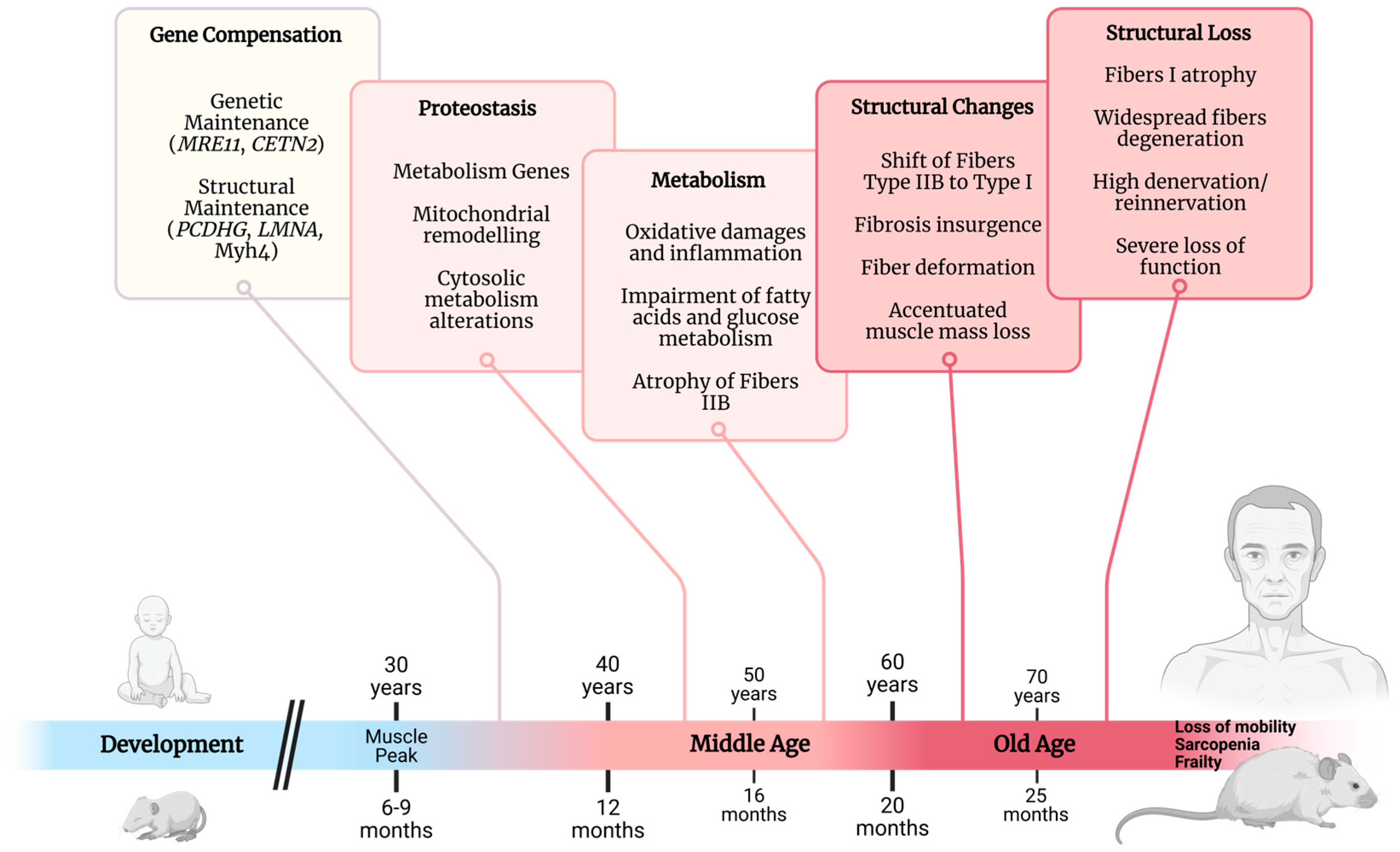
Molecular Framework of the Onset and Progression of Skeletal Muscle Aging
Abstract
1. Introduction
2. Myonuclear Resistance to Aging
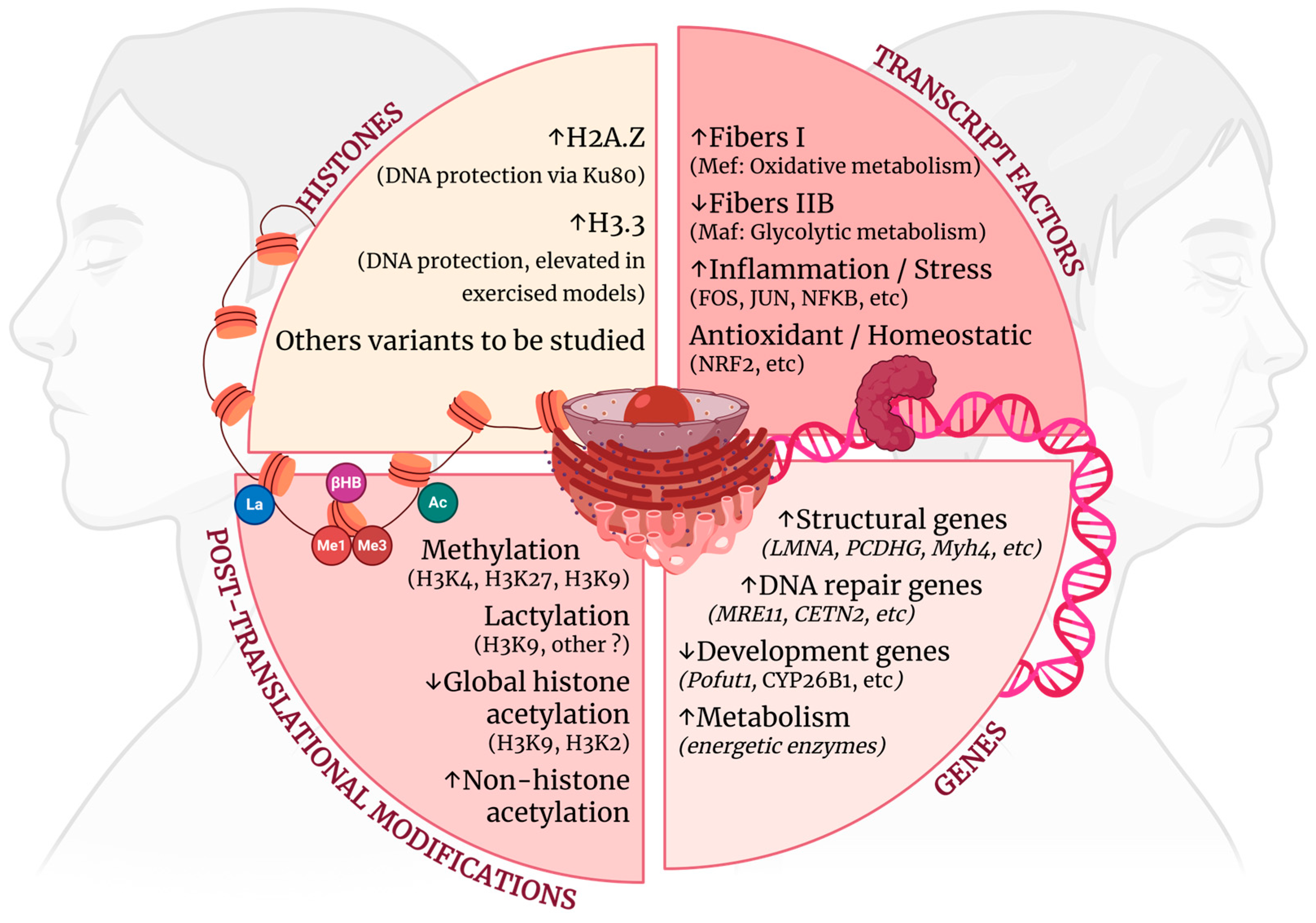
2.1. Genes Associated with Early Muscle Aging
2.2. Transcript Factors Drive Muscle Aging
2.3. Histone Adaptations
2.4. Post-Translational Changes
3. Homeostatic Impairment
3.1. Mitochondrial Adaptations
3.2. Mitochondrial Dynamics
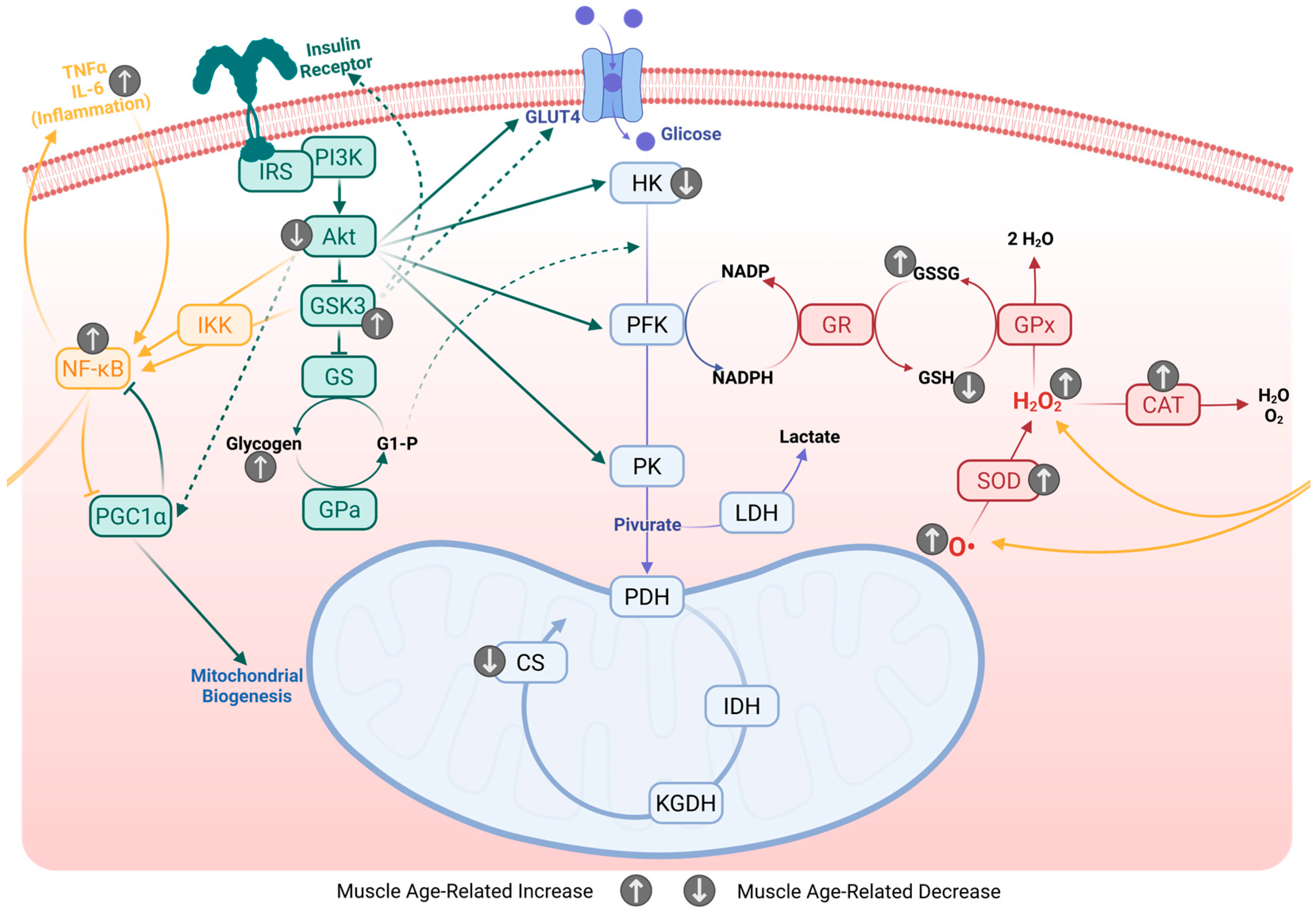
3.3. Insulin Sensitivity and Glucose Intolerance
3.4. Energetic Deviations from Homeostatic Metabolism
3.5. Antioxidant System
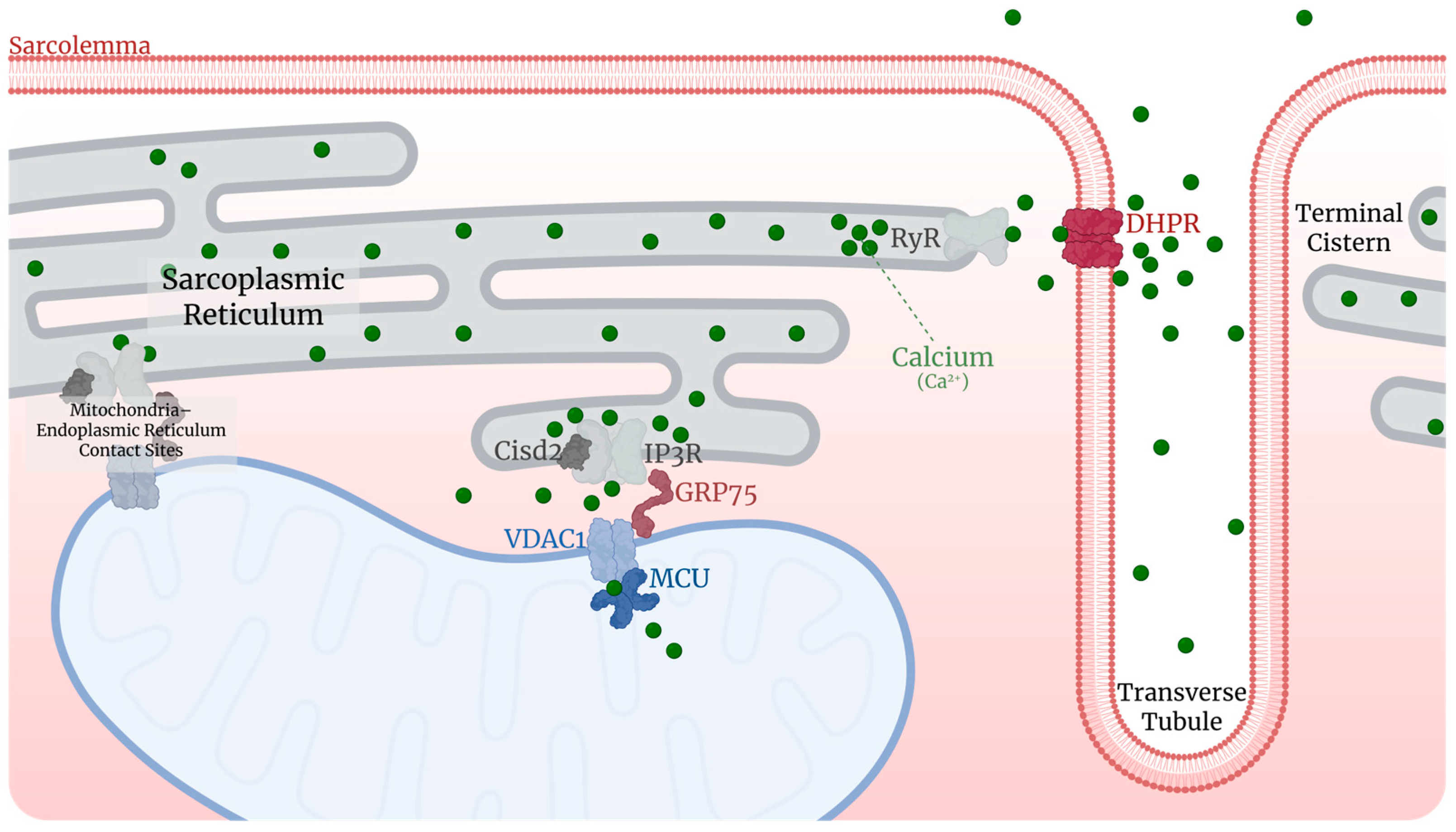
3.6. Ionic Homeostasis
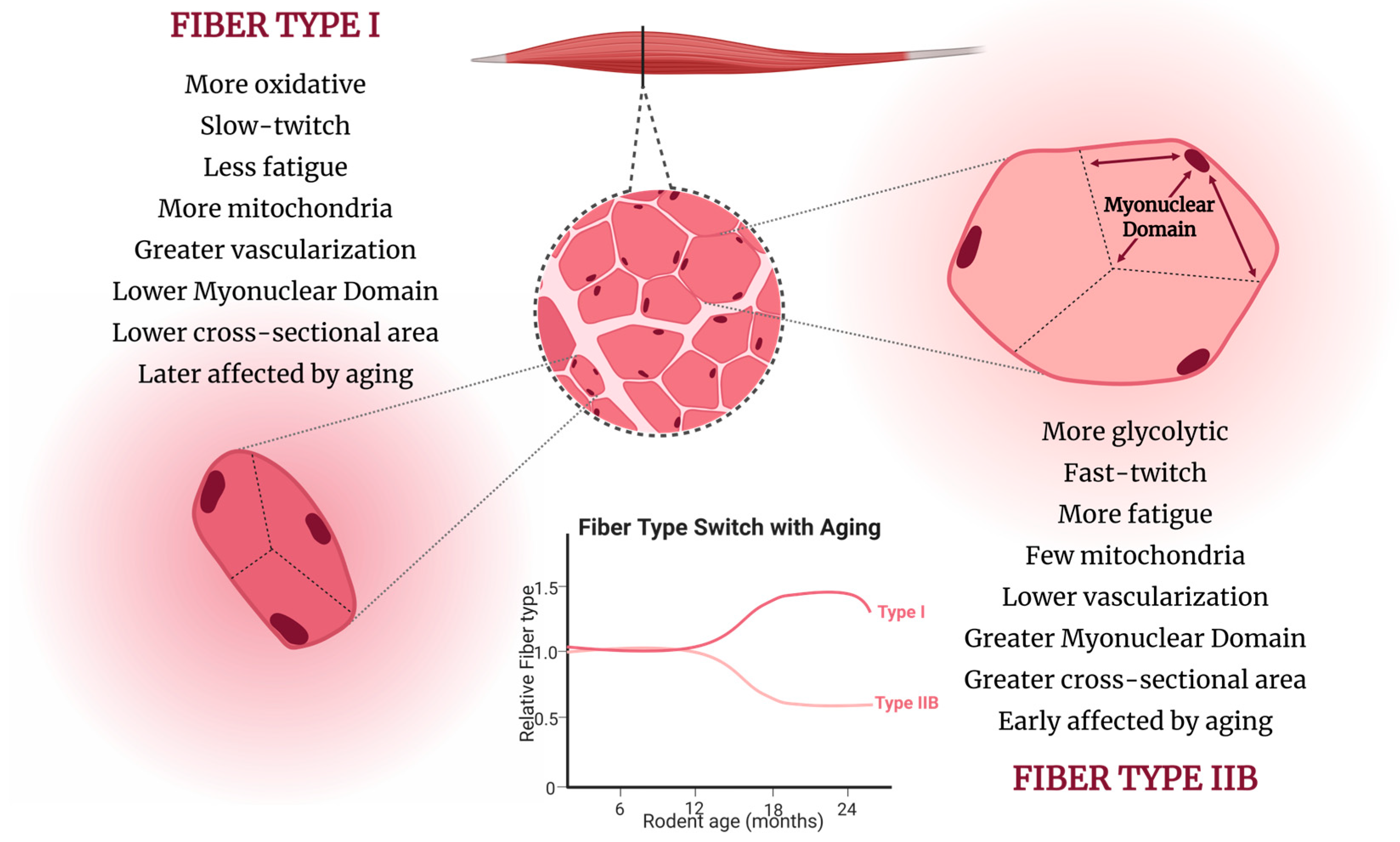
4. Structural Adaptations
4.1. Myonuclear Architecture
4.2. Muscle Vascularization and Oxygen Supply
4.3. Neuromuscular Deterioration

4.4. Immune Aging in Muscle
5. Advanced-Age Skeletal Muscle
6. Search Method Strategy
- (i)
- Original experimental studies reporting molecular, metabolic, or structural changes in skeletal muscle associated with aging;
- (ii)
- Studies focusing on young adults and middle-aged rodents or humans;
- (iii)
- Reviews that provided relevant mechanistic insights.
- (i)
- Studies exclusively comparing young vs. very old/sarcopenic groups without addressing early alterations;
- (ii)
- Studies that did not involve mouse, rat, or human models.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AChR | Acetylcholine receptor |
| AP-1 | Activator protein 1 (FOS/JUN heterodimer transcription factor) |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| ATF4 | Activating transcription factor 4 |
| ATP | Adenosine triphosphate |
| Bmal1 | Brain and muscle ARNT-like 1 |
| CAT | Catalase |
| Ccl2 | C-C motif chemokine ligand 2 |
| CETN2 | Centrin-2 |
| CLOCK | Circadian locomotor output cycles kaput |
| CoQ | Coenzyme Q |
| CPT1B | Carnitine palmitoyltransferase 1B |
| CS | Citrate synthase |
| CuZnSOD | Copper-zinc superoxide dismutase (cytosolic) |
| CYP26B1 | Cytochrome P450 family 26 subfamily B member 1 |
| DHPR | Dihydropyridine receptor (calcium channel) |
| DRP1 | Dynamin-related protein 1 |
| EDL | Extensor digitorum longus |
| EMRE | Essential MCU regulator |
| ENO3 | Enolase 3 |
| ERK1 | Extracellular signal-regulated kinase 1 |
| EZH2 | Enhancer of zeste homolog 2 |
| FABP3 | Fatty-acid-binding protein 3 |
| FDB | Flexor digitorum brevis |
| FIS1 | Mitochondrial fission 1 protein |
| FoxO | Forkhead box O |
| GLUT4 | Glucose transporter type 4 |
| GPx | Glutathione peroxidase |
| GPCPD1 | Glycerophosphocholine phosphodiesterase 1 |
| GR | Glutathione reductase |
| GSH | Glutathione reduced |
| GSK3 | Glycogen synthase kinase 3 |
| GS | Glycogen synthase |
| GSSG | Glutathione oxidized |
| HDAC | Histone deacetylase |
| H2A.Z | Histone variant H2A.Z |
| H3.1 | Histone H3.1 |
| H3.2 | Histone H3.2 |
| H3.3 | Histone H3.3 |
| HFD | High-fat diet |
| HK | Hexokinase |
| HNE | 4-hydroxynonenal |
| ICAM | Intercellular adhesion molecule |
| IDH | Isocitrate dehydrogenase |
| IFNγ | Interferon gamma |
| IGF-1 | Insulin-like growth factor 1 |
| IKK | IκB kinase |
| IL6 | Interleukin 6 |
| IKB | Inhibitor of NF-κB |
| INPPL1 | Inositol polyphosphate phosphatase like 1 (SHIP2) |
| IRS | Insulin receptor substrate |
| JNK | c-Jun N-terminal kinase |
| KGDH | Alpha-ketoglutarate dehydrogenase |
| Ku80 | XRCC5 DNA repair protein (Ku heterodimer subunit) |
| LDH | Lactate dehydrogenase |
| LMNA | Pre-lamin-A/C |
| Lrp4 | Low-density lipoprotein receptor-related protein 4 |
| MAF | Musculoaponeurotic Fibrosarcomatranscription factor family |
| MANF | Mesencephalic astrocyte-derived neurotrophic factor |
| MAST2 | Microtubule associated serine/threonine kinase 2 |
| MCAM | Cell adhesion molecule (mentioned in context of muscle aging) |
| MCU | Mitochondrial calcium uniporter |
| MCUb | Mitochondrial calcium uniporter dominant-negative subunit b |
| MEF2 | Myocyte enhancer factor 2 |
| MERC | Mitochondria–sarcoplasmic reticulum contact coverage |
| Mfn | Mitofusin |
| MHC | Myosin heavy chain |
| MICOS | Mitochondrial contact site and cristae organizing system complex |
| Mll2 | KMT2B—Lysine methyltransferase 2B |
| MND | Myonuclear domain |
| MnSOD | Manganese superoxide dismutase (mitochondrial) |
| mo | Months-old |
| MRE11 | MRE11 homolog, double-strand break repair nuclease |
| MTF2 | Metal response element-binding transcription factor 2 |
| MuSK | Muscle-specific kinase |
| NAD+ | Nicotinamide adenine dinucleotide (oxidized form) |
| NADH | Nicotinamide adenine dinucleotide (reduced form) |
| NF-κB | Nuclear factor kappa B |
| NMJ | Neuromuscular junction |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| Opa1 | Mitochondrial Dynamin Like GTPase; also known as ‘Optic atrophy 1’ |
| PARP-1 | Poly(ADP-ribose) polymerase 1 |
| PCDHG | Protocadherin gamma cluster |
| PDH | Pyruvate dehydrogenase |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| PDK2 | Phosphoinositide-dependent kinase 2 |
| PFKM | Muscular phosphofructokinase |
| PGAM2 | Phosphoglycerate mutase 2 |
| PGK1 | Phosphoglycerate kinase 1 |
| PGM1/2 | Phosphoglucomutase 1 and 2 |
| PI3K | Phosphoinositide 3-kinase |
| PIP3 | Phosphatidylinositol (3,4,5)-trisphosphate |
| PK | Pyruvate kinase |
| PKM1 | Pyruvate kinase muscle isozyme 1 |
| Pofut1 | Protein O-fucosyltransferase 1 |
| ROS | Reactive oxygen species |
| RyR | Ryanodine receptor |
| SDH | Succinate dehydrogenase |
| SIRT | Sirtuin |
| SOD | Superoxide dismutase |
| SR | Sarcoplasmic reticulum |
| TNFα | Tumor necrosis factor alpha |
| VLCAD | Very-long-chain acyl-CoA dehydrogenase |
| WRN | Werner syndrome helicase |
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Scott, A.J.; Ellison, M.; Sinclair, D.A. The Economic Value of Targeting Aging. Nat. Aging 2021, 1, 616–623. [Google Scholar] [CrossRef]
- Börsch, A.; Ham, D.J.; Mittal, N.; Tintignac, L.A.; Migliavacca, E.; Feige, J.N.; Rüegg, M.A.; Zavolan, M. Molecular and Phenotypic Analysis of Rodent Models Reveals Conserved and Species-Specific Modulators of Human Sarcopenia. Commun. Biol. 2021, 4, 194. [Google Scholar] [CrossRef]
- Arnal-Forné, M.; Molina-García, T.; Ortega, M.; Marcos-Garcés, V.; Molina, P.; Ferrández-Izquierdo, A.; Sepulveda, P.; Bodí, V.; Ríos-Navarro, C.; Ruiz-Saurí, A. Changes in Human Skin Composition Due to Intrinsic Aging: A Histologic and Morphometric Study. Histochem. Cell Biol. 2024, 162, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, J.; Du, L.; Ma, Y.; Liu, W.; Ye, R.; Yang, Y.; Xu, H. Analysis of Multi-Part Phenotypic Changes in Skin to Characterize the Trajectory of Skin Aging in Chinese Women. Clin. Cosmet. Investig. Dermatol. 2022, 15, 631–642. [Google Scholar] [CrossRef]
- Pletnikova, O.; Rudow, G.L.; Hyde, T.M.; Kleinman, J.E.; Ali, S.Z.; Bharadwaj, R.; Gangadeen, S.; Crain, B.J.; Fowler, D.R.; Rubio, A.I.; et al. Alzheimer Lesions in the Autopsied Brains of People 30 to 50 Years of Age. Cogn. Behav. Neurol. 2015, 28, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kok, E.; Haikonen, S.; Luoto, T.; Huhtala, H.; Goebeler, S.; Haapasalo, H.; Karhunen, P.J. Apolipoprotein E–Dependent Accumulation of Alzheimer Disease–Related Lesions Begins in Middle Age. Ann. Neurol. 2009, 65, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Li, H.; Hameed, S.; Ting, S.K.S. Clinical Manifestations of Early-Onset Dementia With Lewy Bodies Compared With Late-Onset Dementia With Lewy Bodies and Early-Onset Alzheimer Disease. JAMA Neurol. 2022, 79, 702. [Google Scholar] [CrossRef]
- Fujisawa, C.; Saji, N.; Takeda, A.; Kato, T.; Nakamura, A.; Sakurai, K.; Asanomi, Y.; Ozaki, K.; Takada, K.; Umegaki, H.; et al. Early-Onset Alzheimer Disease Associated With Neuromyelitis Optica Spectrum Disorder. Alzheimer Dis. Assoc. Disord. 2023, 37, 85–87. [Google Scholar] [CrossRef]
- Lachman, M.E. Mind the Gap in the Middle: A Call to Study Midlife. Res. Hum. Dev. 2015, 12, 327–334. [Google Scholar] [CrossRef]
- Infurna, F.J.; Gerstorf, D.; Lachman, M.E. Midlife in the 2020s: Opportunities and Challenges. Am. Psychol. 2020, 75, 470–485. [Google Scholar] [CrossRef]
- Poganik, J.R.; Zhang, B.; Baht, G.S.; Tyshkovskiy, A.; Deik, A.; Kerepesi, C.; Yim, S.H.; Lu, A.T.; Haghani, A.; Gong, T.; et al. Biological Age Is Increased by Stress and Restored upon Recovery. Cell Metab. 2023, 35, 807–820.e5. [Google Scholar] [CrossRef]
- Keshavarz, M.; Xie, K.; Bano, D.; Ehninger, D. Aging—What It Is and How to Measure It. Mech. Ageing Dev. 2023, 213, 111837. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E. Attenuation of Ca2+-Activated ATPase and Shortening Velocity in Hypertrophied Fast Twitch Skeletal Muscle from Aged Japanese Quail. Exp. Gerontol. 2002, 37, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Adelnia, F.; Ubaida-Mohien, C.; Moaddel, R.; Shardell, M.; Lyashkov, A.; Fishbein, K.W.; Aon, M.A.; Spencer, R.G.; Ferrucci, L. Proteomic Signatures of in Vivo Muscle Oxidative Capacity in Healthy Adults. Aging Cell 2020, 19, e13124. [Google Scholar] [CrossRef]
