Endothelial Dysfunction as the Common Pathway Linking Obesity, Hypertension and Atherosclerosis
Abstract
1. Introduction
2. The Endothelium and Endothelial NO Synthase: Structure, Function, and Physiological Significance
2.1. Structure and Functions of the Endothelium
2.2. Endothelial NO Synthase and Endothelium-Derived Hyperpolarizing Factor in Vascular Tone Regulation
3. Mechanisms of Endothelial Dysfunction
3.1. Endothelial Dysfunction vs. Endothelial Activation
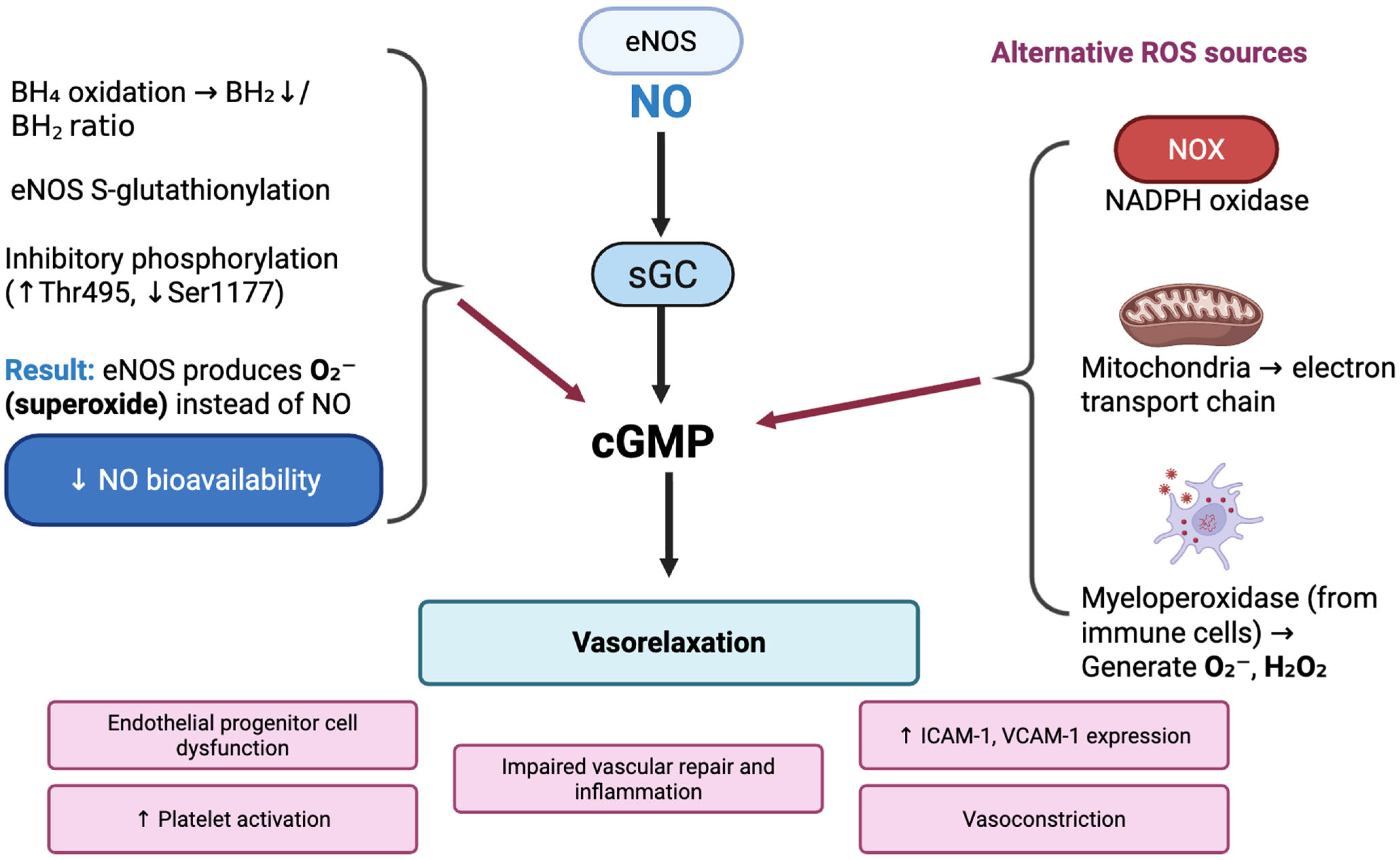
3.2. Oxidative Stress and NO Pathway Impairment
3.3. Inflammatory Pathways and Mediators
3.4. The Impact of Adipocytokines and Chemokines
4. Endothelial Dysfunction in Cardiometabolic Diseases
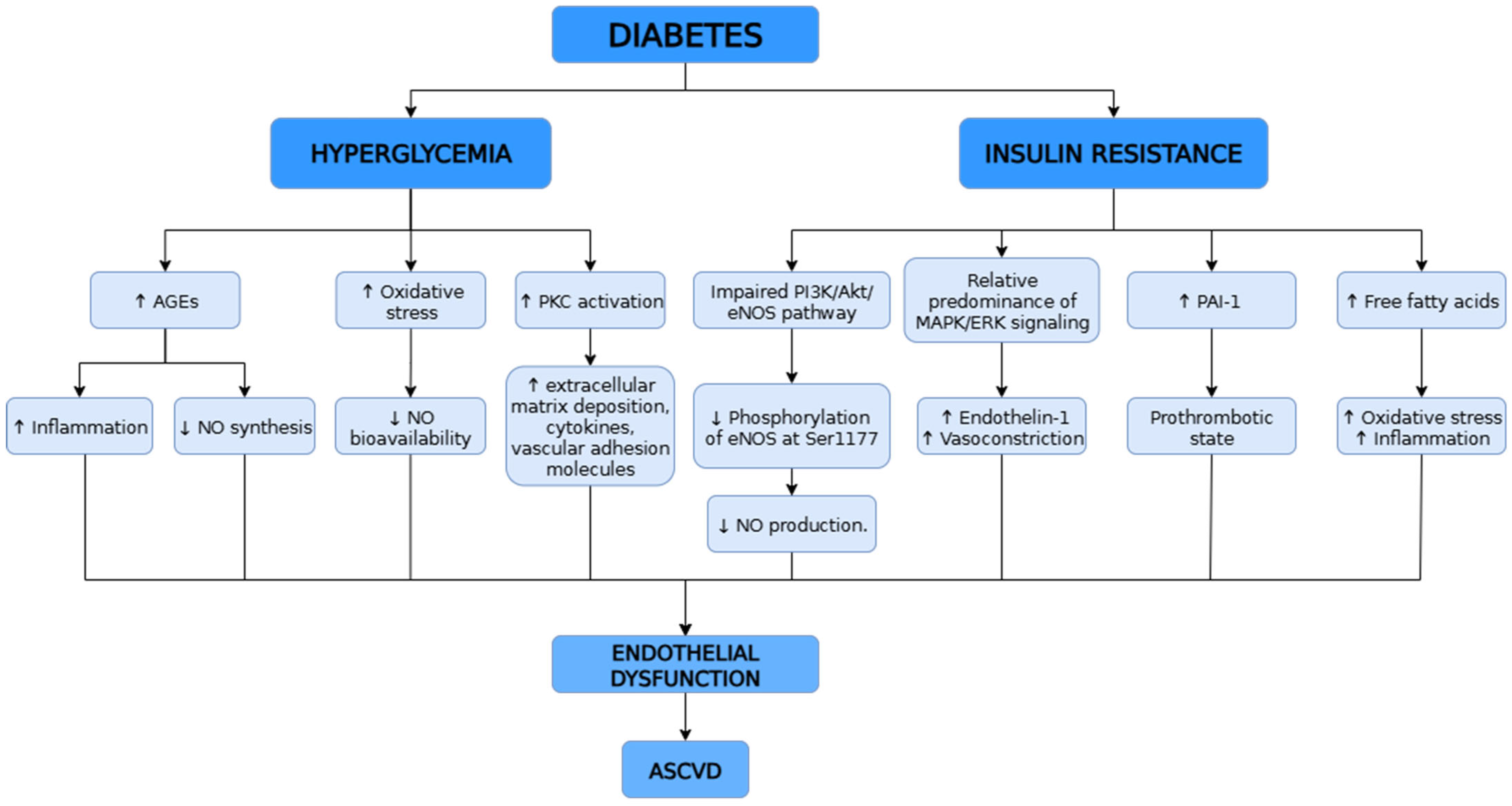
4.1. Diabetes and Insulin Resistance
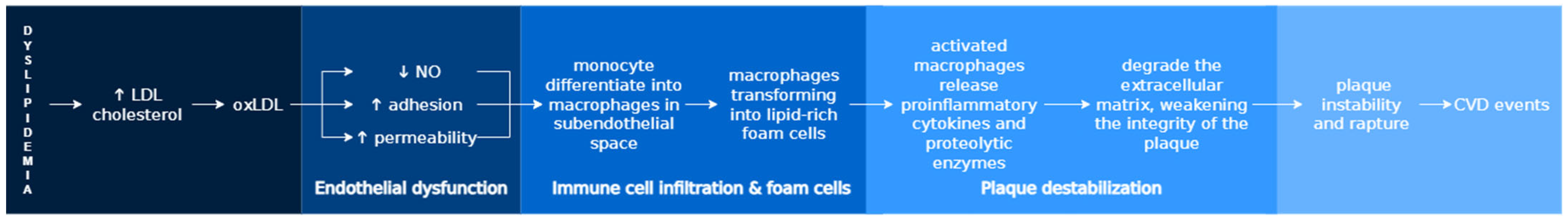
4.2. Dyslipidemia and Atherosclerosis
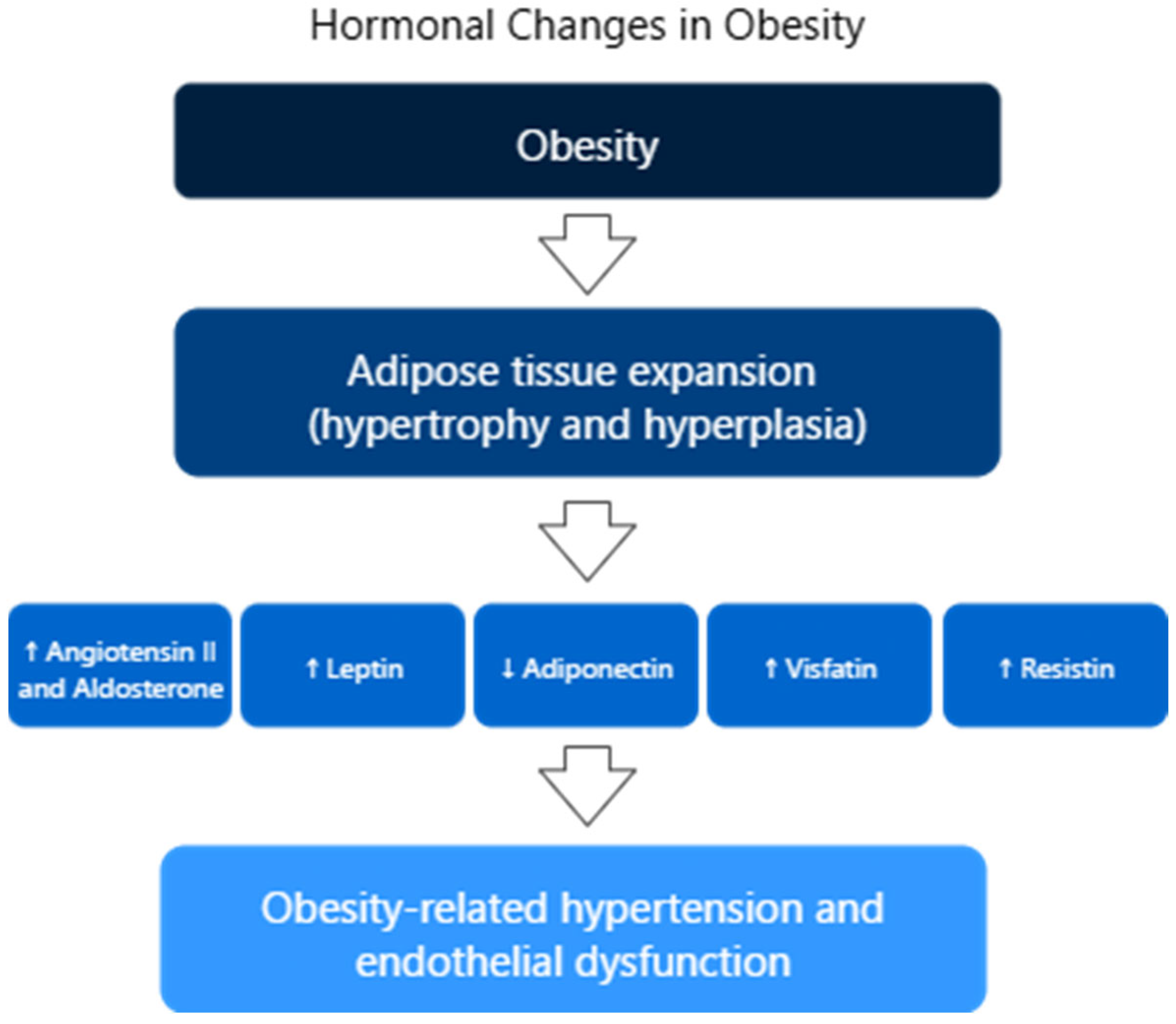
4.3. Hypertension and Obesity
4.4. Chronic Low-Grade Inflammation as a Link Between Endothelial Dysfunction and Metabolic Disorders
5. Biomarkers of Endothelial Dysfunction
5.1. Molecular and Biochemical Indicators
5.1.1. Cell Adhesion Molecules
5.1.2. Acute Phase Proteins and Inflammatory Cytokines
| Category | Biomarker | Source/Nature | Mechanism/Role | Clinical Relevance |
|---|---|---|---|---|
| Cell Adhesion Molecules | ICAM-1 | Endothelial cells [94] | Mediates leukocyte adhesion/transmigration via β2 integrins; upregulated by TNF-α, IL-1β, IL-6, IFN-γ, oxidative stress [94] | Marker of vascular inflammation, CAD risk [94] |
| VCAM-1 | Endothelial cells [95] | Binds leukocyte integrins; promotes transendothelial leukocyte migration; induced by TNF-α, ROS, oxLDL, shear stress [95] | Associated with vascular injury, immune cell recruitment [95] | |
| Soluble CAMs | Circulating forms [96] | Surrogate marker of endothelial activation [90] | Measurable in plasma/serum [96] | |
| Acute Phase Proteins | CRP | Hepatocytes [1] | Reduces eNOS expression, NO bioavailability [1] | Predictor of endothelial dysfunction, CAD, mortality [1,97] |
| SAA | Hepatocytes, macrophages [1] | Proinflammatory, pro-atherogenic [1] | CVD risk prediction [1,98] | |
| Fibrinogen | Hepatocytes [101] | Promotes clot formation and modulates inflammation; elevated levels reduce NO-mediated vasodilation and increase arterial stiffness, especially during inflammation or stress [102,103] | Strong independent predictor of mortality and adverse CV outcomes in CAD [103] | |
| Cytokines | TNF-α | Immune cells [1] | Induces CAM and cytokine expression; decreases NO by inhibiting eNOS and increasing ROS, leading to peroxynitrite formation and impaired vasodilation [86] | Linked to endothelial dysfunction in hypertension, ↑ carotid IMT, ↑ CAD risk, and foam cell formation [1,100] |
| IL-6 | Immune cells [1] | Acute phase mediator, plaque instability [1] | Predicts CAD events [1] | |
| IL-8 | Immune cells [1] | Chemoattractant for neutrophils/T cells [1] | Elevated in CAD [1] | |
| IL-18 | Macrophages [1] | Induces other cytokines, plaque destabilization [1] | Independent CAD risk marker [1] | |
| Vasoactive/ Regularoty Molecules | ADMA | Endogenous molecule [104] | Inhibits NOS, reduces NO, promotes oxidative stress [104] | Predictor of CAD severity, vascular remodeling [1,104,105] |
| vWF | Endothelial cells, megakaryocytes [106] | Platelet adhesion, Factor VIII stabilization [107] | Marker of vascular injury, atherosclerosis [106,107,108] | |
| Endothelin-1 | Endothelial cells, cardiomyocytes [109] | Potent vasoconstrictor, proinflammatory [109] | Predictor of mortality in AHF, CAD [109,110] | |
| Oxidative Stress Molecules | oxLDL | Modified LDL [97] | Activates LOX-1 → CAMs ↑, inflammation [98] | CVD risk in chronic inflammation [98,111] |
| MPO | Neutrophiles, monocytes [112] | Generates oxidants, reduces NO, destabilizes plaques [113] | Marker of CAD [112,113,114] |
5.1.3. Vasoactive and Endothelial Regulatory Molecules
5.1.4. Oxidative Stress Markers
5.2. Imaging and Functional Assessment Techniques
5.2.1. Flow-Mediated Dilation
5.2.2. Peripheral Arterial Tonometry & Reactive Hyperemia Index
