Molecular Biomarkers and Therapeutic Approach of Patients with Diabetes and Obstructive Sleep Apnea
Abstract
1. Introduction
2. Molecular Markers of OSA and T2DM
2.1. Hypoxia-Inducible Factor-1α (HIF-1α)
2.2. Sirtuin 1 (SIRT1)
2.3. MicroRNAs
2.3.1. MicroRNA-181a
2.3.2. MicroRNA-199a
2.4. Inflammatory Markers
2.4.1. Tumor Necrosis Factor-α
2.4.2. Interleukin-6
2.4.3. C-Reactive Protein
2.5. Adipokine Markers
2.5.1. Adiponectin
2.5.2. Leptin
2.5.3. Resistin
2.5.4. Chemerin
2.5.5. Omentin-1
2.6. Other Biomarkers
2.6.1. Melatonin
2.6.2. Orexin
2.6.3. Ghrelin
3. Management of OSA in T2DM Patients
3.1. Gold Standard Treatment
3.2. Current and Emerging Therapeutic Interventions
4. Anti-Diabetic Drugs Used in OSA: Molecular Mechanisms and Clinical Evidence
4.1. GLP-1 Receptor Agonists
4.1.1. Molecular Mechanisms of Action of GLP-1RAs
- ○
- Core Molecular Signaling Pathways
- ○
- Weight Loss-Mediated Mechanisms
- ○
- Central Nervous System (CNS) and Respiratory Control
- ○
- Upper Airway Muscle Tone Enhancement
- ○
- Anti-inflammatory and Cytoprotective Effects
- ○
- Pulmonary-Specific: Mechanisms
4.1.2. Clinical Evidence
4.2. SGLT2 Inhibitors (SGLT2i)
4.2.1. Molecular Mechanisms of Action of SGLT2i
- ○
- Core Molecular Signaling Pathways
- ○
- Weight loss mechanisms
- ○
- Cardiovascular risk reduction mechanisms
4.2.2. Clinical Evidence
4.3. Metformin
4.3.1. Molecular Mechanisms of Action of Metformin
4.3.2. Clinical Evidence
5. Future Therapeutic Research Directions
6. Materials and Methods
- ○
- Molecular mechanisms: Grouped by biomarker categories (transcription factors, inflammatory markers, adipokines, hormonal factors)
- ○
- Therapeutic interventions: Organized by drug class with emphasis on mechanism of action and clinical evidence
- ○
- Clinical implications: Integration of molecular insights with therapeutic potential
7. Essential Considerations
Limitations
- ○
- Possible selection bias in the literature identification despite a comprehensive search strategy;
- ○
- Lack of formal statistical analysis due to study heterogeneity;
- ○
- Potential publication bias favoring positive results;
- ○
- Heterogeneity in OSA diagnostic criteria and severity classification across studies;
- ○
- Limited long-term follow-up data for many interventions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gomase, V.G.; Deshmukh, P.; Lekurwale, V.Y. Obstructive Sleep Apnea and Its Management: A Narrative Review. Cureus 2023, 15, e37359. [Google Scholar] [CrossRef]
- Reutrakul, S.; Mokhlesi, B. Obstructive Sleep Apnea and Diabetes. Chest 2017, 152, 1070–1086. [Google Scholar] [CrossRef]
- Akset, M.; Poppe, K.G.; Kleynen, P.; Bold, I.; Bruyneel, M. Endocrine Disorders in Obstructive Sleep Apnoea Syndrome: A Bidirectional Relationship. Clin. Endocrinol. 2023, 98, 3–13. [Google Scholar] [CrossRef]
- Lévy, P.; Naughton, M.T.; Tamisier, R.; Cowie, M.R.; Bradley, T.D. Sleep Apnoea and Heart Failure. Eur. Respir. J. 2022, 59, 2101640. [Google Scholar] [CrossRef] [PubMed]
- Kalaydzhiev, P.; Poroyliev, N.; Somleva, D.; Ilieva, R.; Markov, D.; Kinova, E.; Goudev, A. Sleep Apnea in Patients with Exacerbated Heart Failure and Overweight. Sleep Med. X 2023, 5, 100065. [Google Scholar] [CrossRef] [PubMed]
- Song, S.O.; He, K.; Narla, R.R.; Kang, H.G.; Ryu, H.U.; Boyko, E.J. Metabolic Consequences of Obstructive Sleep Apnea Especially Pertaining to Diabetes Mellitus and Insulin Sensitivity. Diabetes Metab. J. 2019, 43, 144–155. [Google Scholar] [CrossRef]
- Shih, N.C.; Wei, J.C.C. Comparative Analysis of Diabetes Risk in Patients with Obstructive Sleep Apnea Undergoing Different Treatment Approaches. J. Otolaryngol. Head Neck Surg. 2023, 52, 51. [Google Scholar] [CrossRef]
- Afzal, H.; Butt, N.I.; Ashfaq, F.; Habib, O.; Anser, A.; Aftab, S. Obstructive Sleep Apnea in Type 2 Diabetes Mellitus. Rawal Med. J. 2023, 48, 20. [Google Scholar] [CrossRef]
- Andayeshgar, B.; Janatolmakan, M.; Soroush, A.; Azizi, S.M.; Khatony, A. The Prevalence of Obstructive Sleep Apnea in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Sleep Sci. Pract. 2022, 6, 6. [Google Scholar] [CrossRef]
- Van Dijk, M.; Donga, E.; Van Dijk, J.G.; Lammers, G.J.; Van Kralingen, K.W.; Dekkers, O.M.; Corssmit, E.P.M.; Romijn, J.A. Disturbed Subjective Sleep Characteristics in Adult Patients with Long-Standing Type 1 Diabetes Mellitus. Diabetologia 2011, 54, 1967–1976. [Google Scholar] [CrossRef]
- de Mattos, A.C.M.T.; Campos, Y.S.; Fiorini, V.O.; Sab, Y.; Tavares, B.L.; Velarde, L.G.C.; Lima, G.A.B.; da Cruz Filho, R.A. Relationship between Sleep Disturbances, Lipid Profile and Insulin Sensitivity in Type 1 Diabetic Patients: A Cross-Sectional Study. Arch. Endocrinol. Metab. 2020, 64, 412–417. [Google Scholar] [CrossRef]
- Tan, H.L.; Babwah, F.; Waheed, N.; Butt, M.I. Obstructive Sleep Apnoea and Type 1 Diabetes Mellitus. Br. J. Diabetes Vasc. Dis. 2015, 15, 96. [Google Scholar] [CrossRef]
- Janovsky, C.C.P.S.; Rolim, L.C.d.S.P.; de Sã¡, J.R.; Poyares, D.; Tufik, S.; Silva, A.B.; Dib, S.A. Cardiovascular Autonomic Neuropathy Contributes to Sleep Apnea in Young and Lean Type 1 Diabetes Mellitus Patients. Front. Endocrinol. 2014, 5, 119. [Google Scholar] [CrossRef]
- Reutrakul, S.; Thakkinstian, A.; Anothaisintawee, T.; Chontong, S.; Borel, A.L.; Perfect, M.M.; Janovsky, C.C.P.S.; Kessler, R.; Schultes, B.; Harsch, I.A.; et al. Sleep Characteristics in Type 1 Diabetes and Associations with Glycemic Control: Systematic Review and Meta-Analysis. Sleep Med. 2016, 23, 26–45. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, P.; Wang, L.; Wang, D.; Sun, K.; Ma, Y.; Wang, H.; Xu, C.; Zhang, R.; Zhang, X.; et al. Prevalence of Obstructive Sleep Apnea Syndrome in Hospitalized Patients with Type 2 Diabetes in Beijing, China. J. Diabetes Investig. 2022, 13, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.; Luyster, F.S.; Sereika, S.M.; DiNardo, M.M.; Callan, J.A.; Chasens, E.R. Comorbid Obstructive Sleep Apnea and Insomnia and Its Associations with Mood and Diabetes-Related Distress in Type 2 Diabetes Mellitus. J. Clin. Sleep Med. 2022, 18, 1103–1111. [Google Scholar] [CrossRef]
- Nagayoshi, M.; Punjabi, N.M.; Selvin, E.; Pankow, J.S.; Shahar, E.; Iso, H.; Folsom, A.R.; Lutsey, P.L. Obstructive Sleep Apnea and Incident Type 2 Diabetes. Sleep Med. 2016, 25, 156–161. [Google Scholar] [CrossRef]
- Gabryelska, A.; Chrzanowski, J.; Sochal, M.; Kaczmarski, P.; Turkiewicz, S.; Ditmer, M.; Karuga, F.F.; Czupryniak, L.; Białasiewicz, P. Nocturnal Oxygen Saturation Parameters as Independent Risk Factors for Type 2 Diabetes Mellitus among Obstructive Sleep Apnea Patients. J. Clin. Med. 2021, 10, 3770. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, A.; Jamil, D.I.; Karimi, E.B.; Baghi, V.; Gheshlagh, R.G. Prevalence of Obstructive Sleep Apnea in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2463–2468. [Google Scholar] [CrossRef] [PubMed]
- Worku, A.; Ayele, E.; Alemu, S.; Legese, G.L.; Yimam, S.M.; Kassaw, G.; Diress, M.; Asres, M.S. Obstructive Sleep Apnea Risk and Determinant Factors among Type 2 Diabetes Mellitus Patients at the Chronic Illness Clinic of the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Front. Endocrinol. 2023, 14, 1151124. [Google Scholar] [CrossRef]
- Siddiquee, A.T.; Kim, S.; Thomas, R.J.; Lee, M.H.; Lee, S.K.; Shin, C. Obstructive Sleep Apnoea and Long-Term Risk of Incident Diabetes in the Middle-Aged and Older General Population. ERJ Open Res. 2023, 9, 00401-2022. [Google Scholar] [CrossRef]
- Wang, C.; Tan, J.; Miao, Y.; Zhang, Q. Obstructive Sleep Apnea, Prediabetes and Progression of Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Diabetes Investig. 2022, 13, 1396–1411. [Google Scholar] [CrossRef]
