Silica Spheres Functionalized with Silver and Bismuth Nanoparticles—Antibacterial Activity Against Clinically Relevant Bacterial Pathogens
Abstract
1. Introduction
2. Results and Discussion
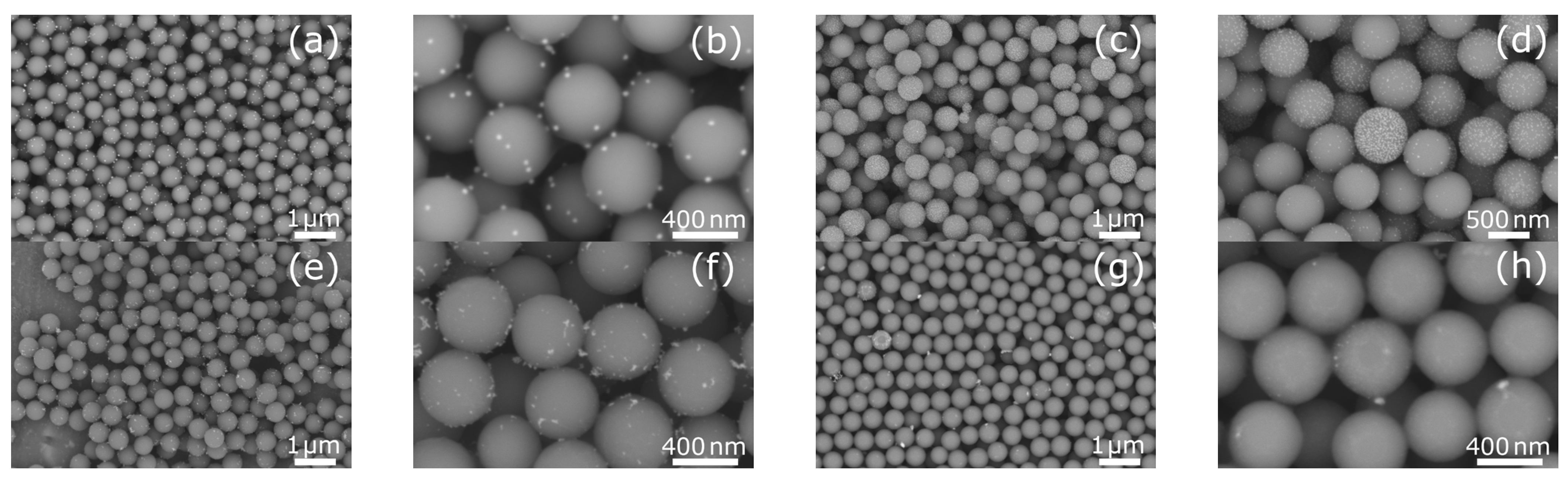
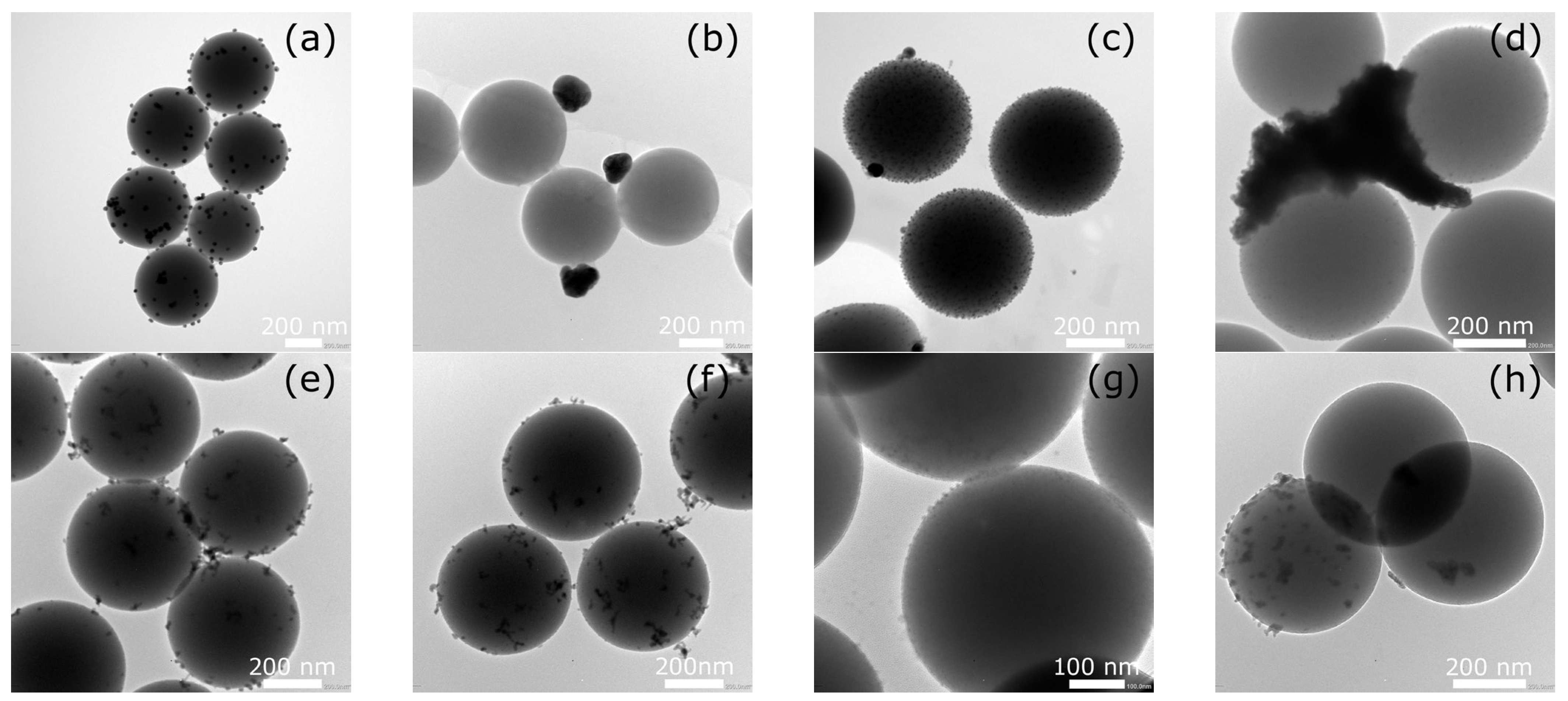
2.1. Morphological Characterization of Silica Spheres Coated with Silver and Bismuth Nanoparticles
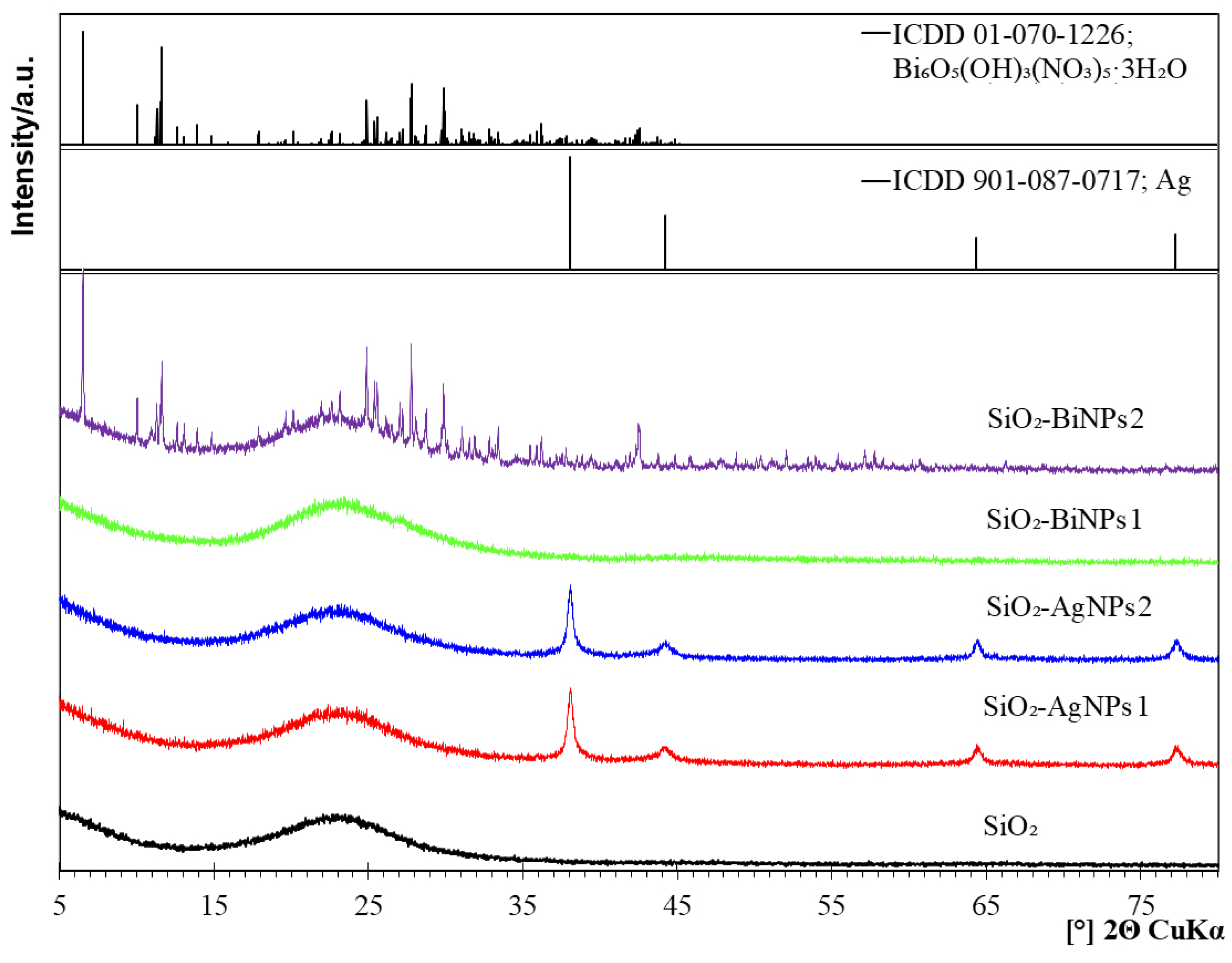
2.2. Phase Composition
2.3. Zeta Potential and Suspension Stability
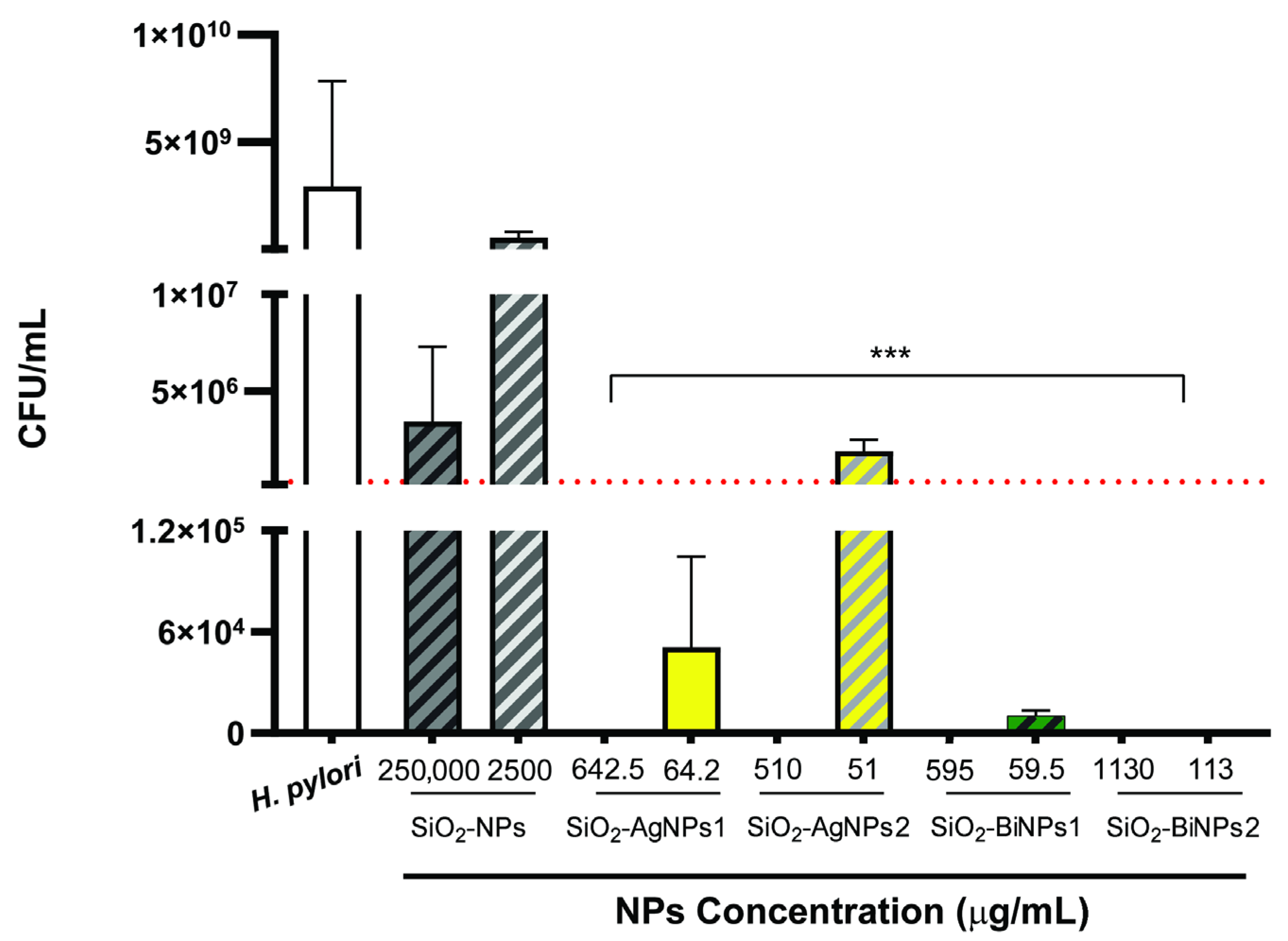
2.4. Antibacterial Efficacy of Nanoparticles
3. Materials and Methods