- Ubaida-Mohien, C.; Lyashkov, A.; Gonzalez-Freire, M.; Tharakan, R.; Shardell, M.; Moaddel, R.; Semba, R.D.; Chia, C.W.; Gorospe, M.; Sen, R.; et al. Discovery Proteomics in Aging Human Skeletal Muscle Finds Change in Spliceosome, Immunity, Proteostasis and Mitochondria. eLife 2019, 8, e49874. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Fiebig, R.; Chandwaney, R.; Ji, L.L. Aging and Exercise Training in Skeletal Muscle: Responses of Glutathione and Antioxidant Enzyme Systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 267, R439–R445. [Google Scholar] [CrossRef]
- Lima, M.C.D.A.M.; Zazula, M.F.; Martins, L.F.; Carvalhal, S.R.; Guimarães, A.T.B.; Fernandes, L.C.; Naliwaiko, K. How Soon Do Metabolic Alterations and Oxidative Distress Precede the Reduction of Muscle Mass and Strength in Wistar Rats in Aging Process? Biogerontology 2024, 25, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Kurochkina, N.S.; Orlova, M.A.; Vigovskiy, M.A.; Zgoda, V.G.; Vepkhvadze, T.F.; Vavilov, N.E.; Makhnovskii, P.A.; Grigorieva, O.A.; Boroday, Y.R.; Philippov, V.V.; et al. Age-related Changes in Human Skeletal Muscle Transcriptome and Proteome Are More Affected by Chronic Inflammation and Physical Inactivity than Primary Aging. Aging Cell 2024, 23, e14098. [Google Scholar] [CrossRef]
- Shavlakadze, T.; Xiong, K.; Mishra, S.; McEwen, C.; Gadi, A.; Wakai, M.; Salmon, H.; Stec, M.J.; Negron, N.; Ni, M.; et al. Age-Related Gene Expression Signatures from Limb Skeletal Muscles and the Diaphragm in Mice and Rats Reveal Common and Species-Specific Changes. Skelet. Muscle 2023, 13, 11. [Google Scholar] [CrossRef]
- Worm, C.; Schambye, M.E.R.; Mkrtchyan, G.V.; Veviorskiy, A.; Shneyderman, A.; Ozerov, I.V.; Zhavoronkov, A.; Bakula, D.; Scheibye-Knudsen, M. Defining the Progeria Phenome. Aging 2024, 16, 2026–2046. [Google Scholar] [CrossRef] [PubMed]
- Eynon, N.; Jacques, M.; Seale, K.; Voisin, S.; Lysenko, A.; Grolaux, R.; Jones-Freeman, B.; Lamon, S.; Levinger, I.; Bauer, C.; et al. DNA Methylation Ageing Atlas Across 17 Human Tissues. bioRxiv, 2025; preprint. [Google Scholar] [CrossRef]
- Lai, Y.; Ramírez-Pardo, I.; Isern, J.; An, J.; Perdiguero, E.; Serrano, A.L.; Li, J.; García-Domínguez, E.; Segalés, J.; Guo, P.; et al. Multimodal Cell Atlas of the Ageing Human Skeletal Muscle. Nature 2024, 629, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Hangelbroek, R.W.J.; Fazelzadeh, P.; Tieland, M.; Boekschoten, M.V.; Hooiveld, G.J.E.J.; Van Duynhoven, J.P.M.; Timmons, J.A.; Verdijk, L.B.; De Groot, L.C.P.G.M.; Van Loon, L.J.C.; et al. Expression of Protocadherin Gamma in Skeletal Muscle Tissue Is Associated with Age and Muscle Weakness. J. Cachexia Sarcopenia Muscle 2016, 7, 604–614. [Google Scholar] [CrossRef]
- Vrtačnik, P.; Merino, L.G.; Subhash, S.; Helgadóttir, H.T.; Bardin, M.; Stefani, F.; Wang, D.; Chen, P.; Franco, I.; Revêchon, G.; et al. Induced Somatic Mutation Accumulation during Skeletal Muscle Regeneration Reduces Muscle Strength. Nat. Aging 2025, 5, 1739–1749. [Google Scholar] [CrossRef]
- Borgesius, N.Z.; De Waard, M.C.; Van Der Pluijm, I.; Omrani, A.; Zondag, G.C.M.; Van Der Horst, G.T.J.; Melton, D.W.; Hoeijmakers, J.H.J.; Jaarsma, D.; Elgersma, Y. Accelerated Age-Related Cognitive Decline and Neurodegeneration, Caused by Deficient DNA Repair. J. Neurosci. 2011, 31, 12543–12553. [Google Scholar] [CrossRef]
- Guedj, A.; Geiger-Maor, A.; Benyamini, H.; Nevo, Y.; Elgavish, S.; Galun, E.; Amsalem, H.; Rachmilewitz, J. Early Age Decline in DNA Repair Capacity in the Liver: In Depth Profile of Differential Gene Expression. Aging 2016, 8, 3131–3146. [Google Scholar] [CrossRef]
- Zahn, J.M.; Sonu, R.; Vogel, H.; Crane, E.; Mazan-Mamczarz, K.; Rabkin, R.; Davis, R.W.; Becker, K.G.; Owen, A.B.; Kim, S.K. Transcriptional Profiling of Aging in Human Muscle Reveals a Common Aging Signature. PLoS Genet. 2006, 2, 115. [Google Scholar] [CrossRef]
- Shavlakadze, T.; Morris, M.; Fang, J.; Wang, S.X.; Zhu, J.; Zhou, W.; Tse, H.W.; Mondragon-Gonzalez, R.; Roma, G.; Glass, D.J. Age-Related Gene Expression Signature in Rats Demonstrate Early, Late, and Linear Transcriptional Changes from Multiple Tissues. Cell Rep. 2019, 28, 3263–3273.e3. [Google Scholar] [CrossRef]
- Tumasian, R.A.; Harish, A.; Kundu, G.; Yang, J.-H.; Ubaida-Mohien, C.; Gonzalez-Freire, M.; Kaileh, M.; Zukley, L.M.; Chia, C.W.; Lyashkov, A.; et al. Skeletal Muscle Transcriptome in Healthy Aging. Nat. Commun. 2021, 12, 2014. [Google Scholar] [CrossRef] [PubMed]
- Balagopal, P.; Rooyackers, O.E.; Adey, D.B.; Ades, P.A.; Nair, K.S. Effects of Aging on in Vivo Synthesis of Skeletal Muscle Myosin Heavy-Chain and Sarcoplasmic Protein in Humans. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E790–E800. [Google Scholar] [CrossRef]
- Yoshihara, T.; Machida, S.; Tsuzuki, T.; Kakigi, R.; Chang, S.; Sugiura, T.; Naito, H. Age-Related Changes in Histone Modification in Rat Gastrocnemius Muscle. Exp. Gerontol. 2019, 125, 110658. [Google Scholar] [CrossRef]
- Zygmunt, D.A.; Singhal, N.; Kim, M.-L.; Cramer, M.L.; Crowe, K.E.; Xu, R.; Jia, Y.; Adair, J.; Martinez-Pena, Y.; Valenzuela, I.; et al. Deletion of Pofut1 in Mouse Skeletal Myofibers Induces Muscle Aging-Related Phenotypes in Cis and in Trans. Mol. Cell. Biol. 2017, 37, e00426-16. [Google Scholar] [CrossRef]
- Al Jaam, B.; Heu, K.; Pennarubia, F.; Segelle, A.; Magnol, L.; Germot, A.; Legardinier, S.; Blanquet, V.; Maftah, A. Reduced Notch Signalling Leads to Postnatal Skeletal Muscle Hypertrophy in Pofut1cax/cax Mice. Open Biol. 2016, 6, 160211. [Google Scholar] [CrossRef]
- He, S.; Luo, Y.; Ma, W.; Wang, X.; Yan, C.; Hao, W.; Fang, Y.; Su, H.; Lai, B.; Liu, J.; et al. Endothelial POFUT1 Controls Injury-Induced Liver Fibrosis by Repressing Fibrinogen Synthesis. J. Hepatol. 2024, 81, 135–148. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, B.; Lu, P.; Zhang, D.; Wu, B.; Varshney, S.; Del Monte-Nieto, G.; Zhuang, Z.; Charafeddine, R.; Kramer, A.H.; et al. Uncontrolled Angiogenic Precursor Expansion Causes Coronary Artery Anomalies in Mice Lacking Pofut1. Nat. Commun. 2017, 8, 578. [Google Scholar] [CrossRef]
- Waldera Lupa, D.M.; Kalfalah, F.; Safferling, K.; Boukamp, P.; Poschmann, G.; Volpi, E.; Götz-Rösch, C.; Bernerd, F.; Haag, L.; Huebenthal, U.; et al. Characterization of Skin Aging-Associated Secreted Proteins (SAASP) Produced by Dermal Fibroblasts Isolated from Intrinsically Aged Human Skin. J. Investig. Dermatol. 2015, 135, 1954–1968. [Google Scholar] [CrossRef]
- Zhang, N.; Long, L.; Li, G.; Wu, X.; Peng, S.; Jiang, Y.; Xiang, A.; Mao, X.; Huang, H.; Yang, Z. Preliminary Study on the Mechanism of POFUT1 in Colorectal Cancer. Med. Oncol. 2023, 40, 235. [Google Scholar] [CrossRef] [PubMed]
- Sadaki, S.; Tsuji, R.; Hayashi, T.; Watanabe, M.; Iwai, R.; Wenchao, G.; Semenova, E.A.; Sultanov, R.I.; Zhelankin, A.V.; Generozov, E.V.; et al. Large MAF Transcription Factors Reawaken Evolutionarily Dormant Fast-Glycolytic Type IIb Myofibers in Human Skeletal Muscle. Skelet. Muscle 2025, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Chen, Y.; Han, X.; Fu, Y.; Chen, J.; Tan, D.; Shan, X.; Jiang, H. Transcription Factor MAFA Regulates Muscle Growth via Calcium Ion Channels and Receptor Tyrosine Kinase Activation. Int. J. Biol. Macromol. 2025, 321, 146518. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.; Bezprozvannaya, S.; McAnally, J.R.; Cai, C.; Liu, N.; Olson, E.N. A Mechanistic Basis of Fast Myofiber Vulnerability to Neuromuscular Diseases. Cell Rep. 2025, 44, 115959. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ding, C.; Fu, T.; Feng, Z.; Lee, J.-E.; Xiao, L.; Xu, Z.; Yin, Y.; Guo, Q.; Sun, Z.; et al. Histone Methyltransferase MLL4 Controls Myofiber Identity and Muscle Performance through MEF2 Interaction. J. Clin. Investig. 2020, 130, 4710–4725. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Nelson, B.R.; Bezprozvannaya, S.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Requirement of MEF2A, C, and D for Skeletal Muscle Regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 4109–4114. [Google Scholar] [CrossRef]
- Piasecka, A.; Sekrecki, M.; Szcześniak, M.W.; Sobczak, K. MEF2C Shapes the Microtranscriptome during Differentiation of Skeletal Muscles. Sci. Rep. 2021, 11, 3476. [Google Scholar] [CrossRef]
- Massenet, J.; Weiss-Gayet, M.; Bandukwala, H.; Bouchereau, W.; Gobert, S.; Magnan, M.; Hubas, A.; Nusbaum, P.; Desguerre, I.; Gitiaux, C.; et al. Epigenetic Control of Myogenic Identity of Human Muscle Stem Cells in Duchenne Muscular Dystrophy. iScience 2024, 27, 111350. [Google Scholar] [CrossRef]
- Almada, A.E.; Horwitz, N.; Price, F.D.; Gonzalez, A.E.; Ko, M.; Bolukbasi, O.V.; Messemer, K.A.; Chen, S.; Sinha, M.; Rubin, L.L.; et al. FOS Licenses Early Events in Stem Cell Activation Driving Skeletal Muscle Regeneration. Cell Rep. 2021, 34, 108656. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Zhou, Q.; Liu, X.; Qiao, Y.; Xie, T.; Sun, H.; Ong, M.T.-Y.; Wang, H. Multiomics and Cellular Senescence Profiling of Aging Human Skeletal Muscle Uncovers Maraviroc as a Senotherapeutic Approach for Sarcopenia. Nat. Commun. 2025, 16, 6207. [Google Scholar] [CrossRef]
