Berbamine Targets TNFAIP3: A Bioactive Compound Alleviates Oxidative Stress and Inflammation in the Comorbidity of Insomnia and Chronic Obstructive Pulmonary Disease Through Multi-Omics Integration
Abstract
1. Introduction
2. Results
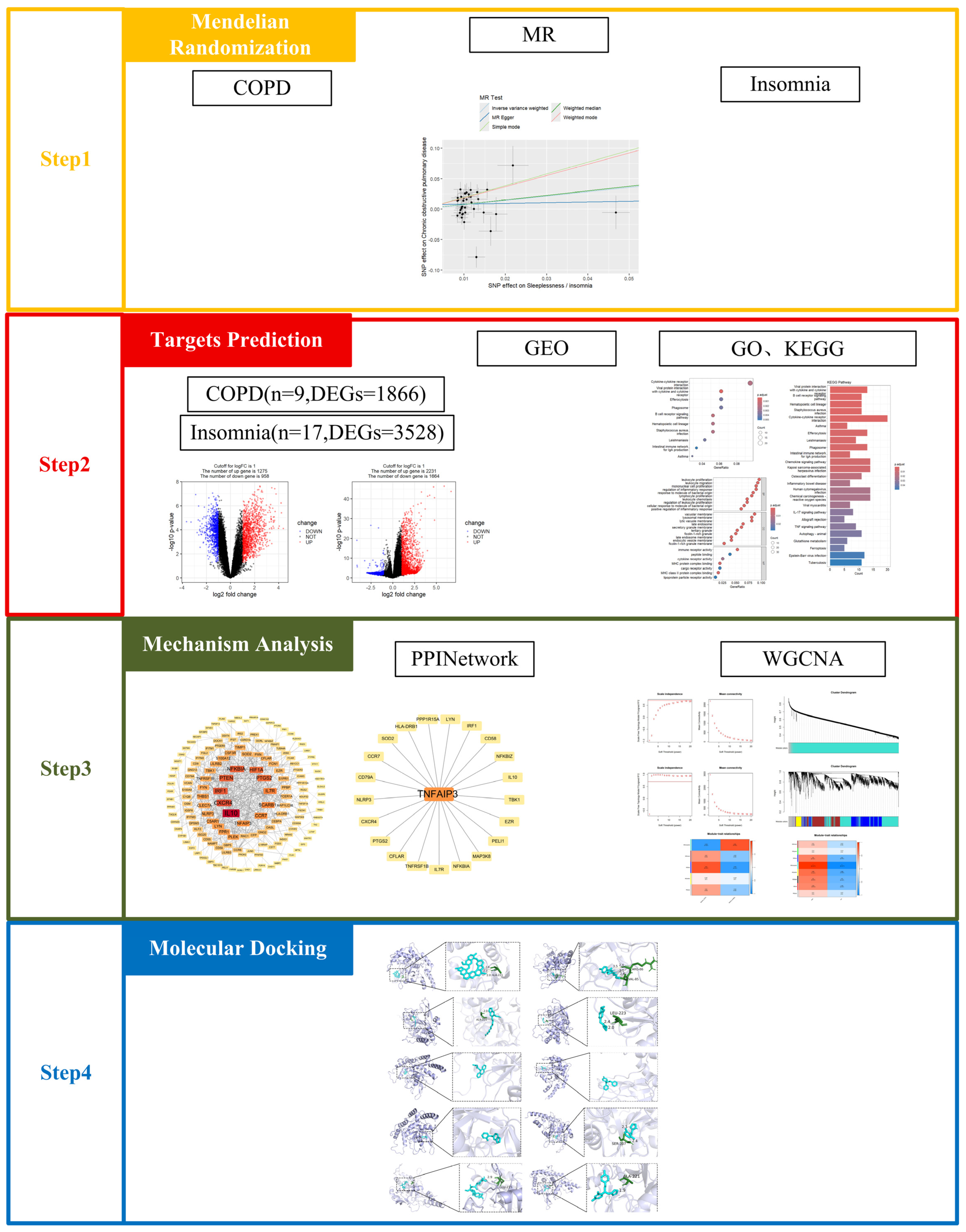
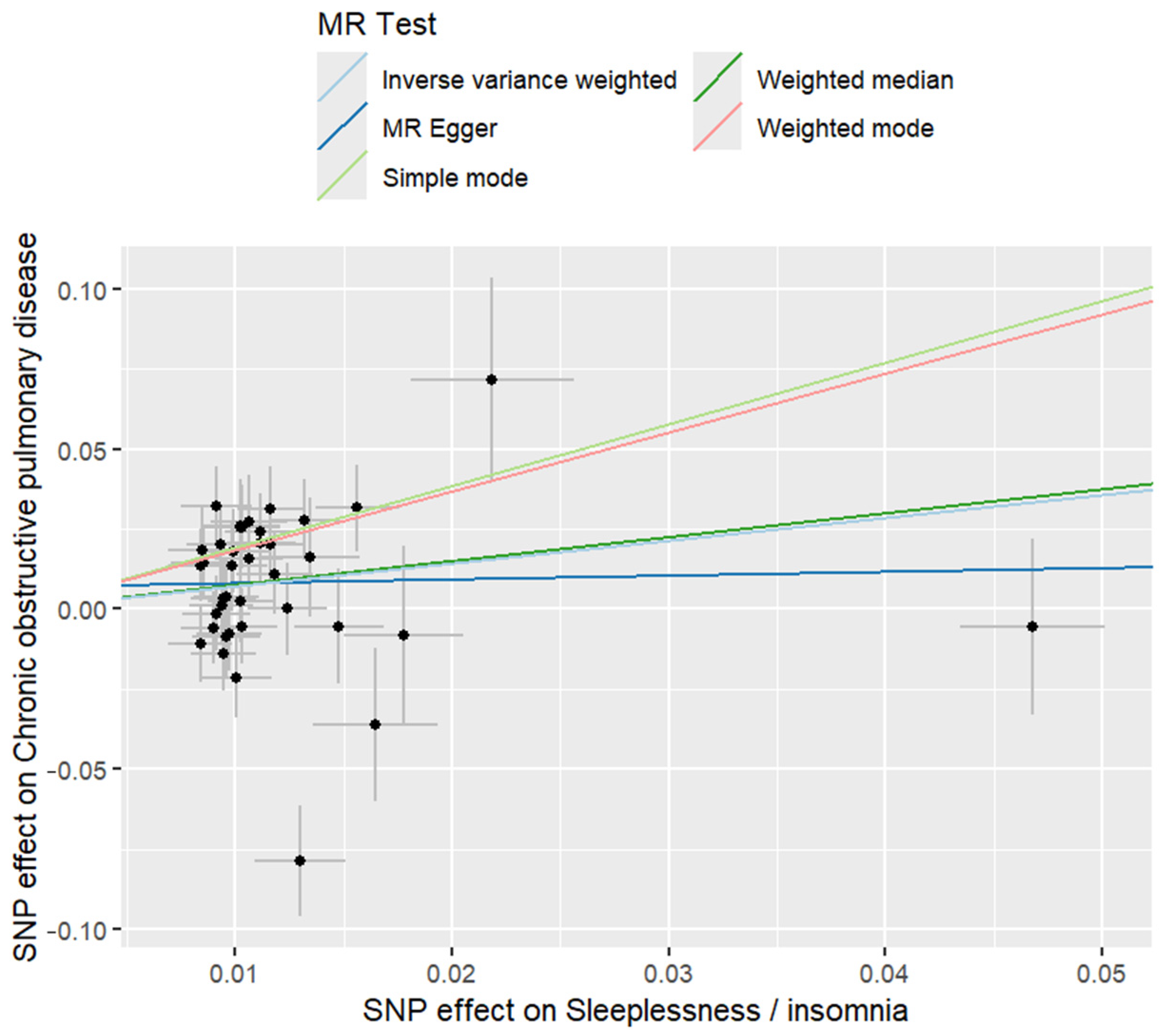
2.1. Mendelian Randomisation
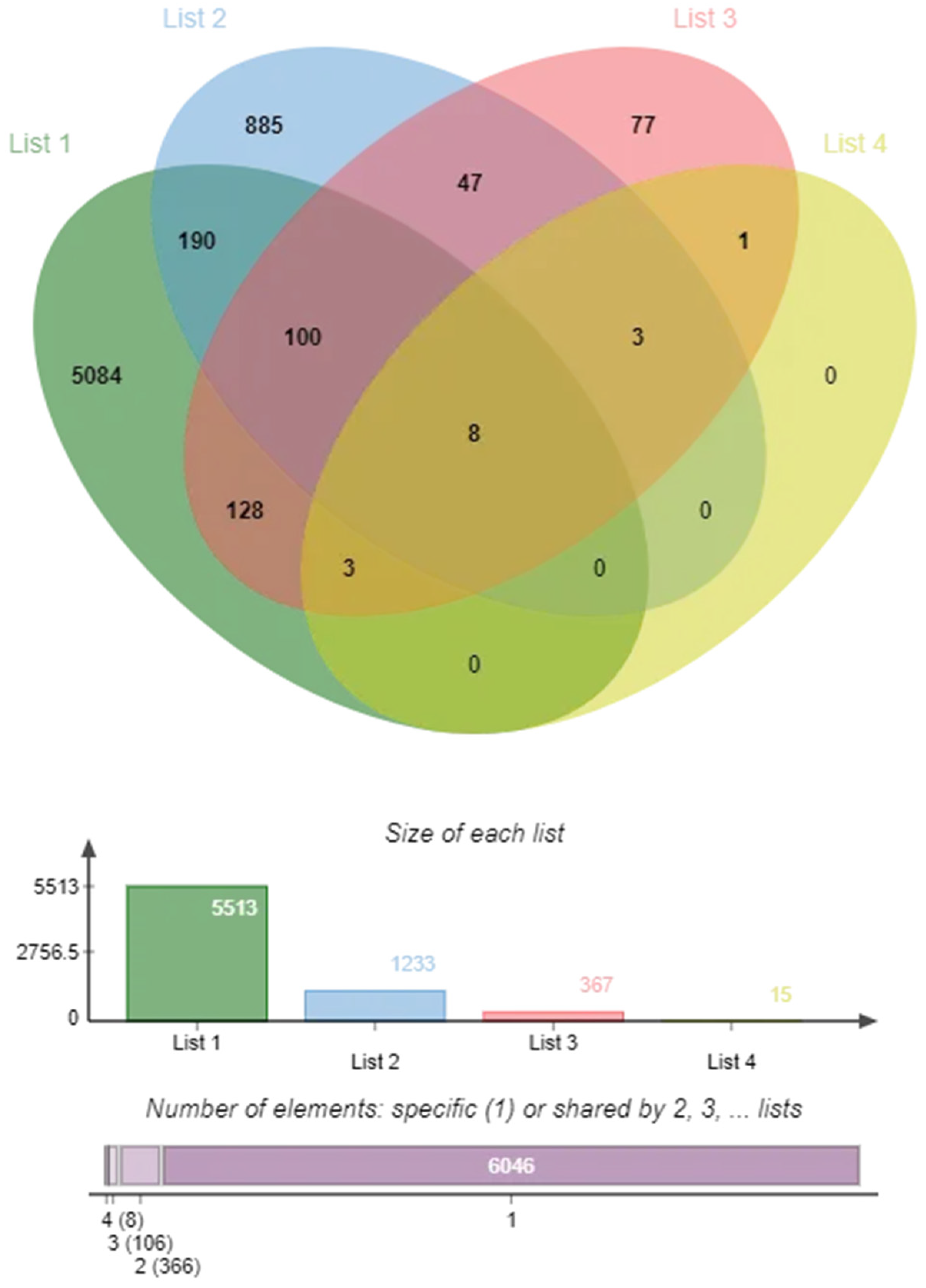
2.2. Targets of COPD and Insomnia
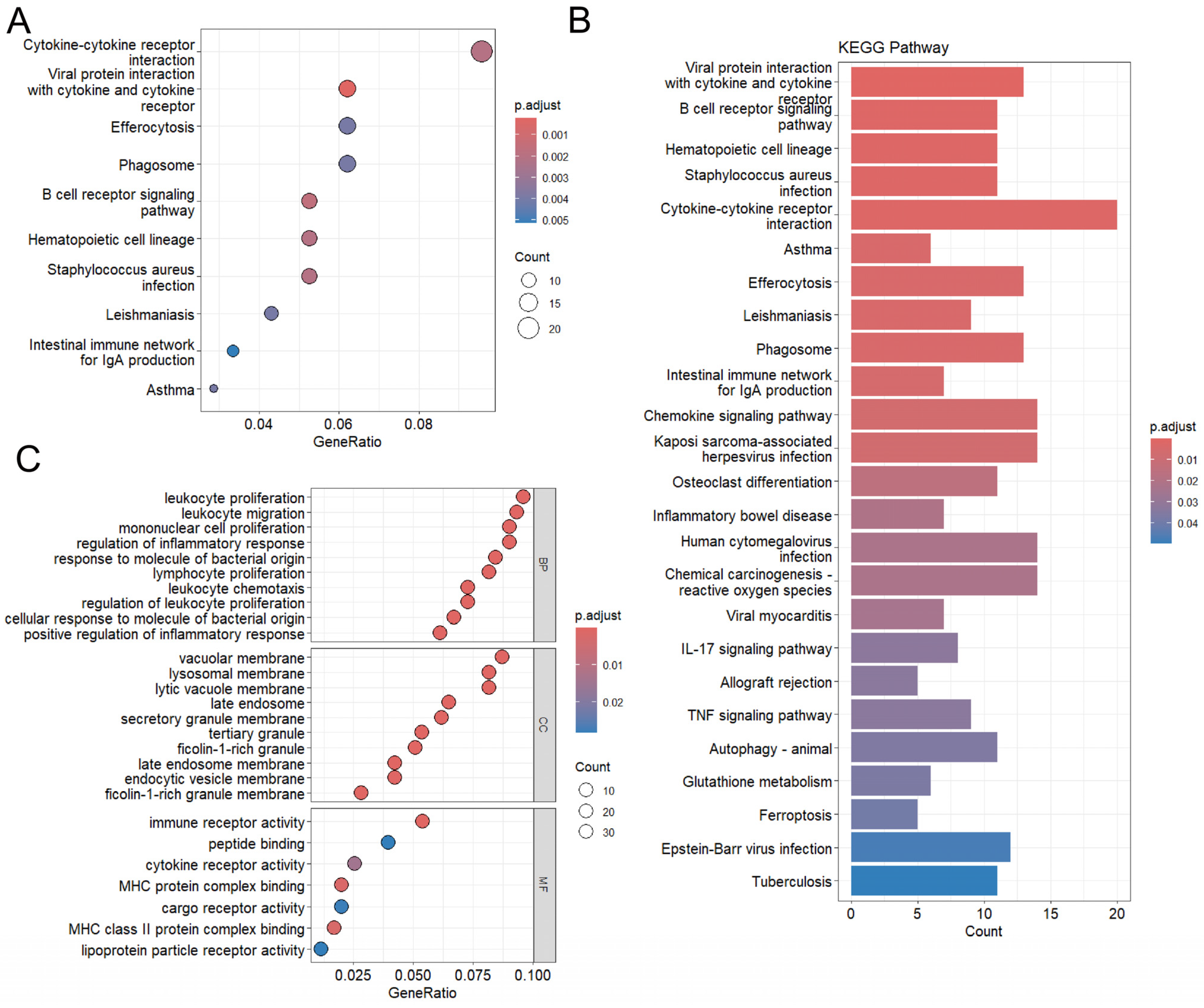
2.3. Functional Enrichment Analysis
2.4. Interaction Network Analysis
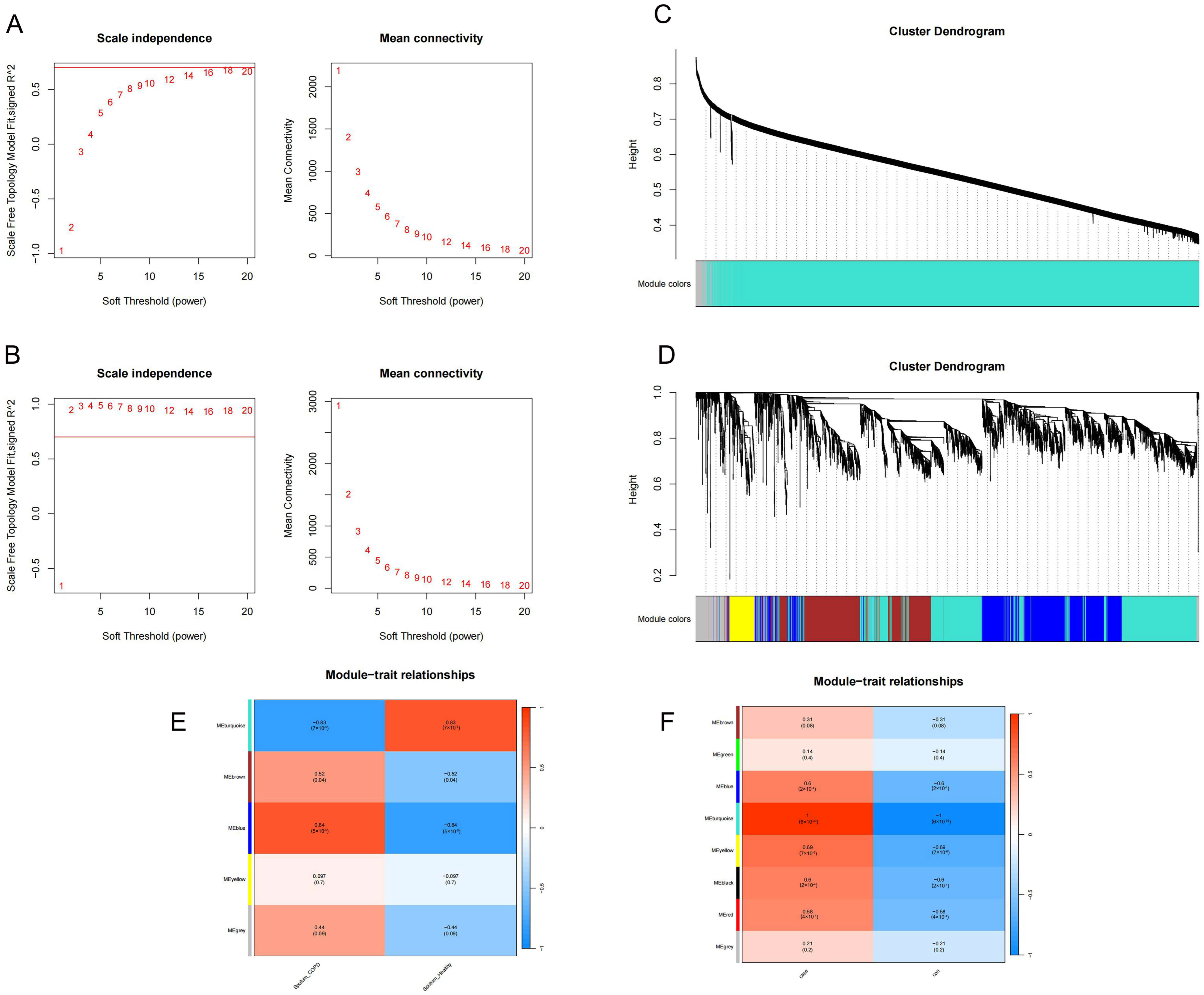
2.5. Identification of Key Modules
2.6. Biomarker Screening
2.7. Drug Signatures
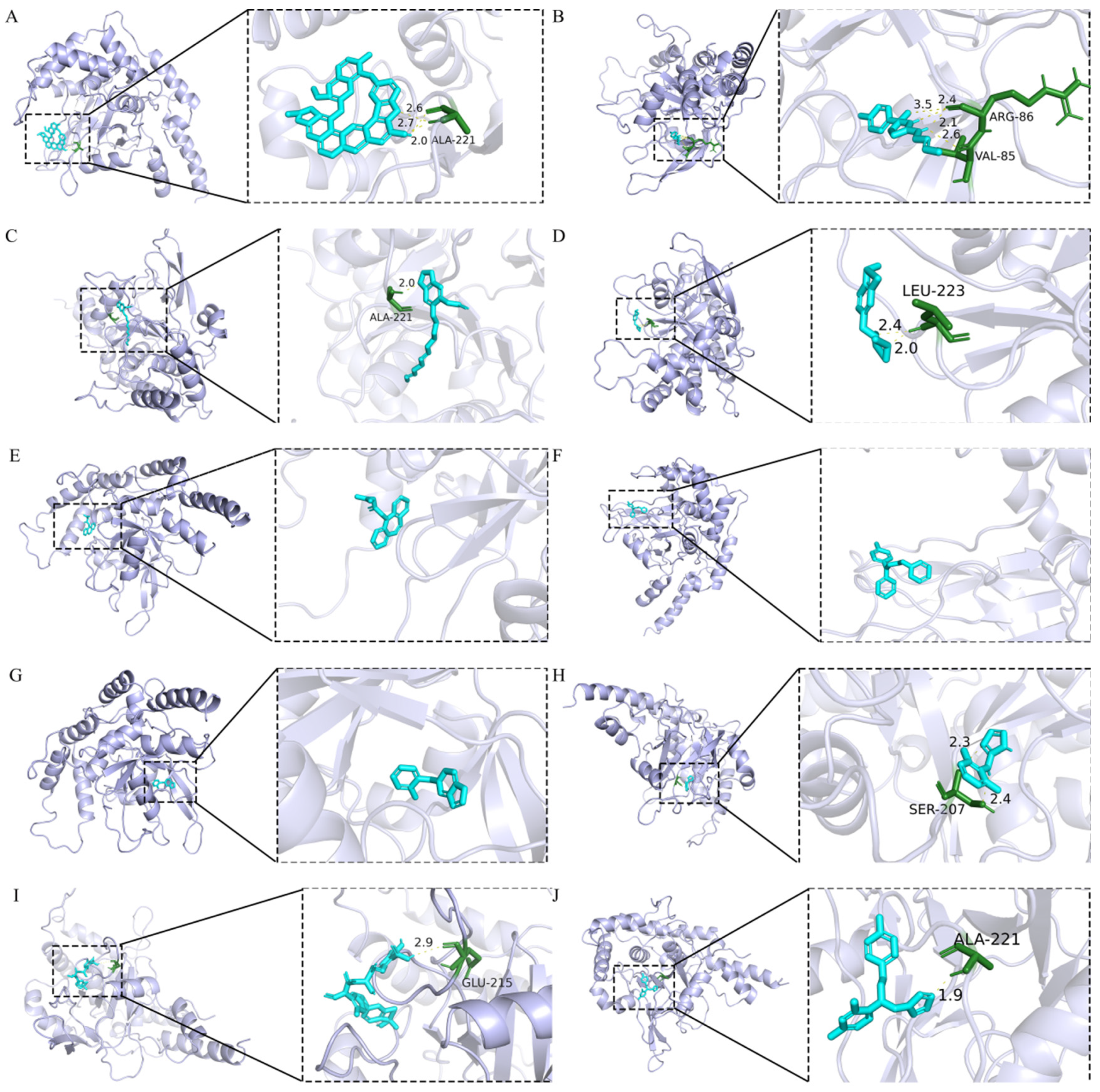
2.8. Molecular Docking Analysis
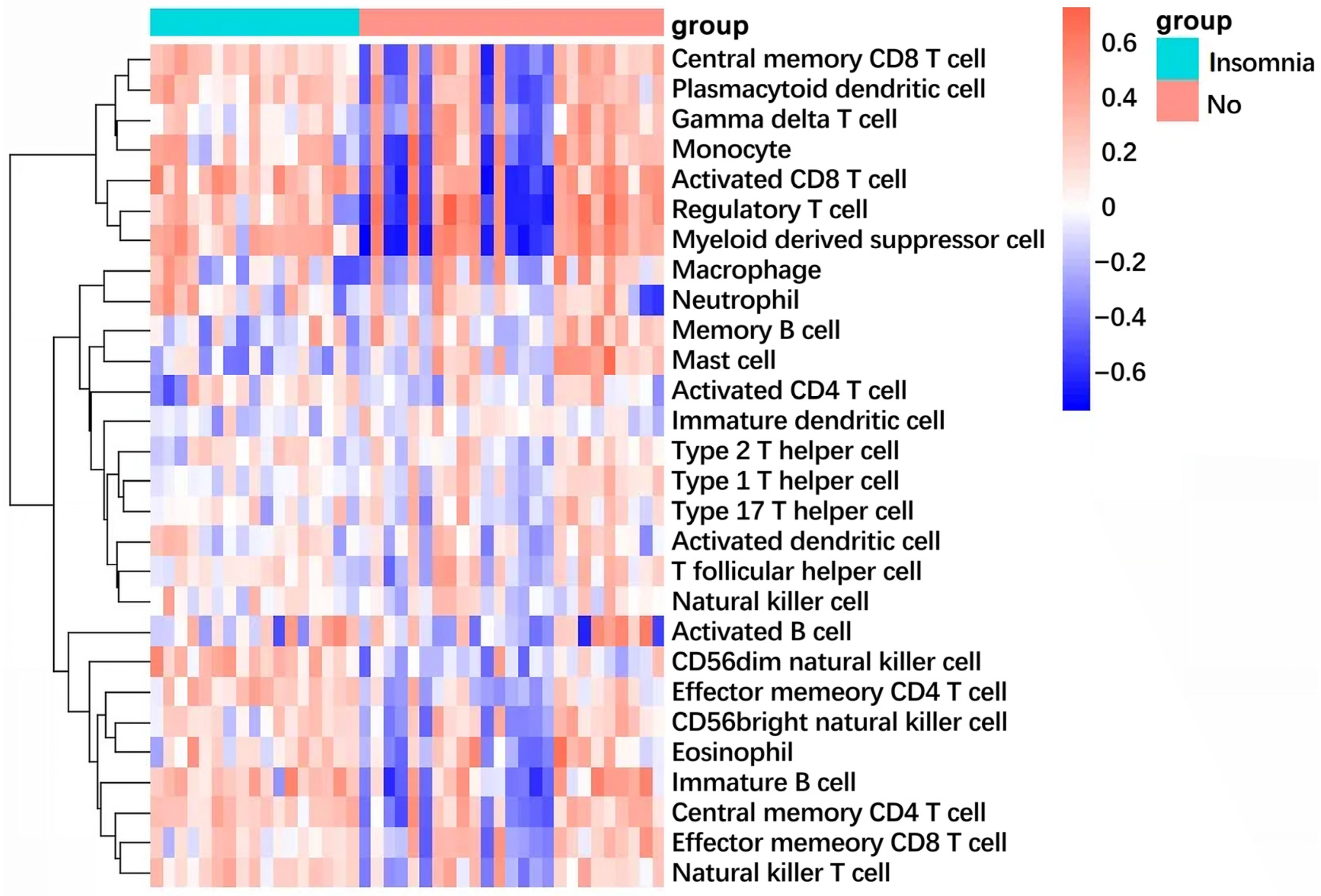
2.9. Immune Infiltration Landscape Reveals Shared Inflammatory Signature
3. Discussion
4. Materials and Methods
4.1. Mendelian Randomisation
4.2. Targets of COPD and Insomnia
4.3. GO/KEGG Enrichment Analysis
4.4. PPI Network Analysis
4.5. WGCNA
4.6. LASSO Regression Analysis
4.7. Drug Repositioning Analysis
4.8. Molecular Docking Analysis
4.9. Immune Infiltration Analysis by Single-Sample GSEA (ssGSEA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COPD | Chronic obstructive pulmonary disease |
| MR | Mendelian randomization |
| PPI | Protein–protein interaction |
| WGCNA | Weighted gene co-expression network analysis |
References
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef]
- Labaki, W.W.; Rosenberg, S.R. Chronic Obstructive Pulmonary Disease. Ann. Intern. Med. 2020, 173, ITC17–ITC32. [Google Scholar] [CrossRef]
- Vanfleteren, L.E.; Beghe, B.; Andersson, A.; Hansson, D.; Fabbri, L.M.; Grote, L. Multimorbidity in COPD, does sleep matter? Eur. J. Intern. Med. 2020, 73, 7–15. [Google Scholar] [CrossRef]
