Computational Analysis of Electron-Donating and Withdrawing Effects on Asymmetric Viologens for Enhanced Electrochromic Performance
Abstract
1. Introduction
2. Results and Discussion
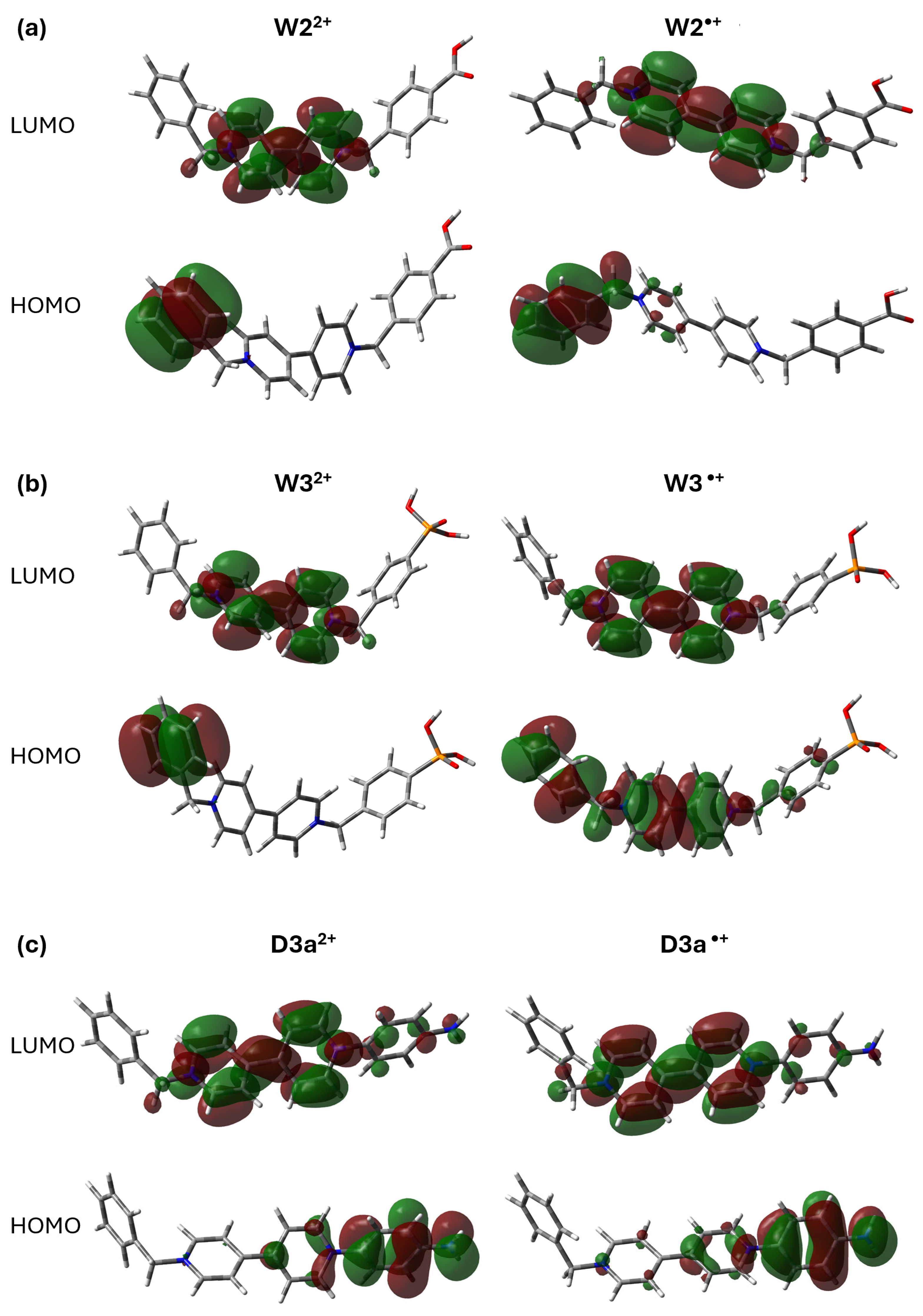
2.1. Electronic Structure and Bonding Analysis of Asymmetric Viologen
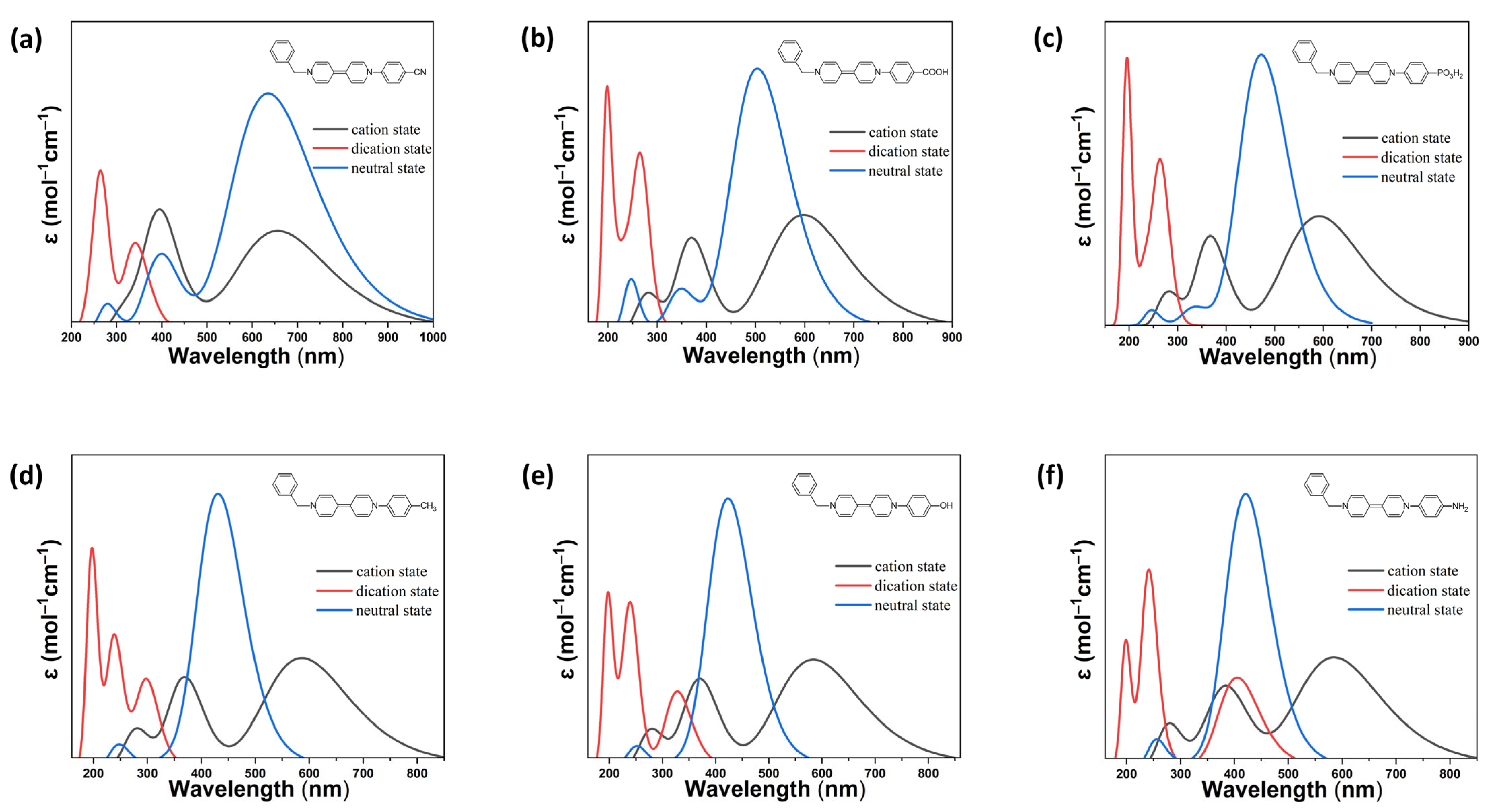
2.2. Optical Behavior of Asymmetric Viologens
2.3. EC Properties
3. Methods and Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nuroldayeva, G.; Balanay, M.P. Flexing the spectrum: Advancements and prospects of flexible electrochromic materials. Polymers 2023, 15, 2924. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhang, M.; Huo, X.; Guo, M. Toward next-generation smart windows: An in-depth analysis of dual-band electrochromic materials and devices. Adv. Opt. Mater. 2024, 12, 2302344. [Google Scholar] [CrossRef]
- Sudarshan, B.; Shantanu, B.; Srivaths, S.; Neethu, S.; Manjunatha, C. Metal oxide based electrochromic materials: Recent advances in synthesis, characterization, and applications. ECS Trans. 2022, 107, 18583–18591. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, L.; Dong, Y.; Zhang, C.; Li, W. Recent advances in electrochromic materials and devices for camouflage applications. Mater. Chem. Front. 2023, 7, 2337–2358. [Google Scholar] [CrossRef]
- Fu, H.; Tang, Y.; Zhan, F.; Zhang, L.; Zhan, W.; Dong, Y.; Li, W.; Zhang, C. Dual polymer electrochromic sunglasses with black to anti-blue-ray conversion based on new anti-blue-ray transparent polymer. Chem. Eng. J. 2023, 461, 141848. [Google Scholar] [CrossRef]
- Gu, C.; Jia, A.-B.; Zhang, Y.-M.; Zhang, S.X.-A. Emerging electrochromic materials and devices for future displays. Chem. Rev. 2022, 122, 14679–14721. [Google Scholar] [CrossRef] [PubMed]
- Shchegolkov, A.V.; Jang, S.-H.; Shchegolkov, A.V.; Rodionov, Y.V.; Sukhova, A.O.; Lipkin, M.S. A brief overview of electrochromic materials and related devices: A nanostructured materials perspective. Nanomaterials 2021, 11, 2376. [Google Scholar] [CrossRef]
