Innovative Therapeutic Approaches Targeting Obesity: Can Flavonoids Improve the Efficacy of Anti-Obesogenic Drugs?
Abstract
1. Introduction
2. Epigenetic Mechanisms of Obesity
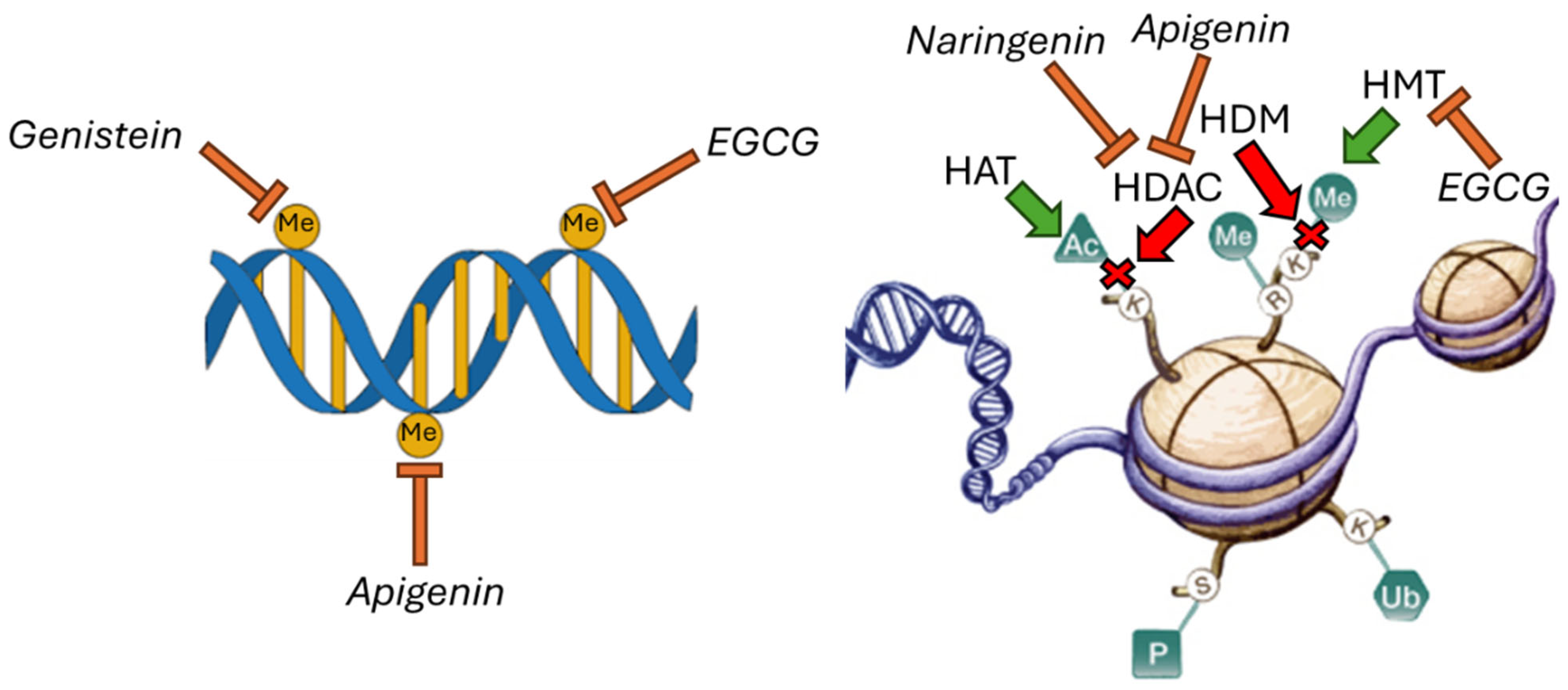
Flavonoids Regulate Epigenetic Mechanisms
3. Anti-Obesity Effects of Flavonoids: Biomarkers and Molecular Pathways
3.1. Anti-Diabesity Activities of Flavonoids
3.2. Anti-Obesity Effects of Flavonoids in Clinical Trials
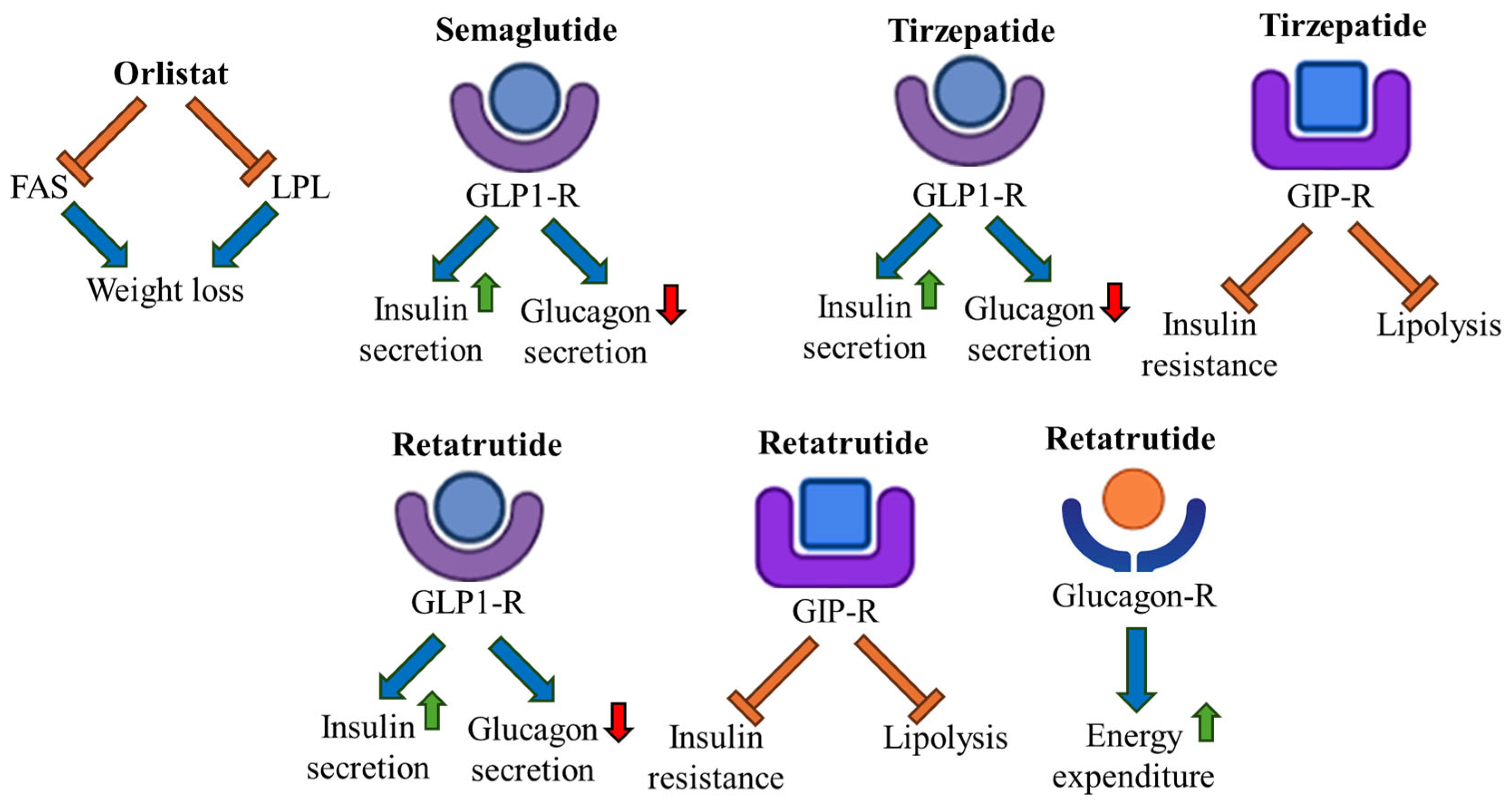
4. Anti-Obesity Drugs
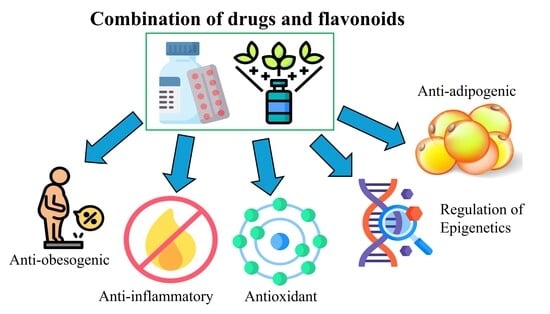
Anti-Obesity Drugs and Flavonoid Combinations: A Novel Perspective
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, A.G.; Williamson, D.F.; Ha, G. Direct medical cost of overweight and obesity in the USA: A quantitative systematic review. Obes. Rev. 2011, 12, 50–61. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27–39. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: A population-based longitudinal study. Int. J. Obes. 2019, 43, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; McAuley, P.A.; Church, T.S.; Milani, R.V.; Blair, S.N. Obesity and cardiovascular diseases. J. Am. Coll. Cardiol. 2014, 63, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Giralt, M.; Villarroya, F. White, Brown, beige/Brite: Different adipose cells for different functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef]
- Kiefer, F.W. The significance of beige and brown fat in humans. Endocr. Connect. 2017, 6, R70–R90. [Google Scholar] [CrossRef]
- Flaticon Website. Available online: https://www.flaticon.com/ (accessed on 23 June 2025).
