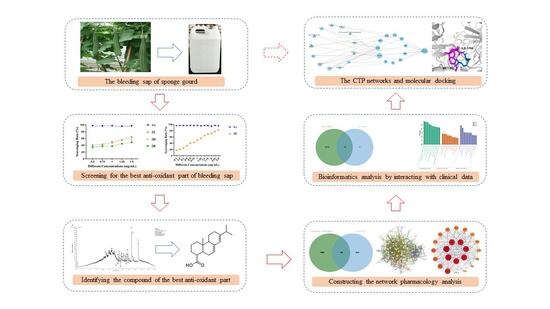
The UHPLC-Orbitrap MS/MS and Network Pharmacology Strategies Reveal the Active Antioxidants of Bleeding Sap from Sponge Gourd in Treating Tuberculosis
Abstract
1. Introduction
2. Results
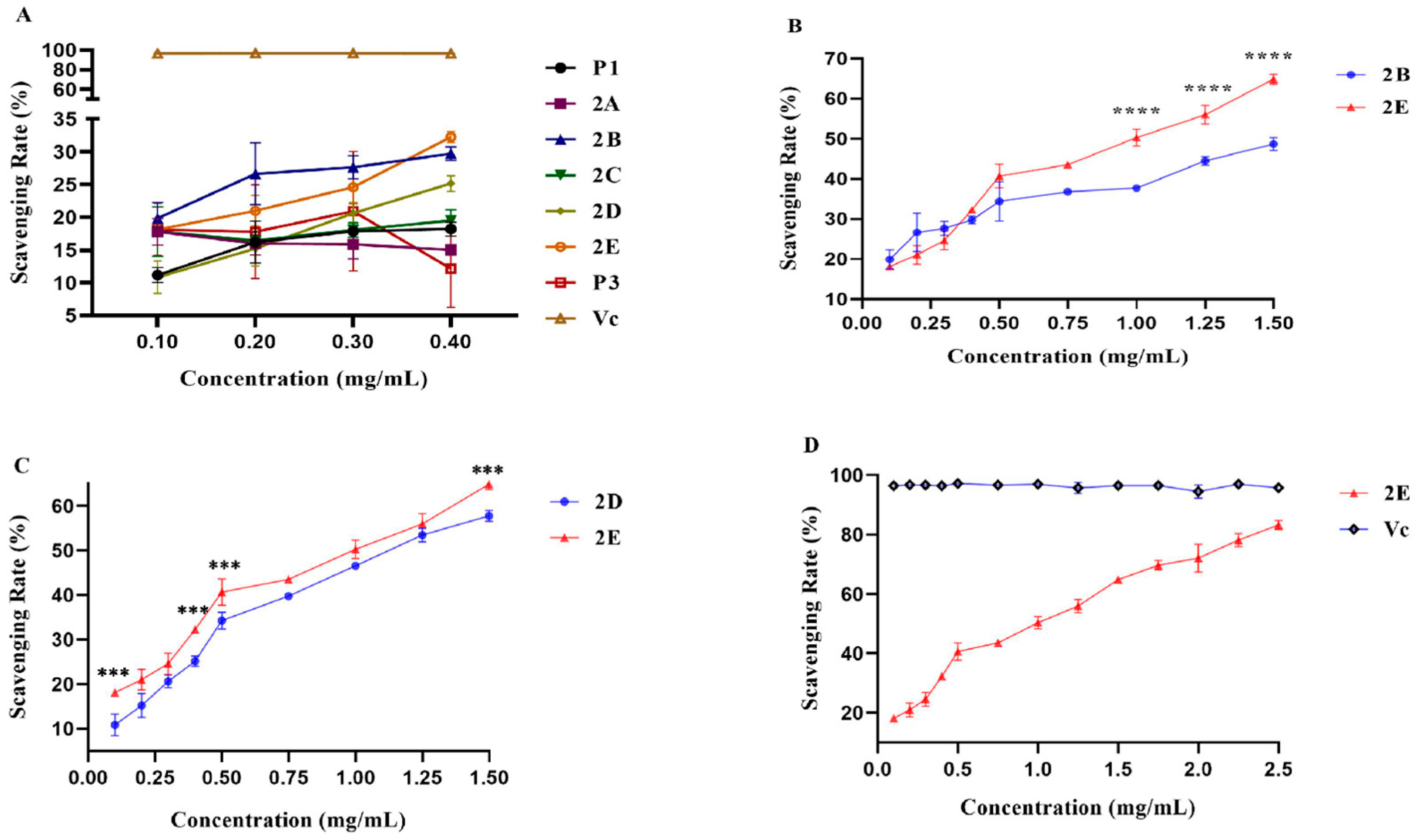
2.1. The Group with the Highest Antioxidant Capacity
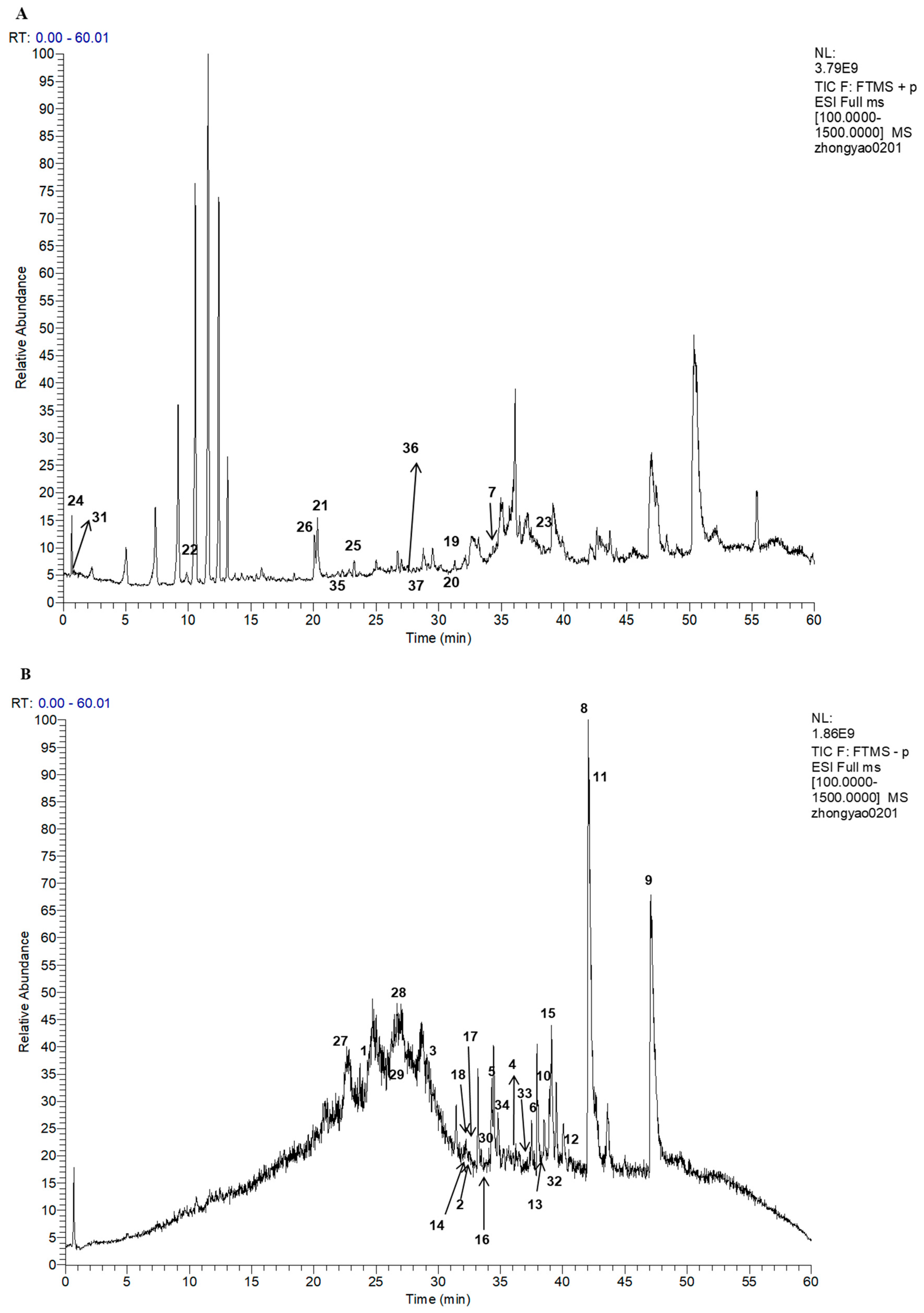
2.2. Compounds Identification
2.2.1. Identification of Fatty Acids and Esters
2.2.2. Identification of Phosphorus-Containing Compounds
2.2.3. Identification of Alkaloids
2.2.4. Identification of Sulfur-Containing Compounds
2.2.5. Identification of Aromatics
2.2.6. Identification of Other Compounds
2.3. Network Pharmacology Analysis
2.3.1. Intersecting Targets Between the Protein Targets of Identified-Compounds and TB-Related Genes
2.3.2. The PPI Network Construction and Screening of Core Targets
2.3.3. Analysis of GO Enrichment
2.3.4. Analysis of KEGG Pathways Enrichment
2.4. Bioinformatics Analysis
2.5. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Materials and Instruments
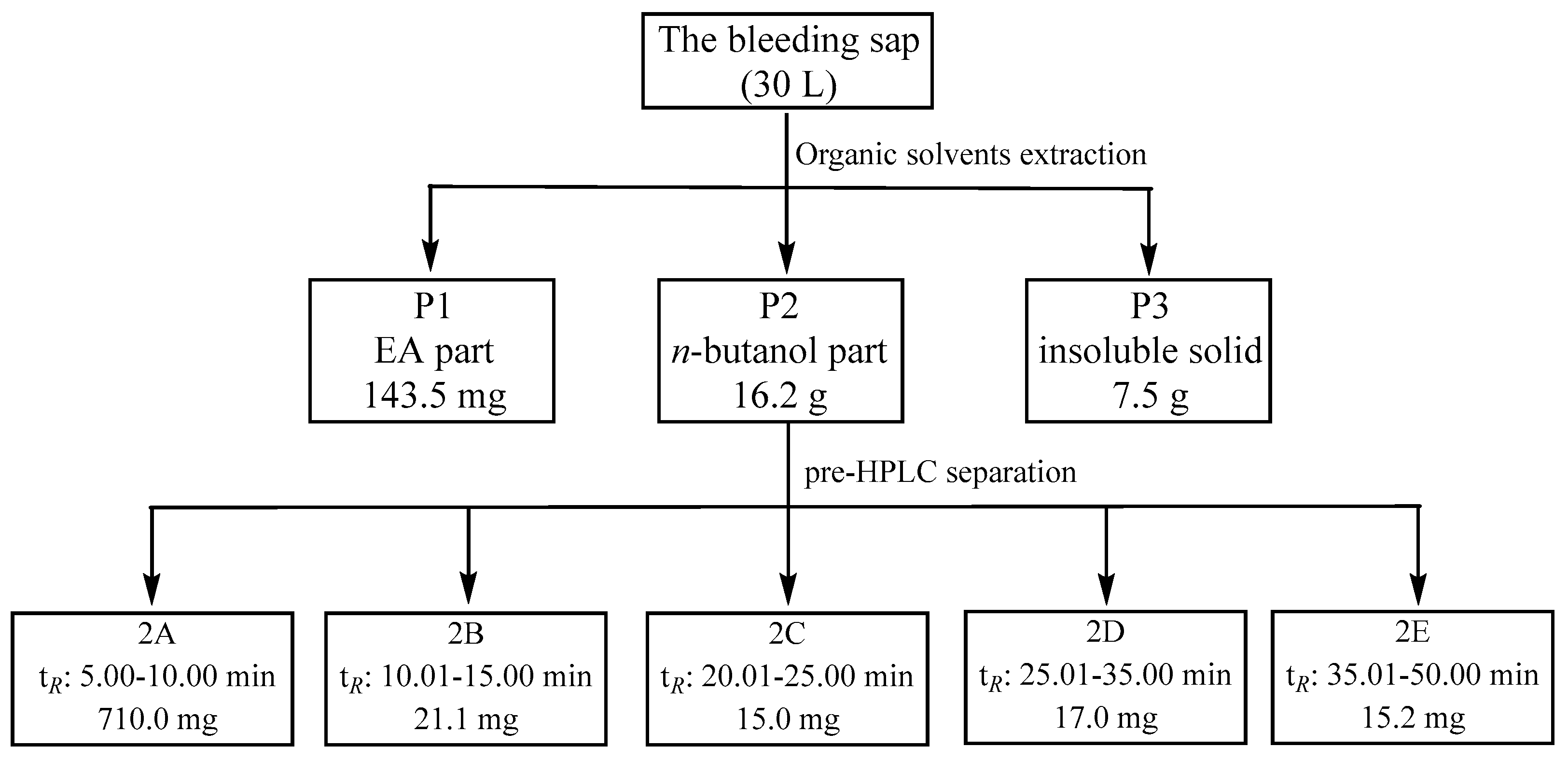
4.2. Organic Solvents Extraction with the Bleeding Sap
4.3. Pre-HPLC Subfraction
4.4. The DPPH Assay
4.5. Conditions for UHPLC-MS/MS
4.6. Network Pharmacology
4.6.1. Collection of the Active Compounds and Screening Their Potential Targets
4.6.2. Prediction of TB Targets
4.6.3. Protein–Protein Interaction Analysis
4.6.4. KEGG and GO Enrichment Analysis
4.7. Bioinformatics Analysis of Tuberculosis
4.7.1. Data Collection
4.7.2. Differentially Expressed Genes and Enrichment Pathways Analysis
4.7.3. Visualization of the Compounds-Targets-Pathways Network
4.8. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.-X.; Feng, Y. Research progress on pharmacological action of different parts of Luffa cylindrica (L.) roem. Sci. Technol. Food Ind. 2021, 42, 355–361. [Google Scholar] [CrossRef]
- Pimple, B.P.; Kadam, P.V.; Patil, M.J. Antidiabetic and antihyperlipidemic activity of Luffa acutangula fruit extracts in streptozotocin induced NIDDM rats. Asian J. Pharm. Clin. Res. 2011, 4, 156–163. [Google Scholar]
- Zhao, X.-M. Supplements to the Compendium of Materia Medica; Hou, R.-Y., Ed.; Original Work Published 1765; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Jadhav, V.B.; Thakare, V.N.; Suralkar, A.A.; Deshpande, D.A.; Naik, S.R. Hepatoprotective activity of Luffa acutangula against CCl4 and rifampicin induced liver toxicity in rats: A biochemical and histopathological evaluation. Indian J. Exp. Biol. 2010, 48, 822–829. [Google Scholar] [PubMed]
- Mishra, S.B.; Mukerjee, A. In vivo and ex vivo evaluation of Luffa acutangula fruit extract and its fractions for hepatoprotective activity in wistar rats. Int. J. Pharm. Sci. Res. 2017, 8, 5227–5233. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.-P.; Shi, M.-X.; Fang, Z.-Z.; Ji, J.-F.; Liao, X.-J.; Hu, X.-S.; Chen, F. The modulation of Luffa cylindrica (L.) roem supplementation on gene expression and amino acid profiles in liver for alleviating hepatic steatosis via gut microbiota in high-fat diet-fed mice: Insight from hepatic transcriptome analysis. J. Nutr. Biochem. 2020, 80, 108365. [Google Scholar] [CrossRef]
- Han, Y.-X.; Zhang, X.-Y.; Qi, R.-J.; Li, X.-M.; Gao, Y.; Zou, Z.-M.; Cai, R.-L.; Qi, Y. Lucyoside B, a triterpenoid saponin from Luffa cylindrica, inhibits the production of inflammatory mediators via both nuclear factor-κB and activator protein-1 pathways in activated macrophages. J. Funct. Foods 2020, 69, 103941. [Google Scholar] [CrossRef]
- Palash, P.; Sangeeta, D.; Narendra, V.; Kirti, M.; Sapna, M.; Anil, K. Anti-inflammatory activity of ethanolic extract of Luffa acutangular. World J. Pharm. Res. 2017, 6, 519–529. [Google Scholar] [CrossRef]
- Vanajothia, R.; Sudha, A.; Manikandan, R.; Rameshthangam, P.; Srinivasan, P. Luffa acutangula and Lippia nodiflora leaf extract induces growth inhibitory effect through induction of apoptosis on human lung cancer cell line. Biomed. Prev. Nutr. 2012, 2, 287–293. [Google Scholar] [CrossRef]
- Abdel-Salam, I.M.; Abou-Bakr, A.A.; Ashour, M. Cytotoxic effect of aqueous ethanolic extract of Luffa cylindrica leaves on cancer stem cells CD44/24 in breast cancer patients with various molecular sub-typesusing tissue samples in vitro. J. Ethnopharmacol. 2019, 238, 111877. [Google Scholar] [CrossRef]
