Autophagy–Lysosome Pathway in Multiple System Atrophy Pathogenesis: The Best Is Yet to Come
Abstract
1. Introduction
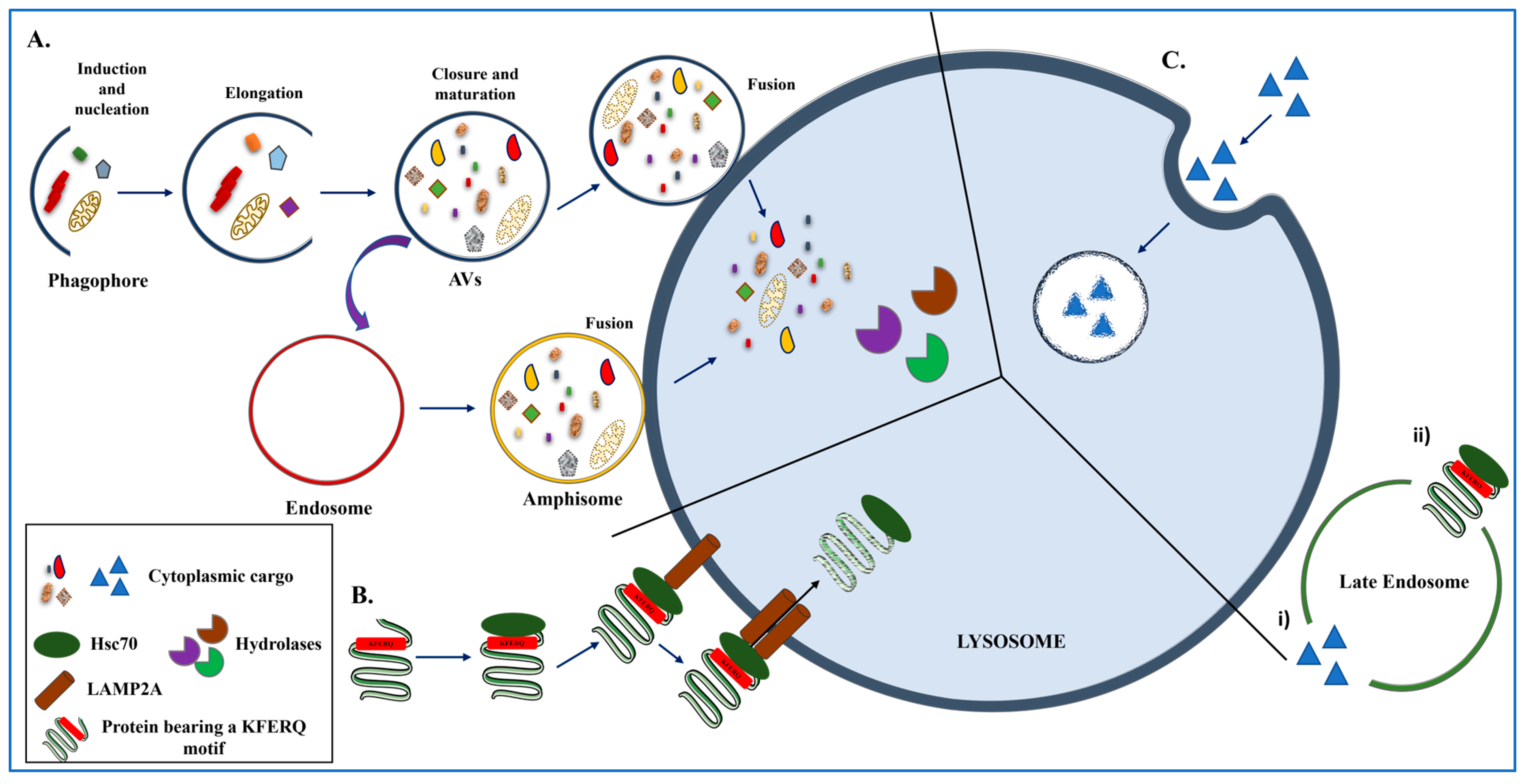
2. The ALP in Action
3. Unraveling the Role of ALP at the Crossroads of MSA Pathophysiology
3.1. Proteolytic Enzymes and αSyn Degradation
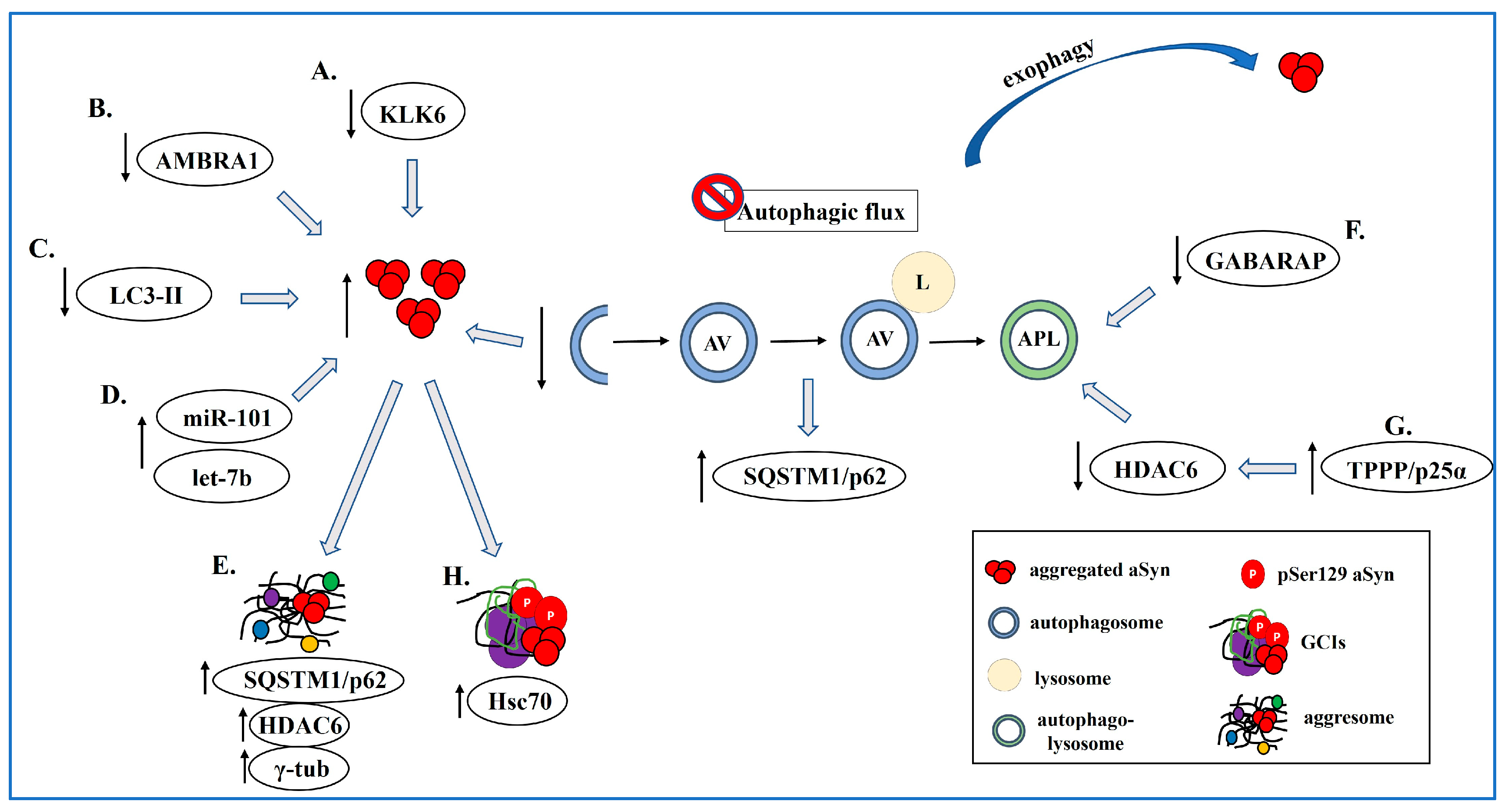
3.2. Autophagy Dysfunction in MSA
3.2.1. The Contribution of Adaptor Proteins and miRNAs
| Strategy/Agent | Primary Target & Intended Effect | Model/System | Reported Outcomes | Translational Status | Reference |
|---|---|---|---|---|---|
| KYP-2047 (prolyl oligopeptidase inhibitor) | PP2A activation → ↑Autophagy | OLN-AS7 cells | ↓ αSyn+ inclusions ↓ pSer129-αSyn ↑ viability | Preclinical (cellular) | [57] |
| Rapamycin/Sirolimus | mTOR inhibition → ↑Macroautophagy | Oligodendroglial cells primary oligodendrocytes αSyn PFFs | Removal of pathological αSyn species; trial: no clinical benefit | Clinical trial (MSA): no benefit (48 weeks) | [58] |
| 17-AAG (Hsp90 inhibitor) | Chaperone reprogramming → ↑Macroautophagy | Primary oligodendrocytes OLN-A53T | ↑ LC3-II puncta ↓ aggregates effect blocked by 3-MA | Preclinical (cellular) | [53] |
| Anti-miR approaches (miR-101, let-7b) | De-repress ALP genes → restore flux | Human MSA striatum (↑miR-101/let-7b), cell models | Predicted ↓ αSyn via restored ALP | Preclinical concept | [56] |