5.2.3. Pulse Wave Analysis
5.2.4. Venous Occlusion Plethysmography
5.2.5. Intracoronary Acetylcholine Provocation Test
5.2.6. Thermodilution or Doppler Flow Wire Measurements
6. Targeted Therapies for Endothelial Dysfunction
6.1. Pharmacological Approaches
6.2. Non-Pharmacological Intervention
6.3. Emerging Targeted Therapies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| ACE | Angiotensin-Converting Enzyme |
| ADMA | Asymmetric Dimethylarginine |
| AGEs | Advanced Glycation End Products |
| AHF | Acute Heart Failure |
| AMPK | AMP-Activated Protein Kinase |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| BAUS | British Association of Urological Surgeons |
| BH2 | Dihydrobiopterin |
| BH4 | Tetrahydrobiopterin |
| CAD | Coronary Artery Disease |
| CAMs | Cell Adhesion Molecules |
| CFR | Coronary Flow Reserve |
| cGMP | Cyclic Guanosine Monophosphate |
| CI | Confidence Interval |
| CRP | C-Reactive Protein |
| CVD | Cardiovascular Disease |
| CVS | Cardiovascular System |
| DM | Diabetes Mellitus |
| ECs | Endothelial Cells |
| EDCFs | Endothelium-Derived Contracting Factors |
| EDHF | Endothelium-Derived Hyperpolarizing Factor in Vascular |
| EDRFs | Endothelium-Derived Relaxing Factors |
| eNOS | Endothelial Nitric Oxide Synthase |
| ET | Endothelin |
| ET-1 | Endothelin-1 |
| FID | Flow-Induced Dilation |
| FMD | Flow-Mediated Dilation |
| FGF21 | Fibroblast Growth Factor 21 |
| HDL | High-Density Lipoprotein |
| H2O2 | Hydrogen Peroxide |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IL-1β | Interleukin-1 Beta |
| IL-17A | Interleukin-17A |
| IL-6 | Interleukin-6 |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription Pathway |
| LDL | Low-Density Lipoprotein |
| LOX-1 | Lectin-Like Oxidized LDL Receptor-1 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| mAChR | Muscarinic Acetylcholine Receptor |
| MPO | Myeloperoxidase |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| NOX2 | NADPH Oxidase 2 |
| O2− | Superoxide Anion |
| ONOO− | Peroxynitrite |
| oxLDL | Oxidized Low-Density Lipoprotein |
| PAF | Platelet-Activating Factor |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| PAT | Peripheral Arterial Tonometry |
| PGH2 | Prostaglandin H2 |
| PGI2 | Prostacyclin |
| PI3K | Phosphoinositide 3-Kinase |
| PKC | Protein Kinase C |
| PKG | Protein Kinase G |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PVAT | Perivascular Adipose Tissue |
| PWA | Pulse Wave Analysis |
| RHI | Reactive Hyperemia Index |
| ROS | Reactive Oxygen Species |
| RR | Relative Risk |
| SAA | Serum Amyloid A |
| sCAMs | Soluble Cell Adhesion Molecules |
| sGC | Soluble Guanylyl Cyclase |
| SMCs | Smooth Muscle Cells |
| SR-A | Scavenger Receptor Class A |
| T2DM | Type 2 Diabetes Mellitus |
| TNF-α | Tumor Necrosis Factor-Alpha |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| vWF | von Willebrand Factor |
| VOP | Vascular Oscillatory Pressure |
References
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Jay Widmer, R.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 43. [Google Scholar] [CrossRef]
- Clarkson, P.; Celermajer, D.S.; Powe, A.J.; Donald, A.E.; Henry, R.M.; Deanfield, J.E. Endothelium-dependent dilatation is impaired in young healthy subjects with a family history of premature coronary disease. Circulation 1997, 96, 3378–3383. [Google Scholar] [CrossRef]
- Lavi, S.; Prasad, A.; Yang, E.H.; Mathew, V.; Simari, R.D.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation 2007, 115, 2621–2627. [Google Scholar] [CrossRef]
- Al Suwaidi, J.; Higano, S.T.; Holmes, D.R.; Lennon, R.; Lerman, A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J. Am. Coll. Cardiol. 2001, 37, 1523–1528. [Google Scholar] [CrossRef]
- Arcaro, G.; Cretti, A.; Balzano, S.; Lechi, A.; Muggeo, M.; Bonora, E.; Bonadonna, R.C. Insulin causes endothelial dysfunction in humans: Sites and mechanisms. Circulation 2002, 105, 576–582. [Google Scholar] [CrossRef]
