The Role of Tenascin-C in Neuroinflammation and Neuroplasticity
Abstract
1. Introduction
2. Neuroinflammation and Neuroplasticity
3. The Structure of TNC and Its Expression Distribution Within the CNS
4. TNC Plays an Important Role in the Development of CNS Diseases
5. The Role and Mechanisms of TNC in Neuroinflammation
5.1. Expression of TNC in Neuroinflammatory Conditions
5.2. Mechanisms of TNC in Modulating Neuroinflammation
6. The Role of TNC in Neuroplasticity
6.1. Effects of TNC on Synapse Formation and Function
6.2. TNC’s Involvement in Neurogenesis and Developmental Processes
6.3. The Role of TNC in Neuronal Morphology and Neural Network Remodeling
6.4. The Bidirectional Regulation Between ECM Remodeling and Neuronal Activity
6.5. The Relationship Between TNC and BDNF
7. The Interplay Between TNC, Neuroinflammation, and Neuroplasticity
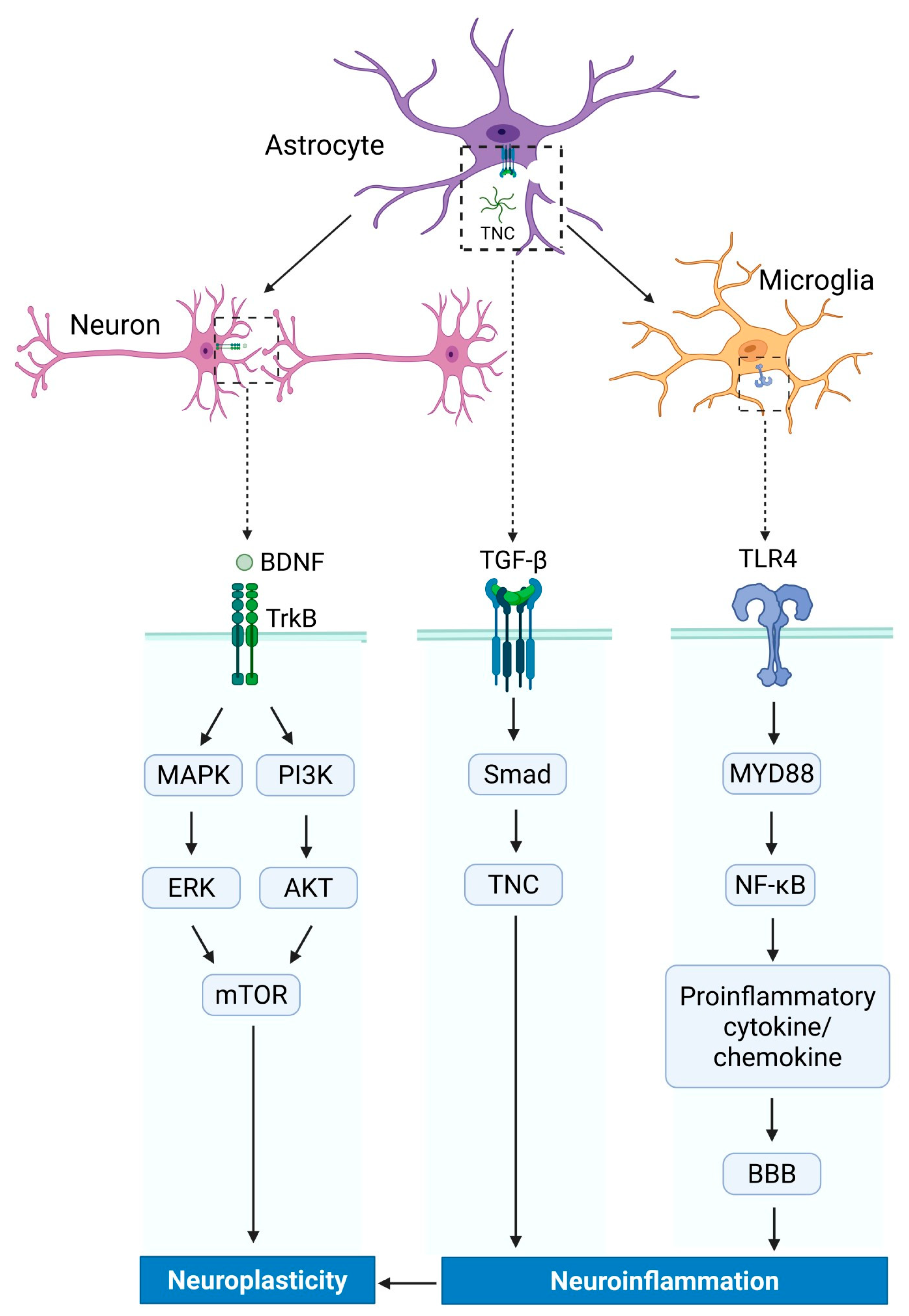
7.1. Signal Pathway Involved in TNC Regulation
7.2. The Balancing Role of TNC in Neuroinflammation and Neuroplasticity
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Roll, L.; Faissner, A. Tenascins in CNS lesions. Semin. Cell Dev. Biol. 2019, 89, 118–124. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K. Tenascin-C in Heart Diseases-The Role of Inflammation. Int. J. Mol. Sci. 2021, 22, 5828. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, F.; Dzaye, O.; Xia, S. Tenascin-C Function in Glioma: Immunomodulation and Beyond. Adv. Exp. Med. Biol. 2020, 1272, 149–172. [Google Scholar] [PubMed]
- Katoh, D.; Kozuka, Y.; Noro, A.; Ogawa, T.; Imanaka-Yoshida, K.; Yoshida, T. Tenascin-C Induces Phenotypic Changes in Fibroblasts to Myofibroblasts with High Contractility through the Integrin αvβ1/Transforming Growth Factor β/SMAD Signaling Axis in Human Breast Cancer. Am. J. Pathol. 2020, 190, 2123–2135. [Google Scholar] [CrossRef]
- Sekeljic, V.; Andjus, P.R. Tenascin-C and its functions in neuronal plasticity. Int. J. Biochem. Cell Biol. 2012, 44, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Chiquet-Ehrismann, R.; Tucker, R.P. Tenascins and the importance of adhesion modulation. Cold Spring Harb. Perspect. Biol. 2011, 3, a004960. [Google Scholar] [CrossRef]
- Chiquet-Ehrismann, R.; Orend, G.; Chiquet, M.; Tucker, R.P.; Midwood, K.S. Tenascins in stem cell niches. Matrix Biol. 2014, 37, 112–123. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, W.; Cai, G.; Ding, Y.; Wei, C.; Li, S.; Yang, Y.; Qin, J.; Liu, D.; Zhang, H.; et al. Myofiber necroptosis promotes muscle stem cell proliferation via releasing Tenascin-C during regeneration. Cell Res. 2020, 30, 1063–1077. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Liu, H. Tenascin-C: A Key Regulator in Angiogenesis during Wound Healing. Biomolecules 2022, 12, 1689. [Google Scholar] [CrossRef]
- Fu, Z.; Zhu, G.; Luo, C.; Chen, Z.; Dou, Z.; Chen, Y.; Zhong, C.; Su, S.; Liu, F. Matricellular protein tenascin C: Implications in glioma progression, gliomagenesis, and treatment. Front. Oncol. 2022, 12, 971462. [Google Scholar] [CrossRef]
- Zuliani-Alvarez, L.; Piccinini, A.M. A virological view of tenascin-C in infection. Am. J. Physiol. Cell Physiol. 2023, 324, C1–C9. [Google Scholar] [CrossRef]
- Marzeda, A.M.; Midwood, K.S. Internal Affairs: Tenascin-C as a Clinically Relevant, Endogenous Driver of Innate Immunity. J. Histochem. Cytochem. 2018, 66, 289–304. [Google Scholar] [CrossRef]
- Glotzbach, K.; Faissner, A. Substrate-bound and soluble domains of tenascin-C regulate differentiation, proliferation and migration of neural stem and progenitor cells. Front. Cell Neurosci. 2024, 18, 1357499. [Google Scholar] [CrossRef]
- Accogli, A.; Addour-Boudrahem, N.; Srour, M. Neurogenesis, neuronal migration, and axon guidance. Handb. Clin. Neurol. 2020, 173, 25–42. [Google Scholar]
- Wiemann, S.; Reinhard, J.; Faissner, A. Immunomodulatory role of the extracellular matrix protein tenascin-C in neuroinflammation. Biochem. Soc. Trans. 2019, 47, 1651–1660. [Google Scholar] [CrossRef]
- Tucic, M.; Stamenkovic, V.; Andjus, P. The Extracellular Matrix Glycoprotein Tenascin C and Adult Neurogenesis. Front. Cell Dev. Biol. 2021, 9, 674199. [Google Scholar] [CrossRef]
- Ding, J.; Lian, J.; Wang, J.; Yang, S.; Li, H.; Shen, H.; Sun, Q.; Li, X.; Chen, G. The role of Tenascin C in intracerebral hemorrhage-induced secondary brain injury in rats via induction of neuronal cell death and neuroinflammation. J. Chem. Neuroanat. 2022, 125, 102147. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Wang, T.; An, C.D.; Jiang, C.Y.; Zhao, J.; Li, S. Brain-derived neurotrophic factor: A mediator of inflammation-associated neurogenesis in Alzheimer’s disease. Rev. Neurosci. 2016, 27, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lu, Y.; Fang, W.; Huang, Y.; Li, Q.; Xu, Z. Neurodegenerative diseases and neuroinflammation-induced apoptosis. Open Life Sci. 2025, 20, 20221051. [Google Scholar] [CrossRef]