| Neurosin/KLK6 augmentation (R80Q) | Proteolysis of αSyn (extracellular/aggregated) | MBP-αSyn transgenic mouse | ↓ Aggregated αSyn in oligodendrocytes/astrocytes ↑ microglial uptake | Preclinical (in vivo) | [45] |
| UCH-L1 inhibition | DUB modulation → ↑Autophagy | Oligodendroglial cell models | Prevents αSyn aggregate formation via autophagy activation | Preclinical (cellular) | [59] |
3.2.2. Aggresome-Related Mechanisms
3.2.3. Defective Autophagy Maturation
3.2.4. αSyn and ALP Impairment in MSA Pathogenesis
3.3. The Contribution of Astrocytes
3.4. TPPP/p25α and Autophagy Inhibition
4. Targeting the ALP to Counteract MSA-like Pathology
4.1. Macroautophagy in MSA
4.2. A Role of CMA in Oligodendroglial αSyn Clearance
5. A Role of the Proteasome in MSA Pathogenesis
5.1. Proteasomal Degradation of Oligodendroglial αSyn
5.2. UPS-Mediated Regulation of TPPP/p25α
6. Conclusions—Future Perspectives
- Combinatorial therapies combining autophagy enhancers with anti-inflammatory agents or neuroprotectants (e.g., antioxidants or mitochondrial function boosters).
- Use of patient-derived biological fluids (blood, serum, plasma) to identify peripheral biomarkers of autophagy dysfunction that may mirror brain pathology in MSA.
