Gamma-Aminobutyric Acid (GABA) as a Dietary Strategy for Enhancing Temperature Stress Resilience in Aquaculture Species
Abstract
1. Introduction
2. Physiological Adaptations of Fish to Temperature Stress
2.1. Nervous System-Mediated Endocrine Adaptation
| Species | Temperature | Duration | Tissues and Organs Assessed | Effects | Conclusion | References |
|---|---|---|---|---|---|---|
| Alosa sapidissima | 24 °C (Low), 27 °C (Mid), and 30 °C (High) | NS | Gills, liver | SOD  CAT  CORT  MDA  ALP  LDH  | Temperature stress altered the structural characteristics of the gills, induced increased activities of the antioxidant enzymes SOD and CAT and the secretion of cortisol to increase heat tolerance. | [43] |
| Dicentrarchus labrax | 8 °C and 32 °C | 30 days | Plasma, Blood, liver | TG  Lactate  Liver size  GLU  Erythrocyte ~ | Temperature fluctuation caused significant and continuing changes in hemato-biochemical indicators and erythrocyte peripheral and nuclear structure | [44] |
| Cyprinus carpio | 32 °C | 48 h | Liver, intestine, gill, Blood | SOD  CAT  GPx  NBT  LZM  MDA  | Exposure to thermal stress increased oxidative stress and diminished the immunity of common carp reared in hyper salinity conditions | [45] |
| Notothenia coriiceps and Notothenia rossii | 4 °C | 1–6 days | Gill, Liver, and Brain | GPx  SOD  MTLP  LPO  GSH  GCL  Erythrocytes  | Sensitivity to ocean warming by tissue oxidative damage associated with thermal stress | [46] |
| Oncorhynchus mykiss | 25 °C | NS | spleen, serum | MRB  SOD  MDA  hsp47  | Heat shock causes cell injury, induces oxidative damage and promotes SERPINH1 mRNA | [47] |
| Oncorhynchus mykiss | 25 °C | 12 h | gill, liver, spleen, heart, brain, and head kidney | CORT  hsp40  hsp90β  | Cells may be damaged under heat stress with increasing AKP activity and elevating CORT | [20] |
| Sander lucioperca | 28 °C, 32 °C, 34 °C and 36 °C | 2 h | blood, liver, brain, gills, heart, spleen, stomach, intestine, and muscle tissue | SOD  CAT  GPx  TBARS  H2O2  ALT  AST  LDL  HDL  TG  CHO  | Heat stress significantly affected the physiological and biochemical activities of pikeperch. Our results showed that heat stress elevated the levels of ROS, then caused antioxidant defenses and an immune response | [48] |
| Oncorhynchus mykiss | 25 °C | for 2, 4, 8, and 12 h | gill | hsp30  | hsp30 mRNA expression in rainbow trout was induced by heat stress and responded in a time- and tissue-specific manner. | [49] |
 Represent an increase in the corresponding parameters;
Represent an increase in the corresponding parameters;  Represents a decrease in the corresponding parameters.
Represents a decrease in the corresponding parameters. 2.2. Antioxidant Adaptation Mechanisms
2.3. Molecular and Metabolic Adaptations
2.4. Hematological and Biochemical Parameters Adaptations
2.5. Immune System Adaptation
2.6. Adaptive Shifts in Ionic Balance
2.7. Growth and Reproductive Adaptation
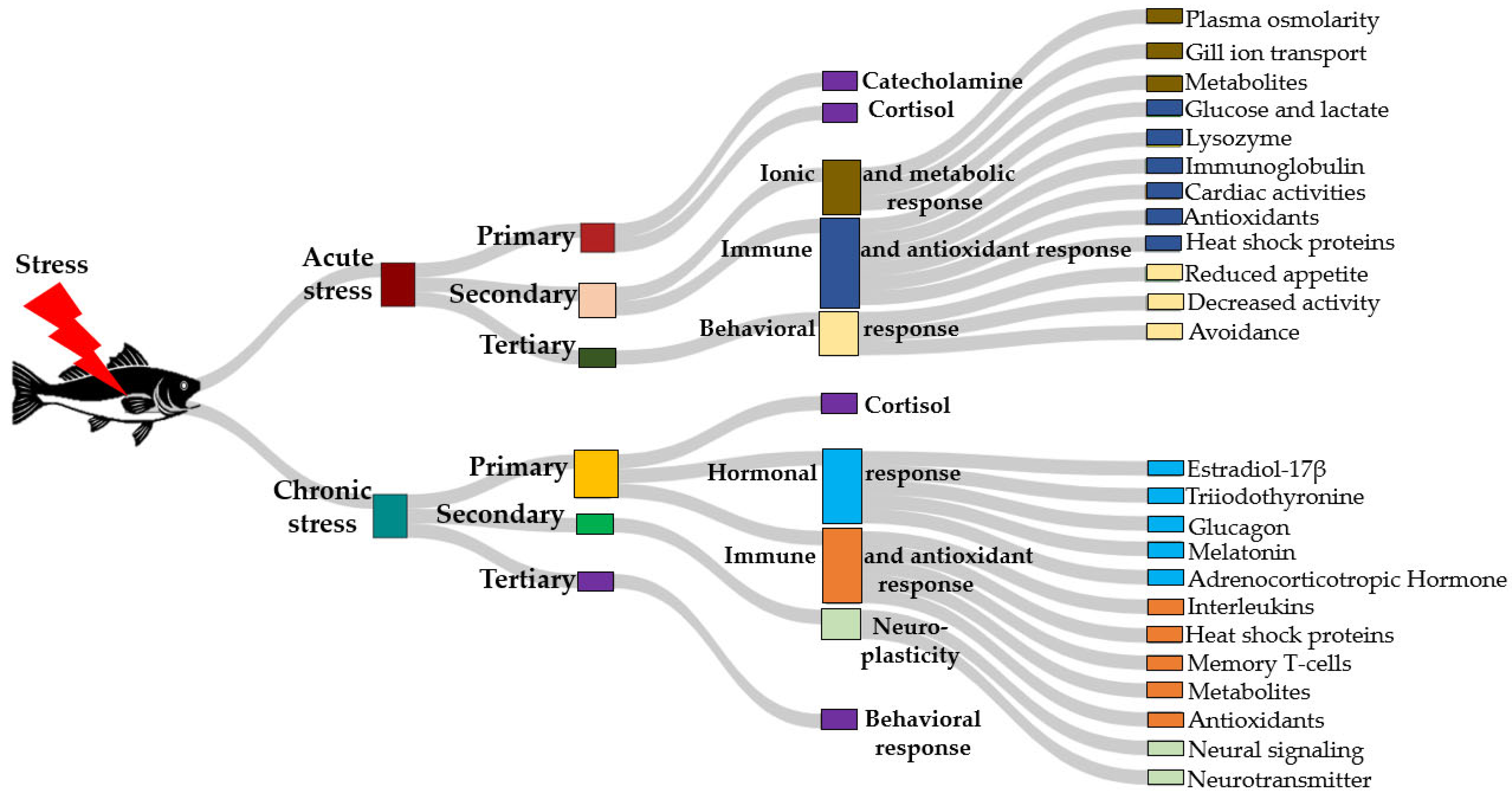
3. The Role of GABA in Stress Mitigation and Implications for Temperature Stress
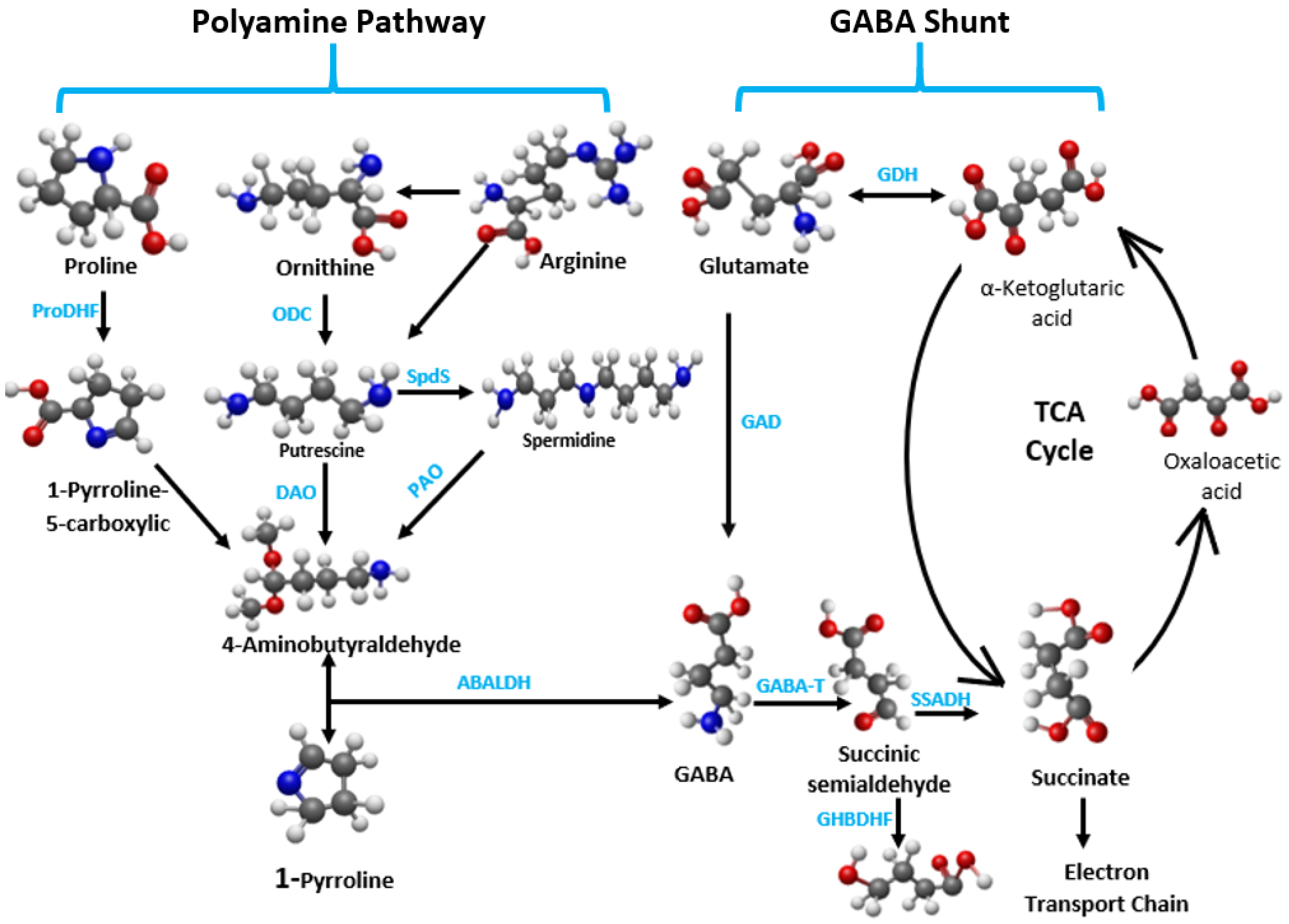
3.1. GABA’s Role in Stress Mitigation
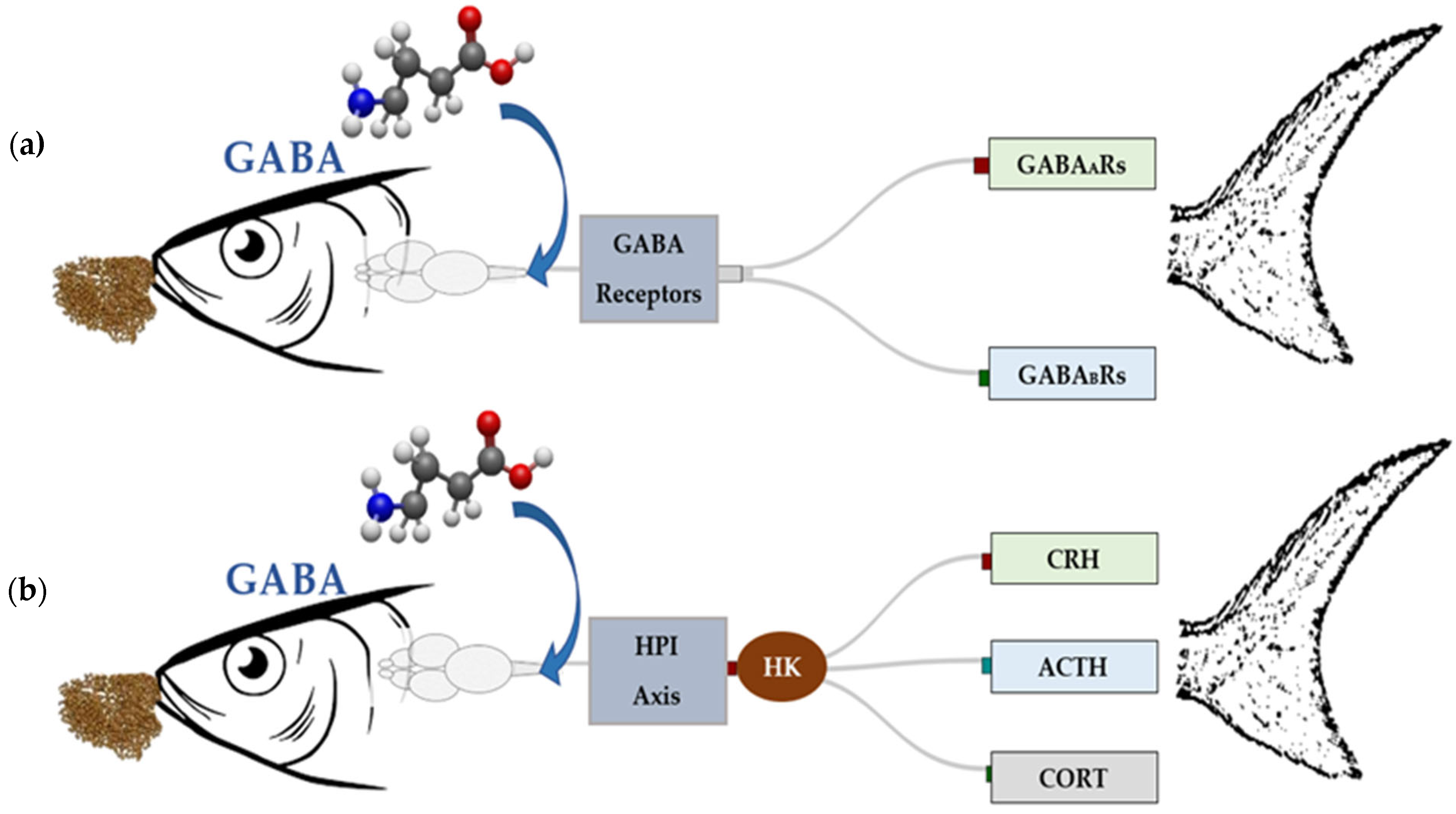
3.2. GABA-Mediated Signaling Pathways in Temperature Stress Regulation

3.3. GABA’s Role in Non-Temperature Stress Conditions and Its Potentials for Temperature Stress Mitigation in Aquaculture
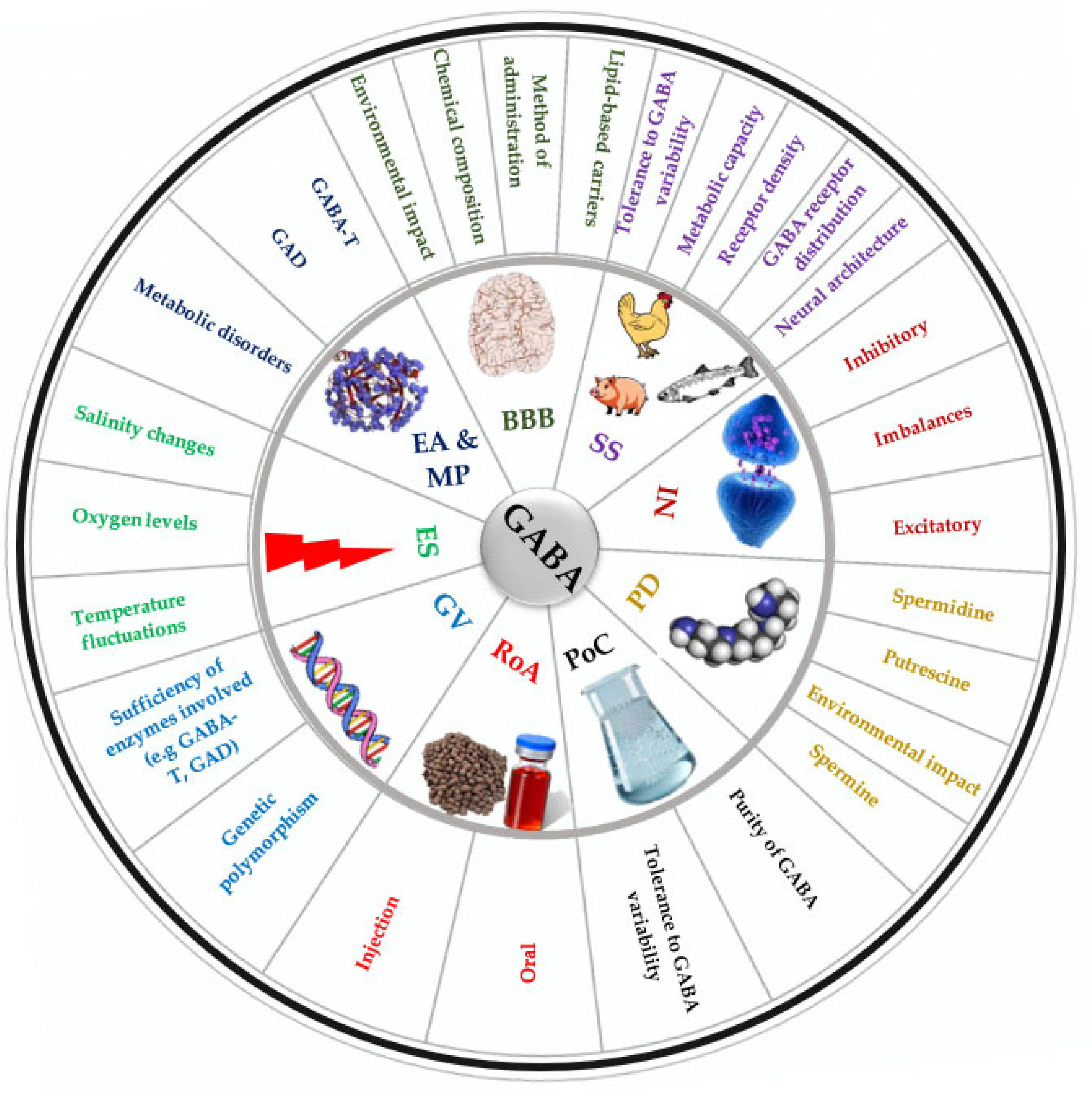
4. Factors Influencing GABA-Mediated Stress Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| ACP | Acid phosphatase |
| ACTH | Adrenocorticotropic hormone |
| ADC | Arginine decarboxylase |
| AKP | Alkaline phosphatase |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AREs | Antioxidant response elements |
| AST | Aspartate transaminase |
| BBB | Blood–Brain Barrier |
| cAMP | cyclic adenosine monophosphate |
| CAT | Catalase |
| CHO | Cholesterol |
| CNS | Central nervous system |
| CORT | Cortisol |
| CRH | Corticotropin-releasing hormone |
| DA | Dopamine |
| DAO | Diamine oxidase |
| E2 | Estradiol-17β |
| EA | Enzyme Activity |
| Ebs | Erythroblasts |
| ECA | Erythrocytic cellular abnormalities |
| ENA | Erythrocytic nuclear abnormalities |
| ER | Endoplasmic reticulum |
| ES | Environmental Stress |
| GABA | Gamma-aminobutyric acid |
| GAD | Glutamate decarboxylase |
| GCL | Glutamate cysteine ligase |
| GDP | Guanosine diphosphate |
| GH1 | Growth hormone 1 |
| GIRK | G-protein–gated inwardly rectifying potassium |
| GLU | Glucose |
| GPx | Glutathione peroxidase |
| GR | Glucocorticoid receptor |
| GSH | Glutathione |
| GST | Glutathione-S-transferase |
| GV | Genetic Variation |
| GTP | Guanosine triphosphate |
| HDL | High density lipoprotein |
| HSF1 | Heat shock factor 1 |
| HPA | Hypothalamus–pituitary–adrenal |
| HPI | Hypothalamus–pituitary–interrenal |
| hsp | Heat shock protein |
| IRF | Interferon regulatory factors |
| LDH | Lactate dehydrogenase |
| LDL | Low density lipoprotein |
| LNCs | Lipid-core nanocapsules |
| LPO | Lipid peroxidation |
| LSZ | Serum lysozyme |
| LZM | Lysozyme |
| MDA | Malondialdehyde |
| MDH | Malate dehydrogenase |
| MP | Metabolic Pathway |
| MRB | Macrophage respiratory burst |
| MTLP | Metallothionein-like proteins |
| NBT | Nitroblue tetrazolium |
| NE | Noradrenaline |
| NI | Neurotransmitter Interaction |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| ODC | Ornithine decarboxylase |
| PAO | Polyamine oxidase |
| PD | Polyamine degradation |
| PI3K | Phosphatidylinositol 3-kinase |
| PKA | Protein kinase A |
| PoC | Purity of Chemical |
| RoA | Route of Administration |
| ROS | Reactive oxygen species |
| RS & M | Receptor Sensitivity and Mechanisms |
| SAM | Sympathetic–adrenal–medullary |
| SOD | Superoxide dismutase |
| SS | Species Specificity |
| TBARS | Thiobarbituric acid |
| TG | Triglycerides |
| TGF-β1 | Transforming growth factor |
| TNF-α | Tumor necrosis factor-alpha |
| VGCCs | Voltage-gated calcium channels |
| WBC | White blood cell |
References