- Mukhara, D.; Oh, U.; Neigh, G.N. Neuroinflammation. Handb. Clin. Neurol. 2020, 175, 235–259. [Google Scholar] [PubMed]
- Silvia, B.; Annamaria, V.; Teresa, R. Emerging Molecular Mechanisms of Neuroinflammation in Seizure Disorders. Inflamm. Epilepsy: New Vistas 2021, 18, 21–43. [Google Scholar]
- Niranjan, R. Recent advances in the mechanisms of neuroinflammation and their roles in neurodegeneration. Neurochem. Int. 2018, 120, 13–20. [Google Scholar] [CrossRef]
- Villasana-Salazar, B.; Vezzani, A. Neuroinflammation microenvironment sharpens seizure circuit. Neurobiol. Dis. 2023, 178, 106027. [Google Scholar] [CrossRef] [PubMed]
- Balosso, S.; Ravizza, T.; Vezzani, A. Ictogenic and Epileptogenic Mechanisms of Neuroinflammation. In Acute Encephalopathy and Encephalitis in Infancy and Its Related Disorders; Yamanouchi, H., Moshé, S.L., Okumura, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 23–31. [Google Scholar]
- Jorfi, M.; Maaser-Hecker, A.; Tanzi, R.E. The neuroimmune axis of Alzheimer’s disease. Genome Med. 2023, 15, 6. [Google Scholar] [CrossRef]
- Cervellati, C.; Trentini, A.; Pecorelli, A.; Valacchi, G. Inflammation in Neurological Disorders: The Thin Boundary Between Brain and Periphery. Antioxid. Redox Signal 2020, 33, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Haikal, C.; Weissert, R. Editorial: Aging, peripheral inflammation, and neurodegeneration. Front. Aging Neurosci. 2024, 16, 1529026. [Google Scholar] [CrossRef] [PubMed]
- Najera-Maldonado, J.M.; Salazar, R.; Alvarez-Fitz, P.; Acevedo-Quiroz, M.; Flores-Alfaro, E.; Hernandez-Sotelo, D.; Espinoza-Rojo, M.; Ramirez, M. Phenolic Compounds of Therapeutic Interest in Neuroprotection. J. Xenobiot. 2024, 14, 227–246. [Google Scholar] [CrossRef]
- Sayfitdinkhuzhaev, Z.F.; Zhukova, N.G.; Baidanova, A.N. Neuroinflammation as an Integral Component of Neurodegeneration in Parkinson’s Disease. Pers. Psychiatry Neurol. 2024, 4, 26–33. [Google Scholar] [CrossRef]
- Khoshtinat Nikkhoi, S.; Yang, G.; Owji, H.; Grizotte-Lake, M.; Cohen, R.I.; Gil Gonzalez, L.; Massumi, M.; Hatefi, A. Bispecific immune cell engager enhances the anticancer activity of CD16+ NK cells and macrophages in vitro, and eliminates cancer metastasis in NK humanized NOG mice. J. Immunother. Cancer 2024, 12, e008295. [Google Scholar] [CrossRef]
- Leonard, B.E.; Wegener, G. Inflammation, insulin resistance and neuroprogression in depression. Acta Neuropsychiatr. 2020, 32, 1–9. [Google Scholar] [CrossRef]
- Singh, P.; Vasundhara, B.; Das, N.; Sharma, R.; Kumar, A.; Datusalia, A.K. Metabolomics in Depression: What We Learn from Preclinical and Clinical Evidences. Mol. Neurobiol. 2025, 62, 718–741. [Google Scholar] [CrossRef]
- Dan, B. Neuroscience underlying rehabilitation: What is neuroplasticity? Dev. Med. Child. Neurol. 2019, 61, 1240. [Google Scholar] [CrossRef]
- Innocenti, G.M. Defining neuroplasticity. Handb. Clin. Neurol. 2022, 184, 3–18. [Google Scholar]
- Dita, M.; Bubuioc, L. Neuroplasticity—The metamorphosis of the human brain. Vector Eur. 2024, 1, 188–192. [Google Scholar] [CrossRef]
- Kloos, A.; Gomes-Osman, J.; Boyd, L. Harnessing Neuroplasticity for Functional Recovery. J. Neurol. Phys. Ther. 2020, 44, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro-Jauregui, M.H.; Munoz-Sanchez, S.; Rojas-Hernandez, J.; Alonso-Orozco, A.I.; Vega-Flores, G.; Tapia-de-Jesus, A.; Leal-Galicia, P. A Comprehensive Overview of Stress, Resilience, and Neuroplasticity Mechanisms. Int. J. Mol. Sci. 2025, 26, 3028. [Google Scholar] [CrossRef]
- Strekalova, T.; Sun, M.; Sibbe, M.; Evers, M.; Dityatev, A.; Gass, P.; Schachner, M. Fibronectin domains of extracellular matrix molecule tenascin-C modulate hippocampal learning and synaptic plasticity. Mol. Cell Neurosci. 2002, 21, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Gottschling, C.; Faissner, A.; Manahan-Vaughan, D. Intrinsic cellular and molecular properties of in vivo hippocampal synaptic plasticity are altered in the absence of key synaptic matrix molecules. Hippocampus 2017, 27, 920–933. [Google Scholar] [CrossRef]
- Cybulska-Klosowicz, A.; Zakrzewska, R.; Pyza, E.; Kossut, M.; Schachner, M. Reduced plasticity of cortical whisker representation in adult tenascin-C-deficient mice after vibrissectomy. Eur. J. Neurosci. 2004, 20, 1538–1544. [Google Scholar] [CrossRef]
- Bukalo, O.; Schachner, M.; Dityatev, A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 2001, 104, 359–369. [Google Scholar] [CrossRef]
- Andrey, F.I.; Ekaterina, V.I.; Tatiana, P.K.; Svetlana, A.Z.; Igor, V.O. EEG-Correlates of Neuroinflammation and Neuroplasticity Processes in Patients with Depressive-Delusional Conditions. In Advances in Intelligent Systems and Computing; Springer: Berlin/Heidelberg, Germany, 2021; pp. 632–637. [Google Scholar]
