Combined Lactiplantibacillus plantarum CRL1506 and MPL16 Nasal Priming More Effectively Modulates Respiratory Antiviral Innate Immunity than Single Strains
Abstract
1. Introduction
2. Results
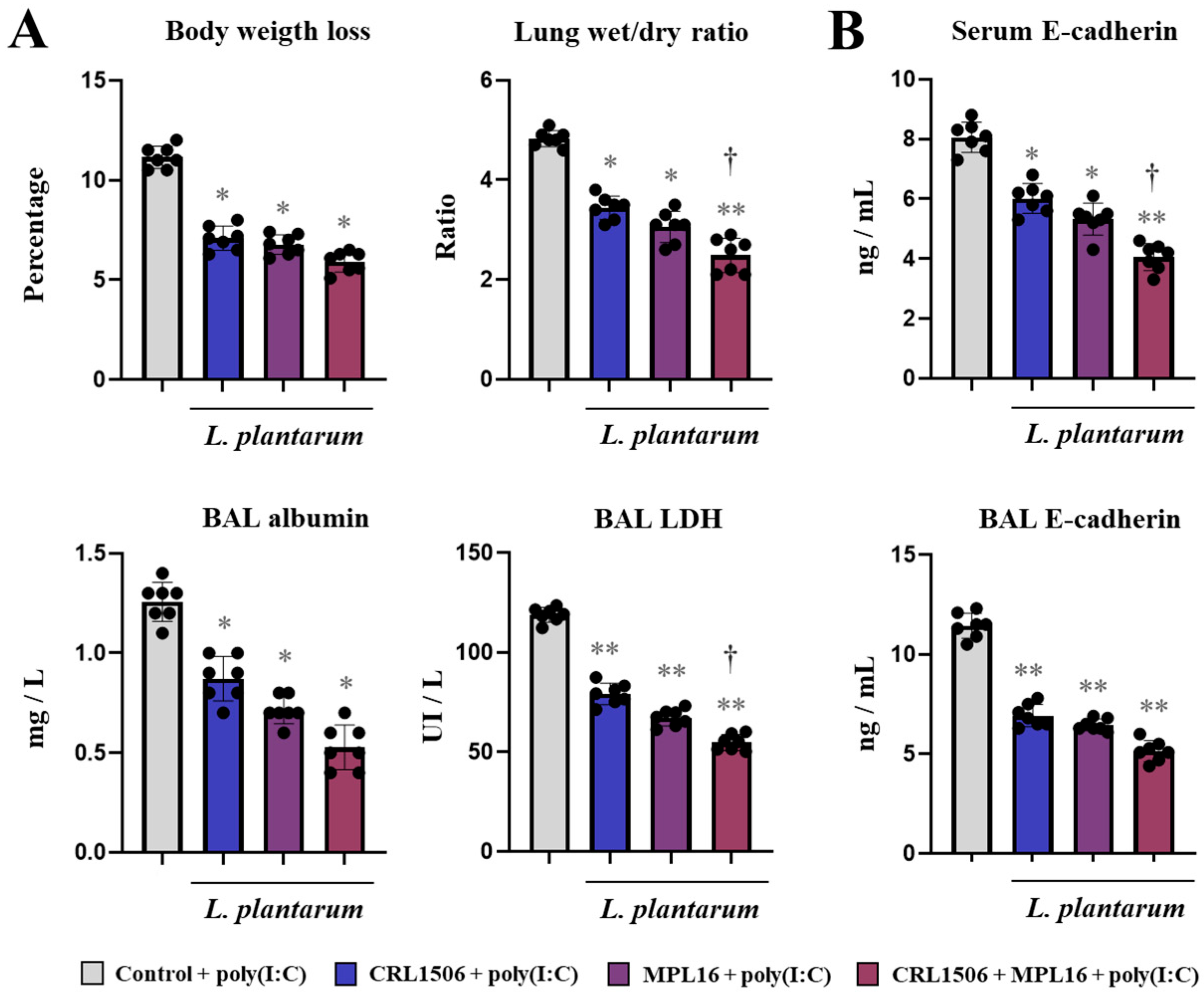
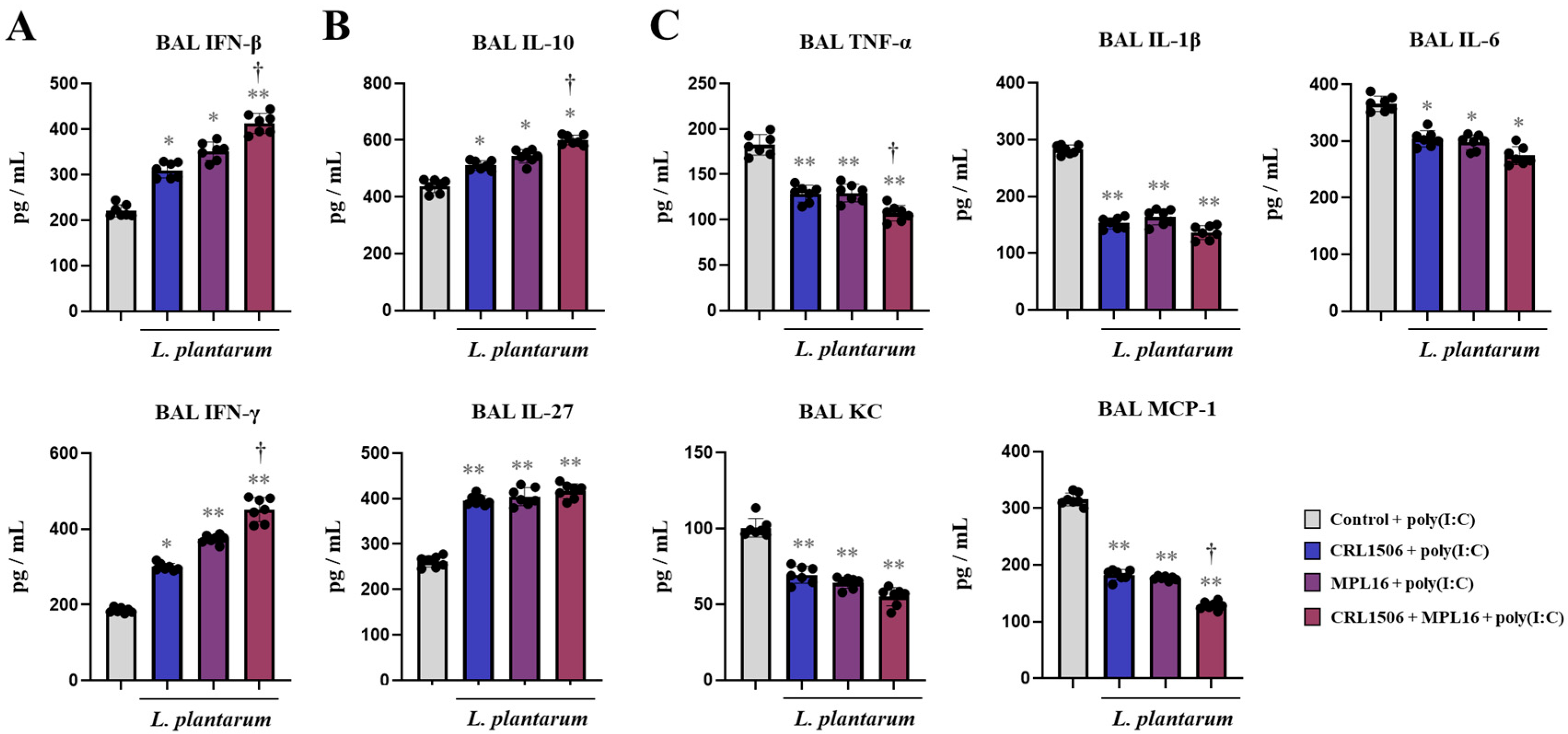
