Heart Under Pressure: Divergent Cardiac Molecules Responses to Azathioprine and Anti-TNF Therapy in Ulcerative Colitis
Abstract
1. Introduction
2. Results
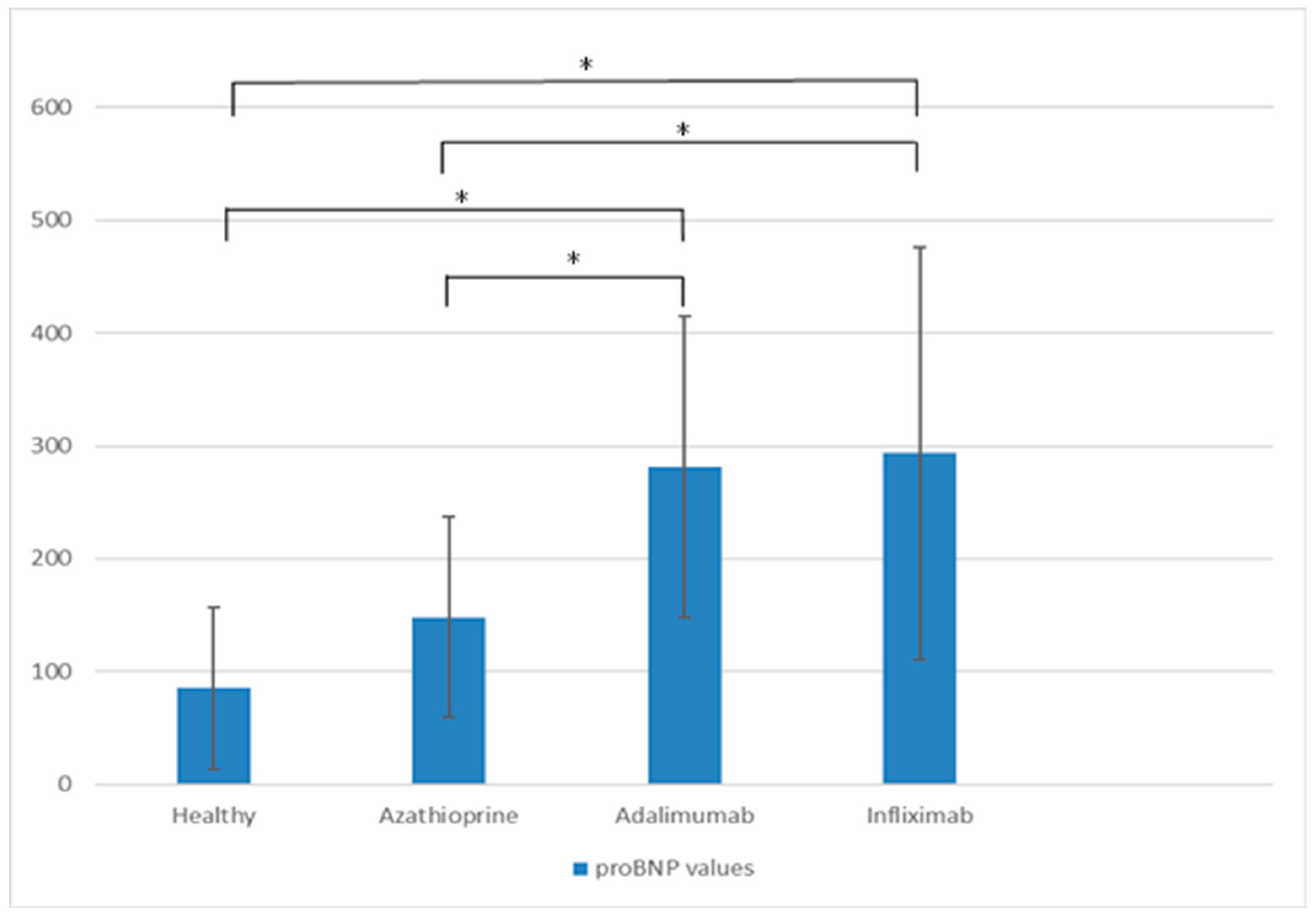
2.1. Short-Term Effects
2.2. Long-Term Effects
3. Discussion
3.1. Short-Term Effects on Cardiac Biomarkers
3.2. Long-Term Use of Immunosuppressants and Anti-TNF Agents: Future Implications
3.2.1. Azathioprine
3.2.2. Infliximab and Adalimumab
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults: A Review. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef]
- Spiceland, C.M.; Lodhia, N. Endoscopy in Inflammatory Bowel Disease: Role in Diagnosis, Management, and Treatment. World J. Gastroenterol. 2018, 24, 4014–4020. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- DeRoche, T.C.; Xiao, S.Y.; Liu, X. Histological Evaluation in Ulcerative Colitis. Gastroenterol. Rep. 2014, 2, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Canavese, G.; Villanacci, V.; Antonelli, E.; Cadei, M.; Sapino, A.; Rocca, R.; Daperno, M.; Suriani, R.; Di Santo, M.G.; Cassoni, P.; et al. Eosinophilia-Associated Basal Plasmacytosis: An Early and Sensitive Histologic Feature of Inflammatory Bowel Disease. APMIS 2017, 125, 179–183. [Google Scholar] [CrossRef]
- Villanacci, V.; Reggiani-Bonetti, L.; Salviato, T.; Leoncini, G.; Cadei, M.; Albarello, L.; Caputo, A.; Aquilano, M.C.; Battista, S.; Parente, P. Histopathology of IBD Colitis. A Practical Approach from the Pathologists of the Italian Group for the Study of the Gastrointestinal Tract (GIPAD). Pathologica 2021, 113, 39–53. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Gardezi, S.A.; Sachdeva, N.; Rampurawala, I.M.; Ranasinghe, A.; Shehzad, M.U.; Gill, K.; Qureshi, R.; Gupta, A.; Hasan, A.; Farhan, M.; et al. Trends and Disparities in Inflammatory Bowel Disease and Cardiovascular Disease-Related Mortality in the United States from 1999 to 2023: A CDC WONDER Analysis. Int. J. Cardiol. Cardiovasc. Risk Prev. 2025, 26, 200438. [Google Scholar] [CrossRef]
- Feng, W.; Chen, G.; Cai, D.; Zhao, S.; Cheng, J.; Shen, H. Inflammatory Bowel Disease and Risk of Ischemic Heart Disease: An Updated Meta-Analysis of Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005892. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Wang, Z.Q.; Huang, H.; Li, J.; Zhang, X.; Ma, C.; Chen, Z. Inflammatory Bowel Disease and Cardiovascular Disease Incidence and Mortality: A Meta-Analysis. Eur. J. Prev. Cardiol. 2018, 25, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yao, J.; Olén, O.; Halfvarson, J.; Bergman, D.; Ebrahimi, F.; Rosengren, A.; Sundström, J.; Ludvigsson, J.F. Risk of Heart Failure in Inflammatory Bowel Disease: A Swedish Population-Based Study. Eur. Heart J. 2024, 45, 2493–2504. [Google Scholar] [CrossRef]
