Preclinical Evidence of Withania somnifera and Cordyceps spp.: Neuroprotective Properties for the Management of Alzheimer’s Disease
Abstract
1. Alzheimer’s Disease: Pathophysiology and Current Therapies
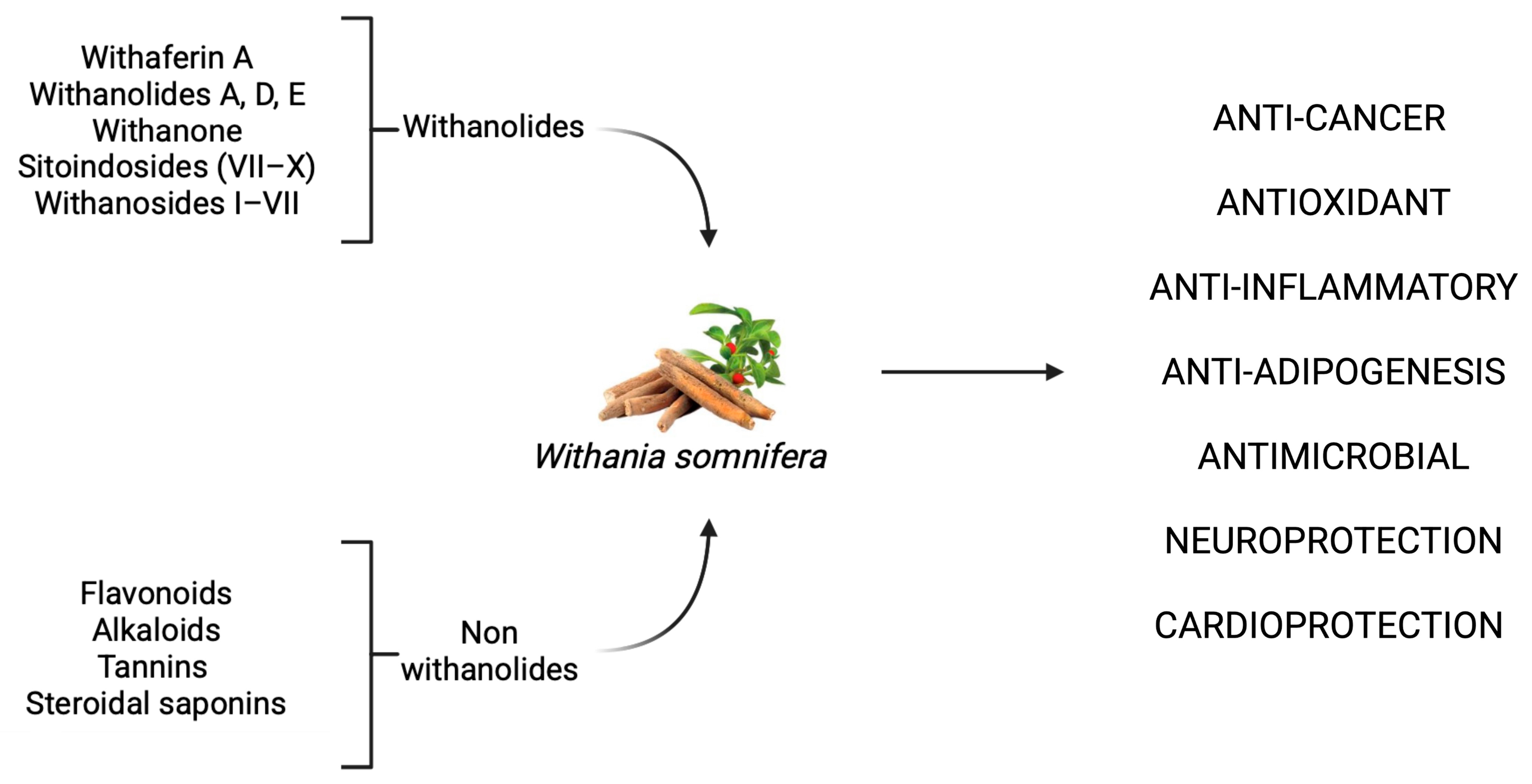
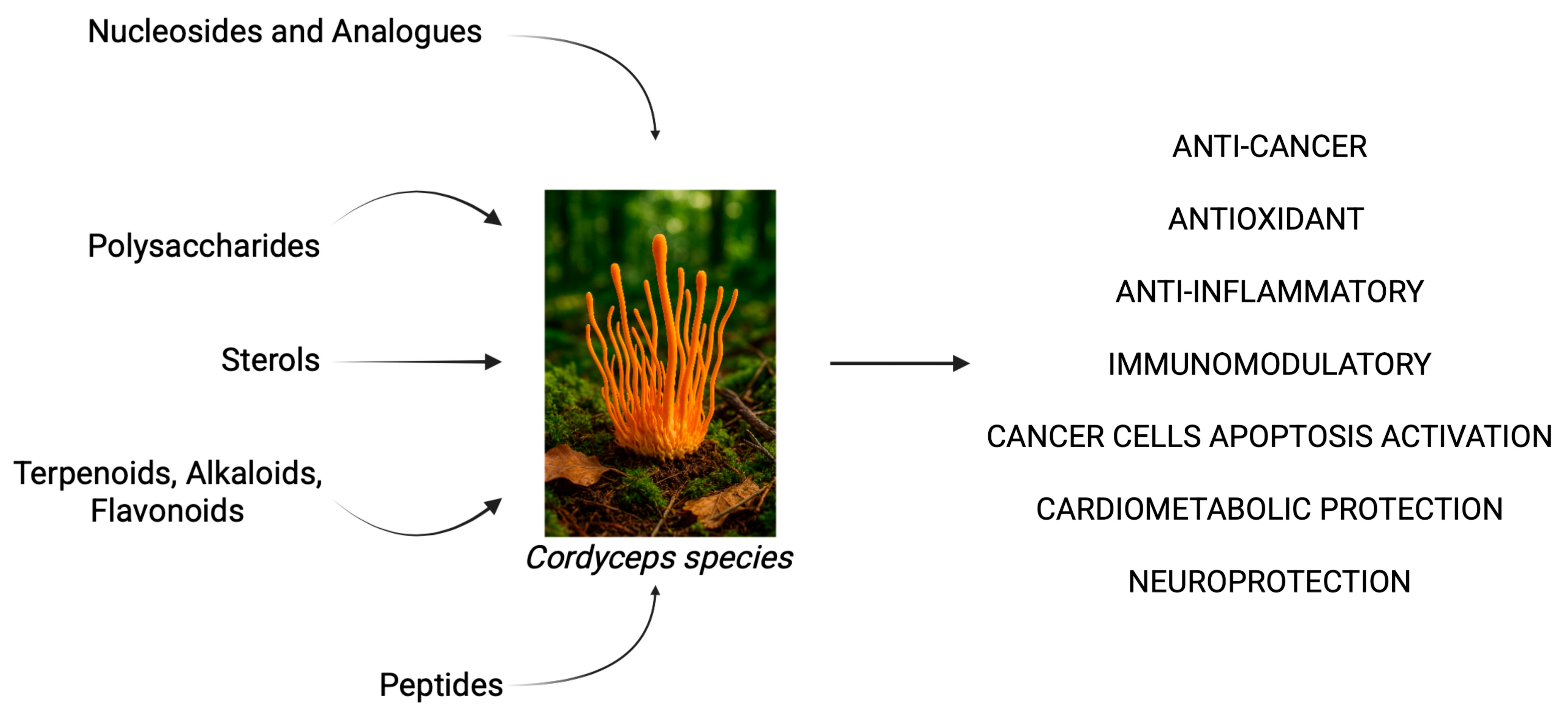
2. W. somnifera and C. spp.: Bioactive Components and Major Mechanisms of Action
2.1. W. somnifera: Pharmacological Profile and Therapeutic Targets
2.1.1. W. somnifera as a Promising Anti-Cancer Agent: Mechanistic Evidence Across Tumor Models
2.1.2. Anti-Inflammatory Mechanisms of W. somnifera: Modulation of NF-κB, Nrf2, and Cytokine Pathways
2.1.3. Neuroprotective Effects of W. somnifera: Molecular Mechanisms and Therapeutic Potential in Neurodegenerative Disorders
2.2. Cordyceps spp. as a Source of Bioactive Compounds in Inflammation, Cancer, and Neurodegeneration
3. Emerging Role of Phytotherapeutic Compounds from W. somnifera and C. spp. In Alzheimer’s Disease
3.1. Neuroprotective Properties of W. somnifera Extract and Bioactive Constituents in Preclinical Model of Alzheimer’s Disease
3.2. Neuroprotective Properties of C. spp. Extract and Bioactive Constituents in Preclinical Model of Alzheimer’s Disease
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association Report. 2024 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y. Tau and Neuroinflammation in Alzheimer’s Disease: Interplay Mechanisms and Clinical Translation. J. Neuroinflammation 2023, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of Pro-Inflammatory Cytokines Released from Microglia in Neurodegenerative Diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Day, C.M.; Abdella, S.; Garg, S. Alzheimer’s Disease Current Therapies, Novel Drug Delivery Systems and Future Directions for Better Disease Management. J. Control. Release 2024, 367, 402–424. [Google Scholar] [CrossRef]
- Piekarz, J.; Picheta, N.; Burdan, O.; Kurek, M.; Chrościńska-Krawczyk, M. Phytotherapy in Alzheimer’s Disease—A Narrative Review. Biomedicines 2024, 12, 1812. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A Comprehensive Review on Ethnopharmacology, Pharmacotherapeutics, Biomedicinal and Toxicological Aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Szeląg, M.; Szklanny, D.; Judasz, E.; Kaczmarek, G.; et al. Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics 2023, 15, 1057. [Google Scholar] [CrossRef]
- Lacombe, J.; Cretignier, T.; Meli, L.; Wijeratne, E.M.K.; Veuthey, J.L.; Cuendet, M.; Gunatilaka, A.A.L.; Zenhausern, F. Withanolide D Enhances Radiosensitivity of Human Cancer Cells by Inhibiting DNA Damage Non-Homologous End Joining Repair Pathway. Front. Oncol. 2020, 9, 1468. [Google Scholar] [CrossRef]
- Issa, M.E.; Wijeratne, E.M.K.; Gunatilaka, A.A.L.; Cuendet, M. Withanolide D Exhibits Similar Cytostatic Effect in Drug-Resistant and Drug-Sensitive Multiple Myeloma Cells. Front. Pharmacol. 2017, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Sultana, T.; Okla, M.K.; Ahmed, M.; Akhtar, N.; Al-Hashimi, A.; Abdelgawad, H. Ihsan-Ul-haq Withaferin A: From Ancient Remedy to Potential Drug Candidate. Molecules 2021, 26, 7696. [Google Scholar] [CrossRef]
- Bashir, A.; Nabi, M.; Tabassum, N.; Afzal, S.; Ayoub, M. An Updated Review on Phytochemistry and Molecular Targets of Withania somnifera (L.) Dunal (Ashwagandha). Front. Pharmacol. 2023, 14, 1049334. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mathew, S.O.; Aharwal, R.P.; Tulli, H.S.; Mohan, C.D.; Sethi, G.; Ahn, K.S.; Webber, K.; Sandhu, S.S.; Bishayee, A. Withaferin A: A Pleiotropic Anticancer Agent from the Indian Medicinal Plant Withania somnifera (L.) Dunal. Pharmaceuticals 2023, 16, 160. [Google Scholar] [CrossRef]