- Oh, J.; Sinha, I.; Tan, K.Y.; Rosner, B.; Dreyfuss, J.M.; Gjata, O.; Tran, P.; Shoelson, S.E.; Wagers, A.J. Age-Associated NF-κB Signaling in Myofibers Alters the Satellite Cell Niche and Re-Strains Muscle Stem Cell Function. Aging 2016, 8, 2871–2896. [Google Scholar] [CrossRef]
- Buford, T.W.; Cooke, M.B.; Manini, T.M.; Leeuwenburgh, C.; Willoughby, D.S. Effects of Age and Sedentary Lifestyle on Skeletal Muscle NF- B Signaling in Men. J. Gerontol. A. Biol. Sci. Med. Sci. 2010, 65A, 532–537. [Google Scholar] [CrossRef]
- Fu, P.; Gong, L.; Yang, L.; Tang, S.; Ma, F. Weight Bearing Training Alleviates Muscle Atrophy and Pyroptosis of Middle-Aged Rats. Front. Endocrinol. 2023, 14, 1202686. [Google Scholar] [CrossRef]
- Pryce, B.R.; Oles, A.; Talbert, E.E.; Romeo, M.J.; Vaena, S.; Sharma, S.; Spadafora, V.; Tolliver, L.; Mahvi, D.A.; Morgan, K.A.; et al. Muscle Inflammation Is Regulated by NF-κB from Multiple Cells to Control Distinct States of Wasting in Cancer Cachexia. Cell Rep. 2024, 43, 114925. [Google Scholar] [CrossRef]
- Benjamin, D.I.; Brett, J.O.; Both, P.; Benjamin, J.S.; Ishak, H.L.; Kang, J.; Kim, S.; Chung, M.; Arjona, M.; Nutter, C.W.; et al. Multiomics Reveals Glutathione Metabolism as a Driver of Bimodality during Stem Cell Aging. Cell Metab. 2023, 35, 472–486.e6. [Google Scholar] [CrossRef]
- Huang, D.-D.; Fan, S.-D.; Chen, X.-Y.; Yan, X.-L.; Zhang, X.-Z.; Ma, B.-W.; Yu, D.-Y.; Xiao, W.-Y.; Zhuang, C.-L.; Yu, Z. Nrf2 Deficiency Exacerbates Frailty and Sarcopenia by Impairing Skeletal Muscle Mitochondrial Biogenesis and Dynamics in an Age-Dependent Manner. Exp. Gerontol. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Miller, M.J.; Marcotte, G.R.; Basisty, N.; Wehrfritz, C.; Ryan, Z.C.; Strub, M.D.; McKeen, A.T.; Stern, J.I.; Nath, K.A.; Rasmussen, B.B.; et al. The Transcription Regulator ATF4 Is a Mediator of Skeletal Muscle Aging. GeroScience 2023, 45, 2525–2543. [Google Scholar] [CrossRef]
- Penniman, C.M.; Bhardwaj, G.; Nowers, C.J.; Brown, C.U.; Junck, T.L.; Boyer, C.K.; Jena, J.; Fuqua, J.D.; Lira, V.A.; O’Neill, B.T. Loss of FoxOs in Muscle Increases Strength and Mitochondrial Function during Aging. J. Cachexia Sarcopenia Muscle 2023, 14, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, C.; Yang, N.; Desdín-Micó, G.; Alonso-Calleja, A.; Vílchez-Acosta, A.; Pico, S.; Parras, A.; Piao, Y.; Schoenfeldt, L.; Luo, S.; et al. Loss of H3K9 Trimethylation Leads to Premature Aging. bioRxiv, 2024; preprint. [Google Scholar] [CrossRef] [PubMed]
- Belotti, E.; Lacoste, N.; Iftikhar, A.; Simonet, T.; Papin, C.; Osseni, A.; Streichenberger, N.; Mari, P.-O.; Girard, E.; Graies, M.; et al. H2A.Z Is Involved in Premature Aging and DSB Repair Initiation in Muscle Fibers. Nucleic Acids Res. 2024, 52, 3031–3049. [Google Scholar] [CrossRef]
- Meng, F.; He, J.; Zhang, X.; Lyu, W.; Wei, R.; Wang, S.; Du, Z.; Wang, H.; Bi, J.; Hua, X.; et al. Histone Lactylation Antagonizes Senescence and Skeletal Muscle Aging by Modulating Aging-Related Pathways. Adv. Sci. 2025, 12, 2412747. [Google Scholar] [CrossRef]
- Creighton, S.D.; Stefanelli, G.; Brimble, M.A.; Hategan, L.; Collins, E.; Zakaria, J.; Vislavski, S.; Reda, A.; McLean, T.A.; Walters, B.J.; et al. Sex-specific Accumulation and Therapeutic Effect of the Histone Variant H2A.Z in Alzheimer’s Disease. Alzheimers Dement. 2023, 19, e064832. [Google Scholar] [CrossRef]
- Luo, J.Q.; Hategan, L.A.; Creighton, S.; Hua, S.; McLean, T.A.B.; Dahi, T.A.; Jin, Z.; Pirri, F.; Winston, S.M.; Reeves, I.L.; et al. Opposing Effects of Histone H2A.Z on Memory, Transcription and Pathology in Male and Female Alzheimer’s Disease Mice and Patients. bioRxiv, 2025; preprint. [Google Scholar] [CrossRef]
- Didier, N.; Hourdé, C.; Amthor, H.; Marazzi, G.; Sassoon, D. Loss of a Single Allele for Ku80 Leads to Progenitor Dysfunction and Accelerated Aging in Skeletal Muscle. EMBO Mol. Med. 2012, 4, 910–923. [Google Scholar] [CrossRef]
- Masuzawa, R.; Rosa Flete, H.K.; Shimizu, J.; Kawano, F. Age-Related Histone H3.3 Accumulation Associates with a Repressive Chromatin in Mouse Tibialis Anterior Muscle. J. Physiol. Sci. 2024, 74, 41. [Google Scholar] [CrossRef]
- Bouchereau, W.; Chenane, L.; Lessard, L.; Weiss-gayet, M.; Cardona, Y.; Mounier, R.; Gallay, L.; Allenbach, Y.; Benvéniste, O.; Corpet, A.; et al. Defective Plasticity in Dermatomyositis Patients Muscle Stem Cells Is Associated with Sustained Intrinsic Inflammatory Signaling and Disruption of the Histone H3.3 Chromatin Loading Pathway. bioRxiv, 2025; preprint. [Google Scholar] [CrossRef]
- Posavec Marjanović, M.; Hurtado-Bagès, S.; Lassi, M.; Valero, V.; Malinverni, R.; Delage, H.; Navarro, M.; Corujo, D.; Guberovic, I.; Douet, J.; et al. MacroH2A1.1 Regulates Mitochondrial Respiration by Limiting Nuclear NAD+ Consumption. Nat. Struct. Mol. Biol. 2017, 24, 902–910. [Google Scholar] [CrossRef]
- Pazienza, V.; Panebianco, C.; Rappa, F.; Memoli, D.; Borghesan, M.; Cannito, S.; Oji, A.; Mazza, G.; Tamburrino, D.; Fusai, G.; et al. Histone macroH2A1.2 Promotes Metabolic Health and Leanness by Inhibiting Adipogenesis. Epigenetics Chromatin 2016, 9, 45. [Google Scholar] [CrossRef]
- Olecka, M.; Van Bömmel, A.; Best, L.; Haase, M.; Foerste, S.; Riege, K.; Dost, T.; Flor, S.; Witte, O.W.; Franzenburg, S.; et al. Nonlinear DNA Methylation Trajectories in Aging Male Mice. Nat. Commun. 2024, 15, 3074. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, T.; Saito, C.; Kawano, F. Early High-Fat Feeding Improves Histone Modifications of Skeletal Muscle at Middle-Age in Mice. Lab. Anim. Res. 2020, 36, 25. [Google Scholar] [CrossRef] [PubMed]
- De Lima Camillo, L.P.; Asif, M.H.; Horvath, S.; Larschan, E.; Singh, R. Histone Mark Age of Human Tissues and Cell Types. Sci. Adv. 2025, 11, eadk9373. [Google Scholar] [CrossRef] [PubMed]
- Goldsworthy, M.; Absalom, N.L.; Schröter, D.; Matthews, H.C.; Bogani, D.; Moir, L.; Long, A.; Church, C.; Hugill, A.; Anstee, Q.M.; et al. Mutations in Mll2, an H3K4 Methyltransferase, Result in Insulin Resistance and Impaired Glucose Tolerance in Mice. PLoS ONE 2013, 8, e61870. [Google Scholar] [CrossRef]
- Oyabu, M.; Ohira, Y.; Fujita, M.; Yoshioka, K.; Kawaguchi, R.; Kubo, A.; Hatazawa, Y.; Yukitoshi, H.; Ortuste Quiroga, H.P.; Horii, N.; et al. Dnmt3a Overexpression Disrupts Skeletal Muscle Homeostasis, Promotes an Aging-like Phenotype, and Reduces Metabolic Elasticity. iScience 2025, 28, 112144. [Google Scholar] [CrossRef]
- Bano, S.; More, S.; Mongad, D.S.; Khalique, A.; Dhotre, D.P.; Bhat, M.K.; Seshadri, V. Prolonged Exposure to Insulin Might Cause Epigenetic Alteration Leading to Insulin Resistance. FEBS Open Bio 2025, 15, 81–93. [Google Scholar] [CrossRef]
- Shimizu, J.; Kawano, F. DNA Hypermethylation Preceded by H3K27 Trimethylation Is Linked to Downregulation of Gene Expression in Disuse Muscle Atrophy in Male Mice. Physiol. Rep. 2025, 13, e70317. [Google Scholar] [CrossRef] [PubMed]
- Moqri, M.; Cipriano, A.; Simpson, D.J.; Rasouli, S.; Murty, T.; De Jong, T.A.; Nachun, D.; De Sena Brandine, G.; Ying, K.; Tarkhov, A.; et al. PRC2-AgeIndex as a Universal Biomarker of Aging and Rejuvenation. Nat. Commun. 2024, 15, 5956. [Google Scholar] [CrossRef] [PubMed]
- Perino, M.; Van Mierlo, G.; Karemaker, I.D.; Van Genesen, S.; Vermeulen, M.; Marks, H.; Van Heeringen, S.J.; Veenstra, G.J.C. MTF2 Recruits Polycomb Repressive Complex 2 by Helical-Shape-Selective DNA Binding. Nat. Genet. 2018, 50, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Voisin, S.; Jacques, M.; Landen, S.; Harvey, N.R.; Haupt, L.M.; Griffiths, L.R.; Gancheva, S.; Ouni, M.; Jähnert, M.; Ashton, K.J.; et al. Meta-analysis of Genome-wide DNA Methylation and Integrative Omics of Age in Human Skeletal Muscle. J. Cachexia Sarcopenia Muscle 2021, 12, 1064–1078. [Google Scholar] [CrossRef]
- Montavon, T.; Shukeir, N.; Erikson, G.; Engist, B.; Onishi-Seebacher, M.; Ryan, D.; Musa, Y.; Mittler, G.; Meyer, A.G.; Genoud, C.; et al. Complete Loss of H3K9 Methylation Dissolves Mouse Heterochromatin Organization. Nat. Commun. 2021, 12, 4359. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Yeo, D.; Kang, C.; Ji, L.L. Aging Alters Acetylation Status in Skeletal and Cardiac Muscles. GeroScience 2020, 42, 963–976. [Google Scholar] [CrossRef]
- Frederick, D.W.; Loro, E.; Liu, L.; Davila, A.; Chellappa, K.; Silverman, I.M.; Quinn, W.J.; Gosai, S.J.; Tichy, E.D.; Davis, J.G.; et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab. 2016, 24, 269–282. [Google Scholar] [CrossRef]