- Sun, F.; Eom, J.H.; Kim, D.Y.; Pande, G.K.; Ju, H.; Chae, H.G.; Park, J.S. Large-area flexible electrochromic devices with high-performance and low-power consumption enabled by hydroxyhexyl viologen-substituted polyhedral oligomeric silsesquioxane. ACS Sustain. Chem. Eng. 2023, 11, 5756–5763. [Google Scholar] [CrossRef]
- Li, S.L.; Shen, Y.; Yang, W.; Wang, Y.J.; Qi, Z.; Zhang, J.; Zhang, X.M. A photo-responsive charge-assisted hydrogen-bonded organic network with ultra-stable viologen radicals. Chin. J. Chem. 2022, 40, 351–356. [Google Scholar] [CrossRef]
- Yin, T.; Duanmu, J.; Liu, L. Viologen-based aqueous organic redox flow batteries: Materials synthesis, properties, and cell performance. J. Mater. Chem. A 2024, 12, 15519–15540. [Google Scholar] [CrossRef]
- Chorol, S.; Saini, P.; Mukhopadhyay, P. Synthesis and properties of electron-deficient and electron-rich redox-active ionic π-systems. Chem. Rec. 2022, 22, e202200172. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, H.; Han, Y.; Liu, J. Molecular engineering of π-extended viologens consisting of quinoxaline-based bridges for tunable electrochromic devices. J. Mol. Struct. 2022, 1262, 133073. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, J. Structures and photochromic behavior of viologen-based compounds: Insights from Mn(II) and Cd(II) complexes. Inorg. Chem. Acta 2024, 572, 122265. [Google Scholar] [CrossRef]
- Balamurugan, G.; Park, J.S. Metallo-terpyridine modified asymmetric viologen exhibiting remarkable optical memory effect in single-layered electrochromic devices. Electrochim. Acta 2021, 382, 138308. [Google Scholar] [CrossRef]
- Qian, C.; Wang, P.; Guo, X.; Jiang, C.; Liu, P. High-contrast energy-efficient flexible electrochromic devices based on viologen derivatives and their application in smart windows and electrochromic displayers. Sol. Energy Mater. Sol. Cells 2024, 266, 112669. [Google Scholar] [CrossRef]
- Sydam, R.; Silori, G.K.; Hsu, H.-C.; Ho, K.-C. Dual-use synthesis of an asymmetric anthraquinone heptyl viologen (AQHV) for solution and gel-polymer electrolyte-based electrochromic devices. J. Mater. Chem. C 2025, 13, 11342–11356. [Google Scholar] [CrossRef]