- Mallipeddi, H.; Thyagarajan, A.; Sahu, R.P. Implications of Withaferin-A for Triple-Negative Breast Cancer Chemoprevention. Biomed. Pharmacother. 2021, 134, 111124. [Google Scholar] [CrossRef]
- Khazal, K.F.; Hill, D.L. Withania somnifera Extract Reduces the Invasiveness of MDA-MB-231 Breast Cancer and Inhibits Cytokines Associated with Metastasis. J. Cancer Metastasis Treat. 2015, 1, 94–100. [Google Scholar] [CrossRef]
- Macharia, J.M.; Káposztás, Z.; Bence, R.L. Medicinal Characteristics of Withania somnifera L. in Colorectal Cancer Management. Pharmaceuticals 2023, 16, 915. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Marynarki Wojennej Gdańsku Klaudia Bogusz, S.; Baran, N.; Maksymowicz, M.; Bielak, A.; Zakonu Bonifratrów św Jana Grandego Krakowie, S.; Nowak, A.; Cywka, Ł.; Szwed, W.; Szpital Specjalistyczny Olsztynie Piotr Machowiec, W.; et al. Benefits of Withania somnifera (Ashwagandha) Supplementation in Obesity. J. Educ. Health Sport. 2023, 35, 11–22. [Google Scholar] [CrossRef]
- Munagala, R.; Kausar, H.; Munjal, C.; Gupta, R.C. Withaferin A Induces P53-Dependent Apoptosis by Repression of HPV Oncogenes and Upregulation of Tumor Suppressor Proteins in Human Cervical Cancer Cells. Carcinogenesis 2011, 32, 1697–1705. [Google Scholar] [CrossRef]
- Mayola, E.; Gallerne, C.; Esposti, D.D.; Martel, C.; Pervaiz, S.; Larue, L.; Debuire, B.; Lemoine, A.; Brenner, C.; Lemaire, C. Withaferin A Induces Apoptosis in Human Melanoma Cells through Generation of Reactive Oxygen Species and Down-Regulation of Bcl-2. Apoptosis 2011, 16, 1014–1027. [Google Scholar] [CrossRef]
- Heyninck, K.; Lahtela-Kakkonen, M.; Van Der Veken, P.; Haegeman, G.; Berghe, W. Vanden Withaferin A Inhibits NF-KappaB Activation by Targeting Cysteine 179 in IKKβ. Biochem. Pharmacol. 2014, 91, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Takada, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides Potentiate Apoptosis, Inhibit Invasion, and Abolish Osteoclastogenesis through Suppression of Nuclear Factor-KappaB (NF-KappaB) Activation and NF-KappaB-Regulated Gene Expression. Mol. Cancer Ther. 2006, 5, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, L.; Checker, R.; Sharma, D.; Thoh, M.; Patil, A.; Degani, M.; Gota, V.; Sandur, S.K. Thiol Dependent NF-ΚB Suppression and Inhibition of T-Cell Mediated Adaptive Immune Responses by a Naturally Occurring Steroidal Lactone Withaferin A. Toxicol. Appl. Pharmacol. 2015, 289, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Basudkar, V.; Gujrati, G.; Ajgaonkar, S.; Gandhi, M.; Mehta, D.; Nair, S. Emerging Vistas for the Nutraceutical Withania somnifera in Inflammaging. Pharmaceuticals 2024, 17, 597. [Google Scholar] [CrossRef]
- Palliyaguru, D.L.; Chartoumpekis, D.V.; Wakabayashi, N.; Skoko, J.J.; Yagishita, Y.; Singh, S.V.; Kensler, T.W. Withaferin A Induces Nrf2-Dependent Protection against Liver Injury: Role of Keap1-Independent Mechanisms. Free. Radic. Biol. Med. 2016, 101, 116–128. [Google Scholar] [CrossRef]
- Um, H.J.; Min, K.j.; Kim, D.E.; Kwon, T.K. Withaferin A Inhibits JAK/STAT3 Signaling and Induces Apoptosis of Human Renal Carcinoma Caki Cells. Biochem. Biophys. Res. Commun. 2012, 427, 24–29. [Google Scholar] [CrossRef]
- Guo, R.; Gan, L.; Lau, W.B.; Yan, Z.; Xie, D.; Gao, E.; Christopher, T.A.; Lopez, B.L.; Ma, X.; Wang, Y. Withaferin A Prevents Myocardial Ischemia/Reperfusion Injury by Upregulating AMP-Activated Protein Kinase-Dependent B-Cell Lymphoma2 Signaling. Circ. J. 2019, 83, 1726–1736. [Google Scholar] [CrossRef]
