Advances in Cytoplasmic Male Sterility in Sugar Beet from Mitochondrial Genome Structural Dynamics and Nuclear-Cytoplasmic Coordination
Abstract
1. Introduction
2. Advances in Sugar Beet Genomics Research
2.1. Advances in Nuclear Genome Research of Sugar Beet
2.2. Advances in Chloroplast Genome Research in Sugar Beet
2.3. Advances in Mitochondrial Genome Research in Sugar Beet
3. Advances in Cytoplasmic Male Sterility Research in Sugar Beet
3.1. Discovery and Types of CMS in Sugar Beet
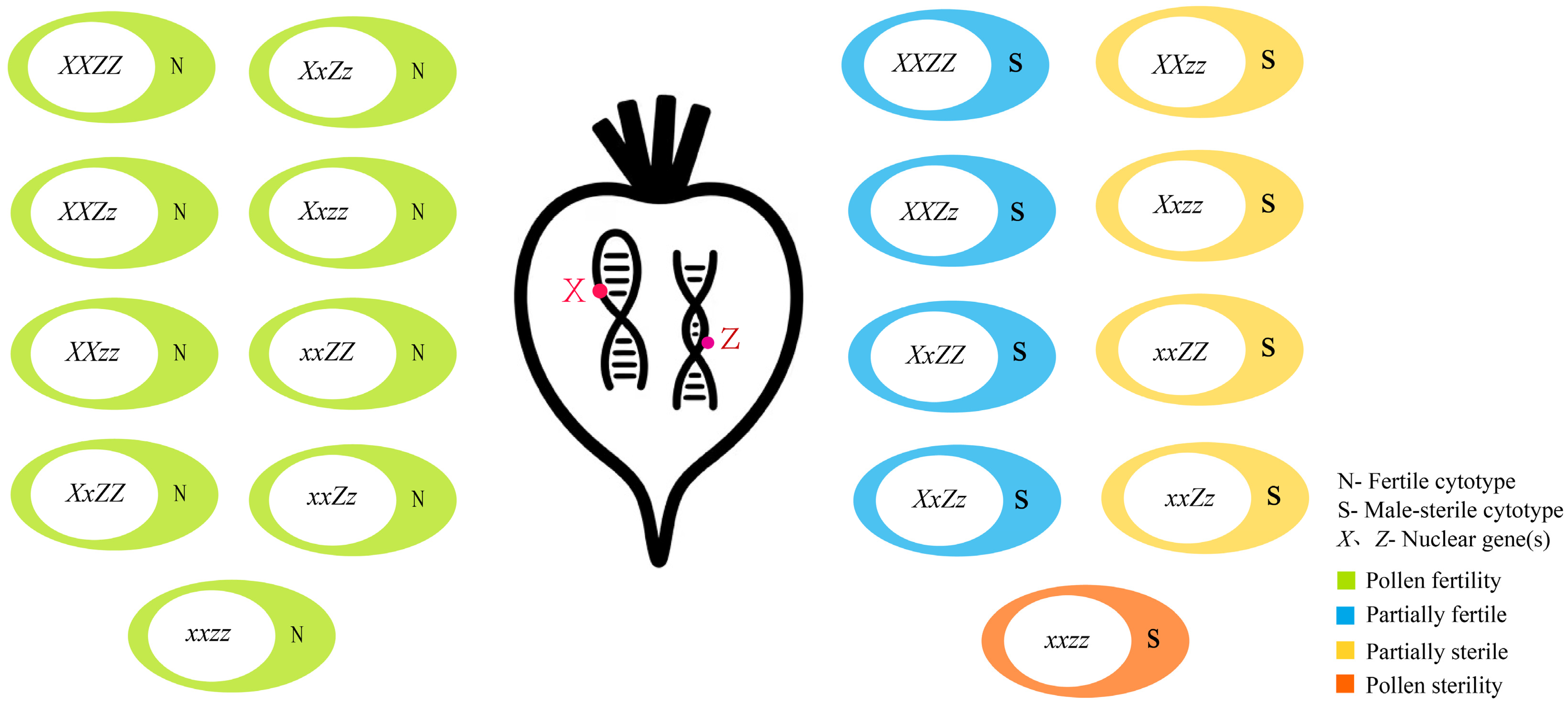
3.2. Mechanisms of Major CMS Types in Sugar Beet
3.3. Mitochondrial Genes Associated with Cytoplasmic Male Sterility in Sugar Beet
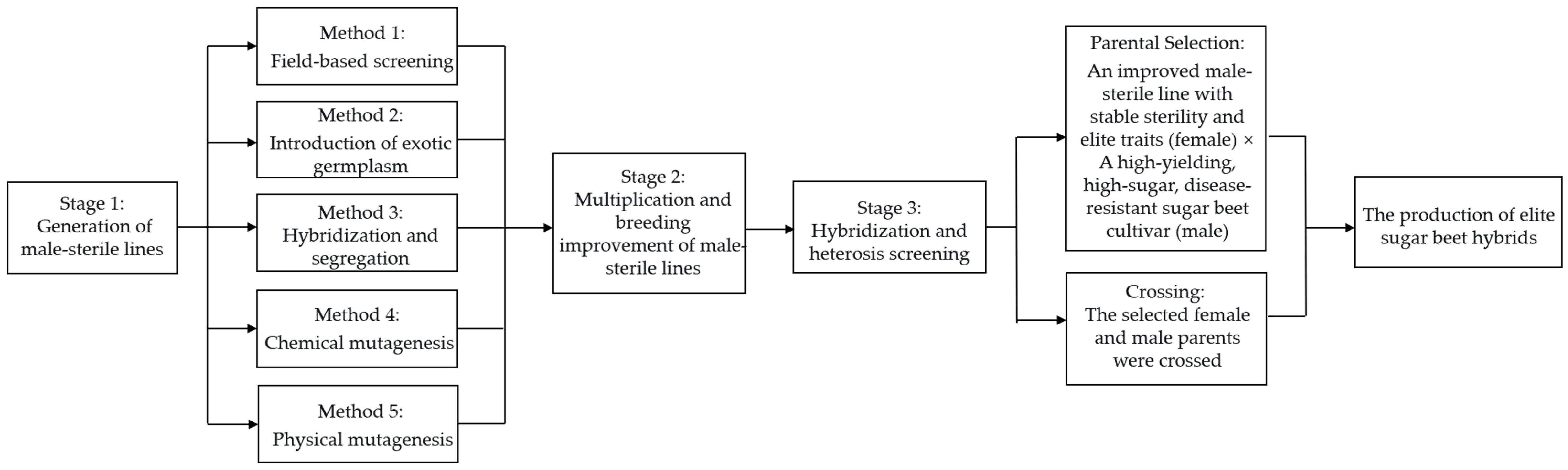
3.4. Research and Application Advances in Fertility Restorer Genes in Sugar Beet
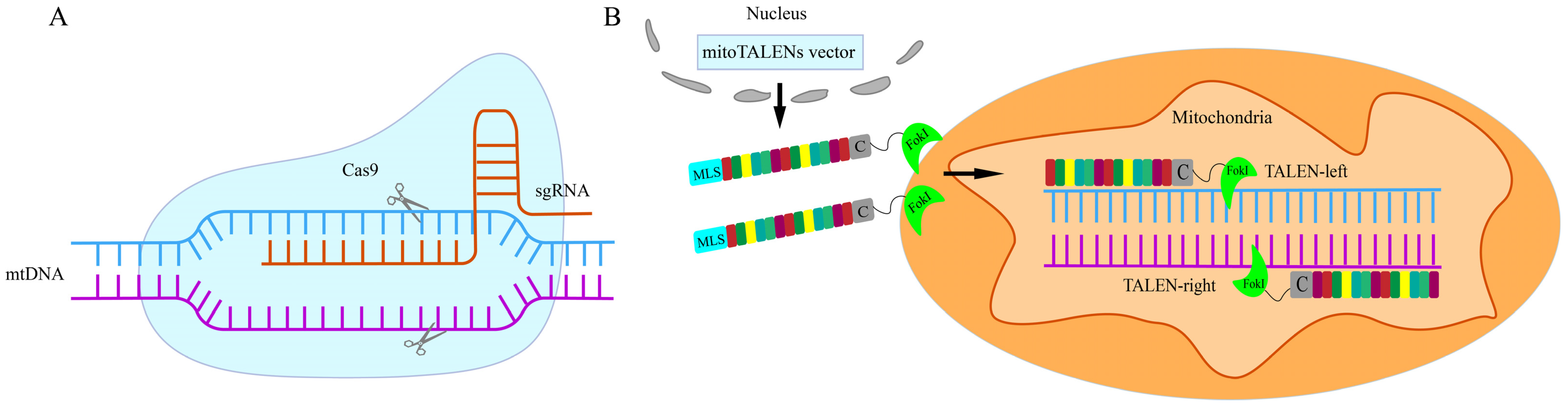
4. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| CMS | Cytoplasmic male sterility |
| GMS | Genic male sterility |
| Rf | Restorer-of-fertility |
| CRISPR/Cas | Clustered regularly interspaced short palindromic repeats/CRISPR-associated systems |
| QTL | Quantitative trait loci |
| LSC | Large single-copy |
| SSC | Small single-copy |
| IRs | Inverted repeats |
| SNPs | Single nucleotide polymorphisms |
| InDels | Insertions/deletions |
| ORFs | Open reading frames |
| MAS | Marker-assisted selection |
| mtDNA | Mitochondrial DNA |
| ZFNs | Zinc finger nucleases |
| TALENs | Transcription activator-like effector nucleases |
| RVDs | Repeat variable diresidues |
| sgRNA | Single-guide RNA |
| MTS | Mitochondrial targeting signal |
| NLS | N-terminal nuclear localization signal |
| CBEs | Cytosine base editors |
| ABEs | Adenine base editors |
| VNTR | Variable number tandem repeat |
References
- Karakotov, S.D.; Apasov, I.V.; Nalbandyan, A.A.; Vasilchenko, E.N.; Fedulova, T.P. Modern issues of sugar beet (Beta vulgaris L.) hybrid breeding. Br. J. Pharmacol. 2021, 25, 394. [Google Scholar] [CrossRef]
- Nan, M.Y. Study on Molecular Mechanism of Differential Development of Nucleo-Cytoplasmic Interaction in Sugarbeet. Master’s Dissertation, Inner Mongolia Agricultural University, Hohhot, China, 2018. [Google Scholar]
- Arakawa, T.; Uchiyama, D.; Ohgami, T.; Ohgami, R.; Murata, T.; Honma, Y.; Kubo, T. A fertility-restoring genotype of beet (Beta vulgaris L.) is composed of a weak restorer-of-fertility gene and a modifier gene tightly linked to the Rf1 locus. PLoS ONE 2018, 13, e0198409. [Google Scholar] [CrossRef] [PubMed]