- Maulu, S.; Hasimuna, O.J.; Haambiya, L.H.; Monde, C.; Musuka, C.G.; Makorwa, T.H.; Munganga, B.P.; Phiri, K.J.; Nsekanabo, J.D. Climate change effects on aquaculture production: Sustainability implications, mitigation, and adaptations. Front. Sustain. Food Syst. 2021, 5, 609097. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Wang, S.; Li, E.; Luo, Z.; Li, X.; Liu, Z.; Li, W.; Wang, X.; Qin, J.G.; Chen, L. Dietary yeast culture can protect against chronic heat stress by improving the survival, antioxidant capacity, immune response, and gut health of juvenile Chinese mitten crab (Eriocheir sinensis). Aquaculture 2025, 596, 741910. [Google Scholar] [CrossRef]
- Huang, Z.H.; Ma, A.J.; Wang, X.A. The immune response of turbot, Scophthalmus maximus (L.), skin to high water temperature. J. Fish Dis. 2011, 34, 619–627. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; King, H.R. Temperature and salmonid reproduction: Implications for aquaculture. J. Fish Biol. 2010, 76, 69–85. [Google Scholar] [CrossRef]
- Agarwal, D.; Shanmugam, S.A.; Kathirvelpandian, A.; Eswaran, S.; Rather, M.A.; Rakkannan, G. Unraveling the impact of climate change on fish physiology: A focus on temperature and salinity dynamics. J. Appl. Ichthyol. 2024, 2024, 5782274. [Google Scholar] [CrossRef]
- Korean National Institute of Fisheries Science (NIFS). Annual Report on Fisheries Research and Development; Korean National Institute of Fisheries Science: Busan, Republic of Korea, 2023.
- Choi, C.Y. Environmental stress-related gene expression and blood physiological responses in olive flounder (Paralichthys olivaceus) exposed to osmotic and thermal stress. Anim. Cells Syst. 2010, 14, 17–23. [Google Scholar] [CrossRef]
- Han, Y.; Koshio, S.; Jiang, Z.; Ren, T.; Ishikawa, M.; Yokoyama, S.; Gao, J. Interactive effects of dietary taurine and glutamine on growth performance, blood parameters, and oxidative status of Japanese flounder Paralichthys olivaceus. Aquaculture 2014, 434, 348–354. [Google Scholar] [CrossRef]
- Abisha, R.; Krishnani, K.K.; Sukhdhane, K.; Verma, A.K.; Brahmane, M.; Chadha, N.K. Sustainable development of climate-resilient aquaculture and culture-based fisheries through adaptation of abiotic stresses: A review. J. Water Clim. Change 2022, 13, 2671–2689. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aquac. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Bae, J.; Hamidoghli, A.; Farris, N.W.; Olowe, O.S.; Choi, W.; Lee, S.; Won, S.; Ohh, M.; Lee, S.H.; Bai, S.C. Dietary γ-aminobutyric acid (GABA) promotes growth and resistance to Vibrio alginolyticus in whiteleg shrimp Litopenaeus vannamei. Aquac. Nutr. 2022, 1, 9105068. [Google Scholar] [CrossRef]
- Farris, N.W.; Hamidoghli, A.; Bae, J.; Won, S.; Choi, W.; Biró, J.; Lee, S.H.; Bai, S.C. Dietary supplementation with γ-aminobutyric acid improves growth, digestive enzyme activity, non-specific immunity and disease resistance against Streptococcus iniae in juvenile olive flounder, Paralichthys olivaceus. Animals 2022, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.W.; Li, Y.T.; Zhou, W.W.; Tian, L.X.; Li, Y.M.; Zeng, S.L.; Liu, Y.J. Effect of γ-aminobutyric acid supplementation on growth performance, endocrine hormone and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei, fed low fishmeal diet. Aquac. Nutr. 2017, 23, 54–62. [Google Scholar] [CrossRef]
- Ruenkoed, S.; Nontasan, S.; Phudkliang, J.; Phudinsai, P.; Pongtanalert, P.; Panprommin, D.; Mongkolwit, K.; Wangkahart, E. Effect of dietary gamma aminobutyric acid (GABA) modulated the growth performance, immune and antioxidant capacity, digestive enzymes, intestinal histology and gene expression of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2023, 141, 109056. [Google Scholar] [CrossRef]
- Ogun, A.O.; Kim, H.; Yoon, S.; Lee, S.; Jeon, H.; Aulia, D.; Hur, J.; Lee, S. Effects of Dietary Gamma-Aminobutyric Acid (GABA) Inclusion on Acute Temperature Stress Responses in Juvenile Olive Flounder (Paralichthys olivaceus). Animals 2025, 15, 809. [Google Scholar] [CrossRef]
- Haque, R.; Singha, K.P.; Karmakar, S. Environmental Stressors on Fish and It’s Adaptation Physiology; ICAR: Mumbai, India, 2019; p. 400061. [Google Scholar]
- Faught, E.; Schaaf, M.J. Molecular mechanisms of the stress-induced regulation of the inflammatory response in fish. Gen. Comp. Endocrinol. 2024, 345, 114387. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.I.; Jalil, S.U.; Ansari, S.A.; Hasanuzzaman, M. GABA shunt: A key-player in mitigation of ROS during stress. Plant Growth Regul. 2021, 94, 131–149. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Wang, Y.N.; Kang, Y.J.; Wang, J.F.; Shi, H.N.; Huang, J.Q.; Jiang, L. Effects of heat stress on serum cortisol, alkaline phosphatase activity and heat shock protein 40 and 90β mRNA expression in rainbow trout Oncorhynchus mykiss. Biologia 2016, 71, 109–115. [Google Scholar] [CrossRef]
- Zhang, C.; He, J.; Wang, X.; Su, R.; Huang, Q.; Qiao, F.; Qin, C.; Qin, J.; Chen, L. Dietary gamma-aminobutyric acid (GABA) improves non-specific immunity and alleviates lipopolysaccharide (LPS)-induced immune overresponse in juvenile Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2022, 124, 480–489. [Google Scholar] [CrossRef]
- Bae, J.; Moniruzzaman, M.; Je, H.W.; Lee, S.; Choi, W.; Min, T.; Kim, K.W.; Bai, S.C. Evaluation of gamma-aminobutyric acid (GABA) as a functional feed ingredient on growth performance, immune enhancement, and disease resistance in olive flounder (Paralichthys olivaceus) under high stocking density. Antioxidants 2024, 13, 647. [Google Scholar] [CrossRef]
- Braithwaite, V.A.; Ebbesson, L.O. Pain and stress responses in farmed fish. Rev. Sci. Tech. 2014, 33, 245–253. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Donaldson, M.R.; Cooke, S.J.; Patterson, D.A.; Macdonald, J.S. Cold shock and fish. J. Fish Biol. 2008, 73, 1491–1530. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef]
- Yada, T.; Tort, L. Stress and disease resistance: Immune system and immunoendocrine interactions. Fish Physiol. 2016, 35, 365–403. [Google Scholar]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J. World Aquacult. Soc. 2022, 53, 314–366. [Google Scholar] [CrossRef]
