Comparative Characterization of Tumor Microenvironments in Monophasic and Biphasic Synovial Sarcomas
Abstract
1. Introduction
2. Results
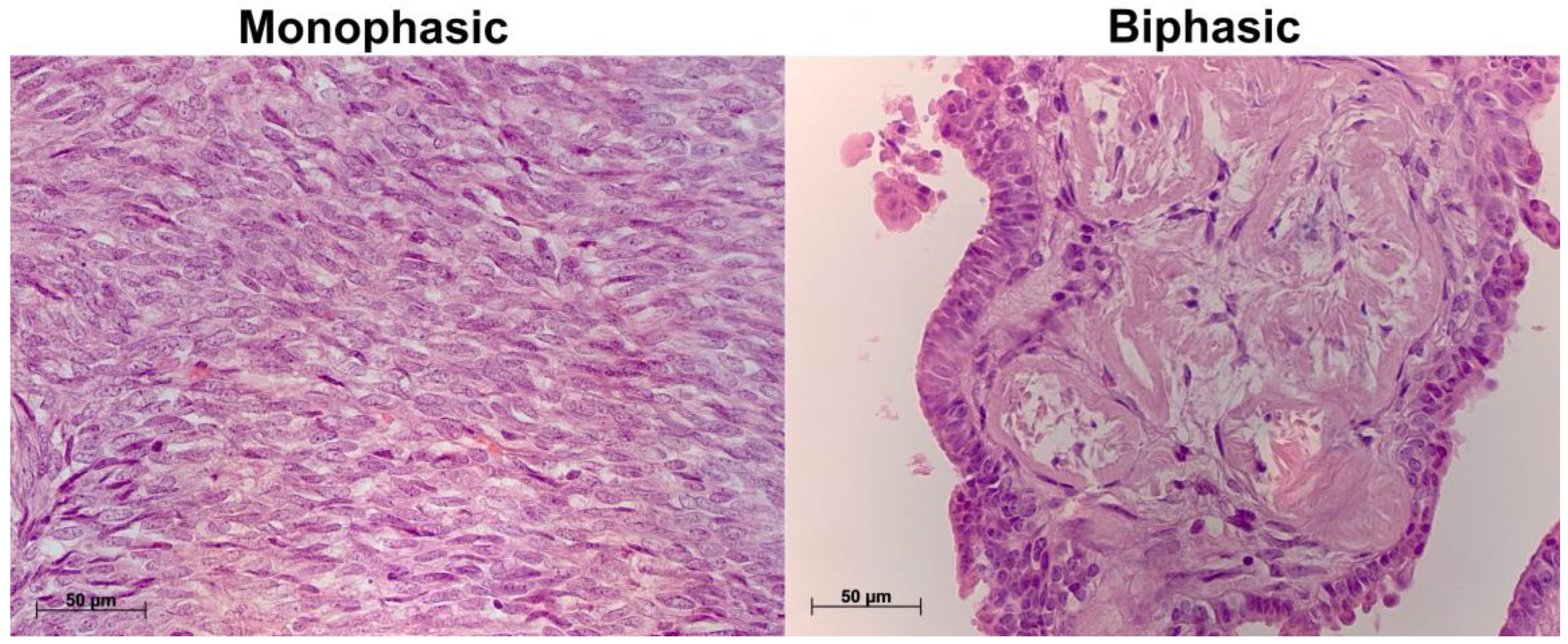
2.1. Histological Characterization of Biphasic and Monophasic Synovial Sarcomas
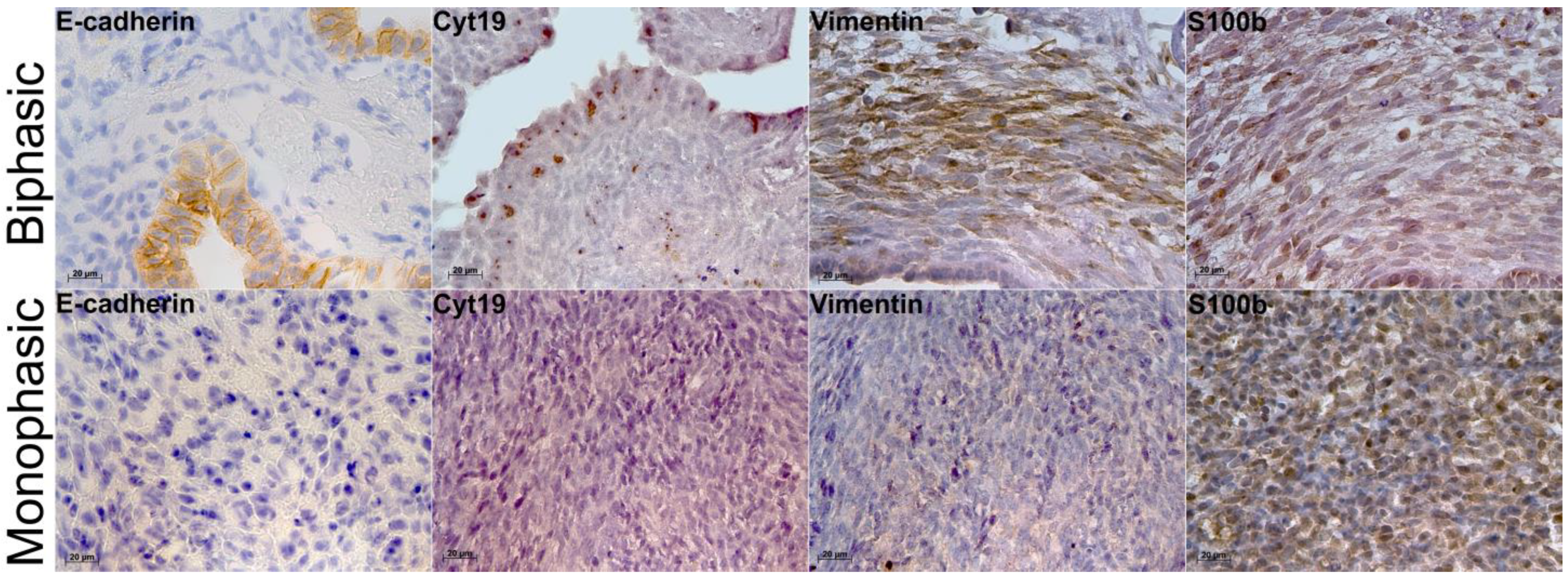
2.2. Immunohistochemistry and Molecular Characterization of Biphasic and Monophasic Sarcomas
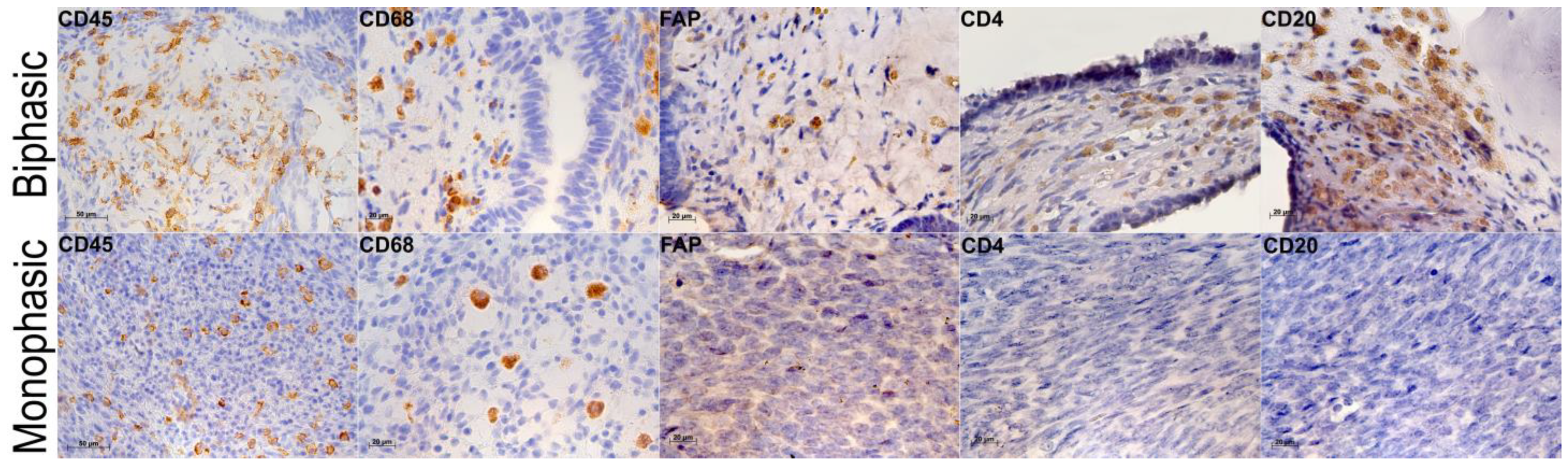
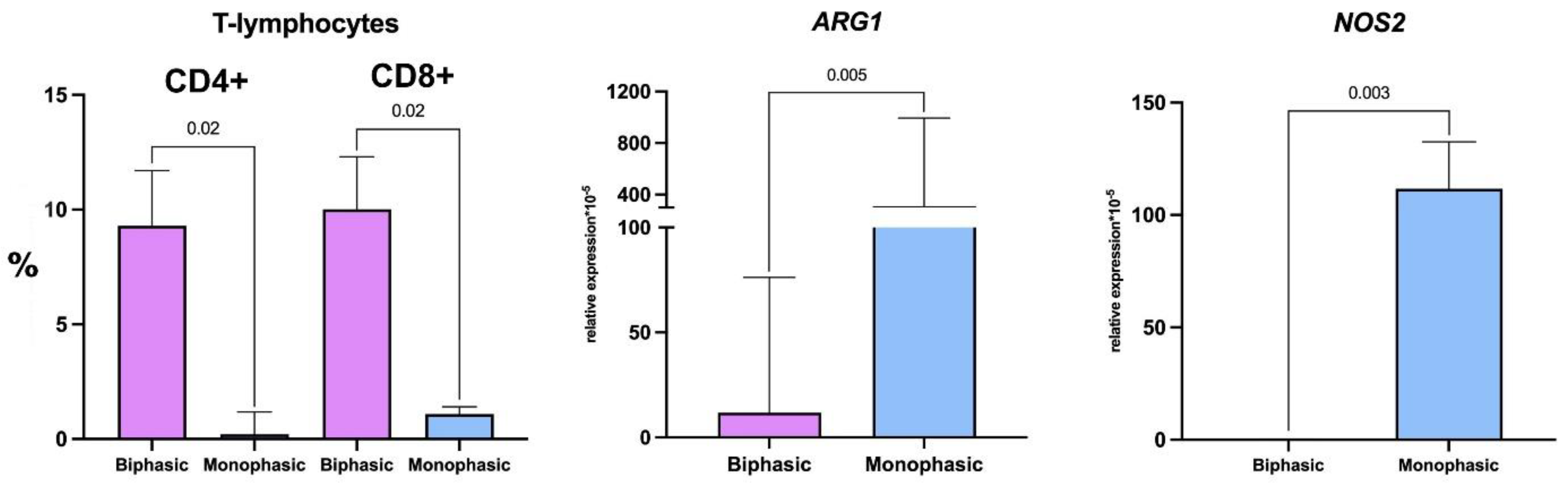
2.3. Tumor Microenvironments in Biphasic and Monophasic Synovial Sarcomas
3. Discussion
4. Materials and Methods
4.1. Characteristics of Patients Whose Material Was Used in the Study
- Male or female over 18 years of age;
- Morphologically verified diagnosis of synovial sarcoma of soft tissues, SS18::SSX translocation-positive;
- Karnofsky index ≥ 70;
- No unhealthy habits;
- No chronic diseases in acute/decompensated phase;
- No aggravated oncological history;
- No previous history of anti-tumor medications and radiation therapy.