- Sun, M.K.; Alkon, D.L. Alzheimer’s therapeutic development: Shifting neurodegeneration to neuroregeneration. Trends Pharmacol. Sci. 2024, 45, 197–209. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Tu, J.L.; Li, X.H.; Hua, Q.; Liu, W.Z.; Liu, Y.; Pan, B.X.; Hu, P.; Zhang, W.H. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav. Immun. 2021, 91, 505–518. [Google Scholar] [CrossRef]
- Sun, S.R.; Zhao, J.N.; Bi, P.W.; Zhang, H.Y.; Li, G.X.; Yan, J.Z.; Li, Y.F.; Yin, Y.Y.; Cheng, H. Pharmacologically activating BDNF/TrkB signaling exerted rapid-acting antidepressant-like effects through improving synaptic plasticity and neuroinflammation. Metab. Brain Dis. 2025, 40, 158. [Google Scholar] [CrossRef]
- Calderone, A.; Latella, D.; Cardile, D.; Gangemi, A.; Corallo, F.; Rifici, C.; Quartarone, A.; Calabro, R.S. The Role of Neuroinflammation in Shaping Neuroplasticity and Recovery Outcomes Following Traumatic Brain Injury: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 11708. [Google Scholar] [CrossRef] [PubMed]
- El-Khatib, S.M.; Vagadia, A.R.; Le, A.C.D.; Baulch, J.E.; Ng, D.Q.; Du, M.; Johnston, K.G.; Tan, Z.; Xu, X.; Chan, A.; et al. BDNF augmentation reverses cranial radiation therapy-induced cognitive decline and neurodegenerative consequences. Acta Neuropathol. Commun. 2024, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Dual role of microglia in Alzheimer’s disease and polarizing effect of cytokines. Theor. Nat. Sci. 2025, 76, 58–61. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, H.; Jia, D.; Zhao, J.; Xu, C.; Liu, J.; Cui, Y.; Zhang, J.; Wang, M.; Chen, M.; et al. Cognitive impairment in Alzheimer’s disease FAD(4T) mouse model: Synaptic loss facilitated by activated microglia via C1qA. Life Sci. 2024, 340, 122457. [Google Scholar] [CrossRef]
- Chelluboina, B.; Chokkalla, A.K.; Mehta, S.L.; Morris-Blanco, K.C.; Bathula, S.; Sankar, S.; Park, J.S.; Vemuganti, R. Tenascin-C induction exacerbates post-stroke brain damage. J. Cereb. Blood Flow. Metab. 2022, 42, 253–263. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Ma, R.; Sun, J.Q.; Peng, M.; Yuan, J.; Lai, N.; Liu, J.; Xia, D. Tenascin-C Facilitates Microglial Polarization via TLR4/MyD88/NF-kappaB Pathway Following Subarachnoid Hemorrhage. J. Inflamm. Res. 2025, 18, 3555–3570. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Shiba, M.; Kawakita, F.; Liu, L.; Shimojo, N.; Imanaka-Yoshida, K.; Yoshida, T.; Suzuki, H. Deficiency of tenascin-C and attenuation of blood-brain barrier disruption following experimental subarachnoid hemorrhage in mice. J. Neurosurg. 2016, 124, 1693–1702. [Google Scholar] [CrossRef]
- Abdullah, Z.; Bayraktutan, U. Suppression of PKC-alpha attenuates TNF-alpha-evoked cerebral barrier breakdown via regulations of MMP-2 and plasminogen-plasmin system. Biochim. Biophys. Acta 2016, 1862, 1354–1366. [Google Scholar] [CrossRef]
- Okada, T.; Suzuki, H. The Role of Tenascin-C in Tissue Injury and Repair After Stroke. Front. Immunol. 2020, 11, 607587. [Google Scholar] [CrossRef] [PubMed]
- Zhanhu, Z.; Yongjun, W.; Bin, Y.U.; Fei, D.; Xiaosong, G.U. Analysis of the expression and function of TNC in the proximal stump nerve following sciatic nerve transection. Med. J. Commun. 2012, 26, 401–403. [Google Scholar]
- Liu, L.; Fujimoto, M.; Nakano, F.; Nishikawa, H.; Okada, T.; Kawakita, F.; Imanaka-Yoshida, K.; Yoshida, T.; Suzuki, H. Deficiency of Tenascin-C Alleviates Neuronal Apoptosis and Neuroinflammation After Experimental Subarachnoid Hemorrhage in Mice. Mol. Neurobiol. 2018, 55, 8346–8354. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Chen, X.; Fang, Y.; Chen, K.; Peng, W.; Wang, Z.; Guo, K.; Tan, X.; Liang, F.; et al. Hydroxychloroquine attenuates neuroinflammation following traumatic brain injury by regulating the TLR4/NF-kappaB signaling pathway. J. Neuroinflamm. 2022, 19, 71. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Lou, L.; Yang, K.C.; Wang, H.B.; Xu, Y.; Lu, G.; He, H.Y. Correlation of tenascin-C concentrations in serum with outcome of traumatic brain injury in humans. Clin. Chim. Acta 2017, 472, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Huangfu, X.Q.; Tao, B.; Zhong, G.J.; Le, Z.D. Serum tenascin-C predicts severity and outcome of acute intracerebral hemorrhage. Clin. Chim. Acta 2018, 481, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Theocharidis, U.; Roll, L.; Faissner, A. The expression of tenascin-C in neural stem/progenitor cells is stimulated by the growth factors EGF and FGF-2, but not by TGFbeta1. Cell Tissue Res. 2021, 385, 659–674. [Google Scholar] [CrossRef]
- Xie, K.; Liu, Y.; Hao, W.; Walter, S.; Penke, B.; Hartmann, T.; Schachner, M.; Fassbender, K. Tenascin-C deficiency ameliorates Alzheimer’s disease-related pathology in mice. Neurobiol. Aging 2013, 34, 2389–2398. [Google Scholar] [CrossRef]