3.1. Synthesis of Silica Spheres
3.2. Surface Modification of Silica Spheres
3.3. Deposition of Silver Nanoparticles
3.4. Deposition of Bismuth Nanoparticles
3.5. Morphology and Composition
3.6. Antibacterial Activity Testing
3.7. Preparation of Nanoparticles Suspensions for Biological Testing
3.8. Minimum Inhibitory Concentration (MIC) Determination
3.9. Minimum Bactericidal Concentration (MBC) Determination
3.10. Cytotoxicity Against Gastric Cell Lines
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| APTES | (3-Aminopropyl)triethoxysilane |
| BiNPs | Bismuth nanoparticles |
| CFU | Colony-forming unit |
| CLSI | Clinical and Laboratory Standards Institute |
| EDS | Energy-dispersive X-ray spectroscopy |
| EtOH | Ethanol |
| FCC | Face-centered cubic |
| FBS | Fetal bovine serum |
| H2O2 | Hydrogen peroxide |
| ICP-OES | Inductively coupled plasma–optical emission spectrometry |
| ISO | International Organization for Standardization |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| MDR | Multidrug resistant |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| NH4OH | Ammonium hydroxide |
| NPs | Nanoparticles |
| PBS | Phosphate-buffered saline |
| PES | Polyethersulfone |
| PVP | Polyvinylpyrrolidone |
| ROS | Reactive oxygen species |
| RPMI | Roswell Park Memorial Institute medium |
| SEM | Scanning electron microscopy |
| SiO2 | Silica |
| TEOS | Tetraethoxysilane |
| TEM | Transmission electron microscopy |
| VRE | Vancomycin-resistant Enterococcus |
| WHO | World Health Organization |
| XRD | X-ray diffraction |
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4378521/ (accessed on 1 September 2025).
- WHO. Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 1 September 2025).
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf. (accessed on 1 September 2025).