- Lee, H.S.; Heo, C.U.; Song, Y.H.; Lee, K.; Choi, C.I. Naringin promotes fat browning mediated by UCP1 activation via the AMPK signaling pathway in 3T3-L1 adipocytes. Arch. Pharm. Res. 2023, 46, 192–205. [Google Scholar] [CrossRef]
- Matsukawa, T.; Villareal, M.O.; Motojima, H.; Isoda, H. Increasing cAMP levels of preadipocytes by cyanidin-3-glucoside treatment induces the formation of beige phenotypes in 3T3-L1 adipocytes. J. Nutr. Biochem. 2017, 40, 77–85. [Google Scholar] [CrossRef]
- Aziz, S.A.; Wakeling, L.A.; Miwa, S.; Alberdi, G.; Hesketh, J.E.; Ford, D. Metabolic programming of a beige adipocyte phenotype by genistein. Mol. Nutr. Food Res. 2017, 61, 1600574. [Google Scholar] [CrossRef]
- Lone, J.; Parray, H.A.; Yun, J.W. Nobiletin induces brown adipocyte-like phenotype and ameliorates stress in 3T3-L1 adipocytes. Biochimie 2018, 146, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.M.; Li, A.N.; Cai, Y.; Chen, J.J.; Xu, Y.; Sui, L.H.; Wang, J.L.; Jin, P.; Wang, K.S.; Yang, Z.C. Skip participates in formation and lipid metabolism of beige adipose and might mediate the effects of SIRT1 activator BTM-0512 on beige remodeling. Biochem. Biophys. Res. Commun. 2021, 537, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, K.; Ma, Y.; Qin, C.; Dong, C.; Jin, P.; Wu, Y.; Xiong, X.; Li, N.; Hu, C.; et al. Resveratrol derivative BTM-0512 mitigates obesity by promoting beige remodeling of subcutaneous preadipocytes. Acta Biochim. Biophys. Sin. 2017, 49, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Rauch, B.; Balla, N.; Ujlaki, G.; Kis, G.; Rahman, O.A.; Kristof, E.; Sipos, A.; Antal, M.; Toth, A.; et al. Olaparib induces browning of in vitro cultures of human primary white adipocytes. Biochem. Pharmacol. 2019, 167, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, J.; Yang, H.; Li, G.; Li, H.; Deng, Z.; Zhang, B. Propolis polyphenols: A review on the composition and anti-obesity mechanism of different types of propolis polyphenols. Front. Nutr. 2023, 10, 1066789. [Google Scholar] [CrossRef]
- Park, H.K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metab. Clin. Exp. 2015, 64, 24–34. [Google Scholar] [CrossRef]
- Aragones, G.; Ardid-Ruiz, A.; Ibars, M.; Suarez, M.; Blade, C. Modulation of leptin resistance by food compounds. Mol. Nutr. Food Res. 2016, 60, 1789–1803. [Google Scholar] [CrossRef]
- Castorina, S.; Barresi, V.; Luca, T.; Privitera, G.; De Geronimo, V.; Lezoche, G.; Cinti, S. Gastric ghrelin cells in obese patients are hyperactive. Int. J. Obes. 2021, 45, 184–194. [Google Scholar] [CrossRef]
- Berg, A.H.; Combs, T.P.; Scherer, P.E. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002, 13, 84–89. [Google Scholar] [CrossRef]
- Mahankali, V.B.; Velraja, S.; Parvathi, V.D.; Ramasamy, S. Key Players in the Complex Pathophysiology of Obesity: A Cross-Talk Between the Obesogenic Genes and Unraveling the Metabolic Pathway of Action of Capsaicin and Orange Peel. Appl. Biochem. Biotechnol. 2025, 197, 649–666, Correction in Appl. Biochem. Biotechnol. 2024, 196, 6582. [Google Scholar] [CrossRef]
- Lim, S.; Quon, M.J.; Koh, K.K. Modulation of adiponectin as a potential therapeutic strategy. Atherosclerosis 2014, 233, 721–728. [Google Scholar] [CrossRef]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Ki-mura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef]
- Makarewicz, A.; Jamka, M.; Geltz, J.; Smidowicz, A.; Kokot, M.; Kaczmarek, N.; Madry, E.; Walkowiak, J. Comparison of the Effect of Endurance, Strength, and Endurance-Strength Training on Inflammatory markers and Adipokines levels in Overweight and Obese Adults: Systematic Review and Meta-Analysis of Randomised Trials. Healthcare 2022, 10, 1098. [Google Scholar] [CrossRef]
- Shah, M.A.; Harris, M.; Faheem, H.I.; Hamid, A.; Yousaf, R.; Rasul, A.; Shah, G.M.; Khalil, A.A.K.; Wahab, A.; Khan, H.; et al. Cross-Talk between Obesity and Diabetes: Introducing Polyphenols as an Effective Phytomedicine to Combat the Dual Sword Diabesity. Curr. Pharm. Des. 2022, 28, 1523–1542. [Google Scholar] [CrossRef]
- Rosenzweig, T.; Braiman, L.; Bak, A.; Alt, A.; Kuroki, T.; Sampson, S.R. Differential effects of tumor necrosis factor-alpha on protein kinase C isoforms alpha and delta mediate inhibition of insulin receptor signaling. Diabetes 2002, 51, 1921–1930. [Google Scholar] [CrossRef]
- Unamuno, X.; Gomez-Ambrosi, J.; Rodriguez, A.; Becerril, S.; Fruhbeck, G.; Catalan, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Cock, T.A.; Houten, S.M.; Auwerx, J. Peroxisome proliferator-activated receptor-[gamma]: Too much of a good thing causes harm. EMBO Rep. 2004, 5, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Lazar, M.A. The many faces of PPARgamma. Cell 2005, 123, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Sysoeva, V.Y.; Lazarev, M.A.; Kulebyakin, K.Y.; Semina, E.V.; Rubina, K.A. Molecular and Cellular Mechanisms Governing Adipogenic Differentiation. Russ. J. Dev. Biol. 2023, 54, S10–S22. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Wang, Y.X.; Zhou, K.; Xie, H.L.; Ren, Z.; Liu, H.T.; Ou, Y.S.; Zhou, Z.X.; Jiang, Z.S. Biological functions of CRTC2 and its role in metabolism--related diseases. J. Cell Commun. Signal. 2023, 17, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Mosetti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124–147. [Google Scholar] [CrossRef]
- Li, X.; Zheng, L.; Zhang, B.; Deng, Z.Y.; Luo, T. The Structure Basis of Phytochemicals as Metabolic Signals for Combating Obesity. Front. Nutr. 2022, 9, 913883. [Google Scholar] [CrossRef]