- Sweed, R.A.; El Wahab, N.H.A.; El Hooshy, M.S.; Morsy, E.Y.; Shetta, D.M. Obstructive Sleep Apnea in Patients with Type 2 Diabetes Mellitus in Egyptian Population. Egypt. J. Bronchol. 2023, 17, 55. [Google Scholar] [CrossRef]
- Balkau, B.; Vol, S.; Loko, S.; Andriamboavonjy, T.; Lantieri, O.; Gusto, G.; Meslier, N.; Racineux, J.-L.; Tichet, J. High Baseline Insulin Levels Associated With 6-Year Incident Observed Sleep Apnea. Diabetes Care 2010, 33, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Bahnasy, W.S.; El-Heneedy, Y.A.E.; El-Seidy, E.A.S.; Labib, N.A.A.; Ibrahim, I.S.E. Sleep Disturbances in Diabetic Peripheral Neuropathy Patients: A Clinical and Polysomnographic Study. Egypt. J. Neurol. Psychiatr. Neurosurg. 2018, 54, 23. [Google Scholar] [CrossRef]
- Huang, L.E.; Bunn, H.F. Hypoxia-Inducible Factor and Its Biomedical Relevance. J. Biol. Chem. 2003, 278, 19575–19578. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.-B.; Okamoto, K.; Pereira, T.; Brismar, K.; Poellinger, L. Hyperglycemia Regulates Hypoxia-Inducible Factor-1α Protein Stability and Function. Diabetes 2004, 53, 3226–3232. [Google Scholar] [CrossRef]
- Shao, Y.; Lv, C.; Yuan, Q.; Wang, Q. Levels of Serum 25(OH)VD3, HIF-1 α, VEGF, VWF, and IGF-1 and Their Correlation in Type 2 Diabetes Patients with Different Urine Albumin Creatinine Ratio. J. Diabetes Res. 2016, 2016, 1925424. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.J.; Bishopric, N.H.; Wu, X.; Peterson, C.A.; Webster, K.A. Hypoxia Regulates β-Enolase and Pyruvate Kinase-M Promoters by Modulating Sp1/Sp3 Binding to a Conserved GC Element. J. Biol. Chem. 1998, 273, 26087–26093. [Google Scholar] [CrossRef]
- Semenza, G.L.; Jiang, B.-H.; Leung, S.W.; Passantino, R.; Concordet, J.-P.; Maire, P.; Giallongo, A. Hypoxia Response Elements in the Aldolase A, Enolase 1, and Lactate Dehydrogenase A Gene Promoters Contain Essential Binding Sites for Hypoxia-Inducible Factor 1. J. Biol. Chem. 1996, 271, 32529–32537. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor 1 Is a Basic-Helix-Loop-Helix-PAS Heterodimer Regulated by Cellular O2 Tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Badawi, Y.; Shi, H. Relative Contribution of Prolyl Hydroxylase-Dependent and -Independent Degradation of HIF-1alpha by Proteasomal Pathways in Cerebral Ischemia. Front. Neurosci. 2017, 11, 239. [Google Scholar] [CrossRef]
- Gabryelska, A.; Białasiewicz, P. Association between Excessive Daytime Sleepiness, REM Phenotype and Severity of Obstructive Sleep Apnea. Sci. Rep. 2020, 10, 34. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Kai, J.; Wang, F.; Wang, Z.; Shao, J.; Tan, S.; Chen, A.; Zhang, F.; Wang, S.; et al. HIF-1α-Upregulated LncRNA-H19 Regulates Lipid Droplet Metabolism through the AMPKα Pathway in Hepatic Stellate Cells. Life Sci. 2020, 255, 117818. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Finger, E.C.; Krieg, A.J.; Wu, C.; Diep, A.N.; Lagory, E.L.; Wei, K.; McGinnis, L.M.; Yuan, J.; Kuo, C.J.; et al. Cross-Talk between Hypoxia and Insulin Signaling through Phd3 Regulates Hepatic Glucose and Lipid Metabolism and Ameliorates Diabetes. Nat. Med. 2013, 19, 1325–1330. [Google Scholar] [CrossRef]
- Rankin, E.B.; Rha, J.; Selak, M.A.; Unger, T.L.; Keith, B.; Liu, Q.; Haase, V.H. Hypoxia-Inducible Factor 2 Regulates Hepatic Lipid Metabolism. Mol. Cell Biol. 2009, 29, 4527–4538. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Roth, P.H.; Fang, H.M.; Wang, G.L. Transcriptional Regulation of Genes Encoding Glycolytic Enzymes by Hypoxia-Inducible Factor 1. J. Biol. Chem. 1994, 269, 23757–23763. [Google Scholar] [CrossRef] [PubMed]
- Minchenko, A.; Leshchinsky, I.; Opentanova, I.; Sang, N.; Srinivas, V.; Armstead, V.; Caro, J. Hypoxia-Inducible Factor-1-Mediated Expression of the 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase-3 (PFKFB3) Gene: Its Possible Role in the Warburg Effect. J. Biol. Chem. 2002, 277, 6183–6187. [Google Scholar] [CrossRef] [PubMed]
- Hackenbeck, T.; Huber, R.; Schietke, R.; Knaup, K.X.; Monti, J.; Wu, X.; Klanke, B.; Frey, B.; Gaipl, U.; Wullich, B.; et al. The GTPase RAB20 Is a HIF Target with Mitochondrial Localization Mediating Apoptosis in Hypoxia. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1–13. [Google Scholar] [CrossRef]
- Li, Y.; Miao, L.Y.; Xiao, Y.L.; Huang, M.; Yu, M.; Meng, K.; Cai, H.R. Hypoxia Induced High Expression of Thioredoxin Interacting Protein (TXNIP) in Non-Small Cell Lung Cancer and Its Prognostic Effect. Asian Pac. J. Cancer Prev. 2015, 16, 2953–2958. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Soni, S.; Padwad, Y.S. HIF-1 in Cancer Therapy: Two Decade Long Story of a Transcription Factor. Acta Oncol. 2017, 56, 503–515. [Google Scholar] [CrossRef]
- Graven, K.K.; Yu, Q.; Pan, D.; Roncarati, J.S.; Farber, H.W. Identification of an Oxygen Responsive Enhancer Element in the Glyceraldehyde-3-Phosphate Dehydrogenase Gene. Biochim. Biophys. Acta Gene Struct. Expr. 1999, 1447, 208–218. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Beasley, N.; Watson, P.H.; Campo, L.; Chia, S.K.; English, R.; Pastorek, J.; Sly, W.S.; Ratcliffe, P.; Harris, A.L. Expression of the Hypoxia-Inducible and Tumor-Associated Carbonic Anhydrases in Ductal Carcinoma in Situ of the Breast. Am. J. Pathol. 2001, 158, 1011–1019. [Google Scholar] [CrossRef]
- Gabryelska, A.; Karuga, F.F.; Szmyd, B.; Białasiewicz, P. HIF-1α as a Mediator of Insulin Resistance, T2DM, and Its Complications: Potential Links With Obstructive Sleep Apnea. Front. Physiol. 2020, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Makino, Y.; Mizumoto, K.; Isoe, T.; Takeda, Y.; Watanabe, J.; Fujita, Y.; Takiyama, Y.; Abiko, A.; Haneda, M. Loss of HIF-1α Impairs GLUT4 Translocation and Glucose Uptake by the Skeletal Muscle Cells. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1065–E1076. [Google Scholar] [CrossRef] [PubMed]
- Görgens, S.W.; Benninghoff, T.; Eckardt, K.; Springer, C.; Chadt, A.; Melior, A.; Wefers, J.; Cramer, A.; Jensen, J.; Birkeland, K.I.; et al. Hypoxia in Combination With Muscle Contraction Improves Insulin Action and Glucose Metabolism in Human Skeletal Muscle via the HIF-1a Pathway. Diabetes 2017, 66, 2800–2807. [Google Scholar] [CrossRef]
- Thomas, A.; Belaidi, E.; Moulin, S.; Horman, S.; van der Zon, G.C.; Viollet, B.; Levy, P.; Bertrand, L.; Pepin, J.-L.; Godin-Ribuot, D.; et al. Chronic Intermittent Hypoxia Impairs Insulin Sensitivity but Improves Whole-Body Glucose Tolerance by Activating Skeletal Muscle AMPK. Diabetes 2017, 66, 2942–2951. [Google Scholar] [CrossRef]
- Alhawiti, N.M.; Al Mahri, S.; Aziz, M.A.; Malik, S.S.; Mohammad, S. TXNIP in Metabolic Regulation: Physiological Role and Therapeutic Outlook. Curr. Drug Targets 2017, 18, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Karuga, F.F.; Kaczmarski, P.; Sochal, M.; Szmyd, B.; Białasiewicz, P.; Gabryelska, A. Cross-Sectional Analysis of Hypoxia-Regulated MiRNA-181a, MiRNA-199a, HIF-1α, and SIRT1 in the Development of Type 2 Diabetes in Patients with Obstructive Sleep Apnea—Preliminary Study. J. Clin. Med. 2024, 13, 7644. [Google Scholar] [CrossRef]
- Santamaria-Martos, F.; Benítez, I.; Ortega, F.; Zapater, A.; Giron, C.; Pinilla, L.; Pascual, L.; Cortijo, A.; Dalmases, M.; Fernandez-Real, J.M.; et al. Circulating MicroRNA Profile as a Potential Biomarker for Obstructive Sleep Apnea Diagnosis. Sci. Rep. 2019, 9, 13456. [Google Scholar] [CrossRef]
- Karuga, F.F.; Jaromirska, J.; Malicki, M.; Sochal, M.; Szmyd, B.; Białasiewicz, P.; Strzelecki, D.; Gabryelska, A. The Role of MicroRNAs in Pathophysiology and Diagnostics of Metabolic Complications in Obstructive Sleep Apnea Patients. Front. Mol. Neurosci. 2023, 16, 1208886. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; You, L.; Shi, C.; Yang, L.; Pang, L.; Cui, X.; Ji, C.; Zheng, W.; Guo, X. Expression of MiR-199a-3p in Human Adipocytes Is Regulated by Free Fatty Acids and Adipokines. Mol. Med. Rep. 2016, 14, 1180–1186. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Alzamil, H. Elevated Serum TNF- α Is Related to Obesity in Type 2 Diabetes Mellitus and Is Associated with Glycemic Control and Insulin Resistance. J. Obes. 2020, 2020, 5076858. [Google Scholar] [CrossRef]