- Liu, W.; Huang, S.; Li, Y.; Zhang, K.; Zheng, X. Suppressive effect of glycyrrhizic acid against lipopolysaccharide-induced neuroinflammation and cognitive impairment in C57 mice via toll-like receptor 4 signaling pathway. Food Nutr. Res. 2019, 63, 1516. [Google Scholar] [CrossRef]
- Hanmin, C.; Xiangyue, Z.; Lenahan, C.; Ling, W.; Yibo, O.; Yue, H. Pleiotropic Role of Tenascin-C in Central Nervous System Diseases: From Basic to Clinical Applications. Front. Neurol. 2020, 11, 576230. [Google Scholar] [CrossRef]
- Jakovcevski, I.; Miljkovic, D.; Schachner, M.; Andjus, P.R. Tenascins and inflammation in disorders of the nervous system. Amino Acids 2013, 44, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, A.; Stamenkovic, V.; Poleksic, J.; Hamad, M.I.K.; Reiss, G.; Jakovcevski, I.; Andjus, P.R. The Role of Tenascin-C on the Structural Plasticity of Perineuronal Nets and Synaptic Expression in the Hippocampus of Male Mice. Biomolecules 2024, 14, 508. [Google Scholar] [CrossRef]
- Mueller-Buehl, C.; Pakusch, J.; Bader, V.; Winklhofer, K.F.; Mark, M.D.; Faissner, A. Combined loss of brevican, neurocan, tenascin-C and tenascin-R leads to impaired fear retrieval due to perineuronal net loss. Sci. Rep. 2025, 15, 5528. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yoshida, T.; Sudo, A. Tenascin-C in Osteoarthritis and Rheumatoid Arthritis. Front. Immunol. 2020, 11, 577015. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.P.; Chiquet-Ehrismann, R. Tenascin-C: Its functions as an integrin ligand. Int. J. Biochem. Cell Biol. 2015, 65, 165–168. [Google Scholar] [CrossRef]
- Loustau, T.; Abou-Faycal, C.; Erne, W.; Zur Wiesch, P.A.; Ksouri, A.; Imhof, T.; Morgelin, M.; Li, C.; Mathieu, M.; Salome, N.; et al. Modulating tenascin-C functions by targeting the MAtrix REgulating MOtif, “MAREMO”. Matrix Biol. 2022, 108, 20–38. [Google Scholar] [CrossRef]
- Midwood, K.; Sacre, S.; Piccinini, A.M.; Inglis, J.; Trebaul, A.; Chan, E.; Drexler, S.; Sofat, N.; Kashiwagi, M.; Orend, G.; et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 2009, 15, 774–780. [Google Scholar] [CrossRef]
- Zuliani-Alvarez, L.; Marzeda, A.M.; Deligne, C.; Schwenzer, A.; McCann, F.E.; Marsden, B.D.; Piccinini, A.M.; Midwood, K.S. Mapping tenascin-C interaction with toll-like receptor 4 reveals a new subset of endogenous inflammatory triggers. Nat. Commun. 2017, 8, 1595. [Google Scholar] [CrossRef]
- Caldeira, J.; Sousa, A.; Sousa, D.M.; Barros, D. Extracellular matrix constitution and function for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 29–72. [Google Scholar]
- Bhome, R.; Bullock, M.D.; Al Saihati, H.A.; Goh, R.W.; Primrose, J.N.; Sayan, A.E.; Mirnezami, A.H. A top-down view of the tumor microenvironment: Structure, cells and signaling. Front. Cell Dev. Biol. 2015, 3, 33. [Google Scholar] [CrossRef]
- Midwood, K.S.; Orend, G. The role of tenascin-C in tissue injury and tumorigenesis. J. Cell Commun. Signal 2009, 3, 287–310. [Google Scholar] [CrossRef]
- Murdamoothoo, D.; Schwenzer, A.; Kant, J.; Rupp, T.; Marzeda, A.; Midwood, K.; Orend, G. Investigating cell-type specific functions of tenascin-C. Methods Cell Biol. 2018, 143, 401–428. [Google Scholar]
- Albacete-Albacete, L.; Sanchez-Alvarez, M.; Del Pozo, M.A. Extracellular Vesicles: An Emerging Mechanism Governing the Secretion and Biological Roles of Tenascin-C. Front. Immunol. 2021, 12, 671485. [Google Scholar] [CrossRef]
- Chiquet-Ehrismann, R. Tenascins. Int. J. Biochem. Cell Biol. 2004, 36, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Abedsaeidi, M.; Hojjati, F.; Tavassoli, A.; Sahebkar, A. Biology of Tenascin C and its Role in Physiology and Pathology. Curr. Med. Chem. 2024, 31, 2706–2731. [Google Scholar] [CrossRef]
- Moritz, S.; Lehmann, S.; Faissner, A.; von Holst, A. An induction gene trap screen in neural stem cells reveals an instructive function of the niche and identifies the splicing regulator sam68 as a tenascin-C-regulated target gene. Stem Cells 2008, 26, 2321–2331. [Google Scholar] [CrossRef]
- Nakamura, K.; Watanabe, Y.; Boitet, C.; Satake, S.; Iida, H.; Yoshihi, K.; Ishii, Y.; Kato, K.; Kondoh, H. Wnt signal-dependent antero-posterior specification of early-stage CNS primordia modeled in EpiSC-derived neural stem cells. Front. Cell Dev. Biol. 2023, 11, 1260528. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.R.; Hwang, I.; Ahn, S.Y.; Chang, Y.S.; Park, W.S.; Ahn, J.Y. Neuron-specific expression of p48 Ebp1 during murine brain development and its contribution to CNS axon regeneration. BMB Rep. 2017, 50, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Chiquet-Ehrismann, R.; Chiquet, M. Tenascins: Regulation and putative functions during pathological stress. J. Pathol. 2003, 200, 488–499. [Google Scholar] [CrossRef]