- Mateus, A.P.; Costa, R.; Gisbert, E.; Pinto, P.I.S.; Andree, K.B.; Estévez, A.; Power, D.M. Thermal imprinting modifies bone homeostasis in cold-challenged sea bream (Sparus aurata L.). J. Exp. Biol. 2017, 220, 3442–3454. [Google Scholar] [CrossRef] [PubMed]
- Amiel, J.J.; Bao, S.; Shine, R. The effects of incubation temperature on the development of the cortical forebrain in a lizard. Anim. Cogn. 2017, 20, 117–125. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, S. The neurobiology of climate change. Sci. Nat. 2018, 105, 11. [Google Scholar] [CrossRef]
- Bonga, W.; Sjoerd, E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Weyts, F.A.A.; Cohen, N.; Flik, G.; Verburg-van Kemenade, B.M.L. Interactions between the immune system and the hypothalamo-pituitary-interrenal axis in fish. Fish Shellfish Immunol. 1999, 9, 1–20. [Google Scholar] [CrossRef]
- Pérez-Casanova, J.C.; Afonso, L.O.B.; Johnson, S.C.; Currie, S.; Gamperl, A.K. The stress and metabolic responses of juvenile Atlantic cod Gadus morhua L. to an acute thermal challenge. J. Fish Biol. 2008, 72, 899–916. [Google Scholar] [CrossRef]
- Goikoetxea, A.; Sadoul, B.; Blondeau-Bidet, E.; Aerts, J.; Blanc, M.O.; Parrinello, H.; Barrachina, C.; Pratlong, M.; Geffroy, B. Genetic pathways underpinning hormonal stress responses in fish exposed to short- and long-term warm ocean temperatures. Ecol. Indic. 2021, 120, 106937. [Google Scholar] [CrossRef]
- Panase, P.; Saenphet, S.; Saenphet, K. Biochemical and physiological responses of Nile tilapia Oreochromis niloticus Lin subjected to cold shock of water temperature. Aquac. Rep. 2018, 11, 17–23. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, D.; Zhao, C.; Xiao, Z.; Xu, S.; Xiao, Y.; Wang, Y.; Liu, Q.; Li, J. The expression pattern of hsp70 plays a critical role in thermal tolerance of marine demersal fish: Multilevel responses of Paralichthys olivaceus and its hybrids (P. olivaceus♀ × P. dentatus♂) to chronic and acute heat stress. Mar. Environ. Res. 2017, 129, 386–395. [Google Scholar] [CrossRef]
- Vornanen, M. The temperature dependence of electrical excitability in fish hearts. J. Exp. Biol. 2016, 219, 1941–1952. [Google Scholar] [CrossRef]
- Anttila, K.; Couturier, C.S.; Øverli, Ø.; Johnsen, A.; Marthinsen, G.; Nilsson, G.E.; Farrell, A.P. Atlantic salmon show capability for cardiac acclimation to warm temperatures. Nat. Commun. 2014, 5, 4252. [Google Scholar] [CrossRef]
- Gamperl, A.K.; Ajiboye, O.O.; Zanuzzo, F.S.; Sandrelli, R.M.; Peroni, E.D.F.C.; Beemelmanns, A. The impacts of increasing temperature and moderate hypoxia on the production characteristics, cardiac morphology and haematology of Atlantic salmon (Salmo salar). Aquaculture 2020, 519, 734874. [Google Scholar] [CrossRef]
- Skeeles, M.R.; Winkler, A.C.; Duncan, M.I.; James, N.C.; van der Walt, K.A.; Potts, W.M. The use of internal heart rate loggers in determining cardiac breakpoints of fish. J. Therm. Biol. 2020, 89, 102524. [Google Scholar] [CrossRef] [PubMed]
- Haverinen, J.; Vornanen, M. Depression of heart rate in fish at critically high temperatures is due to atrioventricular block. bioRxiv 2020. [Google Scholar] [CrossRef]
- Luo, M.; Zhu, W.; Liang, Z.; Feng, B.; Xie, X.; Li, Y.; Liu, Y.; Shi, X.; Fu, J.; Miao, L.; et al. High-temperature stress response: Insights into the molecular regulation of American shad (Alosa sapidissima) using a multi-omics approach. Sci. Total Environ. 2024, 916, 170329. [Google Scholar] [CrossRef]
- Islam, M.J.; Slater, M.J.; Bögner, M.; Zeytin, S.; Kunzmann, A. Extreme ambient temperature effects in European seabass, Dicentrarchus labrax: Growth performance and hemato-biochemical parameters. Aquaculture 2020, 522, 735093. [Google Scholar] [CrossRef]
- Dawood, M.A.; Alkafafy, M.; Sewilam, H. The antioxidant responses of gills, intestines and livers and blood immunity of common carp (Cyprinus carpio) exposed to salinity and temperature stressors. Fish Physiol. Biochem. 2022, 48, 397–408. [Google Scholar] [CrossRef]
- Klein, R.D.; Borges, V.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the Antarctic fish Notothenia coriiceps and Notothenia rossii. J. Therm. Biol. 2017, 68, 110–118. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Li, Z.; Shi, H.; Kang, Y.; Wang, J.; Huang, J.; Jiang, L. Effects of heat stress on respiratory burst, oxidative damage and SERPINH1 (HSP47) mRNA expression in rainbow trout Oncorhynchus mykiss. Fish Physiol. Biochem. 2016, 42, 701–710. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Wang, G.; Chen, Y.; Guo, J.; Pan, C.; Liu, E.; Ling, Q. Physicochemical changes in liver and Hsc70 expression in pikeperch Sander lucioperca under heat stress. Ecotoxicol. Environ. Saf. 2019, 181, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, H.; Liu, Z.; Wang, J.; Huang, J. Effect of heat stress on heat shock protein 30 (Hsp30) mRNA expression in rainbow trout (Oncorhynchus mykiss). Turk. J. Fish. Aquat. Sci. 2019, 19, 681–688. [Google Scholar]
- Kim, J.H.; Kim, S.K.; Hur, Y.B. Temperature-mediated changes in stress responses, acetylcholinesterase, and immune responses of juvenile olive flounder Paralichthys olivaceus in a bio-floc environment. Aquaculture 2019, 506, 453–458. [Google Scholar] [CrossRef]
- Giroux, M.; Gan, J.; Schlenk, D. The effects of bifenthrin and temperature on the endocrinology of juvenile Chinook salmon. Environ. Toxicol. Chem. 2019, 38, 852–861. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The concept of stress in fish. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 1–34. [Google Scholar]
- Shastak, Y.; Pelletier, W. Captivating colors, crucial roles: Astaxanthin’s antioxidant impact on fish oxidative stress and reproductive performance. Animals 2023, 13, 3357. [Google Scholar] [CrossRef]