- Budhiraja, R.; Parthasarathy, S.; Budhiraja, P.; Habib, M.P.; Wendel, C.; Quan, S.F. Insomnia in patients with COPD. Sleep 2012, 35, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, H.; Arzt, M.; Andreas, S.; Plate, T.; Ribera, A.; Seoane, B.; Watz, H.; Kirsten, A.M. Aclidinium bromide improves symptoms and sleep quality in COPD: A pilot study. Eur. Respir. J. 2017, 49, 1700485. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, M.R.; Martin, J.L.; Kleerup, E.C.; Schneider, H.; Mitchell, M.N.; Hansel, N.N.; Sundar, K.; Schotland, H.; Basner, R.C.; Wells, J.M.; et al. Sleep disruption as a predictor of quality of life among patients in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS). Sleep 2018, 41, zsy044. [Google Scholar] [CrossRef]
- Shorofsky, M.; Bourbeau, J.; Kimoff, J.; Jen, R.; Malhotra, A.; Ayas, N.; Tan, W.C.; Aaron, S.D.; Sin, D.D.; Road, J.; et al. Impaired Sleep Quality in COPD Is Associated with Exacerbations: The CanCOLD Cohort Study. Chest 2019, 156, 852–863. [Google Scholar] [CrossRef]
- Li, X.; Feng, S.; Yang, Y.; Liang, Z.; Song, A.; Chen, J.; Guo, Z.; Chen, Z.; Miao, C.; Yang, H.; et al. Association Between Airway Mucus Plugs and Risk of Moderate-to-Severe Exacerbations in Patients with COPD: Results from a Chinese Prospective Cohort Study. Chest 2025, 168, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q.; Sun, X.W.; Zhang, L.; Ding, Y.J.; Li, H.P.; Yan, Y.R.; Lin, Y.N.; Zhou, J.P.; Li, Q.Y. Impact of insomnia and obstructive sleep apnea on the risk of acute exacerbation of chronic obstructive pulmonary disease. Sleep Med. Rev. 2021, 58, 101444. [Google Scholar] [CrossRef]
- Hegerl, U.; Mergl, R. Depression and suicidality in COPD: Understandable reaction or independent disorders? Eur. Respir. J. 2014, 44, 734–743. [Google Scholar] [CrossRef]