- Owen, F.V. Cytoplasmically inherited male-sterility in sugar beets. J. Agric. Res. 1945, 71, 423–440. [Google Scholar]
- Dohm, J.C.; Minoche, A.E.; Holtgräwe, D.; Capella-Gutiérrez, S.; Zakrzewski, F.; Tafer, H.; Himmelbauer, H. The genome of the recently domesticated crop plant sugar beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L. Origin of Eukaryotic Cells: Evidence and Research Implications for a Theory of the Origin and Evolution of Microbial, Plant, and Animal Cells on the Precambrian Earth; Yale University Press: New Haven, CT, USA, 1970. [Google Scholar]
- Wu, Z.Q.; Liao, X.Z.; Zhang, X.N.; Tembrock, L.R.; Broz, A. Genomic architectural variation of plant mitochondria—A review of multichromosomal structuring. J. Syst. Evol. 2022, 60, 160–168. [Google Scholar] [CrossRef]
- McGrath, J.M.; Funk, A.; Galewski, P.; Ou, S.; Townsend, B.; Davenport, K.; Dorn, K.M. A contiguous de novo genome assembly of sugar beet EL10 (Beta vulgaris L.). DNA Res. 2023, 30, dsac033. [Google Scholar] [CrossRef]
- Lehner, R.; Blazek, L.; Minoche, A.E.; Dohm, J.C.; Himmelbauer, H. Assembly and characterization of the genome of chard (Beta vulgaris ssp. vulgaris var. cicla). J. Biotechnol. 2012, 333, 67–76. [Google Scholar] [CrossRef]
- Rodríguez del Río, Á.; Minoche, A.E.; Zwickl, N.F.; Friedrich, A.; Liedtke, S.; Schmidt, T.; Dohm, J.C. Genomes of the wild beets Beta patula and Beta vulgaris ssp. maritima. Plant J. 2019, 99, 1242–1253. [Google Scholar] [CrossRef]
- Sandell, F.L.; Stralis-Pavese, N.; McGrath, J.M.; Schulz, B.; Himmelbauer, H.; Dohm, J.C. Genomic distances reveal relationships of wild and cultivated beets. Nat. Commun. 2022, 13, 2021, Erratum in Nat. Commun. 2024, 15, 1078. [Google Scholar] [CrossRef]
- Tehseen, M.M.; Wyatt, N.A.; Bolton, M.D.; Fugate, K.K.; Preister, L.S.; Yang, S.; Chu, C. Genetic drift, historic migration, and limited gene flow contributing to the subpopulation divergence in wild sea beet (Beta vulgaris ssp. maritima (L.) Arcang). PLoS ONE 2024, 19, e0308626. [Google Scholar] [CrossRef]
- La, V.H.; Chu, D.H.; Ha, T.Q.; Tran, T.T.H.; Tong, V.H.; Tran, V.T.; Cao, P.B. SWEET gene family in sugar beet (Beta vulgaris): Genome-wide survey, phylogeny and expression analysis. Pak. J. Biol. Agric. Sci. 2022, 25, 387–395. [Google Scholar]