- Fadhlaoui, M.; Couture, P. Combined effects of temperature and metal exposure on the fatty acid composition of cell membranes, antioxidant enzyme activities and lipid peroxidation in yellow perch (Perca flavescens). Aquat. Toxicol. 2016, 180, 45–55. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Oxidative stress in fish: A review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, Z.; Bai, Y.; Xu, S. Redox state and metabolic responses to severe heat stress in lenok Brachymystax lenok (Salmonidae). Front. Mol. Biosci. 2023, 10, 1156310. [Google Scholar] [CrossRef]
- Clotfelter, E.D.; Lapidus, S.J.H.; Brown, A.C. The effects of temperature and dissolved oxygen on antioxidant defences and oxidative damage in the fathead minnow Pimephales promelas. J. Fish Biol. 2013, 82, 1086–1092. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Extreme winter cold-induced osmoregulatory, metabolic, and physiological responses in European seabass (Dicentrarchus labrax) acclimatized at different salinities. Sci. Total Environ. 2021, 771, 145202. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Henjes, J.; Slater, M.J. Can dietary manipulation mitigate extreme warm stress in fish? The case of European seabass, Dicentrarchus labrax. Aquaculture 2021, 545, 737153. [Google Scholar] [CrossRef]
- Almeida, J.R.; Gravato, C.; Guilhermino, L. Effects of temperature in juvenile seabass (Dicentrarchus labrax L.) biomarker responses and behaviour: Implications for environmental monitoring. Estuaries Coasts 2015, 38, 45–55. [Google Scholar] [CrossRef]
- Zannat, M.M.; Hossain, F.; Rahman, U.O.; Rohani, M.F.; Shahjahan, M. An overview of climate-driven stress responses in striped catfish (Pangasianodon hypophthalmus)—Prospects in aquaculture. Asian J. Med. Biol. Res. 2023, 9, 70–88. [Google Scholar] [CrossRef]
- Nakano, K.; Iwama, G.K. The 70-kDa heat shock protein response in two intertidal sculpins, Oligocottus maculosus and O. snyderi: Relationship of hsp70 and thermal tolerance. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Werner, I.; Viant, M.R.; Rosenblum, E.S.; Gantner, A.S.; Tjeerdema, R.S.; Johnson, M.L. Cellular responses to temperature stress in steelhead trout (Oncorhynchus mykiss) parr with different rearing histories. Fish Physiol. Biochem. 2006, 32, 261–273. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.S. A review on the involvement of heat shock proteins (extrinsic chaperones) in response to stress conditions in aquatic organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Barbosa, V.; Alves, R.; Custódio, A.; Anacleto, P.; Repolho, T.; Ferreira, P.P.; Rosa, R.; Marques, A.; Diniz, M. Ecophysiological responses of juvenile seabass (Dicentrarchus labrax) exposed to increased temperature and dietary methylmercury. Sci. Total Environ. 2017, 586, 551–558. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, S.K.; Priyam, M.; Siddik, M.A.; Kumar, N.; Mishra, P.K.; Gupta, K.K.; Sarkar, B.; Sharma, T.R.; Pattanayak, A. Immunomodulation by dietary supplements: A preventive health strategy for sustainable aquaculture of tropical freshwater fish, Labeo rohita (Hamilton, 1822). Rev. Aquac. 2021, 13, 2364–2394. [Google Scholar] [CrossRef]
- Mofizur Rahman, M.; Baek, H.J. Evaluation of erythrocyte morphometric indices in juvenile red-spotted grouper, Epinephelus akaara under elevated water temperature. Dev. Reprod. 2019, 23, 345–353. [Google Scholar] [CrossRef]
- Ashaf-Ud-Doulah, M.; Shahjahan, M.; Islam, S.M.M.; Al-Emran, M.; Rahman, M.S.; Hossain, M.A.R. Thermal stress causes nuclear and cellular abnormalities of peripheral erythrocytes in Indian major carp, rohu Labeo rohita. J. Therm. Biol. 2019, 86, 102450. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, M.; Kitahashi, T.; Ando, H. Temperature affects sexual maturation through the control of kisspeptin, kisspeptin receptor, GnRH, and GTH subunit gene expression in the grass puffer during the spawning season. Gen. Comp. Endocrinol. 2017, 243, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, M.; Uddin, M.H.; Bain, V.; Haque, M.M. Increased water temperature altered hemato-biochemical parameters and structure of peripheral erythrocytes in striped catfish Pangasianodon hypophthalmus. Fish Physiol. Biochem. 2018, 44, 1309–1318. [Google Scholar] [CrossRef]
- Ariful, I.; Uddin, M.H.; Uddin, M.J.; Shahjahan, M. Temperature changes influenced the growth performance and physiological functions of Thai pangas Pangasianodon hypophthalmus. Aquac. Rep. 2019, 13, 100179. [Google Scholar] [CrossRef]
- Roychowdhury, P.; Aftabuddin, M.; Pati, M.K. Thermal stress altered growth performance and metabolism and induced anemia and liver disorder in Labeo rohita. Aquac. Res. 2020, 51, 1406–1414. [Google Scholar] [CrossRef]
- Dalvi, R.S.; Das, T.; Debnath, D.; Yengkokpam, S.; Baruah, K.; Tiwari, L.R.; Pal, A.K. Metabolic and cellular stress responses of catfish, Horabagrus brachysoma (Günther) acclimated to increasing temperatures. J. Therm. Biol. 2017, 65, 32–40. [Google Scholar] [CrossRef]
- Makrinos, D.L.; Bowden, T.J. Natural environmental impacts on teleost immune function. Fish Shellfish Immunol. 2016, 53, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.J. Modulation of the immune system of fish by their environment. Fish Shellfish Immunol. 2008, 25, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Pascoli, F.; Lanzano, G.S.; Negrato, E.; Poltronieri, C.; Trocino, A.; Radaelli, G.; Bertotto, D. Seasonal effects on hematological and innate immune parameters in sea bass Dicentrarchus labrax. Fish Shellfish Immunol. 2011, 31, 1081–1087. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defense molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Mashoof, S.; Criscitiello, M.F. Fish immunoglobulins. Biology 2016, 5, 45. [Google Scholar] [CrossRef]
- Álvarez, C.A.; Guzmán, F.; Cárdenas, C.; Marshall, S.H.; Mercado, L. Antimicrobial activity of trout hepcidin. Fish Shellfish Immunol. 2014, 41, 93–101. [Google Scholar] [CrossRef]
- Chen, S.; Ma, X.; Wu, D.; Yang, D.; Zhang, Y.; Liu, Q. Scophthalmus maximus interleukin-1β limits Edwardsiella piscicida colonization in vivo. Fish Shellfish Immunol. 2019, 95, 277–286. [Google Scholar] [CrossRef]