- Laue, J.; Reierth, E.; Melbye, H. When should acute exacerbations of COPD be treated with systemic corticosteroids and antibiotics in primary care: A systematic review of current COPD guidelines. NPJ Prim. Care Respir. Med. 2015, 25, 15002. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef]
- Chen, Y.W.; HajGhanbari, B.; Road, J.D.; Coxson, H.O.; Camp, P.G.; Reid, W.D. Reliability and validity of the Brief Pain Inventory in individuals with chronic obstructive pulmonary disease. Eur. J. Pain. 2018, 22, 1718–1726. [Google Scholar] [CrossRef]
- Volpato, E.; Farver-Vestergaard, I.; Brighton, L.J.; Peters, J.; Verkleij, M.; Hutchinson, A.; Heijmans, M.; von Leupoldt, A. Nonpharmacological management of psychological distress in people with COPD. Eur. Respir. Rev. 2023, 32, 220170. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, Z.; Fang, R.; Li, X.; Li, W. Giardia duodenalis induces extrinsic pathway of apoptosis in intestinal epithelial cells through activation of TNFR1 and K63 de-ubiquitination of RIP1 in vitro. Microb. Pathog. 2020, 149, 104315. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, Y.; Kang, Y.; He, L.; Zhu, B.; Zhang, W.; Lu, Y.; Wu, Q.; Xu, D.; Shi, L. MicroRNA-127 Promotes Anti-microbial Host Defense through Restricting A20-Mediated De-ubiquitination of STAT3. iScience 2020, 23, 100763. [Google Scholar] [CrossRef]
- Zhou, M.; He, J.; Shi, Y.; Liu, X.; Luo, S.; Cheng, C.; Ge, W.; Qu, C.; Du, P.; Chen, Y. ABIN3 Negatively Regulates Necroptosis-induced Intestinal Inflammation Through Recruiting A20 and Restricting the Ubiquitination of RIPK3 in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 99–114. [Google Scholar] [CrossRef]