- Shete, R.V.; Pawashe, P.M.; Kore, K.J.; Otari, K.V. Protective role of Luffa cylindrica linn. against erythromycin estolate induced hepatotoxicity. J. Curr. Pharma Res. 2011, 1, 315–319. [Google Scholar] [CrossRef]
- Sharma, N.K.; Priyanka; Jha, K.K.; Singh, H.K.; Shrivastava, A.K. Hepatoprotective activity of Luffa cylindrica (L.) M. J. Roem. leaf extract in paracetamol intoxicated rats. Indian J. Nat. Prod. Resour. 2014, 5, 143–148. [Google Scholar]
- Bulbul, I.J.; Zulfiker, A.H.; Hamid, K.; Khatun, M.H.; Begum, Y. Comparative study of in vitro antioxidant, antibacterial and cytotoxic activity of two Bangladeshi medicinal plants-Luffa cylindrica L. and Luffa acutangular. Pharmacogn. J. 2011, 3, 59–66. [Google Scholar] [CrossRef]
- Li, J.; Fu, Y.-M.; Zhu, W.-J.; Zhang, S.-M.; Yan, Y.-X.; Yan, L. Effects of Retinervus luffae fructus on serum lipid level in experimental hyperlipidemia rats. Chin. J. Pathophysiol. 2004, 20, 1264–1266. [Google Scholar]
- Li, X.-L.; Li, J.; Zhu, W.-J.; Zhang, W.-H. Effects of Retinervus luffae fructus on mRNA expression of low-density lipoprotein receptor in hyperlipidemia mice. Chin. J. Pathophysiol. 2009, 25, 1156–1159. [Google Scholar]
- Fang, M.-Z.; Song, W.-M.; Xu, Y.; Liu, D.-J.; Wang, R.-T.; Fang, C.-Y. Effect of aerobic exercise combined with Retinervus Luffae fructus on AQP2 mRNA expression in kidney of diabetic rat model. Shandong Med. J. 2013, 53, 4–6. [Google Scholar] [CrossRef]
- Pu, X.-H.; Kang, B.; Han, H.-R.; Fang, C.-Y.; Xu, L.-L. Effects of Luffae on cardiac function in chronic heart failure rats. Lishizhen Med. Mater. Med. Res. 2011, 22, 1020–1022. [Google Scholar] [CrossRef]
- Fang, C.-Y.; Pu, X.-H.; Sun, W.-Q.; Xu, L.-L.; Han, H.-R.; Wang, H.; Kang, B. Effect of Luffae on myocardial calmodulin mRNA expression in chronic heart failure rats. Chin. J. Gerontol. 2013, 33, 2588–2590. [Google Scholar] [CrossRef]
- Kou, J.-P.; Zhuang, S.-F.; Tang, X.-J.; Tong, C.-N.; Yan, Y.-Q. Preliminary studies of Luffa-extract on allergy and acute inflammation. J. China Pharm. Univ. 2001, 32, 293–295. [Google Scholar]
- Chen, W.-W.; Liang, D.; Li, H.-T.; Liang, X.-Z.; Liu, H.-G.; Li, Y. Study on the mechanism of extracts in vine of Luffa on the treatment of allergic rhinitis. J. Guangxi Univ. Chin. Med. 2015, 18, 1–3. [Google Scholar]
- Wang, M.-L.; Tang, Z.-Z.; Chen, M.-M.; Wang, Y.-Y.; Zhao, J.-F.; Lu, C.-M. Study on the antioxidant activity and its stability of bleeding sap from Luffa cylindrica roem. Food Sci. Technol. 2009, 34, 149–154. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Tang, Z.-Z.; Chen, M.-M.; Wang, M.-L.; Zhao, J.-F.; Lu, C.-M. Preliminary study on antioxidants in bleeding sap from Luffa cylindrica roem. Sci. Technol. Food Ind. 2009, 30, 84–86+89. [Google Scholar] [CrossRef]
- Lin, J.-K.; Chen, M.-H.; Xie, J.-M.; Zhao, R.; Ye, X.-M.; Shi, X.-L.; Wang, Z.-R. Purification and characterization of two luffaculins, ribosome-inactivating proteins from seeds of Luffa acutangular. Chin. J. Biochem. Mol. Biol. 2002, 18, 609–613. [Google Scholar]
- Long, J.; Hou, X.-M.; Zhang, G.-H.; Chen, M.-H.; Zheng, Y.-T. Anti-HIV-1 activities of luffaculin 1. Chin. J. Nat. Med. 2008, 6, 372–376. [Google Scholar] [CrossRef]
- Xie, J.-M.; Xu, Y.; Chen, M.-H.; Xu, J.-H.; Yu, C.-X.; Zhao, R.; Wu, G.-H.; Lin, J.-Z. Differentiation-inducing effect of luffaculin-2 on B16 melanoma cells. J. Fujian Med. Univ. 2005, 39, 349–352. [Google Scholar]
- Du, Q.-Z.; Xu, Y.-J.; Li, L.; Zhao, Y.; Jerz, G.; Winterhalter, P. Antioxidant constituents in the fruits of Luffa cylindrica (L.) roem. J. Agric. Food. Chem. 2006, 54, 4186–4190. [Google Scholar] [CrossRef]
- Du, M.-H.; Zhang, C.-Y. Optimization of ethanol extraction process of total flavonoids from pumpkin leaves. Sci. Technol. Food Ind. 2007, 28, 148–150. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, D.; Dong, L. Determination of the content of polysaccharide in Retinervus luffac fructus produced in Henan province with phenol-sulfuric acid. J. Xinxiang Med. Coll. 2008, 25, 36–38. [Google Scholar]
- Zhou, W.-P. Extraction and contents analysis of oleanolic acid from stem Luffa and leaf Luffa. J. Guangxi Univ. Sci. Technol. 2014, 25, 86–89. [Google Scholar] [CrossRef]