- Favero, G.; Paganelli, C.; Buffoli, B.; Rodella, L.F.; Rezzani, R. Endothelium and its alterations in cardiovascular diseases: Life style intervention. BioMed Res. Int. 2014, 2014, 801896. [Google Scholar] [CrossRef]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood J. Am. Soc. Hematol. 1998, 91, 3527–3561. [Google Scholar]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arnal, J.F.; Dinh-Xuan, A.T.; Pueyo, M.; Darblade, B.; Rami, J. Endothelium-derived nitric oxide and vascular physiology and pathology. Cell. Mol. Life Sci. 1999, 55, 1078–1087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, X.R.; Harraz, O.F. Mechanosensing by vascular endothelium. Annu. Rev. Physiol. 2024, 86, 71–97. [Google Scholar] [CrossRef]
- Pasut, A.; Lama, E.; Van Craenenbroeck, A.H.; Kroon, J.; Carmeliet, P. Endothelial cell metabolism in cardiovascular physiology and disease. Nat. Rev. Cardiol. 2025, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef]
- Sessa, W.C. Regulation of endothelial derived nitric oxide in health and disease. Memórias Inst. Oswaldo Cruz 2005, 100, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M.; Vanhoutte, P.M. Endothelium-derived hyperpolarizing factor: Where are we now? Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.A.; Rizvi, S.H.; Lyons, R.; Behrooz, L.; Hamburg, N.M. Vascular Dysfunction in Diabetes and Pharmacotherapeutic Opportunities: A Focus on Endothelial Cell Health. Am. J. Physiol. Heart Circ. Physiol. 2025, 329, H705–H718. [Google Scholar] [CrossRef]
- Józkowiak, M.; Niebora, J.; Domagała, D.; Data, K.; Partyńska, A.; Kulus, M.; Kotrych, K.; Podralska, M.; Górska, A.; Chwiłkowska, A.; et al. New Insights into Endothelial Cell Physiology and Pathophysiology. Biomed. Pharmacother. 2025, 190, 118415. [Google Scholar] [CrossRef]
- Rahimi, M.; Faridi, L.; Nikniaz, L.; Daneshvar, S.; Naseri, A.; Taban-Sadeghi, M.; Manaflouyan, H.; Shahabi, J.; Sarrafzadegan, N. Effect of Endothelial Adhesion Molecules on Atrial Fibrillation: A Systematic Review and Meta-Analysis. Heart Int. 2022, 16, 75–84. [Google Scholar] [CrossRef]
- Patel, R.B.; Colangelo, L.A.; Reiner, A.P.; Gross, M.D.; Jacobs, D.R., Jr.; Launer, L.J.; Lima, J.A.; Lloyd-Jones, D.M.; Shah, S.J. Cellular Adhesion Molecules in Young Adulthood and Cardiac Function in Later Life. J. Am. Coll. Cardiol. 2020, 75, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Macías, C.; Villaescusa, R.; del Valle, L.; Boffil, V.; Cordero, G.; Hernández, A.; Hernández, P.; Ballester, J.M. Moléculas de Adhesión Endoteliales ICAM-1, VCAM-1 y E-Selectin en Pacientes con Síndrome Coronario Agudo [Endothelial Adhesion Molecules ICAM-1, VCAM-1 and E-Selectin in Patients with Acute Coronary Syndrome]. Rev. Esp. Cardiol. 2003, 56, 137–144. [Google Scholar] [CrossRef]
- Segers, V.F.M.; Bringmans, T.; De Keulenaer, G.W. Endothelial Dysfunction at the Cellular Level in Three Dimensions: Severity, Acuteness, and Distribution. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H398–H413. [Google Scholar] [CrossRef]
- Kopaliani, I.; Elsaid, B.; Speier, S.; Deussen, A. Immune and Metabolic Mechanisms of Endothelial Dysfunction. Int. J. Mol. Sci. 2024, 25, 13337. [Google Scholar] [CrossRef]
- Habas, K.; Shang, L. Alterations in Intercellular Adhesion Molecule 1 (ICAM-1) and Vascular Cell Adhesion Molecule 1 (VCAM-1) in Human Endothelial Cells. Tissue Cell 2018, 54, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef]
- Pautz, A.; Li, H.; Kleinert, H. Regulation of NOS Expression in Vascular Diseases. Front. Biosci. 2021, 26, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Solanki, K.; Rajpoot, S.; Bezsonov, E.E.; Orekhov, A.N.; Saluja, R.; Wary, A.; Axen, C.; Wary, K.; Baig, M.S. The Expanding Roles of Neuronal Nitric Oxide Synthase (NOS1). PeerJ 2022, 10, e13651. [Google Scholar] [CrossRef]
- Mazuryk, O.; Gurgul, I.; Oszajca, M.; Polaczek, J.; Kieca, K.; Bieszczad-Żak, E.; Martyka, T.; Stochel, G. Nitric Oxide Signaling and Sensing in Age-Related Diseases. Antioxidants 2024, 13, 1213. [Google Scholar] [CrossRef]
- Carlström, M.; Weitzberg, E.; Lundberg, J.O. Nitric Oxide Signaling and Regulation in the Cardiovascular System: Recent Advances. Pharmacol. Rev. 2024, 76, 1038–1062. [Google Scholar] [CrossRef]