- Jamil, K.; Jayaraman, A.; Ahmad, J.; Joshi, S.; Yerra, S.K. TNF-Alpha −308G/A and −238G/A Polymorphisms and Its Protein Network Associated with Type 2 Diabetes Mellitus. Saudi J. Biol. Sci. 2017, 24, 1195–1203. [Google Scholar] [CrossRef]
- Patel, R.; Palit, S.P.; Rathwa, N.; Ramachandran, A.V.; Begum, R. Genetic Variants of Tumor Necrosis Factor-α and Its Levels: A Correlation with Dyslipidemia and Type 2 Diabetes Susceptibility. Clin. Nutr. 2019, 38, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Bowker, N.; Shah, R.L.; Sharp, S.J.; Luan, J.; Stewart, I.D.; Wheeler, E.; Ferreira, M.A.R.; Baras, A.; Wareham, N.J.; Langenberg, C.; et al. Meta-Analysis Investigating the Role of Interleukin-6 Mediated Inflammation in Type 2 Diabetes. EBioMedicine 2020, 61, 103062. [Google Scholar] [CrossRef]
- Kreiner, F.F.; Kraaijenhof, J.M.; von Herrath, M.; Hovingh, G.K.K.; von Scholten, B.J. Interleukin 6 in Diabetes, Chronic Kidney Disease, and Cardiovascular Disease: Mechanisms and Therapeutic Perspectives. Expert. Rev. Clin. Immunol. 2022, 18, 377–389. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Abd-Elrahman, M.Z.; Bahriz, R.; Albehairy, A. Inflammatory Cytokine and Plasma C-Reactive Protein Response to Ketoacidosis in Adults with Type 1 Diabetes: Egyptian Multicenter Study. Egypt. J. Intern. Med. 2020, 32, 10. [Google Scholar] [CrossRef]
- Lam, D.C.L.; Lam, K.S.L.; Ip, M.S.M. Obstructive Sleep Apnoea, Insulin Resistance and Adipocytokines. Clin. Endocrinol. 2015, 82, 165–177. [Google Scholar] [CrossRef]
- Chen, B.; Lam, K.S.L.; Wang, Y.; Wu, D.; Lam, M.C.; Shen, J.; Wong, L.; Hoo, R.L.C.; Zhang, J.; Xu, A. Hypoxia Dysregulates the Production of Adiponectin and Plasminogen Activator Inhibitor-1 Independent of Reactive Oxygen Species in Adipocytes. Biochem. Biophys. Res. Commun. 2006, 341, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Jensen, J.S.; Bjerre, M.; Pedersen, S.H.; Frystyk, J.; Flyvbjerg, A.; Galatius, S.; Jeppesen, J.; Mogelvang, R. Adiponectin, Type 2 Diabetes and Cardiovascular Risk. Eur. J. Prev. Cardiol. 2015, 22, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Polotsky, V.Y. Leptin and Leptin Resistance in the Pathogenesis of Obstructive Sleep Apnea: A Possible Link to Oxidative Stress and Cardiovascular Complications. Oxid. Med. Cell Longev. 2018, 2018, 5137947. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, Cardiovascular Diseases and Type 2 Diabetes Mellitus. Acta Pharmacol. Sin. 2018, 39, 1176–1188. [Google Scholar] [CrossRef]
- Abed, B.A.; Farhan, L.O.; Dawood, A.S. Relationship between Serum Nesfatin-1, Adiponectin, Resistin Concentration, and Obesity with Type 2 Diabetes Mellitus. Baghdad Sci. J. 2023, 21, 117–123. [Google Scholar] [CrossRef]
- Cherneva, R.V.; Georgiev, O.B.; Petrova, D.S.; Mondeshki, T.L.; Ruseva, S.R.; Cakova, A.D.; Mitev, V.I. Resistin—The Link between Adipose Tissue Dysfunction and Insulin Resistance in Patients with Obstructive Sleep Apnea. J. Diabetes Metab. Disord. 2013, 12, 5. [Google Scholar] [CrossRef]
- Watts, S.W. Trash Talk by Fat. Hypertension 2015, 66, 466–468. [Google Scholar] [CrossRef]
- Kurt, O.K.; Tosun, M.; Alcelik, A.; Yilmaz, B.; Talay, F. Serum Omentin Levels in Patients with Obstructive Sleep Apnea. Sleep Breath. 2014, 18, 391–395. [Google Scholar] [CrossRef]
- Zhang, D.; Pang, X.; Huang, R.; Gong, F.; Zhong, X.; Xiao, Y. Adiponectin, Omentin, Ghrelin, and Visfatin Levels in Obese Patients with Severe Obstructive Sleep Apnea. Biomed. Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- She, N.; Liu, N.; Ren, X.; Liu, H. Association between Omentin and Obstructive Sleep Apnea: A Meta-analysis. Clin. Respir. J. 2023, 17, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Von Frankenberg, A.D.; Reis, A.F.; Gerchman, F. Relationships between Adiponectin Levels, the Metabolic Syndrome, and Type 2 Diabetes: A Literature Review. Arch. Endocrinol. Metab. 2017, 61, 614–622. [Google Scholar] [CrossRef]
- Sun, Q.; Yan, B.; Yang, D.; Guo, J.; Wang, C.; Zhang, Q.; Shi, Y.; Shi, X.; Tian, G.; Liang, X. Serum Adiponectin Levels Are Positively Associated With Diabetic Peripheral Neuropathy in Chinese Patients With Type 2 Diabetes. Front. Endocrinol. 2020, 11, 567959. [Google Scholar] [CrossRef]
- Achari, A.; Jain, S. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Huang, K.; Liang, Y.; Ma, Y.; Wu, J.; Luo, H.; Yi, B. The Variation and Correlation of Serum Adiponectin, Nesfatin-1, IL-6, and TNF-α Levels in Prediabetes. Front. Endocrinol. 2022, 13, 774272. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The Role of Leptin/Adiponectin Ratio in Metabolic Syndrome and Diabetes. Horm. Mol. Biol. Clin. Investig. 2014, 18, 37–45. [Google Scholar] [CrossRef]
- Ciriello, J.; Moreau, J.M.; Caverson, M.M.; Moranis, R. Leptin: A Potential Link Between Obstructive Sleep Apnea and Obesity. Front. Physiol. 2022, 12, 767318. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, J. Effects of Different Obesity-Related Adipokines on the Occurrence of Obstructive Sleep Apnea. Endocr. J. 2020, 67, 485–500. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Li, Y.; Zhang, S.; Cai, X.; Zhang, R.; Gong, S.; Han, X.; Ji, L. Serum Leptin, Resistin, and Adiponectin Levels in Obese and Non-Obese Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Medicine 2020, 99, e19052. [Google Scholar] [CrossRef] [PubMed]
- Vilariño-García, T.; Polonio-González, M.; Pérez-Pérez, A.; Ribalta, J.; Arrieta, F.; Aguilar, M.; Obaya, J.; Gimeno-Orna, J.; Iglesias, P.; Navarro, J.; et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 2338. [Google Scholar] [CrossRef]
- Mirza, S.; Hossain, M.; Mathews, C.; Martinez, P.; Pino, P.; Gay, J.L.; Rentfro, A.; McCormick, J.B.; Fisher-Hoch, S.P. Type 2-Diabetes Is Associated with Elevated Levels of TNF-Alpha, IL-6 and Adiponectin and Low Levels of Leptin in a Population of Mexican Americans: A Cross-Sectional Study. Cytokine 2012, 57, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, Y.; Gavrilova, O.; Yakar, S.; Jou, W.; Pack, S.; Asghar, Z.; Wheeler, M.B.; LeRoith, D. Leptin Improves Insulin Resistance and Hyperglycemia in a Mouse Model of Type 2 Diabetes. Endocrinology 2005, 146, 4024–4035. [Google Scholar] [CrossRef]
- Bidulescu, A.; Dinh, P.C.; Sarwary, S.; Forsyth, E.; Luetke, M.C.; King, D.B.; Liu, J.; Davis, S.K.; Correa, A. Associations of Leptin and Adiponectin with Incident Type 2 Diabetes and Interactions among African Americans: The Jackson Heart Study. BMC Endocr. Disord. 2020, 20, 31. [Google Scholar] [CrossRef]
- Ernst, M.C.; Sinal, C.J. Chemerin: At the Crossroads of Inflammation and Obesity. Trends Endocrinol. Metab. 2010, 21, 660–667. [Google Scholar] [CrossRef]
- Feng, X.; Li, P.; Zhou, C.; Jia, X.; Kang, J. Elevated Levels of Serum Chemerin in Patients with Obstructive Sleep Apnea Syndrome. Biomarkers 2012, 17, 248–253. [Google Scholar] [CrossRef]
- Senthilkumar, G.P.; Anithalekshmi, M.S.; Yasir, M.; Parameswaran, S.; Muthu Packirisamy, R.; Bobby, Z. Role of Omentin 1 and IL-6 in Type 2 Diabetes Mellitus Patients with Diabetic Nephropathy. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Karel, P.; Schilperoord, M.; Reichman, L.J.A.; Krabbe, J.G. The Dark Side of Apnea: Altered 24-Hour Melatonin Secretion in Obstructive Sleep Apnea (OSAS) Is Disease Severity Dependent. Sleep Breath. 2024, 28, 1751–1759. [Google Scholar] [CrossRef]