- Guo, M.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. The effects of IL-1β, IL-8, G-CSF, and TNF-α as molecular adjuvants on the immune response to an E. tarda subunit vaccine in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2018, 77, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.; Takemura, A.; Tsuchiya, M. Effects of changes in environmental factors on the non-specific immune response of Nile tilapia, Oreochromis niloticus L. Aquac. Res. 2005, 36, 391–397. [Google Scholar]
- Sun, J.L.; Zhao, L.L.; Liao, L.; Tang, X.H.; Cui, C.; Liu, Q.; He, K.; Ma, J.-D.; Jin, L.; Yan, T.; et al. Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity, and promotion of cell apoptosis. Fish Shellfish Immunol. 2020, 98, 923–936. [Google Scholar] [CrossRef]
- Imsland, A.K.; Gunnarsson, S.; Foss, A.; Stefansson, S.O. Gill Na+, K+-ATPase activity, plasma chloride and osmolality in juvenile turbot (Scophthalmus maximus) reared at different temperatures and salinities. Aquaculture 2003, 218, 671–683. [Google Scholar] [CrossRef]
- Gradil, A.M.; Wright, G.M.; Speare, D.J.; Wadowska, D.W.; Purcell, S.; Fast, M.D. The effects of temperature and body size on immunological development and responsiveness in juvenile shortnose sturgeon (Acipenser brevirostrum). Fish Shellfish Immunol. 2014, 40, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Chen, Y.C.; Chang, C.H.; Tsai, S.C.; Lee, T.H. Seawater-acclimation abates cold effects on Na+, K+-ATPase activity in gills of the juvenile milkfish, Chanos chanos. Aquaculture 2015, 446, 67–73. [Google Scholar] [CrossRef]
- Strydom, N.A.; Whitfield, A.K.; Paterson, A.W. Influence of altered freshwater flow regimes on abundance of larval and juvenile Gilchristella aestuaria (Pisces: Clupeidae) in the upper reaches of two South African estuaries. Mar. Freshw. Res. 2002, 53, 431. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; McCormick, S.D. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon (Salmo salar) smolts in freshwater and seawater. J. Fish Biol. 2018, 93, 550–559. [Google Scholar] [CrossRef]
- Christensen, E.A.F.; Svendsen, M.B.S.; Steffensen, J.F. Plasma osmolality and oxygen consumption of perch (Perca fluviatilis) in response to different salinities and temperatures. J. Fish Biol. 2017, 90, 819–833. [Google Scholar] [CrossRef]
- Evans, T.G.; Kültz, D. The cellular stress response in fish exposed to salinity fluctuations. J. Exp. Zool. A Ecol. Integr. Physiol. 2020, 333, 421–435. [Google Scholar] [CrossRef]
- Gonzalez, R.J.; McDonald, D.G. Ionoregulatory responses to temperature change in two species of freshwater fish. Fish Physiol. Biochem. 2000, 22, 311–317. [Google Scholar] [CrossRef]
- Lorin-Nebel, C.; Boulo, V.; Bodinier, C.; Charmantier, G. The Na+/K+/2Cl− cotransporter in the sea bass Dicentrarchus labrax during ontogeny: Involvement in osmoregulation. J. Exp. Biol. 2006, 209, 4908–4922. [Google Scholar] [CrossRef]
- Stewart, H.A.; Aboagye, D.L.; Ramee, S.W.; Allen, P.J. Effects of acute thermal stress on acid–base regulation, haematology, ion-osmoregulation and aerobic metabolism in channel catfish (Ictalurus punctatus). Aquac. Res. 2019, 50, 2133–2141. [Google Scholar] [CrossRef]
- Chadwick, J.G.; McCormick, S.D. Upper thermal limits of growth in brook trout and their relationship to stress physiology. J. Exp. Biol. 2017, 220, 3976–3987. [Google Scholar] [CrossRef] [PubMed]
- Giffard-Mena, I.; Lorin-Nebel, C.; Charmantier, G.; Castille, R.; Boulo, V. Adaptation of the sea-bass (Dicentrarchus labrax) to fresh water: Role of aquaporins and Na+/K+-ATPases. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 150, 332–338. [Google Scholar] [CrossRef] [PubMed]
- L’Honoré, T.; Farcy, E.; Blondeau-Bidet, E.; Lorin-Nebel, C. Inter-individual variability in freshwater tolerance is related to transcript level differences in gill and posterior kidney of European sea bass. Gene 2020, 741, 144547. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Bögner, M.; Meyer, A.; Thiele, R.; Slater, M.J. Metabolic and molecular stress responses of European seabass, Dicentrarchus labrax at low and high temperature extremes. Ecol. Indic. 2020, 112, 106118. [Google Scholar] [CrossRef]
- Hu, Y.C.; Chu, K.F.; Hwang, L.Y.; Lee, T.H. Cortisol regulation of Na+, K+-ATPase β1 subunit transcription via the pre-receptor 11β-hydroxysteroid dehydrogenase 1-like (11β-Hsd1L) in gills of hypothermal freshwater milkfish, Chanos chanos. J. Steroid Biochem. Mol. Biol. 2019, 192, 105381. [Google Scholar] [CrossRef]
- Foss, A.; Imsland, A.K.D.; van de Vis, H.; Abbink, W.; Lambooij, B.; Roth, B. Physiological response of temperature shocks in turbot and sole. J. Appl. Aquac. 2019, 31, 34–47. [Google Scholar] [CrossRef]
- Bernard, B.; Mandiki, S.N.M.; Duchatel, V.; Rollin, X.; Kestemont, P. A temperature shift on the migratory route similarly impairs hypo-osmoregulatory capacities in two strains of Atlantic salmon (Salmo salar L.) smolts. Fish Physiol. Biochem. 2019, 45, 1245–1260. [Google Scholar] [CrossRef]
- Fu, K.K.; Fu, C.; Qin, Y.L.; Bai, Y.; Fu, S.J. The thermal acclimation rate varied among physiological functions and temperature regimes in a common cyprinid fish. Aquaculture 2018, 495, 393–401. [Google Scholar] [CrossRef]
- Person-Le Ruyet, J.; Mahe, K.; Le Bayon, N.; Le Delliou, H. Effects of temperature on growth and metabolism in a Mediterranean population of European sea bass, Dicentrarchus labrax. Aquaculture 2004, 237, 269–280. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Geffroy, B.; Wedekind, C. Effects of global warming on sex ratios in fishes. J. Fish Biol. 2020, 97, 596–606, Erratum in J. Fish Biol. 2021, 98, 1495. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, M.; Clota, F.; Sadoul, B.; Blanc, M.; Blondeau-Bidet, E.; Bégout, M.; Cousin, X.; Geffroy, B. Low temperature has opposite effects on sex determination in a marine fish at the larval/postlarval and juvenile stages. Ecol. Evol. 2020, 10, 13825–13835. [Google Scholar] [CrossRef] [PubMed]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Rapid metabolic adaptation in European sea bass (Dicentrarchus labrax) juveniles fed different carbohydrate sources after heat shock stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 145, 73–81. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Berdal, B.; Blust, R.; Brix, O.; Colosimo, A.; De Wachter, B.; Giuliani, A.; Johansen, T.; Fischer, T.; Knust, R.; et al. Climate-induced temperature effects on growth performance, fecundity and recruitment in marine fish: Developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus). Cont. Shelf Res. 2001, 21, 1975–1997. [Google Scholar] [CrossRef]