- Yan, K.; Wu, C.; Ye, Y.; Li, L.; Wang, X.; He, W.; Ren, S.; Xu, Y. A20 inhibits osteoclastogenesis via TRAF6-dependent autophagy in human periodontal ligament cells under hypoxia. Cell Prolif. 2020, 53, e12778. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, J.J.; Huang, H.I.; Kanayama, M.; Youssef, N.; Jin, Y.J.; Reyes, E.Y.; Abram, C.L.; Yang, S.; Lowell, C.A.; et al. The Ubiquitin-Modifying Enzyme A20 Terminates C-Type Lectin Receptor Signals and Is a Suppressor of Host Defense against Systemic Fungal Infection. Infect. Immun. 2020, 88, 10–1128. [Google Scholar] [CrossRef]
- Goyal, A.; Chopra, V.; Garg, K.; Sharma, S. Mechanisms coupling the mTOR pathway to chronic obstructive pulmonary disease (COPD) pathogenesis. Cytokine Growth Factor Rev. 2025, 82, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Potteti, H.R.; Venkareddy, L.K.; Noone, P.M.; Ankireddy, A.; Tamatam, C.R.; Mehta, D.; Tiruppathi, C.; Reddy, S.P. Nrf2 Regulates Anti-Inflammatory A20 Deubiquitinase Induction by LPS in Macrophages in Contextual Manner. Antioxidants 2021, 10, 847. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Turner, J.H. Proinflammatory mediators alter expression of nuclear factor kappa B-regulating deubiquitinases in sinonasal epithelial cells. Int. Forum Allergy Rhinol. 2015, 5, 583–589. [Google Scholar] [CrossRef]
- Ma, N.; Ji, X.; Shi, Y.; Wang, Q.; Wu, J.; Cui, X.; Niu, W. Adverse childhood experiences and mental health disorder in China: A nationwide study from CHARLS. J. Affect. Disord. 2024, 355, 22–30. [Google Scholar] [CrossRef]
- Hedström, A.K.; Hössjer, O.; Bellocco, R.; Ye, W.; Trolle, L.Y.; Åkerstedt, T. Insomnia in the context of short sleep increases suicide risk. Sleep 2021, 44, zsaa245. [Google Scholar] [PubMed]
- Papaioannou, A.I.; Hillas, G.; Loukides, S.; Vassilakopoulos, T. Mortality prevention as the centre of COPD management. ERJ Open Res. 2024, 10, 00850–2023. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Zhai, S.; Tao, S.; Wu, X.; Zou, L.; Yang, Y.; Xie, Y.; Li, T.; Zhang, D.; Qu, Y.; Tao, F. Associations of Sleep Insufficiency and Chronotype with Inflammatory Cytokines in College Students. Nat. Sci. Sleep 2021, 13, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Sogkas, G.; Witte, T. The link between rheumatic disorders and inborn errors of immunity. EBioMedicine 2023, 90, 104501. [Google Scholar] [CrossRef]