- Maccallini, C.; Budriesi, R.; De Filippis, B.; Amoroso, R. Advancements in the Research of New Modulators of Nitric Oxide Synthases Activity. Int. J. Mol. Sci. 2024, 25, 8486. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, B. Endothelial Dysfunction: Molecular Mechanisms and Clinical Implications. MedComm 2024, 5, e651. [Google Scholar] [CrossRef] [PubMed]
- Dri, E.; Lampas, E.; Lazaros, G.; Lazarou, E.; Theofilis, P.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Endothelial Dysfunction. Life 2023, 13, 1420. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamagata, K. Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Bisserier, M. Experimental animal models and patient-derived platforms to bridge preclinical discovery and translational therapeutics in pulmonary arterial hypertension. J. Transl. Med. 2025, 23, 665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahalmani, V.; Sinha, S.; Prakash, A.; Medhi, B. Translational research: Bridging the gap between preclinical and clinical research. Indian J. Pharmacol. 2022, 54, 393–396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The Interplay between Cytokines, Inflammation, and Antioxidants: Mechanistic Insights and Therapeutic Potentials of Various Antioxidants and Anti-Cytokine Compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101 Pt B, 107598. [Google Scholar] [CrossRef] [PubMed]
- Caverzán, M.D.; Beaugé, L.; Oliveda, P.M.; Cesca González, B.; Bühler, E.M.; Ibarra, L.E. Exploring Monocytes-Macrophages in Immune Microenvironment of Glioblastoma for the Design of Novel Therapeutic Strategies. Brain Sci. 2023, 13, 542. [Google Scholar] [CrossRef] [PubMed]
- Brüser, L.; Teichmann, E.; Hinz, B. Effect of Flavonoids on MCP-1 Expression in Human Coronary Artery Endothelial Cells and Impact on MCP-1-Dependent Migration of Human Monocytes. Int. J. Mol. Sci. 2023, 24, 16047. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Imanishi, T.; Tsujioka, H.; Akasaka, T. Endothelial progenitor cells dysfunction and senescence: Contribution to oxidative stress. Curr. Cardiol. Rev. 2008, 4, 275–286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, C.P.; Lin, F.Y.; Huang, P.H.; Chen, Y.L.; Chen, W.C.; Chen, H.Y.; Huang, Y.C.; Liao, W.L.; Huang, H.C.; Liu, P.L.; et al. Endothelial progenitor cell dysfunction in cardiovascular diseases: Role of reactive oxygen species and inflammation. Biomed. Res. Int. 2013, 2013, 845037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geto, Z.; Molla, M.D.; Challa, F.; Belay, Y.; Getahun, T. Mitochondrial Dynamic Dysfunction as a Main Triggering Factor for Inflammation Associated Chronic Non-Communicable Diseases. J. Inflamm. Res. 2020, 13, 97–107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinkova-Kostova, A.T.; Copple, I.M. Advances and challenges in therapeutic targeting of NRF2. Trends Pharmacol. Sci. 2023, 44, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J. Leptin and Atherosclerosis. Atherosclerosis 2006, 189, 47–60. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Wu, B.H.; Liang, T.T.; Liu, Z.; Ju, W.; Wang, Y.; Wen, Y.T.; Liu, M.C.; Du, J.H. Leptin Activates the JAK/STAT Pathway to Promote Angiogenesis in RF/6A Cells in vitro. Int. J. Ophthalmol. 2022, 15, 554–559. [Google Scholar] [CrossRef]
- Gianoli, S.; Tang, J.; Odegard, K.C.; Yuki, K.; Koutsogiannaki, S. Harnessing Adiponectin for Sepsis: Current Knowledge, Clinical Insights and Future Therapies. Crit. Care 2025, 29, 300. [Google Scholar] [CrossRef]
- Jung, H.N.; Jung, C.H. The Role of Anti-Inflammatory Adipokines in Cardiometabolic Disorders: Moving beyond Adiponectin. Int. J. Mol. Sci. 2021, 22, 13529. [Google Scholar] [CrossRef]