- Cao, J.; Gong, Y.; Zou, M.; Li, H.; Chen, S.; Ma, C. Genome-Wide Identification and Salt Stress Response Analysis of the MADS-box Transcription Factors in Sugar Beet. Physiol. Plant. 2024, 176, e70001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, L.; Chen, W.; Fu, Z.; Zhao, S.; E, Y.; Zheng, W. Integration of mRNA and miRNA analysis reveals the molecular mechanisms of sugar beet (Beta vulgaris L.) response to salt stress. Sci. Rep. 2023, 13, 22074. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, Y.; Gong, Y.; Ding, G.; Zhao, C.; Li, Y. Genomic characterization of bZIP gene family and patterns of gene regulation on Cercospora beticola Sacc resistance in sugar beet (Beta vulgaris L.). Front Genet. 2024, 15, 1430589. [Google Scholar] [CrossRef]
- Sielemann, K.; Pucker, B.; Orsini, E.; Elashry, A.; Schulte, L.; Viehöver, P.; Holtgräwe, D. Genomic characterization of a nematode tolerance locus in sugar beet. BMC Genom. 2023, 24, 748. [Google Scholar] [CrossRef]
- Todd, O.E.; Simpson, S.; Scheffler, B.; Dorn, K.M. A fully phased, chromosome-scale genome of sugar beet line FC309 enables the discovery of Fusarium yellows resistance QTL. DNA Res. 2025, 32, dsae032. [Google Scholar] [CrossRef]
- Schmidt, N.; Maiwald, S.; Mann, L.; Weber, B.; Seibt, K.M.; Breitenbach, S.; Heitkam, T. BeetRepeats: Reference sequences for genome and polymorphism annotation in sugar beet and wild relatives. BMC Res. Notes 2024, 17, 351. [Google Scholar] [CrossRef]
- Li, J.; Ni, Y.; Lu, Q.; Chen, H.; Liu, C. PMGA: A plant mitochondrial genome annotator. Plant Commun. 2025, 6, 101191. [Google Scholar] [CrossRef]
- Wang, J.; Kan, S.; Liao, X.; Zhou, J.; Tembrock, L.R.; Daniell, H.; Wu, Z. Plant organellar genomes: Much done, much more to do. Trends Plant Sci. 2024, 29, 754–769. [Google Scholar] [CrossRef]
- Nielsen, A.Z.; Ziersen, B.; Jensen, K.; Lassen, L.M.; Olsen, C.E.; Møller, B.L.; Jensen, P.E. Redirecting photosynthetic reducing power toward bioactive natural product synthesis. ACS Synth. Biol. 2013, 2, 308–315. [Google Scholar] [CrossRef]
- Pei, W. Chloroplast Genome Characteristics and Codon Usage in the Genus Sium. Master’s Dissertation, Kunming Medical University, Kunming, China, 2022. [Google Scholar]
- Li, H.; Cao, H.; Cai, Y.F.; Wang, J.H.; Qu, S.P.; Huang, X.Q. The complete chloroplast genome sequence of sugar beet (Beta vulgaris ssp. vulgaris). Mitochondrial DNA 2014, 25, 209–211. [Google Scholar] [CrossRef]
- Sielemann, K.; Pucker, B.; Schmidt, N.; Viehöver, P.; Weisshaar, B.; Heitkam, T.; Holtgräwe, D. Complete pan-plastome sequences enable high resolution phylogenetic classification of sugar beet and closely related crop wild relatives. BMC Genom. 2022, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- De Marchis, F.; Bellucci, M. Plastid transformation in sugar beet: An important industrial crop. In Chloroplast Biotechnology: Methods and Protocols; Springer: New York, NY, USA, 2021; pp. 283–290. [Google Scholar]
