Chitosan-Based Nanoencapsulation of Mānuka Oil for Periodontal Treatment
Abstract
1. Introduction
2. Essential Oils as Anti-Inflammatory and Antimicrobial Agents for Periodontal Treatment
2.1. Anti-Inflammatory Effects of Essential Oils
2.2. Antimicrobial Properties of Essential Oils Against Oral-Related Bacteria
| Essential Oil | Antimicrobial Mechanism | Major Active Components | References | ||
|---|---|---|---|---|---|
| Tea tree | Disrupts cellular homeostasis. Inhibits the respiratory activity. Affects membrane integrity. | Terpinen-4-ol Terpinolene α-Terpineol 1,8-Cineole γ-Terpinene | [26,51] | ||
| Eucalyptus | Damages the bacterial cell wall. Alters physiological function. Disrupts membrane integrity and permeability, leading to leakage of intracellular constituents. | 1,8-Cineole Citronellal Endo-borneol α-Terpineol Rosifoliol | [52,53] | ||
| Lavender | Interacts with bacterial membrane-associated proteins and metabolic enzymes. Disrupts cytoplasmic membrane and leads to the leakage of intracellular contents. | Carvacrol Linalool Linalyl acetate | [54,55,56] | ||
| Rosemary | Disrupts bacterial lipid bilayer. Leads to the loss of membrane integrity and cellular material. | α-Terpineol Terpinen-4-ol 1,8-Cineole | [57,58] | ||
| Mānuka | Disrupts bacterial cell membranes. Alters cell morphology. Causes the lysis of bacterial cells. | Leptospermone Isoleptospermone Flavesone Grandiflorone | [38,47] | ||
| MIC (%) | Mānuka | Tea Tree | Eucalyptus | Lavender | Rosemary |
| S. mutans | 0.25; 0.08; 0.06 | 1.0; 1.0; 0.26 | 1.0; >1.0 | >1.0 | >1.0 |
| S. aureus | 0.05; 0.02–0.05; 0.03 | 0.1 | 4 | 3.2 | 0.03; 0.125 |
| P. gingivalis | 0.03 | 0.13; 0.065 | 0.5 | 0.5 | 1.0 |
| A. actinomycetemcomitans | 0.03 | 0.5; 0.029 | 0.5 | 0.5 | 0.5 |
| F. nucleatum | 0.03 | 0.06; 0.085 | 0.13 | 0.25; 0.4 | 0.5 |
| References | [39,45,47,59,60,61] | [39,59,62,63] | [39,59,64] | [39,63,65] | [39,66,67] |
| MBC (%) | Mānuka | Tea Tree | Eucalyptus | Lavender | Rosemary |
| S. mutans | 0.25; 2.5 | 1.0; 1.042 | 1.0 | >1.0 | >1.0 |
| S. aureus | 0.09 | 0.2 | 4 | 6.4 | 0.1; 0.250 |
| P. gingivalis | 0.06 | 0.5; 0.065 | 0.5 | >1.0 | 1.0 |
| A. actinomycetemcomitans | 0.13 | 0.5; 0.052 | 0.5 | >1.0 | 1.0 |
| F. nucleatum | 0.03 | 0.25; 0.169 | 0.5 | >1.0 | 0.5 |
| References | [39,45,60] | [39,62,63] | [39,64] | [39,63] | [39,66,67] |
2.3. Mānuka Oil
2.3.1. Chemical Compounds of Mānuka Oil
2.3.2. Biocompatibility, Safety, and Efficacy: In Vitro, In Vivo, and Clinical Trial
3. Essential Oil Encapsulation with Nano-Techniques
3.1. Non-Encapsulation vs. Encapsulation of Essential Oils
3.2. Nanoencapsulation of Essential Oils
3.2.1. Nanotubes
3.2.2. Nanofibers
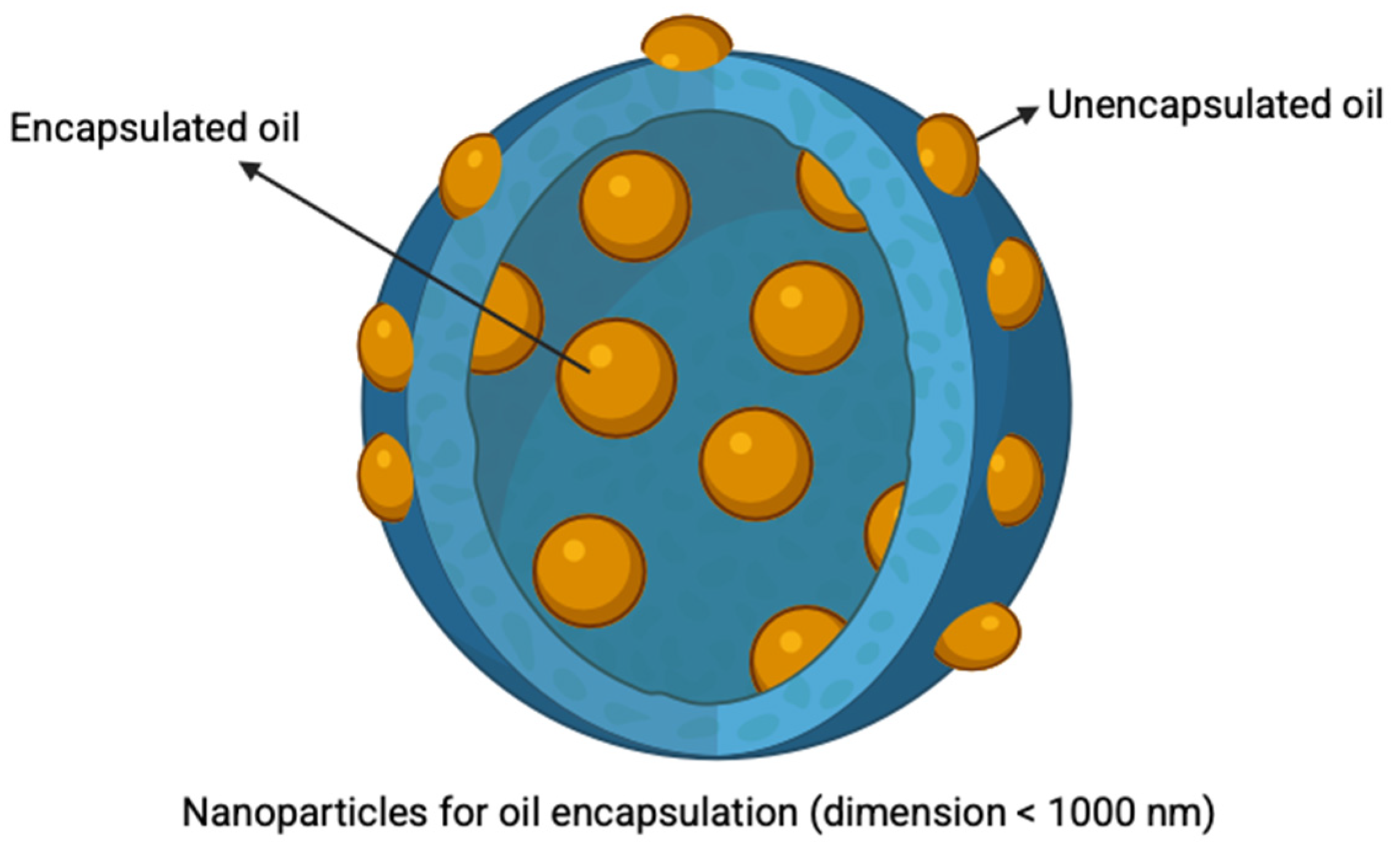
3.2.3. Nanoparticles
4. Methods for Nanoparticle Synthesis for Oil Encapsulation
4.1. Emulsification
4.2. Nanoprecipitation
4.3. Ionotropic Gelation
4.4. Microfluidics
5. Polymers Used in Nanoparticle Synthesis to Deliver Essential Oils
6. Chitosan
6.1. Chitosan Characteristics
6.2. Biocompatibility
6.3. Antimicrobial Properties
6.4. Muco-Adhesive Properties
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 6 November 2024).
