antioxidant defense. Ann. N. Y. Acad. Sci. 2002, 959, 82–92. [Google Scholar] [CrossRef]
- Knekt, P.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Aromaa, A. Does sauna bathing protect against dementia? Prev. Med. Rep. 2020, 20, 101221. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, T.; Kunutsor, S.; Kauhanen, J.; Laukkanen, J.A. Sauna bathing is inversely associated with dementia and Alzheimer’s disease in middle-aged Finnish men. Age Ageing 2017, 46, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Öhman, H.; Savikko, N.; Strandberg, T.E.; Kautiainen, H.; Raivio, M.M.; Laakkonen, M.L.; Tilvis, R.; Pitkälä, K.H. Effects of Exercise on Cognition: The Finnish Alzheimer Disease Exercise Trial: A Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2016, 64, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef]
- Patharapankal, E.J.; Ajiboye, A.L.; Mattern, C.; Trivedi, V. Nose-to-Brain (N2B) Delivery: An Alternative Route for the Delivery of Biologics in the Management and Treatment of Central Nervous System Disorders. Pharmaceutics 2023, 16, 66. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Alexander, A.; Saraf, S. Nose-to-brain drug delivery approach: A key to easily accessing the brain for the treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 2102–2104. [Google Scholar] [CrossRef]
- Wong, C.Y.J.; Baldelli, A.; Hoyos, C.M.; Tietz, O.; Ong, H.X.; Traini, D. Insulin Delivery to the Brain via the Nasal Route: Unraveling the Potential for Alzheimer’s Disease Therapy. Drug Deliv. Transl. Res. 2024, 14, 1776–1793. [Google Scholar] [CrossRef] [PubMed]
- Li, B.S.Y.; Chan, C.W.H.; Li, M.; Wong, I.K.Y.; Yu, Y.H.U. Effectiveness and Safety of Aromatherapy in Managing Behavioral and Psychological Symptoms of Dementia: A Mixed-Methods Systematic Review. Dement. Geriatr. Cogn. Dis. Extra 2021, 11, 273–297. [Google Scholar] [CrossRef]
- Cha, H.; Kim, S.; Seo, M.S.; Kim, H.S. Effects of olfactory stimulation on cognitive function and behavior problems in older adults with dementia: A systematic literature review. Geriatr. Nurs. 2021, 42, 1210–1217. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Hatvate, N.T.; Naren, P.; Khan, S.; Chavda, V.P.; Balar, P.C.; Gandhi, J.; Khatri, D.K. Essential oils for clinical aromatherapy: A comprehensive review. J. Ethnopharmacol. 2024, 330, 118180. [Google Scholar] [CrossRef]
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Hancianu, M. Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid β(1-42) rat model of Alzheimer’s disease. Physiol. Behav. 2013, 120, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Hanashima, Y.; Mizutani, I.; Koike, K. The effect of inhalation of essential oil from Rosmarinus officinalis on scopolamine--induced Alzheimer’s type dementia model mice. Flavour. Fragr. J. 2018, 33, 230–234. [Google Scholar] [CrossRef]
- Okuda, M.; Fujita, Y.; Takada-Takatori, Y.; Sugimoto, H.; Urakami, K. Aromatherapy improves cognitive dysfunction in senescence-accelerated mouse prone 8 by reducing the level of amyloid beta and tau phosphorylation. PLoS ONE 2020, 15, e0240378. [Google Scholar] [CrossRef]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 1: An introduction to thermal therapy. Crit. Rev. Biomed. Eng. 2006, 34, 459–489. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Zhou, J.; Zhang, L.; Xue, J.; Tao, W. Non-Invasive Thermal Therapy for Tissue Engineering and Regenerative Medicine. Small 2022, 18, e2107705. [Google Scholar] [CrossRef]