2.1. Nasal Administration of Lactiplantibacillus plantarum Strains Improve TLR3-Mediated Respiratory Innate Antiviral Immune Response in Mice
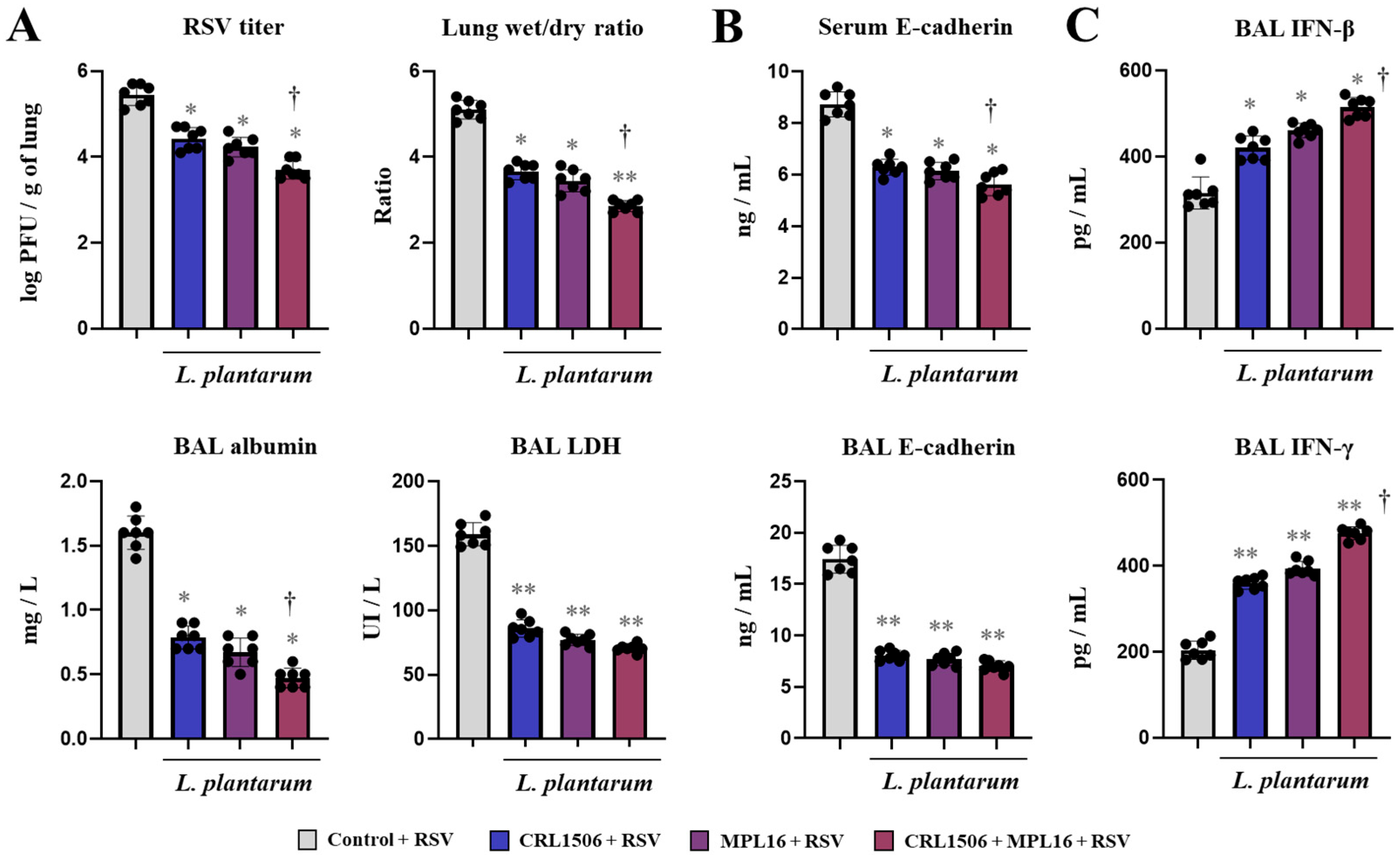
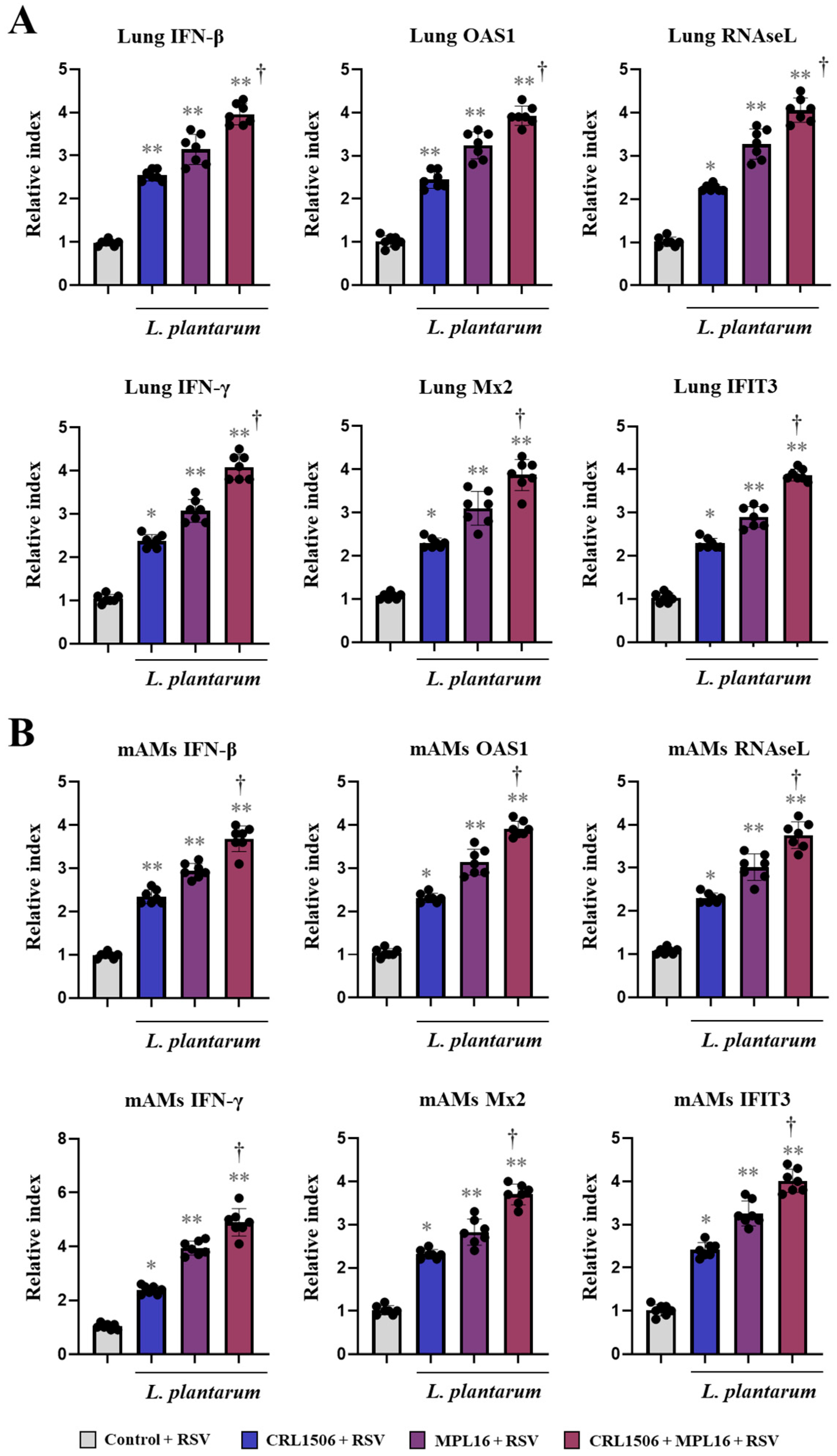
2.2. Nasal Administration