- Hemat Jouy, S.; Mohan, S.; Scichilone, G.; Mostafa, A.; Mahmoud, A.M. Adipokines in the Crosstalk between Adipose Tissues and Other Organs: Implications in Cardiometabolic Diseases. Biomedicines 2024, 12, 2129. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Luo, J.; He, Z.; Li, Q.; Lv, M.; Cai, Y.; Ke, W.; Niu, X.; Zhang, Z. Adipokines in Atherosclerosis: Unraveling Complex Roles. Front. Cardiovasc. Med. 2023, 10, 1235953. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Deshmukh, B.; Ramteke, P.; Bhati, F.K.; Bhat, M.K. Resistin: A Journey from Metabolism to Cancer. Transl. Oncol. 2021, 14, 101178. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Koka, S.; Boini, K.M. Understanding the Role of Adipokines in Cardiometabolic Dysfunction: A Review of Current Knowledge. Biomolecules 2025, 15, 612. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, E.; Deng, L.; Zhu, Y.; Lu, X.; Li, X.; Li, F.; Yan, Y.; Han, J.Y.; Li, Y.; et al. Immunological Roles for Resistin and Related Adipokines in Obesity-Associated Tumors. Int. Immunopharmacol. 2024, 142 Pt A, 112911. [Google Scholar] [CrossRef]
- Roy, P.K.; Islam, J.; Lalhlenmawia, H. Prospects of Potential Adipokines as Therapeutic Agents in Obesity-Linked Atherogenic Dyslipidemia and Insulin Resistance. Egypt. Heart J. 2023, 75, 24. [Google Scholar] [CrossRef]
- Jarmukhanov, Z.; Mukhanbetzhanov, N.; Kozhakhmetov, S.; Nurgaziyev, M.; Sailybayeva, A.; Bekbossynova, M.; Kushugulova, A. The association between the gut microbiota metabolite trimethylamine N-oxide and heart failure. Front. Microbiol. 2024, 15, 1440241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How gut microbiota contributes to heart failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Shan, Q.Y.; Wu, X.; Miao, H.; Zhao, Y.Y. Gut microbiota regulates oxidative stress and inflammation: A double-edged sword in renal fibrosis. Cell. Mol. Life Sci. 2024, 81, 480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sagmeister, A.; Matter, C.M.; Stähli, B.E.; Scharl, M. The Gut-Heart Axis: Effects of Intestinal Microbiome Modulation on Cardiovascular Disease-Ready for Therapeutic Interventions? Int. J. Mol. Sci. 2024, 25, 13529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takeda, Y.; Matoba, K.; Sekiguchi, K.; Nagai, Y.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Endothelial Dysfunction in Diabetes. Biomedicines 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, J.; Lin, A.; Chi, J.; Hao, H.; Chen, H.; Liu, Z. Oxidative Stress, Endothelial Dysfunction, and N-Acetylcysteine in Type 2 Diabetes Mellitus. Antioxid. Redox Signal. 2024, 40, 968–989. [Google Scholar] [CrossRef]
- Merdler, I.; Arbel, Y. Type II Diabetes Mellitus and Endothelial Dysfunction: What Can We Do? Isr. Med. Assoc. J. 2021, 23, 121–122. [Google Scholar] [PubMed]
- Clyne, A.M. Endothelial Response to Glucose: Dysfunction, Metabolism, and Transport. Biochem. Soc. Trans. 2021, 49, 313–325. [Google Scholar] [CrossRef]
- Yang, D.R.; Wang, M.Y.; Zhang, C.L.; Wang, Y. Endothelial Dysfunction in Vascular Complications of Diabetes: A Comprehensive Review of Mechanisms and Implications. Front. Endocrinol. 2024, 15, 1359255. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Endothelial Function in Dyslipidemia: Roles of LDL-Cholesterol, HDL-Cholesterol and Triglycerides. Cells 2023, 12, 1293. [Google Scholar] [CrossRef]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K.; Leduc, Q. Transatlantic Network on Atherothrombosis Inflammation in Atherosclerosis: From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Drożdż, D.; Drożdż, M.; Wójcik, M. Endothelial Dysfunction as a Factor Leading to Arterial Hypertension. Pediatr. Nephrol. 2023, 38, 2973–2985. [Google Scholar] [CrossRef]
- Bleakley, C.; Hamilton, P.K.; Pumb, R.; Harbinson, M.; McVeigh, G.E. Endothelial Function in Hypertension: Victim or Culprit? J. Clin. Hypertens. 2015, 17, 651–654. [Google Scholar] [CrossRef]
- Tang, E.H.; Vanhoutte, P.M. Endothelial Dysfunction: A Strategic Target in the Treatment of Hypertension? Pflügers Arch.-Eur. J. Physiol. 2010, 459, 995–1004. [Google Scholar] [CrossRef]
- Engin, A. Endothelial Dysfunction in Obesity. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 960, pp. 345–379. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, M.; Kyler, K.; Xu, J. Adipose Tissue–Endothelial Cell Interactions in Obesity-Induced Endothelial Dysfunction. Front. Cardiovasc. Med. 2021, 8, 681581. [Google Scholar] [CrossRef] [PubMed]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Manzano-Pech, L.; Rubio-Ruíz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative Stress and Its Association with Cardiometabolic Disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, C.; Chen, K.; Gao, Z.; Bao, T.; Dong, L.; Zhao, L.; Tong, X.; Li, X. Common Mechanisms Underlying Diabetic Vascular Complications: Focus on the Interaction of Metabolic Disorders, Immuno-Inflammation, and Endothelial Dysfunction. Cell Commun. Signal. 2023, 21, 298. [Google Scholar] [CrossRef]