- Ogunbanjo, O.R.; Awotoye, O.O.; Jayeoba, F.M.; Jeminiwa, S.W. Nutritional analysis of selected cucurbitaceae species. Univ. J. Plant Sci. 2016, 4, 1–3. [Google Scholar] [CrossRef]
- Song, S.-Q.; Liu, G.-Z.; Liu, H.; Gao, S.-G. Overview of research on the medicinal value of Loofah. Chin. Agric. Sci. Bull. 2004, 20, 166–167+169. [Google Scholar]
- Xu, J.; Wang, W.; Li, T.-T. Application of combined detection of Mycobacterium tuberculosis-specific IFN-γ and IL-2 in the differential diagnosis of pulmonary tuberculosis and bacterial pneumonia. Chin. J. Infect. Control 2024, 23, 1173–1177. [Google Scholar] [CrossRef]
- Azeez, M.A.; Bello, O.S.; Adedeji, A.O. Traditional and medicinal uses of Luffa cylindrica: A review. J. Med. Plants Stud. 2013, 1, 102–111. [Google Scholar]
- Pyrzynska, K.; Pękal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Methods 2013, 5, 4288–4295. [Google Scholar] [CrossRef]
- Perez de Souza, L.; Alseekh, S.; Scossa, F.; Fernie, A.R. Ultra-high-performance liquid chromatography high-resolution mass spectrometry variants for metabolomics research. Nat. Methods 2021, 18, 733–746. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.-Y.; Zheng, J.-H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.-Y.; Jin, X.; Ma, Y.-Y.; Yang, Y.; Li, J.-J.; Liang, L.-Y.; Liu, R.; Li, Z. A comprehensive application: Molecular docking and network pharmacology for the prediction of bioactive constituents and elucidation of mechanisms of action in component-based Chinese medicine. Comput. Biol. Chem. 2021, 90, 107402. [Google Scholar] [CrossRef]
- Liang, H.; Ruan, H.; Ouyang, Q.; Lai, L.-H. Herb-target interaction network analysis helps to disclose molecular mechanism of traditional Chinese medicine. Sci. Rep. 2016, 6, 36767. [Google Scholar] [CrossRef]
- Kerwin, J.L.; Wiens, A.M. Identification of fatty acids by electrospray mass spectrometry and tandem mass spectrometry. J. Mass Spectrom. 1996, 31, 184–192. [Google Scholar] [CrossRef]
- Uhlig, S.; Negård, M.; Heldal, K.K.; Straumfors, A.; Madsø, L.; Bakke, B.; Eduard, W. Profiling of 3-hydroxy fatty acids as environmental markers of endotoxin using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2016, 1434, 119–126. [Google Scholar] [CrossRef]
- Jiang, R.-Q.; Jiao, Y.; Zhang, P.; Liu, Y.; Wang, X.; Huang, Y.; Zhang, Z.-J.; Xu, F.-G. Twin derivatization strategy for high-coverage quantification of free fatty acids by liquid chromatography–tandem mass spectrometry. Anal. Chem. 2017, 89, 12223–12230. [Google Scholar] [CrossRef]
- Kim, H.; Kim, G.B.; Choi, M.S.; Kim, I.S.; Gye, M.C.; Yoo, H.H. Liquid chromatography-tandem mass spectrometric analysis of acetyl tributyl citrate for migration testing of food contact materials. Microchem. J. 2018, 139, 475–479. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Abdelaziz, S.; Hassan, W.H.B.; El-Sayed, M.A. Phytochmical and biological characterization of Tephrosia nubica Boiss. growing in Saudi Arabia. Arab. J. Chem. 2020, 13, 9216–9230. [Google Scholar] [CrossRef]
- Villalobos Solis, M.I.; Patel, A.; Orsat, V.; Singh, J.; Lefsrud, M. Fatty acid profiling of the seed oils of some varieties of field peas (Pisum sativum) by RP-LC/ESI-MS/MS: Towards the development of an oilseed pea. Food Chem. 2013, 139, 986–993. [Google Scholar] [CrossRef]
- Oliw, E.H.; Su, C.; Skogström, T.; Benthin, G. Analysis of novel hydroperoxides and other metabolites of oleic, linoleic, and linolenic acids by liquid chromatography-mass spectrometry with ion trap MSn. Lipids 1998, 33, 843–852. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Zhao, H.-S.; Zhao, X.; Jia, J.; Ma, Q.; Zhang, S.-C.; Zhang, X.-R.; Chiba, H.; Hui, S.-P.; Ma, X.-X. Identification and quantitation of C=C location isomers of unsaturated fatty acids by epoxidation reaction and tandem mass spectrometry. Anal. Chem. 2017, 89, 10270–10278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, Y.-Z.; Chen, J.-G.; Zhang, J.-Y.; Chen, L.; Wang, B.-C.; Wu, C.-Y.; Yuan, Y.-Z. Identification and direct determination of fatty acids profile in oleic acid by HPLC-CAD and MS-IT-TOF. J. Pharm. Biomed. Anal. 2021, 204, 114238. [Google Scholar] [CrossRef] [PubMed]
- Pertami, S.B.; Yunitasari, E.; Budiono, B.; Yulifah, B.; Astuti, N.P.; Herawati, T.; Arifah, S.N.; Atho’illah, M.F. Bioactive compounds and antioxidant activity in Greek-style yoghurt infused with shield aralia leaves. Nat. Life Sci. Commun. 2024, 23, e2024045. [Google Scholar] [CrossRef]