- Logie, E.; Berghe, W. Vanden Tackling Chronic Inflammation with Withanolide Phytochemicals-A Withaferin a Perspective. Antioxidants 2020, 9, 1107. [Google Scholar] [CrossRef]
- Lerose, V.; Ponticelli, M.; Benedetto, N.; Carlucci, V.; Lela, L.; Tzvetkov, N.T.; Milella, L. Withania somnifera (L.) Dunal, a Potential Source of Phytochemicals for Treating Neurodegenerative Diseases: A Systematic Review. Plants 2024, 13, 771. [Google Scholar] [CrossRef]
- Khalil, H.M.A.; Eliwa, H.A.; El-Shiekh, R.A.; Al-Mokaddem, A.K.; Hassan, M.; Tawfek, A.M.; El-Maadawy, W.H. Ashwagandha (Withania somnifera) Root Extract Attenuates Hepatic and Cognitive Deficits in Thioacetamide-Induced Rat Model of Hepatic Encephalopathy via Induction of Nrf2/HO-1 and Mitigation of NF-ΚB/MAPK Signaling Pathways. J. Ethnopharmacol. 2021, 277, 114141. [Google Scholar] [CrossRef]
- NeuroReport. Available online: https://journals.lww.com/neuroreport/abstract/2002/10070/axon__or_dendrite_predominant_outgrowth_induced_by.5.aspx (accessed on 8 May 2025).
- Baitharu, I.; Jain, V.; Deep, S.N.; Shroff, S.; Sahu, J.K.; Naik, P.K.; Ilavazhagan, G. Withanolide A Prevents Neurodegeneration by Modulating Hippocampal Glutathione Biosynthesis during Hypoxia. PLoS ONE 2014, 9, e105311. [Google Scholar] [CrossRef]
- Sinadinos, C.; Quraishe, S.; Sealey, M.; Samson, P.B.; Mudher, A.; Wyttenbach, A. Low Endogenous and Chemical Induced Heat Shock Protein Induction in a 0N3Rtau-Expressing Drosophila Larval Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 33, 1117–1133. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.S.R.; Yndart Arias, A.; Jayant, R.D.; Kaushik, A.; Geiger, J.; Nair, M.N. Withaferin A Suppresses Beta Amyloid in APP Expressing Cells: Studies for Tat and Cocaine Associated Neurological Dysfunctions. Front. Aging Neurosci. 2018, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Wadhwa, R.; Thakur, M.K. Assessment of Cholinergic Properties of Ashwagandha Leaf-Extract in the Amnesic Mouse Brain. Ann. Neurosci. 2016, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Neuritic Regeneration and Synaptic Reconstruction Induced by Withanolide A. Br. J. Pharmacol. 2005, 144, 961. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The Genus Cordyceps: An Extensive Review of Its Traditional Uses, Phytochemistry and Pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, N.; An, S.S.A. Unique Bioactives from Zombie Fungus (Cordyceps) as Promising Multitargeted Neuroprotective Agents. Nutrients 2023, 16, 102. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Guo, Z.; Li, Q.; Zhang, L.; Zhao, L.; Zhou, X. Immunomodulatory Effect of Cordyceps Militaris Polysaccharide on RAW 264.7 Macrophages by Regulating MAPK Signaling Pathways. Molecules 2024, 29, 3408. [Google Scholar] [CrossRef]
- Lee, S.Y.; Debnath, T.; Kim, S.K.; Lim, B.O. Anti-Cancer Effect and Apoptosis Induction of Cordycepin through DR3 Pathway in the Human Colonic Cancer Cell HT-29. Food Chem. Toxicol. 2013, 60, 439–447. [Google Scholar] [CrossRef]
- Thepmalee, C.; Jenkham, P.; Ramwarungkura, B.; Suwannasom, N.; Khoothiam, K.; Thephinlap, C.; Sawasdee, N.; Panya, A.; Yenchitsomanus, P.T. Enhancing Cancer Immunotherapy Using Cordycepin and Cordyceps Militaris Extract to Sensitize Cancer Cells and Modulate Immune Responses. Sci. Rep. 2024, 14, 21907. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, H.; Ma, H.; Wang, Y. Effects of Cordyceps Cicadae Polysaccharide on Gut Microbiota, the Intestinal Mucosal Barrier, and Inflammation in Diabetic Mice. Metabolites 2025, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Zhao, K.J.; Ji, Z.N.; Song, Z.H.; Dong, T.T.X.; Lo, C.K.; Cheung, J.K.H.; Zhu, S.Q.; Tsim, K.W.K. A Polysaccharide Isolated from Cordyceps Sinensis, a Traditional Chinese Medicine, Protects PC12 Cells against Hydrogen Peroxide-Induced Injury. Life Sci. 2003, 73, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Song, D.; Wu, J.; Zhang, W. Protective Effect of a Polysaccharide Isolated from a Cultivated Cordyceps Mycelia on Hydrogen Peroxide-Induced Oxidative Damage in PC12 Cells. Phytother. Res. 2011, 25, 675–680. [Google Scholar] [CrossRef]