- Kim, T.; Feng, Y.; O’Connor, J.P.; Stoddart, J.F.; Young, R.M.; Wasielewski, M.R. Coherent vibronic wavepackets show structure-directed charge flow in host–guest donor–acceptor complexes. J. Am. Chem. Soc. 2023, 145, 8389–8400. [Google Scholar] [CrossRef]
- Shi, Y.-S.; Xiao, T.; Yang, D.-D.; Zheng, X.-J. Incorporation of a one-dimensional electron-rich chain in viologen-based coordination polymers for super photochromic properties and improved optical applications in polyvinyl alcohol film. Inorg. Chem. Front. 2024, 11, 3200–3210. [Google Scholar] [CrossRef]
- Ambrose, B.; Naresh, R.; Kathiresan, M.; Ulaganathan, M.; Ragupathy, P. Highly stable asymmetric viologen as an anolyte for aqueous organic and halide-based redox flow batteries. Energy Tech. 2023, 11, 2201046. [Google Scholar] [CrossRef]
- Ambrose, B.; Krishnan, M.; Ramamurthy, K.; Kathiresan, M. Recent advances in molecular engineering for viologen-based electrochromic materials: A mini-review. Adv. Photonics Res. 2024, 5, 2400016. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.M.; Moon, H.C. Asymmetric molecular modification of viologens for highly stable electrochromic devices. RSC Adv. 2020, 10, 394–401. [Google Scholar] [CrossRef]
- Cai, G.; Eh, A.L.-S.; Ji, L.; Lee, P.S. Recent advances in electrochromic smart fenestration. Adv. Sustain. Syst. 2017, 1, 1700074. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Displays 2006, 27, 2–18. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. Viologen-based electrochromic materials and devices. J. Mater. Chem. C 2019, 7, 4622–4637. [Google Scholar] [CrossRef]
- Striepe, L.; Baumgartner, T. Viologens and their application as functional materials. Chem. Eur. J. 2017, 23, 16924–16940. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zheng, C.; Wang, L.; Lu, C.; Zhang, B.; Chen, Y.; Li, M.; Zhai, G.; Zhuang, X. Viologen-inspired functional materials: Synthetic strategies and applications. J. Mater. Chem. A 2019, 7, 23337–23360. [Google Scholar] [CrossRef]
- Hashemi, D.; Ma, X.; Ansari, R.; Kim, J.; Kieffer, J. Design principles for the energy level tuning in donor/acceptor conjugated polymers. Phys. Chem. Chem. Phys. 2019, 21, 789–799. [Google Scholar] [CrossRef]