- Application of patient iPSC-derived oligodendrocytes and 3D organoids to investigate autophagy-related mechanisms in MSA development.
- Screening of autophagy-modulating compounds in these humanized models to uncover novel ALP-targeted therapeutic opportunities.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortiz, J.F.; Betté, S.; Tambo, W.; Tao, F.; Cozar, J.C.; Isaacson, S. Multiple System Atrophy—Cerebellar Type: Clinical Picture and Treatment of an Often-Overlooked Disorder. Cureus 2020, 12, e10741. [Google Scholar] [CrossRef]
- Papp, M.I.; Kahn, J.E.; Lantos, P.L. Glial Cytoplasmic Inclusions in the CNS of Patients with Multiple System Atrophy (Striatonigral Degeneration, Olivopontocerebellar Atrophy and Shy-Drager Syndrome). J. Neurol. Sci. 1989, 94, 79–100. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. Alpha-Synuclein in Filamentous Inclusions of Lewy Bodies from Parkinson’s Disease and Dementia with Lewy Bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Lantos, P.L. Papp-Lantos Inclusions and the Pathogenesis of Multiple System Atrophy: An Update. Acta Neuropathol. 2010, 119, 657–667. [Google Scholar] [CrossRef]
- Trojanowski, J.Q.; Revesz, T. Neuropathology Working Group on MSA Proposed Neuropathological Criteria for the Post Mortem Diagnosis of Multiple System Atrophy. Neuropathol. Appl. Neurobiol. 2007, 33, 615–620. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Yoshimoto, M.; Tsuji, S.; Takahashi, H. Alpha-Synuclein Immunoreactivity in Glial Cytoplasmic Inclusions in Multiple System Atrophy. Neurosci. Lett. 1998, 249, 180–182. [Google Scholar] [CrossRef]
- Tu, P.H.; Galvin, J.E.; Baba, M.; Giasson, B.; Tomita, T.; Leight, S.; Nakajo, S.; Iwatsubo, T.; Trojanowski, J.Q.; Lee, V.M. Glial Cytoplasmic Inclusions in White Matter Oligodendrocytes of Multiple System Atrophy Brains Contain Insoluble Alpha-Synuclein. Ann. Neurol. 1998, 44, 415–422. [Google Scholar] [CrossRef]
- Djelloul, M.; Holmqvist, S.; Boza-Serrano, A.; Azevedo, C.; Yeung, M.S.; Goldwurm, S.; Frisén, J.; Deierborg, T.; Roybon, L. Alpha-Synuclein Expression in the Oligodendrocyte Lineage: An In Vitro and In Vivo Study Using Rodent and Human Models. Stem Cell Rep. 2015, 5, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.W.; Johnson, J.M.; Solano, S.M.; Hollingsworth, Z.R.; Standaert, D.G.; Young, A.B. Absence of Alpha-Synuclein mRNA Expression in Normal and Multiple System Atrophy Oligodendroglia. J. Neural Transm. 2005, 112, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.F.; Rey, N.L.; Bousset, L.; Melki, R.; Brundin, P.; Angot, E. Alpha-Synuclein Transfers from Neurons to Oligodendrocytes. Glia 2014, 62, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Danzer, K.M.; Kranich, L.R.; Ruf, W.P.; Cagsal-Getkin, O.; Winslow, A.R.; Zhu, L.; Vanderburg, C.R.; McLean, P.J. Exosomal Cell-to-Cell Transmission of Alpha Synuclein Oligomers. Mol. Neurodegener. 2012, 7, 42. [Google Scholar] [CrossRef]
- Emmanouilidou, E.; Melachroinou, K.; Roumeliotis, T.; Garbis, S.D.; Ntzouni, M.; Margaritis, L.H.; Stefanis, L.; Vekrellis, K. Cell-Produced Alpha-Synuclein Is Secreted in a Calcium-Dependent Manner by Exosomes and Impacts Neuronal Survival. J. Neurosci. 2010, 30, 6838–6851. [Google Scholar] [CrossRef] [PubMed]
- Masuda-Suzukake, M.; Nonaka, T.; Hosokawa, M.; Oikawa, T.; Arai, T.; Akiyama, H.; Mann, D.M.A.; Hasegawa, M. Prion-like Spreading of Pathological α-Synuclein in Brain. Brain 2013, 136, 1128–1138. [Google Scholar] [CrossRef]
- Mavroeidi, P.; Arvanitaki, F.; Karakitsou, A.-K.; Vetsi, M.; Kloukina, I.; Zweckstetter, M.; Giller, K.; Becker, S.; Sorrentino, Z.A.; Giasson, B.I.; et al. Endogenous Oligodendroglial Alpha-Synuclein and TPPP/P25α Orchestrate Alpha-Synuclein Pathology in Experimental Multiple System Atrophy Models. Acta Neuropathol. 2019, 138, 415–441. [Google Scholar] [CrossRef]