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, L.S.; Guo, X. Improvement of mechanical properties, microscopic structures, and antibacterial activity by Ag/ZnO nanocomposite powder for glaze-decorated ceramic. J. Adv. Ceram. 2017, 6, 269–278. [Google Scholar] [CrossRef]
- Yang, W.; Shen, C.; Ji, Q.; An, H.; Wang, J.; Liu, Q.; Zhang, Z. Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology 2009, 20, 85102. [Google Scholar] [CrossRef] [PubMed]
- Rosário, J.d.S.; Moreira, F.H.; Rosa, L.H.F.; Guerra, W.; Silva-Caldeira, P.P. Biological Activities of Bismuth Compounds: An Overview of the New Findings and the Old Challenges Not Yet Overcome. Molecules 2023, 28, 5921. [Google Scholar] [CrossRef]
- Wang, R.; Lai, T.P.; Gao, P.; Zhang, H.; Ho, P.L.; Woo, P.C.Y.; Ma, G.; Kao, R.Y.T.; Li, H.; Sun, H. Bismuth antimicrobial drugs serve as broad-spectrum metallo-β-lactamase inhibitors. Nat. Commun. 2018, 9, 439. [Google Scholar] [CrossRef]
- Soukup, C.R.M.; Duffin, R.N.; Burke, K.J.; Meagher, L.; Andrews, P.C. The antibacterial activity and selectivity of bismuth(III) tris(8-hydroxyquinolinates). J. Inorg. Biochem. 2025, 266, 112836. [Google Scholar] [CrossRef]
- Gonçalves, Â.; Matias, M.; Salvador, J.A.R.; Silvestre, S. Bioactive Bismuth Compounds: Is Their Toxicity a Barrier to Therapeutic Use? Int. J. Mol. Sci. 2024, 25, 1600. [Google Scholar] [CrossRef]
- Griffith, D.M.; Li, H.; Werrett, M.V.; Andrews, P.C.; Sun, H. Medicinal Chemistry and Biomedical Applications of Bismuth-based Compounds and Nanoparticles. Chem. Soc. Rev. 2021, 50, 12037–12069. [Google Scholar] [CrossRef]
- Shahbazi, M.A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The versatile biomedical applications of bismuth-based nanoparticles. Chem. Soc. Rev. 2020, 49, 1253–1273. [Google Scholar] [CrossRef]
- Mahfouz, A.Y.; Abed, N.N.; Abd-EL-Aziz, A.S.; Fathy, R.M. Green synthesis of gamma rays-induced melanin-based bismuth oxide nanoparticles for evaluation of the antibacterial and anti-virulence activities against extra-intestinal pathogenic bacteria. World J. Microbiol. Biotechnol. 2025, 41, 319. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.D.; Benin, B.M.; Abeydeera, N.; Kim, M.-H.; Huang, S.D. Bi2O3 nanoparticles exhibit potent broad-spectrum antimicrobial activity and the ability to overcome Ag-, ciprofloxacin- and meropenem-resistance in P. aeruginosa: The next silver bullet of metal antimicrobials? Biomater. Sci. 2022, 10, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Ma, S.; Kong, J.; Luo, X.; Xie, J.; Zhou, Z.; Bai, X. Recent progress on bismuth-based light-triggered antibacterial nanocomposites: Synthesis, characterization, optical properties and bactericidal applications. Sci. Total. Environ. 2024, 915, 170125. [Google Scholar] [CrossRef]
- Ivask, A.; Elbadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef]
- Takeda, E.; Xu, W.; Terakawa, M.; Niidome, T. Tailored structure and antibacterial properties of silica-coated silver nanoplates by pulsed laser irradiation. ACS Omega 2022, 7, 7251–7256. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of Silica Nanoparticles by Sol-Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review. J. Nanomater. 2012, 2012, 132424. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Yang, W.; Gao, S.; Sun, C.; Li, Q. Mesoporous silica-protected silver nanoparticle disinfectant with controlled Ag+ ion release, efficient magnetic separation, and effective antibacterial activity. Nanoscale Adv. 2019, 1, 840–848. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Wang, Y.; Chen, C.; Gu, H.; Chai, Y.; Wang, Y. Silver nanoparticles-decorated and mesoporous silica coated single-walled carbon nanotubes with an enhanced antibacterial activity for killing drug-resistant bacteria. Nano Res. 2020, 13, 389–400. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Lazarini, F. The crystal structure of a bismuth basic nitrate, Bi6O5(OH)3](NO3)5·3H2O. Acta Cryst. 1978, B34, 3169–3173. [Google Scholar] [CrossRef]