- Hosoda, R.; Nakashima, R.; Yano, M.; Iwahara, N.; Asakura, S.; Nojima, I.; Saga, Y.; Kunimoto, R.; Horio, Y.; Kuno, A. Resveratrol, a SIRT1 Activator, Attenuates Aging-Associated Alterations in Skeletal Muscle and Heart in Mice. J. Pharmacol. Sci. 2023, 152, 112–122. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The Histone Deacetylase Inhibitor Butyrate Improves Metabolism and Reduces Muscle Atrophy during Aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wu, G.; Liu, K.; Chen, Q.; Tao, J.; Liu, H.; Shen, M. Lactate Promotes Myogenesis via Activating H3K9 Lactylation-dependent Up-regulation of Neu2 Expression. J. Cachexia Sarcopenia Muscle 2023, 14, 2851–2865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lan, X.; Ke, H.; Xu, S.; Huang, C.; Wang, J.; Wang, X.; Huang, T.; Wu, X.; Chen, M.; et al. Histone Β-hydroxybutyrylation Is Critical in Reversal of Sarcopenia. Aging Cell 2024, 23, e14284. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, S.; Wei, X.; Liu, K.; Peng, Y.; Yu, M.; Chen, J.; Zhu, J.; Huang, K.; Pan, S. β-Hydroxybutyrate Inhibits FOXO3a by Histone H3K9 β-Hydroxybutyrylation to Ameliorate Stroke-Related Sarcopenia. J. Funct. Foods 2024, 120, 106365. [Google Scholar] [CrossRef]
- Tian, Q.; Moore, A.Z.; Oppong, R.; Ding, J.; Zampino, M.; Fishbein, K.W.; Spencer, R.G.; Ferrucci, L. Mitochondrial DNA Copy Number and Heteroplasmy Load Correlate with Skeletal Muscle Oxidative Capacity by P31 MR Spectroscopy. Aging Cell 2021, 20, e13487. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A.; Contreras-Hernández, I.; Castro-Sepúlveda, M.; Campos, C.A.; Figueroa, R.; Tevy, M.F.; Eisner, V.; Casas, M.; Jaimovich, E. Muscle Function Decline and Mitochondria Changes in Middle Age Precede Sarcopenia in Mice. Aging 2018, 10, 34–55. [Google Scholar] [CrossRef]
- Moore, T.M.; Zhou, Z.; Strumwasser, A.R.; Cohn, W.; Lin, A.J.; Cory, K.; Whitney, K.; Ho, T.; Ho, T.; Lee, J.L.; et al. Age-induced Mitochondrial DNA Point Mutations Are Inadequate to Alter Metabolic Homeostasis in Response to Nutrient Challenge. Aging Cell 2020, 19, e13166. [Google Scholar] [CrossRef]
- Holmes, M.; Koutakis, P.; Ismaeel, A. Aging Alters Gastrocnemius Muscle Hemoglobin Oxygen Saturation (StO2) Characteristics in Healthy Individuals. Eur. J. Appl. Physiol. 2022, 122, 1509–1520. [Google Scholar] [CrossRef]
- Landers-Ramos, R.Q.; Merriman, K.; Chartier, G.L.; Parks, J.; Park, H.; Knuth, N.D. Comparison of Skeletal Muscle Oxidative Function in Young, Middle-Aged, and Older Males and Females with Similar Physical Activity Levels. J. Appl. Physiol. 2024, 136, 618–629. [Google Scholar] [CrossRef]
- Sergi, D.; Angelini, S.; Spaggiari, R.; Castaldo, F.; Zuliani, G.; Sanz, J.M.; Passaro, A.; The PANGEA Study Group; Dalla Nora, E.; Brombo, G.; et al. Advanced Glycation End-Product Intake Predicts Insulin Resistance in a Sex-Dependent Fashion. Eur. J. Nutr. 2025, 64, 162. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Nuevo-Tapioles, C.; Herrero Martín, J.C.; Pereira, M.P.; Serrano Sanz, S.; Ramírez De Molina, A.; Cuezva, J.M.; Formentini, L. Dysfunctional Oxidative Phosphorylation Shunts Branched-chain Amino Acid Catabolism onto Lipogenesis in Skeletal Muscle. EMBO J. 2020, 39, e103812. [Google Scholar] [CrossRef]
- Granata, C.; Caruana, N.J.; Botella, J.; Jamnick, N.A.; Huynh, K.; Kuang, J.; Janssen, H.A.; Reljic, B.; Mellett, N.A.; Laskowski, A.; et al. High-Intensity Training Induces Non-Stoichiometric Changes in the Mitochondrial Proteome of Human Skeletal Muscle without Reorganisation of Respiratory Chain Content. Nat. Commun. 2021, 12, 7056. [Google Scholar] [CrossRef]
- Zhang, T.-R.; Chiang, C.-H.; Hsu, T.-C.; Wang, C.-Y.; Chen, C.-Y. Age and Dietary Restriction Modulate Mitochondrial Quality in Quadriceps Femoris Muscle of Male Mice. Biogerontology 2024, 25, 447–459. [Google Scholar] [CrossRef]
- Torii, K.; Sugiyama, S.; Takagi, K.; Satake, T.; Ozawa, T. Age-Related Decrease in Respiratory Muscle Mitochondrial Function in Rats. Am. J. Respir. Cell Mol. Biol. 1992, 6, 88–92. [Google Scholar] [CrossRef]
- Koonen, D.P.Y.; Sung, M.M.Y.; Kao, C.K.C.; Dolinsky, V.W.; Koves, T.R.; Ilkayeva, O.; Jacobs, R.L.; Vance, D.E.; Light, P.E.; Muoio, D.M.; et al. Alterations in Skeletal Muscle Fatty Acid Handling Predisposes Middle-Aged Mice to Diet-Induced Insulin Resistance. Diabetes 2010, 59, 1366–1375. [Google Scholar] [CrossRef]
- Kadoguchi, T.; Shimada, K.; Miyazaki, T.; Kitamura, K.; Kunimoto, M.; Aikawa, T.; Sugita, Y.; Ouchi, S.; Shiozawa, T.; Yokoyama-Nishitani, M.; et al. Promotion of Oxidative Stress Is Associated with Mitochondrial Dysfunction and Muscle Atrophy in Aging Mice. Geriatr. Gerontol. Int. 2020, 20, 78–84. [Google Scholar] [CrossRef]
- Gutiérrez-Casado, E.; Khraiwesh, H.; López-Domínguez, J.A.; Montero-Guisado, J.; López-Lluch, G.; Navas, P.; De Cabo, R.; Ramsey, J.J.; González-Reyes, J.A.; Villalba, J.M. The Impact of Aging, Calorie Restriction and Dietary Fat on Autophagy Markers and Mitochondrial Ultrastructure and Dynamics in Mouse Skeletal Muscle. J. Gerontol. Ser. A 2019, 74, 760–769. [Google Scholar] [CrossRef]
- Tsentsevitsky, A.N.; Sibgatullina, G.V.; Odoshivkina, Y.G.; Khuzakhmetova, V.F.; Tokmakova, A.R.; Ponomareva, A.A.; Salnikov, V.V.; Zakirjanova, G.F.; Petrov, A.M.; Bukharaeva, E.A. Functional and Structural Changes in Diaphragm Neuromuscular Junctions in Early Aging. Int. J. Mol. Sci. 2024, 25, 8959. [Google Scholar] [CrossRef] [PubMed]
- Vue, Z.; Garza-Lopez, E.; Neikirk, K.; Katti, P.; Vang, L.; Beasley, H.; Shao, J.; Marshall, A.G.; Crabtree, A.; Murphy, A.C.; et al. 3D Reconstruction of Murine Mitochondria Exhibits Changes in Structure Across Aging Linked to the MICOS Complex. Aging Cell 2023, 22, e14009. [Google Scholar] [CrossRef] [PubMed]
- Goulding, R.P.; Charlton, B.T.; Breedveld, E.A.; Van Der Laan, M.; Strating, A.R.; Noort, W.; Kolodyazhna, A.; Appelman, B.; Van Vugt, M.; Grootemaat, A.E.; et al. Skeletal Muscle Mitochondrial Fragmentation Predicts Age-associated Decline in Physical Capacity. Aging Cell 2025, 24, e14386. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gong, Y.; Hu, W.; Mao, Y.; Wang, T.; Sun, Z.; Su, X.; Fu, G.; Wang, Y.; Lai, D. Ultrastructural and Proteomic Profiling of Mitochondria-Associated Endoplasmic Reticulum Membranes Reveal Aging Signatures in Striated Muscle. Cell Death Dis. 2022, 13, 296. [Google Scholar] [CrossRef]
- Sayed, R.K.A.; De Leonardis, E.C.; Guerrero-Martínez, J.A.; Rahim, I.; Mokhtar, D.M.; Saleh, A.M.; Abdalla, K.E.H.; Pozo, M.J.; Escames, G.; López, L.C.; et al. Identification of Morphological Markers of Sarcopenia at Early Stage of Aging in Skeletal Muscle of Mice. Exp. Gerontol. 2016, 83, 22–30. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.-P.; Picard, M.; Pelletier, F.S.-J.; Sgarioto, N.; Auger, M.-J.; Vallée, J.; Robitaille, R.; St-Pierre, D.H.; Gouspillou, G. Mitochondrial Morphology Is Altered in Atrophied Skeletal Muscle of Aged Mice. Oncotarget 2015, 6, 17923–17937. [Google Scholar] [CrossRef]
- Brandt, T.; Mourier, A.; Tain, L.S.; Partridge, L.; Larsson, N.-G.; Kühlbrandt, W. Changes of Mitochondrial Ultrastructure and Function during Ageing in Mice and Drosophila. eLife 2017, 6, e24662. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Cell Senescence, Rapamycin and Hyperfunction Theory of Aging. Cell Cycle 2022, 21, 1456–1467. [Google Scholar] [CrossRef]
- Petersen, K.F.; Morino, K.; Alves, T.C.; Kibbey, R.G.; Dufour, S.; Sono, S.; Yoo, P.S.; Cline, G.W.; Shulman, G.I. Effect of Aging on Muscle Mitochondrial Substrate Utilization in Humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11330–11334. [Google Scholar] [CrossRef] [PubMed]
- Tábara, L.-C.; Segawa, M.; Prudent, J. Molecular Mechanisms of Mitochondrial Dynamics. Nat. Rev. Mol. Cell Biol. 2025, 26, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, D.; Sorianello, E.; Segalés, J.; Irazoki, A.; Ruiz-Bonilla, V.; Sala, D.; Planet, E.; Berenguer-Llergo, A.; Muñoz, J.P.; Sánchez-Feutrie, M.; et al. Mfn2 Deficiency Links Age-related Sarcopenia and Impaired Autophagy to Activation of an Adaptive Mitophagy Pathway. EMBO J. 2016, 35, 1677–1693. [Google Scholar] [CrossRef]
- Scudese, E.; Marshall, A.G.; Vue, Z.; Exil, V.; Rodriguez, B.I.; Demirci, M.; Vang, L.; López, E.G.; Neikirk, K.; Shao, B.; et al. 3 D Mitochondrial Structure in Aging Human Skeletal Muscle: Insights Into MFN -2-Mediated Changes. Aging Cell 2025, 24, e70054. [Google Scholar] [CrossRef]
- Chen, H.; Chomyn, A.; Chan, D.C. Disruption of Fusion Results in Mitochondrial Heterogeneity and Dysfunction. J. Biol. Chem. 2005, 280, 26185–26192. [Google Scholar] [CrossRef]
- Baker, N.; Wade, S.; Triolo, M.; Girgis, J.; Chwastek, D.; Larrigan, S.; Feige, P.; Fujita, R.; Crist, C.; Rudnicki, M.A.; et al. The Mitochondrial Protein OPA1 Regulates the Quiescent State of Adult Muscle Stem Cells. Cell Stem Cell 2022, 29, 1315–1332.e9. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017, 25, 1374–1389.e6. [Google Scholar] [CrossRef] [PubMed]