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of Gut Microbiota Biotransformation on Dietary Tannins and Human Health Implications. Microorganisms 2021, 9, 965. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Liao, Y.; Weng, Z.; Chen, Y.; Ouchi, T.; Fan, Y.; Zhao, Z.; Li, L. Multi-omics approach reveals TGF-β signaling-driven senescence in periodontium stem cells. J. Adv. Res. 2024, 76, 387–403. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M.; Zhu, L.; Chen, L.; Zhang, H.; Huang, Z.; Zhou, H. Tubeimoside I Inhibits the Proliferation of Liver Cancer Through Inactivating NF-κB Pathway by Regulating TNFAIP3 Expression. Drug Des. Devel Ther. 2025, 19, 1895–1908. [Google Scholar] [CrossRef]
- Xie, Y.; He, Q.; Chen, H.; Lin, Z.; Xu, Y.; Yang, C. Crocin ameliorates chronic obstructive pulmonary disease-induced depression via PI3K/Akt mediated suppression of inflammation. Eur. J. Pharmacol. 2019, 862, 172640. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.A.; Huang, T.L.; Huang, K.W.; Hung, Y.Y. TNFAIP3 mRNA Level Is Associated with Psychological Anxiety in Major Depressive Disorder. Neuroimmunomodulation 2017, 24, 271–275. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Oshima, S.; Takahara, M.; Maeyashiki, C.; Nemoto, Y.; Kobayashi, M.; Nibe, Y.; Nozaki, K.; Nagaishi, T.; Okamoto, R.; et al. TNFAIP3 promotes survival of CD4 T cells by restricting MTOR and promoting autophagy. Autophagy 2015, 11, 1052–1062. [Google Scholar] [CrossRef]