- Dahibhate, N.L.; Dwivedi, P.; Kumar, K. GC-MS and UHPLC-HRMS based metabolite profiling of Bruguiera gymnorhiza reveals key bioactive compounds. S. Afr. J. Bot. 2022, 149, 1044–1048. [Google Scholar] [CrossRef]
- Lesage, D.; Virelizier, H.; Tabet, J.C.; Jankowski, C.K. Study of mass spectrometric fragmentations of tributyl phosphate via collision-induced dissociation. Rapid Commun. Mass Spectrom. 2001, 15, 1947–1956. [Google Scholar] [CrossRef]
- Santín, G.; Eljarrat, E.; Barceló, D. Simultaneous determination of 16 organophosphorus flame retardants and plasticizers in fish by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1441, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bacaloni, A.; Cavaliere, C.; Foglia, P.; Nazzari, M.; Samperi, R.; Lagana, A. Liquid chromatography/tandem mass spectrometry determination of organophosphorus flame retardants and plasticizers in drinking and surface waters. Rapid Commun. Mass Spectrom. 2007, 21, 1123–1130. [Google Scholar] [CrossRef]
- Yusa`, V.; Lo’pez, A.; Dualde, P.; Pardo, O.; Fochi, I.; Miralles, P.; Coscollá, C. Identification of 24 unknown substances (NIAS/IAS) from food contact polycarbonate by LC-Orbitrap Tribrid HRMS-DDMS3: Safety assessment. Int. J. Anal. Chem. 2021, 2021, 6654611. [Google Scholar] [CrossRef]
- Dmitrovic, J.; Durden, D.A. A new approach to the analysis of nicarbazin and ionophores in eggs by HPLC/MS/MS. J. AOAC Int. 2011, 94, 428–435. [Google Scholar] [CrossRef]
- Kim, N.S.; Moon, S.H.; Choi, H.S.; Lee, J.H.; Park, S.; Kang, H. Simultaneous separation and determination of 20 potential adulterant antigout and antiosteoporosis pharmaceutical compounds in herbal food products using LC with electrospray ionization MS/MS and LC with quadrupole-time-of-flight MS. J. Sep. Sci. 2020, 43, 2750–2765. [Google Scholar] [CrossRef]
- Lyon, P.A.; Stebbings, W.L.; Crow, F.W.; Tomer, K.B.; Lippstreu, D.L.; Gross, M.L. Analysis of anionic surfactants by mass spectrometry/mass spectrometry with fast atom bombardment. Anal. Chem. 1984, 56, 8–13. [Google Scholar] [CrossRef]
- Conboy, J.J.; Henion, J.D.; Martin, M.W.; Zweigenbaum, J.A. Ion chromatography/mass spectrometry for the determination of organic ammonium and sulfate compounds. Anal. Chem. 1990, 62, 800–807. [Google Scholar] [CrossRef]
- Ogunbiyi, O.D.; Cappelini, L.T.D.; Monem, M.; Mejias, E.; George, F.; Gardinali, P.; Bagner, D.M.; Quinete, N. Innovative non-targeted screening approach using high-resolution mass spectrometry for the screening of organic chemicals and identification of specific tracers of soil and dust exposure in children. J. Hazard. Mater. 2024, 469, 134025. [Google Scholar] [CrossRef]
- Jonker, W.; Ballesteros-Gómez, A.; Hamers, T.; Somsen, G.W.; Lamoree, M.H.; Kool, J. Highly selective screening of estrogenic compounds in consumer-electronics plastics by liquid chromatography in parallel combined with nano fractionation-bioactivity detection and mass spectrometry. Environ. Sci. Technol. 2016, 50, 12385–12393. [Google Scholar] [CrossRef]
- Li, Z.-K.; Xue, F.; Xu, L.-G.; Peng, C.-F.; Kuang, H.; Ding, T.; Xu, C.-L.; Sheng, C.-Y.; Gong, Y.-X.; Wang, L.-B. Simultaneous determination of nine types of phthalate residues in commercial milk products using HPLC-ESI-MS-MS. J. Chromatogr. Sci. 2011, 49, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Tintaru, A.; Labed, V.; Charles, L. Structural characterisation of degradation products formed upon di-n-butyl phthalate radiolysis by high-performance liquid chromatography electrospray tandem mass spectrometry. Eur. J. Mass Spectrom. 2010, 16, 595–603. [Google Scholar] [CrossRef]
- Sunyer-Caldú, A.; Peiró, A.; Díaz, M.; Ibáñez, L.; Gil-Solsona, R.; Gago-Ferrero, P.; Diaz-Cruz, M.S. Target analysis and suspect screening of UV filters, parabens and other chemicals used in personal care products in human cord blood: Prenatal exposure by mother-fetus transfer. Environ. Int. 2023, 173, 107834. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-J.; Yan, W.-Y.; He, M.-H.; Huang, J.-L.; Wu, Y.-Q.; Ye, L.; Zhang, R.-R. Determination of abietic acid compounds in poultry by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry coupled with dispersive solid phase extraction. Food Mach. 2023, 39, 75–79. [Google Scholar] [CrossRef]