- Jheng, H.-F.; Tsai, P.-J.; Guo, S.-M.; Kuo, L.-H.; Chang, C.-S.; Su, I.-J.; Chang, C.-R.; Tsai, Y.-S. Mitochondrial Fission Contributes to Mitochondrial Dysfunction and Insulin Resistance in Skeletal Muscle. Mol. Cell. Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Fix, D.K.; VanderVeen, B.N.; Counts, B.R.; Carson, J.A. Regulation of Skeletal Muscle DRP-1 and FIS-1 Protein Expression by IL-6 Signaling. Oxid. Med. Cell. Longev. 2019, 2019, 8908457. [Google Scholar] [CrossRef] [PubMed]
- Vue, Z.; Neikirk, K.; Vang, L.; Garza-Lopez, E.; Christensen, T.A.; Shao, J.; Lam, J.; Beasley, H.K.; Marshall, A.G.; Crabtree, A.; et al. Three-Dimensional Mitochondria Reconstructions of Murine Cardiac Muscle Changes in Size across Aging. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H965–H982. [Google Scholar] [CrossRef]
- Dall’Agnese, A.; Platt, J.M.; Zheng, M.M.; Friesen, M.; Dall’Agnese, G.; Blaise, A.M.; Spinelli, J.B.; Henninger, J.E.; Tevonian, E.N.; Hannett, N.M.; et al. The Dynamic Clustering of Insulin Receptor Underlies Its Signaling and Is Disrupted in Insulin Resistance. Nat. Commun. 2022, 13, 7522. [Google Scholar] [CrossRef]
- Arias, E.B.; Gosselin, L.E.; Cartee, G.D. Exercise Training Eliminates Age-Related Differences in Skeletal Muscle Insulin Receptor and IRS-1 Abundance in Rats. J. Gerontol. A. Biol. Sci. Med. Sci. 2001, 56, B449–B455. [Google Scholar] [CrossRef]
- Martineau, L.C.; Chadan, S.G.; Parkhouse, W.S. Age-Associated Alterations in Cardiac and Skeletal Muscle Glucose Transporters, Insulin and IGF-1 Receptors, and PI3-Kinase Protein Contents in the C57BL/6 Mouse. Mech. Ageing Dev. 1999, 106, 217–232. [Google Scholar] [CrossRef]
- Toyoshima, Y.; Nakamura, K.; Taguchi, Y.; Tokita, R.; Takeuchi, S.; Osawa, H.; Teramoto, N.; Sugihara, H.; Yoshizawa, F.; Yamanouchi, K.; et al. Deletion of IRS-1 Leads to Growth Failure and Insulin Resistance with Downregulation of Liver and Muscle Insulin Signaling in Rats. Sci. Rep. 2025, 15, 649. [Google Scholar] [CrossRef]
- Pasini, E.; Le Douairon Lahaye, S.; Flati, V.; Assanelli, D.; Corsetti, G.; Speca, S.; Bernabei, R.; Calvani, R.; Marzetti, E. Effects of Treadmill Exercise and Training Frequency on Anabolic Signaling Pathways in the Skeletal Muscle of Aged Rats. Exp. Gerontol. 2012, 47, 23–28. [Google Scholar] [CrossRef]
- Sasako, T.; Umehara, T.; Soeda, K.; Kaneko, K.; Suzuki, M.; Kobayashi, N.; Okazaki, Y.; Tamura-Nakano, M.; Chiba, T.; Accili, D.; et al. Deletion of Skeletal Muscle Akt1/2 Causes Osteosarcopenia and Reduces Lifespan in Mice. Nat. Commun. 2022, 13, 5655. [Google Scholar] [CrossRef]
- Quirós Cognuck, S.; Reis, W.L.; Silva, M.; Debarba, L.K.; Mecawi, A.S.; De Paula, F.J.A.; Rodrigues Franci, C.; Elias, L.L.K.; Antunes-Rodrigues, J. Sex Differences in Body Composition, Metabolism-related Hormones, and Energy Homeostasis during Aging in Wistar Rats. Physiol. Rep. 2020, 8, e14597. [Google Scholar] [CrossRef]
- Marcella, B.M.; Hockey, B.L.; Braun, J.L.; Whitley, K.C.; Geromella, M.S.; Baranowski, R.W.; Watson, C.J.F.; Silvera, S.; Hamstra, S.I.; Wasilewicz, L.J.; et al. GSK3 Inhibition Improves Skeletal Muscle Function and Whole-Body Metabolism in Male Mouse Models of Duchenne Muscular Dystrophy. Nat. Commun. 2024, 15, 10210. [Google Scholar] [CrossRef]
- He, L.; Fei, D.L.; Nagiec, M.J.; Mutvei, A.P.; Lamprakis, A.; Kim, B.Y.; Blenis, J. Regulation of GSK3 Cellular Location by FRAT Modulates mTORC1-Dependent Cell Growth and Sensitivity to Rapamycin. Proc. Natl. Acad. Sci. USA 2019, 116, 19523–19529. [Google Scholar] [CrossRef]
- Eshima, H.; Shahtout, J.L.; Siripoksup, P.; Pearson, M.J.; Mahmassani, Z.S.; Ferrara, P.J.; Lyons, A.W.; Maschek, J.A.; Peterlin, A.D.; Verkerke, A.R.; et al. Lipid Hydroperoxides Promote Sarcopenia through Carbonyl Stress. eLife 2023, 12, e85289. [Google Scholar] [CrossRef]
- Yamauchi, S.; Sugiura, Y.; Yamaguchi, J.; Zhou, X.; Takenaka, S.; Odawara, T.; Fukaya, S.; Fujisawa, T.; Naguro, I.; Uchiyama, Y.; et al. Mitochondrial Fatty Acid Oxidation Drives Senescence. Sci. Adv. 2024, 10, eado5887. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lee, S.H.; Jung, Y.; Lee, Y.; Yoon, J.H.; Choi, J.Y.; Hwang, C.Y.; Son, Y.H.; Park, S.S.; Hwang, G.-S.; et al. FABP3-Mediated Membrane Lipid Saturation Alters Fluidity and Induces ER Stress in Skeletal Muscle with Aging. Nat. Commun. 2020, 11, 5661. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Lara, M.A.; Dommerholt, M.B.; Zhang, W.; Blankestijn, M.; Wolters, J.C.; Abegaz, F.; Gerding, A.; Van Der Veen, Y.T.; Thomas, R.; Van Os, R.P.; et al. Age-Related Susceptibility to Insulin Resistance Arises from a Combination of CPT1B Decline and Lipid Overload. BMC Biol. 2021, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Cikes, D.; Leutner, M.; Cronin, S.J.F.; Novatchkova, M.; Pfleger, L.; Klepochová, R.; Lair, B.; Lac, M.; Bergoglio, C.; Viguerie, N.; et al. Gpcpd1–GPC Metabolic Pathway Is Dysfunctional in Aging and Its Deficiency Severely Perturbs Glucose Metabolism. Nat. Aging 2024, 4, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Kareem, H.R. Microspectrophotometric Quantification of the Skeletal Muscle Glycogen Contents with Aging. Iraqi J. Med. Sci. 2016, 10. [Google Scholar]
- Hernandez, A.; Truckenbrod, L.; Federico, Q.; Campos, K.; Moon, B.; Ferekides, N.; Hoppe, M.; D’Agostino, D.; Burke, S. Metabolic Switching Is Impaired by Aging and Facilitated by Ketosis Independent of Glycogen. Aging 2020, 12, 7963–7984. [Google Scholar] [CrossRef]
- Nielsen, J.; Suetta, C.; Hvid, L.G.; Schrøder, H.D.; Aagaard, P.; Ørtenblad, N. Subcellular Localization-Dependent Decrements in Skeletal Muscle Glycogen and Mitochondria Content Following Short-Term Disuse in Young and Old Men. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E1053–E1060. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Jung, H.; Shin, H.; Kim, Y.; Yim, H.; Chung, H.; Lim, I.K.; Yoon, G. Enhanced Glycogenesis Is Involved in Cellular Senescence via GSK3/GS Modulation. Aging Cell 2008, 7, 894–907. [Google Scholar] [CrossRef]
- Zhou, Q.; Kerbl-Knapp, J.; Zhang, F.; Korbelius, M.; Kuentzel, K.B.; Vujić, N.; Akhmetshina, A.; Hörl, G.; Paar, M.; Steyrer, E.; et al. Metabolomic Profiles of Mouse Tissues Reveal an Interplay between Aging and Energy Metabolism. Metabolites 2021, 12, 17. [Google Scholar] [CrossRef]
- De Tata, V.; Cavallini, G.; Masiello, P.; Pollera, M.; Gori, Z.; Bergamini, E. Age-Related Changes in Muscle Glycogen Metabolism. Aging Clin. Exp. Res. 1991, 3, 408–410. [Google Scholar] [CrossRef]
- De Coster, W.; De Reuck, J.; Sieben, G.; Eecken, H.V. Early Ultrastructural Changes in Aging Rat Gastrocnemius Muscle: A Stereologic Study. Muscle Nerve 1981, 4, 111–116. [Google Scholar] [CrossRef]
- Larkin, L.M.; Horwitz, B.A.; Eiffert, K.C.; McDonald, R.B. Adrenergic Stimulated Skeletal Muscle Glycogenolysis in Perfused Hindlimbs of Young and Old Male Fischer 344 Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 266, R749–R755. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Yang, W.; Liao, Z.-Y.; Wu, Y.-X.; Fan, Z.; Guo, A.; Yu, J.; Chen, Q.-N.; Wu, J.-H.; Zhou, J.; et al. MICU3 Regulates Mitochondrial Ca2+-Dependent Antioxidant Response in Skeletal Muscle Aging. Cell Death Dis. 2021, 12, 1115. [Google Scholar] [CrossRef]
- Lee, S.-R.; Khamoui, A.V.; Jo, E.; Park, B.-S.; Zourdos, M.C.; Panton, L.B.; Ormsbee, M.J.; Kim, J.-S. Effects of Chronic High-Fat Feeding on Skeletal Muscle Mass and Function in Middle-Aged Mice. Aging Clin. Exp. Res. 2015, 27, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.Z.; Caturegli, G.; Metter, E.J.; Makrogiannis, S.; Resnick, S.M.; Harris, T.B.; Ferrucci, L. Difference in Muscle Quality over the Adult Life Span and Biological Correlates in the Baltimore Longitudinal Study of Aging. J. Am. Geriatr. Soc. 2014, 62, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, I.; Itagaki, A.; Saito, T.; Lee, S. Responses to Oxidative Stress and Antioxidant Capacity in Rats at Different Growth Stages. J. Phys. Ther. Sci. 2023, 35, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Mijares, A.; Allen, P.D.; Lopez, J.R. Senescence Is Associated With Elevated Intracellular Resting [Ca2 +] in Mice Skeletal Muscle Fibers. An in Vivo Study. Front. Physiol. 2021, 11, 601189. [Google Scholar] [CrossRef] [PubMed]
- Mårtensson, J.; Meister, A. Mitochondrial Damage in Muscle Occurs after Marked Depletion of Glutathione and Is Prevented by Giving Glutathione Monoester. Proc. Natl. Acad. Sci. USA 1989, 86, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Mosoni, L.; Breuillé, D.; Buffière, C.; Obled, C.; Mirand, P.P. Age-Related Changes in Glutathione Availability and Skeletal Muscle Carbonyl Content in Healthy Rats. Exp. Gerontol. 2004, 39, 203–210. [Google Scholar] [CrossRef]
- Lass, A.; Kwong, L.; Sohal, R.S. Mitochondrial Coenzyme Q Content and Aging. BioFactors 1999, 9, 199–205. [Google Scholar] [CrossRef]
- Quiles, J.L.; Ochoa, J.J.; Battino, M.; Gutierrez-Rios, P.; Nepomuceno, E.A.; Frías, M.L.; Huertas, J.R.; Mataix, J. Life-long Supplementation with a Low Dosage of Coenzyme Q10 in the Rat: Effects on Antioxidant Status and DNA Damage. BioFactors 2005, 25, 73–86. [Google Scholar] [CrossRef]