- Woloszynska, M. Heteroplasmy and stoichiometric complexity of plant mitochondrial genomes—Though this be madness, yet there’s method in’t. Exp. Bot. 2010, 61, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Kubo, T.; Mikami, T. The Owen mitochondrial genome in sugar beet (Beta vulgaris L.): Possible mechanisms of extensive rearrangements and the origin of the mitotype-unique regions. Theor. Appl. Genet. 2006, 113, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Darracq, A.; Varré, J.S.; Maréchal-Drouard, L.; Courseaux, A.; Castric, V.; Saumitou-Laprade, P.; Touzet, P. Structural and content diversity of mitochondrial genome in beet: A comparative genomic analysis. Genome Biol. Evol. 2011, 3, 723–736. [Google Scholar] [CrossRef]
- Yamamoto, M.P.; Shinada, H.; Onodera, Y.; Komaki, C.; Mikami, T.; Kubo, T. A male sterility-associated mitochondrial protein in wild beets causes pollen disruption in transgenic plants. Plant J. 2008, 54, 1027–1036. [Google Scholar] [CrossRef]
- Ducos, E.; Touzet, P.; Boutry, M. The male sterile G cytoplasm of wild beet displays modified mitochondrial respiratory complexes. Plant J. 2001, 26, 171–180. [Google Scholar] [CrossRef]
- Meyer, E.H.; Lehmann, C.; Boivin, S.; Brings, L.; De Cauwer, I.; Bock, R.; Touzet, P. CMS-G from Beta vulgaris ssp. maritima is maintained in natural populations despite containing an atypical cytochrome c oxidase. Biochem. J. 2018, 475, 759–773. [Google Scholar] [CrossRef]
- Kubota, K.; Oishi, M.; Taniguchi, E.; Akazawa, A.; Matsui, K.; Kitazaki, K.; Kubo, T. Mitochondrial phylogeny and distribution of cytoplasmic male sterility-associated genes in Beta vulgaris. PLoS ONE 2024, 19, e0308551. [Google Scholar] [CrossRef]
- Jun, L.Y. Molecular Identification of the Fertility of Owen-Sugar Beet and Research on Molecular Assisted Breeding. Master’s Dissertation, Harbin Institute of Technology, Harbin, China, 2015. [Google Scholar]
- Kanomata, Y.; Hayakawa, R.; Kashikura, J.; Satoh, K.; Matsuhira, H.; Kuroda, Y.; Kubo, T. Nuclear and mitochondrial DNA polymorphisms suggest introgression contributed to garden beet (Beta vulgaris L.) domestication. Genet. Resour. Crop Evol. 2022, 69, 271–283. [Google Scholar] [CrossRef]
- Mikami, T.; Yamamoto, M.P.; Matsuhira, H.; Kitazaki, K.; Kubo, T. Molecular basis of cytoplasmic male sterility in beets: An overview. Plant Genet. Resour.-C 2011, 9, 284–287. [Google Scholar] [CrossRef]