4.2. Morphological Characterization of the Tumors: Immunohistochemistry
4.3. Flow Cytometry
4.4. Reverse Transcription Real-Time Polymerase Chain Reaction Assay
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SS | Synovial sarcomas |
| TAM | Tumor-associated macrophage |
| FAP | Fibroblast activation protein |
| EGFR | Epidermal growth factor receptor |
| PDGFRL | Platelet-derived growth factor receptor-like |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A |
| PDL1 | Programmed death ligand 1 |
| IHC | Immunohistochemistry |
| ICI | Immune checkpoint inhibitors |
References
- Oike, N.; Kawashima, H.; Ogose, A.; Hotta, T.; Hatano, H.; Ariizumi, T.; Sasaki, T.; Yamagishi, T.; Umezu, H.; Endo, N. Prognostic impact of the tumor immune microenvironment in synovial sarcoma. Cancer Sci. 2018, 109, 3043–3054. [Google Scholar] [CrossRef]
- Brodin, B.; Haslam, K.; Yang, K.; Bartolazzi, A.; Xie, Y.; Starborg, M.; Lundeberg, J.; Larsson, O. Cloning and characterization of spliced fusion transcript variants of synovial sarcoma: SYT/SSX4, SYT/SSX4v, and SYT/SSX2v. Possible regulatory role of the fusion gene product in wild type SYT expression. Gene 2001, 268, 173–182. [Google Scholar] [CrossRef]
- Banito, A.; Li, X.; Laporte, A.N.; Roe, J.S.; Sanchez-Vega, F.; Huang, C.H.; Dancsok, A.R.; Hatzi, K.; Chen, C.-C.; Tschaharganeh, D.F.; et al. The SS18-SSX oncoprotein hijacks KDM2B-PRC1.1 to drive synovial sarcoma. Cancer Cell 2018, 33, 527–541, Erratum in Cancer Cell 2018, 34, 346–348. [Google Scholar] [CrossRef]
- Xing, Z.; Wei, L.; Jiang, X.; Conroy, J.; Glenn, S.; Bshara, W.; Yu, T.; Pao, A.; Tanaka, S.; Kawai, A.; et al. Analysis of mutations in primary and metastatic synovial sarcoma. Oncotarget 2018, 9, 36878. [Google Scholar] [PubMed]
- Choi, J.H.; Ro, J.Y. The 2020 WHO classification of tumors of soft tissue: Selected changes and new entities. Adv. Anat. Pathol. 2021, 28, 44–58. [Google Scholar]
- Xiong, L.; Chen, Z.; Zhou, Y.; Li, H.; Xiao, T. The survival and prognosis analysis of synovial sarcoma subtypes: A Surveillance, Epidemiology, and End Results population-based analysis. Int. Orthop. 2020, 44, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Sun, T.; Hou, B.; Hong, G.; Mallampati, S.; Sun, H.; Zhou, X.; Zhou, C.; Zhang, H.; et al. Survival changes in patients with synovial sarcoma, 1983–2012. J. Cancer 2017, 8, 1759. [Google Scholar] [CrossRef] [PubMed]
- Guillou, L.; Benhattar, J.; Bonichon, F.; Gallagher, G.; Terrier, P.; Stauffer, E.; Somerhausen, N.d.S.A.; Michels, J.-J.; Jundt, G.; Vince, D.R.; et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: A multicenter, retrospective analysis. J. Clin. Oncol. 2004, 22, 4040–4050. [Google Scholar] [CrossRef]
- Ladanyi, M.; Antonescu, C.R.; Leung, D.H.; Woodruff, J.M.; Kawai, A.; Healey, J.H.; Brennan, M.F.; A Bridge, J.; Neff, J.R.; Barr, F.G.; et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: A multi-institutional retrospective study of 243 patients. Cancer Res. 2002, 62, 135–140. [Google Scholar]
- Gyorki, D.E.; Bae, S.; Smith, R.C.; Caruso, D.A.; Coker, D.; Connolly, E.A.; Desai, J.; Johnston, A.; Lawless, A.K.; Lazarakis, S.; et al. Update of clinical practice guidelines for the management of patients with sarcoma. ANZ J. Surg. 2025, 95, 289–292. [Google Scholar] [CrossRef]