- Joshi, D.; Garg, T.; Goyal, A.K.; Rath, G. Advanced drug delivery approaches against periodontitis. Drug Deliv. 2016, 23, 363–377. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial interactions and successions during plaque development. Periodontology 2000 2006, 42, 47–79. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontology 2000 2017, 75, 152–188. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000 2018, 76, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Chatzopoulos, G.S.; Koidou, V.P.; Tsalikis, L. Local drug delivery in the treatment of furcation defects in periodontitis: A systematic review. Clin. Oral Investig. 2023, 27, 955–970. [Google Scholar] [CrossRef]
- Nahas, P.; Houeis, S.; Chamboredon, R.; Heysselaer, D.; Zeinoun, T.; Nammour, S. Assessment of the periodontal cementum ablation depth during root planing by an Er:YAG laser at different energy densities: An ex vivo study. Dent. J. 2023, 11, 116. [Google Scholar] [CrossRef]
- García-Gargallo, M.; Zurlohe, M.; Montero, E.; Alonso, B.; Serrano, J.; Sanz, M.; Herrera, D. Evaluation of new chlorhexidine-and cetylpyridinium chloride-based mouthrinse formulations adjunctive to scaling and root planing: Pilot study. Int. J. Dent. Hyg. 2017, 15, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Cugini, M.A.; Dibart, S.; Smith, C.; Kent, R.L., Jr.; Socransky, S.S. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J. Clin. Periodontol. 1997, 24, 324–334. [Google Scholar] [CrossRef]
- de Oliveira, R.P.; de Melo Alencar, C.; Silva, F.A.; Magno, M.B.; Maia, L.C.; Silva, C.M. Effect of desensitizing agents on dentin hypersensitivity after non-surgical periodontal therapy: A systematic review and meta-analysis. J. Dent. 2020, 103, 103498. [Google Scholar] [CrossRef] [PubMed]
- Vinel, A.; Al Halabi, A.; Roumi, S.; Le Neindre, H.; Millavet, P.; Simon, M.; Cuny, C.; Barthet, J.-S.; Barthet, P.; Laurencin-Dalicieux, S. Non-surgical periodontal treatment: SRP and innovative therapeutic approaches. In Periodontitis: Advances in Experimental Research; Santi-Rocca, J., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 303–327. [Google Scholar]
- Kwon, T.; Lamster, I.B.; Levin, L. Current concepts in the management of periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.K.; Parikh, J.K. Advances and trends in encapsulation of essential oils. Int. J. Pharm. 2023, 635, 122668. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic devices: A tool for nanoparticle synthesis and performance evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- Abedini-Nassab, R.; Pouryosef Miandoab, M.; Şaşmaz, M. Microfluidic synthesis, control, and sensing of magnetic nanoparticles: A review. Micromachines 2021, 12, 768. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. A systematic review of the anti-inflammatory and immunomodulatory properties of 16 essential oils of herbs. Evid. Based Complement. Alternat. Med. 2020, 2020, 8878927. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Ferrante, A.; Prager, R.; Riley, T.; Carson, C.; Finlay-Jones, J.; Hart, P. The water-soluble components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm. Res. 2001, 50, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Ho, C.L.; Li, L.H.; Weng, Y.C.; Hua, K.F.; Ju, T.C. Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through reducing MAPK and NF-κB pathways. BMC Complement. Med. Ther. 2020, 20, 200. [Google Scholar] [CrossRef]
- Arooj, B.; Asghar, S.; Saleem, M.; Khalid, S.H.; Asif, M.; Chohan, T.; Khan, I.U.; Zubair, H.M.; Yaseen, H.S. Anti-inflammatory mechanisms of eucalyptol rich Eucalyptus globulus essential oil alone and in combination with flurbiprofen. Inflammopharmacology 2023, 31, 1849–1862. [Google Scholar] [CrossRef]
- Zuzarte, M.; Francisco, V.; Neves, B.; Liberal, J.; Cavaleiro, C.; Canhoto, J.; Salgueiro, L.; Cruz, M.T. Lavandula viridis L’Hér. essential oil inhibits the inflammatory response in macrophages through blockade of NF-KB signaling cascade. Front. Pharmacol. 2021, 12, 695911. [Google Scholar] [CrossRef]
- But, V.M.; Bulboacă, A.E.; Rus, V.; Ilyés, T.; Gherman, M.L.; Bolboacă, S.D. Anti-inflammatory and antioxidant efficacy of lavender oil in experimentally induced thrombosis. Thromb. J. 2023, 21, 85. [Google Scholar] [CrossRef]
- Karaji, Z.G.; Fathi, M.; Mirnasori, R.; van der Zee, E.A. Swimming exercise and clove oil can improve memory by molecular responses modification and reduce dark cells in rat model of Alzheimer’s disease. Exp. Gerontol. 2023, 177, 112192. [Google Scholar] [CrossRef]
- Pandey, V.K.; Srivastava, S.; Ashish; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: A comprehensive review. Heliyon 2024, 10, e22437. [Google Scholar] [CrossRef]
- Zaia, M.G.; Cagnazzo, T.; Feitosa, K.A.; Soares, E.G.; Faccioli, L.H.; Allegretti, S.M.; Afonso, A.; Anibal Fde, F. Anti-Inflammatory properties of menthol and menthone in schistosoma mansoni infection. Front. Pharmacol. 2016, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.A.; Radfar, M.; Goudarzi, Z. Peppermint as a promising treatment agent in inflammatory conditions: A comprehensive systematic review of literature. Phytother. Res. 2024, 38, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H.; et al. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-α-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Carlson, R.; von Fraunhofer, J. Mānuka oil vs. oral pathogens: An overview. EC Dent. Sci. 2022, 21, 148–154. [Google Scholar]
- Takarada, K.; Kimizuka, R.; Takahashi, N.; Honma, K.; Okuda, K.; Kato, T. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol. Immunol. 2004, 19, 61–64. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3083632, Leptospermone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Leptospermone (accessed on 25 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 59518515, Isoleptospermone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Isoleptospermone (accessed on 25 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 15800949, Flavesone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Flavesone (accessed on 25 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3014646, 4-Cyclohexene-1,3-dione, 5-hydroxy-2,2,6,6-tetramethyl-4-(1-oxo-3-phenylpropyl). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3014646 (accessed on 25 February 2025).