- Marchbank, T.; Ojobo, E.; Playford, C.J.; Playford, R.J. Reparative Properties of the Traditional Chinese Medicine Cordyceps Sinensis (Chinese Caterpillar Mushroom) Using HT29 Cell Culture and Rat Gastric Damage Models of Injury. Br. J. Nutr. 2011, 105, 1303–1310. [Google Scholar] [CrossRef]
- Gladen-Kolarsky, N.; Monestime, O.; Bollen, M.; Choi, J.; Yang, L.; Magaña, A.A.; Maier, C.S.; Soumyanath, A.; Gray, N.E. Withania somnifera (Ashwagandha) Improves Spatial Memory, Anxiety and Depressive-like Behavior in the 5xFAD Mouse Model of Alzheimer’s Disease. Antioxidants 2024, 13, 1164. [Google Scholar] [CrossRef]
- Bhaskar, M.; Chintamaneni, M. Withania somnifera and Eclipta Alba Ameliorate Oxidative Stress Induced Mitochondrial Dysfunction in an Animal Model of Alzheimer’s Disease. Am. J. Phytomedicine Clin. Ther. 2014, 2, 140–152, ISSN 2321-2748. [Google Scholar]
- Afewerky, H.K.; Li, H.; Zhang, T.; Li, X.; Mahaman, Y.A.R.; Duan, L.; Qin, P.; Zheng, J.; Pei, L.; Lu, Y. Sodium–Calcium Exchanger Isoform-3 Targeted Withania somnifera (L.) Dunal Therapeutic Intervention Ameliorates Cognition in the 5xFAD Mouse Model of Alzheimer’s Disease. Sci. Rep. 2022, 12, 1537. [Google Scholar] [CrossRef]
- Sehgal, N.; Gupta, A.; Valli, R.K.; Joshi, S.D.; Mills, J.T.; Hamel, E.; Khanna, P.; Jain, S.C.; Thakur, S.S.; Ravindranath, V. Withania somnifera Reverses Alzheimer’s Disease Pathology by Enhancing Low-Density Lipoprotein Receptor-Related Protein in Liver. Proc. Natl. Acad. Sci. USA 2012, 109, 3510–3515. [Google Scholar] [CrossRef]
- Visweswari, G.; Visweswari, G.; Christopher, R.; Rajendra, W. Withania somnifera against Glutamate Excitotoxicity and Neuronal Cell Loss in a Scopolamine-Induced Rat Model of Alzheimer’s Disease. Eur. J. Biol. Res. 2021, 11, 156–167. [Google Scholar] [CrossRef]
- Abdullah, F.; Nour, N.; Mansouri, R. The Neuroprotective Effect of Lycopodium and Withania somnifera on Alzheimer’s Disease Induced by Aluminum Chloride in Rats. Eur. Online J. Nat. Soc. Sci. 2022, 11, 852–861, ISSN 1805-3602. [Google Scholar]
- Visweswari, G.; Christopher, R.; Rajendra, W. Dose-dependent effect of Withania somnifera on the cholinergic system in scopolamine-induced alzheimer’s disease in rats. Int. J. Pharm. Sci. Res. 2014, 5, 4240–4248. [Google Scholar] [CrossRef]
- Pandey, A.; Bani, S.; Dutt, P.; Kumar Satti, N.; Avtar Suri, K.; Nabi Qazi, G. Multifunctional Neuroprotective Effect of Withanone, a Compound from Withania somnifera Roots in Alleviating Cognitive Dysfunction. Cytokine 2018, 102, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Withanoside IV and Its Active Metabolite, Sominone, Attenuate Aβ(25-35)-Induced Neurodegeneration. Eur. J. Neurosci. 2006, 23, 1417–1426. [Google Scholar] [CrossRef]