- Qiu, X.; Hou, C.; Yang, Z.; Wang, Q.; Li, L. Inflammatory Bowel Disease and Risk of Coronary Heart Disease: A Mendelian Randomization Study. Wien Klin Wochenschr. 2022, 134, 779–787. [Google Scholar] [CrossRef]
- Avouac, J.; Gossec, L.; Soubrier, M.; Dougados, M. The Cardiovascular Safety of Tumour Necrosis Factor Inhibitors in Arthritic Conditions: A Structured Review with Recommendations. Autoimmun. Rev. 2020, 19, 102–110. [Google Scholar] [CrossRef]
- Bragagni, G.; Brogna, R.; Franceschetti, P.; Zoli, G. Cardiac Involvement in Crohn’s Disease: Echocardiographic Study. J. Gastroenterol. Hepatol. 2007, 22, 18–22. [Google Scholar] [CrossRef]
- Matsuura, E.; Atzeni, F.; Sarzi-Puttini, P.; Turiel, M.; Lopez, L.R.; Nurmohamed, M.T. Is Atherosclerosis an Autoimmune Disease? BMC Med. 2014, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.G.; Symmons, D.P.M. What Effects Might Anti-TNFα Treatment Be Expected to Have on Cardiovascular Morbidity and Mortality in Rheumatoid Arthritis? A Review of the Role of TNFα in Cardiovascular Pathophysiology. Rheumatology 2007, 46, 1234–1242. [Google Scholar] [CrossRef]
- Licordari, R.; Correale, M.; Bonanno, S.; Beltrami, M.; Ciccarelli, M.; Micari, A.; Palazzuoli, A.; Dattilo, G. Beyond Natriuretic Peptides: Unveiling the Power of Emerging Biomarkers in Heart Failure. Biomolecules 2024, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Gaggin, H.K.; Januzzi, J.L. Biomarkers and Diagnostics in Heart Failure. Clin. Chim. Acta 2013, 1832, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Wolsk, E.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.; Lewis, E.F.; Maggioni, A.P.; McMurray, J.J.V.; Probstfield, J.L.; et al. Risk Estimates of Imminent Cardiovascular Death and Heart Failure Hospitalization Are Improved Using Serial Natriuretic Peptide Measurements in Patients with Coronary Artery Disease and Type 2 Diabetes. J. Am. Heart Assoc. 2022, 11, e021327. [Google Scholar] [CrossRef]
- Riestra, S. P090. Evaluation of NT-proBNP in Inflammatory Bowel Disease. J. Crohns Colitis 2015, 9 (Suppl. S1), S123. [Google Scholar]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef]
- Tomáš, Ľ.; Lazúrová, I.; Pundová, L.; Oetterová, M.; Zakuciová, M.; Petrášová, D.; Studenčan, M. Acute and Long-Term Effect of Infliximab on Humoral and Echocardiographic Parameters in Patients with Chronic Inflammatory Diseases. Clin. Rheumatol. 2013, 32, 61–66. [Google Scholar] [CrossRef]
- Lang, C.C.; Choy, A.M.; Turner, K.; Tobin, R.; Coutie, W.; Struthers, A.D. The Effect of Intravenous Saline Loading on Plasma Levels of Brain Natriuretic Peptide in Man. J. Hypertens. 1993, 11, 737–741. [Google Scholar] [CrossRef]
- Mallick, B.; Malik, S. Use of Azathioprine in Ulcerative Colitis: A Comprehensive Review. Cureus 2022, 14, e24874. [Google Scholar] [CrossRef]