- Schwarz, L.; Goldbaum, O.; Bergmann, M.; Probst-Cousin, S.; Richter-Landsberg, C. Involvement of Macroautophagy in Multiple System Atrophy and Protein Aggregate Formation in Oligodendrocytes. J. Mol. Neurosci. 2012, 47, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Asi, Y.T.; Simpson, J.E.; Heath, P.R.; Wharton, S.B.; Lees, A.J.; Revesz, T.; Houlden, H.; Holton, J.L. Alpha-Synuclein mRNA Expression in Oligodendrocytes in MSA. Glia 2014, 62, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Mavroeidi, P.; Arvanitaki, F.; Vetsi, M.; Becker, S.; Vlachakis, D.; Jensen, P.H.; Stefanis, L.; Xilouri, M. Autophagy Mediates the Clearance of Oligodendroglial SNCA/Alpha-Synuclein and TPPP/p25A in Multiple System Atrophy Models. Autophagy 2022, 18, 2104–2133. [Google Scholar] [CrossRef]
- Kaji, S.; Maki, T.; Kinoshita, H.; Uemura, N.; Ayaki, T.; Kawamoto, Y.; Furuta, T.; Urushitani, M.; Hasegawa, M.; Kinoshita, Y.; et al. Pathological Endogenous α-Synuclein Accumulation in Oligodendrocyte Precursor Cells Potentially Induces Inclusions in Multiple System Atrophy. Stem Cell Rep. 2018, 10, 356–365. [Google Scholar] [CrossRef]
- Wong, E.; Cuervo, A.M. Autophagy Gone Awry in Neurodegenerative Diseases. Nat. Neurosci. 2010, 13, 805–811. [Google Scholar] [CrossRef]
- Böing, C.; Di Fabrizio, M.; Burger, D.; Bol, J.G.J.M.; Huisman, E.; Rozemuller, A.J.M.; van de Berg, W.D.J.; Stahlberg, H.; Lewis, A.J. Distinct Ultrastructural Phenotypes of Glial and Neuronal Alpha-Synuclein Inclusions in Multiple System Atrophy. Brain 2024, 147, 3727–3741. [Google Scholar] [CrossRef]
- Higashi, S.; Moore, D.J.; Minegishi, M.; Kasanuki, K.; Fujishiro, H.; Kabuta, T.; Togo, T.; Katsuse, O.; Uchikado, H.; Furukawa, Y.; et al. Localization of MAP1-LC3 in Vulnerable Neurons and Lewy Bodies in Brains of Patients with Dementia with Lewy Bodies. J. Neuropathol. Exp. Neurol. 2011, 70, 264–280. [Google Scholar] [CrossRef]
- Pickford, F.; Masliah, E.; Britschgi, M.; Lucin, K.; Narasimhan, R.; Jaeger, P.A.; Small, S.; Spencer, B.; Rockenstein, E.; Levine, B.; et al. The Autophagy-Related Protein Beclin 1 Shows Reduced Expression in Early Alzheimer Disease and Regulates Amyloid Beta Accumulation in Mice. J. Clin. Investig. 2008, 118, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Stefanis, L.; Fredenburg, R.; Lansbury, P.T.; Sulzer, D. Impaired Degradation of Mutant Alpha-Synuclein by Chaperone-Mediated Autophagy. Science 2004, 305, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Emr, S.D. Autophagy as a Regulated Pathway of Cellular Degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Tanji, K.; Odagiri, S.; Maruyama, A.; Mori, F.; Kakita, A.; Takahashi, H.; Wakabayashi, K. Alteration of Autophagosomal Proteins in the Brain of Multiple System Atrophy. Neurobiol. Dis. 2013, 49, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Füllgrabe, J.; Klionsky, D.J.; Joseph, B. The Return of the Nucleus: Transcriptional and Epigenetic Control of Autophagy. Nat. Rev. Mol. Cell Biol. 2014, 15, 65–74. [Google Scholar] [CrossRef]
- Akkoc, Y.; Gozuacik, D. MicroRNAs as Major Regulators of the Autophagy Pathway. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118662. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Orenstein, S.J.; Cuervo, A.M. Chaperone-Mediated Autophagy: Molecular Mechanisms and Physiological Relevance. Semin. Cell Dev. Biol. 2010, 21, 719–726. [Google Scholar] [CrossRef]
- Chiang, H.L.; Terlecky, S.R.; Plant, C.P.; Dice, J.F. A Role for a 70-Kilodalton Heat Shock Protein in Lysosomal Degradation of Intracellular Proteins. Science 1989, 246, 382–385. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F. A Receptor for the Selective Uptake and Degradation of Proteins by Lysosomes. Science 1996, 273, 501–503. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F.; Knecht, E. A Population of Rat Liver Lysosomes Responsible for the Selective Uptake and Degradation of Cytosolic Proteins. J. Biol. Chem. 1997, 272, 5606–5615. [Google Scholar] [CrossRef] [PubMed]
- Agarraberes, F.A.; Dice, J.F. A Molecular Chaperone Complex at the Lysosomal Membrane Is Required for Protein Translocation. J. Cell Sci. 2001, 114, 2491–2499. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Kaushik, S.; Varticovski, L.; Cuervo, A.M. The Chaperone-Mediated Autophagy Receptor Organizes in Dynamic Protein Complexes at the Lysosomal Membrane. Mol. Cell. Biol. 2008, 28, 5747–5763. [Google Scholar] [CrossRef]