- Blay, J.Y.; von Mehren, M.; Jones, R.L.; Martin-Broto, J.; Stacchiotti, S.; Bauer, S.; Nathenson, M. Synovial sarcoma: Characteristics, challenges, and evolving therapeutic strategies. ESMO Open 2023, 8, 101618. [Google Scholar] [CrossRef]
- Pan, D.; Kobayashi, A.; Jiang, P.; Ferrari de Andrade, L.; Tay, R.E.; Luoma, A.M.; Tsoucas, D.; Qiu, X.; Lim, K.; Rao, P.; et al. A major chromatin regulator determines resistance of tumor cells to T cell–mediated killing. Science 2018, 359, 770–775. [Google Scholar] [CrossRef]
- Lai, J.P.; Robbins, P.F.; Raffeld, M.; Aung, P.P.; Tsokos, M.; Rosenberg, S.A.; Miettinen, M.M.; Lee, C.-C.R. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: Significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod. Pathol. 2012, 25, 854–858. [Google Scholar] [CrossRef]
- Jerby-Arnon, L.; Neftel, C.; Shore, M.E.; Weisman, H.R.; Mathewson, N.D.; McBride, M.J.; Haas, B.; Izar, B.; Volorio, A.; Boulay, G.; et al. Opposing immune and genetic mechanisms shape oncogenic programs in synovial sarcoma. Nat. Med. 2021, 27, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.F.; Kassim, S.H.; Tran, T.L.; Crystal, J.S.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Dudley, M.E.; Wunderlich, J.R.; Sherry, R.M.; et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1–reactive T-cell receptor: Long-term follow-up and correlates with response. Clin. Cancer Res. 2015, 21, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Umakoshi, M.; Nakamura, A.; Tsuchie, H.; Li, Z.; Kudo-Asabe, Y.; Miyabe, K.; Ito, Y.; Yoshida, M.; Nagasawa, H.; Okada, K.; et al. Macrophage numbers in the marginal area of sarcomas predict clinical prognosis. Sci. Rep. 2023, 13, 1290. [Google Scholar] [CrossRef] [PubMed]
- Goff, P.H.; Riolobos, L.; LaFleur, B.J.; Spraker, M.B.; Seo, Y.D.; Smythe, K.S.; Campbell, J.S.; Pierce, R.H.; Zhang, Y.; He, Q.; et al. Neoadjuvant therapy induces a potent immune response to sarcoma, dominated by myeloid and B cells. Clin. Cancer Res. 2022, 28, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Luk, S.J.; IJsselsteijn, M.E.; Somarakis, A.; Acem, I.; de Bruijn, I.B.; Szuhai, K.; Bovee, J.V.M.G.; de Miranda, N.F.C.C.; Falkenburg, J.H.F.; Heemskerk, M.H.M. Immunological differences between monophasic and biphasic synovial sarcoma with implications for immunotherapy. Cancer Immunol. Immunother. 2024, 74, 31. [Google Scholar] [CrossRef]
- Kallen, M.E.; Hornick, J.L. The 2020 WHO classification: What’s new in soft tissue tumor pathology? Am. J. Surg. Pathol. 2021, 45, e1–e23. [Google Scholar] [CrossRef]
- Saito, T.; Oda, Y.; Sugimachi, K.; Kawaguchi, K.I.; Tamiya, S.; Tanaka, K.; Matsuda, S.; Sakamoto, A.; Iwamoto, Y.; Tsuneyoshi, M. E-cadherin gene mutations frequently occur in synovial sarcoma as a determinant of histological features. Am. J. Pathol. 2001, 159, 2117–2124. [Google Scholar] [CrossRef]
- Smith, T.A.; Machen, S.K.; Fisher, C.; Goldblum, J.R. Usefulness of cytokeratin subsets for distinguishing monophasic synovial sarcoma from malignant peripheral nerve sheath tumor. Am. J. Clin. Pathol. 1999, 112, 641–648. [Google Scholar] [CrossRef]
- Ali, R.M.; Seerwan, K.; Ali, S.M.; Abdullah, A.M.; Hawrami, O.; Hussein, D.M.; Mohammed, B.J.; Karim, M.; Abdullah, F.; Abdalla, B.A.; et al. Primary pancreatic synovial sarcoma: Report of a rare case and review of the literature. Med. Int. 2023, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Batth, I.S.; Li, S. Discovery of cell-surface vimentin (CSV) as a sarcoma target and development of CSV-targeted IL12 immune therapy. In Current Advances in Osteosarcoma: Clinical Perspectives: Past, Present and Future; Springer: Cham, Switzerland, 2020; pp. 169–178. [Google Scholar]