- Xing, D.; Jin, Y.; Sun, D.; Liu, Y.; Cai, B.; Gao, C.; Cui, Y.; Jin, B. Protective effect of TNFAIP3 on testosterone production in Leydig cells under an aging inflammatory microenvironment. Arch. Gerontol. Geriatr. 2024, 117, 105274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Q.; Sun, W.; Zhou, X.; Fu, D.; Mao, L. New Insights into the Role of NLRP3 Inflammasome in Pathogenesis and Treatment of Chronic Obstructive Pulmonary Disease. J. Inflamm. Res. 2021, 14, 4155–4168. [Google Scholar] [CrossRef]
- Chan, S.M.H.; Selemidis, S.; Vlahos, R. The Double-Edged Sword of ROS in Muscle Wasting and COPD: Insights from Aging-Related Sarcopenia. Antioxidants 2024, 13, 882. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Kovacova, V.; Mondockova, V.; Zemanova, N.; Babikova, M.; Biro, R.; Ciernikova, S.; Omelka, R. Honey: A Promising Therapeutic Supplement for the Prevention and Management of Osteoporosis and Breast Cancer. Antioxidants 2023, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Xia, J.; Benner, M.J.; Hancock, R.E. NetworkAnalyst—Integrative approaches for protein-protein interaction network analysis and visual exploration. Nucleic Acids Res. 2014, 42, W167–W174. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Yu, H.; Du, X.; Zhao, Q.; Yin, C.; Song, W. Weighted gene Co-expression network analysis (WGCNA) reveals a set of hub genes related to chlorophyll metabolism process in chlorella (Chlorella vulgaris) response androstenedione. Environ. Pollut. 2022, 306, 119360. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.H.; Hong, S.; Fang, J.; Zhang, F.; Liu, G.X.; Seo, J.; Zhang, W.B. Lasso Proteins: Modular Design, Cellular Synthesis, and Topological Transformation. Angew. Chem. Int. Ed. Engl. 2020, 59, 19153–19161. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Qi, L.; Sun, Z.; Liu, J.; Lv, Z.; Chen, L.; Huang, B.; Zhu, S.; Liu, Y.; Li, Y. Evaluation and analysis of incidence and risk factors of lower extremity venous thrombosis after urologic surgeries: A prospective two-center cohort study using LASSO-logistic regression. Int. J. Surg. 2021, 89, 105948. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, J.E.; Xie, Z.; Marino, G.B.; Nguyen, N.; Clarke, D.J.B.; Ma’ayan, A. Enrichr-KG: Bridging enrichment analysis across multiple libraries. Nucleic Acids Res. 2023, 51, W168–W179. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Lu, X.J. DSSR-enabled innovative schematics of 3D nucleic acid structures with PyMOL. Nucleic Acids Res. 2020, 48, e74. [Google Scholar] [CrossRef]


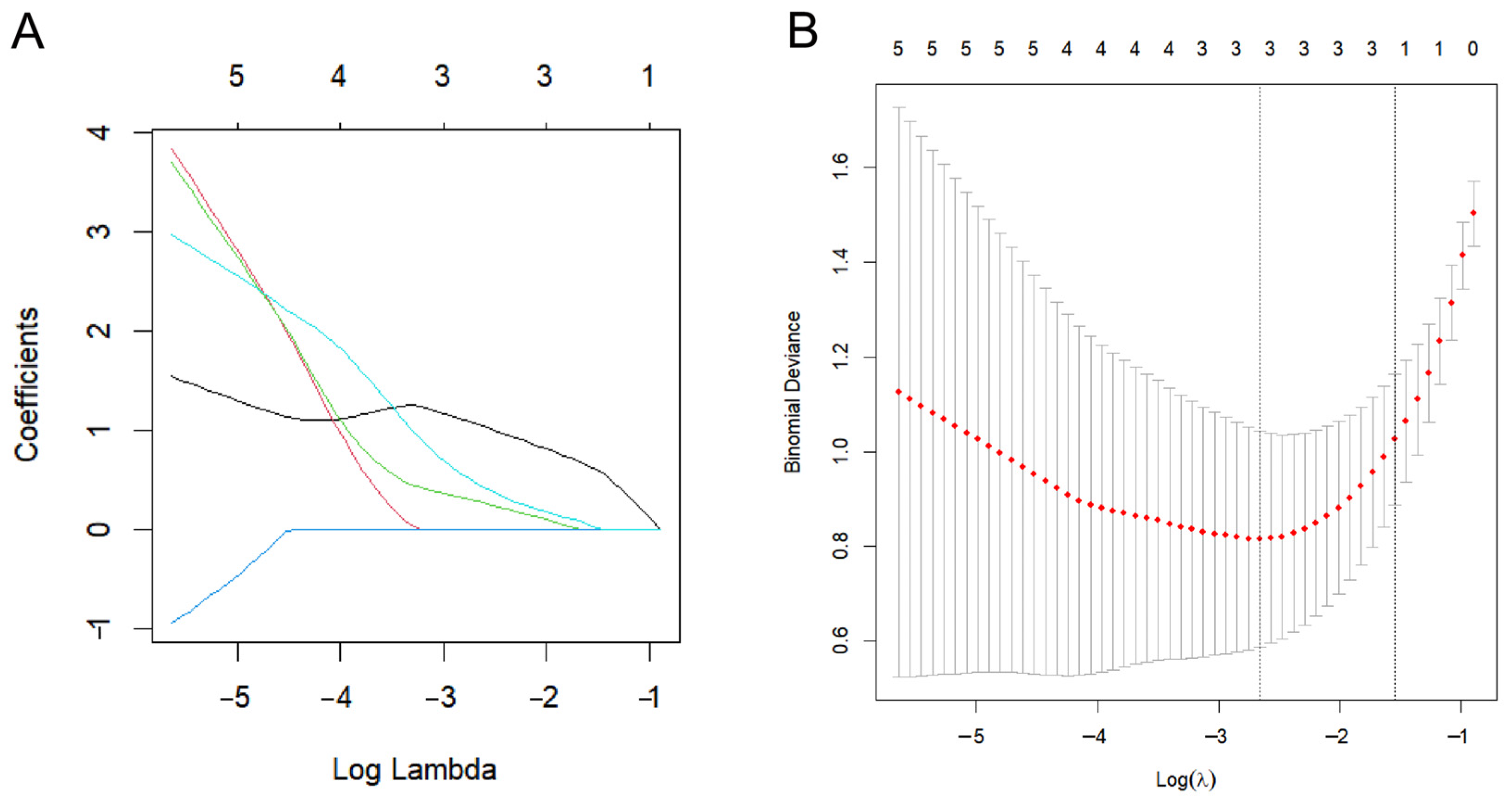

| Gene | Coef |
|---|---|
| CXCR4 | 1.05523161222664 |
| IRF1 | 0.279011336498418 |
| TNFAIP3 | 0.4521141338732 |
| Term | p | Adjusted p | OR | Combined Score |
|---|---|---|---|---|
| BERBAMINE CTD 00001312 | 5.499852259717758 × 10−4 | 0.012660698449782595 | 19,989.00 | 150,029.8210287992 |
| tolbutamide CTD 00006903 | 5.999842544083076 × 10−4 | 0.012660698449782595 | 19,988.00 | 148,283.11962915692 |
| PIPERONYL BUTOXIDE CTD 00006567 | 5.999842544083076 × 10−4 | 0.012660698449782595 | 19,988.00 | 148,283.11962915692 |
| fenthion CTD 00005965 | 8.999788927950273 × 10−4 | 0.012660698449782595 | 19,982.00 | 140,136.54844084635 |
| trimipramine MCF7 UP | 9.999772468562472 × 10−4 | 0.012660698449782595 | 19,980.00 | 138,017.40508704726 |
| cloperastine MCF7 UP | 0.0010499764455398322 | 0.012660698449782595 | 19,979.00 | 137,035.71221916509 |
| ticlopidine MCF7 UP | 0.001099975657412352 | 0.012660698449782595 | 19,978.00 | 136,099.47030191717 |
| clonidine CTD 00005689 | 0.001249973366379199 | 0.012660698449782595 | 19,975.00 | 133,525.54486996148 |
| lasalocid PC3 UP | 0.001249973366379199 | 0.012660698449782595 | 19,975.00 | 133,525.54486996148 |
| econazole MCF7 UP | 0.0012999726251831402 | 0.012660698449782595 | 19,974.00 | 132,735.46073187632 |