- Christensen, A.N.; Lebech, B. Investigation of the crystal structure of a basic bismuth(III) nitrate with the composition [Bi6O4(OH)4]0.54(1)[Bi6O5(OH)3]0.46(1)(NO3)5.54(1). Dalton Trans. 2012, 41, 1971–1980. [Google Scholar] [CrossRef]
- Prasad, S.S.; Ratha, I.; Adarsh, T.; Anand, A.; Sinha, P.K.; Diwan, P.; Annapurna, K.; Biswas, K. In Vitro bioactivity and antibacterial properties of bismuth oxide modified bioactive glasses. J. Mater. Res. 2018, 33, 178–190. [Google Scholar] [CrossRef]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 2022, 33, 3. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; Bispo de Jesus, M.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Yang, E.H.; Chen, W.Y.; Chiang, H.C.; Li, C.H.; Wu, I.H.; Chen, P.J.; Wu, C.T.; Tsai, Y.C.; Cheng, W.C.; Huang, C.J.; et al. 10-Day versus 14-day bismuth quadruple therapy for first-line eradication of Helicobacter pylori infection: A randomised, open-label, non-inferiority trial. EClinicalMedicine 2024, 70, 102529. [Google Scholar] [CrossRef]
- Katelaris, P.; Hunt, R.; Bazzoli, F.; Cohen, H.; Fock, K.M.; Gemilyan, M.; Malfertheiner, P.; Mégraud, F.; Piscoya, A.; Quach, D.; et al. Helicobacter pylori World Gastroenterology Organization Global Guideline. J. Clin. Gastroenterol. 2023, 57, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Pinho, A.S.; Pereira, R.; Pereira, M.; Rai, A.; Ferreira, L.; Martins, M.C.L.; Parreira, P. Cholesterol Functionalized Nanoparticles Are Effective against Helicobacter pylori, the Gastric Bug: A Proof-of-Concept Study. Adv. Healthc. Mater. 2025, 14, 2404065. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.R.; Chitas, R.; Parreira, P.; Martins, M.C.L. How to manage Helicobacter pylori infection beyond antibiotics: The bioengineering quest. Appl. Mater. Today 2024, 37, 102123. [Google Scholar] [CrossRef]
- Asgari, S.; Nikkam, N.; Saniee, P. Metallic Nanoparticles as promising tools to eradicate H. pylori: A comprehensive review on recent advancements. Talanta Open 2022, 6, 100129. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices Part 5, Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Chitas, R.; Nunes, C.; Reis, S.; Parreira, P.; Martins, M.C.L. How Charge, Size and Protein Corona Modulate the Specific Activity of Nanostructured Lipid Carriers (NLC) against Helicobacter pylori. Pharmaceutics 2022, 14, 2745. [Google Scholar] [CrossRef]
- Parreira, P.; Magalhaes, A.; Goncalves, I.C.; Gomes, J.; Vidal, R.; Reis, C.A.; Leckband, D.E.; Martins, M.C. Effect of surface chemistry on bacterial adhesion, viability, and morphology. J. Biomed. Mater. Res. A 2011, 99A, 344–353. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 10th ed.; CLSI; CLSI Document M07-A10; Wayne, PA, USA, 2015. [Google Scholar]
- Parreira, P.; Soares, B.I.G.; Freire, C.S.R.; Silvestre, A.J.D.; Reis, C.A.; Martins, M.C.L.; Duarte, M.F. Eucalyptus spp. outer bark extracts inhibit Helicobacter pylori growth: In vitro studies. Ind. Crops Prod. 2017, 105, 207–214. [Google Scholar] [CrossRef]
- CLSI Guideline M26-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999.
- Fonseca, D.R.; Alves, P.M.; Neto, E.; Custódio, B.; Guimarães, S.; Moura, D.; Annis, F.; Martins, M.; Gomes, A.; Teixeira, C.; et al. One-Pot Microfluidics to Engineer Chitosan Nanoparticles Conjugated with Antimicrobial Peptides Using “Photoclick” Chemistry: Validation Using the Gastric Bacterium Helicobacter pylori. ACS Appl. Mater. Interfaces 2024, 16, 14533–14547. [Google Scholar] [CrossRef]
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12, Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.