- Khan, M.A.; Srivastava, V.; Kabir, M.; Samal, M.; Insaf, A.; Ibrahim, M.; Zahiruddin, S.; Ahmad, S. Development of Synergy-Based Combination for Learning and Memory Using in Vitro, in Vivo and TLC-MS-Bioautographic Studies. Front. Pharmacol. 2021, 12, 678611. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R.; Wane, D. An Investigation into the Stress-Relieving and Pharmacological Actions of an Ashwagandha (Withania somnifera) Extract: A Randomized, Double-Blind, Placebo-Controlled Study. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Srivastav, A.K.; Sur, T.K.; Chaudhuri, S.; Wang, Y.; Biswas, T.K. Effects of Withania somnifera Extract in Chronically Stressed Adults: A Randomized Controlled Trial. Nutrients 2024, 16, 1293. [Google Scholar] [CrossRef]
- He, M.T.; Lee, A.Y.; Kim, J.H.; Park, C.H.; Shin, Y.S.; Cho, E.J. Protective Role of Cordyceps Militaris in Aβ1–42-Induced Alzheimer’s Disease in Vivo. Food Sci. Biotechnol. 2018, 28, 865. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Lee, C.L. Cordyceps Cicadae NTTU 868 Mycelium with The Addition of Bioavailable Forms of Magnesium from Deep Ocean Water Prevents the Aβ40 and Streptozotocin-Induced Memory Deficit via Suppressing Alzheimer’s Disease Risk Factors and Increasing Magnesium Uptake of Brain. Fermentation 2021, 7, 39. [Google Scholar] [CrossRef]
- Jiao, L.; Yu, Z.; Zhong, X.; Yao, W.; Xing, L.; Ma, G.; Shen, J.; Wu, Y.; Du, K.; Liu, J.; et al. Cordycepin Improved Neuronal Synaptic Plasticity through CREB-Induced NGF Upregulation Driven by MG-M2 Polarization: A Microglia-Neuron Symphony in AD. Biomed. Pharmacother. 2023, 157, 114054. [Google Scholar] [CrossRef]
- Jin, D.Q.; Park, B.C.; Lee, J.S.; Choi, H.D.; Lee, Y.S.; Yang, J.H.; Kim, J.A. Mycelial Extract of Cordyceps Ophioglossoides Prevents Neuronal Cell Death and Ameliorates β-Amyloid Peptide-Induced Memory Deficits in Rats. Biol. Pharm. Bull. 2004, 27, 1126–1129. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Huang, W.-M.; Tang, P.-C.; Sun, Y.; Zhang, X.; Qiu, L.; Yu, B.-C.; Hong, Y.-X.; He, Y.; Ge, X.-Q. Anti-Inflammatory and Neuroprotective Effects of Natural Cordycepin in Rotenone-Induced PD Models through Inhibiting Drp1-Mediated Mitochondrial Fission. Neurotoxicology 2021, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Wang, K.; Luo, C.; Huang, Y.; Misilimu, D.; Wen, H.; Jin, P.; Li, C.; Gong, Y.; Gao, Y. Cordycepin Confers Long-Term Neuroprotection via Inhibiting Neutrophil Infiltration and Neuroinflammation after Traumatic Brain Injury. J. Neuroinflammation 2021, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Jung, E.S.; Choi, E.K.; Sin, H.S.; Ha, K.C.; Chae, S.W. Immunomodulatory Effects of a Mycelium Extract of Cordyceps (Paecilomyces Hepiali; CBG-CS-2): A Randomized and Double-Blind Clinical Trial. BMC Complement. Altern. Med. 2019, 19, 77. [Google Scholar] [CrossRef] [PubMed]
| Num | Year | Ref. Country | Paper Title | Animal Model (Species; Gender) | Intervention | Control | Results |
|---|---|---|---|---|---|---|---|
| 1 | 2024 | [46]; USA and Canada | W. somnifera (ashwagandha) improves spatial memory, anxiety, and depressive-like behavior in the 5× FAD mouse model of Alzheimer’s disease. | Mice (5× FAD; male and female) | Administration of aqueous extract of dried W. somnifera at 0, 0.5, or 2.5 mg/mL via drinking water ad libitum for 4 weeks. Behavioral testing was conducted in the final week of treatment. | Wild-type mice administered drinking water |
|
| 2 | 2014 | [47]; India | W. somnifera and Eclipta alba ameliorate oxidative stress-induced mitochondrial dysfunction in an animal model of Alzheimer’s disease. | Albino rats (Wistar; male) | 60 rats were divided into ten groups: a control group, a toxicant group (carboxymethyl cellulose), two standard drug-treated groups (donepezil and Piracetam), three groups treated with a methanolic extract of W. somnifera (at doses of 50, 100, and 200 mg/kg), and three groups treated with methanolic extract of Eclipta alba (at doses of 50, 100, and 200 mg/kg). The intervention lasted for 8 days. On the 8th day, animals were subjected to scopolamine treatment and induction of oxidative stress. | Albino rats administered carboxymethyl cellulose |