- Reinhard, J.; Brosicke, N.; Theocharidis, U.; Faissner, A. The extracellular matrix niche microenvironment of neural and cancer stem cells in the brain. Int. J. Biochem. Cell Biol. 2016, 81, 174–183. [Google Scholar] [CrossRef]
- Kreja, L.; Liedert, A.; Schlenker, H.; Brenner, R.E.; Fiedler, J.; Friemert, B.; Durselen, L.; Ignatius, A. Effects of mechanical strain on human mesenchymal stem cells and ligament fibroblasts in a textured poly(L-lactide) scaffold for ligament tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2575–2582. [Google Scholar] [CrossRef]
- Dzyubenko, E.; Manrique-Castano, D.; Pillath-Eilers, M.; Vasileiadou, P.; Reinhard, J.; Faissner, A.; Hermann, D.M. Tenascin-C restricts reactive astrogliosis in the ischemic brain. Matrix Biol. 2022, 110, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Suzuki, H.; Fukai, F. Involvement of integrin-activating peptides derived from tenascin-C in colon cancer progression. World J. Gastrointest. Oncol. 2021, 13, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, X.; Su, C.; You, Y.; Jiang, Z.; Fan, Q.; Zhu, D. The role of tenascin-C in tumor microenvironments and its potential as a therapeutic target. Front. Cell Dev. Biol. 2025, 13, 1554312. [Google Scholar] [CrossRef] [PubMed]
- Salviano-Silva, A.; Wollmann, K.; Brenna, S.; Reimer, R.; Neumann, J.E.; Dottermusch, M.; Woythe, L.; Maire, C.L.; Puig, B.; Schuller, U.; et al. Extracellular Vesicles Carrying Tenascin-C are Clinical Biomarkers and Improve Tumor-Derived DNA Analysis in Glioblastoma Patients. ACS Nano 2025, 19, 9844–9859. [Google Scholar] [CrossRef]
- Suzuki, H.; Fujimoto, M.; Kawakita, F.; Liu, L.; Nakatsuka, Y.; Nakano, F.; Nishikawa, H.; Okada, T.; Kanamaru, H.; Imanaka-Yoshida, K.; et al. Tenascin-C in brain injuries and edema after subarachnoid hemorrhage: Findings from basic and clinical studies. J. Neurosci. Res. 2020, 98, 42–56. [Google Scholar] [CrossRef]
- Suzuki, H.; Kawakita, F.; Nakajima, H.; Oinaka, H.; Nampei, M.; Suzuki, Y. Tenascin-C as a Target for Intervention in Delayed Cerebral Ischemia After Subarachnoid Hemorrhage. Acta Neurochir. Suppl. 2025, 136, 11–17. [Google Scholar]
- Zachariassen, M.; Thomsen, M.M.; Hillig, T.; Trier-Petersen, P.; Jensen, A.V.; Friis-Hansen, L.J.; Brandt, C.T. Tenascin-C in patients with central nervous system infections. J. Neuroimmunol. 2024, 392, 578373. [Google Scholar] [CrossRef]
- Suzuki, H.; Kanamaru, K.; Shiba, M.; Fujimoto, M.; Kawakita, F.; Imanaka-Yoshida, K.; Yoshida, T.; Taki, W. Tenascin-C is a possible mediator between initial brain injury and vasospasm-related and -unrelated delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Acta Neurochir. Suppl. 2015, 120, 117–121. [Google Scholar]
- Hamza, O.; Kiss, A.; Kramer, A.M.; Trojanek, S.; Abraham, D.; Acar, E.; Nagel, F.; Tretter, V.E.; Kitzwogerer, M.; Podesser, B.K. Tenascin C promotes valvular remodeling in two large animal models of ischemic mitral regurgitation. Basic Res. Cardiol. 2020, 115, 76. [Google Scholar] [CrossRef]
- Tajiri, K.; Yonebayashi, S.; Li, S.; Ieda, M. Immunomodulatory Role of Tenascin-C in Myocarditis and Inflammatory Cardiomyopathy. Front. Immunol. 2021, 12, 624703. [Google Scholar] [CrossRef]
- van Kralingen, C.; Kho, D.T.; Costa, J.; Angel, C.E.; Graham, E.S. Exposure to inflammatory cytokines IL-1beta and TNFalpha induces compromise and death of astrocytes; implications for chronic neuroinflammation. PLoS ONE 2013, 8, e84269. [Google Scholar] [CrossRef]
- Uddin, M.J.; Li, C.S.; Joe, Y.; Chen, Y.; Zhang, Q.; Ryter, S.W.; Chung, H.T. Carbon Monoxide Inhibits Tenascin-C Mediated Inflammation via IL-10 Expression in a Septic Mouse Model. Mediat. Inflamm. 2015, 2015, 613249. [Google Scholar] [CrossRef]
- Domaingo, A.; Jokesch, P.; Schweiger, A.; Gschwandtner, M.; Gerlza, T.; Koch, M.; Midwood, K.S.; Kungl, A.J. Chemokine Binding to Tenascin-C Influences Chemokine-Induced Immune Cell Migration. Int. J. Mol. Sci. 2023, 24, 14694. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, D.; Adzic, M.; Peric, M.; Reiss, G.; Milosevic, M.; Andjus, P.R.; Jakovcevski, I. Tenascin-C fibronectin D domain is involved in the fine-tuning of glial response to CNS injury in vitro. Front. Cell Dev. Biol. 2022, 10, 952208. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, Z.; Rakkar, K.; Bath, P.M.; Bayraktutan, U. Inhibition of TNF-alpha protects in vitro brain barrier from ischaemic damage. Mol. Cell Neurosci. 2015, 69, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, C.; Allen, N.J.; Susman, M.W.; O’Rourke, N.A.; Park, C.Y.; Ozkan, E.; Chakraborty, C.; Mulinyawe, S.B.; Annis, D.S.; Huberman, A.D.; et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009, 139, 380–392. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Midwood, K.S.; Varga, J. Tenascin-C in fibrosis in multiple organs: Translational implications. Semin. Cell Dev. Biol. 2022, 128, 130–136. [Google Scholar] [CrossRef]