- Ramracheya, R.D.; Muller, D.S.; Squires, P.E.; Brereton, H.; Sugden, D.; Huang, G.C.; Amiel, S.A.; Jones, P.M.; Persaud, S.J. Function and Expression of Melatonin Receptors on Human Pancreatic Islets. J. Pineal Res. 2008, 44, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Parmar, N.; Pramanik Palit, S.; Rathwa, N.; Ramachandran, A.V.; Begum, R. Diabetes Mellitus and Melatonin: Where Are We? Biochimie 2022, 202, 2–14. [Google Scholar] [CrossRef]
- Gestreau, C.; Bévengut, M.; Dutschmann, M. The Dual Role of the Orexin/Hypocretin System in Modulating Wakefulness and Respiratory Drive. Curr. Opin. Pulm. Med. 2008, 14, 512–518. [Google Scholar] [CrossRef]
- Polito, R.; Francavilla, V.C.; Ambrosi, A.; Tartaglia, N.; Tafuri, D.; Monda, M.; Messina, A.; Sessa, F.; Di Maio, G.; Ametta, A.; et al. The Orexin-A Serum Levels Are Strongly Modulated by Physical Activity Intervention in Diabetes Mellitus Patients. J. Hum. Sport Exerc. 2020, 15, 244–251. [Google Scholar] [CrossRef]
- Zarifkar, M.; Noshad, S.; Shahriari, M.; Afarideh, M.; Khajeh, E.; Karimi, Z.; Ghajar, A.; Esteghamati, A. Inverse Association of Peripheral Orexin-A with Insulin Resistance in Type 2 Diabetes Mellitus: A Randomized Clinical Trial. Rev. Diabet. Stud. 2017, 14, 301–310. [Google Scholar] [CrossRef]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors That Regulate Feeding Behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Lotfy, M.; D’Souza, C.; Alseiari, S.M.; Alsaadi, A.A.; Qahtan, S.A. Hypocretin/Orexin Modulates Body Weight and the Metabolism of Glucose and Insulin. Diabetes Metab. Res. Rev. 2020, 36, e3229. [Google Scholar] [CrossRef]
- Mohammadi, I.; Sadeghi, M.; Tajmiri, G.; Brühl, A.B.; Sadeghi Bahmani, L.; Brand, S. Evaluation of Blood Levels of Omentin-1 and Orexin-A in Adults with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Life 2023, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.; Pradhan, G.; Zhou, Y.; Xue, B.; Sun, Y. Diverse and Complementary Effects of Ghrelin and Obestatin. Biomolecules 2022, 12, 517. [Google Scholar] [CrossRef]
- Dezaki, K.; Sone, H.; Yada, T. Ghrelin Is a Physiological Regulator of Insulin Release in Pancreatic Islets and Glucose Homeostasis. Pharmacol. Ther. 2008, 118, 239–249. [Google Scholar] [CrossRef]
- Pardak, P.; Filip, R.; Woliński, J. The Impact of Sleep-Disordered Breathing on Ghrelin, Obestatin, and Leptin Profiles in Patients with Obesity or Overweight. J. Clin. Med. 2022, 11, 2032. [Google Scholar] [CrossRef]
- Lindqvist, A.; Shcherbina, L.; Prasad, R.B.; Miskelly, M.G.; Abels, M.; Martínez-Lopéz, J.A.; Fred, R.G.; Nergård, B.J.; Hedenbro, J.; Groop, L.; et al. Ghrelin Suppresses Insulin Secretion in Human Islets and Type 2 Diabetes Patients Have Diminished Islet Ghrelin Cell Number and Lower Plasma Ghrelin Levels. Mol. Cell Endocrinol. 2020, 511, 110835. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J. Clin. Sleep Med. 2019, 15, 301–334. [Google Scholar] [CrossRef]
- Liu, T.; Li, W.; Zhou, H.; Wang, Z. Verifying the Relative Efficacy between Continuous Positive Airway Pressure Therapy and Its Alternatives for Obstructive Sleep Apnea: A Network Meta-Analysis. Front. Neurol. 2017, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Uniken Venema, J.A.M.; Doff, M.H.J.; Joffe-Sokolova, D.; Wijkstra, P.J.; van der Hoeven, J.H.; Stegenga, B.; Hoekema, A. Long-Term Obstructive Sleep Apnea Therapy: A 10-Year Follow-up of Mandibular Advancement Device and Continuous Positive Airway Pressure. J. Clin. Sleep Med. 2020, 16, 353–359. [Google Scholar] [CrossRef]
- Li, Z.; Cai, S.; Wang, J.; Chen, R. Predictors of the Efficacy for Daytime Sleepiness in Patients With Obstructive Sleep Apnea With Continual Positive Airway Pressure Therapy: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2022, 13, 911996. [Google Scholar] [CrossRef] [PubMed]
- Amendolara, M.; Di Lecce, V.; Santomasi, C.; Quaranta, V.N.; Portacci, A.; Lazzaretti, I.D.; Cuccaro, L.A.S.; Casparrini, M.; Spierto, S.; Picerno, V.; et al. The Impact of PAP Therapy First Impression on Short-Term Treatment Adherence. Sleep Breath. 2025, 29, 152. [Google Scholar] [CrossRef]
- Missey, F.; Ejneby, M.S.; Ngom, I.; Donahue, M.J.; Trajlinek, J.; Acerbo, E.; Botzanowski, B.; Cassarà, A.M.; Neufeld, E.; Glowacki, E.D.; et al. Obstructive Sleep Apnea Improves with Non-Invasive Hypoglossal Nerve Stimulation Using Temporal Interference. Bioelectron. Med. 2023, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Thuler, E.R.; Rabelo, F.A.W.; Santos Junior, V.; Kayamori, F.; Bianchini, E.M.G. Hypoglossal Nerve Trunk Stimulation: Electromyography Findings during Drug-Induced Sleep Endoscopy: A Case Report. J. Med. Case Rep. 2023, 17, 187. [Google Scholar] [CrossRef]
- Olson, M.D.; Junna, M.R. Hypoglossal Nerve Stimulation Therapy for the Treatment of Obstructive Sleep Apnea. Neurotherapeutics 2021, 18, 91–99. [Google Scholar] [CrossRef]
- Lorenzi-Filho, G.; Almeida, F.R.; Strollo, P.J. Treating OSA: Current and Emerging Therapies beyond CPAP. Respirology 2017, 22, 1500–1507. [Google Scholar] [CrossRef]
- Sultana, R.; Sissoho, F.; Kaushik, V.P.; Raji, M.A. The Case for Early Use of Glucagon-like Peptide-1 Receptor Agonists in Obstructive Sleep Apnea Patients with Comorbid Diabetes and Metabolic Syndrome. Life 2022, 12, 1222. [Google Scholar] [CrossRef]
- Malhotra, A.; Grunstein, R.R.; Fietze, I.; Weaver, T.E.; Redline, S.; Azarbarzin, A.; Sands, S.A.; Schwab, R.J.; Dunn, J.P.; Chakladar, S.; et al. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. N. Engl. J. Med. 2024, 391, 1193–1205, Correction in N. Engl. J. Med. 2024, 391, 15. [Google Scholar] [CrossRef] [PubMed]
- Blackman, A.; Foster, G.D.; Zammit, G.; Rosenberg, R.; Aronne, L.; Wadden, T.; Claudius, B.; Jensen, C.B.; Mignot, E. Effect of Liraglutide 3.0 Mg in Individuals with Obesity and Moderate or Severe Obstructive Sleep Apnea: The SCALE Sleep Apnea Randomized Clinical Trial. Int. J. Obes. 2016, 40, 1310–1319. [Google Scholar] [CrossRef]
- Neeland, I.J.; Eliasson, B.; Kasai, T.; Marx, N.; Zinman, B.; Inzucchi, S.E.; Wanner, C.; Zwiener, I.; Wojeck, B.S.; Yaggi, H.K.; et al. The Impact of Empagliflozin on Obstructive Sleep Apnea and Cardiovascular and Renal Outcomes: An Exploratory Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2020, 43, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Li, S.; Yu, X.; Wei, Q.; Yu, F.; Tong, J. DAHOS Study: Efficacy of Dapagliflozin in Treating Heart Failure with Reduced Ejection Fraction and Obstructive Sleep Apnea Syndrome—A 3-Month, Multicenter, Randomized Controlled Clinical Trial. Eur. J. Clin. Pharmacol. 2024, 80, 771–780. [Google Scholar] [CrossRef]
- Tan, X.; van Egmond, L.; Chapman, C.D.; Cedernaes, J.; Benedict, C. Aiding Sleep in Type 2 Diabetes: Therapeutic Considerations. Lancet Diabetes Endocrinol. 2018, 6, 60–68. [Google Scholar] [CrossRef]
- Soreca, I.; Arnold, N.; Dombrovski, A.Y. Bright Light Therapy for CPAP-Resistant OSA Symptoms. J. Clin. Sleep Med. 2024, 20, 211–219. [Google Scholar] [CrossRef]
- Gabryelska, A.; Turkiewicz, S.; Gajewski, A.; Jaromirska, J.; Strzelecki, D.; Białasiewicz, P.; Chałubiński, M.; Sochal, M. Assessment of Continuous Positive Airway Pressure Effect on the Circadian Clock Signaling Pathway in Obstructive Sleep Apnea Patients. Sci. Rep. 2025, 15, 11273. [Google Scholar] [CrossRef]
- Papaetis, G. GLP-1 Receptor Agonists, SGLT-2 Inhibitors, and Obstructive Sleep Apnoea: Can New Allies Face an Old Enemy? Arch. Med. Sci. Atheroscler. Dis. 2023, 8, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, M.; Hisano, F.; Wakazono, N.; Tsutsumi, K.; Oshida, Y.; Miyata, T. Effect of Treatment With Sodium-Glucose Cotransporter 2 Inhibitor on the Initiation of Continuous Positive Airway Pressure Therapy in Type 2 Diabetic Patients With Obstructive Sleep Apnea Syndrome. J. Clin. Med. Res. 2021, 13, 497–501. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Patel, S.R.; Strollo, P.J.; Cronin, J.; Yee, J.; Pho, H.; Werner, A.; Farkas, R. Aroxybutynin and Atomoxetine (AD109) for the Treatment of Obstructive Sleep Apnea: Rationale, Design and Baseline Characteristics of the Phase 3 Clinical Trials. Contemp. Clin. Trials Commun. 2025, 47, 101538. [Google Scholar] [CrossRef]