- Berova, N.; Bari, L.D.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef]
- Song, I.; You, L.; Chen, K.; Lee, W.-J.; Mei, J. Chiroptical switching of electrochromic polymer thin films. Adv. Mater. 2024, 36, 2307057. [Google Scholar] [CrossRef]
- Pospíšil, L.; Bednárová, L.; Štěpánek, P.; Slavíček, P.; Vávra, J.; Hromadová, M.; Dlouhá, H.; Tarábek, J.; Teplý, F. Intense chiroptical switching in a dicationic helicene-like derivative: Exploration of a viologen-type redox manifold of a non-racemic helquat. J. Am. Chem. Soc. 2014, 136, 10826–10829. [Google Scholar] [CrossRef]
- Saielli, G. TD-DFT prediction of the intermolecular charge-transfer UV-Vis spectra of viologen salts in solution. Appl. Sci. 2020, 10, 8108. [Google Scholar] [CrossRef]
- Autschbach, J.; Jorge, F.E.; Ziegler, T. Density functional calculations on electronic circular dichroism spectra of chiral transition metal complexes. Inorg. Chem. 2003, 42, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.W.; Wang, S.-X.; Soo, D.X.Y.; Xu, J. Viologen-based electrochromic materials: From small molecules, polymers and composites to their applications. Polymers 2019, 11, 1839. [Google Scholar] [CrossRef] [PubMed]
- Nachimuthu, S.; Shie, W.-R.; Liaw, D.-J.; Romashko, R.V.; Jiang, J.-C. Theoretical study of electrochemical and electrochromic properties of novel viologen derivatives: Effects of donors and π-conjugation. J. Phys. Chem. B 2019, 123, 4735–4744. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Send, R.; Kühn, M.; Furche, F. Assessing excited state methods by adiabatic excitation energies. J. Chem. Theory Comput. 2011, 7, 2376–2386. [Google Scholar] [CrossRef]
- Jacquemin, D.; Perpète, E.A. On the basis set convergence of TD-DFT oscillator strengths: Dinitrophenylhydrazones as a case study. J. Mol. Struct.THEOCHEM 2007, 804, 31–34. [Google Scholar] [CrossRef]
- Martin, R.L. Natural transition orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Yu, J.; Su, N.Q.; Yang, W. Describing chemical reactivity with frontier molecular orbitalets. JACS Au 2022, 2, 1383–1394. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.1.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016.