- Nakamura, A.; Morise, J.; Yabuno-Nakagawa, K.; Hashimoto, Y.; Takematsu, H.; Oka, S. Site-specific HNK-1 epitope on alternatively spliced fibronectin type-III repeats in tenascin-C promotes neurite outgrowth of hippocampal neurons through contactin-1. PLoS ONE 2019, 14, e0210193. [Google Scholar] [CrossRef]
- Manrique-Castano, D.; Dzyubenko, E.; Borbor, M.; Vasileiadou, P.; Kleinschnitz, C.; Roll, L.; Faissner, A.; Hermann, D.M. Tenascin-C preserves microglia surveillance and restricts leukocyte and, more specifically, T cell infiltration of the ischemic brain. Brain Behav. Immun. 2021, 91, 639–648. [Google Scholar] [CrossRef]
- Dityatev, A.; Schachner, M.; Sonderegger, P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci. 2010, 11, 735–746. [Google Scholar] [CrossRef]
- Evers, M.R.; Salmen, B.; Bukalo, O.; Rollenhagen, A.; Bosl, M.R.; Morellini, F.; Bartsch, U.; Dityatev, A.; Schachner, M. Impairment of L-type Ca2+ channel-dependent forms of hippocampal synaptic plasticity in mice deficient in the extracellular matrix glycoprotein tenascin-C. J. Neurosci. 2002, 22, 7177–7194. [Google Scholar] [CrossRef]
- Treloar, H.B.; Ray, A.; Dinglasan, L.A.; Schachner, M.; Greer, C.A. Tenascin-C is an inhibitory boundary molecule in the developing olfactory bulb. J. Neurosci. 2009, 29, 9405–9416. [Google Scholar] [CrossRef] [PubMed]
- Gottschling, C.; Wegrzyn, D.; Denecke, B.; Faissner, A. Elimination of the four extracellular matrix molecules tenascin-C, tenascin-R, brevican and neurocan alters the ratio of excitatory and inhibitory synapses. Sci. Rep. 2019, 9, 13939. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.R.; Czvitkovich, S.; Dassie, E.; Vogelaar, C.F.; Faissner, A.; Blits, B.; Gage, F.H.; ffrench-Constant, C.; Fawcett, J.W. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J. Neurosci. 2009, 29, 5546–5557. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Apeksha, A.; Norenberg, M.D. Role of Matricellular Proteins in Disorders of the Central Nervous System. Neurochem. Res. 2017, 42, 858–875. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Dityatev, A. Crosstalk between glia, extracellular matrix and neurons. Brain Res. Bull. 2018, 136, 101–108. [Google Scholar] [CrossRef]
- Rigato, F.; Garwood, J.; Calco, V.; Heck, N.; Faivre-Sarrailh, C.; Faissner, A. Tenascin-C promotes neurite outgrowth of embryonic hippocampal neurons through the alternatively spliced fibronectin type III BD domains via activation of the cell adhesion molecule F3/contactin. J. Neurosci. 2002, 22, 6596–6609. [Google Scholar] [CrossRef]
- Carceller, H.; Guirado, R.; Ripolles-Campos, E.; Teruel-Marti, V.; Nacher, J. Perineuronal Nets Regulate the Inhibitory Perisomatic Input onto Parvalbumin Interneurons and gamma Activity in the Prefrontal Cortex. J. Neurosci. 2020, 40, 5008–5018. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, P.; Khoshneviszadeh, M.; Jandke, S.; Schreiber, S.; Dityatev, A. Interplay between perivascular and perineuronal extracellular matrix remodelling in neurological and psychiatric diseases. Eur. J. Neurosci. 2021, 53, 3811–3830. [Google Scholar] [CrossRef] [PubMed]
- Mascio, G.; Notartomaso, S.; Martinello, K.; Liberatore, F.; Bucci, D.; Imbriglio, T.; Cannella, M.; Antenucci, N.; Scarselli, P.; Lattanzi, R.; et al. A Progressive Build-up of Perineuronal Nets in the Somatosensory Cortex Is Associated with the Development of Chronic Pain in Mice. J. Neurosci. 2022, 42, 3037–3048. [Google Scholar] [CrossRef]
- Lensjø, K.K.; Christensen, A.C.; Tennøe, S.; Fyhn, M.; Hafting, T. Differential Expression and Cell-Type Specificity of Perineuronal Nets in Hippocampus, Medial Entorhinal Cortex, and Visual Cortex Examined in the Rat and Mouse. eNeuro 2017, 4, ENEURO.0379-16.2017. [Google Scholar] [CrossRef]
- Beddows, C.A.; Shi, F.; Horton, A.L.; Dalal, S.; Zhang, P.; Ling, C.C.; Yong, V.W.; Loh, K.; Cho, E.; Karagiannis, C.; et al. Pathogenic hypothalamic extracellular matrix promotes metabolic disease. Nature 2024, 633, 914–922. [Google Scholar] [CrossRef]
- Francos-Quijorna, I.; Sanchez-Petidier, M.; Burnside, E.R.; Badea, S.R.; Torres-Espin, A.; Marshall, L.; de Winter, F.; Verhaagen, J.; Moreno-Manzano, V.; Bradbury, E.J. Chondroitin sulfate proteoglycans prevent immune cell phenotypic conversion and inflammation resolution via TLR4 in rodent models of spinal cord injury. Nat. Commun. 2022, 13, 2933. [Google Scholar] [CrossRef]