- Wada, H.; Goto, H.; Hagiwara, S.; Yamamoto, Y. Redox Status Of Coenzyme Q10 Is Associated With Chronological Age. J. Am. Geriatr. Soc. 2007, 55, 1141–1142. [Google Scholar] [CrossRef]
- Passi, S.; De Pità, O.; Puddu, P.; Littarru, G.P. Lipophilic Antioxidants in Human Sebum and Aging. Free Radic. Res. 2002, 36, 471–477. [Google Scholar] [CrossRef]
- Meza-Torres, C.; Reyes-Torres, I.; Bui Thanh, T.; Campos-Silva, C.; Rodriguez-Bies, E.; Navas, P.; López-Lluch, G. Evolution of COQ-Synthome Transcripts and CoQ Levels in Mice Tissues Along Aging: Effect of Resveratrol and Exercise. Antioxidants 2025, 14, 800. [Google Scholar] [CrossRef]
- Inoue, R.; Miura, M.; Yanai, S.; Nishimune, H. Coenzyme Q10 Supplementation Improves the Motor Function of Middle-Aged Mice by Restoring the Neuronal Activity of the Motor Cortex. Sci. Rep. 2023, 13, 4323. [Google Scholar] [CrossRef]
- Fernández-Portero, C.; Amián, J.G.; Bella, R.D.L.; López-Lluch, G.; Alarcón, D. Coenzyme Q10 Levels Associated With Cognitive Functioning and Executive Function in Older Adults. J. Gerontol. Ser. A 2023, 78, 1–8. [Google Scholar] [CrossRef]
- Hernández-Camacho, J.D.; Vicente-García, C.; Ardila-García, L.; Padilla-Campos, A.; López-Lluch, G.; Santos-Ocaña, C.; Zammit, P.S.; Carvajal, J.J.; Navas, P.; Fernández-Ayala, D.J.M. Prenatal and Progressive Coenzyme Q10 Administration to Mitigate Muscle Dysfunction in Mitochondrial Disease. J. Cachexia Sarcopenia Muscle 2024, 15, 2402–2416. [Google Scholar] [CrossRef]
- Nakazawa, H.; Ikeda, K.; Shinozaki, S.; Yasuhara, S.; Yu, Y.; Martyn, J.A.J.; Tompkins, R.G.; Yorozu, T.; Inoue, S.; Kaneki, M. Coenzyme Q10 Protects against Burn-induced Mitochondrial Dysfunction and Impaired Insulin Signaling in Mouse Skeletal Muscle. FEBS Open Bio 2019, 9, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Vertechy, M.; Cooper, M.B.; Ghirardi, O.; Ramacci, M.T. Antioxidant Enzyme Activities in Heart and Skeletal Muscle of Rats of Different Ages. Exp. Gerontol. 1989, 24, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Sullivan-Gunn, M.J.; Lewandowski, P.A. Elevated Hydrogen Peroxide and Decreased Catalase and Glutathione Peroxidase Protection Are Associated with Aging Sarcopenia. BMC Geriatr. 2013, 13, 104. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Di Filippo, E.S.; Mancinelli, R.; Doria, C.; Rotini, A.; Fanò-Illic, G.; Fulle, S. Low Intensity Exercise Training Improves Skeletal Muscle Regeneration Potential. Front. Physiol. 2015, 6, 399. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.; Bejma, J.; Ookawara, T.; Ohno, H.; Ji, L.L. Superoxide Dismutase Gene Expression in Skeletal Muscle: Fiber-Specific Effect of Age. Mech. Ageing Dev. 2000, 116, 33–45. [Google Scholar] [CrossRef]
- Pandya, C.D.; Lee, B.; Toque, H.A.; Mendhe, B.; Bragg, R.T.; Pandya, B.; Atawia, R.T.; Isales, C.; Hamrick, M.; Caldwell, R.W.; et al. Age-Dependent Oxidative Stress Elevates Arginase 1 and Uncoupled Nitric Oxide Synthesis in Skeletal Muscle of Aged Mice. Oxid. Med. Cell. Longev. 2019, 2019, 1704650. [Google Scholar] [CrossRef]
- Lammi-Keefe, C.J.; Swan, P.B.; Hegarty, P.V.J. Copper-Zinc and Manganese Superoxide Dismutase Activities in Cardiac and Skeletal Muscles during Aging in Male Rats. Gerontology 1984, 30, 153–158. [Google Scholar] [CrossRef]
- Van Remmen, H.; Ikeno, Y.; Hamilton, M.; Pahlavani, M.; Wolf, N.; Thorpe, S.R.; Alderson, N.L.; Baynes, J.W.; Epstein, C.J.; Huang, T.-T.; et al. Life-Long Reduction in MnSOD Activity Results in Increased DNA Damage and Higher Incidence of Cancer but Does Not Accelerate Aging. Physiol. Genomics 2003, 16, 29–37. [Google Scholar] [CrossRef]
- Braun, J.L.; Messner, H.N.; Cleverdon, R.E.G.; Baranowski, R.W.; Hamstra, S.I.; Geromella, M.S.; Stuart, J.A.; Fajardo, V.A. Heterozygous SOD2 Deletion Selectively Impairs SERCA Function in the Soleus of Female Mice. Physiol. Rep. 2022, 10, e15285. [Google Scholar] [CrossRef]
- Lee, S.; Van Remmen, H.; Csete, M. Sod2 Overexpression Preserves Myoblast Mitochondrial Mass and Function, but Not Muscle Mass with Aging. Aging Cell 2009, 8, 296–310. [Google Scholar] [CrossRef]
- Gupta, S.V.; Campos, L.; Schmidt, K.H. Mitochondrial Superoxide Dismutase Sod2 Suppresses Nuclear Genome Instability during Oxidative Stress. Genetics 2023, 225, iyad147. [Google Scholar] [CrossRef]
- Su, Y.; Claflin, D.R.; Huang, M.; Davis, C.S.; Macpherson, P.C.D.; Richardson, A.; Van Remmen, H.; Brooks, S.V. Deletion of Neuronal CuZnSOD Accelerates Age-Associated Muscle Mitochondria and Calcium Handling Dysfunction That Is Independent of Denervation and Precedes Sarcopenia. Int. J. Mol. Sci. 2021, 22, 10735. [Google Scholar] [CrossRef]
- Zhang, Y.; Davis, C.; Sakellariou, G.K.; Shi, Y.; Kayani, A.C.; Pulliam, D.; Bhattacharya, A.; Richardson, A.; Jackson, M.J.; McArdle, A.; et al. CuZnSOD Gene Deletion Targeted to Skeletal Muscle Leads to Loss of Contractile Force but Does Not Cause Muscle Atrophy in Adult Mice. FASEB J. 2013, 27, 3536–3548. [Google Scholar] [CrossRef]
- Lustgarten, M.S.; Jang, Y.C.; Liu, Y.; Qi, W.; Qin, Y.; Dahia, P.L.; Shi, Y.; Bhattacharya, A.; Muller, F.L.; Shimizu, T.; et al. MnSOD Deficiency Results in Elevated Oxidative Stress and Decreased Mitochondrial Function but Does Not Lead to Muscle Atrophy during Aging: Increased Mitochondrial Oxidative Stress Does Not Lead to Muscle Atrophy. Aging Cell 2011, 10, 493–505. [Google Scholar] [CrossRef]
- Jiménez-Moreno, R.; Wang, Z.-M.; Gerring, R.C.; Delbono, O. Sarcoplasmic Reticulum Ca2+ Release Declines in Muscle Fibers from Aging Mice. Biophys. J. 2008, 94, 3178–3188. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Y.; Ding, Z.; Zhao, Y.; Chen, T.; Ge, W.; Zhang, J. Iron Accumulation with Age Alters Metabolic Pattern and Circadian Clock Gene Expression through the Reduction of AMP-Modulated Histone Methylation. J. Biol. Chem. 2022, 298, 101968. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-M.; Messi, M.L.; Delbono, O. Sustained Overexpression of IGF-1 Prevents Age-Dependent Decrease in Charge Movement and Intracellular Ca2+ in Mouse Skeletal Muscle. Biophys. J. 2002, 82, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-M.; Messi, M.L.; Delbono, O. L-Type Ca2+ Channel Charge Movement and Intracellular Ca2+ in Skeletal Muscle Fibers from Aging Mice. Biophys. J. 2000, 78, 1947–1954. [Google Scholar] [CrossRef]
- De Luca, A.; Mambrini, M.; Conte Camerino, D. Changes in Membrane Ionic Conductances and Excitability Characteristics of Rat Skeletal Muscle during Aging. Pflüg. Arch. 1990, 415, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Fodor, J.; Al-Gaadi, D.; Czirják, T.; Oláh, T.; Dienes, B.; Csernoch, L.; Szentesi, P. Improved Calcium Homeostasis and Force by Selenium Treatment and Training in Aged Mouse Skeletal Muscle. Sci. Rep. 2020, 10, 1707. [Google Scholar] [CrossRef]
- Gafnp’, A.; Yuh, K.-C.M. A Comparative Study of the Ca2+-Mg2+ Dependent ATPase from Skeletal Muscles of Young, Adult and Old Rats. Mech. Ageing Dev. 1989, 49, 105–117. [Google Scholar] [CrossRef]
- Allen, R.J.; Kronemberger, A.; Shi, Q.; Pope, M.; Cuadra-Muñoz, E.; Son, W.; Song, L.-S.; Anderson, E.J.; Pereira, R.O.; Lira, V.A. Altered Relaxation and Mitochondria-Endoplasmic Reticulum Contacts Precede Major (Mal)Adaptations in Aging Skeletal Muscle and Are Prevented by Exercise. bioRxiv, 2025; preprint. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, Z.; Wu, C.; Teng, Y.; Liao, C.; Kao, C.; Chen, L.; Lin, C.; Tsai, T. Comparative Proteomic Profiling Reveals a Role for Cisd2 in Skeletal Muscle Aging. Aging Cell 2018, 17, e12705. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Chen, Y.-F.; Wang, C.-H.; Kao, C.-H.; Zhuang, H.-W.; Chen, C.-C.; Chen, L.-K.; Kirby, R.; Wei, Y.-H.; Tsai, S.-F.; et al. A Persistent Level of Cisd2 Extends Healthy Lifespan and Delays Aging in Mice. Hum. Mol. Genet. 2012, 21, 3956–3968. [Google Scholar] [CrossRef]
- Huo, J.; Prasad, V.; Grimes, K.M.; Vanhoutte, D.; Blair, N.S.; Lin, S.-C.; Bround, M.J.; Bers, D.M.; Molkentin, J.D. MCUb Is an Inducible Regulator of Calcium-Dependent Mitochondrial Metabolism and Substrate Utilization in Muscle. Cell Rep. 2023, 42, 113465. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Huo, J.; Bround, M.J.; Boyer, J.G.; Schwanekamp, J.A.; Ghazal, N.; Maxwell, J.T.; Jang, Y.C.; Khuchua, Z.; Shi, K.; et al. The Mitochondrial Calcium Uniporter Underlies Metabolic Fuel Preference in Skeletal Muscle. JCI Insight 2018, 3, e121689. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xiong, L.; Chen, J.; Liu, Q.; Wang, F.; Fu, X.; Huo, J.; Bu, Y.; Chen, S.; Liu, Q. Sirtuin 3 Modulation by High Phosphates: A Potential Mechanism in Muscle Aging and Sarcopenia. Am. J. Transl. Res. 2025, 17, 4187–4197. [Google Scholar] [CrossRef]