| Sample | Metal | Modification Description | Planned [wt.%] | Obtained * [wt.%] |
|---|---|---|---|---|
| SiO2-AgNPs1 | Ag | Silica spheres with silver nanoparticles, no APTES | 3.0 | 2.04 |
| SiO2-AgNPs2 | Ag | Silica spheres with silver nanoparticles, with APTES | 3.0 | 2.57 |
| SiO2-BiNPs1 | Bi | Silica spheres with bismuth nanoparticles, no APTES | 3.0 | 2.38 |
| SiO2-BiNPs2 | Bi | Silica spheres with bismuth nanoparticles, with APTES | 5.0 | 4.52 |
| Sample | Tested Strains | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | S. aureus ATCC 43300 MRSA | S. epidermidis ATCC 28212 | E. faecium VRE ATCC 700221 | E. faecalis ATCC 29212 | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | ||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| SiO2-AgNPs1 Ag 1020 µg mL−1 | c2 | c1 | c2 | c1 | c3 | c1 | c1–c2 | >c1 | c1 | >c1 | c1 | >c1 | >c1 | not tested |
| 255.0 | 510.0 | 255.0 | 510.0 | 127.5 | 510.0 | 382.5 | 510.0 | 510.0 | 510.0 | 510.0 | 510.0 | >510.0 | not tested | |
| SiO2-AgNPs2 Ag 1285 µg mL−1 | c2 | c1 | c2 | c1 | c3 | c1 | c2 | >c1 | c2 | >c1 | c1 | >c1 | >c1 | not tested |
| 321.2 | 642.5 | 321.2 | 642.5 | 160.6 | 642.5 | 321.2 | 642.5 | 321.2 | 642.5 | 642.5 | 642.5 | >642.5 | not tested | |
| SiO2-BiNPs1 Bi 1190 µg mL−1 | c2 | >c1 | c2 | c1 | c4 | c2–c3 | c2 | >c1 | c2 | c1 | c1 | >c1 | >c1 | not tested |
| 297.5 | >595 | 297.5 | >595 | 74.375 | 297.5–148.75 | 297.5 | >595 | 297.5 | 595 | 595 | >595 | >595 | not tested | |
| SiO2-BiNPs2 Bi 2260 µg mL−1 | c3 | c2 | c3 | c1 | c5 | c3 | c2 | >c1 | c2–c3 | c1 | c1 | >c1 | >c1 | not tested |
| 282.5 | 565 | 282.5 | 1130 | 70.625 | 282.5 | 565 | 1130 | 423.75 | 1130 | 1130 | 1130 | >1130 | not tested | |
| Control | No inhibition | No inhibition | No inhibition | No inhibition | No inhibition | No inhibition | No inhibition | No inhibition | No inhibition | No inhibition | No inhibition | No inhibition | No inhibition | not tested |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajek, M.; Klesiewicz, K.; Biegun-Żurowska, M.; Parreira, P.; Ziąbka, M.; Różycka, A.; Rapacz-Kmita, A. Silica Spheres Functionalized with Silver and Bismuth Nanoparticles—Antibacterial Activity Against Clinically Relevant Bacterial Pathogens. Int. J. Mol. Sci. 2025, 26, 10203. https://doi.org/10.3390/ijms262010203
Gajek M, Klesiewicz K, Biegun-Żurowska M, Parreira P, Ziąbka M, Różycka A, Rapacz-Kmita A. Silica Spheres Functionalized with Silver and Bismuth Nanoparticles—Antibacterial Activity Against Clinically Relevant Bacterial Pathogens. International Journal of Molecular Sciences. 2025; 26(20):10203. https://doi.org/10.3390/ijms262010203
Chicago/Turabian StyleGajek, Marcin, Karolina Klesiewicz, Maria Biegun-Żurowska, Paula Parreira, Magdalena Ziąbka, Agnieszka Różycka, and Alicja Rapacz-Kmita. 2025. "Silica Spheres Functionalized with Silver and Bismuth Nanoparticles—Antibacterial Activity Against Clinically Relevant Bacterial Pathogens" International Journal of Molecular Sciences 26, no. 20: 10203. https://doi.org/10.3390/ijms262010203
APA StyleGajek, M., Klesiewicz, K., Biegun-Żurowska, M., Parreira, P., Ziąbka, M., Różycka, A., & Rapacz-Kmita, A. (2025). Silica Spheres Functionalized with Silver and Bismuth Nanoparticles—Antibacterial Activity Against Clinically Relevant Bacterial Pathogens. International Journal of Molecular Sciences, 26(20), 10203. https://doi.org/10.3390/ijms262010203