- Douglas, M.H.; van Klink, J.W.; Smallfield, B.M.; Perry, N.B.; Anderson, R.E.; Johnstone, P.; Weavers, R.T. Essential oils from New Zealand mānuka: Triketone and other chemotypes of Leptospermum scoparium. Phytochemistry 2004, 65, 1255–1264. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, Y.-K.; Jang, Y.-S.; Lee, M.-H. Enhancement of biofunctionalization by loading mānuka oil on TiO2 nanotubes. Nanomaterials 2022, 12, 569. [Google Scholar] [CrossRef]
- Song, S.-Y.; Hyun, J.-E.; Kang, J.-H.; Hwang, C.-Y. In vitro antibacterial activity of the mānuka essential oil from Leptospermum scoparium combined with Tris-EDTA against Gram-negative bacterial isolates from dogs with otitis externa. Vet. Dermatol. 2020, 31, 81-e6. [Google Scholar] [CrossRef]
- Alnaimat, S.; Wainwright, M.; Jaber, S.; Amasha, R. Mechanism of the antibacterial action of (Leptospermum scoparium) oil on methicillin-resistant Staphylococcus aureus (MRSA) and E. coli. In Proceedings of the 2nd Mediterranean Symposium on Medicinal and Aromatic Plants (MESMAP-2), Antalya, Turkey, 22–25 April 2015; pp. 22–25. [Google Scholar]
- van Klink, J.W.; Larsen, L.; Perry, N.B.; Weavers, R.T.; Cook, G.M.; Bremer, P.J.; MacKenzie, A.D.; Kirikae, T. Triketones active against antibiotic-resistant bacteria: Synthesis, structure–activity relationships, and mode of action. Bioorg. Med. Chem. 2005, 13, 6651–6662. [Google Scholar] [CrossRef]
- Jeong, E.-Y.; Lee, M.-J.; Lee, H.-S. Antimicrobial activities of leptospermone isolated from Leptospermum scoparium seeds and structure–activity relationships of its derivatives against foodborne bacteria. Food Sci. Biotechnol. 2018, 27, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Mathew, C.; Tesfaye, W.; Rasmussen, P.; Peterson, G.M.; Bartholomaeus, A.; Sharma, M.; Thomas, J. Mānuka oil-a review of antimicrobial and other medicinal properties. Pharmaceuticals 2020, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 2004, 53, 1081–1085. [Google Scholar] [CrossRef]
- He, Y.; Sang, S.; Tang, H.; Ou, C. In vitro mechanism of antibacterial activity of eucalyptus essential oil against specific spoilage organisms in aquatic products. J. Food Process. Preserv. 2022, 46, e16349. [Google Scholar] [CrossRef]
- Ameur, E.; Sarra, M.; Yosra, D.; Mariem, K.; Nabil, A.; Lynen, F.; Larbi, K.M. Chemical composition of essential oils of eight Tunisian Eucalyptus species and their antibacterial activity against strains responsible for otitis. BMC Complement. Med. Ther. 2021, 21, 209. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Speranza, B.; Guerrieri, A.; Racioppo, A.; Bevilacqua, A.; Campaniello, D.; Corbo, M.R. Sage and lavender essential oils as potential antimicrobial agents for foods. Microbiol. Res. 2023, 14, 1089–1113. [Google Scholar] [CrossRef]
- Stamova, S.; Ermenlieva, N.; Tsankova, G.; Georgieva, E. Antimicrobial activity of lavender essential oil from Lavandula angustifolia mill.: In vitro and in silico evaluation. Antibiotics 2025, 14, 656. [Google Scholar]
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Soliman, M.M.; Elsaba, Y.M.; Soliman, M.S.A.; Ahmed, E.Z. Composition and antimicrobial activity of Rosmarinus officinalis L. and Artemisia monosperma L. leaf essential oils and methanolic extracts from plants grown in normal and saline habitats in Egypt. Sci. Rep. 2024, 14, 7342. [Google Scholar] [CrossRef] [PubMed]
- Filoche, S.K.; Soma, K.; Sissons, C.H. Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol. Immunol. 2005, 20, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Pedonese, F.; Longo, E.; Torracca, B.; Najar, B.; Fratini, F.; Nuvoloni, R. Antimicrobial and anti-biofilm activity of mānuka essential oil against Listeria monocytogenes and Staphylococcus aureus of food origin. Ital. J. Food Saf. 2022, 11, 10039. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A novel interpretation of the fractional inhibitory concentration index: The case Origanum vulgare L. and Leptospermum scoparium J. R. et G. Forst essential oils against Staphylococcus aureus strains. Microbiol. Res. 2017, 195, 11–17. [Google Scholar] [CrossRef]
- Kulik, E.; Lenkeit, K.; Meyer, J. Antimicrobial effects of tea tree oil (Melaleuca alternifolia) on oral microorganisms. Schweiz. Monatsschr. Zahnmed. 2000, 110, 125–130. [Google Scholar]
- Thosar, N.; Basak, S.; Bahadure, R.N.; Rajurkar, M. Antimicrobial efficacy of five essential oils against oral pathogens: An in vitro study. Eur. J. Dent. 2013, 7, S071–S077. [Google Scholar] [CrossRef]
- Aldoghaim, F.S.; Flematti, G.R.; Hammer, K.A. Antimicrobial activity of several cineole-rich western Australian Eucalyptus essential oils. Microorganisms 2018, 6, 122. [Google Scholar] [CrossRef]
- Rosner, O.; Livne, S.; Bsharat, M.; Dviker, S.; Jeffet, U.; Matalon, S.; Sterer, N. Lavandula angustifolia essential oil inhibits the ability of Fusobacterium nucleatum to produce volatile sulfide compounds, a Key components in oral malodor. Molecules 2024, 29, 2982. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G.; Liu, X.-L. Chemical composition and antimicrobial activity of the essential oil of rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Fu, Y.; Zu, Y.; Chen, L.; Shi, X.; Wang, Z.; Sun, S.; Efferth, T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Res. 2007, 21, 989–994. [Google Scholar] [CrossRef]
- Bil, G.; Kapferer, E.; Koch, A.; Sedmak, C. Between Māori and modern? The case of mānuka honey. In Appreciating Local Knowledge; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2016; pp. 61–76. [Google Scholar]