- Saftig, P.; Klumperman, J. Lysosome Biogenesis and Lysosomal Membrane Proteins: Trafficking Meets Function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, J.; Bao, J. Microautophagy: Lesser-Known Self-Eating. Cell. Mol. Life Sci. 2012, 69, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Schuck, S. Microautophagy—Distinct Molecular Mechanisms Handle Cargoes of Many Sizes. J. Cell Sci. 2020, 133, jcs246322. [Google Scholar] [CrossRef]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of Cytosolic Proteins by Late Endosomes. Dev. Cell 2011, 20, 131–139. [Google Scholar] [CrossRef]
- Cerri, S.; Blandini, F. Role of Autophagy in Parkinson’s Disease. Curr. Med. Chem. 2019, 26, 3702–3718. [Google Scholar] [CrossRef]
- Olanow, C.W.; McNaught, K.S.P. Ubiquitin-Proteasome System and Parkinson’s Disease. Mov. Disord. 2006, 21, 1806–1823. [Google Scholar] [CrossRef]
- Jellinger, K.A. Multiple System Atrophy: An Oligodendroglioneural Synucleinopathy1. J. Alzheimer’s Dis. 2018, 62, 1141–1179. [Google Scholar] [CrossRef]
- Iwata, A.; Maruyama, M.; Akagi, T.; Hashikawa, T.; Kanazawa, I.; Tsuji, S.; Nukina, N. Alpha-Synuclein Degradation by Serine Protease Neurosin: Implication for Pathogenesis of Synucleinopathies. Hum. Mol. Genet. 2003, 12, 2625–2635. [Google Scholar] [CrossRef]
- Tatebe, H.; Watanabe, Y.; Kasai, T.; Mizuno, T.; Nakagawa, M.; Tanaka, M.; Tokuda, T. Extracellular Neurosin Degrades α-Synuclein in Cultured Cells. Neurosci. Res. 2010, 67, 341–346. [Google Scholar] [CrossRef]
- Kasai, T.; Tokuda, T.; Yamaguchi, N.; Watanabe, Y.; Kametani, F.; Nakagawa, M.; Mizuno, T. Cleavage of Normal and Pathological Forms of Alpha-Synuclein by Neurosin in Vitro. Neurosci. Lett. 2008, 436, 52–56. [Google Scholar] [CrossRef]
- Spencer, B.; Valera, E.; Rockenstein, E.; Trejo-Morales, M.; Adame, A.; Masliah, E. A Brain-Targeted, Modified Neurosin (Kallikrein-6) Reduces α-Synuclein Accumulation in a Mouse Model of Multiple System Atrophy. Mol. Neurodegener. 2015, 10, 48. [Google Scholar] [CrossRef]
- Kiely, A.P.; Miners, J.S.; Courtney, R.; Strand, C.; Love, S.; Holton, J.L. Exploring the Putative Role of Kallikrein-6, Calpain-1 and Cathepsin-D in the Proteolytic Degradation of α-Synuclein in Multiple System Atrophy. Neuropathol. Appl. Neurobiol. 2019, 45, 347–360. [Google Scholar] [CrossRef]
- Sung, J.Y.; Park, S.M.; Lee, C.-H.; Um, J.W.; Lee, H.J.; Kim, J.; Oh, Y.J.; Lee, S.-T.; Paik, S.R.; Chung, K.C. Proteolytic Cleavage of Extracellular Secreted {alpha}-Synuclein via Matrix Metalloproteinases. J. Biol. Chem. 2005, 280, 25216–25224. [Google Scholar] [CrossRef] [PubMed]
- Miners, J.S.; Love, S. Endothelin-Converting Enzymes Degrade α-Synuclein and Are Reduced in Dementia with Lewy Bodies. J. Neurochem. 2017, 141, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Hamamichi, S.; Caldwell, K.A.; Caldwell, G.A.; Yacoubian, T.A.; Wilson, S.; Xie, Z.-L.; Speake, L.D.; Parks, R.; Crabtree, D.; et al. Lysosomal Enzyme Cathepsin D Protects against Alpha-Synuclein Aggregation and Toxicity. Mol. Brain 2008, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, D.; Dodson, M.; Ouyang, X.; Boyer-Guittaut, M.; Liang, Q.; Ballestas, M.E.; Fineberg, N.; Zhang, J. Over-Expression of an Inactive Mutant Cathepsin D Increases Endogenous Alpha-Synuclein and Cathepsin B Activity in SH-SY5Y Cells. J. Neurochem. 2014, 128, 950–961. [Google Scholar] [CrossRef]
- Cullen, V.; Lindfors, M.; Ng, J.; Paetau, A.; Swinton, E.; Kolodziej, P.; Boston, H.; Saftig, P.; Woulfe, J.; Feany, M.B.; et al. Cathepsin D Expression Level Affects Alpha-Synuclein Processing, Aggregation, and Toxicity in Vivo. Mol. Brain 2009, 2, 5. [Google Scholar] [CrossRef]
- Miki, Y.; Tanji, K.; Mori, F.; Tatara, Y.; Utsumi, J.; Sasaki, H.; Kakita, A.; Takahashi, H.; Fimia, G.M.; Wakabayashi, K. AMBRA1, a Novel α-Synuclein-Binding Protein, Is Implicated in the Pathogenesis of Multiple System Atrophy. Brain Pathol. 2018, 28, 28–42. [Google Scholar] [CrossRef]
- Riedel, M.; Goldbaum, O.; Schwarz, L.; Schmitt, S.; Richter-Landsberg, C. 17-AAG Induces Cytoplasmic Alpha-Synuclein Aggregate Clearance by Induction of Autophagy. PLoS ONE 2010, 5, e8753. [Google Scholar] [CrossRef]