- Kersten, S. Peroxisome proliferator activated receptors and obesity. Eur. J. Pharmacol. 2002, 440, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Hwang, E.S.; Mcmanus, M.T.; Amsterdam, A.; Tian, Y.; Kalmukova, R.; Mueller, E.; Benjamin, T.; Spiegelman, B.M.; Sharp, P.A.; et al. Taz, a transcriptional modulator of mesenchymal stem cell differentiation. Science 2005, 309, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Erickson, R.L.; Gerin, I.; DeRose, P.M.; Bajnok, L.; Longo, K.A.; Misek, D.E.; Kuick, R.; Hanash, S.M.; Atkins, K.B.; et al. Microarray analyses during adipogenesis: Understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor α in adipocyte metabolism. Mol. Cell. Biol. 2002, 22, 5989–5999. [Google Scholar] [CrossRef]
- Kim, S.K.; Kong, C.S. Anti-adipogenic effect of dioxinodehydroeckol via AMPK activation in 3T3-L1 adipocytes. Chem. Biol. Interact. 2010, 186, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Hawley, S.A. AMP-activated protein kinase: The energy charge hypothesis revisited. Bioessays 2001, 23, 1112–1119. [Google Scholar] [CrossRef]
- Li, H.X.; Xiao, L.; Wang, C.; Gao, J.L.; Zhai, Y.G. Epigenetic regulation of adipocyte differentiation and adipogenesis. J. Zhejiang Univ. Sci. B 2010, 11, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Yoon, K.S.; Ha, J.; Kang, I.; Choe, W. Crosstalk between Antioxidants and Adipogenesis: Mechanistic Pathways and Their Roles in Metabolic Health. Antioxidants 2025, 14, 203–228. [Google Scholar] [CrossRef]
- Corrales, P.; Vidal-Puig, A.; Medina-Gomez, G. PPARs and Metabolic Disorders Associated with Challenged Adipose Tissue Plasticity. Int. J. Mol. Sci. 2018, 19, 2124–2139. [Google Scholar] [CrossRef]
- Dama, A.; Shpati, K.; Daliu, P.; Dumur, S.; Gorica, E.; Santini, A. Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation. Nutrients 2024, 16, 507–524. [Google Scholar] [CrossRef]
- Rios-Hoyo, A.; Cortes, M.J.; Rios-Ontiveros, H.; Meaney, E.; Ceballos, G.; Gutierrez-Salmean, G. Obesity, Metabolic Syndrome, and Dietary Therapeutical Approaches with a Special Focus on Nutraceuticals (Polyphenols): A Mini-Review. Int. J. Vitam. Nutr. Res. 2014, 84, 113–123. [Google Scholar] [CrossRef]
- Salvamani, S.; Gunasekaran, B.; Shaharuddin, N.A.; Ahmad, S.A.; Shukor, M.Y. Antiartherosclerotic Effects of Plant Flavonoids. Biomed. Res. Int. 2014, 2014, 480258. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Chen, F.; Zhou, B. Epigenetics in obesity: Mechanisms and advances in therapies based on natural products. Pharmacol. Res. Perspect. 2024, 12, e1171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Lin, Z.J.; Li, C.C.; Lin, X.; Shan, S.K.; Guo, B.; Zheng, M.H.; Li, F.; Yuan, L.Q.; Li, Z.H. Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal Transduct. Target Ther. 2023, 8, 98–124. [Google Scholar] [CrossRef] [PubMed]
- King, S.E.; Skinner, M.K. Epigenetic transgenerational inheritance of obesity susceptibility. Trends Endocrinol. Metab. 2020, 31, 478–494. [Google Scholar] [CrossRef]
- Casado-Pelaez, M.; Bueno-Costa, A.; Esteller, M. Single cell cancer epigenetics. Trends Cancer 2022, 8, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef]
- Milagro, F.I.; Campión, J.; García-Díaz, D.F.; Goyenechea, E.; Paternain, L.; Martínez, J.A. High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J. Physiol. Biochem. 2009, 65, 1–9. [Google Scholar] [CrossRef]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341–1361. [Google Scholar] [CrossRef]
- Millán-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone post-translational modifications–cause and con-sequence of genome function. Nat. Rev. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef]
- Avery, L.B.; Bumpus, N.N. Valproic acid is a novel activator of AMP-activated protein kinase and decreases liver mass, hepatic fat accumulation, and serum glucose in obese mice. Mol. Pharmacol. 2014, 85, 1–10. [Google Scholar] [CrossRef]
- Lewis, E.C.; Blaabjerg, L.; Storling, J.; Ronn, S.G.; Mascagni, P.; Dinarello, C.A.; Mandrup-Poulsen, T. The oral histone deacetylase inhibitor ITF2357 reduces cytokines and protects islet beta cells in vivo and in vitro. Mol. Med. 2011, 17, 369–377. [Google Scholar] [CrossRef]
- Lam, H.N.; Lin, S.P.; Nguyen, D.H.N.; Chen, C.M.; Su, C.T.; Fang, T.C.; Li, S.C. Integrative Roles of Functional Foods, Microbiotics, Nutrigenetics, and Nutrigenomics in Managing Type 2 Diabetes and Obesity. Nutrients 2025, 17, 608–634. [Google Scholar] [CrossRef] [PubMed]
- Dincer, Y.; Yuksel, S. Antiobesity effects of phytochemicals from an epigenetic perspective. Nutrition 2021, 84, 111119. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, Y.; Ling, F.; Guan, Y.; Zhang, D.; Zhu, Q.; Liu, J.; Wu, Y.; Niu, Y. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 2020, 19, e13199. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.; Heilmann, K.; Danesi, F.; Bordoni, A.; Gerhauser, C. Modulation of adipocyte differentiation and proadipogenic gene expression by sulforaphane, genistein, and docosahexaenoic acid as a first step to counteract obesity. Oxid. Med. Cell Longev. 2018, 2018, 1617202. [Google Scholar] [CrossRef]
- Sun, Y.S.; Qu, W. Dietary apigenin promotes lipid catabolism, thermogenesis, and browning in adipose tissues of HFD-fed mice. Food Chem. Toxicol. 2019, 133, 110780. [Google Scholar] [CrossRef]
- de la Garza, A.L.; Etxeberria, U.; Palacios-Ortega, S.; Haslberger, A.G.; Aumueller, E.; Milagro, F.I.; Martinez, J.A. Modulation of hyperglycemia and TNFα-mediated inflammation by helichrysum and grapefruit extracts in diabetic db/db mice. Food Funct. 2014, 5, 2120–2128. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Zhong, M.; Chen, Y.; Su, W.; Li, P. Epigenetic regulation by naringenin and naringin: A literature review focused on the mechanisms underlying its pharmacological effects. Fitoterapia 2025, 181, 106353. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, A.G. Epigenetics Tutorial. BioSocial Methods Collaborative, University of Michigan Institute for Social Research. 2014. Available online: https://biosocialmethods.isr.umich.edu/research-support/videos-tutorials/epigenetics-tutorial/ (accessed on 24 June 2025).