| Structures | 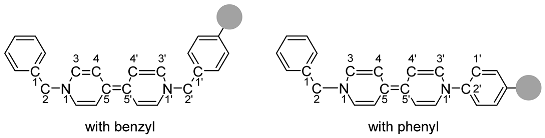 | |||||
|---|---|---|---|---|---|---|
| Bond Lengths (Å) | Torsion Angles (Degrees) | |||||
| N1-C2 | C5-C5′ | N1′-C2′ | C1-C2-N1-C3 | C4-C5-C5′-C4′ | C3′-N1′-C2′-C1′ | |
| (a) Neutral state | ||||||
| W1 | 1.445 | 1.370 | 1.441 | 86.01 | 0.11 | 84.92 |
| W2 | 1.444 | 1.370 | 1.442 | 85.81 | 0.10 | 85.36 |
| W3 | 1.445 | 1.370 | 1.442 | 85.96 | 0.11 | 86.05 |
| D1 | 1.444 | 1.370 | 1.445 | 85.62 | 0.05 | 85.63 |
| D2 | 1.444 | 1.370 | 1.445 | 85.64 | 0.06 | 85.31 |
| D3 | 1.444 | 1.371 | 1.454 | 85.85 | 0.23 | 60.05 |
| W1a | 1.447 | 1.373 | 1.395 | 86.88 | 1.30 | 29.38 |
| W2a | 1.447 | 1.373 | 1.398 | 86.84 | 1.30 | 29.80 |
| W3a | 1.447 | 1.373 | 1.398 | 86.86 | 0.64 | 30.24 |
| D1a | 1.445 | 1.372 | 1.411 | 86.10 | 0.54 | 36.92 |
| D2a | 1.445 | 1.371 | 1.413 | 86.10 | 0.91 | 40.62 |
| D3a | 1.444 | 1.371 | 1.415 | 86.07 | 0.47 | 42.33 |
| (b) Radical cation state | ||||||
| W1 | 1.467 | 1.424 | 1.464 | 88.46 | 0.08 | 88.52 |
| W2 | 1.481 | 1.424 | 1.474 | 88.44 | 0.01 | 88.55 |
| W3 | 1.481 | 1.424 | 1.475 | 49.92 | 0.15 | 56.26 |
| D1 | 1.466 | 1.424 | 1.467 | 88.37 | 0.07 | 88.35 |
| D2 | 1.466 | 1.424 | 1.467 | 88.41 | 0.05 | 88.53 |
| D3 | 1.478 | 1.424 | 1.489 | 47.61 | 0.07 | 38.46 |
| W1a | 1.483 | 1.425 | 1.433 | 45.06 | 2.37 | 49.42 |
| W2a | 1.482 | 1.424 | 1.435 | 45.89 | 2.35 | 49.52 |
| W3a | 1.482 | 1.424 | 1.436 | 49.53 | 0.98 | 50.34 |
| D1a | 1.481 | 1.424 | 1.483 | 47.06 | 0.92 | 53.64 |
| D2a | 1.480 | 1.424 | 1.439 | 47.02 | 0.87 | 56.59 |
| D3a | 1.479 | 1.424 | 1.439 | 47.83 | 0.87 | 56.62 |
| (c) Dicationic state | ||||||
| W1 | 1.526 | 1.487 | 1.519 | 4.01 | 42.35 | 19.99 |
| W2 | 1.526 | 1.487 | 1.519 | 4.18 | 42.29 | 23.39 |
| W3 | 1.526 | 1.487 | 1.523 | 3.86 | 42.14 | 10.38 |
| D1 | 1.525 | 1.487 | 1.528 | 3.45 | 42.43 | 0.04 |
| D2 | 1.525 | 1.487 | 1.532 | 3.17 | 42.18 | 5.08 |
| D3 | 1.524 | 1.487 | 1.541 | 5.84 | 42.17 | 1.55 |
| W1a | 1.527 | 1.487 | 1.457 | 1.07 | 41.88 | 56.74 |
| W2a | 1.526 | 1.487 | 1.457 | 2.12 | 41.78 | 55.40 |
| W3a | 1.526 | 1.487 | 1.457 | 3.54 | 42.26 | 55.48 |
| D1a | 1.525 | 1.486 | 1.454 | 4.85 | 41.86 | 52.60 |
| D2a | 1.525 | 1.486 | 1.449 | 1.18 | 40.75 | 50.60 |
| D3a | 1.524 | 1.484 | 1.440 | 3.87 | 39.94 | 45.06 |
| Cpds | State | λ | f | Transition Assignment (%) | HOMO | LUMO | GH-L |
|---|---|---|---|---|---|---|---|
| W12+ | S0 → S5 | 244 | 0.511 | H-2 → L = 19 | −9.01 | −2.55 | 6.46 |