- Dubinsky, M.C. Azathioprine, 6-Mercaptopurine in Inflammatory Bowel Disease: Pharmacology, Efficacy, and Safety. Clin. Gastroenterol. Hepatol. 2004, 2, 731–743. [Google Scholar] [CrossRef]
- Ariyaratnam, J.; Subramanian, V. Association between Thiopurine Use and Nonmelanoma Skin Cancers in Patients with Inflammatory Bowel Disease: A Meta-Analysis. Am. J. Gastroenterol. 2014, 109, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, D.S.; Lewis, J.D.; Beaugerie, L.; Tierney, A.; Brensinger, C.M.; Gisbert, J.P.; Loftus, E.V.; Peyrin-Biroulet, L.; Blonski, W.C.; Van Domselaar, M.; et al. Risk of Lymphoma in Patients with Inflammatory Bowel Disease Treated with Azathioprine and 6-Mercaptopurine: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 847–858.e4. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.D. Azathioprine-Induced Pericarditis in a Patient with Ulcerative Colitis. Can. J. Gastroenterol. 1997, 11, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.S.; Harpool, J.; Lloyd, S.G. Azathioprine-Induced Acute Fulminant Eosinophilic Myocarditis. J. Am. Coll. Cardiol. 2019, 73 (Suppl. S1), 1542. [Google Scholar] [CrossRef]
- Turow, A.; Yong, T.Y.; Fok, J.S.; Li, J.Y. Azathioprine Hypersensitivity Presenting as Cardiogenic Shock and Sweet’s Syndrome in a Patient with Microscopic Polyangiitis. Intern. Med. 2012, 51, 1889–1892. [Google Scholar] [CrossRef]
- Su, S.; Wang, Y.M.; Zaborniak, K.; Hamza, S.; Jassal, D.S.; Blouw, M. Severe Distributive Shock, Neutrophilic Dermatosis, and ST-Elevation Myocardial Infarction in the Setting of Azathioprine Hypersensitivity Syndrome. Allergy Asthma Clin. Immunol. 2024, 20, 39. [Google Scholar] [CrossRef]
- Fan, Z.; He, Y.; Sun, W.; Li, Z.; Ye, C.; Wang, C. Clinical Characteristics, Diagnosis and Management of Sweet Syndrome Induced by Azathioprine. Clin. Exp. Med. 2023, 23, 3581–3587. [Google Scholar] [CrossRef]
- Dogan, P.; Grbovic, E.; Inci, S.; Bayraktar, F.; Cagli, K. Azathioprine-Induced Atrial Fibrillation. Intractable Rare Dis. Res. 2015, 4, 207–209. [Google Scholar] [CrossRef]
- Tapner, M.J.; Jones, B.E.; Wu, W.M.; Farrell, G.C. Toxicity of low dose azathioprine and 6-mercaptopurine in rat hepatocytes. Roles of xanthine oxidase and mitochondrial injury. J Hepatol. 2004, 40, 454–463. [Google Scholar] [CrossRef]
- Al Maruf, A.; Wan, L.; O’Brien, P.J. Evaluation of Azathioprine-Induced Cytotoxicity in an In Vitro Rat Hepatocyte System. BioMed Res. Int. 2014, 2014, 379748. [Google Scholar] [CrossRef]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T. Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, Double-Blind, Placebo-Controlled, Pilot Trial of Infliximab, a Chimeric Monoclonal Antibody to Tumor Necrosis Factor-Alpha, in Patients with Moderate-to-Severe Heart Failure: Results of the Anti-TNF Therapy Against Congestive Heart Failure (ATTACH) Trial. Circulation 2003, 107, 3133–3140. [Google Scholar]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.H.; Lomer, M.C.