|
| 3 | 2022 | [48]; China | Sodium–calcium exchanger isoform-3-targeted W. somnifera (L.) Dunal therapeutic intervention ameliorates cognition in the 5× FAD mouse model of Alzheimer’s disease. | Mice (5× FAD; male) | Animals were divided into four groups: control group (1 mL of saline 0.9%/day), low-dose treatment group (200 mg/kg/day of methanol extract of W. somnifera), high-dose treatment group (400 mg/kg/day of methanol extract of W. somnifera), and a positive control group (200 mg/kg day of resveratrol). | 5× FAD mice treated with 1 mL of saline 0.9% |
|
| 4 | 2012 | [49]; India | W. somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. | Mice (APP/PS1, APPSwInd (J20 line), WT; male and female) | Transgenic mice were divided into two groups: one receiving W. somnifera’s extract (1 g/kg) in ethanol and the control group receiving an equivalent volume of ethanol daily via oral administration for 7–30 days. | Transgenic mice administered drinking water |
|
| 5 | 2021 | [50]; India | W. somnifera against glutamate excitotoxicity and neuronal cell loss in a scopolamine-induced rat model of Alzheimer’s disease. | Rats (Wistar albino; male) | Rats were divided into five groups: control; scopolamine-induced Alzheimer’s model (2 mg/kg intraperitoneally) + normal saline administered orally; scopolamine-induced Alzheimer’s model + donepezil hydrochloride (5 mg/kg) administered orally. The intervention lasted for 30 days. Rats were then decapitated and their brains removed for analysis. | Scopolamine-induced Alzheimer’s model of Wistar albino male rats |
|
| 6 | 2022 | [51]; Saudi Arabi | The neuroprotective effect of Lycopodium and W. somnifera on Alzheimer’s disease induced by aluminum chloride in rats. | Rats (Wistar albino; female) | Rats were divided into five groups: the control administered with distilled water; the AD group (aluminum chloride 175 mg/kg) treated with a water extract of Lycopodium (50 mg/kg); the AD group (aluminum chloride 175 mg/kg) treated with a water extract of W. somnifera (200 mg/kg); the AD group (aluminum chloride 175 mg/kg) treated with W. somnifera (200 mg/kg) and Lycopodium extract (50 mg/kg). | Aluminum chloride-induced Alzheimer’s model of Wistar albino female rats |
|
| 7 | 2022 | [48]; China | Aβ1–42-related sodium–calcium exchanger isoform-3 downregulation in models of Alzheimer’s disease: therapeutic significance of W. somnifera root extract. | Mice (5× FAD; male and female) | 5× FAD mice were administered 300 mg/kg/day of W. somnifera’s methanolic extract via oral gavage for 30 days. | 5× FAD mice administered with vehicle |
|
| 8 | 2014 | [52]; India | Dose-dependent effect of W. somnifera on the cholinergic system in scopolamine-induced Alzheimer’s disease in rats. | Rats (Wistar; male) | Rats were divided into groups: a normal control; a scopolamine-induced Alzheimer’s control (2 mg/kg per b.w. intraperitoneally); and experimental groups treated with methanol or aqueous extracts (100, 200, 300 mg/kg, orally) or donepezil (5 mg/kg, orally). Treatments lasted 10 days to evaluate their potential neuroprotective effects against scopolamine-induced cognitive impairment. | Scopolamine-induced Alzheimer’s male Wistar rats |
|