- Cameron, D.; Welch, A.A.; Adelnia, F.; Bergeron, C.M.; Reiter, D.A.; Dominguez, L.J.; Brennan, N.A.; Fishbein, K.W.; Spencer, R.G.; Ferrucci, L. Age and Muscle Function Are More Closely Associated With Intracellular Magnesium, as Assessed by 31P Magnetic Resonance Spectroscopy, Than With Serum Magnesium. Front. Physiol. 2019, 10, 1454. [Google Scholar] [CrossRef]
- DeRuisseau, K.C.; Park, Y.-M.; DeRuisseau, L.R.; Cowley, P.M.; Fazen, C.H.; Doyle, R.P. Aging-Related Changes in the Iron Status of Skeletal Muscle. Exp. Gerontol. 2013, 48, 1294–1302. [Google Scholar] [CrossRef]
- Tricarico, D.; Petruzzi, R.; Camerino, D.C. Changes of the Biophysical Properties of Calcium-Activated Potassium Channels of Rat Skeletal Muscle Fibres during Aging. Pflügers Arch. Eur. J. Physiol. 1997, 434, 822–829. [Google Scholar] [CrossRef]
- Alves, F.M.; Kysenius, K.; Caldow, M.K.; Hardee, J.P.; Crouch, P.J.; Ayton, S.; Bush, A.I.; Lynch, G.S.; Koopman, R. Iron Accumulation in Skeletal Muscles of Old Mice Is Associated with Impaired Regeneration after Ischaemia–Reperfusion Damage. J. Cachexia Sarcopenia Muscle 2021, 12, 476–492. [Google Scholar] [CrossRef]
- Kumar, A.; Vaca-Dempere, M.; Mortimer, T.; Deryagin, O.; Smith, J.G.; Petrus, P.; Koronowski, K.B.; Greco, C.M.; Segalés, J.; Andrés, E.; et al. Brain-Muscle Communication Prevents Muscle Aging by Maintaining Daily Physiology. Science 2024, 384, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among Skeletal Muscle Mass Indices Derived from Height-, Weight-, and Body Mass Index-Adjusted Models in Assessing Sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef]
- Degens, H. Determinants of Skeletal Muscle Hypertrophy and the Attenuated Hypertrophic Response at Old Age. J. Sports Med. Doping Stud. 2012, s1, 003. [Google Scholar] [CrossRef]
- Horwath, O.; Moberg, M.; Edman, S.; Philp, A.; Apró, W. Ageing Leads to Selective Type II Myofibre Deterioration and Denervation Independent of Reinnervative Capacity in Human Skeletal Muscle. Exp. Physiol. 2025, 110, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J.; Taylor, C.C.; Sj, M. What Is the Cause of the Ageing Atrophy? Total Number, Size and Proportion of Different Fiber Types Studied in Whole Vastus Lateralis Muscle from 15- to 83-Year-Old Men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Moro, T.; Brightwell, C.R.; Volpi, E.; Rasmussen, B.B.; Fry, C.S. Resistance Exercise Training Promotes Fiber Type-Specific Myonuclear Adaptations in Older Adults. J. Appl. Physiol. 2020, 128, 795–804. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, F.; Gong, M.; Jin, L.; Ren, X.; Liu, K.; Lu, J. Lifelong Treadmill Training Improves Muscle Function Detected by a Modified Grip Strength Test during Aging in BALB/c Mice. Life Sci. 2020, 251, 117603. [Google Scholar] [CrossRef]
- Bruusgaard, J.C.; Liestøl, K.; Gundersen, K. Distribution of Myonuclei and Microtubules in Live Muscle Fibers of Young, Middle-Aged, and Old Mice. J. Appl. Physiol. 2006, 100, 2024–2030. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kouzaki, K.; Sasaki, K.; Nakazato, K. Alterations in Neuromuscular Junction Morphology with Ageing and Endurance Training Modulate Neuromuscular Transmission and Myofibre Composition. J. Physiol. 2025, 603, 107–125. [Google Scholar] [CrossRef]
- Rice, K.M.; Manne, N.D.P.K.; Gadde, M.K.; Paturi, S.; Arvapalli, R.; Blough, E. Differential Regulation of Apoptosis in Slow and Fast Twitch Muscles of Aged Female F344BN Rats. AGE 2015, 37, 30. [Google Scholar] [CrossRef]
- Kanehisa, H.; Ikegawa, S.; Tsunoda, N.; Fukunaga, T. Cross-Sectional Areas of Fat and Muscle in Limbs During Growth and Middle Age. Int. J. Sports Med. 1994, 15, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of Sarcopenia: Prevalence, Risk Factors, and Consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Bildsoe, H.; Hughes, S.M. Evidence That Satellite Cell Decrement Contributes to Preferential Decline in Nuclear Number from Large Fibres during Murine Age-Related Muscle Atrophy. J. Cell Sci. 2005, 118, 4813–4821. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Smeets, J.S.J.; Van Kranenburg, J.; Kies, A.K.; Van Loon, L.J.C.; Verdijk, L.B. Changes in Myonuclear Domain Size Do Not Precede Muscle Hypertrophy during Prolonged Resistance-type Exercise Training. Acta Physiol. 2016, 216, 231–239. [Google Scholar] [CrossRef]
- Snijders, T.; Holwerda, A.M.; Van Loon, L.J.C.; Verdijk, L.B. Myonuclear Content and Domain Size in Small versus Larger Muscle Fibres in Response to 12 Weeks of Resistance Exercise Training in Older Adults. Acta Physiol. 2021, 231, e13599. [Google Scholar] [CrossRef]
- Brook, M.S.; Wilkinson, D.J.; Tarum, J.; Mitchell, K.W.; Lund, J.L.; Phillips, B.E.; Szewczyk, N.J.; Kadi, F.; Greenhaff, P.L.; Smith, K.; et al. Neither Myonuclear Accretion nor a Myonuclear Domain Size Ceiling Is a Feature of the Attenuated Hypertrophic Potential of Aged Human Skeletal Muscle. GeroScience 2023, 45, 451–462. [Google Scholar] [CrossRef]
- Schuenke, M.D.; Brooks, N.E.; Hikida, R.S. Interactions of Aging, Overload, and Creatine Supplementation in Rat Plantaris Muscle. J. Aging Res. 2011, 2011, 393416. [Google Scholar] [CrossRef]
- Rahmati, M.; McCarthy, J.J.; Malakoutinia, F. Myonuclear Permanence in Skeletal Muscle Memory: A Systematic Review and Meta-analysis of Human and Animal Studies. J. Cachexia Sarcopenia Muscle 2022, 13, 2276–2297. [Google Scholar] [CrossRef]
- Brooks, N.E.; Schuenke, M.D.; Hikida, R.S. Ageing Influences Myonuclear Domain Size Differently in Fast and Slow Skeletal Muscle of Rats. Acta Physiol. 2009, 197, 55–63. [Google Scholar] [CrossRef]
- Kavazis, A.N.; DeRuisseau, K.C.; Gordon, D.M. The Senescent Rat Diaphragm Does Not Exhibit Age-Related Changes in Caspase Activities, DNA Fragmentation, or Myonuclear Domain. Eur. J. Appl. Physiol. 2012, 112, 3983–3990. [Google Scholar] [CrossRef]
- Cristea, A.; Qaisar, R.; Edlund, P.K.; Lindblad, J.; Bengtsson, E.; Larsson, L. Effects of Aging and Gender on the Spatial Organization of Nuclei in Single Human Skeletal Muscle Cells. Aging Cell 2010, 9, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.A.; Hammond, K.G.; Stec, M.J.; Bickel, C.S.; Windham, S.T.; Tuggle, S.C.; Bamman, M.M. Quantification and Characterization of Grouped Type I Myofibers in Human Aging. Muscle Nerve 2018, 57, E52–E59. [Google Scholar] [CrossRef] [PubMed]
- Hansson, K.-A.; Eftestøl, E. Scaling of Nuclear Numbers and Their Spatial Arrangement in Skeletal Muscle Cell Size Regulation. Mol. Biol. Cell 2023, 34, pe3. [Google Scholar] [CrossRef] [PubMed]
- Sakita, M.; Murakami, S.; Fujino, H. Age-Related Morphological Regression of Myelinated Fibers and Capillary Architecture of Distal Peripheral Nerves in Rats. BMC Neurosci. 2016, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.W.; Aussieker, T.; Kruger, C.Q.; Gorissen, S.H.M.; Van Loon, L.J.C.; Snijders, T. Muscle Fiber Capillarization Is Associated with Various Indices of Skeletal Muscle Mass in Healthy, Older Men. Exp. Gerontol. 2021, 143, 111161. [Google Scholar] [CrossRef]
- Ripoll, E.; Sillau, A.H.; Banchero, N. Changes in the Capillarity of Skeletal Muscle in the Growing Rat. Pflügers Arch. Eur. J. Physiol. 1979, 380, 153–158. [Google Scholar] [CrossRef]
- Degens, H.; Deveci, D.; Botto-Van Bemden, A.; Hoofd, L.J.C.; Egginton, S. Maintenance of Heterogeneity of Capillary Spacing Is Essential for Adequate Oxygenation in the Soleus Muscle of the Growing Rat. Microcirculation 2006, 13, 467–476. [Google Scholar] [CrossRef]
- Hoang, D.-H.; Bouvière, J.; Galvis, J.; Moullé, P.; Migliavacca, E.; Juban, G.; Liot, S.; Stuelsatz, P.; Le Grand, F.; Feige, J.N.; et al. Immune Aging Impairs Muscle Regeneration via Macrophage-Derived Anti-Oxidant Selenoprotein P. EMBO Rep. 2025, 26, 4153–4179. [Google Scholar] [CrossRef]
- Fukada, K.; Kajiya, K. Age-Related Structural Alterations of Skeletal Muscles and Associated Capillaries. Angiogenesis 2020, 23, 79–82. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Lapp, S.; Dvoretskiy, S.; Garcia, G.; Kim, M.; Tannehill, A.; Daniels, L.; Boppart, M.D. Optimization of a Pericyte Therapy to Improve Muscle Recovery after Limb Immobilization. J. Appl. Physiol. 2022, 132, 1020–1030. [Google Scholar] [CrossRef]
- Gordon, J.A.; Hoffman, J.R.; Stout, J.R.; Fukuda, D.H.; Coker, N.A.; Varanoske, A.N.; Arroyo, E.; Wells, A.J.; Gepner, Y. Differences in Muscle Oxygenation between Young and Middle-Aged Recreationally Active Men during High-Volume Resistance Exercise. Kinesiology 2019, 51, 3–11. [Google Scholar] [CrossRef]