- Spolidoro, M.; Putignano, E.; Munafo, C.; Maffei, L.; Pizzorusso, T. Inhibition of matrix metalloproteinases prevents the potentiation of nondeprived-eye responses after monocular deprivation in juvenile rats. Cereb. Cortex 2012, 22, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.; Lensjo, K.K.; Dinh, T.; Yang, S.; Andrews, M.R.; Hafting, T.; Fyhn, M.; Fawcett, J.W.; Dick, G. Aggrecan Directs Extracellular Matrix-Mediated Neuronal Plasticity. J. Neurosci. 2018, 38, 10102–10113. [Google Scholar] [CrossRef]
- Eyileten, C.; Sharif, L.; Wicik, Z.; Jakubik, D.; Jarosz-Popek, J.; Soplinska, A.; Postula, M.; Czlonkowska, A.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D. The Relation of the Brain-Derived Neurotrophic Factor with MicroRNAs in Neurodegenerative Diseases and Ischemic Stroke. Mol. Neurobiol. 2021, 58, 329–347. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, J.; Xu, B.; Yang, J.; Wu, Z.; Cheng, L. TrkB/BDNF signaling pathway and its small molecular agonists in CNS injury. Life Sci. 2024, 336, 122282. [Google Scholar] [CrossRef]
- Legutko, D.; Kuzniewska, B.; Kalita, K.; Yasuda, R.; Kaczmarek, L.; Michaluk, P. BDNF signaling requires Matrix Metalloproteinase-9 during structural synaptic plasticity. bioRxiv 2024. [Google Scholar] [CrossRef]
- Fujita, M.; Yamamoto, T.; Iyoda, T.; Fujisawa, T.; Nagai, R.; Kudo, C.; Sasada, M.; Kodama, H.; Fukai, F. Autocrine Production of PDGF Stimulated by the Tenascin-C-Derived Peptide TNIIIA2 Induces Hyper-Proliferation in Glioblastoma Cells. Int. J. Mol. Sci. 2019, 20, 3183. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, F.; Cheng, D.; Wang, Z.; Xing, N.; Yuan, J.; Zhang, W.; Xing, F. Free heme induces neuroinflammation and cognitive impairment by microglial activation via the TLR4/MyD88/NF-kappaB signaling pathway. Cell Commun. Signal 2024, 22, 16. [Google Scholar] [CrossRef]
- Kawai, T.; Ikegawa, M.; Ori, D.; Akira, S. Decoding Toll-like receptors: Recent insights and perspectives in innate immunity. Immunity 2024, 57, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.; Lee, J.H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Merk, D.; Cox, F.F.; Jakobs, P.; Promel, S.; Altschmied, J.; Haendeler, J. Dose-Dependent Effects of Lipopolysaccharide on the Endothelium-Sepsis versus Metabolic Endotoxemia-Induced Cellular Senescence. Antioxidants 2024, 13, 443. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Zhai, L.; Sheng, X.; Zheng, W.; Chu, H.; Zhang, G. Atorvastatin Attenuates Cognitive Deficits and Neuroinflammation Induced by Abeta(1-42) Involving Modulation of TLR4/TRAF6/NF-kappaB Pathway. J. Mol. Neurosci. 2018, 64, 363–373. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Sakai, Y. Transforming Growth Factor-beta Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int. J. Mol. Sci. 2019, 20, 5822. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Dietrich, H.K.; Axtell, R.C.; Williams, A.M.; Egusquiza, R.; Wai, K.M.; Koshy, A.A.; Buckwalter, M.S. Astrocytic TGF-beta signaling limits inflammation and reduces neuronal damage during central nervous system Toxoplasma infection. J. Immunol. 2014, 193, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zhang, J.; Shen, M.; Zhang, J. Silencing of Tenascin-C Inhibited Inflammation and Apoptosis Via PI3K/Akt/NF-kappaB Signaling Pathway in Subarachnoid Hemorrhage Cell Model. J. Stroke Cerebrovasc. Dis. 2020, 29, 104485. [Google Scholar] [CrossRef] [PubMed]
- Antonijevic, M.; Dallemagne, P.; Rochais, C. Indirect influence on the BDNF/TrkB receptor signaling pathway via GPCRs, an emerging strategy in the treatment of neurodegenerative disorders. Med. Res. Rev. 2025, 45, 274–310. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Gao, D. Naringenin alleviates cognitive dysfunction in rats with cerebral ischemia/reperfusion injury through up-regulating hippocampal BDNF-TrkB signaling: Involving suppression in neuroinflammation and oxidative stress. Neuroreport 2024, 35, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ge, M.-m.; Zhang, L.-Q.; Yuan, X.-M.; Han, S.-Y.; Manyande, A.; Tian, Y.-K.; Tian, X. Dysfunction of the BDNF-TrkB signaling pathway contributes to learning and memory impairments induced by neuroinflammation in mice. Authorea 2022, 505, 21–33. [Google Scholar]
- De Laporte, L.; Rice, J.J.; Tortelli, F.; Hubbell, J.A. Tenascin C promiscuously binds growth factors via its fifth fibronectin type III-like domain. PLoS ONE 2013, 8, e62076. [Google Scholar] [CrossRef]
- Liu, Y.; Paauwe, M.; Nixon, A.B.; Hawinkels, L. Endoglin Targeting: Lessons Learned and Questions That Remain. Int. J. Mol. Sci. 2020, 22, 147. [Google Scholar] [CrossRef]
- Zhao, Y.; Gui, W.; Niu, F.; Chong, S. The MAPK Signaling Pathways as a Novel Way in Regulation and Treatment of Parasitic Diseases. Diseases 2019, 7, 9. [Google Scholar] [CrossRef]
- Maier, S.; Lutz, R.; Gelman, L.; Sarasa-Renedo, A.; Schenk, S.; Grashoff, C.; Chiquet, M. Tenascin-C induction by cyclic strain requires integrin-linked kinase. Biochim. Biophys. Acta 2008, 1783, 1150–1162. [Google Scholar] [CrossRef]