| 9 | 2018 | [53]; India | Multifunctional neuroprotective effect of withanone, a compound from W. somnifera roots, in alleviating cognitive dysfunction. | Rats (Wistar; male) | Rats were divided into six groups: sham control; CSF control group (bilateral ICV injection 10 μL on each side); STZ control group (ICV injection of STZ 10 μL on each side); three drug-treated groups (5 or 10 or 20 mg/kg of withanone, orally). The drug treatment lasted for 21 days after the surgery. | Rats in the STZ control group (ICV injection of STZ 10 μL on each side) |
|
| 10 | 2006 | [54]; Japan | Withanoside IV and its active metabolite, sominone, attenuate Aβ(25–35)-induced neurodegeneration. | Mice (ddY; male) | Mice were divided into two groups: the treatment group (25 nmol of Aβ(25–35) injected into the right ventricle); and the control group (vehicle injected into the right ventricle). Seven days after intra-cerebrovascular injection, the treatment group received withanoside IV (10 µmol/kg/day) administered once daily for 13 days. The vehicle group received 0.5% Arabic gum solution administered orally on the same schedule. | Mice in the vehicle group were administered with 0.5% Arabic gum solution after intra-cerebrovascular injection of Aβ(25–35) |
|
| 11 | 2021 | [55]; India | Development of synergy-based combination for learning and memory using in vitro, in vivo, and TLC-MS-Bioautographic studies. | Rats (Wistar; male) | Rats were divided into nine groups: control group (10 mL 0.5% CMC/rat); negative control (scopolamine 2 mg/kg); treatment groups 3 to 6 received either the selected high or low dose of an aqueous extract of W. somnifera or Myristica fragans together with scopolamine; treatment group 7 was administered with the high dose of the W. somnifera extract in combination with the low dose of Myristica fragans together with scopolamine; treatment group 8 was administered with the high dose of Myristica fragans in combination with the low dose of W. somnifera together with scopolamine; the positive control group (Pyracetam 200 mg/kg) received scopolamine. The treatment lasted for 37 days, and behavioral tests were carried out between days 30 and 36. | Wistar male rats were administered with scopolamine |
|
| Num | Year | Ref. Country | Paper Title | Animal Model (Specie; Gender) | Experimental Design and Intervention | Control | Results |
|---|---|---|---|---|---|---|---|
| 1 | 2018 | [58]; Korea | Protective role of C. s militaris in in vivo Aβ1–42-induced Alzheimer’s disease. | Mice (IR; male) | Mice were divided into the following groups: normal group (water; Aβ1–42 intra-cerebrovascularly injected control group (3 μL/3 min); Aβ1–42 injection + ethanol extract of C. militaris (100 mg/kg/day) group; Aβ1–42 injections + C. mimlitaris ethanol extract (200 mg/kg/day) group; Aβ1–42 injections + donepezil (5 mg/kg/day) group. Aβ1–42 injections took place on day 3 of the experimental schedule, while the oral administration of C. spp. extract or donepezil started on day 8 and continued till day 23. Behavioral and cognitive functions were evaluated between days 15 and 23. Sacrifices took place on day 24 of the experiment. | Male IRC mice with Aβ1–42 intra-cerebrovascularly injected (3 μL/3 min) | C. militaris extract at 100 and 200 mg/kg/day led to significant improvements in cognitive and behavioral functions in treated mice. Space perceptive ability, objective cognitive ability, and cognitive abilities related to the Morris water maze test all improved in relation to the treatment. |