- Weiss, S.W.; Goldblum, J.R.; Folpe, A.L. Enzinger and Weiss’s Soft Tissue Tumors; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Karamchandani, J.R.; Nielsen, T.O.; van de Rijn, M.; West, R.B. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 445–450. [Google Scholar] [CrossRef]
- Nagayama, S.; Katagiri, T.; Tsunoda, T.; Hosaka, T.; Nakashima, Y.; Araki, N.; Kusuzaki, K.; Nakayama, T.; Tsuboyama, T.; Nakamura, T.; et al. Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res. 2002, 62, 5859–5866. [Google Scholar]
- Ishibe, T.; Nakayama, T.; Aoyama, T.; Nakamura, T.; Toguchida, J. Neuronal differentiation of synovial sarcoma and its therapeutic application. Clin. Orthop. Relat. Res®. 2008, 466, 2147–2155. [Google Scholar] [CrossRef]
- Allander, S.V.; Illei, P.B.; Chen, Y.; Antonescu, C.R.; Bittner, M.; Ladanyi, M.; Meltzer, P.S. Expression profiling of synovial sarcoma by cDNA microarrays: Association of ERBB2, IGFBP2, and ELF3 with epithelial differentiation. Am. J. Pathol. 2002, 161, 1587–1595. [Google Scholar] [CrossRef]
- Singh, S.K.; Clarke, I.D.; Hide, T.; Dirks, P.B. Cancer stem cells in nervous system tumors. Oncogene 2004, 23, 7267–7273. [Google Scholar] [CrossRef]
- Fang, D.; Nguyen, T.K.; Leishear, K.; Finko, R.; Kulp, A.N.; Hotz, S.; Van Belle, P.A.; Xu, X.; Elder, D.E.; Herlyn, M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005, 65, 9328–9337. [Google Scholar] [CrossRef]
- Staege, M.S.; Hutter, C.; Neumann, I.; Foja, S.; Hattenhorst, U.E.; Hansen, G.; Afar, D.; Burdach, S.E.G. DNA microarrays reveal relationship of Ewing family tumors to both endothelial and fetal neural crest-derived cells and define novel targets. Cancer Res. 2004, 64, 8213–8221. [Google Scholar] [CrossRef] [PubMed]
- May, W.A.; Gishizky, M.L.; Lessnick, S.L.; Lunsford, L.B.; Lewis, B.C.; Delattre, O.; Zucman, J.; Thomas, G.; Denny, C.T. Ewing sarcoma 11; 22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc. Natl. Acad. Sci. USA 1993, 90, 5752–5756. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, F.K.; Chang, K.T.; Stichel, D.; Banito, A.; Jones, D.T.; Heilig, C.E.; Fröhling, S.; Sahm, F.; Stenzinger, A.; Hartmann, W.; et al. Endometrial stromal sarcomas with BCOR-rearrangement harbor MDM2 amplifications. J. Pathol. Clin. Res. 2020, 6, 178–184. [Google Scholar] [CrossRef]
- Salgado, C.M.; Alaggio, R.; Ciolfi, A.; Zin, A.; Camassei, F.D.; Pedace, L.; Milano, G.M.; Serra, A.; Di Giannatale, A.; Mastronuzzi, A.; et al. Pediatric BCOR-altered tumors from soft tissue/kidney display specific DNA methylation profiles. Mod. Pathol. 2023, 36, 100039. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.; Frigerio, S.; Behnke, S.; Senn, B.; Odermatt, B.; Zimmermann, D.R.; Moch, H. Mutations in the tyrosine kinase domain of the EGFR gene are rare in synovial sarcoma. Mod. Pathol. 2006, 19, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Mock, H.; Sauter, G.; Buchholz, N.; Gasser, T.C.; Bubendorf, L.; Waldman, F.M.; Mihatsch, M.J. Epidermal growth factor receptor expression is associated with rapid tumor cell proliferation in renal cell carcinoma. Hum. Pathol. 1997, 28, 1255–1259. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Hsu, F.D.; O’Connell, J.X.; Gilks, C.B.; Sorensen, P.H.; Linn, S.; West, R.B.; Liu, C.L.; Botstein, D.; Brown, P.O.; et al. Tissue microarray validation of epidermal growth factor receptor and SALL2 in synovial sarcoma with comparison to tumors of similar histology. Am. J. Pathol. 2003, 163, 1449–1456. [Google Scholar] [CrossRef]