- Biro, S.; Masuda, A.; Kihara, T.; Tei, C. Clinical implications of thermal therapy in lifestyle-related diseases. Exp. Biol. Med. 2003, 228, 1245–1249. [Google Scholar] [CrossRef]
- Hunt, A.P.; Minett, G.M.; Gibson, O.R.; Kerr, G.K.; Stewart, I.B. Could Heat Therapy Be an Effective Treatment for Alzheimer’s and Parkinson’s Diseases? A Narrative Review. Front. Physiol. 2020, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Von Schulze, A.T.; Deng, F.; Morris, J.K.; Geiger, P.C. Heat therapy: Possible benefits for cognitive function and the aging brain. J. Appl. Physiol. 2020, 129, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Abreu, M.M. Hyperthermia and targeting heat shock proteins: Innovative approaches for neurodegenerative disorders and Long COVID. Front. Neurosci. 2025, 19, 1475376. [Google Scholar] [CrossRef]
- Tundo, G.R.; Sbardella, D.; Ciaccio, C.; Grasso, G.; Gioia, M.; Coletta, A.; Polticelli, F.; Di Pierro, D.; Milardi, D.; Van Endert, P.; et al. Multiple functions of insulin-degrading enzyme: A metabolic crosslight? Crit. Rev. Biochem. Mol. Biol. 2017, 52, 554–582. [Google Scholar] [CrossRef]
- Kuo, Y.Y.; Chen, W.T.; Lin, G.B.; Chen, Y.M.; Liu, H.H.; Chao, C.Y. Thermal cycling-hyperthermia ameliorates Aβ25–35-induced cognitive impairment in C57BL/6 mice. Neurosci. Lett. 2023, 810, 137337. [Google Scholar] [CrossRef]
- Mearow, K.M.; Dodge, M.E.; Rahimtula, M.; Yegappan, C. Stress-mediated signaling in PC12 cells—The role of the small heat shock protein, Hsp27, and Akt in protecting cells from heat stress and nerve growth factor withdrawal. J. Neurochem. 2002, 83, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Kuo, Y.Y.; Lin, G.B.; Lu, C.H.; Hsu, H.P.; Sun, Y.K.; Chao, C.Y. Thermal cycling protects SH-SY5Y cells against hydrogen peroxide and β-amyloid-induced cell injury through stress response mechanisms involving Akt pathway. PLoS ONE 2020, 15, e0240022. [Google Scholar] [CrossRef]
- Yin, G.; Li, L.Y.; Qu, M.; Luo, H.B.; Wang, J.Z.; Zhou, X.W. Upregulation of AKT attenuates amyloid-β-induced cell apoptosis. J. Alzheimer’s Dis. 2011, 25, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Moosavi, M.; Zarifkar, A.; Rastegar, K.; Maghsoudi, N. The Interplay of Akt and ERK in Aβ Toxicity and Insulin-Mediated Protection in Primary Hippocampal Cell Culture. J. Mol. Neurosci. 2015, 57, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Kommaddi, R.P.; Gowaikar, R.; Haseena, P.A.; Diwakar, L.; Singh, K.; Mondal, A. Akt activation ameliorates deficits in hippocampal-dependent memory and activity-dependent synaptic protein synthesis in an Alzheimer’s disease mouse model. J. Biol. Chem. 2024, 300, 105619. [Google Scholar] [CrossRef]
- Hannuksela, M.L.; Ellahham, S. Benefits and risks of sauna bathing. Am. J. Med. 2001, 110, 118–126. [Google Scholar] [CrossRef]
- Bain, A.R.; Nybo, L.; Ainslie, P.N. Cerebral Vascular Control and Metabolism in Heat Stress. Compr. Physiol. 2015, 5, 1345–1380. [Google Scholar] [CrossRef]
- Prieur, E.A.K.; Jadavji, N.M. Assessing Spatial Working Memory Using the Spontaneous Alternation Y-maze Test in Aged Male Mice. Bio Protoc. 2019, 9, e3162. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, 55718. [Google Scholar] [CrossRef]
- Navarro, D.; Gasparyan, A.; Martí Martínez, S.; Díaz Marín, C.; Navarrete, F.; García Gutiérrez, M.S.; Manzanares, J. Methods to Identify Cognitive Alterations from Animals to Humans: A Translational Approach. Int. J. Mol. Sci. 2023, 24, 7653. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, D.K.; Chung, B.R.; Kim, H.V.; Kim, Y. Intracerebroventricular Injection of Amyloid-β Peptides in Normal Mice to Acutely Induce Alzheimer-like Cognitive Deficits. J. Vis. Exp. 2016, 109, 53308. [Google Scholar] [CrossRef]