- Scarpa, E.S.; Antonelli, A.; Balercia, G.; Sabatelli, S.; Maggi, F.; Caprioli, G.; Giacchetti, G.; Micucci, M. Antioxidant, Anti-Inflammatory, Anti-Diabetic, and Pro-Osteogenic Activities of Polyphenols for the Treatment of Two Different Chronic Diseases: Type 2 Diabetes Mellitus and Osteoporosis. Biomolecules 2024, 14, 836–865. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, Y.H.; Son, S.W.; Moon, E.Y.; Pyo, S.; Um, S.H. Fisetin induces Sirt1 expression while inhibiting early adipogenesis in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2015, 467, 638–644, Correction in Biochem. Biophys. Res. Commun. 2018, 506, 306. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, N.; Nobushi, Y.; Wada, T.; Sonoda, K.; Okazaki, Y.; Tsutsumi, S.; Park, Y.K.; Kurokawa, M.; Shimba, S.; Yasukawa, K. Inhibitory effects of compounds isolated from the dried branches and leaves of murta (Myrceugenia euosma) on lipid accumulation in 3T3-L1 cells. J. Nat. Med. 2016, 70, 502–509. [Google Scholar] [CrossRef]
- Tang, S.; Shi, Z.; Qiao, X.; Zhuang, Z.; Ding, Y.; Wu, Y.; Ding, Z.; Huang, Y. Carya cathayensis leaf extract attenuates ectopic fat deposition in liver, abdomen and aortic arch in ovariectomized rats fed a high-fat diet. Phytomedicine 2021, 82, 153447. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Z.; Wang, X.; Huang, B.; Zhang, W.; Sha, Y.; Pang, W. A natural small molecule pinocembrin resists high-fat diet-induced obesity through GPR120-ERK1/2 pathway. J. Nutr. Biochem. 2025, 135, 109772. [Google Scholar] [CrossRef]
- San, H.T.; Khine, H.E.E.; Sritularak, B.; Prompetchara, E.; Chaotham, C.; Che, C.T.; Likhitwitayawuid, K. Pinostrobin: An Adipogenic Suppressor from Fingerroot (Boesenbergia rotunda) and Its Possible Mechanisms. Foods 2022, 11, 3024–3039. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhonden, T.; Simal-Gandara, J.; Kopustinskiene, D.M.; Bernatoniene, J.; Pourbagher-Shahri, A.M.; Samarghandian, S. Promising Protective Effects of Chrysin in Cardiometabolic Diseases. Curr Drug Targets 2022, 23, 458–470. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, Y.; Ham, H.; Park, Y.; Jeong, H.S.; Lee, J. Nobiletin suppresses adipogenesis by regulating the expression of adipogenic transcription factors and the activation of AMP-activated protein kinase (AMPK). J. Agric. Food Chem. 2011, 59, 12843–12849. [Google Scholar] [CrossRef]
- Gautam, J.; Khedgikar, V.; Kushwaha, P.; Choudhary, D.; Nagar, G.K.; Dev, K.; Dixit, P.; Singh, D.; Maurya, R.; Trivedi, R. Formononetin, an isoflavone, activates AMP-activated protein kinase/β-catenin signalling to inhibit adipogenesis and rescues C57BL/6 mice from high-fat diet-induced obesity and bone loss. Br. J. Nutr. 2017, 117, 645–661. [Google Scholar] [CrossRef]
- Guo, L.; Kang, J.S.; Kang, N.J.; Je, B.I.; Lee, Y.J.; Park, Y.H.; Choi, Y.W. Pelargonidin suppresses adipogenesis in 3T3-L1 cells through inhibition of PPAR-γ signaling pathway. Arch. Biochem. Biophys. 2020, 686, 108365. [Google Scholar] [CrossRef]
- Saulite, L.; Jekabsons, K.; Klavins, M.; Muceniece, R.; Riekstina, U. Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes. Phytomedicine 2019, 53, 86–95. [Google Scholar] [CrossRef]
- Park, M.; Sharma, A.; Lee, H.J. Anti-Adipogenic Effects of Delphinidin-3-O-β-Glucoside in 3T3-L1 Preadipocytes and Primary White Adipocytes. Molecules 2019, 24, 1848–1859. [Google Scholar] [CrossRef]
- Kongthitilerd, P.; Barras, E.; Rong, W.; Thibodeaux, A.; Rigdon, M.; Yao, S.; Adisakwattana, S.; Suantawee, T.; Cheng, H. Cyanidin inhibits adipogenesis in 3T3-L1 preadipocytes by activating the PLC-IP3 pathway. Biomed. Pharmacother. 2023, 162, 114677. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Lee, H.S.; Jung, J.I.; Kim, S.M.; Kim, N.Y.; Seo, T.S.; Bae, J.S.; Kim, E.J. Cyanidin-3-O-galactoside-enriched Aronia melanocarpa extract attenuates weight gain and adipogenic pathways in high-fat diet-induced obese C57BL/6 mice. Nutrients 2019, 11, 1190–1205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, S.T.; Yang, X.; You, P.P.; Zhang, W. Myricetin suppresses differentiation of 3 T3-L1 preadipocytes and enhances lipolysis in adipocytes. Nutr. Res. 2015, 35, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.J.; Lee, M.K.; Im, J.H.; Kim, B.S.; Lee, H.I. Anti-obesity effects of ethanol extract of green Citrus junos peel enriched in naringin and hesperidin in vitro and in vivo. Nutr. Res. Pract. 2025, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, Y. Effects of Isorhamnetin on Adipocyte Mitochondrial Biogenesis and AMPK Activation. Molecules 2018, 23, 1853–1864. [Google Scholar] [CrossRef]