- Long, L.S. The Research on Characteristics of Male Cytoplasm Sterile Molecule of Beet and the Applied Research on Breeding. Doctoral Dissertation, Heilongjiang Bayi Agricultural University, Daqing, China, 2010. [Google Scholar]
- Mikami, T.; Kishima, Y.; Sugiura, M.; Kinoshita, T. Organelle genome diversity in sugar beet with normal and different sources of male sterile cytoplasms. Theor. Appl. Genet. 1985, 71, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Saumitou-Laprade, P.; Rouwendal, G.J.A.; Cuguen, J.; Krens, F.A.; Michaelis, G. Different CMS sources found in Beta vulgaris ssp. maritima: Mitochondrial variability in wild populations revealed by a rapid screening procedure. Theor. Appl. Genet. 1993, 85, 529–535. [Google Scholar] [CrossRef] [PubMed]
- You, C.D.; Jie, C.; Jia, N.; Hong, D.C.; Chen, B. Proteomic analysis of florescence differentiation between cytoplasmic male sterile line and maintainer in sugar beet. J. Harbin Inst. Technol. 2014, 46, 69–74. [Google Scholar]
- Nishizawa, S.; Kubo, T.; Mikami, T. Variable number of tandem repeat loci in the mitochondrial genomes of beets. Curr. Genet. 2000, 37, 34–38. [Google Scholar] [CrossRef]
- Min, W.J. Comparative Analysis of Whole-Genome DNA Methylation Between Cyptoplasmic Male Sterility Line and Maintainer Line of Sugar Beet. Master’s Dissertation, Harbin Institute of Technology, Harbin, China, 2022. [Google Scholar]
- Yang, H.; Xue, Y.; Li, B.; Lin, Y.; Li, H.; Guo, Z.; Tang, J. The chimeric gene atp6c confers cytoplasmic male sterility in maize by impairing the assembly of the mitochondrial ATP synthase complex. Mol. Plant 2022, 15, 872–886. [Google Scholar] [CrossRef]
- Havird, J.C.; Forsythe, E.S.; Williams, A.M.; Werren, J.H.; Dowling, D.K.; Sloan, D.B. Selfish mitonuclear conflict. Curr. Biol. 2019, 29, R496–R511. [Google Scholar] [CrossRef]
- Touzet, P. Mitochondrial genome evolution and gynodioecy. Adv. Bot. Res. 2012, 63, 71–98. [Google Scholar]
- Hurst, L.D.; Atlan, A.; Bengtsson, B.O. Genetic conflict. Q. Rev. Biol. 1996, 71, 317–364. [Google Scholar] [CrossRef]
- JIvanov, M.K.; Dymshits, G.M. Cytoplasmic male sterility and restoration of pollen fertility in higher plants. Russ. J. Genet. 2007, 43, 354–368. [Google Scholar] [CrossRef]
- Powling, A.; Ellis, T.H.N. Studies on the organelle genomes of sugarbeet with male-fertile and male-sterile cytoplasms. Theor. Appl. Genet. 1983, 65, 323–328. [Google Scholar] [CrossRef]