- Odagiri, S.; Tanji, K.; Mori, F.; Kakita, A.; Takahashi, H.; Wakabayashi, K. Autophagic Adapter Protein NBR1 Is Localized in Lewy Bodies and Glial Cytoplasmic Inclusions and Is Involved in Aggregate Formation in α-Synucleinopathy. Acta Neuropathol. 2012, 124, 173–186. [Google Scholar] [CrossRef]
- Sailer, A.; Scholz, S.W.; Nalls, M.A.; Schulte, C.; Federoff, M.; Price, T.R.; Lees, A.; Ross, O.A.; Dickson, D.W.; Mok, K.; et al. A Genome-Wide Association Study in Multiple System Atrophy. Neurology 2016, 87, 1591–1598. [Google Scholar] [CrossRef]
- Valera, E.; Spencer, B.; Mott, J.; Trejo, M.; Adame, A.; Mante, M.; Rockenstein, E.; Troncoso, J.C.; Beach, T.G.; Masliah, E.; et al. MicroRNA-101 Modulates Autophagy and Oligodendroglial Alpha-Synuclein Accumulation in Multiple System Atrophy. Front. Mol. Neurosci. 2017, 10, 329. [Google Scholar] [CrossRef]
- Cui, H.; Kilpeläinen, T.; Zouzoula, L.; Auno, S.; Trontti, K.; Kurvonen, S.; Norrbacka, S.; Hovatta, I.; Jensen, P.H.; Myöhänen, T.T. Prolyl Oligopeptidase Inhibition Reduces Alpha-Synuclein Aggregation in a Cellular Model of Multiple System Atrophy. J. Cell. Mol. Med. 2021, 25, 9634–9646. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.-A.; Martinez, J.; Millar Vernetti, P.; Ma, T.; Perez, M.A.; Zhong, J.; Qian, Y.; Dutta, S.; Maina, K.N.; Siddique, I.; et al. mTOR Inhibition with Sirolimus in Multiple System Atrophy: A Randomized, Double-Blind, Placebo-Controlled Futility Trial and 1-Year Biomarker Longitudinal Analysis. Mov. Disord. 2022, 37, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Pukaß, K.; Richter-Landsberg, C. Inhibition of UCH-L1 in Oligodendroglial Cells Results in Microtubule Stabilization and Prevents α-Synuclein Aggregate Formation by Activating the Autophagic Pathway: Implications for Multiple System Atrophy. Front. Cell. Neurosci. 2015, 9, 163. [Google Scholar] [CrossRef]
- Terni, B.; Rey, M.J.; Boluda, S.; Torrejón-Escribano, B.; Sabate, M.P.; Calopa, M.; van Leeuwen, F.W.; Ferrer, I. Mutant Ubiquitin and P62 Immunoreactivity in Cases of Combined Multiple System Atrophy and Alzheimer’s Disease. Acta Neuropathol. 2007, 113, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.A.; Ward, C.L.; Kopito, R.R. Aggresomes: A Cellular Response to Misfolded Proteins. J. Cell Biol. 1998, 143, 1883–1898. [Google Scholar] [CrossRef]
- Kopito, R.R. Aggresomes, Inclusion Bodies and Protein Aggregation. Trends Cell Biol. 2000, 10, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Kovacs, J.J.; McLaurin, A.; Vance, J.M.; Ito, A.; Yao, T.P. The Deacetylase HDAC6 Regulates Aggresome Formation and Cell Viability in Response to Misfolded Protein Stress. Cell 2003, 115, 727–738. [Google Scholar] [CrossRef]
- Miki, Y.; Mori, F.; Tanji, K.; Kakita, A.; Takahashi, H.; Wakabayashi, K. Accumulation of Histone Deacetylase 6, an Aggresome-Related Protein, Is Specific to Lewy Bodies and Glial Cytoplasmic Inclusions. Neuropathology 2011, 31, 561–568. [Google Scholar] [CrossRef]
- Chiba, Y.; Takei, S.; Kawamura, N.; Kawaguchi, Y.; Sasaki, K.; Hasegawa-Ishii, S.; Furukawa, A.; Hosokawa, M.; Shimada, A. Immunohistochemical Localization of Aggresomal Proteins in Glial Cytoplasmic Inclusions in Multiple System Atrophy. Neuropathol. Appl. Neurobiol. 2012, 38, 559–571. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H.-Q.; Zhu, X.; Zhang, L.; Albanesi, J.; Levine, B.; Yin, H. GABARAPs Regulate PI4P-Dependent Autophagosome:Lysosome Fusion. Proc. Natl. Acad. Sci. USA 2015, 112, 7015–7020. [Google Scholar] [CrossRef]
- Makioka, K.; Yamazaki, T.; Takatama, M.; Nakazato, Y.; Okamoto, K. Activation and Alteration of Lysosomes in Multiple System Atrophy. NeuroReport 2012, 23, 270–276. [Google Scholar] [CrossRef]
- Pukaß, K.; Goldbaum, O.; Richter-Landsberg, C. Mitochondrial Impairment and Oxidative Stress Compromise Autophagosomal Degradation of α-Synuclein in Oligodendroglial Cells. J. Neurochem. 2015, 135, 194–205. [Google Scholar] [CrossRef]