| Molecule | Free Energy (kcal/mol) | Molecule | Free Energy (kcal/mol) |
|---|---|---|---|
| Berbamine | −9.25 | Fenthion | −6.08 |
| Tolbutamide | −8.68 | Trimipramine | −5.98 |
| Ticlopidine | −8.02 | Clonidine | −5.76 |
| Econazole | −7.1 | Piperonyl | −4.93 |
| Cloperastine | −6.58 | Lasalocid | −1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Jiang, S.; Liu, Z.; Liu, X.; Lu, T.; Liu, X. Berbamine Targets TNFAIP3: A Bioactive Compound Alleviates Oxidative Stress and Inflammation in the Comorbidity of Insomnia and Chronic Obstructive Pulmonary Disease Through Multi-Omics Integration. Int. J. Mol. Sci. 2025, 26, 10227. https://doi.org/10.3390/ijms262010227
Deng X, Jiang S, Liu Z, Liu X, Lu T, Liu X. Berbamine Targets TNFAIP3: A Bioactive Compound Alleviates Oxidative Stress and Inflammation in the Comorbidity of Insomnia and Chronic Obstructive Pulmonary Disease Through Multi-Omics Integration. International Journal of Molecular Sciences. 2025; 26(20):10227. https://doi.org/10.3390/ijms262010227
Chicago/Turabian StyleDeng, Xinliao, Shuaiyu Jiang, Ziyi Liu, Xinyu Liu, Tao Lu, and Xiaodan Liu. 2025. "Berbamine Targets TNFAIP3: A Bioactive Compound Alleviates Oxidative Stress and Inflammation in the Comorbidity of Insomnia and Chronic Obstructive Pulmonary Disease Through Multi-Omics Integration" International Journal of Molecular Sciences 26, no. 20: 10227. https://doi.org/10.3390/ijms262010227
APA StyleDeng, X., Jiang, S., Liu, Z., Liu, X., Lu, T., & Liu, X. (2025). Berbamine Targets TNFAIP3: A Bioactive Compound Alleviates Oxidative Stress and Inflammation in the Comorbidity of Insomnia and Chronic Obstructive Pulmonary Disease Through Multi-Omics Integration. International Journal of Molecular Sciences, 26(20), 10227. https://doi.org/10.3390/ijms262010227