- Azemard, C.; Menager, M.; Vieillescazes, C. Analysis of diterpenic compounds by GC-MS/MS: Contribution to the identification of main conifer resins. Anal. Bioanal. Chem. 2016, 408, 6599–6612. [Google Scholar] [CrossRef] [PubMed]
- Patyra, A.; Dudek, M.K.; Kiss, A.K. LC-DAD-ESI-MS/MS and NMR analysis of conifer wood specialized metabolites. Cells 2022, 11, 3332. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Hu, J.-Y.; Jia, A.; Peng, H.; Wu, S.-M.; Dong, Z.-M. Determination and occurrence of retinoic acids and their 4-oxo metabolites in Liaodong Bay, China, and its adjacent rivers. Environ. Toxicol. Chem. 2010, 29, 2491–2497. [Google Scholar] [CrossRef]
- Kane, M.A.; Folias, A.E.; Wang, C.; Napoli, J.L. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal. Chem. 2008, 80, 1702–1708. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Gibson, C.R.; Brown, C.M. Identification of diethylene glycol monobutyl ether as a source of contamination in an ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 2003, 14, 1247–1249. [Google Scholar] [CrossRef]
- Blokland, M.H.; Zoontjes, P.W.; Sterk, S.S.; Stephany, R.W.; Zweigenbaum, J.; van Ginkel, L.A. Confirmatory analysis of trenbolone using accurate mass measurement with LC/TOF-MS. Anal. Chim. Acta 2008, 618, 86–93. [Google Scholar] [CrossRef]
- Thevis, M.; Guddat, S.; Schänzer, W. Doping control analysis of trenbolone and related compounds using liquid chromatography–tandem mass spectrometry. Steroids 2009, 74, 315–321. [Google Scholar] [CrossRef]
- Gao, Q.; Han, Z.Y.; Tian, D.F.; Liu, G.-L.; Wang, Z.-Y.; Lin, J.-F.; Chang, Z.; Zhang, D.-D.; Xie, Y.-Z.; Sun, Y.-K.; et al. Xinglou Chengqi Decoction improves neurological function in experimental stroke mice as evidenced by gut microbiota analysis and network pharmacology. Chin. J. Nat. Med. 2021, 19, 881–899. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2023 [R/OL]; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Alashavi, H.; Daher, M.; Chorgoliani, D.; Saflo, M.; Zeidan, M.; Huseyinibrahim, F.; Ismail, E.; Yousef, A.R.H.; Ayat, K.; Elobayd, E.; et al. Descriptive epidemiology of the tuberculosis service delivery project beneficiaries in northwest Syria: 2019–2020. Front. Public Health 2021, 9, 672114. [Google Scholar] [CrossRef]
- Quenard, F.; Fournier, P.E.; Drancourt, M.; Brouqui, P. Role of second-line injectable antituberculosis drugs in the treatment of MDR/XDR tuberculosis. Int. J. Antimicrob. Agents 2017, 50, 252–254. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, K.; Wu, C. Study on preparing juice of towel gourd into solid powder by spray drying and it’s bactriostasis. Nor. Horticul. 2012, 2012, 186–188. [Google Scholar]
- Wang, X.-L.; Ding, Q.; Sun, H.-J.; Li, D.-D.; Zhang, P.-P.; He, P.-C.; He, S.-D.; Cao, X.-D. Chemical composition of bleeding sap of sponge gourd and in vitro antioxidant activity. Hans J. Food Nutr. Sci. 2015, 4, 101–110. [Google Scholar] [CrossRef]
- Song, M.-T.; Ruan, J.; Zhang, R.-Y.; Deng, J.; Ma, Z.-Q.; Ma, S.-P. Astragaloside IV ameliorates neuroinflammation-induced depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3 inflammasome axis. Acta Pharmacol. Sin. 2018, 39, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Zhang, S.-X.; Wu, H.-J.; Rong, X.-L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Bocher, V.; Pineda-Torra, I.; Fruchart, J.C.; Staels, B. PPARs: Transcription factors controlling lipid and lipoprotein metabolism. Ann. N. Y. Acad. Sci. 2002, 967, 7–18. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Kawada, T.; Goto, T.; Kim, C.-S.; Taimatsu, A.; Egawa, K.; Yamamoto, T.; Jisaka, M.; Nishimura, K.; Yokota, K.; et al. Abietic acid activates peroxisome proliferator-activated receptor-γ (PPARγ) in RAW264.7 macrophages and 3T3-L1 adipocytes to regulate gene expression involved in inflammation and lipid metabolism. FEBS Lett. 2003, 550, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.E.; Carneiro, A.B.; Silva, A.R.; Bozza, P.T. PPARγ expression and function in mycobacterial infection: Roles in lipid metabolism, immunity, and bacterial killing. PPAR Res. 2012, 2012, 383829. [Google Scholar] [CrossRef]