- Kwon, O.S.; Jung, S.H.; Yang, B.S. Topical administration of mānuka oil prevents UV-B irradiation-induced cutaneous photoaging in mice. Evid. Based Complement. Alternat. Med. 2013, 2013, 930857. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Yan, S.-H.; Yen, M.-Y.; Wu, P.-F.; Liao, W.-T.; Huang, T.-S.; Wen, Z.-H.; Wang, H.-M.D. Investigations of kanuka and mānuka essential oils for in vitro treatment of disease and cellular inflammation caused by infectious microorganisms. J. Microbiol. Immunol. Infect. 2016, 49, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; Brennan, N.J.; Van Klink, J.W.; Harris, W.; Douglas, M.H.; McGimpsey, J.A.; Smallfield, B.M.; Anderson, R.E. Essential oils from New Zealand mānuka and kanuka: Chemotaxonomy of Leptospermum. Phytochemistry 1997, 44, 1485–1494. [Google Scholar] [CrossRef]
- Alsaud, N.; Shahbaz, K.; Farid, M. Evaluation of deep eutectic solvents in the extraction of β-caryophyllene from New Zealand mānuka leaves (Leptospermum scoparium). Chem. Eng. Res. Des. 2021, 166, 97–108. [Google Scholar] [CrossRef]
- Bass, N.I.; Parekh, M.Y.; Satyal, P.; Soni, S.; Jacob, J.A.; Mack, J.P.; Lobo, D.E. Mānuka essential oil triggers apoptosis and activation of c-Jun N-terminal kinase in fibroblasts and fibrosarcoma cells. Molecules 2024, 29, 5168. [Google Scholar] [CrossRef]
- Reichling, J.; Koch, C.; Stahl-Biskup, E.; Sojka, C.; Schnitzler, P. Virucidal activity of a β-triketone-rich essential oil of Leptospermum scoparium (mānuka oil) against HSV-1 and HSV-2 in cell culture. Planta Med. 2005, 71, 1123–1127. [Google Scholar] [CrossRef]
- Bhuyan, B.K.; Fraser, T.J.; Day, K.J. Cell proliferation kinetics and drug sensitivity of exponential and stationary populations of cultured L1210 cells. Cancer Res. 1977, 37, 1057–1063. [Google Scholar]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Lauten, J.D.; Boyd, L.; Hanson, M.B.; Lillie, D.; Gullion, C.; Madden, T.E. A clinical study: Melaleuca, mānuka, calendula and green tea mouth rinse. Phytother. Res. 2005, 19, 951–957. [Google Scholar] [CrossRef]
- Maddocks-Jennings, W.; Wilkinson, J.M.; Cavanagh, H.M.; Shillington, D. Evaluating the effects of the essential oils Leptospermum scoparium (mānuka) and Kunzea ericoides (kanuka) on radiotherapy induced mucositis: A randomized, placebo controlled feasibility study. Eur. J. Oncol. Nurs. 2009, 13, 87–93. [Google Scholar] [CrossRef]
- Campo Research PTE Ltd. Mānuka Oil Extract Leptospermum scoparium; US Library of Congress: Washington, DC, USA, 2015. [Google Scholar]
- Albuquerque, P.M.; Azevedo, S.G.; de Andrade, C.P.; D’Ambros, N.C.d.S.; Pérez, M.T.M.; Manzato, L. Biotechnological applications of nanoencapsulated essential oils: A review. Polymers 2022, 14, 5495. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Badri, W.; Dumas, E.; Ghnimi, S.; Elaissari, A.; Saurel, R.; Gharsallaoui, A. Nanoencapsulation of essential oils as natural food antimicrobial agents: An overview. Appl. Sci. 2021, 11, 5778. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Ther. 2019, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y. Nanomaterials at work in biomedical research. Nat. Mater. 2008, 7, 758–760. [Google Scholar] [CrossRef]
- Wu, W.; Ichihara, G.; Suzuki, Y.; Izuoka, K.; Oikawa-Tada, S.; Chang, J.; Sakai, K.; Miyazawa, K.; Porter, D.; Castranova, V.; et al. Dispersion method for safety research on manufactured nanomaterials. Ind. Health 2014, 52, 54–65. [Google Scholar] [CrossRef]
- Trotta, F.; Mele, A. Nanomaterials: Classification and properties. In Nanosponges: Synthesis and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–26. [Google Scholar]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomedicine 2015, 11, 1689–1694. [Google Scholar] [CrossRef]
- Medina, C.; Santos-Martinez, M.J.; Radomski, A.; Corrigan, O.I.; Radomski, M.W. Nanoparticles: Pharmacological and toxicological significance. Br. J. Pharmacol. 2007, 150, 552–558. [Google Scholar] [CrossRef]
- Ashraf, H.; Gul, H.; Jamil, B.; Saeed, A.; Pasha, M.; Kaleem, M.; Khan, A.S. Synthesis, characterization, and evaluation of the antifungal properties of tissue conditioner incorporated with essential oils-loaded chitosan nanoparticles. PLoS ONE 2022, 17, e0273079. [Google Scholar] [CrossRef]
- Ullah, N.; Amin, A.; Farid, A.; Selim, S.; Rashid, S.A.; Aziz, M.I.; Kamran, S.H.; Khan, M.A.; Rahim Khan, N.; Mashal, S.; et al. Development and evaluation of essential oil-based nanoemulgel formulation for the treatment of oral bacterial infections. Gels 2023, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Sawatphakdee, G.; Yostawonkul, J.; Oontawee, S.; Rodprasert, W.; Sawangmake, C.; Kornsuthisopon, C.; Yata, T.; Tabtieang, S.P.; Nowwarote, N.; Pirarat, N. Feasibility of nanostructured lipid carrier loaded with alpha-mangostin and clove oil for canine periodontal therapy. Animals 2024, 14, 2084. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, B.S.; Mohanty, M.; Behera, S. PLGA-lecithin nanocarrier encapsulating curcuma caesia oil in a mucoadhesive gel: Efficacy analysis against periodontal infections. J. Microencapsul. 2025, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Lee, J.-S.; Lee, H.G. Nanoencapsulation of grapefruit seed extract and cinnamon oil for oral health: Preparation, in vitro, and clinical antimicrobial activities. J. Agric. Food Chem. 2023, 71, 5646–5654. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Kim, H.-E.; Han, S.-J.; Choi, J.-S. Antibacterial and antibiofilm activities of cinnamon essential oil nanoemulsion against multi-species oral biofilms. Sci. Rep. 2021, 11, 5911. [Google Scholar] [CrossRef]
- Batista, D.G.; Sganzerla, W.G.; da Silva, L.R.; Vieira, Y.G.S.; Almeida, A.R.; Dominguini, D.; Ceretta, L.; Pinheiro, A.C.; Bertoldi, F.C.; Becker, D.; et al. Antimicrobial and cytotoxic potential of eucalyptus essential oil-based nanoemulsions for mouthwashes application. Antibiotics 2024, 13, 942. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Li, J.; Tang, M.; Chen, H.; Wang, G.; Guo, J.; Gui, S. Microemulsion-thermosensitive gel composites as in situ-forming drug reservoir for periodontitis tissue repair through alveolar bone and collagen regeneration strategy. Pharm. Dev. Technol. 2023, 28, 30–39. [Google Scholar] [CrossRef]