- Bonavent, J.F.; Bessone, L.; Geny, A.; Berville, A.; Denizot, J.P.; Brian, C. A possible origin for the sugar beet cytoplasmic male sterility source Owen. Genome 1989, 32, 322–327. [Google Scholar] [CrossRef]
- Yoshida, Y.; Matsunaga, M.; Cheng, D.; Xu, D.; Honma, Y.; Mikami, T.; Kubo, T. Mitochondrial minisatellite polymorphisms in fodder and sugar beets reveal genetic bottlenecks associated with domestication. Biol. Plant 2012, 56, 369–372. [Google Scholar] [CrossRef]
- Fénart, S.; Touzet, P.; Arnaud, J.F.; Cuguen, J. Emergence of gynodioecy in wild beet (Beta vulgaris ssp. maritima L.): A genealogical approach using chloroplastic nucleotide sequences. Proc. R. Soc. B Biol. Sci. 2006, 273, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Majewska-Sawka, A.; Sadoch, Z. Cytoplazmatyczna męska sterylność roślin-mechanizmy biologiczne i molekularne. Kosmos 2003, 52, 413–423. [Google Scholar]
- Chen, Z.; Zhao, N.; Li, S.; Grover, C.E.; Nie, H.; Wendel, J.F.; Hua, J. Plant mitochondrial genome evolution and cytoplasmic male sterility. Crit. Rev. Plant Sci. 2017, 36, 55–69. [Google Scholar] [CrossRef]
- Wasiak, M. Genetyczne podstawy cytoplazmatyczno-jądrowej męskiej sterylności (CMS) u roślin oraz jej wykorzystanie w hodowli. Praca przeglądowa. J. Agron. Crop Sci. 2019, 74, 15–30. [Google Scholar] [CrossRef]
- Weng, J.; Wang, H.; Cheng, D.; Liu, T.; Zeng, D.; Dai, C.; Luo, C. The Effects of DNA Methylation on Cytoplasmic Male Sterility in Sugar Beet. Int. J. Mol. Sci. 2024, 25, 1118. [Google Scholar] [CrossRef]
- Yamamoto, M.P.; Kubo, T.; Mikami, T. The 5′-leader sequence of sugar beet mitochondrial atp6 encodes a novel polypeptide that is characteristic of Owen cytoplasmic male sterility. Mol. Genet. Genom. 2005, 273, 342–349. [Google Scholar] [CrossRef]
- Dewey, R.; Timothy, D.H.; Levings, C.S. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc. Natl Acad. Sci. USA 1987, 84, 5374–5378. [Google Scholar] [CrossRef]
- Schnable, P.S.; Wise, R.P. The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci. 1998, 3, 175–180. [Google Scholar] [CrossRef]
- Honma, Y.; Taguchi, K.; Hiyama, H.; Yui-Kurino, R.; Mikami, T.; Kubo, T. Molecular mapping of restorer-of-fertility 2 gene identified from a sugar beet (Beta vulgaris L. ssp. vulgaris) homozygous for the non-restoring restorer-of-fertility 1 allele. Theor. Appl. Genet. 2014, 127, 2567–2574. [Google Scholar] [CrossRef]
- Arakawa, T.; Kagami, H.; Katsuyama, T.; Kitazaki, K.; Kubo, T. A lineage-specific paralog of Oma1 evolved into a gene family from which a suppressor of male sterility-inducing mitochondria emerged in plants. Genome Biol. Evol. 2020, 12, 2314–2327. [Google Scholar] [CrossRef]