- Arnett, E.; Weaver, A.M.; Woodyard, K.C.; Montoya, M.J.; Li, M.; Hoang, K.V.; Hayhurst, A.; Azad, A.K.; Schlesinger, L.S. PPARγ is critical for Mycobacterium tuberculosis induction of Mcl-1 and limitation of human macrophage apoptosis. PLOS Pathog. 2018, 14, e1007100. [Google Scholar] [CrossRef]
- Ye, Y.-T.; Liu, J.; Guo, Y.; Gao, Y.-J.; Rao, J.-Y.; Su, R.-G.; Zhang, L.; Huang, Z.-K.; Luo, Q.; Li, J.-M. PPARγ ameliorates Mycobacterium tuberculosis H37Ra-induced foamy macrophage formation via the ABCG1-dependent cholesterol efflux pathway in THP-1 macrophages. Front. Microbiol. 2022, 13, 829870. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Sánchez-Álvarez, M.; Fajardo, A.; Kapetanovic, R.; Steiner, B.; Dutra, F.; Moreira, L.; López, J.A.; Campo, R.; Marí, M.; et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science 2020, 370, eaay8085. [Google Scholar] [CrossRef] [PubMed]
- Vats, D.; Rani, G.; Arora, A.; Sharma, V.; Rathore, I.; Mubeen, S.A.; Singh, A. Tuberculosis and T cells: Impact of T cell diversity in tuberculosis infection. Tuberculosis 2024, 149, 102567. [Google Scholar] [CrossRef]
- Song, C.-H.; Lee, J.-S.; Lee, S.-H.; Lim, K.; Kim, H.-J.; Park, J.-K.; Paik, T.-H.; Jo, E.-K. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J. Clin. Immunol. 2003, 23, 194–201. [Google Scholar] [CrossRef]
- Pattanaik, K.P.; Ganguli, G.; Naik, S.K.; Sonawane, A. Mycobacterium tuberculosis EsxL induces TNF-α secretion through activation of TLR2 dependent MAPK and NF-κB pathways. Mol. Immunol. 2021, 130, 133–141. [Google Scholar] [CrossRef]
- Sun, J.-X.; Zhang, Q.-W.; Yang, G.-Z.; Li, Y.-H.; Fu, Y.; Zheng, Y.-Y.; Jiang, X. The licorice flavonoid isoliquiritigenin attenuates Mycobacterium tuberculosis-induced inflammation through Notch1/NF-κB and MAPK signaling pathways. J. Ethnopharmacol. 2022, 294, 115368. [Google Scholar] [CrossRef]
- Singh, B.; Pahuja, I.; Yadav, P.; Shaji, A.; Chaturvedi, S.; Ranganathan, A.; Dwivedi, V.P.; Das, G. Adjunct therapy with all-trans-retinoic acid improves therapeutic efficacy through immunomodulation while treating tuberculosis with antibiotics in mice. J. Infect. Dis. 2024, 229, 1509–1518. [Google Scholar] [CrossRef]
- Thummuri, D.; Guntuku, L.; Challa, V.S.; Ramavat, R.N.; Naidu, V.G.M. Abietic acid attenuates RANKL induced osteoclastogenesis and inflammation associated osteolysis by inhibiting the NF-KB and MAPK signaling. J. Cell. Physiol. 2018, 234, 443–453. [Google Scholar] [CrossRef]
- Ye, H.-L.; Wang, J.; Mao, J.-D.; Pan, D.-B. Abietic acid ameliorates pancreatic injury in acute pancreatitis by modulating MAPK pathway. Trop. J. Pharm. Res. 2023, 22, 1573–1578. [Google Scholar] [CrossRef]
- Yang, R.-R.; Zheng, J.-H.; Qin, J.; Liu, S.-J.; Liu, X.-Y.; Gu, Y.-Y.; Yang, S.-Y.; Du, J.; Li, S.-G.; Chen, B.; et al. Dibutyl phthalate affects insulin synthesis and secretion by regulating the mitochondrial apoptotic pathway and oxidative stress in rat insulinoma cells. Ecotoxicol. Environ. Saf. 2023, 249, 114396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, J.; Cheng, J.; Wu, Z. Dibutyl phthalate-induced activation of ROS and ERK1/2 causes hepatic and renal damage in Kunming mice. Hum. Exp. Toxicol. 2019, 38, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-M.; Huang, C.; Ma, X.; Wu, R.; Zhu, W.-W.; Li, X.-T.; Liang, Z.-F.; Deng, F.-F.; Wu, J.-S.; Geng, S.-S. Phthalates promote prostate cancer cell proliferation through activation of ERK5 and p38. Environ. Toxicol. Pharmacol. 2018, 63, 29–33. [Google Scholar] [CrossRef]
- Wan, X.-W.; Cui, X.-D.; Wang, X.; Feng, M.-Y.; Wei, S.-N.; Yu, J.; Cheng, S.; Luo, H.; Hu, J.-X. Di-n-butyl phthalate induces toxicity in male fetal mouse testicular development by regulating the MAPK signaling pathway. Toxicol. Appl. Pharmacol. 2024, 486, 116933. [Google Scholar] [CrossRef]
- GeneCards. Available online: https://www.genecards.org/ (accessed on 12 August 2025).
- The GEO Database. Available online: https://www.ncbi.nlm.nih.gov/geo/ (accessed on 12 August 2025).
- Venny 2.1.0. Available online: https://bioinfogp.cnb.csic.es/tools/venny// (accessed on 12 August 2025).
- The Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 12 August 2025).