- Angilletta, M.J.; Huey, R.B.; Frazier, M.R. Thermodynamic effects on organismal performance: Is hotter better? Physiol. Biochem. Zool. 2010, 83, 197–206. [Google Scholar] [CrossRef]
- Neuheimer, A.B.; Thresher, R.E.; Lyle, J.M.; Semmens, J.M. Tolerance limit for fish growth exceeded by warming waters. Nat. Clim. Change 2011, 1, 110–113. [Google Scholar] [CrossRef]
- Wade, N.M.; Clark, T.D.; Maynard, B.T.; Atherton, S.; Wilkinson, R.J.; Smullen, R.P.; Taylor, R.S. Effects of an unprecedented summer heatwave on the growth performance, flesh colour and plasma biochemistry of marine cage-farmed Atlantic salmon (Salmo salar). J. Therm. Biol. 2019, 80, 64–74. [Google Scholar] [CrossRef]
- Seebacher, F.; Post, E. Climate change impacts on animal migration. Clim. Change Responses 2015, 2, 5. [Google Scholar] [CrossRef]
- Servili, A.; Canario, A.V.M.; Mouchel, O.; Muñoz-Cueto, J.A. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 2020, 291, 113439. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.A.; Chalde, T.; Elisio, M.; Strüssmann, C.A. Effects of global warming on fish reproductive endocrine axis, with special emphasis in pejerrey Odontesthes bonariensis. Gen. Comp. Endocrinol. 2013, 192, 45–54. [Google Scholar] [CrossRef] [PubMed]
- King, H.; Pankhurst, N. Ovarian growth and plasma sex steroid and vitellogenin profiles during vitellogenesis in Tasmanian female Atlantic salmon (Salmo salar). Aquaculture 2003, 219, 797–813. [Google Scholar] [CrossRef]
- Żarski, D.; Horváth, Á.; Bernáth, G.; Krejszeff, S.; Radóczi, J.; Palińska-Żarska, K.; Bokor, Z.; Kupren, K.; Urbányi, B. Stimulation of ovulation and spermiation. In Controlled Reproduction of Wild Eurasian Perch: A Hatchery Manual; Springer: Cham, Switzerland, 2017; pp. 33–40. [Google Scholar]
- Cejko, B.I.; Krejszeff, S.; Judycka, S.; Targońska, K.; Kucharczyk, D. Effect of different treatment agents and post-treatment latency times on spermiation stimulation of northern pike (Esox lucius) under controlled conditions. Theriogenology 2019, 142, 260–267. [Google Scholar] [CrossRef]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, B.; Liu, J.; Guo, Z.; Kou, Y.; Lu, W.; Sun, J.; Li, Y. Enhancing resilience to chronic ammonia stress in crucian carp (Carassius carassius) through dietary gamma-aminobutyric acid (GABA) supplementation: Effects on growth performance, immune function, hepatotoxicity, and apoptosis. Aquac. Rep. 2024, 37, 102259. [Google Scholar] [CrossRef]
- Cho, Y.R.; Chang, J.Y.; Chang, H.C. Production of γ-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from Kimchi and its neuroprotective effect on neuronal cells. J. Microbiol. Biotechnol. 2007, 17, 104–109. [Google Scholar]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of oral gamma-aminobutyric acid (GABA) administration on stress and sleep in humans: A systematic review. Front. Neurosci. 2020, 14, 559962. [Google Scholar] [CrossRef]
- Varghese, T.; Rejish Boonstra, V.J.; Anand, G.; Dasgupta, S.; Pal, A.K. Dietary GABA enhances hypoxia tolerance of a bottom-dwelling carp, Cirrhinus mrigala, by modulating HIF-1α, thyroid hormones and metabolic responses. Fish Physiol. Biochem. 2020, 46, 199–212. [Google Scholar] [CrossRef]
- DeMorrow, S. Role of the hypothalamic–pituitary–adrenal axis in health and disease. Int. J. Mol. Sci. 2018, 19, 986. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.M.; Antunes-Rodrigues, J.; Franci, C.R.; Anselmo-Franci, J.A.; Karanth, S.; Rettori, V. Role of the hypothalamic-pituitary-adrenal axis in the control of the response to stress and infection. Braz. J. Med. Biol. Res. 2000, 33, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Ramirez, L.A. Development of an Early Life Stress Model in Larval Zebrafish and Analysis of Stress-Induced Transcriptomic Changes in Hypothalamic Cells. Doctoral Dissertation, Heidelberg University, Heidelberg, Germany, 2018. [Google Scholar]
- De Marco, R.J.; Groneberg, A.H.; Yeh, C.M.; Castillo Ramirez, L.A.; Ryu, S. Optogenetic elevation of endogenous glucocorticoid level in larval zebrafish. Front. Neural Circuits 2013, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Giordano, R.; Pellegrino, M.; Picu, A.; Bonelli, L.; Balbo, M.; Berardelli, R.; Lanfranco, F.; Ghigo, E. Neuroregulation of the hypothalamus-pituitary-adrenal (HPA) axis in humans: Effects of GABA-, mineralocorticoid-, and GH-secretagogue-receptor modulation. Sci. World J. 2006, 6, 1–11. [Google Scholar] [CrossRef]
- Paragliola, R.M.; Papi, G.; Pontecorvi, A.; Corsello, S.M. Treatment with synthetic glucocorticoids and the hypothalamus-pituitary-adrenal axis. Int. J. Mol. Sci. 2017, 18, 2201. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Duman, R.S. Cortical GABAergic dysfunction in stress and depression: New insights for therapeutic interventions. Front. Cell. Neurosci. 2019, 13, 448587. [Google Scholar] [CrossRef]
- Ochoa-de la Paz, L.D.; Gulias-Cañizo, R.; Ruíz-Leyja, E.D.; Sánchez-Castillo, H.; Parodí, J. The role of GABA neurotransmitter in the human central nervous system, physiology, and pathophysiology. Rev. Mex. Neurocienc. 2021, 22, 67–76. [Google Scholar] [CrossRef]
- Umaru, I.J.; Innocent, N.; Umaru, K.I. Review: Neurochemical aspects of mental health and neurological diseases. Afr. J. Biochem. Mol. Biol. Res. 2024, 1, 202–257. [Google Scholar] [CrossRef]
- Sieghart, W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv. Pharmacol. 2006, 54, 231–263. [Google Scholar]
- Vithlani, M.; Terunuma, M.; Moss, S.J. The dynamic modulation of GABAA receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol. Rev. 2011, 91, 1009–1028. [Google Scholar] [CrossRef]