- Williamson, J.R.; Hoffmann, P.L.; Kohrt, W.M.; Spina, R.J.; Coggan, A.R.; Holloszy, O. Endurance Exercise Training Decreases Capillary Basement Membrane Width in Older Nondiabetic and Diabetic Adults. J. Appl. Physiol. 1996, 80, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Bigler, M.; Koutsantonis, D.; Odriozola, A.; Halm, S.; Tschanz, S.A.; Zakrzewicz, A.; Weichert, A.; Baum, O. Morphometry of Skeletal Muscle Capillaries: The Relationship between Capillary Ultrastructure and Ageing in Humans. Acta Physiol. 2016, 218, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Guarente, L. SIRT1 Mediates Central Circadian Control in the SCN by a Mechanism That Decays with Aging. Cell 2013, 153, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Kamp, D.; Krause, V.; Butz, M.; Schnitzler, A.; Pollok, B. Changes of Cortico-Muscular Coherence: An Early Marker of Healthy Aging? Age 2013, 35, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Mejia Maza, A.; Jarvis, S.; Lee, W.C.; Cunningham, T.J.; Schiavo, G.; Secrier, M.; Fratta, P.; Sleigh, J.N.; Fisher, E.M.C.; Sudre, C.H. NMJ-Analyser Identifies Subtle Early Changes in Mouse Models of Neuromuscular Disease. Sci. Rep. 2021, 11, 12251. [Google Scholar] [CrossRef]
- Aliya, B.; Mohiuddin, M.; Choi, J.J.; Jeong, G.; Kang, I.; Castels, H.; Jones, C.; Jang, Y.C. Comparison of Neuromuscular Junction Dynamics Following Ischemic and Aged Skeletal Muscle. bioRxiv, 2021; preprint. [Google Scholar] [CrossRef]
- Li, Y.; Patel, M.; Baroudi, J.; Wu, M.; Gatti, S.; Liang, M.; Wipf, P.; Badawi, Y.; Meriney, S.D. A Cross-sectional Study of Ageing at the Mouse Neuromuscular Junction and Effects of an Experimental Therapeutic Approach for Dynapenia. J. Physiol. 2023, 601, 4135–4150. [Google Scholar] [CrossRef]
- Valdez, G.; Tapia, J.C.; Kang, H.; Clemenson, G.D.; Gage, F.H.; Lichtman, J.W.; Sanes, J.R. Attenuation of Age-Related Changes in Mouse Neuromuscular Synapses by Caloric Restriction and Exercise. Proc. Natl. Acad. Sci. USA 2010, 107, 14863–14868. [Google Scholar] [CrossRef]
- Cheng, A.; Morsch, M.; Murata, Y.; Ghazanfari, N.; Reddel, S.W.; Phillips, W.D. Sequence of Age-Associated Changes to the Mouse Neuromuscular Junction and the Protective Effects of Voluntary Exercise. PLoS ONE 2013, 8, e67970. [Google Scholar] [CrossRef]
- Hastings, R.L.; Avila, M.F.; Suneby, E.; Juros, D.; O’Young, A.; Peres da Silva, J.; Valdez, G. Cellular and Molecular Evidence That Synaptic Schwann Cells Contribute to Aging of Mouse Neuromuscular Junctions. Aging Cell 2023, 22, e13981. [Google Scholar] [CrossRef]
- Carnio, S.; LoVerso, F.; Baraibar, M.A.; Longa, E.; Khan, M.M.; Maffei, M.; Reischl, M.; Canepari, M.; Loefler, S.; Kern, H.; et al. Autophagy Impairment in Muscle Induces Neuromuscular Junction Degeneration and Precocious Aging. Cell Rep. 2014, 8, 1509–1521. [Google Scholar] [CrossRef]
- Vaughan, S.K.; Stanley, O.L.; Valdez, G. Impact of Aging on Proprioceptive Sensory Neurons and Intrafusal Muscle Fibers in Mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2017, 72, 771–779. [Google Scholar] [CrossRef]
- Kawai-Takaishi, M.; Hosoyama, T. Muscle Spindle Afferent Neurons Preferentially Degenerate with Aging. Sci. Rep. 2025, 15, 23946. [Google Scholar] [CrossRef] [PubMed]
- Petrany, M.J.; Swoboda, C.O.; Sun, C.; Chetal, K.; Chen, X.; Weirauch, M.T.; Salomonis, N.; Millay, D.P. Single-Nucleus RNA-Seq Identifies Transcriptional Heterogeneity in Multinucleated Skeletal Myofibers. Nat. Commun. 2020, 11, 6374. [Google Scholar] [CrossRef] [PubMed]
- Ham, A.S.; Lin, S.; Tse, A.; Thürkauf, M.; McGowan, T.J.; Jörin, L.; Oliveri, F.; Rüegg, M.A. Single-Nuclei Sequencing of Skeletal Muscle Reveals Subsynaptic-Specific Transcripts Involved in Neuromuscular Junction Maintenance. Nat. Commun. 2025, 16, 2220. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, H.; Dong, X.; Hui, T.; Yan, M.; Ren, D.; Zou, S.; Wang, S.; Fei, E.; Zhang, W.; et al. Deficiency of Skeletal Muscle Agrin Contributes to the Pathogenesis of Age-Related Sarcopenia in Mice. Cell Death Dis. 2024, 15, 201. [Google Scholar] [CrossRef]
- Jaime, D.; Fish, L.A.; Madigan, L.A.; Xi, C.; Piccoli, G.; Ewing, M.D.; Blaauw, B.; Fallon, J.R. The MuSK-BMP Pathway Maintains Myofiber Size in Slow Muscle through Regulation of Akt-mTOR Signaling. Skelet. Muscle 2024, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Juros, D.; Hastings, R.L.; Pendragon, A.; Kay, J.; Valdez, G. Loss of MEGF10 Decreases the Number of Perisynaptic Schwann Cells and Innervation of Neuromuscular Junctions in Aging Mice. J. Peripher. Nerv. Syst. 2025, 30, e70014. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Chand, K.K.; Hammond, L.A.; Lavidis, N.A.; Noakes, P.G. Functional Decline at the Aging Neuromuscular Junction Is Associated with Altered Laminin-A4 Expression. Aging 2017, 9, 880–899. [Google Scholar] [CrossRef]
- Duran-Jimenez, B.; Dobler, D.; Moffatt, S.; Rabbani, N.; Streuli, C.H.; Thornalley, P.J.; Tomlinson, D.R.; Gardiner, N.J. Advanced Glycation End Products in Extracellular Matrix Proteins Contribute to the Failure of Sensory Nerve Regeneration in Diabetes. Diabetes 2009, 58, 2893–2903. [Google Scholar] [CrossRef]
- Zhao, H.; Iyama, R.; Kurogi, E.; Hayashi, T.; Egawa, T. Direct and Acute Effects of Advanced Glycation End Products on Proteostasis in Isolated Mouse Skeletal Muscle. Physiol. Rep. 2024, 12, e16121. [Google Scholar] [CrossRef]
- Liu, N.; Butcher, J.T.; Nakano, A.; Del Campo, A. Changes in Macrophage Immunometabolism as a Marker of Skeletal Muscle Dysfunction across the Lifespan. Aging 2023, 15, 4035–4050. [Google Scholar] [CrossRef]
- Tobin, S.W.; Alibhai, F.J.; Wlodarek, L.; Yeganeh, A.; Millar, S.; Wu, J.; Li, S.; Weisel, R.D.; Li, R. Delineating the Relationship between Immune System Aging and Myogenesis in Muscle Repair. Aging Cell 2021, 20, e13312. [Google Scholar] [CrossRef] [PubMed]
- Sousa, N.S.; Bica, M.; Brás, M.F.; Sousa, A.C.; Antunes, I.B.; Encarnação, I.A.; Costa, T.M.; Martins, I.B.; Barbosa-Morais, N.L.; Sousa-Victor, P.; et al. The Immune Landscape of Murine Skeletal Muscle Regeneration and Aging. Cell Rep. 2024, 43, 114975. [Google Scholar] [CrossRef]
- Reidy, P.T.; McKenzie, A.I.; Mahmassani, Z.S.; Petrocelli, J.J.; Nelson, D.B.; Lindsay, C.C.; Gardner, J.E.; Morrow, V.R.; Keefe, A.C.; Huffaker, T.B.; et al. Aging Impairs Mouse Skeletal Muscle Macrophage Polarization and Muscle-Specific Abundance during Recovery from Disuse. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E85–E98. [Google Scholar] [CrossRef]
- Wan, X.; Ji, Y.; Wang, R.; Yang, H.; Cao, X.; Lu, S. Association between Systemic Immune-Inflammation Index and Sarcopenic Obesity in Middle-Aged and Elderly Chinese Adults: A Cross-Sectional Study and Mediation Analysis. Lipids Health Dis. 2024, 23, 230. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, L.; Zhang, D.; Chen, Z. Association between Systemic Immune-Inflammation Index and Low Muscle Mass in US Adults: A Cross-Sectional Study. BMC Public Health 2023, 23, 1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wehling-Henricks, M.; Welc, S.S.; Fisher, A.L.; Zuo, Q.; Tidball, J.G. Aging of the Immune System Causes Reductions in Muscle Stem Cell Populations, Promotes Their Shift to a Fibrogenic Phenotype, and Modulates Sarcopenia. FASEB J. 2019, 33, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Kedlian, V.R.; Wang, Y.; Liu, T.; Chen, X.; Bolt, L.; Tudor, C.; Shen, Z.; Fasouli, E.S.; Prigmore, E.; Kleshchevnikov, V.; et al. Human Skeletal Muscle Aging Atlas. Nat. Aging 2024, 4, 727–744. [Google Scholar] [CrossRef]
- Kuswanto, W.; Burzyn, D.; Panduro, M.; Wang, K.K.; Jang, Y.C.; Wagers, A.J.; Benoist, C.; Mathis, D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 2016, 44, 355–367. [Google Scholar] [CrossRef]
- Markworth, J.F.; Brown, L.A.; Lim, E.; Castor-Macias, J.A.; Larouche, J.; Macpherson, P.C.D.; Davis, C.; Aguilar, C.A.; Maddipati, K.R.; Brooks, S.V. Metabolipidomic Profiling Reveals an Age-Related Deficiency of Skeletal Muscle pro-Resolving Mediators That Contributes to Maladaptive Tissue Remodeling. Aging Cell 2021, 20, e13393. [Google Scholar] [CrossRef]
- Dalle, C.; Ostermann, A.I.; Konrad, T.; Coudy-Gandilhon, C.; Decourt, A.; Barthélémy, J.-C.; Roche, F.; Féasson, L.; Mazur, A.; Béchet, D.; et al. Muscle Loss Associated Changes of Oxylipin Signatures During Biological Aging: An Exploratory Study From the PROOF Cohort. J. Gerontol. Ser. A 2019, 74, 608–615. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horlem, T.; Carvalhal, S.R.S.; Bonatto, S.J.R.; Fernandes, L.C. Molecular Framework of the Onset and Progression of Skeletal Muscle Aging. Int. J. Mol. Sci. 2025, 26, 10145. https://doi.org/10.3390/ijms262010145
Horlem T, Carvalhal SRS, Bonatto SJR, Fernandes LC. Molecular Framework of the Onset and Progression of Skeletal Muscle Aging. International Journal of Molecular Sciences. 2025; 26(20):10145. https://doi.org/10.3390/ijms262010145
Chicago/Turabian StyleHorlem, Thomas, Stephanie Rubianne Silva Carvalhal, Sandro José Ribeiro Bonatto, and Luiz Cláudio Fernandes. 2025. "Molecular Framework of the Onset and Progression of Skeletal Muscle Aging" International Journal of Molecular Sciences 26, no. 20: 10145. https://doi.org/10.3390/ijms262010145
APA StyleHorlem, T., Carvalhal, S. R. S., Bonatto, S. J. R., & Fernandes, L. C. (2025). Molecular Framework of the Onset and Progression of Skeletal Muscle Aging. International Journal of Molecular Sciences, 26(20), 10145. https://doi.org/10.3390/ijms262010145