- Wang, D.; Li, X.-B.; Li, Y.-F.; Wang, R.-L.; Wang, C.-X.; Li, Y.-W. New molecular mechanisms of quercetin in improving recurrent spontaneous abortion based on in-depth network pharmacology and molecular docking. Front. Chem. 2024, 12, 1407667. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.B.; Baiseitova, A.; Amzeyeva, U.; Shang, X.; Jenis, J. α-Glucosidase Inhibition-Guided Network Pharmacology and Molecular Docking Reveal the Antidiabetic Potential of Cichorium intybus as a Functional Food. Int. J. Mol. Sci. 2025, 26, 9497. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 12 August 2025).
- SwissTargetPrediction. Available online: http://www.swisstargetprediction.ch/ (accessed on 12 August 2025).
- The OMIM. Available online: https://www.omim.org/ (accessed on 12 August 2025).
- DISGENET. Available online: https://disgenet.com/ (accessed on 12 August 2025).
- The STRING database. Available online: https://string-db.org/ (accessed on 12 August 2025).


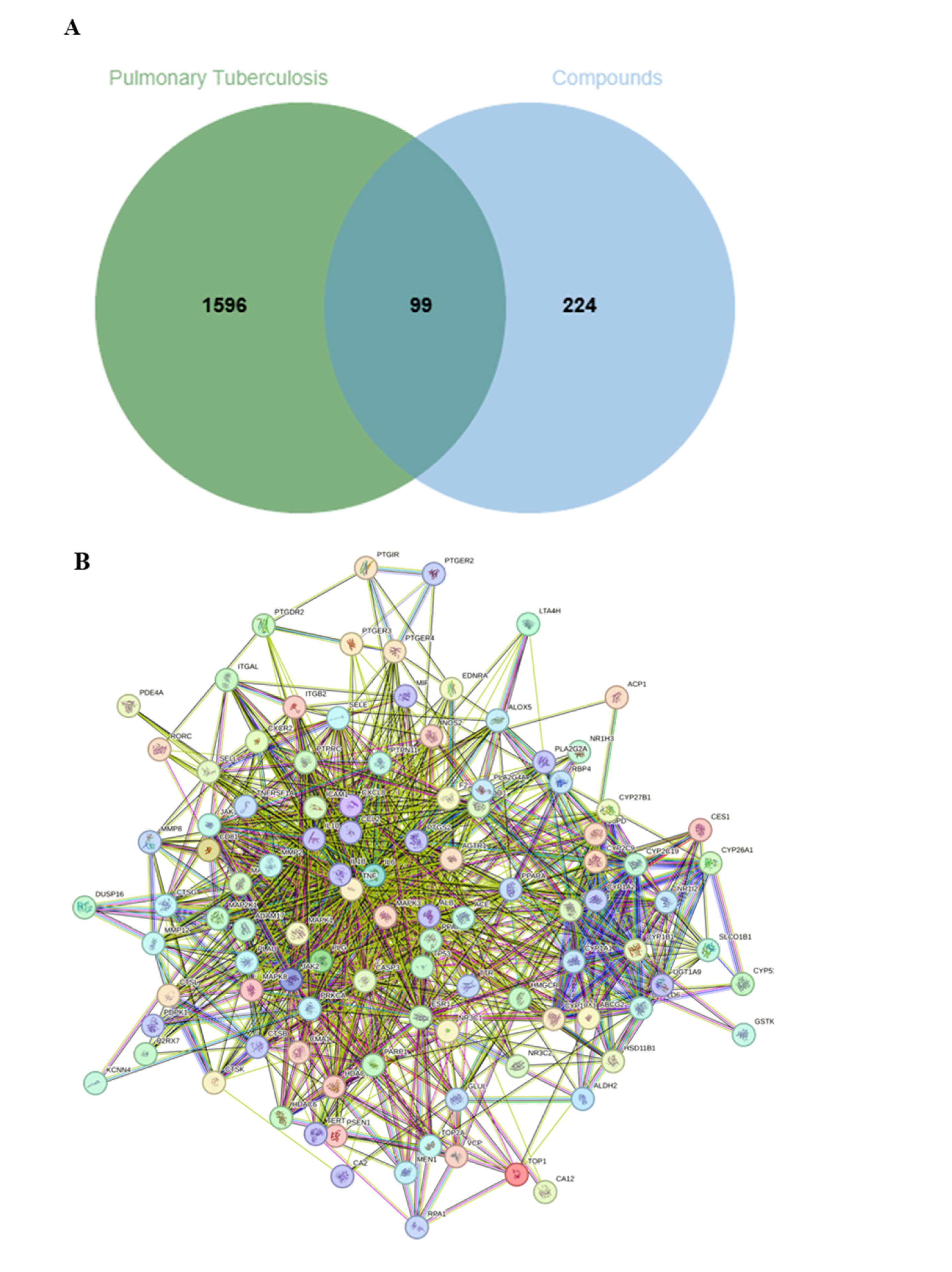
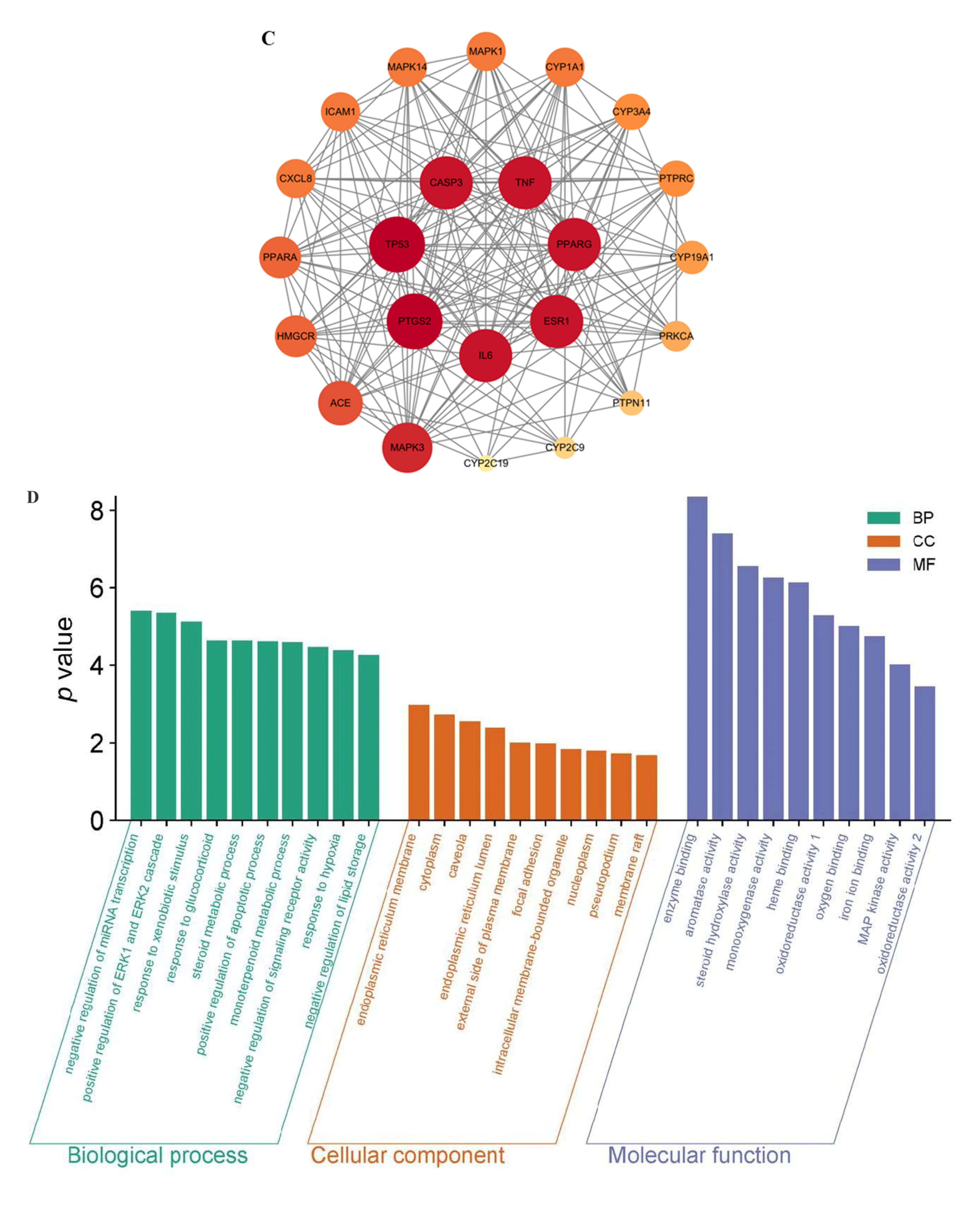
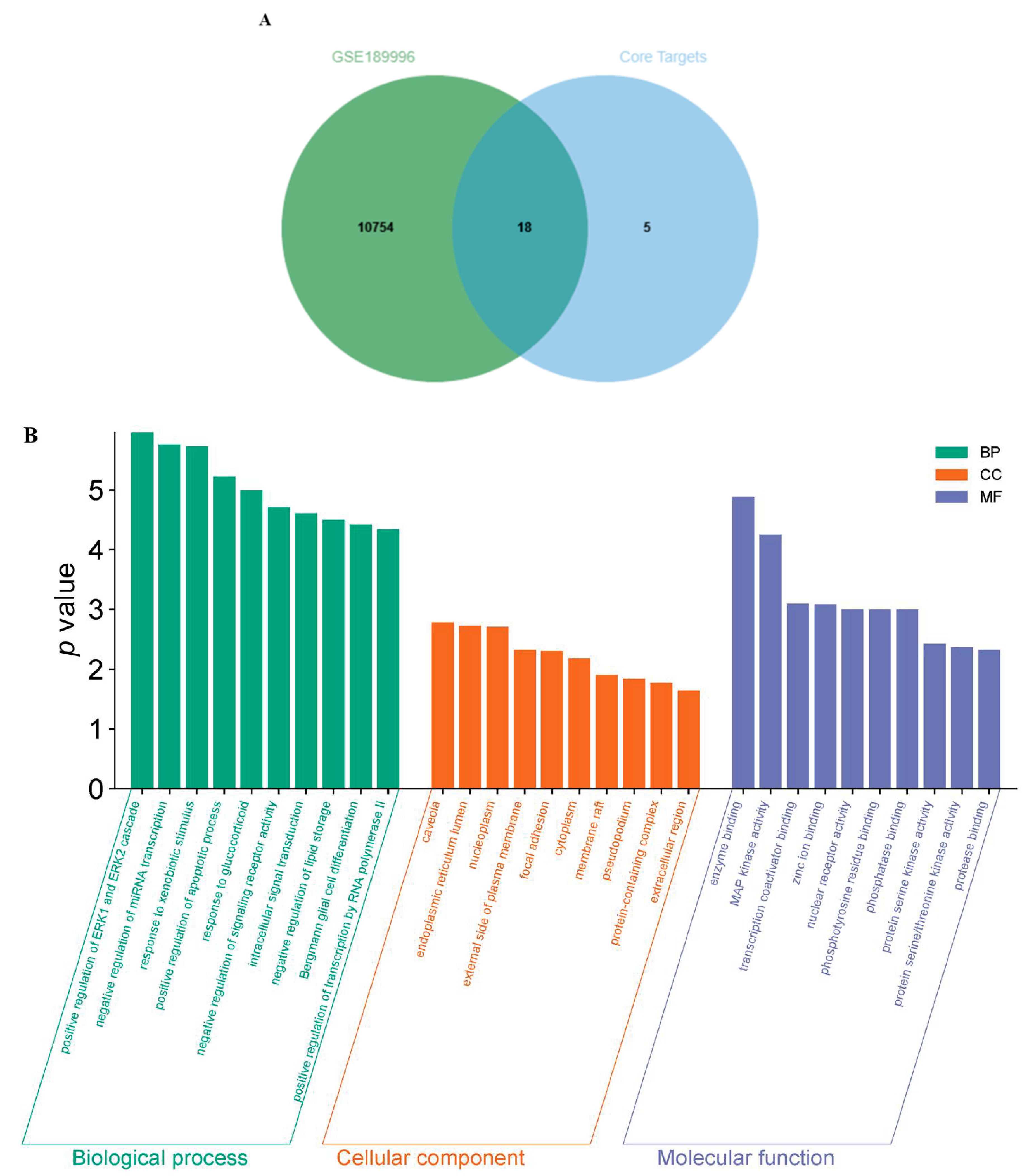

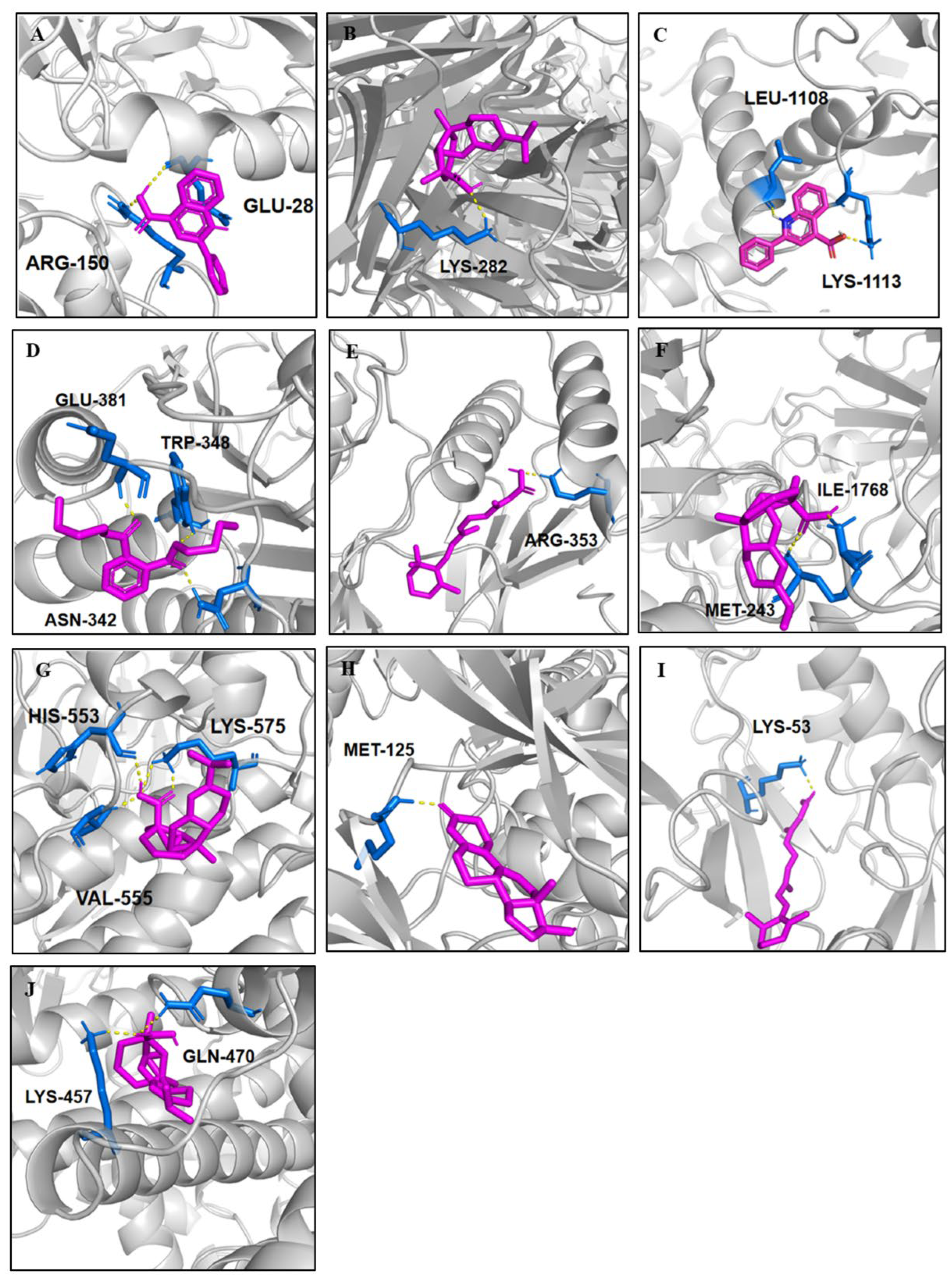
| No. | RT [Min] | mzCloud Best Match | Molecular Ion Peak (m/z) | Reference Ion | Name | Formula | Annot. Delta Mass [ppm] | Fragment Ions (m/z) | References |
|---|---|---|---|---|---|---|---|---|---|
| Fatty acids & esters | |||||||||
| 1 | 24.79 | 99.6 | 157.1232 | [M-H]−1 | Nonanoic acid | C9H18O2 | −1.09 | 139.1128; 113.0973; 59.0137 | [40] |
| 2 | 32.47 | 99.6 | 243.1963 | [M-H]−1 | 3-Hydroxy myristic acid | C14H28O3 | −0.95 | 225.1863; 181.1959; 59.0138; | [41] |
| 3 | 28.82 | 99.2 | 171.1389 | [M-H]−1 | Decanoic acid | C10H20O2 | −0.97 | 153.1285; 127.1129 | [40] |
| 4 | 36.20 | 99.2 | 213.1858 | [M-H]−1 | Tridecylic acid | C13H26O2 | −0.99 | 195.1753; 168.9926 | [42] |
| 5 | 34.52 | 99.1 | 199.1700 | [M-H]−1 | Lauric acid | C12H24O2 | −1.62 | 181.1600; 155.1081; 59.8251 | [40] |
| 6 | 37.48 | 99.1 | 227.2014 | [M-H]−1 | Myristic acid | C14H28O2 | −1.31 | 209.1911; 59.0136 | [40] |
| 7 | 34.35 | 99.1 | 361.2220 | [M+H]+1 | Tributyl citrate | C18H32O7 | −0.17 | 259.1539; 213.0778; 185.0806; 129.0181; 87.0072; 68.9970; | [43] |
| 8 | 42.05 | 98.9 | 255.2325 | [M-H]−1 | Ethyl myristate | C16H32O2 | −1.89 | 237.2226; 59.0138 | [44] |
| 9 | 47.10 | 98.9 | 283.2636 | [M-H]−1 | Stearic acid | C18H36O2 | −2.15 | 265.2538 | [45] |
| 10 | 39.24 | 98.8 | 241.2170 | [M-H]−1 | Pentadecanoic acid | C15H30O2 | −1.34 | 223.2068 | [40] |
| 11 | 42.72 | 98.6 | 281.2482 | [M-H]−1 | Oleic acid | C18H34O2 | −1.43 | 263.2381; 127.0763; 71.0139 | [40,46] |
| 12 | 41.08 | 98.5 | 267.2327 | [M-H]−1 | trans-10-Heptadecenoic acid | C17H32O2 | −1.07 | 249.2218; 83.0503 | [47] |
| 13 | 38.58 | 98.4 | 253.2169 | [M-H]−1 | Palmitoleic acid | C16H30O2 | −1.45 | 235.2069; 71.0138 | [48] |
| 14 | 32.20 | 98.4 | 185.1544 | [M-H]−1 | Undecanoic acid | C11H22O2 | −1.36 | 167.1441 | [40] |
| 15 | 39.62 | 97.7 | 279.2326 | [M-H]−1 | Linoleic Acid | C18H32O2 | −1.35 | 261.2224; 71.0138; 59.0137 | [48] |
| 16 | 33.96 | 95.8 | 297.2433 | [M-H]−1 | (E)-6-hydroxyoctadec-4-enoic acid | C18H34O3 | −0.88 | 279.2329; 183.1398; 155.1079; 141.1284; 71.0486; | [49] |
| 17 | 33.84 | 93.3 | 293.2119 | [M-H]−1 | 13-Hydroxy-9Z,11E,15Z-octadecatrienoic acid | C18H30O3 | −0.96 | 275.2016; 185.1184; 125.0970; 97.0657 | [50] |
| 18 | 32.62 | 93 | 271.2277 | [M-H]−1 | 16-Hydroxyhexadecanoic acid | C16H32O3 | −0.65 | 253.2173; 225.2220; 197.1911 155.1443; 99.0815; 59.0138 | [50] |
| Phosphorus-containing compounds | |||||||||
| 19 | 31.25 | 99.7 | 267.1717 | [M+H]+1 | Tributyl phosphate | C12H27O4P | −1.15 | 267.1716; 211.1093; 155.0466 116.9946; 98.9839; 57.0698; | [51] |
| 20 | 31.21 | 99.6 | 211.1092 | [M+H]+1 | Dibutyl phosphate | C8H19O4P | −0.93 | 155.0466; 98.9840; 7.0698 | [51] |
| 21 | 20.26 | 99.2 | 279.0928 | [M+H]+1 | Triphenylphosphine oxide | C18H15OP | −1.75 | 219.0568; 201.0462; 173.0512 141.0102; 95.0043; 77.0386; | [52] |
| 22 | 9.96 | 99.1 | 183.0779 | [M+H]+1 | Triethyl phosphate | C6H15O4P | −1.13 | 155.0468; 127.0154; 116.9947; 98.9841 | [53] |
| Alkaloids | |||||||||
| 23 | 38.14 | 98.3 | 256.2634 | [M+H]+1 | Hexadecanamide | C16H33NO | −0.25 | 130.1225; 102.0912; 88.0756; | [54] |
| 24 | 0.74 | 94.5 | 125.0708 | [M+H]+1 | 4,6-Dimethyl-2-hydroxypyrimidine | C6H8N2O | −0.82 | 107.0603; 98.0603; 66.0338 | [55] |
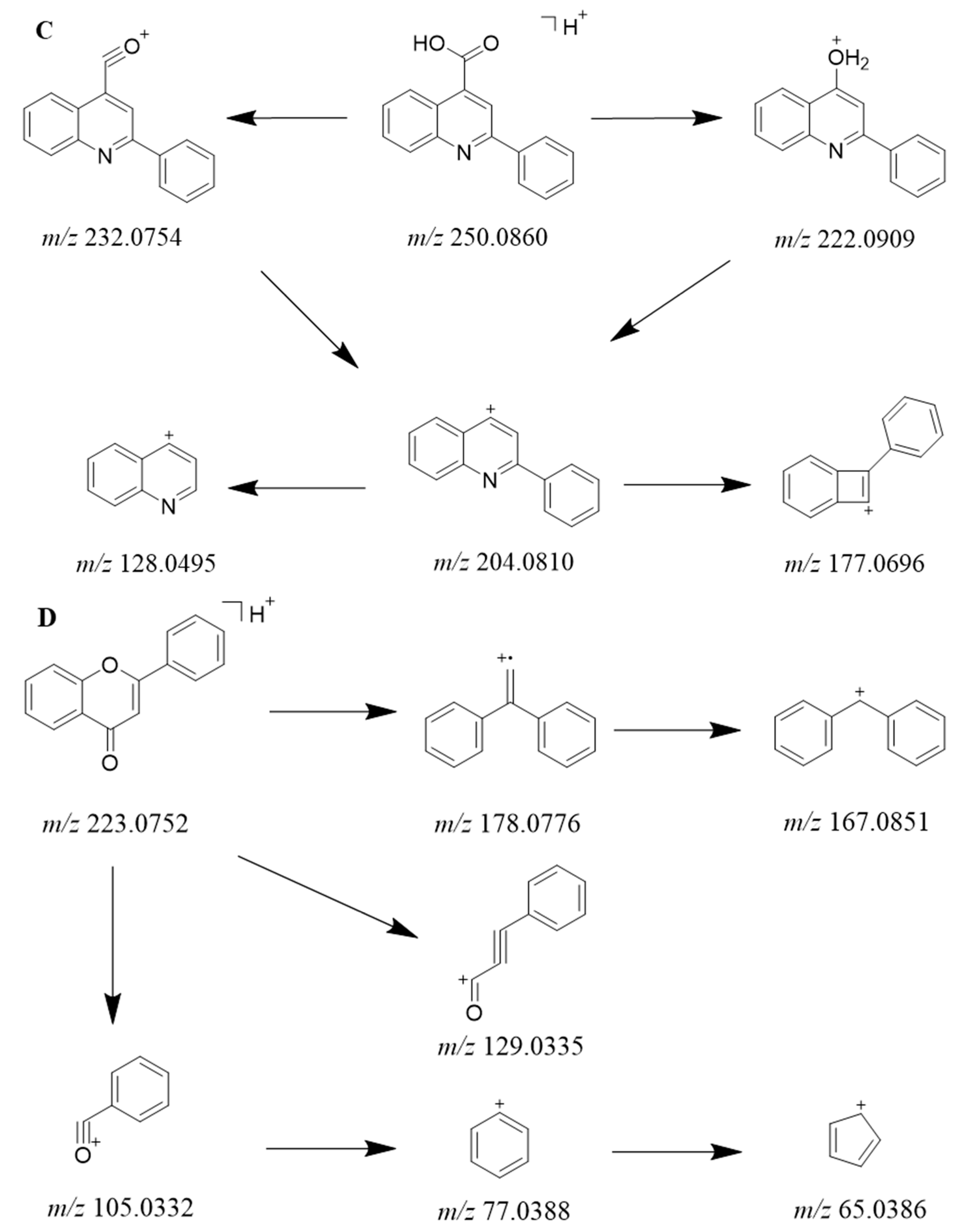
| 25 | 23.27 | 94.1 | 250.0860 | [M+H]+1 | Cinchophen | C16H11NO2 | −0.89 | 232.0754; 222.0909; 204.0810 | [56] |