- Ding, H.; Jin, M.; Liu, D.; Wang, S.; Zhang, J.; Song, X.; Huang, R. Tenascin-C promotes the migration of bone marrow stem cells via toll-like receptor 4-mediated signaling pathways: MAPK, AKT and Wnt. Mol. Med. Rep. 2018, 17, 7603–7610. [Google Scholar] [CrossRef]
- Karati, D.; Mukherjee, S.; Roy, S. A Promising Drug Candidate as Potent Therapeutic Approach for Neuroinflammation and Its In Silico Justification of Chalcone Congeners: A Comprehensive Review. Mol. Neurobiol. 2024, 61, 1873–1891. [Google Scholar] [CrossRef] [PubMed]
- Morellini, F.; Malyshev, A.; Volgushev, M.; Chistiakova, M.; Papashvili, G.; Fellini, L.; Kleene, R.; Schachner, M.; Dityatev, A. Impaired Fear Extinction Due to a Deficit in Ca(2+) Influx Through L-Type Voltage-Gated Ca(2+) Channels in Mice Deficient for Tenascin-C. Front. Integr. Neurosci. 2017, 11, 16. [Google Scholar] [CrossRef]
- Mubuchi, A.; Takechi, M.; Nishio, S.; Matsuda, T.; Itoh, Y.; Sato, C.; Kitajima, K.; Kitagawa, H.; Miyata, S. Assembly of neuron- and radial glial-cell-derived extracellular matrix molecules promotes radial migration of developing cortical neurons. Elife 2024, 12, RP92342. [Google Scholar] [CrossRef]
- Skupien, A.; Konopka, A.; Trzaskoma, P.; Labus, J.; Gorlewicz, A.; Swiech, L.; Babraj, M.; Dolezyczek, H.; Figiel, I.; Ponimaskin, E.; et al. CD44 regulates dendrite morphogenesis through Src tyrosine kinase-dependent positioning of the Golgi. J. Cell Sci. 2014, 127, 5038–5051. [Google Scholar] [CrossRef]
- Martinelli, S.; Anderzhanova, E.A.; Bajaj, T.; Wiechmann, S.; Dethloff, F.; Weckmann, K.; Heinz, D.E.; Ebert, T.; Hartmann, J.; Geiger, T.M.; et al. Stress-primed secretory autophagy promotes extracellular BDNF maturation by enhancing MMP9 secretion. Nat. Commun. 2021, 12, 4643. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, D.; Adzic, M.; Peric, M.; Jakovcevski, I.; Forster, E.; Schachner, M.; Andjus, P.R. Different Functions of Recombinantly Expressed Domains of Tenascin-C in Glial Scar Formation. Front. Immunol. 2020, 11, 624612, Erratum in Front. Immunol. 2021, 12, 672476. [Google Scholar]
- Chen, J.; Joon Lee, H.; Jakovcevski, I.; Shah, R.; Bhagat, N.; Loers, G.; Liu, H.Y.; Meiners, S.; Taschenberger, G.; Kugler, S.; et al. The extracellular matrix glycoprotein tenascin-C is beneficial for spinal cord regeneration. Mol. Ther. 2010, 18, 1769–1777. [Google Scholar] [CrossRef]
- Luo, W.; Li, Y.; Zhao, J.; Niu, R.; Xiang, C.; Zhang, M.; Xiao, C.; Liu, W.; Gu, R. CD44-targeting hyaluronic acid-selenium nanoparticles boost functional recovery following spinal cord injury. J. Nanobiotechnol. 2024, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Eichler, A.; Vlachos, A. Monitoring and Modulating Inflammation-Associated Alterations in Synaptic Plasticity: Role of Brain Stimulation and the Blood-Brain Interface. Biomolecules 2021, 11, 359. [Google Scholar] [CrossRef]


| CNS Disorder | Neuroinflammation Effects | Plasticity Effects |
|---|---|---|
| Stroke | TNC ↑ → TNF-α/IL-1β release, BBB disruption, brain injury ↑ [17,51,52,53,54] | TNC ↓ → apoptosis ↓, tissue repair ↑ [55,56,57] |
| Traumatic Brain Injury (TBI) | CSF TNC ↑ → inflammation ↑ [58,59,60] | TNC ↑ → NSC migration ↑ [1,61] TNC ↑ → synaptic remodeling ↓ [51] |
| Alzheimer’s Disease (AD) | TNC ↑ → Aβ deposition ↑, inflammation ↑, cognitive decline ↑ [62,63] | TNC ↓ → Aβ ↓, synaptic function ↑, neuroprotection ↑ [62,64] |
| Parkinson’s Disease (PD) | TNC activates microglia via TLR4 → dopaminergic degeneration [29,65] | TNC ↓ → excitotoxicity ↓, PNN integrity disrupted → synaptic instability [29,66] |
| Epilepsy | TNC ↑ → seizure threshold ↓ [21,23] | TNC ↓ → PNN degradation ↑ → GABAergic inhibition ↓ → synaptic reorganization ↓ [23,66,67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.-L.; Bao, S.-W.; Huang, M.-X.; Gao, Y.-J.; Lu, H.-J.; Wu, X.-B. The Role of Tenascin-C in Neuroinflammation and Neuroplasticity. Int. J. Mol. Sci. 2025, 26, 10174. https://doi.org/10.3390/ijms262010174
Jin Y-L, Bao S-W, Huang M-X, Gao Y-J, Lu H-J, Wu X-B. The Role of Tenascin-C in Neuroinflammation and Neuroplasticity. International Journal of Molecular Sciences. 2025; 26(20):10174. https://doi.org/10.3390/ijms262010174
Chicago/Turabian StyleJin, Ya-Li, Shi-Wen Bao, Meng-Xuan Huang, Yong-Jing Gao, Huan-Jun Lu, and Xiao-Bo Wu. 2025. "The Role of Tenascin-C in Neuroinflammation and Neuroplasticity" International Journal of Molecular Sciences 26, no. 20: 10174. https://doi.org/10.3390/ijms262010174
APA StyleJin, Y.-L., Bao, S.-W., Huang, M.-X., Gao, Y.-J., Lu, H.-J., & Wu, X.-B. (2025). The Role of Tenascin-C in Neuroinflammation and Neuroplasticity. International Journal of Molecular Sciences, 26(20), 10174. https://doi.org/10.3390/ijms262010174