- Kitazaki, K.; Arakawa, T.; Matsunaga, M.; Yui-Kurino, R.; Matsuhira, H.; Mikami, T.; Kubo, T. Post-translational mechanisms are associated with fertility restoration of cytoplasmic male sterility in sugar beet (Beta vulgaris). Plant J. 2015, 83, 290–299. [Google Scholar] [CrossRef]
- Arakawa, T.; Ue, S.; Sano, C.; Matsunaga, M.; Kagami, H.; Yoshida, Y.; Kubo, T. Identification and characterization of a semi-dominant restorer-of-fertility 1 allele in sugar beet (Beta vulgaris). Theor. Appl. Genet. 2019, 132, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Kubo, T.; Nishizawa, S.; Estiati, A.; Itchoda, N.; Mikami, T. The cytoplasmic male-sterile type and normal type mitochondrial genomes of sugar beet share the same complement of genes of known function but differ in the content of expressed ORFs. Mol. Genet. Genom. 2004, 272, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S. The Comparative Analysis of CMS-Associated Gene in sugar-Beet Mitochondria and the RNA Editing of atp6 Gene. Master’s Dissertation, Harbin Institute of Technology, Harbin, China, 2012. [Google Scholar]
- Roux, F.; Mary-Huard, T.; Barillot, E.; Wenes, E.; Botran, L.; Durand, S.; Budar, F. Cytonuclear interactions affect adaptive traits of the annual plant Arabidopsis thaliana in the field. Proc. Natl. Acad. Sci. USA 2016, 113, 3687–3692. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Matsunaga, M.; Matsui, K.; Itoh, K.; Kuroda, Y.; Matsuhira, H.; Kubo, T. The molecular basis for allelic differences suggests Restorer-of-fertility 1 is a complex locus in sugar beet (Beta vulgaris L.). BMC Plant Biol. 2020, 20, 503. [Google Scholar] [CrossRef]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef]
- Jo, A.; Ham, S.; Lee, G.H.; Lee, Y.I.; Kim, S.; Lee, Y.S.; Lee, Y. Efficient mitochondrial genome editing by CRISPR/Cas9. Biomed. Res. Int. 2015, 2015, 305716. [Google Scholar] [CrossRef]
- Zhou, C.; Okuno, M.; Nakazato, I.; Tsutsumi, N.; Arimura, S.I. Targeted A-to-G base editing in the organellar genomes of Arabidopsis with monomeric programmable deaminases. Plant Physiol. 2024, 194, 2278–2287. [Google Scholar] [CrossRef]