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Rastelli, S.; Granata, A.; Inserra, G.; Empana, J.P.; Boutouyrie, P.; Laurent, S.; Castellino, P. Arterial Stiffness in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Hypertens. 2016, 34, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Miehsler, W.; Novacek, G.; Wenzl, H.; Vogelsang, H.; Knoflach, P.; Kaser, A.; Dejaco, C.; Petritsch, W.; Kapitan, M.; Maier, H.; et al. A decade of infliximab: The Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J. Crohns Colitis. 2010, 4, 221–256. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Coté, T.R.; Cuffe, M.S.; Kramer, J.M.; Braun, M.M. Case Reports of Heart Failure after Therapy with a Tumor Necrosis Factor Antagonist. Ann. Intern. Med. 2003, 138, 807–811. [Google Scholar] [CrossRef]
- Sote, Y.; Green, S.; Maddison, P. Complete Heart Block after Infliximab Therapy. Rheumatology 2008, 47, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Diwan, M.M.; Patel, K.C. A Rare Case of Supraventricular Tachycardia Induced by Infliximab: A Case Report. Cases J. 2009, 2, 147. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Michaud, K. Heart failure in rheumatoid arthritis: Rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am. J. Med. 2004, 116, 305–311. [Google Scholar] [CrossRef]
- Rungoe, C.; Basit, S.; Ranthe, M.F.; Wohlfahrt, J.; Langholz, E.; Jess, T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: A nationwide Danish cohort study. Gut 2013, 62, 689–694. [Google Scholar] [CrossRef]
- Kristensen, L.E.; Danese, S.; Yndestad, A.; Wang, C.; Nagy, E.; Modesto, I.; Rivas, J.; Benda, B. Identification of Two Tofacitinib Subpopulations with Different Relative Risk Versus TNF Inhibitors: An Analysis of the Open Label, Randomised Controlled Study ORAL Surveillance. Ann. Rheum. Dis. 2023, 82, 901–910. [Google Scholar] [CrossRef]
- Peters, M.J.L.; Welsh, P.; McInnes, I.B.; Wolbink, G.; Dijkmans, B.A.C.; Sattar, N.; Nurmohamed, M.T. Tumour Necrosis Factor α Blockade Reduces Circulating N-Terminal Pro-Brain Natriuretic Peptide Levels in Patients with Active Rheumatoid Arthritis: Results from a Prospective Cohort Study. Ann. Rheum. Dis. 2010, 69, 1281–1285. [Google Scholar] [CrossRef]
- Bragagni, G.; Lari, F.; Magenta, G.; Brogna, R.; Zoli, G. Echocardiographic Evaluation of Anti-Tumor Necrosis Fac-tor-Alpha Therapy with Infliximab in Patients without Cardiac Pathologies. Recent. Prog. Med. 2010, 101, 289–292. [Google Scholar]
- Livia, C.; Inglis, S.; Crespo-Díaz, R.; Rizzo, S.; Mahlberg, R.; Bagwell, M.; Hillestad, M. Infliximab Limits Injury in Myo-cardial Infarction. J. Am. Heart Assoc. 2024, 13, e032172. [Google Scholar] [CrossRef] [PubMed]