- Li, H.; Kang, J.H.; Han, J.M.; Cho, M.H.; Chung, Y.J.; Park, K.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Jeong, T.S. Anti-Obesity Effects of Soy Leaf via Regulation of Adipogenic Transcription Factors and Fat Oxidation in Diet-Induced Obese Mice and 3T3-L1 Adipocytes. J. Med. Food 2015, 18, 899–908. [Google Scholar] [CrossRef]
- Byun, M.R.; Jeong, H.; Bae, S.J.; Kim, A.R.; Hwang, E.S.; Hong, J.H. TAZ is required for the osteogenic and anti-adipogenic activities of kaempferol. Bone 2012, 50, 364–372. [Google Scholar] [CrossRef]
- Park, H.J.; Chung, B.Y.; Lee, M.K.; Song, Y.; Lee, S.S.; Chu, G.M.; Kang, S.N.; Song, Y.M.; Kim, G.S.; Cho, J.H. Centipede grass exerts anti-adipogenic activity through inhibition of C/EBPβ, C/EBPα, and PPARγ expression and the AKT signaling pathway in 3T3-L1 adipocytes. BMC Complement. Altern. Med. 2012, 12, 230. [Google Scholar] [CrossRef]
- Bae, C.R.; Park, Y.K.; Cha, Y.S. Quercetin-rich onion peel extract suppresses adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 adipocytes. J. Sci. Food Agric. 2014, 94, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Kang, O.H.; Kim, S.B.; Mun, S.H.; Kang, D.H.; Yang, D.W.; Choi, J.G.; Lee, Y.M.; Kang, D.K.; Lee, H.S.; et al. Quercetin prevents adipogenesis by regulation of transcriptional factors and lipases in OP9 cells. Int. J. Mol. Med. 2015, 35, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, J.S.; Seo, M.S.; Jung, J.W.; Lee, Y.S.; Kang, K.S. Genistein and daidzein repress adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells via Wnt/β-catenin signalling or lipolysis. Cell Prolif. 2010, 43, 594–605. [Google Scholar] [CrossRef]
- Crespillo, A.; Alonso, M.; Vida, M.; Pavon, F.J.; Serrano, A.; Rivera, P.; Romero-Zerbo, Y.; Fernandez-Llebrez, P.; Martinez, A.; Perez-Valero, V.; et al. Reduction of body weight, liver steatosis and ex-pression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 2011, 164, 1899–1915. [Google Scholar] [CrossRef]
- Choi, Y.R.; Shim, J.; Kim, M.J. Genistein: A Novel Potent Anti-Adipogenic and Anti-Lipogenic Agent. Molecules 2020, 25, 2042–2057, Correction in Molecules 2024, 29, 714. [Google Scholar] [CrossRef]
- Lao, W.; Tan, Y.; Jin, X.; Xiao, L.; Kim, J.J.Y.; Qu, X. Comparison of Cytotoxicity and the Anti-Adipogenic Effect of Green Tea Polyphenols with Epigallocatechin-3-Gallate in 3T3-L1 Preadipocytes. Am. J. Chin. Med. 2015, 43, 1177–1190. [Google Scholar] [CrossRef]
- Lee, H.; Bae, S.; Yoon, Y. The anti-adipogenic effects of (-)epigallocatechin gallate are dependent on the WNT/β-catenin pathway. J. Nutr. Biochem. 2013, 24, 1232–1240. [Google Scholar] [CrossRef]
- Wu, L.; Guo, T.; Deng, R.; Liu, L.; Yu, Y. Apigenin Ameliorates Insulin Resistance and Lipid Accumulation by Endoplasmic Reticulum Stress and SREBP-1c/SREBP-2 Pathway in Palmitate-Induced HepG2 Cells and High-Fat Diet-Fed Mice. J. Pharmacol. Exp. Ther. 2021, 377, 146–156. [Google Scholar] [CrossRef]
- Jung, U.J.; Cho, Y.Y.; Choi, M.S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients 2016, 8, 305–320. [Google Scholar] [CrossRef]
- Richard, A.J.; Amini-Vaughan, Z.; Ribnicky, D.M.; Stephens, J.M. Naringenin inhibits adipogenesis and reduces insulin sensitivity and adiponectin expression in adipocytes. Evid. Based Complement. Alternat. Med. 2013, 2013, 549750. [Google Scholar] [CrossRef]
- Sindhwani, R.; Bora, K.S.; Hazra, S. The dual challenge of diabesity: Pathophysiology, management, and future directions. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 4891–4912. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Venkatesan, V. Treatment of “Diabesity”: Beyond Pharmacotherapy. Curr. Drug Targets 2018, 19, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Bukhari, S.A.; Chauhdary, Z.; Akhter, N.; Noreen, R. Effect of resveratrol and quercetin on SFRP4 as a biomarker of diabesity: In silico and in vivo studies. Biochem. Biophys. Res. Commun. 2025, 761, 151748. [Google Scholar] [CrossRef]
- Potenza, M.A.; Iacobazzi, D.; Sgarra, L.; Montagnani, M. The Intrinsic Virtues of EGCG, an Extremely Good Cell Guardian, on Prevention and Treatment of Diabesity Complications. Molecules 2020, 25, 3061–3080. [Google Scholar] [CrossRef]