- Oun, A.A.; Bae, A.Y.; Shin, G.H.; Park, M.-K.; Kim, J.T. Comparative study of oregano essential oil encapsulated in halloysite nanotubes and diatomaceous earth as antimicrobial and antioxidant composites. Appl. Clay Sci. 2022, 224, 106522. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef]
- Lee, M.H.; Park, H.J. Preparation of halloysite nanotubes coated with Eudragit for a controlled release of thyme essential oil. J. Appl. Polym. Sci. 2015, 132, 42771. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Hiwrale, A.; Bharati, S.; Pingale, P.; Rajput, A. Nanofibers: A current era in drug delivery system. Heliyon 2023, 9, e18917. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Cornejo Bravo, J.M.; Serrano Medina, A.; Pérez González, G.L.; Villarreal Gómez, L.J. A summary of electrospun nanofibers as drug delivery system: Drugs loaded and biopolymers used as matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Dadras Chomachayi, M.; Solouk, A.; Akbari, S.; Sadeghi, D.; Mirahmadi, F.; Mirzadeh, H. Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: Thyme essential oil and doxycycline monohydrate release study. J. Biomed. Mater. Res. A 2018, 106, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Râpă, M.; Gaidau, C.; Mititelu-Tartau, L.; Berechet, M.-D.; Berbecaru, A.C.; Rosca, I.; Chiriac, A.P.; Matei, E.; Predescu, A.-M.; Predescu, C. Bioactive collagen hydrolysate-chitosan/essential oil electrospun nanofibers designed for medical wound dressings. Pharmaceutics 2021, 13, 1939. [Google Scholar] [CrossRef] [PubMed]
- Partheniadis, I.; Stathakis, G.; Tsalavouti, D.; Heinämäki, J.; Nikolakakis, I. Essential oil—Loaded nanofibers for pharmaceutical and biomedical applications: A systematic mini-review. Pharmaceutics 2022, 14, 1799. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Drug targeting with nanoparticles. Eur. J. Drug Metab. Pharmacokinet. 1994, 19, 253–256. [Google Scholar] [CrossRef]
- Gimondi, S.; Guimarães, C.F.; Vieira, S.F.; Gonçalves, V.M.; Tiritan, M.E.; Reis, R.L.; Ferreira, H.; Neves, N.M. Microfluidic mixing system for precise PLGA-PEG nanoparticles size control. Nanomedicine 2022, 40, 102482. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Islam, M.R.; Choudhury, Z.S.; Mostafa, A.; Kadir, M.F. Nanotechnology based approaches in cancer therapeutics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043001. [Google Scholar] [CrossRef]
- Mazumdar, S.; Chitkara, D.; Mittal, A. Exploration and insights into the cellular internalization and intracellular fate of amphiphilic polymeric nanocarriers. Acta Pharm. Sin. B 2021, 11, 903–924. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Jang, E.; Lee, K.; Haam, S.; Huh, Y.-M. Delivery of cancer therapeutics using nanotechnology. Pharmaceutics 2013, 5, 294–317. [Google Scholar] [CrossRef] [PubMed]
- Roncari Rocha, G.; Sims, K.R., Jr.; Xiao, B.; Klein, M.I.; Benoit, D.S. Nanoparticle carrier co-delivery of complementary antibiofilm drugs abrogates dual species cariogenic biofilm formation in vitro. J. Oral Microbiol. 2022, 14, 1997230. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.N.; Utra, U.; Alias, A.K.; Tan, T.B.; Tan, C.P.; Yussof, N.S. Physical, morphological and antibacterial properties of lime essential oil nanoemulsions prepared via spontaneous emulsification method. LWT-Food Sci. Technol. 2020, 128, 109388. [Google Scholar] [CrossRef]
- Liang, R.; Xu, S.; Shoemaker, C.F.; Li, Y.; Zhong, F.; Huang, Q. Physical and antimicrobial properties of peppermint oil nanoemulsions. J. Agric. Food Chem. 2012, 60, 7548–7555. [Google Scholar] [CrossRef]
- Parris, N.; Cooke, P.H.; Hicks, K.B. Encapsulation of essential oils in zein nanospherical particles. J. Agric. Food Chem. 2005, 53, 4788–4792. [Google Scholar] [CrossRef]
- Liakos, I.L.; Iordache, F.; Carzino, R.; Scarpellini, A.; Oneto, M.; Bianchini, P.; Grumezescu, A.M.; Holban, A.M. Cellulose acetate—Essential oil nanocapsules with antimicrobial activity for biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 471–479. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of essential oils via nanoprecipitation process: Overview, progress, challenges and prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef]
- Iannitelli, A.; Grande, R.; Stefano, A.D.; Giulio, M.D.; Sozio, P.; Bessa, L.J.; Laserra, S.; Paolini, C.; Protasi, F.; Cellini, L. Potential antibacterial activity of carvacrol-loaded poly(DL-lactide-co-glycolide) (PLGA) nanoparticles against microbial biofilm. Int. J. Mol. Sci. 2011, 12, 5039–5051. [Google Scholar] [CrossRef]
- Fraj, A.; Jaâfar, F.; Marti, M.; Coderch, L.; Ladhari, N. A comparative study of oregano (Origanum vulgare L.) essential oil-based polycaprolactone nanocapsules/microspheres: Preparation, physicochemical characterization, and storage stability. Ind. Crops. Prod. 2019, 140, 111669. [Google Scholar] [CrossRef]
- Pham, M.P.T.; Tran, L.Q.; Le, C.Y.V.; Tran, V.T.; Do, N.H.N.; Le, P.K. Ultrasound-assisted ionic gelation of neem oil-encapsulated chitosan nanoparticles as a promising biopesticide. Chem. Pap. 2024, 78, 1825–1832. [Google Scholar] [CrossRef]
- Astutiningsih, F.; Anggrahini, S.; Fitriani, A.; Supriyadi, S. Optimization of saffron essential oil nanoparticles using chitosan-Arabic gum complex nanocarrier with ionic gelation method. Int. J. Food Sci. 2022, 2022, 4035033. [Google Scholar] [CrossRef]
- Helal, M.A.; Abdel-Gawad, A.M.; Kandil, O.M.; Khalifa, M.M.E.; Morrison, A.A.; Bartley, D.J.; Cave, G.W.V.; Elsheikha, H.M. Microfluidic-based formulation of essential oils-loaded chitosan coated PLGA particles enhances their bioavailability and nematocidal activity. Pharmaceutics 2022, 14, 2030. [Google Scholar] [CrossRef]