| S0 → S7 | 239 | 0.487 | H-2 → L = 17 | ||||
| S0 → S10 | 227 | 0.515 | H-2 → L+1 = 42 | ||||
| S0 → S13 | 199 | 0.658 | H-1 → L+5 = 12; H → L+5 = 31 | ||||
| S0 → S16 | 194 | 0.841 | H-3 → L+1 = 1; H-3 → L+6 = 22 | ||||
| W22+ | S0 → S8 | 279 | 0.578 | H-7 → L+1 = 29; H-2 → L = 11 | −9.01 | −2.54 | 6.47 |
| S0 → S11 | 239 | 0.487 | H-3 → L+1 = 22; H-3 → L+2 = 20 | ||||
| S0 → S14 | 228 | 0.423 | H-1 → L+5 = 14; H → L+5 = 28 | ||||
| S0 → S15 | 194 | 0.841 | H-3 → L+3 = 16; H-3 → L+6 = 6; H-6 → L+3 = 1 | ||||
| W32+ | S0 → S4 | 247 | 0.258 | H-4 → L = 37; H-4 → L+1 = 2 | −9.01 | −2.53 | 6.48 |
| S0 → S7 | 239 | 0.487 | H-7 → L = 25; H-2 → L+1 = 1 | ||||
| S0 → S14 | 239 | 0.474 | H-2 → L+2 = 20; H-3 → L+4 = 5 | ||||
| S0 → S19 | 194 | 0.841 | H-1 → L+8 = 26; H-1 → L+3 = 2 | ||||
| D12+ | S0 → S5 | 247 | 0.265 | H-4 → L = 39; H-1 → L = 3 | −8.66 | −2.49 | 6.17 |
| S0 → S7 | 239 | 0.487 | H-6 → L = 41; H-4 → L = 2 | ||||
| S0 → S15 | 240 | 0.908 | H-2 → L+2 = 20; H-2 → L+3 = 12 | ||||
| S0 → S18 | 194 | 0.841 | H → L+7 = 26 | ||||
| D22+ | S0 → S5 | 246 | 0.313 | H-5 → L = 19; H-4 → L = 18; H-1 → L = 6 | −8.24 | −2.48 | 5.76 |
| S0 → S8 | 239 | 0.748 | H-6 → L = 35; H-5 → L = 1 | ||||
| S0 → S18 | 198 | 0.687 | H-1 → L+2 = 20; H-1 → L+3 = 13 | ||||
| D32+ | S0 → S6 | 250 | 0.945 | H → L+3 = 6; H → L+5 = 9; H → L+6 = 5 | −7.42 | −2.46 | 4.96 |
| S0 → S11 | 239 | 0.625 | H-6 → L = 34; H → L+7 = 2 | ||||
| S0 → S18 | 200 | 0.532 | H-1 → L+3 = 36; H-1 → L+8 = 8 | ||||
| W1a2+ | S0 → S1 | 267 | 0.841 | H-6 → L = 5; H-2 → L = 27 | −9.02 | −2.73 | 6.29 |
| S0 → S7 | 234 | 0.307 | H-6 → L = 39 H-2 → L+1 = 3 | ||||
| S0 → S13 | 199 | 0.592 | H → L+3 = 21; H → L+4 = 10 | ||||
| S0 → S18 | 194 | 0.929 | H-3 → L+2 = 22 | ||||
| W2a2+ | S0 → S1 | 269 | 1.001 | H-3 → L = 33 H-1 → L = 2 | −9.02 | −2.70 | 6.32 |
| S0 → S7 | 234 | 0.345 | H-7 → L = 40; H-3 → L = 2 | ||||
| S0 → S14 | 199 | 0.619 | H → L+1 = 1; H → L+4 = 26 | ||||
| S0 → S16 | 196 | 0.570 | H-3 → L+2 = 4; H-2 → L+2 = 26 | ||||
| W3a2+ | S0 → S1 | 268 | 0.953 | H-3 → L+1 = 3; H-3 → L = 35; H-1 → L = 2 | −9.02 | −2.68 | 6.34 |
| S0 → S7 | 231 | 0.356 | H-8 → L = 31; H-5 → L = 14; H-4 → L = 11 | ||||
| S0 → S13 | 199 | 0.642 | H → L+3 = 31 | ||||
| S0 → S17 | 194 | 0.654 | H-2 → L+2 = 27; H-3 → L+4 = 4 | ||||
| S0 → S20 | 188 | 0.305 | H-4 → L+1 = 11; H → L+8 = 7 | ||||
| D1a2+ | S0 → S1 | 299 | 0.605 | H-6 → L = 1; H → L = 43 | −8.90 | −2.64 | 6.26 |