- Wolfram, S.; Raederstorff, D.; Preller, M.; Wang, Y.; Teixeira, S.R.; Riegger, C.; Weber, P. Epigallocatechin Gallate Supplementation Alleviates Diabetes in Rodents. J. Nutr. 2006, 136, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xia, M.; Zou, T.; Ling, W.; Zhong, R.; Zhang, W. Cyanidin-3-glucoside attenatues obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J. Nutr. Biochem. 2012, 23, 349–360. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Vrablik, M.; Al Rasadi, K.; Banach, M.; Toth, P.P.; Rizzo, M. Nutraceuticals in the management of Dyslipidemia: Which, When, and for Whom? Could Nutraceuticals Help Low-Risk Individuals with Non-optimal Lipid levels? Curr. Atheroscler. Rep. 2021, 23, 57–70. [Google Scholar] [CrossRef]
- Terzo, S.; Amato, A.; Magan-Fernandez, A.; Castellino, G.; Calvi, P.; Chianetta, R.; Giglio, R.V.; Patti, A.M.; Nikolic, D.; Firenze, A.; et al. A Nutraceutical Containing Cholorogenic Acid and Luteolin Improves cardiometabolic parameters in Subjects with Pre-Obesity: A 6-Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 462–471. [Google Scholar] [CrossRef]
- Naeini, F.; Namkhah, Z.; Tutunchi, H.; Rezayat, S.M.; Mansouri, S.; Yaseri, M.; Hosseinzadeh-Attar, M.J. Effects of naringenin supplementation on cardiovascular risk factors in overweight/obese patients with nonalcoholic fatty liver disease: A pilot double-blind, placebo-controlled, randomized clinical trial. Eur. J. Gastroenterol. Hepatol. 2022, 34, 345–353. [Google Scholar] [CrossRef]
- Namkhah, Z.; Naeini, F.; Rezayat, S.M.; Yaseri, M.; Mansouri, S.; Hosseinzadeh-Attar, M.J. Does naringenin supplementation improve lipid profile, severity of hepatic steatosis and probability of liver fibrosis in overweight/obese patients with NAFLD? A randomised, double-blind, placebo-controlled, clinical trial. Int. J. Clin. Pract. 2021, 75, e14852. [Google Scholar] [CrossRef]
- Barajas-Vega, J.L.; Raffoul-Orozco, A.K.; Hernandez-Molina, D.; Avila-Gonzalez, A.E.; Garcia-Cobian, T.A.; Ru-bio-Arellano, E.D.; Ramirez-Lizardo, E.J. Naringin reduces body weight, plasma lipids and increases adiponectin levels in patients with dyslipidemia. Int. J. Vitam. Nutr. Res. 2022, 92, 292–298. [Google Scholar] [CrossRef]
- Dodangeh, S.; Hasani-Ranjbar, S. Old and new anti-obesity drugs. J. Diabetes Metab. Disord. 2024, 24, 16–28. [Google Scholar] [CrossRef]
- Gregg, E.W.; Shaw, J.E. Global health effects of overweight and obesity. N. Engl. J. Med. 2017, 377, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Daneschvar, H.L.; Aronson, M.D.; Smetana, G.W. FDA-approved anti-obesity drugs in the United States. Am. J. Med. 2016, 129, 879.e1–879.e6. [Google Scholar] [CrossRef] [PubMed]
- Radwan, R.M.; Lee, Y.A.; Kotecha, P.; Wright, D.R.; Hernandez, I.; Ramon, R.; Donahoo, W.T.; Chen, Y.; Allen, J.M.; Bian, J.; et al. Regional trends and disparities in newer GLP1 receptor agonist initiation among real-world adult patients eligible for obesity treatment. Diabetes Obese Metab. 2025, 27, 3113–3123. [Google Scholar] [CrossRef]
- Pressley, H.; Cornelio, C.K.; Adams, E.N. Setmelanotide: A novel targeted treatment for monogenic obesity. J. Pharm. Technol. 2022, 38, 368–373. [Google Scholar] [CrossRef]
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef]
- Jakubowska, A.; le Roux, C.W.; Viljoen, A. The Road towards Triple agonists: Glucagon-Like peptide 1, glucose-dependent Insulinotropic polypeptide and glucagon Receptor-An update. Endocrinol. Metab. 2024, 39, 12–22. [Google Scholar] [CrossRef]
- Melson, E.; Miras, A.D.; Papamargaritis, D. Future therapies for obesity. Clin. Med. 2023, 23, 337–346. [Google Scholar] [CrossRef]
- Rehman, K.; Munawar, S.M.; Akash, M.S.H.; Buabeid, M.A.; Chohan, T.A.; Tariq, M.; Jabeen, K.; Arafa, E.S.A. Hesperidin improves insulin resistance via down-regulation of inflammatory responses: Biochemical analysis and in silico validation. PLoS ONE 2020, 15, e0227637, Correction in PLoS ONE 2020, 15, e0229348. [Google Scholar]
- Nishimura, S.; Manabe, I.; Nagai, R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov. Med. 2009, 8, 55–60. [Google Scholar] [PubMed]
- Tilinca, M.C.; Antal, C.; Balint, A.; Salcudean, A.; Varga, A. The newest therapeutically approach of “diabesity” using GLP-1 ra molecules: Impact of the oral formulation. Farmacia 2023, 71, 1–10. [Google Scholar] [CrossRef]
- Allocca, S.; Monda, A.; Messina, A.; Casillo, M.; Sapuppo, W.; Monda, V.; Polito, R.; Di Maio, G.; Monda, M.; La Marra, M. Endocrine and Metabolic Mechanisms Linking Obesity to Type 2 Diabetes: Implications for Targeted Therapy. Healthcare 2025, 13, 1437–1464. [Google Scholar] [CrossRef]
- Zou, C.; Shao, J. Role of adipocytokines in obesity-associated insulin resistance. J. Nutr. Biochem. 2008, 19, 277–286. [Google Scholar] [CrossRef]