- Taherian, P.; Nourbakhsh, M.S.; Mehrizi, A.A.; Hashemi, M. Encapsulation of frankincense essential oil by microfluidic and bulk approaches: A comparative study. Fibers Polym. 2022, 23, 2970–2980. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Weisany, W.; Yousefi, S.; Tahir, N.A.-r.; Golestanehzadeh, N.; McClements, D.J.; Adhikari, B.; Ghasemlou, M. Targeted delivery and controlled released of essential oils using nanoencapsulation: A review. Adv. Colloid Interface Sci. 2022, 303, 102655. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Miastkowska, M.; Michalczyk, A.; Figacz, K.; Sikora, E. Nanoformulations as a modern form of biofungicide. J. Environ. Health Sci. Eng. 2020, 18, 119–128. [Google Scholar] [CrossRef]
- Zhou, Z.; Lin, S.; Yue, T.; Lee, T.-C. Adsorption of food dyes from aqueous solution by glutaraldehyde cross-linked magnetic chitosan nanoparticles. J. Food Eng. 2014, 126, 133–141. [Google Scholar] [CrossRef]
- Dhawan, S.; Singla, A.K.; Sinha, V.R. Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS PharmSciTech 2004, 5, 67. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Zou, D.; Hui, Y.; Nigam, K.; Middelberg, A.P.J.; Zhao, C.-X. Formulation of nanoparticles using mixing-induced nanoprecipitation for drug delivery. Ind. Eng. Chem. Res. 2020, 59, 4134–4149. [Google Scholar] [CrossRef]
- Tanaka, K.; Kuramochi, H.; Maeda, K.; Takahashi, Y.; Osako, M.; Suzuki, G. Size-controlled preparation of polyethylene nanoplastic particles by nanoprecipitation and insights into the underlying mechanisms. ACS Omega 2023, 8, 14470–14477. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters influencing the size of chitosan-TPP nano-and microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; Tohidi, M.M.; Paymard, B.; Lucero-Acuña, A. Chapter 9—Immobilization of essential oils in biopolymeric matrices: Recent approaches for controlled delivery systems. In Studies in Natural Products Chemistry; Rahman, A.-u., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 78, pp. 365–401. [Google Scholar]
- Colombo, S.; Beck-Broichsitter, M.; Bøtker, J.P.; Malmsten, M.; Rantanen, J.; Bohr, A. Transforming nanomedicine manufacturing toward Quality by Design and microfluidics. Adv. Drug Deliv. Rev. 2018, 128, 115–131. [Google Scholar] [CrossRef]
- Lim, J.-M.; Karnik, R. Optimizing the discovery and clinical translation of nanoparticles: Could microfluidics hold the key? Nanomedicine 2014, 9, 1113–1116. [Google Scholar] [CrossRef]
- Kumar, K.; Nightingale, A.M.; Krishnadasan, S.H.; Kamaly, N.; Wylenzinska-Arridge, M.; Zeissler, K.; Branford, W.R.; Ware, E.; deMello, A.J.; deMello, J.C. Direct synthesis of dextran-coated superparamagnetic iron oxide nanoparticles in a capillary-based droplet reactor. J. Mater. Chem. 2012, 22, 4704–4708. [Google Scholar] [CrossRef]
- Hung, L.-H.; Lee, A.P. Microfluidic devices for the synthesis of nanoparticles and biomaterials. J. Med. Biol. Eng. 2007, 27, 1. [Google Scholar]
- Zou, L.; Huang, B.; Zheng, X.; Pan, H.; Zhang, Q.; Xie, W.; Zhao, Z.; Li, X. Microfluidic synthesis of magnetic nanoparticles in droplet-based microreactors. Mater. Chem. Phys. 2022, 276, 125384. [Google Scholar] [CrossRef]
- Dupuis, V.; Cerbu, C.; Witkowski, L.; Potarniche, A.-V.; Timar, M.C.; Żychska, M.; Sabliov, C.M. Nanodelivery of essential oils as efficient tools against antimicrobial resistance: A review of the type and physical-chemical properties of the delivery systems and applications. Drug Deliv. 2022, 29, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Yahya, E.B.; Jummaat, F.; Amirul, A.A.; Adnan, A.S.; Olaiya, N.G.; Abdullah, C.K.; Rizal, S.; Mohamad Haafiz, M.K.; Khalil, H.P.S.A. A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Pharmaceutical and biomedical applications of cellulose nanofibers: A review. Environ. Chem. Lett. 2021, 19, 2043–2055. [Google Scholar] [CrossRef]
- Luis, A.I.S.; Campos, E.V.R.; de Oliveira, J.L.; Guilger-Casagrande, M.; de Lima, R.; Castanha, R.F.; de Castro, V.L.S.S.; Fraceto, L.F. Zein nanoparticles impregnated with eugenol and garlic essential oils for treating fish pathogens. ACS Omega 2020, 5, 15557–15566. [Google Scholar] [CrossRef]
- Gonçalves da Rosa, C.; Zapelini de Melo, A.P.; Sganzerla, W.G.; Machado, M.H.; Nunes, M.R.; Vinicius de Oliveira Brisola Maciel, M.; Bertoldi, F.C.; Manique Barreto, P.L. Application in situ of zein nanocapsules loaded with Origanum vulgare Linneus and Thymus vulgaris as a preservative in bread. Food Hydrocoll. 2020, 99, 105339. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Paula, H.C.B.; Paula, R.C.M.d. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151. [Google Scholar] [CrossRef]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosan–alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef]
- Almeida, K.B.; Ramos, A.S.; Nunes, J.B.B.; Silva, B.O.; Ferraz, E.R.A.; Fernandes, A.S.; Felzenszwalb, I.; Amaral, A.C.F.; Roullin, V.G.; Falcão, D.Q. PLGA nanoparticles optimized by Box-Behnken for efficient encapsulation of therapeutic cymbopogon citratus essential oil. Colloids Surf. B Biointerfaces 2019, 181, 935–942. [Google Scholar] [CrossRef]
- Samling, B.A.; Assim, Z.; Tong, W.-Y.; Leong, C.-R.; Rashid, S.A.; Nik Mohamed Kamal, N.N.S.; Muhamad, M.; Tan, W.-N. Cynometra cauliflora essential oils loaded-chitosan nanoparticles: Evaluations of their antioxidant, antimicrobial and cytotoxic activities. Int. J. Biol. Macromol. 2022, 210, 742–751. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H.; Imran, M. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Khodaiyan, F. The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils. Food Chem. 2020, 309, 125520. [Google Scholar] [CrossRef]
- Yadav, A.; Kujur, A.; Kumar, A.; Singh, P.P.; Gupta, V.; Prakash, B. Encapsulation of bunium persicum essential oil using chitosan nanopolymer: Preparation, characterization, antifungal assessment, and thermal stability. Int. J. Biol. Macromol. 2020, 142, 172–180. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An attractive biocompatible polymer for microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 71853, Chitosan. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chitosan (accessed on 4 May 2025).