| S0 → S7 | 237 | 0.698 | H-6 → L = 44; H → L+1 = 1 | ||||
| S0 → S15 | 199 | 0.587 | H-4 → L+3 = 2; H-2 → L+4 = 12; H-1 → L+3 = 30 | ||||
| S0 → S19 | 192 | 0.528 | H-3 → L+6 = 18; H → L+5 = 15 | ||||
| D2a2+ | S0 → S1 | 328 | 0.561 | H → L = 44; H → L+1 = 4 | −8.44 | −2.63 | 5.81 |
| S0 → S8 | 237 | 0.625 | H-6 → L = 36; H → L+5 = 5 | ||||
| S0 → S15 | 199 | 0.667 | H-2 → L+4 = 12; H-1 → L+2 = 4; H-1 → L+3 = 27 | ||||
| D3a2+ | S0 → S1 | 405 | 0.628 | H → L = 44; H → L+1 = 4 | −7.55 | −2.61 | 4.94 |
| S0 → S10 | 240 | 0.937 | H-7 → L = 4; H-6 → L = 23; H-4 → L = 16 | ||||
| S0 → S18 | 198 | 0.684 | H-2 → L+4 = 12; H-1 → L+2 = 19; H-1 → L+3 = 13 |
| Compound | E1red (V) | E2red (V) | ΔEred | Kc |
|---|---|---|---|---|
| W1 | −0.66 | −1.28 | 0.63 | 3.97 × 104 |
| W2 | −0.68 | −1.28 | 0.60 | 2.51 × 104 |
| W3 | −0.57 | −1.42 | 0.84 | 1.58 × 106 |
| D1 | −0.82 | −1.31 | 0.49 | 3.97 × 103 |
| D2 | −0.76 | −1.28 | 0.35 | 6.30 × 103 |
| D3 | −0.60 | −1.52 | 0.92 | 5.74 × 106 |
| W1a | −0.45 | −1.23 | 0.78 | 4.98 × 105 |
| W2a | −0.47 | −1.26 | 0.79 | 6.22 × 105 |
| W3a | −0.55 | −1.09 | 0.54 | 9.98 × 103 |
| D1a | −0.53 | −1.39 | 0.85 | 1.91 × 106 |
| D2a | −0.53 | −1.41 | 0.88 | 2.72 × 106 |
| D3a | −0.56 | −1.45 | 0.89 | 3.67 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuroldayeva, G.; Balanay, M.P. Computational Analysis of Electron-Donating and Withdrawing Effects on Asymmetric Viologens for Enhanced Electrochromic Performance. Int. J. Mol. Sci. 2025, 26, 10137. https://doi.org/10.3390/ijms262010137
Nuroldayeva G, Balanay MP. Computational Analysis of Electron-Donating and Withdrawing Effects on Asymmetric Viologens for Enhanced Electrochromic Performance. International Journal of Molecular Sciences. 2025; 26(20):10137. https://doi.org/10.3390/ijms262010137
Chicago/Turabian StyleNuroldayeva, Gulzat, and Mannix P. Balanay. 2025. "Computational Analysis of Electron-Donating and Withdrawing Effects on Asymmetric Viologens for Enhanced Electrochromic Performance" International Journal of Molecular Sciences 26, no. 20: 10137. https://doi.org/10.3390/ijms262010137
APA StyleNuroldayeva, G., & Balanay, M. P. (2025). Computational Analysis of Electron-Donating and Withdrawing Effects on Asymmetric Viologens for Enhanced Electrochromic Performance. International Journal of Molecular Sciences, 26(20), 10137. https://doi.org/10.3390/ijms262010137