- Kazlauskas, A. PDGFs and their receptors. Gene 2017, 614, 1–7. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, J.; Wang, Z.; Zha, L. Overexpression of platelet-derived growth factor-B increases the growth, invasion, and angiogenesis of gastric carcinoma cells through protein kinase B. Neoplasma 2013, 60, 605–612. [Google Scholar] [CrossRef]
- Hermanson, M.; Funa, K.; Koopmann, J.; Maintz, D.; Waha, A.; Westermark, B.; Heldin, C.H.; Wiestler, O.D.; Louis, D.N.; Von Deimling, A.; et al. Association of loss of heterozygosity on chromosome 17p with high platelet-derived growth factor α receptor expression in human malignant gliomas. Cancer Res. 1996, 56, 164–171. [Google Scholar]
- MacDonald, T.J.; Brown, K.M.; LaFleur, B.; Peterson, K.; Lawlor, C.; Chen, Y.; Packer, R.J.; Cogen, P.; Stephan, D.A. Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat. Genet. 2001, 29, 143–152, Erratum in Nat. Genet. 2003, 35, 287. [Google Scholar] [CrossRef]
- Katano, M.; Nakamura, M.; Fujimoto, K.; Miyazaki, K.; Morisaki, T. Prognostic value of platelet-derived growth factor-A (PDGF-A) in gastric carcinoma. Ann. Surg. 1998, 227, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Imura, Y.; Nakai, T.; Nakai, S.; Yasuda, N.; Kaneko, K.; Outani, H.; Takenaka, S.; Hamada, K.; Myoui, A.; et al. Therapeutic potential of TAS-115 via c-MET and PDGFRα signal inhibition for synovial sarcoma. BMC Cancer 2017, 17, 334. [Google Scholar] [CrossRef]
- Kozak, K.; Szumera-Cieckiewicz, A.; Rembiszewska, A.; Podgórska, A.; Paterczyk, H.K.; Przybyl, J.; Gos, A.; Switaj, T.; Prochorec-Sobieszek, M.; Rutkowski, P. Programmed death ligand-1 (PD-L1) expression and prognostic value in synovial sarcoma. Ann. Oncol. 2014, 25, iv503. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Perrot, D.; Leroux, A.; Collin, F.; Le Cesne, A.; Coindre, J.-M.; Blay, J.-Y.; Birnbaum, D.; Mamessier, E. PDL1 expression is a poor-prognosis factor in soft-tissue sarcomas. Oncoimmunology 2017, 6, e1278100. [Google Scholar] [CrossRef] [PubMed]
- Saâda-Bouzid, E.; Burel-Vandenbos, F.; Ranchère-Vince, D.; Birtwisle-Peyrottes, I.; Chetaille, B.; Bouvier, C.; Château, M.-C.; Peoc’H, M.; Battistella, M.; Bazin, A.; et al. Prognostic value of HMGA2, CDK4, and JUN amplification in well-differentiated and dedifferentiated liposarcomas. Mod. Pathol. 2015, 28, 1404–1414. [Google Scholar] [CrossRef]
- Li, X.; Seebacher, N.A.; Garbutt, C.; Ma, H.; Gao, P.; Xiao, T.; Hornicek, F.J.; Duan, Z. Inhibition of cyclin-dependent kinase 4 as a potential therapeutic strategy for treatment of synovial sarcoma. Cell Death Dis. 2018, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; De Reyniés, A.; Keung, E.Z.; Chen, T.W.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Jumaniyazova, E.; Lokhonina, A.; Dzhalilova, D.; Kosyreva, A.; Fatkhudinov, T. Immune cells in the tumor microenvironment of soft tissue sarcomas. Cancers 2023, 15, 5760. [Google Scholar] [CrossRef]