| 26 | 20.22 | 93.5 | 225.1958 | [M+H]+1 | N, N-Dicyclohexylurea | C13H24N2O | −1.53 | 143.1179; 100.1120; 83.0855 | [54] |
| Sulfur-containing compounds | |||||||||
| 27 | 22.60 | 99.8 | 265.1476 | [M-H]−1 | Dodecyl sulfate | C12H26O4S | −1.09 | 96.9601; 79.9574 | [57] |
| 28 | 26.98 | 99.6 | 293.1789 | [M-H]−1 | Myristyl sulfate | C14H30O4S | −0.95 | 96.9600; 79.9573 | [58] |
| 29 | 26.52 | 99.3 | 325.1839 | [M-H]−1 | 4-Dodecylbenzenesulfonic acid | C18H30O3S | −1.29 | 239.0745; 225.0588; 197.0260; 183.0119; 170.0040; 79.9574 | [57,58,59] |
| Aromatics | |||||||||
| 30 | 34.23 | 99.7 | 205.1595 | [M-H]−1 | 2,4-Di-tert-butylphenol | C14H22O | −1.55 | 189.1284 | [60] |
| 31 | 0.94 | 99.2 | 279.1590 | [M+H]+1 | Dibutyl phthalate | C16H22O4 | −0.43 | 205.0856; 149.0232; 121.0282 111.0438; 93.0335; 65.0386 | [61,62] |
| 32 | 38.99 | 99 | 339.2318 | [M-H]−1 | 2,2′-Methylenebis(4-methyl-6-tert-butylphenol) | C23H32O2 | −3.54 | 163.1127; 147.0812; 107.0503 | [63] |
| Terpenes | |||||||||
| 33 | 37.59 | 97.9 | 301.2170 | [M-H]−1 | Abietic acid | C20H30O2 | −1.07 | 271.1695; 257.1539 | [64,65,66] |
| 34 | 35.33 | 96.8 | 299.2013 | [M-H]−1 | Tretinoin | C20H28O2 | −1.2 | 265.2418; 255.2118; 175.0765 | [67,68] |
| Flavonoid | |||||||||
| 35 | 22.09 | 98.2 | 223.0753 | [M+H]+1 | Flavone | C15H10O2 | −0.38 | 178.0776; 129.0335; 121.0282; 103.0542; 95.0488; 77.0388 | [69] |
| Ether | |||||||||
| 36 | 27.26 | 97.7 | 219.1952 | [M+H]+1 | Bis(2-butoxyethyl) ether | C12H26O3 | −1.11 | 163.1312; 145.1215; 101.0960; 89.0598; 83.0856; 57.0698; | [70] |
| Steroid | |||||||||
| 37 | 27.32 | 90.8 | 271.1691 | [M+H]+1 | Trenbolone | C18H22O2 | −0.59 | 253.1588; 215.1063; 197.0961; 193.1008; 145.1012; 131.0858; | [71,72] |
| No. | Targeted Protein | Compounds (No. in Table 1) | Binding Energy (kcal/mol) | H-Bonds Number | Binding Site |
|---|---|---|---|---|---|
| 1 | CXCL8 | Cinchophen (25) | −6.12 | 2 | ARG-150; GLU-28 |
| 2 | TNF | Abietic acid (33) | −6.83 | 1 | LYS-282 |
| 3 | ICAM1 | Cinchophen (25) | −6.19 | 2 | LEU-1108; LYS-1113 |
| 4 | CASP3 | Dibutyl phthalate (31) | −5.19 | 3 | GLU-381; TRP-348; ASN-342 |
| 5 | MAPK1 | Tretinoin (34) | −6.67 | 1 | ARG-353 |
| 6 | TP53 | Abietic acid (33) | −6.06 | 2 | MET-243; ILE-1768 |
| 7 | PRKCA | Dibutyl phthalate (31) | −5.64 | 1 | LYS-517 |
| Abietic acid (33) | −8.13 | 4 | HIS-553; VAL-555; LYS-575; LYS-575 | ||
| 8 | MAPK3 | Abietic acid (33) | −6.14 | 2 | ARG-242; ILE-244 |
| Trenbolone (37) | −7.38 | 1 | MET-125 | ||
| 9 | MAPK14 | Tretinoin (34) | −7.89 | 1 | LYS-53 |
| Linoleic acid (15) | −5.02 | 2 | ARG-149; ILE-147 | ||
| Dibutyl phthalate (31) | −5.58 | 1 | MET-109 | ||
| 10 | PPARγ | Oleic acid (11) | −5.04 | 2 | LYS-458; LYS-457 |
| Linoleic acid (15) | −5.78 | 2 | LYS-457; GLN-470 | ||
| Palmitoleic acid (13) | −5.21 | 1 | SER-342 | ||
| Abietic acid (33) | −8.21 | 2 | LYS-457; GLN-470 | ||
| Tretinoin (34) | −7.83 | 2 | LEU-476; TYR-473 | ||
| Stearic acid (9) | −5.85 | 2 | LYS-474; LYS-457 | ||
| trans-10-Heptadecenoic acid (12) | −5.87 | 2 | ARG-288; GLU-343 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Jiang, L.; Dai, Y.; Si, X.; Li, H.; Anjum, K. The UHPLC-Orbitrap MS/MS and Network Pharmacology Strategies Reveal the Active Antioxidants of Bleeding Sap from Sponge Gourd in Treating Tuberculosis. Int. J. Mol. Sci. 2025, 26, 10231. https://doi.org/10.3390/ijms262010231
Zhang D, Jiang L, Dai Y, Si X, Li H, Anjum K. The UHPLC-Orbitrap MS/MS and Network Pharmacology Strategies Reveal the Active Antioxidants of Bleeding Sap from Sponge Gourd in Treating Tuberculosis. International Journal of Molecular Sciences. 2025; 26(20):10231. https://doi.org/10.3390/ijms262010231
Chicago/Turabian StyleZhang, Di, Lu Jiang, Yujiang Dai, Xinxin Si, Huifang Li, and Komal Anjum. 2025. "The UHPLC-Orbitrap MS/MS and Network Pharmacology Strategies Reveal the Active Antioxidants of Bleeding Sap from Sponge Gourd in Treating Tuberculosis" International Journal of Molecular Sciences 26, no. 20: 10231. https://doi.org/10.3390/ijms262010231
APA StyleZhang, D., Jiang, L., Dai, Y., Si, X., Li, H., & Anjum, K. (2025). The UHPLC-Orbitrap MS/MS and Network Pharmacology Strategies Reveal the Active Antioxidants of Bleeding Sap from Sponge Gourd in Treating Tuberculosis. International Journal of Molecular Sciences, 26(20), 10231. https://doi.org/10.3390/ijms262010231