| Azathioprine | Adalimumab | Infliximab | p Value | ||
|---|---|---|---|---|---|
| vs. ADA | vs. IFX | ||||
| proBNP (pg/mL) | 148.29 | 281.38 | 293.68 | 0.000 | 0.001 |
| NT-proBNP (pg/mL) | 126.83 | 270.13 | 276.32 | 0.000 | 0.000 |
| Troponin (ng/mL) | 0.001 | 0.0045 | 0.0067 | 0.000 | 0.000 |
| CK (U/L) | 80.33 | 102.67 | 92.04 | NS | NS |
| CK-MB (U/L) | 27.96 | 43.00 | 24.57 | 0.004 | NS |
| CRP (mg/L) | 101.28 | 58.92 | 70.73 | NS | NS |
| FCP (µg/g) | 486.65 | 1000.73 | 1522 | 0.001 | 0.015 |
| SE (mm/h) | 18.58 | 36.58 | 41.28 | 0.006 | 0.005 |
| Histologic score * | 5.2 | 5.3 | 5.3 | 0.001 | 0.000 |
| Azathioprine | Adalimumab | Infliximab | |
|---|---|---|---|
| proBNP 6 h (pg/mL) | 244.66 | 311.45 | 351.39 |
| NT-proBNP 6 h (pg/mL) | 229.04 | 293.95 | 348.82 |
| Azathioprine | Adalimumab | Infliximab | p Value | ||
|---|---|---|---|---|---|
| vs. ADA | vs. IFX | ||||
| proBNP 3 M (pg/mL) | 326.38 | 127.86 | 144.86 | 0.000 | 0.000 |
| NT-proBNP 3 M (pg/mL) | 248.88 | 201.5 | 130.6 | NS | 0.000 |
| Troponin 3 M (ng/mL) | 0.001 | 0.0073 | 0.009 | 0.000 | 0.000 |
| CK 3 M (U/L) | 196.75 | 77.12 | 62.18 | 0.000 | 0.000 |
| CK-MB 3 M (U/L) | 64.67 | 37.34 | 21.54 | 0.012 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvetković, M.; Simović, S.; Radojević, D.; Maksić, M.; Zdravković, N.; Milošević, B.; Stojanović, B.; Stolić, R.; Todorović, Ž.; Gogić, A.; et al. Heart Under Pressure: Divergent Cardiac Molecules Responses to Azathioprine and Anti-TNF Therapy in Ulcerative Colitis. Int. J. Mol. Sci. 2025, 26, 10160. https://doi.org/10.3390/ijms262010160
Cvetković M, Simović S, Radojević D, Maksić M, Zdravković N, Milošević B, Stojanović B, Stolić R, Todorović Ž, Gogić A, et al. Heart Under Pressure: Divergent Cardiac Molecules Responses to Azathioprine and Anti-TNF Therapy in Ulcerative Colitis. International Journal of Molecular Sciences. 2025; 26(20):10160. https://doi.org/10.3390/ijms262010160
Chicago/Turabian StyleCvetković, Mirjana, Stefan Simović, Dušan Radojević, Mladen Maksić, Nataša Zdravković, Bojan Milošević, Bojan Stojanović, Radojica Stolić, Željko Todorović, Anđela Gogić, and et al. 2025. "Heart Under Pressure: Divergent Cardiac Molecules Responses to Azathioprine and Anti-TNF Therapy in Ulcerative Colitis" International Journal of Molecular Sciences 26, no. 20: 10160. https://doi.org/10.3390/ijms262010160
APA StyleCvetković, M., Simović, S., Radojević, D., Maksić, M., Zdravković, N., Milošević, B., Stojanović, B., Stolić, R., Todorović, Ž., Gogić, A., Zdravković, N., & Zdravković, M. (2025). Heart Under Pressure: Divergent Cardiac Molecules Responses to Azathioprine and Anti-TNF Therapy in Ulcerative Colitis. International Journal of Molecular Sciences, 26(20), 10160. https://doi.org/10.3390/ijms262010160