- Ikeda, H. Cancer immunotherapy in progress—An overview of the past 130 years. Int. Immunol. 2025, 37, 253–260. [Google Scholar] [CrossRef]
- Medina-Ceballos, E.; Giner, F.; Machado, I.; Heras-Morán, B.; Espino, M.; Navarro, S.; Llombart-Bosch, A. The Prognostic Impact of the Tumor Immune Microenvironment in Synovial Sarcoma: An Immunohistochemical Analysis Using Digital Pathology and Conventional Interpretation. J. Pers. Med. 2025, 15, 169. [Google Scholar] [CrossRef]
- Issels, R.D.; Noessner, E.; Lindner, L.H.; Schmidt, M.; Albertsmeier, M.; Blay, J.Y.; Stutz, E.; Xu, Y.; Buecklein, V.; Altendorf-Hofmann, A.; et al. Immune infiltrates in patients with localised high-risk soft tissue sarcoma treated with neoadjuvant chemotherapy without or with regional hyperthermia: A translational research program of the EORTC 62961-ESHO 95 randomised clinical trial. Eur. J. Cancer 2021, 158, 123–132. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Takeya, M.; Komohara, Y. Role of tumor-associated macrophages in human malignancies: Friend or foe? Pathol. Int. 2016, 66, 491–505. [Google Scholar] [CrossRef]
- Robinson, A.; Han, C.Z.; Glass, C.K.; Pollard, J.W. Monocyte regulation in homeostasis and malignancy. Trends Immunol. 2021, 42, 104–119. [Google Scholar] [CrossRef]
- Kwiecień, I.; Rutkowska, E.; Polubiec-Kownacka, M.; Raniszewska, A.; Rzepecki, P.; Domagała-Kulawik, J. Blood monocyte subsets with activation markers in relation with macrophages in non-small cell lung cancer. Cancers 2020, 12, 2513. [Google Scholar] [CrossRef]
- Valdés-Ferrada, J.; Muñoz-Durango, N.; Pérez-Sepulveda, A.; Muñiz, S.; Coronado-Arrázola, I.; Acevedo, F.; Soto, J.A.; Bueno, S.M.; Sánchez, C.; Kalergis, A.M. Peripheral blood classical monocytes and plasma interleukin 10 are associated to neoadjuvant chemotherapy response in breast cancer patients. Front. Immunol. 2020, 11, 1413. [Google Scholar] [CrossRef] [PubMed]
- Cillo, A.R.; Mukherjee, E.; Bailey, N.G.; Onkar, S.; Daley, J.; Salgado, C.; Li, X.; Liu, D.; Ranganathan, S.; Burgess, M.; et al. Ewing sarcoma and osteosarcoma have distinct immune signatures and intercellular communication networks. Clin. Cancer Res. 2022, 28, 4968–4982. [Google Scholar] [CrossRef] [PubMed]
- Recine, F.; Vanni, S.; Bongiovanni, A.; Fausti, V.; Mercatali, L.; Miserocchi, G.; Liverani, C.; Pieri, F.; Casadei, R.; Cavaliere, D.; et al. Clinical and translational implications of immunotherapy in sarcomas. Front. Immunol. 2024, 15, 1378398. [Google Scholar] [CrossRef]
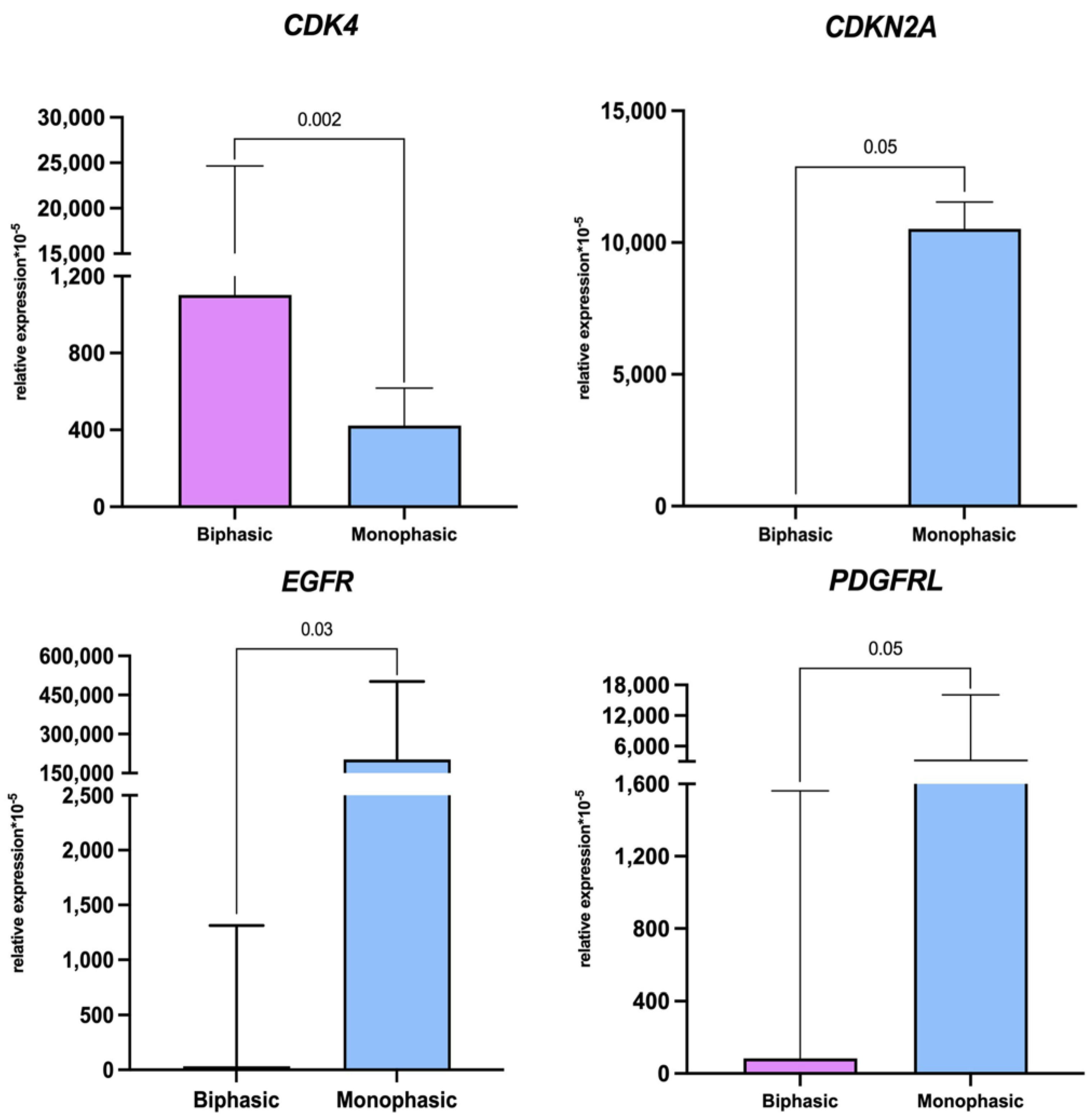
| Gene | Biphasic (n = 2) ×10−5 | Monophasic (n = 7) ×10−5 | p-Value |
|---|---|---|---|
| CDKN2A | 0 (0–0) | 10,520 (9803–11,238) | 0.05 |
| EGFR | 188.0 (32.3–2315) | 243,033 (161,312–392,040) | 0.03 |
| PDGFRL | 694 (84.2–1903.8) | 208,580,433 (26,171,059–1,576,930,097) | 0.05 |
| PDL1 | 3069 (897.4–3069) | 1979 (913.3–5795) | 0.9 |
| CDK4 | 1102 (867.8–24,668) | 423.3 (262.4–617.4) | 0.002 |
| Cell Type/Marker(s) | Biphasic (n = 2) | Monophasic (n = 7) | p-Value | |
|---|---|---|---|---|
| Leukocytes | CD45+ | 33.5 (13.5–53.5) | 21.6 (9.7–33.0) | |
| Monocytes | CD14+ | 10.0 (4.6–15.3) | 9.7 (7.7–24.1) | |
| CD16+ | 32.7 (5.2–60.3) | 3.7 (1.9–13.9) | ||
| Macrophages | CD68+ | 38.6 (17.6–59.6) | 56.4 (30.8–69.0) | |
| CD86+ M1 | 12.9 (5.9–19.9) | 25.0 (18.7–39.31) | ||
| CD163+ M2 | 27.9 (9.8–46.0) | 49.7 (32.2–53.8) | ||