- Kuwabara, K.; Arimura, S.I.; Shirasawa, K.; Ariizumi, T. orf137 triggers cytoplasmic male sterility in tomato. Plant Physiol. 2022, 189, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Arimura, S.I.; Ayabe, H.; Sugaya, H.; Okuno, M.; Tamura, Y.; Tsuruta, Y.; Tsutsumi, N. Targeted gene disruption of ATP synthases 6-1 and 6-2 in the mitochondrial genome of Arabidopsis thaliana by mitoTALENs. Plant J. 2020, 104, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Forner, J.; Kleinschmidt, D.; Meyer, E.H.; Gremmels, J.; Morbitzer, R.; Lahaye, T.; Bock, R. Targeted knockout of a conserved plant mitochondrial gene by genome editing. Nat. Plants 2023, 9, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Su, T.; Zhang, X.; Qiu, L.; Yang, X.; Koizuka, N.; Yang, J. Editing of ORF138 restores fertility of Ogura cytoplasmic male sterile broccoli via mitoTALENs. Plant Biotechnol. J. 2024, 22, 1325–1334. [Google Scholar] [CrossRef]
- Taniguchi, E.; Satoh, K.; Ohkubo, M.; Ue, S.; Matsuhira, H.; Kuroda, Y.; Kitazaki, K. Nuclear DNA segments homologous to mitochondrial DNA are obstacles for detecting heteroplasmy in sugar beet (Beta vulgaris L.). PLoS ONE 2023, 18, e0285430. [Google Scholar] [CrossRef]
- Lan, L.; Hu, H.; Jia, Y.; Zhang, X.; Jia, M.; Li, C.; Wu, Z. Tips for improving genome annotation quality. Genom. Commun. 2025, 2, e005. [Google Scholar] [CrossRef]
- Yang, Y.; Du, W.; Li, Y.; Lei, J.; Pan, W. Recent advances and challenges in de novo genome assembly. Genom. Commun. 2025, 2, e014. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.; Zhang, S.; Gu, X.; Zhao, Y.; Wu, Z.; Liu, D.; Xing, W. Advances in Cytoplasmic Male Sterility in Sugar Beet from Mitochondrial Genome Structural Dynamics and Nuclear-Cytoplasmic Coordination. Int. J. Mol. Sci. 2025, 26, 10175. https://doi.org/10.3390/ijms262010175
Zhong W, Zhang S, Gu X, Zhao Y, Wu Z, Liu D, Xing W. Advances in Cytoplasmic Male Sterility in Sugar Beet from Mitochondrial Genome Structural Dynamics and Nuclear-Cytoplasmic Coordination. International Journal of Molecular Sciences. 2025; 26(20):10175. https://doi.org/10.3390/ijms262010175
Chicago/Turabian StyleZhong, Weiting, Shuo Zhang, Xiaolin Gu, Yanghe Zhao, Zhiqiang Wu, Dali Liu, and Wang Xing. 2025. "Advances in Cytoplasmic Male Sterility in Sugar Beet from Mitochondrial Genome Structural Dynamics and Nuclear-Cytoplasmic Coordination" International Journal of Molecular Sciences 26, no. 20: 10175. https://doi.org/10.3390/ijms262010175
APA StyleZhong, W., Zhang, S., Gu, X., Zhao, Y., Wu, Z., Liu, D., & Xing, W. (2025). Advances in Cytoplasmic Male Sterility in Sugar Beet from Mitochondrial Genome Structural Dynamics and Nuclear-Cytoplasmic Coordination. International Journal of Molecular Sciences, 26(20), 10175. https://doi.org/10.3390/ijms262010175