- Amersfoort, J.; Eelen, G.; Carmeliet, P. Immunomodulation by Endothelial Cells—Partnering up with the Immune System? Nat. Rev. Immunol. 2022, 22, 576–588. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Imran, M.; Ahsan, H. Biomarkers as biomedical bioindicators: Approaches and techniques for the detection, analysis, and validation of novel biomarkers of diseases. Pharmaceutics 2023, 15, 1630. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Stoner, L.; Lucero, A.A.; Palmer, B.R.; Jones, L.M.; Young, J.M.; Faulkner, J. Inflammatory biomarkers for predicting cardiovascular disease. Clin. Biochem. 2013, 46, 1353–1371. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Pickett, J.R.; Wu, Y.; Zacchi, L.F.; Ta, H.T. Targeting endothelial vascular cell adhesion molecule-1 in atherosclerosis: Drug discovery and development of vascular cell adhesion molecule-1-directed novel therapeutics. Cardiovasc. Res. 2023, 119, 2278–2293. [Google Scholar] [CrossRef]
- Milošević, N.; Rütter, M.; David, A. Endothelial cell adhesion molecules—(un)attainable targets for nanomedicines. Front. Med. Technol. 2022, 4, 846065. [Google Scholar] [CrossRef]
- Hubbard, A.K.; Rothlein, R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic. Biol. Med. 2000, 28, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Landis, H.E.; Miller, R.E.; Tizabi, Y. Intercellular adhesion molecule 1 (ICAM-1): An inflammatory regulator with potential implications in ferroptosis and Parkinson’s disease. Cells 2024, 13, 1554. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef]
- Ferraz-Amaro, I.; Ibrahim-Achi, Z.; de Vera-González, A.; González-Delgado, A.; Renuncio-García, M.; Vicente-Rabaneda, E.F.; Ocejo-Vinyals, J.G.; Castañeda, S.; González-Gay, M.Á. Associations between soluble cell adhesion molecules cardiovascular comorbidities in systemic sclerosis: Implications for insulin resistance. J. Clin. Med. 2025, 14, 1467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef]
- Chami, B.; Hossain, F.; Hambly, T.W.; Cai, X.; Aran, R.; Fong, G.; Vellajo, A.; Martin, N.J.J.; Wang, X.; Dennis, J.M.; et al. Serum Amyloid A Stimulates Vascular and Renal Dysfunction in Apolipoprotein EDeficient Mice Fed a Normal Chow Diet. Front. Immunol. 2019, 10, 380. [Google Scholar] [CrossRef]
- Saxton, R.A.; Glassman, C.R.; Garcia, K.C. Emerging principles of cytokine pharmacology and therapeutics. Nat. Rev. Drug Discov. 2023, 22, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.P.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-α in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef]
- Wolberg, A.S. Fibrinogen and fibrin: Synthesis, structure, and function in health and disease. J. Thromb. Haemost. 2023, 21, 3005–3015. [Google Scholar] [CrossRef]
- Ellins, E.A.; Rees, D.A.; Deanfield, J.E.; Steptoe, A.; Halcox, J.P. Increased fibrinogen responses to psychophysiological stress predict future endothelial dysfunction: Implications for cardiovascular disease? Brain Behav. Immun. 2017, 60, 233–239. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, G.; Liu, X. Blood fibrinogen level as a biomarker of adverse outcomes in patients with coronary artery disease: A systematic review and meta-analysis. Medicine 2022, 101, e30117. [Google Scholar] [CrossRef]
- Guo, X.; Xing, Y.; Jin, W. Role of ADMA in the pathogenesis of microvascular complications in type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1183586. [Google Scholar] [CrossRef]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic. Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef]
- Cortes, G.A.; Moore, M.J.; El-Nakeep, S. Physiology, von Willebrand factor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559062/ (accessed on 8 August 2025).