- Mohammed, M.A.; Syeda, J.T.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Richardson, S.W.; Kolbe, H.J.; Duncan, R. Potential of low molecular mass chitosan as a DNA delivery system: Biocompatibility, body distribution and ability to complex and protect DNA. Int. J. Pharm. 1999, 178, 231–243. [Google Scholar] [CrossRef]
- Arancibia, R.; Maturana, C.; Silva, D.; Tobar, N.; Tapia, C.; Salazar, J.C.; Martínez, J.; Smith, P.C. Effects of chitosan particles in periodontal pathogens and gingival fibroblasts. J. Dent. Res. 2013, 92, 740–745. [Google Scholar] [CrossRef]
- Pistone, S.; Goycoolea, F.M.; Young, A.; Smistad, G.; Hiorth, M. Formulation of polysaccharide-based nanoparticles for local administration into the oral cavity. Eur. J. Pharm. Sci. 2017, 96, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Mumuni, M.A.; Kenechukwu, F.C.; Ofokansi, K.C.; Attama, A.A.; Díaz, D.D. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr. Polym. 2020, 229, 115506. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Tago, K.; Nagata, T.; Furuya, M.; Seki, T.; Kato, H.; Morimura, T.; Ohara, N. A 90-day ad libitum administration toxicity study of oligoglucosamine in F344 rats. Food Chem. Toxicol. 2007, 45, 1575–1587. [Google Scholar] [CrossRef]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef]
- Minami, S.; Oh-Oka, M.; Okamoto, Y.; Miyatake, K.; Matsuhashi, A.; Shigemasa, Y.; Fukumoto, Y. Chitosan-inducing hemorrhagic pneumonia in dogs. Carbohydr. Polym. 1996, 29, 241–246. [Google Scholar] [CrossRef]
- Zhu, Y.; Ali, A.; Mulinari dos Santos, G.; Franciscon, J.P.S.; de Molon, R.S.; Goh, C.; Ervolino, E.; Theodoro, L.H.; Shrestha, A. A chitosan-based hydrogel to modulate immune cells and promote periodontitis healing in the high-fat diet-induced periodontitis rat model. Acta Biomater. 2025, 200, 452–463. [Google Scholar] [CrossRef]
- Akncbay, H.; Senel, S.; Ay, Z.Y. Application of chitosan gel in the treatment of chronic periodontitis. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 290–296. [Google Scholar] [CrossRef]
- Boynueğri, D.; Özcan, G.; Şenel, S.; Uç, D.; Uraz, A.; Öğüş, E.; Çakılcı, B.; Karaduman, B. Clinical and radiographic evaluations of chitosan gel in periodontal intraosseous defects: A pilot study. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90B, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; Britto, D.d.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan based bioadhesives for biomedical applications: A review. Carbohydr. Polym. 2022, 282, 119100. [Google Scholar] [CrossRef] [PubMed]
- Pedro, A.S.; Cabral-Albuquerque, E.; Ferreira, D.; Sarmento, B. Chitosan: An option for development of essential oil delivery systems for oral cavity care? Carbohydr. Polym. 2009, 76, 501–508. [Google Scholar] [CrossRef]
- Aksungur, P.; Sungur, A.; Ünal, S.; İskit, A.B.; Squier, C.A.; Şenel, S. Chitosan delivery systems for the treatment of oral mucositis: In vitro and in vivo studies. J. Control. Release 2004, 98, 269–279. [Google Scholar] [CrossRef]
- Özdoğan, A.I.; İlarslan, Y.D.; Kösemehmetoğlu, K.; Akca, G.; Kutlu, H.B.; Comerdov, E.; Iskit, A.B.; Şenel, S. In vivo evaluation of chitosan based local delivery systems for atorvastatin in treatment of periodontitis. Int. J. Pharm. 2018, 550, 470–476. [Google Scholar] [CrossRef]



| Essential Oil | Anti-Inflammation Mechanism | Major Active Components | References |
|---|---|---|---|
| Tea tree | Inhibit LPS-induced production of TNF-α, IL-1β, IL-8 and IL-10. | Terpinen-4-ol α-Terpineol 1,8-Cineole | [25,26] |
| Eucalyptus | Inhibit LPS-induced inflammation by reducing MAPK and NF-κB pathways. | 1,8-Cineole α-Pinene | [27,28] |
| Lavender | Decrease production of inflammatory mediator, including TNF-α, IL-1β, NO and PGE2; block NF-κB pathway. | Linalool Linalyl acetate | [29,30] |
| Clove | Alter NF-κB pathway; reduce production of IL-6 and COX-2. | Eugenol | [31,32] |
| Peppermint | Activate AMPK, ULK1 and NRF2 pathway; down-regulate MAPKs and NF-κB pathways; suppress oxidative stress and production of NO. | Menthol menthone | [33,34,35] |
| Mānuka | Inhibit LPS-induced production of TNF-α, IL-1β, IL-6 and NO; Suppress MAPKs and NF-κB pathways. | α-Pinene | [36,37] |
| β-Triketones | 2D Structure | 3D Structure | |
| Leptospermone [40] | C15H22O4 |  |  |
| Isoleptospermone [41] | C15H22O4 | 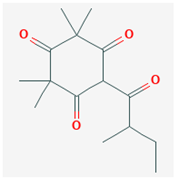 |  |
| Flavesone [42] | C14H20O4 | 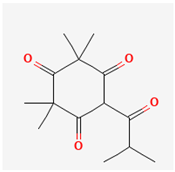 |  |
| Grandiflorone [43] | C19H22O4 | 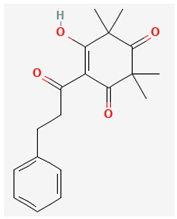 | 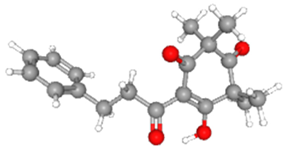 |
| In Vitro Cell Experiment | Mānuka Oil Concentration | Effect | |
|---|---|---|---|
| Normal human fibroblast cells (CUA-4) | 0.05% | Reduced 25% cell viability over 24 h [73]. | |
| Fibrosarcoma cells (HT-1080) | 0.05% | Reduced 56% cell viability over 24 h [73]. | |
| THP-1 cells | 0.1% to 10% | No toxic effect over 48 h [70]. | |
| African green monkey kidney cells (RC-37 cells) | 0.003% | No toxic effect [74]. | |
| 0.004% | 50% decrease in cell viability [74]. | ||
| In Vivo Animal Experiment | Mānuka Oil Concentration | Effect | |
| Rabbit | Femoral bone defect model | 0.5% | Improved bone healing [45]. |
| Hairless mice | Topical application | 10% | Reduced UV-induced cutaneous photoaging [69]. |
| Human Clinical Trial | Mānuka Oil Concentration | Effect | |
| Gargling | 2 drops diluted in water per time, 3–5 times per day | Improvement in treating radiation-induced mucositis [48]. | |
| Mouth rinse | 0.33% | No side effects as reported from hematological test [77]. | |
| Essential Oils | Nanocarriers | Size (nm) | Encapsulation Efficiency (%) | Release Kinetics (h) | Muco-Adhesion or Penetration | Antimicrobial Activity/ Therapeutic Efficacy | Oral Application |
|---|---|---|---|---|---|---|---|
| Oregano and Lemongrass oil [90] | Chitosan nanoparticle | 100–200 | NA | 6 to 24 (distilled water). | NA | In vitro antimicrobial: C. albicans | Tissue conditioner |
| Cinnamon and clove oil [91] | Carbopol 640 Nanoemulgel | 152 | 95.78 (Cinnamon) 96.45 (Clove) | 2 to 24 (Franz diffusion cell with PBS, pH 6.8). | Model: in vitro goat buccal mucosa. Adhesive strength: 42.87 N/cm2 Drug permeation: 54.21% (cinnamon) and 52.72% (clove) over 24 h. | In vitro antimicrobial: P. aeruginosa B. chungangensis S. epidermidis S. aureus C. albicans | Oral bacterial infections |