- Fellner, L.; Buchinger, E.; Brueck, D.; Irschick, R.; Wenning, G.K.; Stefanova, N. Limited Effects of Dysfunctional Macroautophagy on the Accumulation of Extracellularly Derived α-Synuclein in Oligodendroglia: Implications for MSA Pathogenesis. BMC Neurosci. 2018, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Fellner, L.; Gabassi, E.; Haybaeck, J.; Edenhofer, F. Autophagy in α-Synucleinopathies-An Overstrained System. Cells 2021, 10, 3143. [Google Scholar] [CrossRef] [PubMed]
- Puska, G.; Lutz, M.I.; Molnar, K.; Regelsberger, G.; Ricken, G.; Pirker, W.; Laszlo, L.; Kovacs, G.G. Lysosomal Response in Relation to α-Synuclein Pathology Differs between Parkinson’s Disease and Multiple System Atrophy. Neurobiol. Dis. 2018, 114, 140–152. [Google Scholar] [CrossRef]
- Monzio Compagnoni, G.; Kleiner, G.; Samarani, M.; Aureli, M.; Faustini, G.; Bellucci, A.; Ronchi, D.; Bordoni, A.; Garbellini, M.; Salani, S.; et al. Mitochondrial Dysregulation and Impaired Autophagy in iPSC-Derived Dopaminergic Neurons of Multiple System Atrophy. Stem Cell Rep. 2018, 11, 1185–1198. [Google Scholar] [CrossRef]
- Loria, F.; Vargas, J.Y.; Bousset, L.; Syan, S.; Salles, A.; Melki, R.; Zurzolo, C. α-Synuclein Transfer between Neurons and Astrocytes Indicates That Astrocytes Play a Role in Degradation Rather than in Spreading. Acta Neuropathol. 2017, 134, 789–808. [Google Scholar] [CrossRef]
- Rannikko, E.H.; Weber, S.S.; Kahle, P.J. Exogenous α-Synuclein Induces Toll-like Receptor 4 Dependent Inflammatory Responses in Astrocytes. BMC Neurosci. 2015, 16, 57. [Google Scholar] [CrossRef]
- Song, Y.J.C.; Lundvig, D.M.S.; Huang, Y.; Gai, W.P.; Blumbergs, P.C.; Højrup, P.; Otzen, D.; Halliday, G.M.; Jensen, P.H. P25α Relocalizes in Oligodendroglia from Myelin to Cytoplasmic Inclusions in Multiple System Atrophy. Am. J. Pathol. 2007, 171, 1291–1303. [Google Scholar] [CrossRef]
- Ejlerskov, P.; Rasmussen, I.; Nielsen, T.T.; Bergström, A.-L.; Tohyama, Y.; Jensen, P.H.; Vilhardt, F. Tubulin Polymerization-Promoting Protein (TPPP/P25α) Promotes Unconventional Secretion of α-Synuclein through Exophagy by Impairing Autophagosome-Lysosome Fusion. J. Biol. Chem. 2013, 288, 17313–17335. [Google Scholar] [CrossRef]
- Lehotzky, A.; Oláh, J.; Fekete, J.T.; Szénási, T.; Szabó, E.; Győrffy, B.; Várady, G.; Ovádi, J. Co-Transmission of Alpha-Synuclein and TPPP/P25 Inhibits Their Proteolytic Degradation in Human Cell Models. Front. Mol. Biosci. 2021, 8, 666026. [Google Scholar] [CrossRef]
- Jin, H.; Ishikawa, K.; Tsunemi, T.; Ishiguro, T.; Amino, T.; Mizusawa, H. Analyses of Copy Number and mRNA Expression Level of the Alpha-Synuclein Gene in Multiple System Atrophy. J. Med. Dent. Sci. 2008, 55, 145–153. [Google Scholar] [PubMed]
- Richter-Landsberg, C.; Gorath, M.; Trojanowski, J.Q.; Lee, V.M. Alpha-Synuclein Is Developmentally Expressed in Cultured Rat Brain Oligodendrocytes. J. Neurosci. Res. 2000, 62, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, M.; Huh, Y.-J.; Lee, Y.-I. The Impairments of α-Synuclein and Mechanistic Target of Rapamycin in Rotenone-Induced SH-SY5Y Cells and Mice Model of Parkinson’s Disease. Front. Neurosci. 2019, 13, 1028. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.L.; Ravikumar, B.; Atkins, J.; Skepper, J.N.; Rubinsztein, D.C. Alpha-Synuclein Is Degraded by Both Autophagy and the Proteasome. J. Biol. Chem. 2003, 278, 25009–25013. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M.; Galvan, V. Rapamycin and Alzheimer’s Disease: Time for a Clinical Trial? Sci. Transl. Med. 2019, 11, eaar4289. [Google Scholar] [CrossRef] [PubMed]
- Don, A.S.A.; Zheng, X.F.S. Recent Clinical Trials of mTOR-Targeted Cancer Therapies. Rev. Recent Clin. Trials 2011, 6, 24–35. [Google Scholar] [CrossRef]
- Xilouri, M.; Brekk, O.R.; Polissidis, A.; Chrysanthou-Piterou, M.; Kloukina, I.; Stefanis, L. Impairment of Chaperone-Mediated Autophagy Induces Dopaminergic Neurodegeneration in Rats. Autophagy 2016, 12, 2230–2247. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Tasset, I.; Cheng, M.M.; Diaz, A.; Pan, M.-K.; Lieberman, O.J.; Hutten, S.J.; Alcalay, R.N.; Kim, S.; Ximénez-Embún, P.; et al. Mutant Glucocerebrosidase Impairs α-Synuclein Degradation by Blockade of Chaperone-Mediated Autophagy. Sci. Adv. 2022, 8, eabm6393. [Google Scholar] [CrossRef]