| 2 | 2021 | [59]; Taiwan | C. cicadae NTTU 868 mycelium with the addition of bioavailable forms of magnesium from deep ocean water prevents Aβ40 and Streptozotocin-induced memory deficit by suppressing Alzheimer’s disease risk factors and increasing magnesium uptake in the brain. | Rats (Sprague Dawley; male) | Rats were divided into the following groups: normal control (standard chow); vehicle group (intra-cerebrovascular injection of the vehicle); negative control injected with Aβ-STZ solution (24.299 μg Aβ40 + 0.9 mg STZ in 180 μL) in the left lateral ventricle; treatment group administered with Aβ-STZ solution + deep ocean water (0.0976 mL/kg/day); treatment groups administered with Aβ-STZ solution + C. cicadae cultured in ultrapure water (200 mg/kg/day) or deep ocean water (220 mg/kg/day) or in MgCl₂ (137 mg/kg day); treatment groups administered with N6-(2-hydroxyethyl)-adenosine (1.12 mg/kg/day) or polysaccharides (1.5 mg/kg/day). The treatment lasted for 28 days after the intra-cerebrovascular injection, and experiments were conducted during the last week before sacrifice. | The negative control group was injected intra-cerebrovascularly with Aβ-STZ solution (24.299 μg Aβ40 + 0.9 mg STZ in 180 μL) in the left lateral ventricle |
|
| 3 | 2023 | [60]; China | Cordycepin improved neuronal synaptic plasticity through CREB-induced NGF upregulation driven by MG-M2 polarization, a microglia-neuron symphony in AD. | Mice (double-transgenic APP/PS1, C56BL/6; male and female) | Double-transgenic APP/PS1 mice were divided into two groups: the negative control group was administered intragastrically with distilled water; the treated group was administered with q water extract of cordycepin from C. militaris (10 mg/kg/day). C56BL/6 mice served as the positive control group, administered intragastrically with distilled water. The treatment lasted 4 weeks. | Double-transgenic APP/PS1 mice |
|
| 4 | 2004 | [61]; Korea | Mycelial extract of C. spp. ophioglossoides prevents neuronal cell death and ameliorates b-amyloid peptide-induced memory deficits in rats | Rats (Sprague Dawley male) | Rats were divided into three different groups: the negative control group was injected intracranially with Aβ (1 mg/mL) to induce AD-like pathology; the treatment group was administered with methanolic extract of C. spp. ophioglossoides mycelium (100 mg/kg/day) intraperitoneally before Aβ (1 mg/mL) injection; rats in the positive control group received distilled water intracranially. The experiment lasted 3 weeks. | Rats injected intracranially with Aβ (1 mg/mL) | Treatment with the extract of C. spp. ophioglossoides mycelium was effective in preventing pathological alterations in spatial memory and learning capacity induced by Aβ injection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tancreda, G.; Ravera, S.; Panfoli, I. Preclinical Evidence of Withania somnifera and Cordyceps spp.: Neuroprotective Properties for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 5403. https://doi.org/10.3390/ijms26115403
Tancreda G, Ravera S, Panfoli I. Preclinical Evidence of Withania somnifera and Cordyceps spp.: Neuroprotective Properties for the Management of Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(11):5403. https://doi.org/10.3390/ijms26115403
Chicago/Turabian StyleTancreda, Gabriele, Silvia Ravera, and Isabella Panfoli. 2025. "Preclinical Evidence of Withania somnifera and Cordyceps spp.: Neuroprotective Properties for the Management of Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 11: 5403. https://doi.org/10.3390/ijms26115403
APA StyleTancreda, G., Ravera, S., & Panfoli, I. (2025). Preclinical Evidence of Withania somnifera and Cordyceps spp.: Neuroprotective Properties for the Management of Alzheimer’s Disease. International Journal of Molecular Sciences, 26(11), 5403. https://doi.org/10.3390/ijms26115403