- Yang, M.; Crawley, J.N. Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 2009, 48, 8–24. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Sun, S.; Li, J.; Wang, Y.; Dong, J.; Yang, S.; Lou, Y.; Yang, J.; Li, W.; et al. Olfactory Evaluation in Alzheimer’s Disease Model Mice. Brain Sci. 2022, 12, 607. [Google Scholar] [CrossRef]
- Jahn, H. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 2013, 15, 445–454. [Google Scholar] [CrossRef]
- Lu, R.C.; Tan, M.S.; Wang, H.; Xie, A.M.; Yu, J.T.; Tan, L. Heat shock protein 70 in Alzheimer’s disease. BioMed Res. Int. 2014, 2014, 435203. [Google Scholar] [CrossRef]
- Rivera, I.; Capone, R.; Cauvi, D.M.; Arispe, N.; De Maio, A. Modulation of Alzheimer’s amyloid β peptide oligomerization and toxicity by extracellular Hsp70. Cell Stress Chaperones 2018, 23, 269–279. [Google Scholar] [CrossRef]
- Valle-Medina, A.; Calzada-Mendoza, C.C.; Ocharan-Hernández, M.E.; Jiménez-Zamarripa, C.A.; Juárez-Cedillo, T. Heat shock protein 70 in Alzheimer’s disease and other dementias: A possible alternative therapeutic. J. Alzheimer’s Dis. Rep. 2025, 9, 25424823241307021. [Google Scholar] [CrossRef]
- Abdi, A.; Sadraie, H.; Dargahi, L.; Khalaj, L.; Ahmadiani, A. Apoptosis inhibition can be threatening in Aβ-induced neuroinflammation, through promoting cell proliferation. Neurochem. Res. 2011, 36, 39–48. [Google Scholar] [CrossRef]
- Hoshino, T.; Suzuki, K.; Matsushima, T.; Yamakawa, N.; Suzuki, T.; Mizushima, T. Suppression of Alzheimer’s disease-related phenotypes by geranylgeranylacetone in mice. PLoS ONE 2013, 8, e76306. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.G.; Leverenz, J.B.; McMillan, P.J.; Kulstad, J.J.; Ericksen, S.; Roth, R.A.; Schellenberg, G.D.; Jin, L.W.; Kovacina, K.S.; Craft, S. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-epsilon4 allele. Am. J. Pathol. 2003, 162, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Teter, B.; Morihara, T.; Lim, G.P.; Ambegaokar, S.S.; Ubeda, O.J.; Frautschy, S.A.; Cole, G.M. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: Implications for Alzheimer’s disease intervention. J. Neurosci. 2004, 24, 11120–11126. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y. Neuroprotection signaling of nuclear akt in neuronal cells. Exp. Neurobiol. 2014, 23, 200–206. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Cui, W.; Tao, J.; Wang, Z.; Ren, M.; Zhang, Y.; Sun, Y.; Peng, Y.; Li, R. Neuregulin1beta1 antagonizes apoptosis via ErbB4-dependent activation of PI3-kinase/Akt in APP/PS1 transgenic mice. Neurochem. Res. 2013, 38, 2237–2246. [Google Scholar] [CrossRef]
- Magrané, J.; Rosen, K.M.; Smith, R.C.; Walsh, K.; Gouras, G.K.; Querfurth, H.W. Intraneuronal beta-amyloid expression downregulates the Akt survival pathway and blunts the stress response. J. Neurosci. 2005, 25, 10960–10969. [Google Scholar] [CrossRef]
- Maroni, P.; Bendinelli, P.; Tiberio, L.; Rovetta, F.; Piccoletti, R.; Schiaffonati, L. In vivo heat-shock response in the brain: Signalling pathway and transcription factor activation. Brain Res. Mol. Brain Res. 2003, 119, 90–99. [Google Scholar] [CrossRef]
- Jensen, C.S.; Hasselbalch, S.G.; Waldemar, G.; Simonsen, A.H. Biochemical Markers of Physical Exercise on Mild Cognitive Impairment and Dementia: Systematic Review and Perspectives. Front. Neurol. 2015, 6, 187. [Google Scholar] [CrossRef]