- Bettler, B.; Tiao, J.Y. Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol. Ther. 2006, 110, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Jazvinscak Jembrek, M.; Vlainic, J. GABA receptors: Pharmacological potential and pitfalls. Curr. Pharm. Des. 2015, 21, 4943–4959. [Google Scholar] [CrossRef] [PubMed]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Mele, M.; Leal, G.; Duarte, C.B. Role of GABAAR trafficking in the plasticity of inhibitory synapses. J. Neurochem. 2016, 139, 997–1018. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Costa, R.O.; Duarte, C.B. Alterations in GABAA-receptor trafficking and synaptic dysfunction in brain disorders. Front. Cell. Neurosci. 2019, 13, 77. [Google Scholar] [CrossRef]
- Almeida, D.M.; Piazza, J.R.; Stawski, R.S.; Klein, L.C. The Speedometer of Life: Stress, Health and Aging. In Handbook of the Psychology of Aging; Schaie, K.W., Willis, S.L., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 154–196. [Google Scholar]
- Belelli, D.; Peters, J.A.; Phillips, G.D.; Lambert, J.J. The immediate and maintained effects of neurosteroids on GABAA receptors. Curr. Opin. Endocr. Metab. Res. 2022, 24, 100333. [Google Scholar] [CrossRef]
- Jacob, T.C.; Moss, S.J.; Jurd, R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008, 9, 331–343. [Google Scholar] [CrossRef]
- Henley, J.M.; Wilkinson, K.A. AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues Clin. Neurosci. 2013, 15, 11–27. [Google Scholar] [CrossRef]
- Fritschy, J.M.; Brünig, I. Formation and plasticity of GABAergic synapses: Physiological mechanisms and pathophysiological implications. Pharmacol. Ther. 2003, 98, 299–323. [Google Scholar] [CrossRef]
- Oldham, W.M.; Hamm, H.E. Structural basis of function in heterotrimeric G proteins. Q. Rev. Biophys. 2006, 39, 117–166. [Google Scholar] [CrossRef]
- Padgett, C.L.; Slesinger, P.A. GABAB receptor coupling to G-proteins and ion channels. Adv. Pharmacol. 2010, 58, 123–147. [Google Scholar] [PubMed]
- Offermanns, S.; Rosenthal, W. Encyclopedia of Molecular Pharmacology; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Lee, S.; Moniruzzaman, M.; Farris, N.; Min, T.; Bai, S.C. Interactive effect of dietary gamma-aminobutyric acid (GABA) and water temperature on growth performance, blood plasma indices, heat shock proteins and GABAergic gene expression in juvenile olive flounder Paralichthys olivaceus. Metabolites 2023, 13, 619. [Google Scholar] [CrossRef]
- Jin, X.; Qiao, A.; Moskophidis, D.; Mivechi, N.F. Modulation of heat shock factor 1 activity through silencing of Ser303/Ser307 phosphorylation supports a metabolic program leading to age-related obesity and insulin resistance. Mol. Cell. Biol. 2018, 38, e00095-18. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Paramasivan, P.; Kankia, I.H.; Langdon, S.P.; Deeni, Y.Y. Emerging role of nuclear factor erythroid 2-related factor 2 in the mechanism of action and resistance to anticancer therapies. Cancer Drug Resist. 2019, 2, 490. [Google Scholar] [CrossRef]
- Abramov, T.; Suwansaard, S.; da Silva, P.M.; Wang, T.; Dove, M.; O’Connor, W.; Parker, L.; Lovejoy, D.A.; Cummins, S.F.; Elizur, A. Teneurin and TCAP phylogeny and physiology: Molecular analysis, immune activity, and transcriptomic analysis of the stress response in the Sydney rock oyster (Saccostrea glomerata) hemocytes. Front. Endocrinol. 2022, 13, 1664. [Google Scholar] [CrossRef]
- Danielli, N.M.; Trevisan, R.; Mello, D.F.; Fischer, K.; Deconto, V.S.; da Silva Acosta, D.; Bianchini, A.; Bainy, A.C.D.; Dafre, A.L. Upregulating Nrf2-dependent antioxidant defenses in Pacific oysters Crassostrea gigas: Investigating the Nrf2/Keap1 pathway in bivalves. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 195, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Henderson, S.; Miller-Ezzy, P.; Li, X.X.; Qin, J.G. Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish Shellfish Immunol. 2019, 86, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; Hu, C.; Sun, X.; Xu, N. Physiological and transcriptome analysis of γ-aminobutyric acid (GABA) in improving Gracilariopsis lemaneiformis stress tolerance at high temperatures. Algal Res. 2021, 60, 102532. [Google Scholar] [CrossRef]
- Li, X. G-Protein Modulation of Ion Channels and Control of Neuronal Excitability by Light. Doctoral Dissertation, Case Western Reserve University, Cleveland, OH, USA, 2007. [Google Scholar]
- Tsentsevitsky, A.N.; Khaziev, E.F.; Kovyazina, I.V.; Petrov, A.M. GIRK channel as a versatile regulator of neurotransmitter release via L-type Ca2+ channel-dependent mechanism in the neuromuscular junction. Neuropharmacology 2022, 209, 109021. [Google Scholar] [CrossRef]
- O’Neal, T.J. Characterizing the Role of Accumbens Medium Spiny Neurons in Vulnerability to Heroin Addiction. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 2021. [Google Scholar]
- Johnson, C.M.; Cui, N.; Xing, H.; Wu, Y.; Jiang, C. The antitussive cloperastine improves breathing abnormalities in a Rett Syndrome mouse model by blocking presynaptic GIRK channels and enhancing GABA release. Neuropharmacology 2020, 176, 108214. [Google Scholar] [CrossRef] [PubMed]
- Bukharaeva, E.; Khuzakhmetova, V.; Dmitrieva, S.; Tsentsevitsky, A. Adrenoceptors modulate cholinergic synaptic transmission at the neuromuscular junction. Int. J. Mol. Sci. 2021, 22, 4611. [Google Scholar] [CrossRef] [PubMed]
- Errington, A.C. Extrasynaptic GABAA Receptors: A Brief Introduction to Extrasynaptic GABAA Receptors and ‘Tonic’GABAA Receptor-Mediated Inhibition in Physiology and Disease. In Extrasynaptic GABAA Receptors; Springer: New York, NY, USA, 2014; pp. 1–14. [Google Scholar]
- Ryan, R.M.; Ingram, S.L.; Scimemi, A. Regulation of glutamate, GABA and dopamine transporter uptake, surface mobility and expression. Front. Cell. Neurosci. 2021, 15, 670346. [Google Scholar] [CrossRef] [PubMed]
- Švob Štrac, D.; Muck Šeler, D.; Pivac, N. The Effects of GABA and GABAergic Drugs on the HPA Axis Activity; Nova Science Publishers: New York, NY, USA, 2014. [Google Scholar]