- Mason, M.; Welsh, E.J.; Smith, I. Drug Therapy for Obstructive Sleep Apnoea in Adults. Cochrane Database Syst. Rev. 2013, 2013, CD003002. [Google Scholar] [CrossRef]
- Dragonieri, S.; Portacci, A.; Quaranta, V.N.; Carratu, P.; Lazar, Z.; Carpagnano, G.E.; Bikov, A. Therapeutic Potential of Glucagon-like Peptide-1 Receptor Agonists in Obstructive Sleep Apnea Syndrome Management: A Narrative Review. Diseases 2024, 12, 224. [Google Scholar] [CrossRef]
- Janić, M.; Škrgat, S.; Harlander, M.; Lunder, M.; Janež, A.; Pantea Stoian, A.; El-Tanani, M.; Maggio, V.; Rizzo, M. Potential Use of GLP-1 and GIP/GLP-1 Receptor Agonists for Respiratory Disorders: Where Are We At? Medicina 2024, 60, 2030. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, R.; Pei, L.; Zhang, X.; Wei, J.; Wen, Y.; Liu, H.; Ye, H.; Wang, J.; Wang, L. The Relationship between the Use of GLP-1 Receptor Agonists and the Incidence of Respiratory Illness: A Meta-Analysis of Randomized Controlled Trials. Diabetol. Metab. Syndr. 2023, 15, 164. [Google Scholar] [CrossRef]
- Malhotra, A.; Grunstein, R.R.; Azarbarzin, A.; Sands, S.A.; Dang, X.; Chakladar, S.; Dunn, J.P.; Falcon, B.; Bednarik, J. Tirzepatide for sleep-disordered breathing in SURMOUNT-OSA: Time course and association with body weight. Sleep Med. 2025, 10, 106853. [Google Scholar] [CrossRef]
- Jiang, W.; Li, W.; Cheng, J.; Li, W.; Cheng, F. Efficacy and Safety of Liraglutide in Patients with Type 2 Diabetes Mellitus and Severe Obstructive Sleep Apnea. Sleep Breath. 2023, 27, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.; Crilly, S.; O’Mahony, A.; O’Riordan, B.; Traynor, M.; Gitau, R.; McDonald, K.; Ledwidge, M.; O’Shea, D.; Murphy, D.J.; et al. Continuous Positive Airway Pressure but Not GLP1-Mediated Weight Loss Improves Early Cardiovascular Disease in Obstructive Sleep Apnea: A Randomized Proof-of-Concept Study. Ann. Am. Thorac. Soc. 2024, 21, 464–473. [Google Scholar] [CrossRef]
- Sprung, V.S.; Kemp, G.J.; Wilding, J.P.; Adams, V.; Murphy, K.; Burgess, M.; Emegbo, S.; Thomas, M.; Needham, A.J.; Weimken, A.; et al. Randomised, COntrolled Multicentre Trial of 26 Weeks Subcutaneous Liraglutide (a Glucagon-like Peptide-1 Receptor Agonist), with or without ContiNuous Positive Airway Pressure (CPAP), in Patients with Type 2 Diabetes Mellitus (T2DM) and Obstructive Sleep ApnoEa (OSA) (ROMANCE): Study Protocol Assessing the Effects of Weight Loss on the Apnea–Hypnoea Index (AHI). BMJ Open 2020, 10, e038856. [Google Scholar] [CrossRef]
- Gomez-Peralta, F.; Abreu, C.; Castro, J.C.; Alcarria, E.; Cruz-Bravo, M.; Garcia-Llorente, M.J.; Albornos, C.; Moreno, C.; Cepeda, M.; Almodóvar, F. An Association between Liraglutide Treatment and Reduction in Excessive Daytime Sleepiness in Obese Subjects with Type 2 Diabetes. BMC Endocr. Disord. 2015, 15, 78. [Google Scholar] [CrossRef]
- Baser, O.; Lu, Y.; Chen, S.; Chen, S.; Baser, E. Tirzepatide and Semaglutide for the Treatment of Obstructive Sleep Apnea and Obesity: A Retrospective Analysis. Med. Res. Arch. 2024, 13, 1193–1205. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.; Evangelopoulos, A.; Vlahodimitris, I.; Grivakou, E.; Kotsi, E.; Dimitriou, K.; Skourtis, A.; Mourouzis, I. SGLT-2 Inhibitors and the Inflammasome: What’s Next in the 21st Century? Nutrients 2023, 15, 2294. [Google Scholar] [CrossRef]
- Peng, Y.; Qin, D.; Wang, Y.; Xue, L.; Qin, Y.; Xu, X. The Effect of SGLT-2 Inhibitors on Cardiorespiratory Fitness Capacity: A Systematic Review and Meta-Analysis. Front. Physiol. 2023, 13, 1081920. [Google Scholar] [CrossRef]
- Abdelmasih, R.; Abdelmaseih, R.; Thakker, R.; Faluk, M.; Ali, A.; Alsamman, M.M.; Hasan, S.M. Update on the Cardiovascular Benefits of Sodium-Glucose Co-Transporter-2 Inhibitors: Mechanism of Action, Available Agents and Comprehensive Review of Literature. Cardiol. Res. 2021, 12, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Frąk, W.; Hajdys, J.; Radzioch, E.; Szlagor, M.; Młynarska, E.; Rysz, J.; Franczyk, B. Cardiovascular Diseases: Therapeutic Potential of SGLT-2 Inhibitors. Biomedicines 2023, 11, 2085. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V. Molecular Determinants of Renal Glucose Reabsorption. Focus on “Glucose Transport by Human Renal Na+/D-Glucose Cotransporters SGLT1 and SGLT2”. Am. J. Physiol. Cell Physiol. 2011, 300, C6–C8. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.; Vashi, R.; Patel, S. Cerebrovascular Complications of Diabetes: SGLT-2 Inhibitors as a Promising Future Therapeutics. Curr. Drug Targets 2021, 22, 1629–1636. [Google Scholar] [CrossRef]
- Vrhovac, I.; Eror, D.B.; Klessen, D.; Burger, C.; Breljak, D.; Kraus, O.; Radović, N.; Jadrijević, S.; Aleksic, I.; Walles, T.; et al. Localizations of Na+-D-Glucose Cotransporters SGLT1 and SGLT2 in Human Kidney and of SGLT1 in Human Small Intestine, Liver, Lung, and Heart. Pflügers Arch. 2015, 467, 1881–1898. [Google Scholar] [CrossRef]
- Miller, E.M. Elements for Success in Managing Type 2 Diabetes With SGLT-2 Inhibitors: Role of the Kidney in Glucose Homeostasis: Implications for SGLT-2 Inhibition in the Treatment of Type 2 Diabetes Mellitus. J. Fam. Pract. 2017, 66, S3–S5. [Google Scholar]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef]
- Kolesnik, E.; Scherr, D.; Rohrer, U.; Benedikt, M.; Manninger, M.; Sourij, H.; von Lewinski, D. SGLT2 Inhibitors and Their Antiarrhythmic Properties. Int. J. Mol. Sci. 2022, 23, 1678. [Google Scholar] [CrossRef] [PubMed]
- Dharia, A.; Khan, A.; Sridhar, V.S.; Cherney, D.Z.I. SGLT2 Inhibitors: The Sweet Success for Kidneys. Annu. Rev. Med. 2023, 74, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.; McCullough, P.A. Roles for SGLT2 Inhibitors in Cardiorenal Disease. Cardiorenal Med. 2022, 12, 81–93. [Google Scholar] [CrossRef]
- Sy, G.L.L.; Te, M.T.; Payawal, D.A. Effect of SGLT 2 Inhibitors on Reducing Liver Enzymes: A Meta-Analysis. J. Gastroenterol. Hepatol. 2021, 36, 253. [Google Scholar]
- Krishnan, R.; Subramanian, R.; Selvarajan, R. SGLT2 Inhibitor: A Cardio-Renal Metabolic Pill. Int. J. Health Sci. Res. 2023, 13, 133–141. [Google Scholar] [CrossRef]
- Szekeres, Z.; Toth, K.; Szabados, E. The Effects of SGLT2 Inhibitors on Lipid Metabolism. Metabolites 2021, 11, 87. [Google Scholar] [CrossRef]
- Gao, Y.-M.; Feng, S.-T.; Wen, Y.; Tang, T.-T.; Wang, B.; Liu, B.-C. Cardiorenal Protection of SGLT2 Inhibitors—Perspectives from Metabolic Reprogramming. EBioMedicine 2022, 83, 104215. [Google Scholar] [CrossRef]
- Llorens-Cebrià, C.; Molina-Van den Bosch, M.; Vergara, A.; Jacobs-Cachá, C.; Soler, M.J. Antioxidant Roles of SGLT2 Inhibitors in the Kidney. Biomolecules 2022, 12, 143. [Google Scholar] [CrossRef]
- Qiu, M.; Ding, L.-L.; Zhan, Z.-L.; Liu, S.-Y. Use of SGLT2 Inhibitors and Occurrence of Noninfectious Respiratory Disorders: A Meta-Analysis of Large Randomized Trials of SGLT2 Inhibitors. Endocrine 2021, 73, 31–36. [Google Scholar] [CrossRef]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Feder, D.; de Fatima Veiga Gouveia, M.R.; Govato, T.C.P.; Nassis, C.D.Z. SGLT2 Inhibitors and the Mechanisms Involved in Weight Loss. Curr. Pharmacol. Rep. 2020, 6, 346–353. [Google Scholar] [CrossRef]
- Elian, V.I.; Dragomirescu, L.; Cheta, D.M.; Stoian, A.P.; Serafinceanu, C. Weight Loss Improves Metabolic Status in Overweight and Obese Subjects. Diabetes 2014, 63, A659–A660. [Google Scholar]
- Serafinceanu, C.; Crăciun, A.M.; Dobjanschi, C.; Elian, V. Sglt2 Inhibitors—Is the Paradigm in Type 2 Diabetes Mellitus Management Changing? Rom. J. Diabetes Nutr. Metab. Dis. 2014, 21, 261–265. [Google Scholar] [CrossRef]