- Gaballah, H.H.; Zakaria, S.S.; Mwafy, S.E.; Tahoon, N.M.; Ebeid, A.M. Mechanistic insights into the effects of quercetin and/or GLP-1 analogue liraglutide on high-fat diet/streptozotocin-induced type 2 diabetes in rats. Biomed. Pharmacother. 2017, 92, 331–339. [Google Scholar] [CrossRef]
- Eraky, S.M.; Ramadan, N.M.; El-Magd, N.F.A. Antidiabetic effects of quercetin and liraglutide combination through modulation of TXNIP/IRS-1/PI3K pathway. Cell Biochem. Funct. 2022, 40, 90–102. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal. Transduct. Target Ther. 2024, 9, 234–262. [Google Scholar] [CrossRef] [PubMed]
- Oriquat, G.; Masoud, I.M.; Kamel, M.A.; Aboudeya, H.M.; Bakir, M.B.; Shaker, S.A. The Anti-Obesity and Anti-Steatotic Effects of Chrysin in a rat Model of Obesity Mediated through Modulating the Hepatic AMPK/mTOR/lipogenesis Pathways. Molecules 2023, 28, 1734–1752. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, D.S.; Jung, U.J. Efficacy of nobiletin in improving hypercholesterolemia and non-alcoholic fatty liver disease in high-cholesterol diet-fed mice. Nutr. Res. Pract. 2021, 15, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Colletti, A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine 2016, 23, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Giordani, C.; Antonelli, A.; Petrelli, M.; Balercia, G.; Silvetti, F.; Pieroni, A.; Sabbatinelli, J.; Rippo, M.R.; Olivieri, F.; et al. The Combination of Natural Molecules Naringenin, Hesperetin, Curcumin, Polydatin and Quercetin Synergistically Decreases SEMA3E Expression Levels and DPPIV Activity in In Vitro Models of Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 8071–8087. [Google Scholar] [CrossRef] [PubMed]

| Adipocytokines | Biological Activities | Pathologic Alterations in Obesity | References |
|---|---|---|---|
| Leptin | Increase in energy consumption; inhibition of body weight gain | Leptin deficiency and leptin resistance determine an increase in body weight | [17,18] |
| Adiponectin | Increase in insulin sensitivity; induction of fatty acid oxidation; decrease in triglyceride levels; inhibition of gluconeogenesis | Adiponectin decrease determines the development of insulin resistance and metabolic dysfunctions and the reduction in fatty acid oxidation | [20,21,22,23] |
| TNF-α | Induction of inflammation; induction of insulin resistance; induction of gluconeogenesis | TNF-α increase contributes to a chronic inflammatory state and to the development of metabolic dysfunctions | [16,25,26] |
| IL-6 | Induction of inflammation | IL-6 increase contributes to a chronic inflammatory state | [16] |
| IL-8 | Induction of inflammation | IL-8 increase contributes to a chronic inflammatory state | [16] |
| IL-1β | Induction of inflammation | IL-1β increase contributes to a chronic inflammatory state | [16] |
| MCP-1 | Induction of inflammation | MCP-1 increase contributes to a chronic inflammatory state | [16] |
| Flavonoids | Study Type | Epigenetic Regulations | References |
|---|---|---|---|
| EGCG | In vivo | Inhibition of DNA methylation; inhibition of histone methylation | [58] |
| Genistein | In vitro | Inhibition of DNA methylation; modulation of miRNA expression | [59] |
| Apigenin | In vivo | Inhibition of DNA methylation; inhibition of histone deacetylation | [60] |
| Naringenin | In vitro; in vivo | Modulation of miRNA expression; inhibition of histone deacetylation | [61,62] |
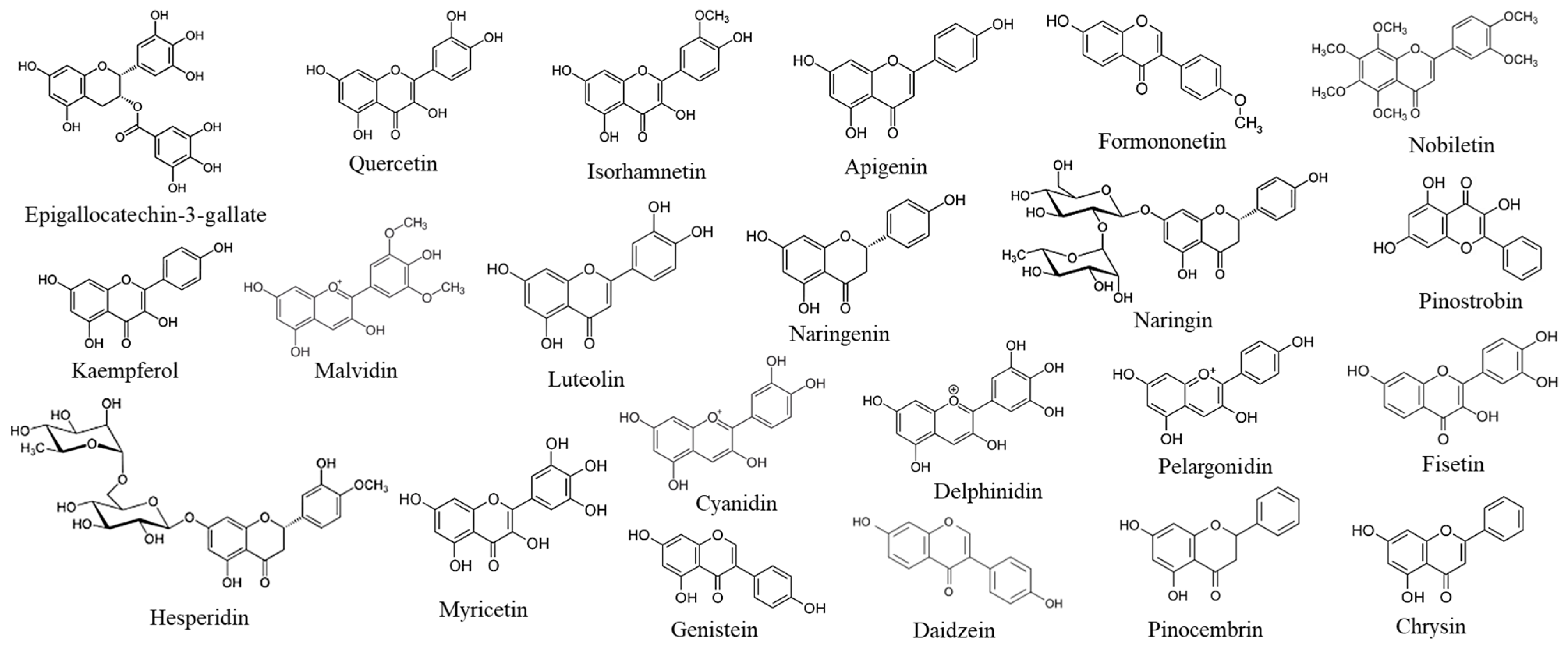
| Flavonoids | Flavonoid Chemical Families | References |
|---|---|---|
| Pelargonidin | Anthocyanins | [43,44,45,46] |
| Malvidin | Anthocyanins | [43,44,45,46] |
| Delphinidin | Anthocyanins | [43,44,45,46] |
| Cyanidin | Anthocyanins | [43,44,45,46] |
| Chrysin | Flavones | [43,44,45,46] |
| Nobiletin | Flavones | [43,44,45,46] |
| Luteolin | Flavones | [43,44,45,46] |
| Apigenin | Flavones | [43,44,45,46] |
| Pinocembrin | Flavanones | [43,44,45,46] |
| Pinostrobin | Flavanones | [43,44,45,46] |
| Hesperidin | Flavanones | [43,44,45,46] |
| Naringin | Flavanones | [43,44,45,46] |
| Naringenin | Flavanones | [43,44,45,46] |
| Formononetin | Isoflavones | [43,44,45,46] |
| Daidzein | Isoflavones | [43,44,45,46] |
| Genistein | Isoflavones | [43,44,45,46] |
| Fisetin | Flavonols | [43,44,45,46] |
| Myricetin | Flavonols | [43,44,45,46] |
| Isorhamnetin | Flavonols | [43,44,45,46] |
| Kaempferol | Flavonols | [43,44,45,46] |
| Quercetin | Flavonols | [43,44,45,46] |
| EGCG | Flavanols | [43,44,45,46] |
| Flavonoids | Study Type | Administration Mode | Biological Activities | Outcomes of Studies | References |