- Lelas, A.; Greinix, H.T.; Wolff, D.; Eissner, G.; Pavletic, S.Z.; Pulanic, D. Von Willebrand factor, factor VIII, and other acute phase reactants as biomarkers of inflammation and endothelial dysfunction in chronic graftversushost disease. Front. Immunol. 2021, 12, 676756. [Google Scholar] [CrossRef] [PubMed]
- Steffes, L.C.; Cheng, P.; Quertermous, T.; Kumar, M.E. von Willebrand factor is produced exclusively by endothelium, not neointima, in occlusive vascular lesions in both pulmonary hypertension and atherosclerosis. Circulation 2022, 146, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Dmour, B.A.; Costache, A.D.; Dmour, A.; Huzum, B.; Duca, Ș.T.; Chetran, A.; Miftode, R.Ș.; Afrăsânie, I.; Tuchiluș, C.; Cianga, C.M.; et al. Could endothelin-1 be a promising neurohormonal biomarker in acute heart failure? Diagnostics 2023, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Dmour, B.-A.; Badescu, M.C.; Tuchiluș, C.; Cianga, C.M.; Constantinescu, D.; Dima, N.; Duca, Ș.T.; Dmour, A.; Costache, A.D.; Cepoi, M.-R.; et al. Can Endothelin-1 Help Address the Diagnostic and Prognostic Challenges in Multimorbid Acute Heart Failure Patients? Life 2025, 15, 628. [Google Scholar] [CrossRef]
- Hong, C.G.; Florida, E.; Li, H.; Parel, P.M.; Mehta, N.N.; Sorokin, A.V. Oxidized low-density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 9, 1023651. [Google Scholar] [CrossRef]
- Amirfakhryan, H.; New, K.J. The role of myeloperoxidase as a biomarker in atherosclerotic cardiovascular disease. Cardiol. Plus 2024, 9, 195–209. [Google Scholar] [CrossRef]
- Cheng, D.; Talib, J.; Stanley, C.P.; Rashid, I.; Michaëlsson, E.; Lindstedt, E.L.; Croft, K.D.; Kettle, A.J.; Maghzal, G.J.; Stocker, R. Inhibition of MPO (myeloperoxidase) attenuates endothelial dysfunction in mouse models of vascular inflammation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1448–1457. [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef]
- Abagnale, L.; Candia, C.; Motta, A.; Galloway, B.; Ambrosino, P.; Molino, A.; Maniscalco, M. Flow-mediated dilation as a marker of endothelial dysfunction in pulmonary diseases: A narrative review. Respir. Med. Res. 2023, 84, 101049. [Google Scholar] [CrossRef]
- Mućka, S.; Miodońska, M.; Jakubiak, G.K.; Starzak, M.; Cieślar, G.; Stanek, A. Endothelial Function Assessment by Flow-Mediated Dilation Method: A Valuable Tool in the Evaluation of the Cardiovascular System. Int. J. Environ. Res. Public Health 2022, 19, 11242. [Google Scholar] [CrossRef]
- Ahn, Y.; Aung, N.; Ahn, H.-S. A Comprehensive Review of Clinical Studies Applying Flow-Mediated Dilation. Diagnostics 2024, 14, 2499. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Schnall, R.P.; Sheffy, J.K.; Penzel, T. Peripheral arterial tonometry–PAT technology. Sleep. Med. Rev. 2022, 61, 101566. [Google Scholar] [CrossRef] [PubMed]
- Quyyumi, A.A.; Almuwaqqat, Z.; Islam, S.J. Clinical investigations of vascular function. In The Vasculome; Galis, Z.S., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 181–196. [Google Scholar] [CrossRef]
- Stoner, L.; Young, J.M.; Fryer, S. Assessments of arterial stiffness and endothelial function using pulse wave analysis. Int. J. Vasc. Med. 2012, 2012, 903107. [Google Scholar] [CrossRef]
- Kuvin, J.T.; Patel, A.R.; Sliney, K.A.; Pandian, N.G.; Sheffy, J.; Schnall, R.P.; Karas, R.H.; Udelson, J.E. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am. Heart J. 2003, 146, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Marti, C.N.; Gheorghiade, M.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Quyyumi, A.A.; Butler, J. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 2012, 60, 1455–1469. [Google Scholar] [CrossRef]
- Drera, A.; Rodella, L.; Brangi, E.; Riccardi, M.; Vizzardi, E. Endothelial Dysfunction in Heart Failure: What Is Its Role? J. Clin. Med. 2024, 13, 2534. [Google Scholar] [CrossRef]
- Ong, P.; Athanasiadis, A.; Sechtem, U. Intracoronary acetylcholine provocation testing for assessment of coronary vasomotor disorders. J. Vis. Exp. 2016, 114, 54295. [Google Scholar] [CrossRef]
- Sueda, S.; Sakaue, T. The need for separate testing with acetylcholine for the assessment of endothelial dysfunction and coronary artery spasm. Eur. Cardiol. 2024, 19, e17. [Google Scholar] [CrossRef]
- Taqueti, V.R. Coronary flow reserve: A versatile tool for interrogating pathophysiology, and a reliable marker of cardiovascular outcomes and mortality. Eur. Heart J. 2022, 43, 1594–1596. [Google Scholar] [CrossRef]
- Mahendiran, T.; Fawaz, S.; Keulards, D.; Viscusi, M.; Everaars, H.; Keeble, T.; Damman, P.; Knaapen, P.; Collet, C.; De Bruyne, B. Simplification of coronary continuous thermodilution. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.1389. [Google Scholar] [CrossRef]
- Allbritton-King, J.D.; García-Cardeña, G. Endothelial cell dysfunction in cardiac disease: Driver or consequence? Front. Cell Dev. Biol. 2023, 11, 1278166. [Google Scholar] [CrossRef]
- Tiefenbacher, C.P.; Friedrich, S.; Bleeke, T.; Vahl, C.; Chen, X.; Niroomand, F. ACE inhibitors and statins acutely improve endothelial dysfunction of human coronary arterioles. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H142–H146. [Google Scholar] [CrossRef]