- Elian, V.; Popovici, V.; Karampelas, O.; Pircalabioru, G.G.; Radulian, G.; Musat, M. Risks and Benefits of SGLT-2 Inhibitors for Type 1 Diabetes Patients Using Automated Insulin Delivery Systems—A Literature Review. Int. J. Mol. Sci. 2024, 25, 1972. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, Q.; Bai, X.-Y.; Zhou, Y.-F.; Zhou, Q.-L.; Zhang, M. Effect of Dapagliflozin on Obstructive Sleep Apnea in Patients with Type 2 Diabetes: A Preliminary Study. Nutr. Diabetes 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Armentaro, G.; Pelaia, C.; Condoleo, V.; Severini, G.; Crudo, G.; De Marco, M.; Pastura, C.A.; Tallarico, V.; Pezzella, R.; Aiello, D.; et al. Effect of SGLT2-Inhibitors on Polygraphic Parameters in Elderly Patients Affected by Heart Failure, Type 2 Diabetes Mellitus, and Sleep Apnea. Biomedicines 2024, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.; Bin Atique, H.; Regmi, N.; Sattar, Y.; Sundus, S.; Ambreen, S.; Lohia, P.; Qureshi, W.T.; Soubani, A. SGLT2 Inhibitors and Sleep Apnea; How Helpful Are the Medications: A Meta-Analysis. Endocr. Metab. Sci. 2021, 2, 100084. [Google Scholar] [CrossRef]
- Sawada, K.; Karashima, S.; Kometani, M.; Oka, R.; Takeda, Y.; Sawamura, T.; Fujimoto, A.; Demura, M.; Wakayama, A.; Usukura, M.; et al. Effect of Sodium Glucose Cotransporter 2 Inhibitors on Obstructive Sleep Apnea in Patients with Type 2 Diabetes. Endocr. J. 2018, 65, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Miyake, T.; Senba, H.; Sakai, T.; Furukawa, E.; Yamamoto, S.; Niiya, T.; Matsuura, B.; Hiasa, Y. The Effectiveness of Dapagliflozin for Sleep-Disordered Breathing among Japanese Patients with Obesity and Type 2 Diabetes Mellitus. Endocr. J. 2018, 65, 953–961. [Google Scholar] [CrossRef]
- Butt, J.H.; Jering, K.; DE Boer, R.A.; Claggett, B.L.; Desai, A.S.; Hernandez, A.F.; Inzucchi, S.E.; Jhund, P.S.; Køber, L.; Kosiborod, M.N.; et al. Heart Failure, Investigator-Reported Sleep Apnea and Dapagliflozin: A Patient-Level Pooled Meta-Analysis of DAPA-HF and DELIVER. J. Card. Fail. 2024, 30, 436–448. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Kopel, J.; Higuchi, K.; Ristic, B.; Sato, T.; Ramachandran, S.; Ganapathy, V. The Hepatic Plasma Membrane Citrate Transporter NaCT (SLC13A5) as a Molecular Target for Metformin. Sci. Rep. 2020, 10, 8536. [Google Scholar] [CrossRef]
- Agius, L.; Ford, B.E.; Chachra, S.S. The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective. Int. J. Mol. Sci. 2020, 21, 3240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Iannantuoni, F.; Gruevska, A.; Muntane, J.; Rocha, M.; Victor, V.M. Mechanisms of Action of Metformin in Type 2 Diabetes: Effects on Mitochondria and Leukocyte-Endothelium Interactions. Redox Biol. 2020, 34, 101517. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on Mechanisms of Action and Repurposing Potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- Johanns, M.; Hue, L.; Rider, M.H. AMPK Inhibits Liver Gluconeogenesis: Fact or Fiction? Biochem. J. 2023, 480, 105–125. [Google Scholar] [CrossRef]
- Cho, K.; Chung, J.Y.; Cho, S.K.; Shin, H.-W.; Jang, I.-J.; Park, J.-W.; Yu, K.-S.; Cho, J.-Y. Antihyperglycemic Mechanism of Metformin Occurs via the AMPK/LXRα/POMC Pathway. Sci. Rep. 2015, 5, 8145. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the Glucoregulatory Mechanisms of Metformin in Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef]
- An, H.; He, L. Current Understanding of Metformin Effect on the Control of Hyperglycemia in Diabetes. J. Endocrinol. 2016, 228, R97–R106. [Google Scholar] [CrossRef]
- Shohrati, M.; Karbasi-Afshar, R.; Saburi, A. Remarks in Metformin and Sleep Disorders in Diabetic Patients. Indian. J. Endocrinol. Metab. 2012, 16, 675. [Google Scholar] [CrossRef]
- Zunica, E.R.M.; Heintz, E.C.; Dantas, W.S.; Hebert, R.C.; Tanksley, M.; Beyl, R.A.; Mader, E.C.; Kirwan, J.P.; Axelrod, C.L.; Singh, P. Effects of Metformin on Glucose Metabolism and Mitochondrial Function in Patients with Obstructive Sleep Apnea: A Pilot Randomized Trial. Physiol. Rep. 2024, 12, e15948. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Rein, L.; Tarima, S.; Woodson, B.T.; Meurer, J.R. The Relationship between Metformin and Obstructive Sleep Apnea. J. Sleep Med. Disord. 2015, 2, 1027. [Google Scholar] [PubMed]
- Kajbaf, F.; Fendri, S.; Basille-Fantinato, A.; Diouf, M.; Rose, D.; Jounieaux, V.; Lalau, J.-D. The Relationship between Metformin Therapy and Sleep Quantity and Quality in Patients with Type 2 Diabetes Referred for Potential Sleep Disorders. Diabet. Med. 2014, 31, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, E.S.; Mackiewicz, M.; Gislason, T.; Teff, K.L.; Pack, A.I. Molecular Signatures of Obstructive Sleep Apnea in Adults: A Review and Perspective. Sleep 2009, 32, 447–470. [Google Scholar] [CrossRef]
- Fernandes, M.; Spanetta, M.; Vetrugno, G.; Nuccetelli, M.; Placidi, F.; Castelli, A.; Manfredi, N.; Izzi, F.; Laganà, G.; Bernardini, S.; et al. The Potential Role of Interleukin-6 in the Association between Inflammation and Cognitive Performance in Obstructive Sleep Apnea. Brain Behav. Immun. Health 2024, 42, 100875. [Google Scholar] [CrossRef]
- Verma, S.; Bhatta, M.; Davies, M.; Deanfield, J.E.; Garvey, W.T.; Jensen, C.; Kandler, K.; Kushner, R.F.; Rubino, D.M.; Kosiborod, M.N. Effects of Once-Weekly Semaglutide 2.4 Mg on C-Reactive Protein in Adults with Overweight or Obesity (STEP 1, 2, and 3): Exploratory Analyses of Three Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trials. EClinicalMedicine 2023, 55, 101737. [Google Scholar] [CrossRef]
- Kang, N.; Oh, S.; Kim, S.-Y.; Ahn, H.; Son, M.; Heo, S.-J.; Byun, K.; Jeon, Y.-J. Anti-Obesity Effects of Ishophloroglucin A from the Brown Seaweed Ishige Okamurae (Yendo) via Regulation of Leptin Signal in Ob/Ob Mice. Algal Res. 2022, 61, 102533. [Google Scholar] [CrossRef]
- Amorim, M.R.; Aung, O.; Mokhlesi, B.; Polotsky, V.Y. Leptin-Mediated Neural Targets in Obesity Hypoventilation Syndrome. Sleep 2022, 45, zsac153. [Google Scholar] [CrossRef] [PubMed]
- Suriyagandhi, V.; Nachiappan, V. Therapeutic Target Analysis and Molecular Mechanism of Melatonin—Treated Leptin Resistance Induced Obesity: A Systematic Study of Network Pharmacology. Front. Endocrinol. 2022, 13, 927576, Correction in Front. Endocrinol. 2025, 16, 1670260. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.C.; Mangoni, A.A.; Zinellu, E.; Pintus, G.; Carru, C.; Fois, A.G.; Pirina, P.; Zinellu, A. Circulating Superoxide Dismutase Concentrations in Obstructive Sleep Apnoea (OSA): A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 1764. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Homayouni-Tabrizi, M.; Amiri, H.; Safari-Faramani, R.; Moradi, M.-T.; Fadaei, R.; Khazaie, H. The Effect of Continuous Positive Airway Pressure on Total Antioxidant Capacity in Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep Breath. 2023, 27, 1237–1245. [Google Scholar] [CrossRef]
- Saruhan, E.; Sertoglu, E.; Unal, Y.; Bek, S.; Kutlu, G. The Role of Antioxidant Vitamins and Selenium in Patients with Obstructive Sleep Apnea. Sleep Breath. 2021, 25, 923–930. [Google Scholar] [CrossRef]
- Bakshi, G.K.; Khurana, S.; Srivastav, S.; Kumar, R.; Chourasia, M.; Bose, S. Integrative Analysis of Candidate MicroRNAs and Gene Targets for OSA Management Using in Silico and In-Vitro Approach. Biotechnol. Notes 2025, 6, 79–88. [Google Scholar] [CrossRef]
- Moriondo, G.; Soccio, P.; Tondo, P.; Scioscia, G.; Sabato, R.; Foschino Barbaro, M.P.; Lacedonia, D. Obstructive Sleep Apnea: A Look towards Micro-RNAs as Biomarkers of the Future. Biology 2023, 12, 66. [Google Scholar] [CrossRef]
 = Activation.
= Activation.
 = Activation.
= Activation.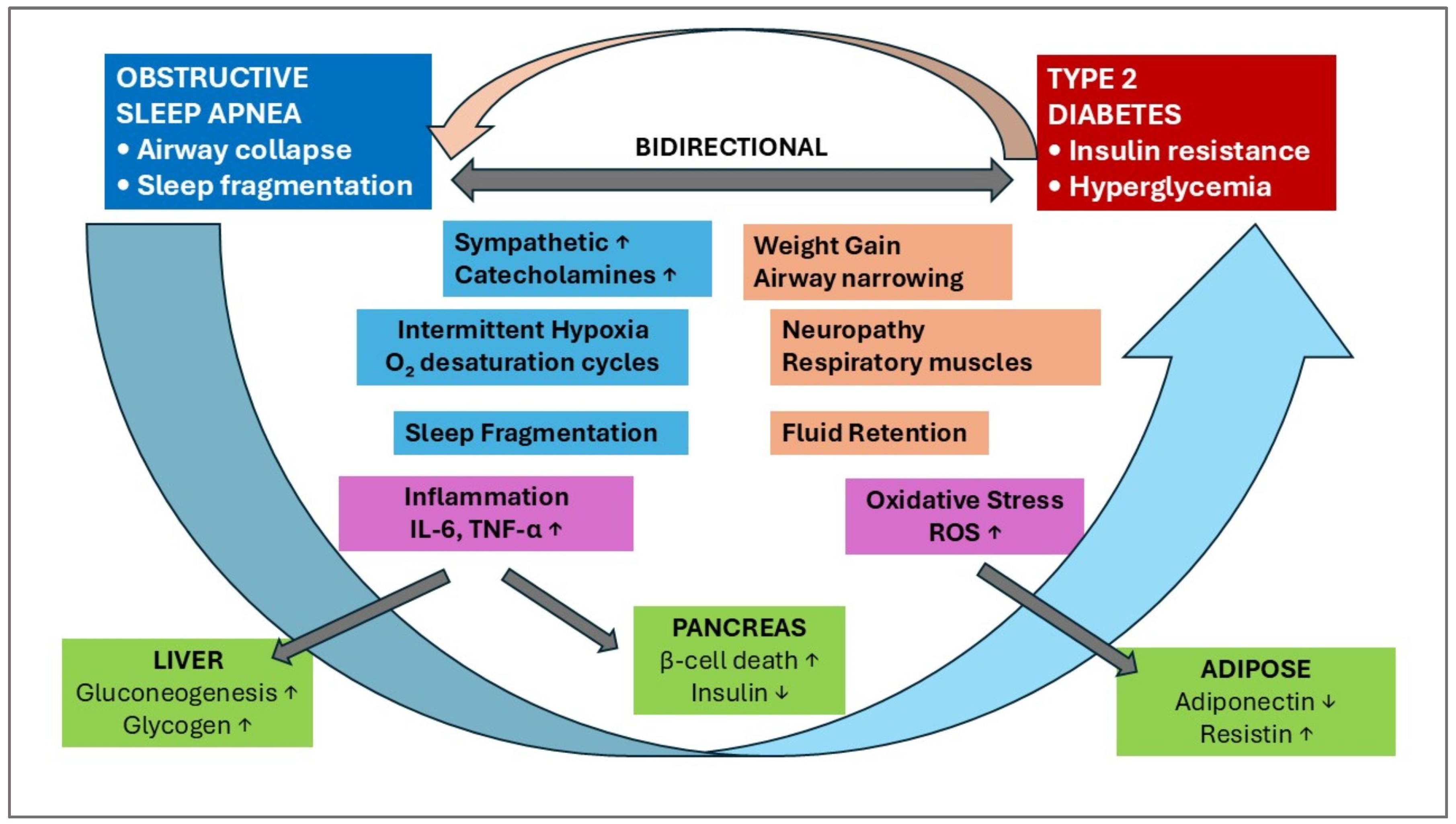
 = Activation;
= Activation;  = Inhibition.