- Wu, W.; Ji, Y.; Wang, Z.; Wu, X.; Li, J.; Gu, F.; Chen, Z.; Wang, Z. The FDA-approved anti-amyloid-β monoclonal antibodies for the treatment of Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Med. Res. 2023, 28, 544. [Google Scholar] [CrossRef]
- Ornish, D.; Madison, C.; Kivipelto, M.; Kemp, C.; McCulloch, C.E.; Galasko, D.; Artz, J.; Rentz, D.; Lin, J.; Norman, K.; et al. Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer’s disease: A randomized, controlled clinical trial. Alzheimer’s Res. Ther. 2024, 16, 122. [Google Scholar] [CrossRef]
- Matsumoto, K.; Honda, K.; Kobayashi, N. Protective effect of heat preconditioning of rat liver graft resulting in improved transplant survival. Transplantation 2001, 71, 862–868. [Google Scholar] [CrossRef]
- Le Bourg, É. Hormesis, aging and longevity. Biochim. Biophys. Acta 2009, 1790, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, É. Characterisation of the positive effects of mild stress on ageing and resistance to stress. Biogerontology 2020, 21, 485–493. [Google Scholar] [CrossRef]
- Guisle, I.; Canet, G.; Pétry, S.; Fereydouni-Forouzandeh, P.; Morin, F.; Kérauden, R.; Whittington, R.A.; Calon, F.; Hébert, S.S.; Planel, E. Sauna-like conditions or menthol treatment reduce tau phosphorylation through mild hyperthermia. Neurobiol. Aging 2022, 113, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Crane, C.R.; Sanders, D.C. Inhalation Toxicology: VIII. Establishing Heat Tolerance Limits for Rats and Mice Subjected to Acute Exposures at Elevated Air Temperatures; DOT/FAA/AM-86/8; Civil Aerospace Medical Institute: Oklahoma City, OK, USA, 1986. [Google Scholar]
- Kassis, S.; Grondin, M.; Averill-Bates, D.A. Heat shock increases levels of reactive oxygen species, autophagy and apoptosis. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118924. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperthermia 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, H.; Yang, H.; Li, F.; He, X.; Gu, Z.; Zhao, M.; Su, L. Reactive oxygen species mediate heat stress-induced apoptosis via ERK dephosphorylation and Bcl-2 ubiquitination in human umbilical vein endothelial cells. Oncotarget 2017, 8, 12902–12916. [Google Scholar] [CrossRef]
- Dalton, P.; Wysocki, C.J. The nature and duration of adaptation following long-term odor exposure. Percept. Psychophys. 1996, 58, 781–792. [Google Scholar] [CrossRef]
- Kato, A.; Touhara, K. Mammalian olfactory receptors: Pharmacology, G protein coupling and desensitization. Cell Mol. Life Sci. 2009, 66, 3743–3753. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Sinding, C.; de Wijk, R.A.; Hummel, T. Habituation and adaptation to odors in humans. Physiol. Behav. 2017, 177, 13–19. [Google Scholar] [CrossRef]
- Kim, K.; Bae, J.; Jin, Y.; Moon, C. Odor habituation can modulate very early olfactory event-related potential. Sci. Rep. 2020, 10, 18117. [Google Scholar] [CrossRef]
- Bandiera, B.; Natale, F.; Rinaudo, M.; Sollazzo, R.; Spinelli, M.; Fusco, S.; Grassi, C. Olfactory stimulation with multiple odorants prevents stress-induced cognitive and psychological alterations. Brain Commun. 2024, 6, fcae390. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Shimatani, K. Effect of olfactory stimulation from aromatherapy on the autonomic nervous activity during aerobic exercises. Sci. Rep. 2024, 14, 11198. [Google Scholar] [CrossRef] [PubMed]
- Scutigliani, E.M.; Liang, Y.; Crezee, H.; Kanaar, R.; Krawczyk, P.M. Modulating the Heat Stress Response to Improve Hyperthermia-Based Anticancer Treatments. Cancers 2021, 13, 1243. [Google Scholar] [CrossRef]