- Kawamoto, Y.; Akiguchi, I.; Shirakashi, Y.; Honjo, Y.; Tomimoto, H.; Takahashi, R.; Budka, H. Accumulation of Hsc70 and Hsp70 in Glial Cytoplasmic Inclusions in Patients with Multiple System Atrophy. Brain Res. 2007, 1136, 219–227. [Google Scholar] [CrossRef]
- Stefanis, L.; Emmanouilidou, E.; Pantazopoulou, M.; Kirik, D.; Vekrellis, K.; Tofaris, G.K. How Is Alpha-Synuclein Cleared from the Cell? J. Neurochem. 2019, 150, 577–590. [Google Scholar] [CrossRef]
- Mori, F.; Nishie, M.; Piao, Y.-S.; Kito, K.; Kamitani, T.; Takahashi, H.; Wakabayashi, K. Accumulation of NEDD8 in Neuronal and Glial Inclusions of Neurodegenerative Disorders. Neuropathol. Appl. Neurobiol. 2005, 31, 53–61. [Google Scholar] [CrossRef]
- Mondal, M.; Conole, D.; Nautiyal, J.; Tate, E.W. UCHL1 as a Novel Target in Breast Cancer: Emerging Insights from Cell and Chemical Biology. Br. J. Cancer 2022, 126, 24–33. [Google Scholar] [CrossRef]
- Lowe, J.; McDermott, H.; Landon, M.; Mayer, R.J.; Wilkinson, K.D. Ubiquitin Carboxyl-Terminal Hydrolase (PGP 9.5) Is Selectively Present in Ubiquitinated Inclusion Bodies Characteristic of Human Neurodegenerative Diseases. J. Pathol. 1990, 161, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Nihira, T.; Ren, Y.-R.; Cao, X.-Q.; Wada, K.; Setsuie, R.; Kabuta, T.; Wada, K.; Hattori, N.; Mizuno, Y.; et al. Effects of UCH-L1 on Alpha-Synuclein over-Expression Mouse Model of Parkinson’s Disease. J. Neurochem. 2009, 108, 932–944. [Google Scholar] [CrossRef]
- Liu, Z.; Meray, R.K.; Grammatopoulos, T.N.; Fredenburg, R.A.; Cookson, M.R.; Liu, Y.; Logan, T.; Lansbury, P.T. Membrane-Associated Farnesylated UCH-L1 Promotes Alpha-Synuclein Neurotoxicity and Is a Therapeutic Target for Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 4635–4640. [Google Scholar] [CrossRef]
- Stefanova, N.; Kaufmann, W.A.; Humpel, C.; Poewe, W.; Wenning, G.K. Systemic Proteasome Inhibition Triggers Neurodegeneration in a Transgenic Mouse Model Expressing Human α-Synuclein under Oligodendrocyte Promoter: Implications for Multiple System Atrophy. Acta Neuropathol. 2012, 124, 51–65. [Google Scholar] [CrossRef][Green Version]
- Lehotzky, A.; Tirián, L.; Tökési, N.; Lénárt, P.; Szabó, B.; Kovács, J.; Ovádi, J. Dynamic Targeting of Microtubules by TPPP/P25 Affects Cell Survival. J. Cell Sci. 2004, 117, 6249–6259. [Google Scholar] [CrossRef] [PubMed]
- Lehotzky, A.; Oláh, J.; Szunyogh, S.; Szabó, A.; Berki, T.; Ovádi, J. Zinc-Induced Structural Changes of the Disordered Tppp/P25 Inhibits Its Degradation by the Proteasome. Biochim. Biophys. Acta 2015, 1852, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Staerz, S.D.; Anamoah, C.; Tepe, J.J. 20S Proteasome Enhancers Prevent Cytotoxic Tubulin Polymerization-Promoting Protein Induced α-Synuclein Aggregation. iScience 2024, 27, 110166. [Google Scholar] [CrossRef] [PubMed]

| Checkpoint/Marker | Oligodendrocytes | Microglia | |
|---|---|---|---|
| Aggresomes | GCIs | ||
| p62/SQSTM1 | + | + | |
| HDAC6 | + | - | |
| γ-tubulin | + | + | |
| NBRI | - | + | |
| LC3-II | ↑ | ||
| Lysosomal status | ↓ GABARAP (maturation) | ↑ activation | |
| αSyn | ↑ aggregation | ↑ uptake and clearance | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroeidi, P.; Xilouri, M. Autophagy–Lysosome Pathway in Multiple System Atrophy Pathogenesis: The Best Is Yet to Come. Int. J. Mol. Sci. 2025, 26, 10204. https://doi.org/10.3390/ijms262010204
Mavroeidi P, Xilouri M. Autophagy–Lysosome Pathway in Multiple System Atrophy Pathogenesis: The Best Is Yet to Come. International Journal of Molecular Sciences. 2025; 26(20):10204. https://doi.org/10.3390/ijms262010204
Chicago/Turabian StyleMavroeidi, Panagiota, and Maria Xilouri. 2025. "Autophagy–Lysosome Pathway in Multiple System Atrophy Pathogenesis: The Best Is Yet to Come" International Journal of Molecular Sciences 26, no. 20: 10204. https://doi.org/10.3390/ijms262010204
APA StyleMavroeidi, P., & Xilouri, M. (2025). Autophagy–Lysosome Pathway in Multiple System Atrophy Pathogenesis: The Best Is Yet to Come. International Journal of Molecular Sciences, 26(20), 10204. https://doi.org/10.3390/ijms262010204