| CD206+ M2 | 19.4 (15.1–23.6) | 44.8 (32.2–57.3) | ||
| T cells | CD4+ helpers | 9.3 (6.9–11.7) | 0.2 (0.08–1.2) | 0.04 |
| CD8+ cytotoxic | 10.0 (7.8–12.3) | 1.1 (0.6–1.4) | 0.04 | |
| Biphasic (n = 2) ×10−5 | Monophasic (n = 7) ×10−5 | p-Value | ||
|---|---|---|---|---|
| Macrophage markers | NOS2 (M1) | 0 (0–0.4) | 112 (28–133) | 0.008 |
| ARG1 (M2) | 22.2 (5.9–76.2) | 311 (229–994) | 0.01 | |
| Parameter | n (%) |
|---|---|
| Total number of patients | 9 (100) |
| Age at diagnosis, years | |
| <20 | 2 (22) |
| ≥20 | 7 (78) |
| Sex | |
| Male | 4 (44) |
| Female | 5 (66) |
| Localization | |
| Upper limbs | 0 (0) |
| Lower limbs | 7 (78) |
| Torso | 2 (22) |
| Head and neck | 0 (0) |
| Disease episode | |
| Newly diagnosed tumor | 6 |
| Relapse | 3 |
| Size of tumor node, cm | |
| ≤5 | 0 (0) |
| >5 | 7 (78) |
| >10 | 1 (11) |
| >15 | 1 (11) |
| Malignancy grade | |
| I | 0 (0) |
| II | 0 (0) |
| III | 9 (100) |
| IV | 0 (0) |
| Presence of distant metastases | |
| No | 7 (78) |
| Yes | 2 (22) |
| Histological subtype | |
| Monophasic sarcoma | 7 (78) |
| Biphasic sarcoma | 2 (22) |
| Gene | Primer | Nucleotide Sequence |
|---|---|---|
| ARG1 | Forward | AAA GGG ACA GCC ACG AGG AG |
| Reverse | GGA TGT CAG CAA AGG GCA GG | |
| NOS2 | Forward | TGC TTT GTG CGG AAT GCC AG |
| Reverse | ATG TGG TCC TCA TCT GGG CG | |
| CDKN2A | Forward | AGT TAC GGT CGG AGG CCG AT |
| Reverse | TGG TTA CTG CCT CTG GTG CC | |
| EGFR | Forward | CCC CCT GAC TCC GTC CAG TA |
| Reverse | CCC AAC TGC GTG AGC TTG TT | |
| PDGFRL | Forward | GCT ACC CTG CGT ATC TGG AC |
| Reverse | ATT CAC CTG TGT CTG CCG AG | |
| PDL1 | Forward | CCT TTG CCT CCA CTC AAT G |
| Reverse | AAC AGG GTG GTT ACA GCG AT | |
| CDK4 | Forward | GTT CGT GAG GTG GCT TTA CTG |
| Reverse | CCA ACT GGT CGG CTT CAG | |
| GAPDH | Forward | TGG TGA AGA CGC CAG TGG A |
| Reverse | GCA CCG TCA AGG CTG AGA AC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosyreva, A.; Jumaniyazova, E.; Sentyabreva, A.; Miroshnichenko, E.; Dzhalilova, D.; Fetisov, T.; Tararykova, A.; Lokhonina, A.; Fatkhudinov, T. Comparative Characterization of Tumor Microenvironments in Monophasic and Biphasic Synovial Sarcomas. Int. J. Mol. Sci. 2025, 26, 10119. https://doi.org/10.3390/ijms262010119
Kosyreva A, Jumaniyazova E, Sentyabreva A, Miroshnichenko E, Dzhalilova D, Fetisov T, Tararykova A, Lokhonina A, Fatkhudinov T. Comparative Characterization of Tumor Microenvironments in Monophasic and Biphasic Synovial Sarcomas. International Journal of Molecular Sciences. 2025; 26(20):10119. https://doi.org/10.3390/ijms262010119
Chicago/Turabian StyleKosyreva, Anna, Enar Jumaniyazova, Alexandra Sentyabreva, Ekaterina Miroshnichenko, Dzhuliia Dzhalilova, Timur Fetisov, Anastasia Tararykova, Anastasiya Lokhonina, and Timur Fatkhudinov. 2025. "Comparative Characterization of Tumor Microenvironments in Monophasic and Biphasic Synovial Sarcomas" International Journal of Molecular Sciences 26, no. 20: 10119. https://doi.org/10.3390/ijms262010119
APA StyleKosyreva, A., Jumaniyazova, E., Sentyabreva, A., Miroshnichenko, E., Dzhalilova, D., Fetisov, T., Tararykova, A., Lokhonina, A., & Fatkhudinov, T. (2025). Comparative Characterization of Tumor Microenvironments in Monophasic and Biphasic Synovial Sarcomas. International Journal of Molecular Sciences, 26(20), 10119. https://doi.org/10.3390/ijms262010119