- Ahmed, K.; Zaidi, S.F.; Rehman, M.U.; Rehman, R.; Kondo, T. Hyperthermia and protein homeostasis: Cytoprotection and cell death. J. Therm. Biol. 2020, 91, 102615. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Tundo, G.R.; Sbardella, D.; Ciaccio, C.; Bianculli, A.; Orlandi, A.; Desimio, M.G.; Arcuri, G.; Coletta, M.; Marini, S. Insulin-degrading enzyme (IDE): A novel heat shock-like protein. J. Biol. Chem. 2013, 288, 2281–2289. [Google Scholar] [CrossRef]
- Vekrellis, K.; Ye, Z.; Qiu, W.Q.; Walsh, D.; Hartley, D.; Chesneau, V.; Rosner, M.R.; Selkoe, D.J. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J. Neurosci. 2000, 20, 1657–1665. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef]
- Tian, Y.; Jing, G.; Zhang, M. Insulin-degrading enzyme: Roles and pathways in ameliorating cognitive impairment associated with Alzheimer’s disease and diabetes. Ageing Res. Rev. 2023, 90, 101999. [Google Scholar] [CrossRef] [PubMed]
- Chovatiya, R.; Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell 2014, 54, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.P.; Lieberman, J. Live or let die: Posttranscriptional gene regulation in cell stress and cell death. Immunol. Rev. 2013, 253, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Mesihovic, A.; Iannacone, R.; Firon, N.; Fragkostefanakis, S. Heat stress regimes for the investigation of pollen thermotolerance in crop plants. Plant Reprod. 2016, 29, 93–105. [Google Scholar] [CrossRef]
- Borgstedt, L.; Bratke, S.; Blobner, M.; Pötzl, C.; Ulm, B.; Jungwirth, B.; Schmid, S. Isoflurane has no effect on cognitive or behavioral performance in a mouse model of early-stage Alzheimer’s disease. Front. Neurosci. 2022, 16, 1033729. [Google Scholar] [CrossRef]
- Hofmann, C.; Sander, A.; Wang, X.X.; Buerge, M.; Jungwirth, B.; Borgstedt, L.; Kreuzer, M.; Kopp, C.; Schorpp, K.; Hadian, K.; et al. Inhalational anesthetics do not deteriorate amyloid-β-derived pathophysiology in Alzheimer’s disease: Investigations on the molecular, neuronal, and behavioral level. J. Alzheimer’s Dis. 2021, 84, 1193–1218. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.-B.; Liu, H.-H.; Kuo, Y.-Y.; Chen, Y.-M.; Hsu, F.-T.; Wang, Y.-W.; Kung, Y.; Ching, C.; Chao, C.-Y. Thermal Cycling Stimulation via Nasal Inhalation Attenuates Aβ25–35-Induced Cognitive Deficits in C57BL/6 Mice. Int. J. Mol. Sci. 2025, 26, 10236. https://doi.org/10.3390/ijms262010236
Lin G-B, Liu H-H, Kuo Y-Y, Chen Y-M, Hsu F-T, Wang Y-W, Kung Y, Ching C, Chao C-Y. Thermal Cycling Stimulation via Nasal Inhalation Attenuates Aβ25–35-Induced Cognitive Deficits in C57BL/6 Mice. International Journal of Molecular Sciences. 2025; 26(20):10236. https://doi.org/10.3390/ijms262010236
Chicago/Turabian StyleLin, Guan-Bo, Hsu-Hsiang Liu, Yu-Yi Kuo, You-Ming Chen, Fang-Tzu Hsu, Yu-Wei Wang, Yi Kung, Chien Ching, and Chih-Yu Chao. 2025. "Thermal Cycling Stimulation via Nasal Inhalation Attenuates Aβ25–35-Induced Cognitive Deficits in C57BL/6 Mice" International Journal of Molecular Sciences 26, no. 20: 10236. https://doi.org/10.3390/ijms262010236
APA StyleLin, G.-B., Liu, H.-H., Kuo, Y.-Y., Chen, Y.-M., Hsu, F.-T., Wang, Y.-W., Kung, Y., Ching, C., & Chao, C.-Y. (2025). Thermal Cycling Stimulation via Nasal Inhalation Attenuates Aβ25–35-Induced Cognitive Deficits in C57BL/6 Mice. International Journal of Molecular Sciences, 26(20), 10236. https://doi.org/10.3390/ijms262010236