= Inhibition.
 = Activation;
= Activation;  = Inhibition.
= Inhibition.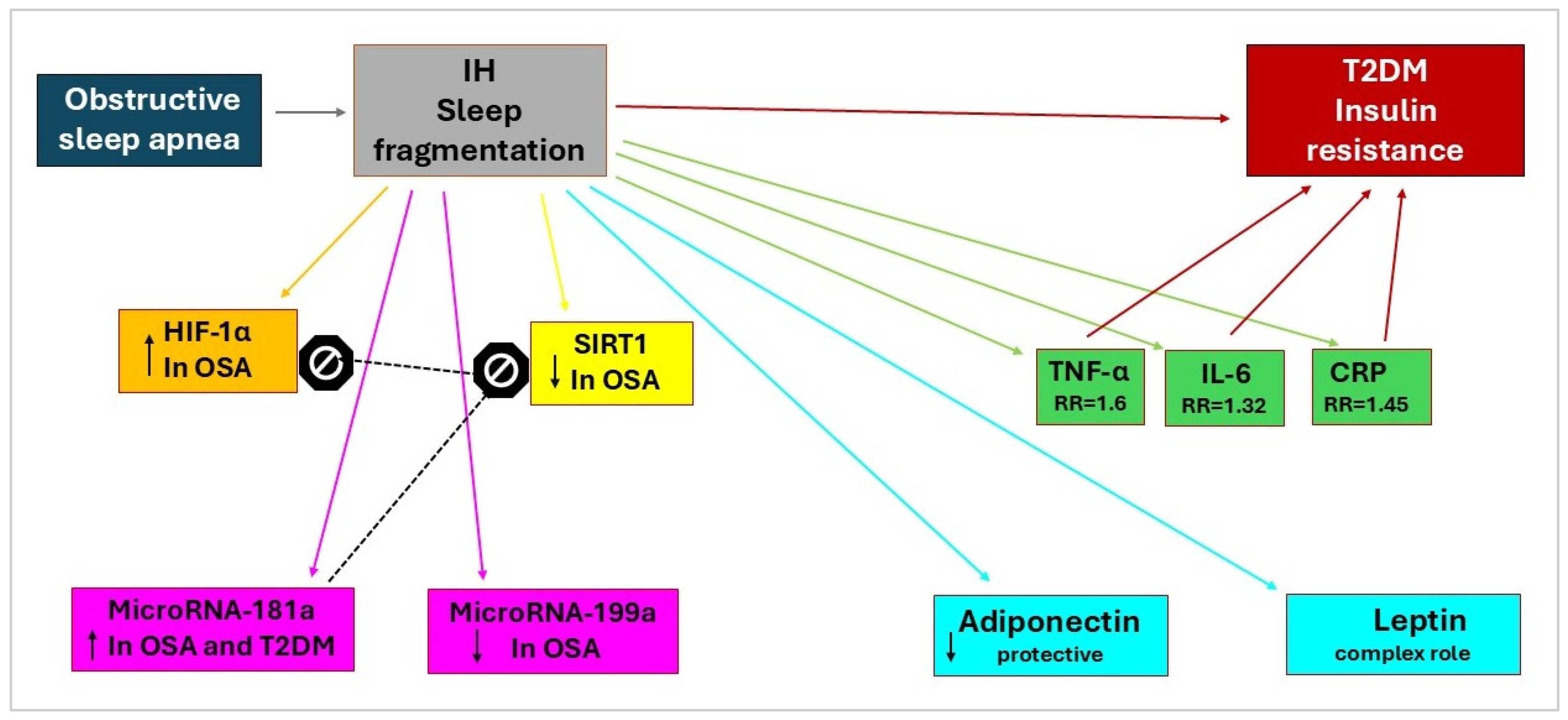

| Adipokine | Sources | Receptor | Actions | Reference |
|---|---|---|---|---|
| Adiponectin | Adipocyte | AdipoR1 and AdipoR2; T-Cadherin | Increases insulin sensitivity; Anti-inflammatory | [62,63] |
| Leptin | White adipose tissue (Obesity gene encoding) | Leptin receptor, (LepR or ObR) | Increases energy consumption; Inhibits fat synthesis; Induces fat decomposition; Inhibits insulin synthesis and secretion. | [64,65] |
| Resistin | Adipose tissue; Immune and epithelial cells | No mention | Inhibits insulin’s ability to stimulate glucose cellular uptake; Pro-inflammatory | [66,67] |
| Chemerin | Adipose tissue | Specific receptor proteins: ChemR23 (CMKLR1), and RARRES2 | Acts in an immune response; Anti-inflammatory; Regulates glucose metabolism. | [68] |
| Omentin-1 | Omental adipose tissue | No mention | Anti-inflammatory; Regulates fat metabolism; Improves insulin sensitivity. | [69,70,71] |
| Therapy Class | Specific Agent | Mechanism | OSA Benefits | T2DM Benefits | Clinical Evidence | FDA Status | Key Studies |
|---|---|---|---|---|---|---|---|
| GLP-1 Receptor Agonists | Tirzepatide | Dual GIP/GLP-1 agonist | 52-week study: significant AHI reduction | Superior glycemic control and weight loss vs. GLP-1 alone | Phase III positive results | Approved for obesity, T2DM, and OSA | [110] |
| Semaglutide | GLP-1 receptor agonist; | AHI reduction, improved sleep quality, and weight loss | Established weight and glycemic; cardiovascular protection | Phase III trials completed | Approved for obesity, T2DM | [111] | |
| Liraglutide | GLP-1 receptor agonist | AHI improvement | Established weight and glycemic benefit | RCT evidence | Approved for obesity, T2DM | [112] | |
| SGLT2 Inhibitors | Empagliflozin | SGLT2 inhibition; natriuretic effects | 65% OSA occurrence reduction; improved SpO2 | Cardiovascular protection; renal benefits, Glycemic control | Meta-analysis evidence | Approved for T2DM, HF, CKD | [113] |
| Dapagliflozin | SGLT2 inhibition; natriuretic effects | Reduced fluid retention; improved AHI | Cardiovascular protection; renal benefits, Glycemic control | Observational studies | Approved for T2DM, HF, CKD | [114] | |
| Chronotherapy | Melatonin | Circadian synchronization; antioxidant effects | Sleep architecture improvement | Insulin sensitivity enhancement | Preclinical evidence | OTC supplement | [115] |
| Light Therapy | Circadian entrainment; PER2 enhancement | Potential sleep quality improvement | Metabolic rhythm restoration | Early studies | Nonpharmacological | [116] | |
| CRY Stabilizers | Clock gene stabilization (TW68) | Potential circadian restoration | Hepatic glucose suppression | Preclinical only | Investigational | [117] | |
| Combinations | GLP-1 + SGLT2 | Synergistic metabolic effects | Additive OSA benefits potential | Enhanced glycemic/CV outcomes | Ongoing trials | Individual approvals | [118] |
| CPAP + GLP-1 /SGLT2 | Mechanical + metabolic intervention | Optimal AHI reduction + weight loss | Comprehensive metabolic control | Limited studies | Standard + approved | [119] | |
| Aroxybutynin +atomoxetine | A selective norepinephrine reuptake inhibitor and a selective antimuscarinic | Activation of the upper airway dilator muscles | No mention | Phase III trials are ongoing | Submitted for approval in OSA | [120] |
| Reference | Primary and Secondary Objectives | Population/Participants | Sample Size | Intervention/Exposure | Outcome Measures | Major Findings |
|---|---|---|---|---|---|---|
| Malhotra A. et al., 2024 [111] | Primary: To evaluate the change in AHI from baseline. Secondary: To assess percent change in AHI, body weight, hypoxic burden, patient-reported sleep impairment and disturbance (PROMIS scales), hsCRP concentration, and SBP. | Adults with moderate-to-severe OSA (AHI ≥ 15 events/h) and obesity (BMI ≥ 30) | 469 (Trial 1: 234 [no PAP], Trial 2: 235 [with PAP]) | Tirzepatide (maximum tolerated dose of 10 mg or 15 mg subcutaneously once weekly) vs. placebo for 52 weeks | Change in AHI, percent change in AHI, percent change in body weight, hypoxic burden, PROMIS-SRI and PROMIS-SD scores, hsCRP concentration, and SBP. | In Trial 1, tirzepatide reduced AHI by −25.3 events/h vs. −5.3 with placebo (difference −20.0, p < 0.001); body weight by −17.7% vs. −1.6%. In Trial 2, AHI was reduced by −29.3 vs. −5.5 (difference −23.8, p < 0.001); body weight by −19.6% vs. −2.3%. Significant improvements in hypoxic burden, PROMIS scores, hsCRP, and SBP |
| Jiang W. et al., 2023 [126] | Primary: To assess liraglutide’s effect on OSA severity in patients with T2DM. Secondary: To evaluate glycemic control, body weight, and safety. | Patients with T2DM and severe OSA | 60 | Liraglutide (1.8 mg/day) vs. control | AHI, HbA1c, body weight, adverse events | Liraglutide reduced AHI by 12.2 events/h (p < 0.001), improved HbA1c, and reduced body weight, with a tolerable safety profile. |
| Blackman A. et al., 2016 [112] | Primary: To evaluate liraglutide’s effect on OSA severity in obese individuals. Secondary: To assess changes in body weight and cardio-metabolic outcomes. | Individuals with obesity and with moderate-to-severe OSA | 359 | Liraglutide (3.0 mg/day) vs. placebo | AHI, body weight, HbA1c, blood pressure | Liraglutide reduced AHI by 12.2 events/h (p = 0.015), body weight by 5.7%, and improved cardiometabolic markers compared to the placebo. |
| O’Donnell C. et al., 2024 [127] | Primary: To compare CPAP and liraglutide on early CV disease markers in OSA. Secondary: To assess changes in AHI and metabolic parameters. | Adults with OSA and obesity | 30 | CPAP vs. liraglutide (3.0 mg/day) | Carotid intima-media thickness, AHI, HbA1c, body weight | CPAP improved cardiovascular markers (p = 0.02) more than liraglutide; however, liraglutide reduced AHI and body weight, with no significant cardiovascular benefit. |
| Sprung et al., 2020 [128] | Primary: To assess liraglutide with or without CPAP on OSA in patients with T2DM. Secondary: To evaluate glycemic control, body weight, and CV risk markers. | Type 2 diabetes patients with OSA | 72 | Liraglutide, CPAP, or both vs. placebo | AHI, HbA1c, body weight, cardiovascular risk markers | Study protocol: designed to assess the combined effects of liraglutide and CPAP; results not reported in this paper. |
| Gomez-Peralta F. et al., 2015 [129] | Primary: To investigate liraglutide’s effect on excessive daytime sleepiness in obese type 2 diabetes patients. Secondary: To assess glycemic control and body weight changes. | Obese patients with type 2 diabetes | 158 | Liraglutide (1.2–1.8 mg/day) | Epworth Sleepiness Scale (ESS), HbA1c, body weight | Liraglutide reduced ESS scores by 2.9 points (p < 0.001), improved HbA1c, and decreased body weight, suggesting benefits for daytime sleepiness. |