- Yu, X.; Hou, W.; Xiao, L. Gamma-Aminobutyric Acid (GABA) Avoids Deterioration of Transport Water Quality, Regulates Plasma Biochemical Indices, Energy Metabolism, and Antioxidant Capacity of Tawny Puffer (Takifugui flavidus) under Transport Stress. Biology 2024, 13, 474. [Google Scholar] [CrossRef]
- Yudin, Y.; Rohacs, T. Inhibitory Gi/O-coupled receptors in somatosensory neurons: Potential therapeutic targets for novel analgesics. Mol. Pain 2018, 14, 1744806918763646. [Google Scholar] [CrossRef]
- Zhang, C.; He, J.; Wang, X.; Yang, Y.; Huang, Q.; Qiao, F.; Shi, Q.; Qin, J.; Chen, L. Gamma-aminobutyric acid enhances hypoxia tolerance of juvenile Chinese mitten crab (Eriocheir sinensis) by regulating respiratory metabolism and alleviating neural excitotoxicity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 260, 109409. [Google Scholar] [CrossRef]
- Verburg-van Kemenade, B.M.L.; Cohen, N.; Chadzinska, M. Neuroendocrine-immune interaction: Evolutionarily conserved mechanisms that maintain allostasis in an ever-changing environment. Dev. Comp. Immunol. 2017, 66, 2–23. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Snigirov, S.; Sylantyev, S. GABAA receptors activate fish feeding behavior via two distinct functional pathways. J. Exp. Biol. 2018, 221, jeb170514. [Google Scholar]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.N.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Carney Almroth, B.; Bresolin de Souza, K.; Jönsson, E.; Sturve, J. Oxidative stress and biomarker responses in the Atlantic halibut after long term exposure to elevated CO2 and a range of temperatures. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 238, 110321. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. γ-aminobutyric acid (GABA): Biosynthesis, role, commercial production, and applications. Stud. Nat. Prod. Chem. 2018, 57, 413–452. [Google Scholar]
- Ncho, C.M.; Jeong, C.; Gupta, V.; Goel, A. The effect of gamma-aminobutyric acid supplementation on growth performances, immune responses, and blood parameters of chickens reared under stressful environment: A meta-analysis. Environ. Sci. Pollut. Res. 2021, 28, 45019–45028. [Google Scholar] [CrossRef] [PubMed]
- Kyprianou, T.-D.; Pörtner, H.O.; Anestis, A.; Kostoglou, B.; Feidantsis, K.; Michaelidis, B. Metabolic and molecular stress responses of gilthead seabream Sparus aurata during exposure to low ambient temperature: An analysis of mechanisms underlying the winter syndrome. J. Comp. Physiol. B 2010, 180, 1005–1018. [Google Scholar] [CrossRef]
| Species | Type of Stress | Route of Administration | Effect | Dosage | Duration of Stress | References |
|---|---|---|---|---|---|---|
| Litopenaeus vannamei | Vibrio alginolyticus challenge | Diet | Survival rate  | 50, 100, 300 mg/kg + 4 g oxytetracyclin | 9 days | [12] |
| Eriocheir sinensis | Fasting stress | Injection |  | 100, 1000 μmol/mL | 48 h | [169] |
| Paralicthyes olivaceus | Streptococcus iniae challenge | Diet |  | CON, with 92 mg/kg GABA content), a positive control with 4 g/kg oxytetracycline (OTC), and five other diets supplemented with 50, 100, 150, 200 and 250 mg/kg | 12 days | [13] |
| Litopenaeus vannamei | Ammonia stress | Diet | SR beginning from day 11  Antioxidants  | 0, 50, 100, 150, 200, 250 mg/kg | 36 h | [14] |
| Cirrhinus mrigala | Hypoxia | Diet | Metabolism during hypoxia  | 0.00%, 0.50%, 0.75%, 1.0% | 72 h | [125] |
| Eriocheir sinensis | Lipopolysaccharide | Diet | Anti-lipopolysaccharide factors and anti-inflammatory signaling pathways  | 0, 40, 80, 160, 320, 640 mg/kg | 24 h | [21] |
| Oreochromis niloticus | Ammonia | Diet | 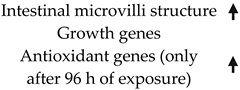 | 0, 200, 500 mg/kg | 96 h | [15] |
| Takifugu flavidus | Transportation stress | Immersion | Plasma Biochemistry  Energy metabolism  Antioxidant capacity  | 0, 5, 50, and 150 mg/L | 3 days | [167] |
| Paralicthyes olivaceus | Density and Edwardsiella tardar challenge | Diet | Immune response  | 0, 150, 200, 250 mg/kg | 48 h | [22] |
| Carassius carassius | Ammonia stress | Diet | Growth impairment  Malondialdehyde  Liver injury  Antioxidant genes  | 100 mg/kg | 56 days | [122] |
| Micropterus salmoides | Ammonia | Immersion and Diet |  | Immersion: 0, 30, 60, 90, 120, and 150 mg/L Diet: 0, 30, 90, and 150 mg/kg | Immersion: 96 h; samples taken every 24 h Diet: 15 days; samples taken every 3 days | [86] |
| Eriocheir sinensis | Hypoxia | Diet | Oxygen consumption  Neural excitotoxicity  Antioxidants  | 0, 250, 500 mg/kg | 24 h | [169] |
 Represent an increase in the corresponding parameter;
Represent an increase in the corresponding parameter;  Represent an increase in the corresponding parameters.
Represent an increase in the corresponding parameters.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogun, A.O.; Moniruzzaman, M.; Jeon, H.; Kim, H.; Aulia, D.; Hur, J.; Yoon, S.; Lee, S.; Min, T.; Lee, S. Gamma-Aminobutyric Acid (GABA) as a Dietary Strategy for Enhancing Temperature Stress Resilience in Aquaculture Species. Int. J. Mol. Sci. 2025, 26, 10233. https://doi.org/10.3390/ijms262010233
Ogun AO, Moniruzzaman M, Jeon H, Kim H, Aulia D, Hur J, Yoon S, Lee S, Min T, Lee S. Gamma-Aminobutyric Acid (GABA) as a Dietary Strategy for Enhancing Temperature Stress Resilience in Aquaculture Species. International Journal of Molecular Sciences. 2025; 26(20):10233. https://doi.org/10.3390/ijms262010233
Chicago/Turabian StyleOgun, Abayomi Oladimeji, Mohammad Moniruzzaman, Hyuncheol Jeon, Haham Kim, Deni Aulia, Junhyeok Hur, Sooa Yoon, Suhyun Lee, Taesun Min, and Seunghyung Lee. 2025. "Gamma-Aminobutyric Acid (GABA) as a Dietary Strategy for Enhancing Temperature Stress Resilience in Aquaculture Species" International Journal of Molecular Sciences 26, no. 20: 10233. https://doi.org/10.3390/ijms262010233
APA StyleOgun, A. O., Moniruzzaman, M., Jeon, H., Kim, H., Aulia, D., Hur, J., Yoon, S., Lee, S., Min, T., & Lee, S. (2025). Gamma-Aminobutyric Acid (GABA) as a Dietary Strategy for Enhancing Temperature Stress Resilience in Aquaculture Species. International Journal of Molecular Sciences, 26(20), 10233. https://doi.org/10.3390/ijms262010233