|---|---|---|---|---|---|
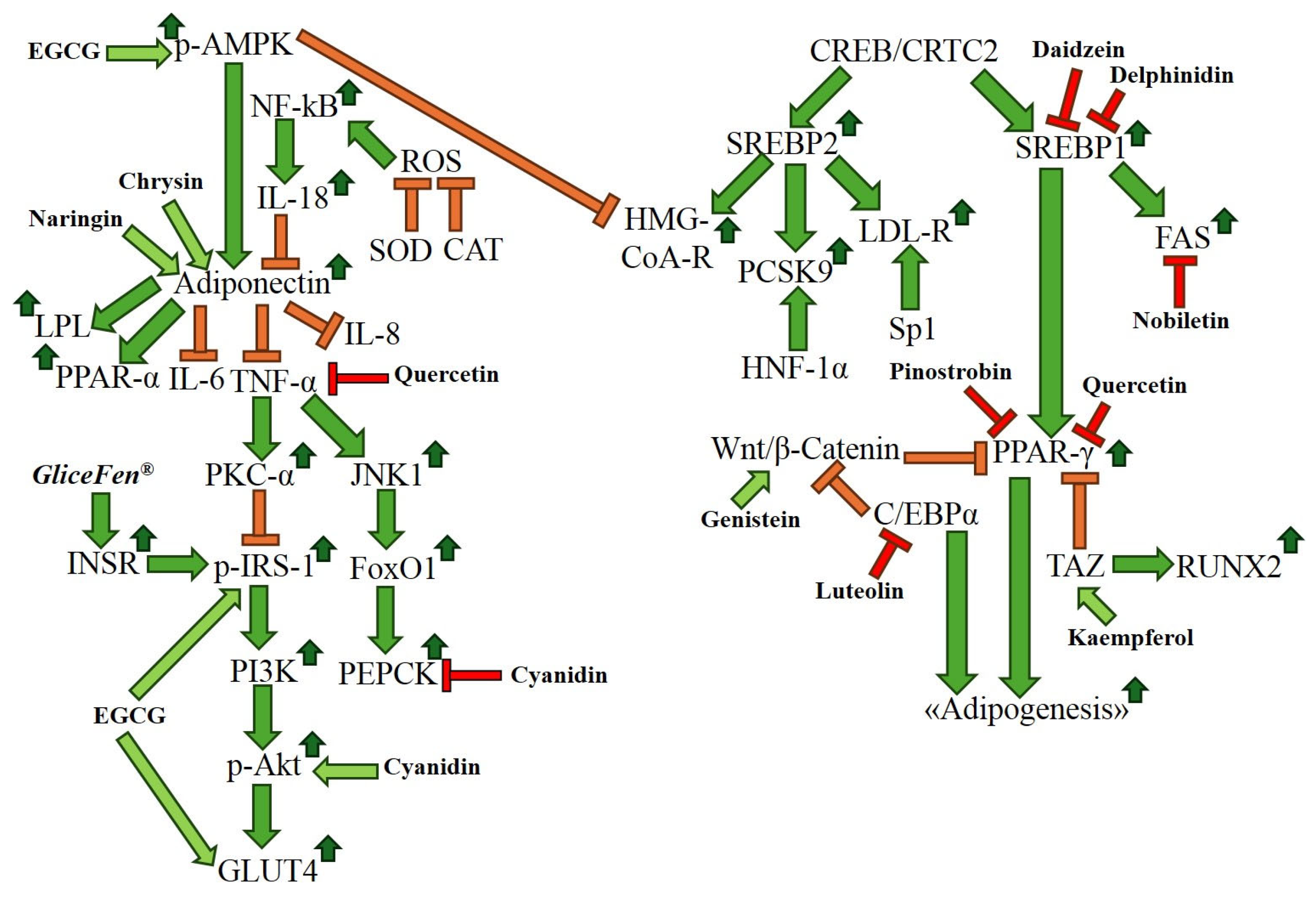
| Fisetin | In vitro | Isolated flavonoid | Anti-adipogenic | Increase: β-catenin Decrease: PPAR-γ | [64,65] |
| Pinocembrin | In vitro; in vivo | Flavonoid-enriched extract; isolated flavonoid | Anti-adipogenic | Decrease: PPAR-γ, SREBP1 | [66,68] |
| Pinostrobin | In vitro; in vivo | Flavonoid-enriched extract; isolated flavonoid | Anti-adipogenic | Decrease: PPAR-γ, SREBP1 | [66,69] |
| Chrysin | In vitro; in vivo | Isolated flavonoid | Anti-adipogenic | Decrease: PPAR-γ | [70] |
| Nobiletin | In vitro | Isolated flavonoid | Anti-adipogenic | Decrease: PPAR-γ, C/EBPα | [71] |
| Formononetin | In vitro | Isolated flavonoid | Anti-adipogenic | Increase: β-catenin Decrease: C/EBPα, PPAR-γ | [72] |
| Pelargonidin | In vitro | Isolated flavonoid | Anti-adipogenic | Decrease: PPAR-γ | [73] |
| Malvidin | In vitro | Isolated flavonoid | Anti-adipogenic | Increase: RUNX-2, BMP-2 | [74] |
| Delphinidin | In vitro | Isolated flavonoid | Anti-adipogenic | Decrease: FABP4, PPAR-γ, SREBP1, C/EBPα | [74,75] |
| Cyanidin | In vitro; in vivo | Flavonoid-enriched extract; isolated flavonoid | Anti-adipogenic | Decrease: PPAR-γ, C/EBPα, SREBP1 | [76,77] |
| Myricetin | In vitro | Isolated flavonoid | Anti-adipogenic | Decrease: PPAR-γ, C/EBPα, SREBP1 | [78] |
| Hesperidin | In vitro; in vivo | Flavonoid-enriched extract | Anti-adipogenic | Decrease: C/EBPα, SREBP1, PPAR-γ | [79] |
| Naringin | In vitro; in vivo | Flavonoid-enriched extract | Anti-adipogenic | Decrease: SREBP1, C/EBPα, PPAR-γ | [79] |
| Isorhamnetin | In vitro | Isolated flavonoid | Anti-adipogenic | Decrease: PPAR-γ | [80] |
| Kaempferol | In vitro | Isolated flavonoid | Anti-adipogenic | Increase: TAZ, RUNX2 Decrease: PPAR-γ, C/EBPα, SREBP1 | [81,82] |
| Luteolin | In vitro | Flavonoid-enriched extract | Anti-adipogenic | Decrease: C/EBPα, PPAR-γ | [83] |
| Quercetin | In vitro | Flavonoid-enriched extract; isolated flavonoid | Anti-adipogenic | Decrease: PPAR-γ, C/EBPα, SREBP1 | [84,85] |
| Daidzein | In vitro; in vivo | Isolated flavonoid | Anti-adipogenic | Increase: Wnt/β-catenin Decrease: PPAR-γ, SREBP1 | [86,87] |
| Genistein | In vitro | Isolated flavonoid | Anti-adipogenic; regulation of epigenetics | Increase: Wnt/β-catenin Decrease: PPAR-γ, C/EBPα, SREBP1, DNMT | [59,86,88] |
| EGCG | In vitro | Isolated flavonoid | Anti-adipogenic; regulation of epigenetics | Increase: Wnt/β-catenin Decrease: PPAR-γ, SREBP1, C/EBPα, DNMT, HMT | [58,89,90] |
| Apigenin | In vitro; in vivo | Isolated flavonoid | Anti-adipogenic; regulation of epigenetics | Decrease: SREBP1, PPAR-γ, DNMT, HDAC | [60,91,92] |
| Naringenin | In vitro | Isolated flavonoid | Anti-adipogenic; regulation of epigenetics | Decrease: PPAR-γ, Stat5A, HDAC | [62,93] |
| Flavonoids | Study Type | Administration Mode | Outcomes of Studies | References |
|---|---|---|---|---|
| Quercetin | In vitro | Isolated flavonoid | Increase: SOD, CAT Decrease: IL-6, TNF-α, SFRP4 | [96] |
| EGCG | In vivo | Isolated flavonoid | Increase: p-AMPK, p-IRS-1, GLUT4 Decrease: PEPCK | [97,98] |
| Cyanidin | In vivo | Isolated flavonoid | Increase: p-Akt Decrease: TNF-α, MCP-1, FAS, SREBP1, PEPCK | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpa, E.-S.; Amatori, S.; Caprioli, G.; Maggi, F.; Moroncini, G.; Balercia, G.; Giacchetti, G. Innovative Therapeutic Approaches Targeting Obesity: Can Flavonoids Improve the Efficacy of Anti-Obesogenic Drugs? Int. J. Mol. Sci. 2025, 26, 10142. https://doi.org/10.3390/ijms262010142
Scarpa E-S, Amatori S, Caprioli G, Maggi F, Moroncini G, Balercia G, Giacchetti G. Innovative Therapeutic Approaches Targeting Obesity: Can Flavonoids Improve the Efficacy of Anti-Obesogenic Drugs? International Journal of Molecular Sciences. 2025; 26(20):10142. https://doi.org/10.3390/ijms262010142
Chicago/Turabian StyleScarpa, Emanuele-Salvatore, Stefano Amatori, Giovanni Caprioli, Filippo Maggi, Gianluca Moroncini, Giancarlo Balercia, and Gilberta Giacchetti. 2025. "Innovative Therapeutic Approaches Targeting Obesity: Can Flavonoids Improve the Efficacy of Anti-Obesogenic Drugs?" International Journal of Molecular Sciences 26, no. 20: 10142. https://doi.org/10.3390/ijms262010142
APA StyleScarpa, E.-S., Amatori, S., Caprioli, G., Maggi, F., Moroncini, G., Balercia, G., & Giacchetti, G. (2025). Innovative Therapeutic Approaches Targeting Obesity: Can Flavonoids Improve the Efficacy of Anti-Obesogenic Drugs? International Journal of Molecular Sciences, 26(20), 10142. https://doi.org/10.3390/ijms262010142