| Clove oil [92] | Nanolipid | 190–220 | 98.95 | 8 to 72 (buffer, no specific information). | Model: in vitro dog canine gingival tissue. Penetration: reaching 100 µm depth within 45 min. | In vitro antimicrobial: E. coli Salmonella spp. K. Pneumoniae B. pyogenes | Periodontal therapy |
| Curcuma caesia oil [93] | PLGA–lecithin nanocarrier | 51.28 | 86.4 | 69.26% over 24 h (PBS, pH 6.8). | Model: in vitro goat buccal mucosa Permeation: 42.79% oil release over 24 h (incorporated in Carbopol 934 P gel). | In vitro antimicrobial: E. coli S. aureus L. acidophilus P. aeruginosa B. subtilis | Periodontal infections |
| Model: in vivo ligature-induced rat periodontitis model (incorporate in Carbopol 934 P gel). Application: topical application with cotton swab. Plasma pharmacokinetic: mean residence time was 4.3 h. | In vivo therapeutic efficacy: reduce inflammatory cells and cellular debris. | ||||||
| Cinnamon oil with grapeseed extract [94] | Chitosan/carrageenan nanoparticle | 357 | NA | NA | Model: in vitro mucin adsorption method. Mucin adsorption: 20–40%. | In vitro antimicrobial: S. mutans S. sobrinus | Mouth rinse |
| Cinnamon oil [95] | Nanoemulsion | NA | Loading: 5% | NA | NA | In vitro antimicrobial: muti-species oral biofilm on bovine enamel. | Dental caries management |
| Eucalyptus oil [96] | Nanoemulsion | 100–180 | NA | NA | NA | In vitro antimicrobial: S. mutans | Mouthwash |
| Clove oil with baicalin [97] | Microemulsion | 26.53 | 93.8 | 4 h (NaCl 0.9% containing 25% w/v PEG400) | NA | In vivo therapeutic efficacy (incorporate in P407/P188 gel): reduce inflammatory response and alveolar bone loss. | Periodontal tissue repair |
| Methods | Encapsulated Essential Oil | Encapsulation Materials | Size (nm) | PDI | References |
|---|---|---|---|---|---|
| Emulsification | Lime | Emulsified in water with Tween 80 as surfactant | 21 | 0.444 | [80,114] |
| 28 | 0.469 | ||||
| 60 | 0.557 | ||||
| Peppermint | Emulsified in starch and water | 180–230 | 0.250–0.340 | [115] | |
| Oregano | Zein | >100 | NA | [116] | |
| Nanoprecipitation | Peppermint | Cellulose acetate | 180 | 0.09–0.27 | [117,118] |
| Cinnamon | 150 | ||||
| Lemongrass | 200 | ||||
| Origanum vulgare | Polycaprolactone (PCL) | 180–230 | 0.130–0.180 | [119,120] | |
| Ionic gelation | Neem | Chitosan | 133–175 | 0.170–0.260 | [121] |
| Saffron | Chitosan–Arabic gum complex | 16–24 | 0.495–0.607 | [122] | |
| Microfluidics | Eugenol, linalool, and geraniol | Chitosan coated PLGA | NA | NA | [123] |
| Frankincense | Chitosan with TPP | 118 | <0.28 | [124] |
| Polymers | Example | Advantages | Limitations |
|---|---|---|---|
| Cellulose | Encapsulated peppermint, cinnamon, and lemongrass essential oils into cellulose acetate-based spherical nanocapsules [117]. | Non-toxic. Biocompatible. Biodegradable. Cost-effective [143,144]. | Polymer without intrinsic antimicrobial properties [145]. |
| Zein | Encapsulated eugenol and garlic essential oils into zein NPs [146]. | Better prevention against oxidation and degradation of the encapsulated compounds [147]. | Further toxicological studies required [142]. |
| Sodium alginate | Encapsulated lippia sidoides essential oil into alginate–cashew gum NPs [148]. | Non-toxic. Biocompatible. Biodegradable. Interacted with agents with different charge [149]. | Polymer without antimicrobial properties [145]. |
| PLGA | Encapsulated Cymbopogon citratus essential oil with PLGA NPs [150]. | Non-toxic. Biocompatible. Biodegradable and easily modified [150]. | High production cost. Low encapsulation efficiency [145]. |
| Chitosan | Encapsulated Cynometra cauliflora essential oil, curry leaf essential oil, and clove essential oil [151,152,153]. | Muco-adhesive. Intrinsic antimicrobial effect. Biodegradable. Biocompatible [152,154]. | No major limitations reported [142]. |
| Bacteria | Molecular Weight of Chitosan (kDa) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 28 | 107 | 224 | 470 | 624 | 746 | 1106 | 1671 | ||
| Gram-positive | S. aureus | >1.0 | - | >0.8 | 0.8 | - | >0.8 | >1.0 | >1.0 |
| Bacillus megaterium | >0.8 | - | >0.5 | 0.5 | - | >0.8 | >0.5 | >0.8 | |
| Listeria monocytogenes | >1.0 | - | >0.8 | 0.8 | - | >0.8 | >1.0 | >1.0 | |
| Lactobacillus brevis | >0.8 | - | >1.0 | 1.0 | - | >1.0 | >0.5 | >0.8 | |
| Gram-negative | E. coli | >1.0 | - | >1.0 | 0.8 | - | >0.8 | >1.0 | >1.0 |
| Pseudomonas fluorescens | >1.0 | - | >0.8 | 0.8 | - | >0.8 | >1.0 | >1.0 | |
| S. typhimurium | >1.0 | - | >1.0 | 0.8 | - | >1.0 | >1.0 | >1.0 | |
| Vibrio parahaemolyticus | >1.0 | - | >1.0 | 0.8 | - | >0.8 | >1.0 | >1.0 | |
| Oral-Related Bacteria | Molecular Weight of Chitosan (kDa) | ||||||||
| 28 | 107 | 224 | 470 | 624 | 746 | 1106 | 1671 | ||
| Gram-positive | S. mutans | - | 5 | - | - | 5 | - | - | - |
| Gram-negative | P. gingivalis | - | 1 | - | - | 1 | - | - | - |
| T. forsythensis | - | 3 | - | - | 1 | - | - | - | |
| P. buccae | - | 1 | - | - | 3 | - | - | - | |
| A. actinomycetemcomitans | - | 3 | - | - | 5 | - | - | - | |
| P. intermedia | - | 3 | - | - | 1 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Duncan, W.J.; Hughes-Medlicott, N.J.; Alhamdani, G.; Coates, D.E. Chitosan-Based Nanoencapsulation of Mānuka Oil for Periodontal Treatment. Int. J. Mol. Sci. 2025, 26, 10201. https://doi.org/10.3390/ijms262010201
Chen C, Duncan WJ, Hughes-Medlicott NJ, Alhamdani G, Coates DE. Chitosan-Based Nanoencapsulation of Mānuka Oil for Periodontal Treatment. International Journal of Molecular Sciences. 2025; 26(20):10201. https://doi.org/10.3390/ijms262010201
Chicago/Turabian StyleChen, Chen, Warwick J. Duncan, Natalie J. Hughes-Medlicott, Ghsaq Alhamdani, and Dawn E. Coates. 2025. "Chitosan-Based Nanoencapsulation of Mānuka Oil for Periodontal Treatment" International Journal of Molecular Sciences 26, no. 20: 10201. https://doi.org/10.3390/ijms262010201
APA StyleChen, C., Duncan, W. J., Hughes-Medlicott, N. J., Alhamdani, G., & Coates, D. E. (2025). Chitosan-Based Nanoencapsulation of Mānuka Oil for Periodontal Treatment. International Journal of Molecular Sciences, 26(20), 10201. https://doi.org/10.3390/ijms262010201