| Baser O. et al., 2024 [130] | Primary: To assess the association between AOMs and the incidence of OSA. Secondary: To compare OSA risk between tirzepatide and semaglutide users. | Patients with obesity (AOM cohort: tirzepatide or semaglutide users; non-AOM cohort: no AOM use) | 105,402 (AOM: 20,384; non-AOM: 85,018) | Tirzepatide or semaglutide vs. no AOM | Incidence of OSA, hazard ratio of OSA | The AOM cohort had a lower incidence of OSA (3.12%) compared to the non-AOM cohort (12.56%, p < 0.001); AOM use reduced the likelihood of OSA by 40% (HR = 0.60, p < 0.0001). Additionally, tirzepatide (2.65%) and semaglutide (3.18%) showed no significant difference (p = 0.1664). |
| Characteristic | GLP-1RAs | SGLT2 Inhibitors |
|---|---|---|
| Weight Loss Mechanism | Central appetite suppression | Peripheral caloric loss |
| Primary Site | CNS/GI tract | Kidney |
| Fluid Effects | Minimal | Diuretic |
| Respiratory Control | Direct CNS effects and indirect | Indirect via metabolic changes |
| Onset of Action | Rapid (days-weeks) | Gradual (weeks-months) |
| Dependency | Receptor-mediated | Non-receptor-mediated |
| Reference | Primary and Secondary Objectives | Population/Participants | Sample Size | Intervention/Exposure | Outcome Measures | Major Findings |
|---|---|---|---|---|---|---|
| Qiu M et al., 2021 [150] | Primary: To assess the association between SGLT2i and noninfectious respiratory disorders. Secondary: To evaluate specific respiratory outcomes. | Patients with T2DM from randomized trials | 42,151 | SGLT2 inhibitors vs. placebo or other therapies | Incidence of noninfectious respiratory disorders | SGLT2i were not associated with an increased risk of noninfectious respiratory disorders (RR, 0.95; 95% CI, 0.84–1.07), suggesting safety in this context. |
| Tang Y et al., 2019 [156] | Primary: To evaluate dapagliflozin’s effect on OSA in T2DM. Secondary: To assess changes in glycemic control and body weight. | Patients with T2DM and OSA | 24 | Dapagliflozin (10 mg/day) | AHI, HbA1c, body weight | Dapagliflozin reduced AHI (p = 0.03), improved glycemic control, and decreased body weight, suggesting potential benefits for OSA in individuals with type 2 diabetes. |
| Armentaro G et al., 2024 [157] | Primary: To assess SGLT2 inhibitors’ effect on OSA parameters in elderly patients. Secondary: To evaluate CV and metabolic outcomes. | Elderly patients with heart failure, T2DM, and OSA | 60 | SGLT2i | AHI, oxygen saturation, CV events | SGLT2i improved AHI and oxygen saturation (p < 0.05), with benefits in cardiovascular and metabolic parameters in elderly patients. |
| Mir T et al., 2021 [158] | Primary: To investigate the effect of SGLT2i on sleep apnea in T2DM. Secondary: To assess safety and metabolic outcomes. | Patients with T2DM and OSA from randomized trials | NA | SGLT2 inhibitors vs. control | AHI, AE, glycemic control | SGLT2i significantly reduced AHI (p < 0.05) and improved glycemic control, indicating a beneficial role in managing sleep apnea. |
| Kusunoki M et al., 2021 [119] | Primary: To assess SGLT2 inhibitors’ effect on CPAP initiation in patients with T2DM and OSA. Secondary: To evaluate glycemic control and body weight. | Patients with T2DM and OSA | 30 | SGLT2i | CPAP initiation rate, HbA1c, body weight | SGLT2i reduced the need for CPAP initiation (p < 0.05), with improvements in HbA1c and body weight, suggesting benefits in the management of OSA. |
| Neeland IJ et al., 2020 [113] | Primary: To evaluate empagliflozin’s effect on OSA in T2DM. Secondary: To assess CV and renal outcomes. | Patients with T2DM and CV disease | 7020 | Empagliflozin vs. placebo | OSA events, CV death, renal outcomes | Empagliflozin reduced OSA events (HR 0.76, 95% CI 0.59–0.98) and improved cardiovascular and renal outcomes, suggesting broader benefits. |
| Sawada K et al., 2018 [159] | Primary: To investigate the SGLT2i effect on OSA severity in T2DM. Secondary: To assess metabolic and anthropometric changes. | Type 2 diabetes patients with OSA | 24 | SGLT2 inhibitors | Apnea-hypopnea index (AHI), body mass index, HbA1c | SGLT2 inhibitors significantly reduced AHI (p = 0.02) and improved BMI and HbA1c, indicating potential therapeutic benefits for OSA. |
| Furukawa S et al., 2018 [160] | Primary: To assess dapagliflozin’s effect on sleep-disordered breathing in obese T2DM. Secondary: To evaluate body weight and glycemic control. | Japanese patients with obesity and T2DM | 30 | Dapagliflozin (5 mg/day) | Apnea-hypopnea index (AHI), body weight, HbA1c | Dapagliflozin reduced AHI (p < 0.05), body weight, and HbA1c, demonstrating its efficacy in improving sleep-disordered breathing. |
| Butt JH et al., 2024 [161] | Primary: To evaluate dapagliflozin’s effect on sleep apnea in heart failure and type 2 diabetes patients. Secondary: To assess CV outcomes. | Heart failure patients with or without T2DM | 11,007 | Dapagliflozin vs. placebo | Sleep apnea events, heart failure hospitalization, CV death | Dapagliflozin reduced sleep apnea events (HR 0.79, 95% CI 0.64–0.97) and improved heart failure and cardiovascular outcomes. |
| Molecular Marker | Clinical Relevance | Therapeutic Target | References |
|---|---|---|---|
| HIF-1α | Increase in OSA patients; correlates with insulin resistance; promotes inflammation. | HIF-1α stabilizers; circadian modulators | [45] |
| TNF-α | Elevated in OSA; correlates with CIH severity | Anti-TNF therapies; adipokine modulators | [177] |
| IL-6 | Acute phase reactant; hepatic glucose production | JAK inhibitors; IL-6 blockers | [178] |
| CRP | Correlates with OSA severity and diabetes risk | Anti-inflammatory agents | [179] |
| Leptin | Resistance in obesity; maintains inflammation despite metabolic dysfunction. | Leptin sensitizers; circadian modulators | [180,181,182] |
| Adiponectin | Reduced in both OSA and T2DM; protective against metabolic dysfunction | Adiponectin receptor agonists | [78] |
| Resistin | Elevated in metabolic dysfunction | Adipokine modulators | [67] |
| ROS/Antioxidants | Activates NF-κB; impairs insulin signaling | Antioxidant supplementation; SOD mimetics | [183,184,185] |
| miRNA-181a | Altered in OSA; links to insulin resistance | miRNA modulators | [186,187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elian, V.; Popovici, V.; Steriade, A.T.; Radulian, G.; Ozon, E.A.; Moroșan, E.; Musat, M. Molecular Biomarkers and Therapeutic Approach of Patients with Diabetes and Obstructive Sleep Apnea. Int. J. Mol. Sci. 2025, 26, 10234. https://doi.org/10.3390/ijms262010234
Elian V, Popovici V, Steriade AT, Radulian G, Ozon EA, Moroșan E, Musat M. Molecular Biomarkers and Therapeutic Approach of Patients with Diabetes and Obstructive Sleep Apnea. International Journal of Molecular Sciences. 2025; 26(20):10234. https://doi.org/10.3390/ijms262010234
Chicago/Turabian StyleElian, Viviana, Violeta Popovici, Alexandru Tudor Steriade, Gabriela Radulian, Emma Adriana Ozon, Elena Moroșan, and Madalina Musat. 2025. "Molecular Biomarkers and Therapeutic Approach of Patients with Diabetes and Obstructive Sleep Apnea" International Journal of Molecular Sciences 26, no. 20: 10234. https://doi.org/10.3390/ijms262010234
APA StyleElian, V., Popovici, V., Steriade, A. T., Radulian, G., Ozon, E. A., Moroșan, E., & Musat, M. (2025). Molecular Biomarkers and Therapeutic Approach of Patients with Diabetes and Obstructive Sleep Apnea. International Journal of Molecular Sciences, 26(20), 10234. https://doi.org/10.3390/ijms262010234