- Rahangdale, S.; Yeh, S.Y.; Malhotra, A.; Veves, A. Therapeutic interventions and oxidative stress in diabetes. Expert. Rev. Endocrinol. Metab. 2009, 4, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Tabit, C.E.; Chung, W.B.; Hamburg, N.M.; Vita, J.A. Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Rev. Endocr. Metab. Disord. 2010, 11, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhou, Y.; Ling, P.; Feng, X.; Luo, S.; Zheng, X.; Little, P.J.; Xu, S.; Weng, J. Metformin in cardiovascular diabetology: A focused review of its impact on endothelial function. Theranostics 2021, 11, 9376–9396. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yue, T.; Chen, Z.; Wu, W.; Xu, S.; Weng, J. Targeting FGF21 in cardiovascular and metabolic diseases: From mechanism to medicine. Int. J. Biol. Sci. 2023, 19, 66. [Google Scholar] [CrossRef]
- An, X.; Sun, W.; Wen, Z.; Duan, L.; Zhang, Y.; Kang, X.; Ji, H.; Sun, Y.; Jiang, L.; Zhao, X.; et al. Comparison of the efficacy and safety of GLP-1 receptor agonists on cardiovascular events and risk factors: A review and network meta-analysis. Diabetes Obes. Metab. 2025, 27, 1735–1751. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Fernandez-Gandara, C.; Garcia-Rios, A.; Rangel-Zuñiga, O.A.; Gutierrez-Mariscal, F.M.; Torres-Peña, J.D.; Marin, C.; Lopez-Moreno, J.; Castaño, J.P.; Delgado-Lista, J.; et al. Mediterranean diet and endothelial function in patients with coronary heart disease: An analysis of the CORDIOPREV randomized controlled trial. PLoS Med. 2020, 17, e1003282. [Google Scholar] [CrossRef]
- Esposito, K.; Ciotola, M.; Giugliano, F.; De Sio, M.; Giugliano, G.; D’armiento, M.; Giugliano, D. Mediterranean diet improves erectile function in subjects with the metabolic syndrome. Int. J. Impot. Res. 2006, 18, 405–410. [Google Scholar] [CrossRef]
- Keogh, J.B.; Brinkworth, G.D.; Noakes, M.; Clifton, P.M. Effects of weight loss from a very-low-calorie diet on endothelial function and markers of cardiovascular disease risk in subjects with abdominal obesity. Am. J. Clin. Nutr. 2008, 87, 567–576. [Google Scholar] [CrossRef]
- Vandercappellen, E.J.; Koster, A.; Savelberg, H.H.C.M.; Eussen, S.J.P.M.; Dagnelie, P.C.; Schaper, N.C.; Schram, M.T.; van der Kallen, C.J.H.; van Greevenbroek, M.M.J.; Wesselius, A.; et al. Sedentary behaviour and physical activity are associated with biomarkers of endothelial dysfunction and low-grade inflammation—Relevance for (pre)diabetes: The Maastricht Study. Diabetologia 2022, 65, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Villani, V.; Buono, R.; Wei, M.; Kumar, S.; Yilmaz, O.H.; Cohen, P.; Sneddon, J.B.; Perin, L.; Longo, V.D. Fasting-mimicking diet promotes Ngn3-driven β-cell regeneration to reverse diabetes. Cell 2017, 168, 775–788.e12. [Google Scholar] [CrossRef] [PubMed]
- von Stebut, E.; Boehncke, W.H.; Ghoreschi, K.; Gori, T.; Kaya, Z.; Thaci, D.; Schäffler, A. IL-17A in Psoriasis and Beyond: Cardiovascular and Metabolic Implications. Front. Immunol. 2020, 10, 3096. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Ant-iinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.C.; Tanguay, J.F.; Wright, S.R.; Duchatelle, V.; Petroni, T.; Grégoire, J.C.; Ibrahim, R.; Heinonen, T.M.; Robb, S.; Bertrand, O.F.; et al. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: Results of the SELECT-ACS trial. J. Am. Coll. Cardiol. 2013, 61, 2048–2055. [Google Scholar] [CrossRef]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in Plaque Psoriasis—Results of Two Phase 3 Trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Prange, K.H.M.; Glass, C.K.; de Winther, M.P.J. Pharmacological Targeting of the CCL2/CCR2 Axis for Atheroprotection: A Meta-Analysis of Preclinical Studies. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e244–e257. [Google Scholar] [CrossRef]
- Alemán-Ruiz, C.; Wang, W.; Dingledine, R.; Varvel, N.H. Pharmacological inhibition of the inflammatory receptor CCR2 relieves the early deleterious consequences of status epilepticus. Sci. Rep. 2023, 13, 5651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vincent, V.; Thakkar, H.; Sen, A.; Bansal, A.; Das, U.S.; Gunasekaran, A.; Bhatla, N.; Velpandian, T.; Singh, A. Adiponectin mediated metabolic and sphingolipid alterations in preventing endothelial dysfunction. Mol. Cell. Biochem. 2025, 480, 4365–4377. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K.; Kwon, Y.; Byeon, S.; Lee, Y.H. AdipoRon, adiponectin receptor agonist, improves vascular function in the mesenteric arteries of type 2 diabetic mice. FASEB J. 2019, 33, 830–835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarska, E.; Bojdo, K.; Frankenstein, H.; Krawiranda, K.; Kustosik, N.; Lisińska, W.; Rysz, J.; Franczyk, B. Endothelial Dysfunction as the Common Pathway Linking Obesity, Hypertension and Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 10096. https://doi.org/10.3390/ijms262010096
Młynarska E, Bojdo K, Frankenstein H, Krawiranda K, Kustosik N, Lisińska W, Rysz J, Franczyk B. Endothelial Dysfunction as the Common Pathway Linking Obesity, Hypertension and Atherosclerosis. International Journal of Molecular Sciences. 2025; 26(20):10096. https://doi.org/10.3390/ijms262010096
Chicago/Turabian StyleMłynarska, Ewelina, Kinga Bojdo, Hanna Frankenstein, Katarzyna Krawiranda, Natalia Kustosik, Wiktoria Lisińska, Jacek Rysz, and Beata Franczyk. 2025. "Endothelial Dysfunction as the Common Pathway Linking Obesity, Hypertension and Atherosclerosis" International Journal of Molecular Sciences 26, no. 20: 10096. https://doi.org/10.3390/ijms262010096
APA StyleMłynarska, E., Bojdo, K., Frankenstein, H., Krawiranda, K., Kustosik, N., Lisińska, W., Rysz, J., & Franczyk, B. (2025). Endothelial Dysfunction as the Common Pathway Linking Obesity, Hypertension and Atherosclerosis. International Journal of Molecular Sciences, 26(20), 10096. https://doi.org/10.3390/ijms262010096