of Lactiplantibacillus plantarum Strains Improve Immunity Against RSV Infection in Mice
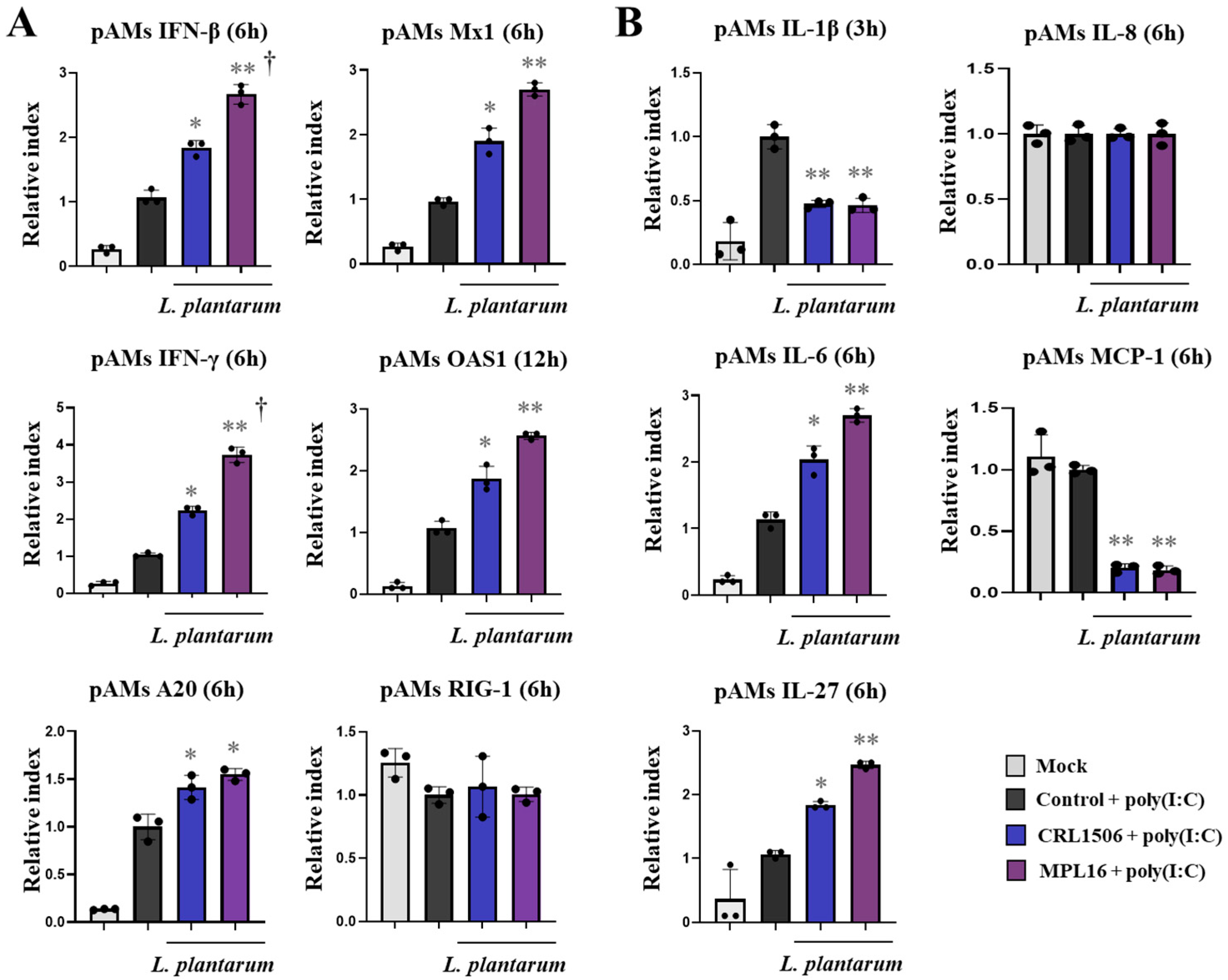
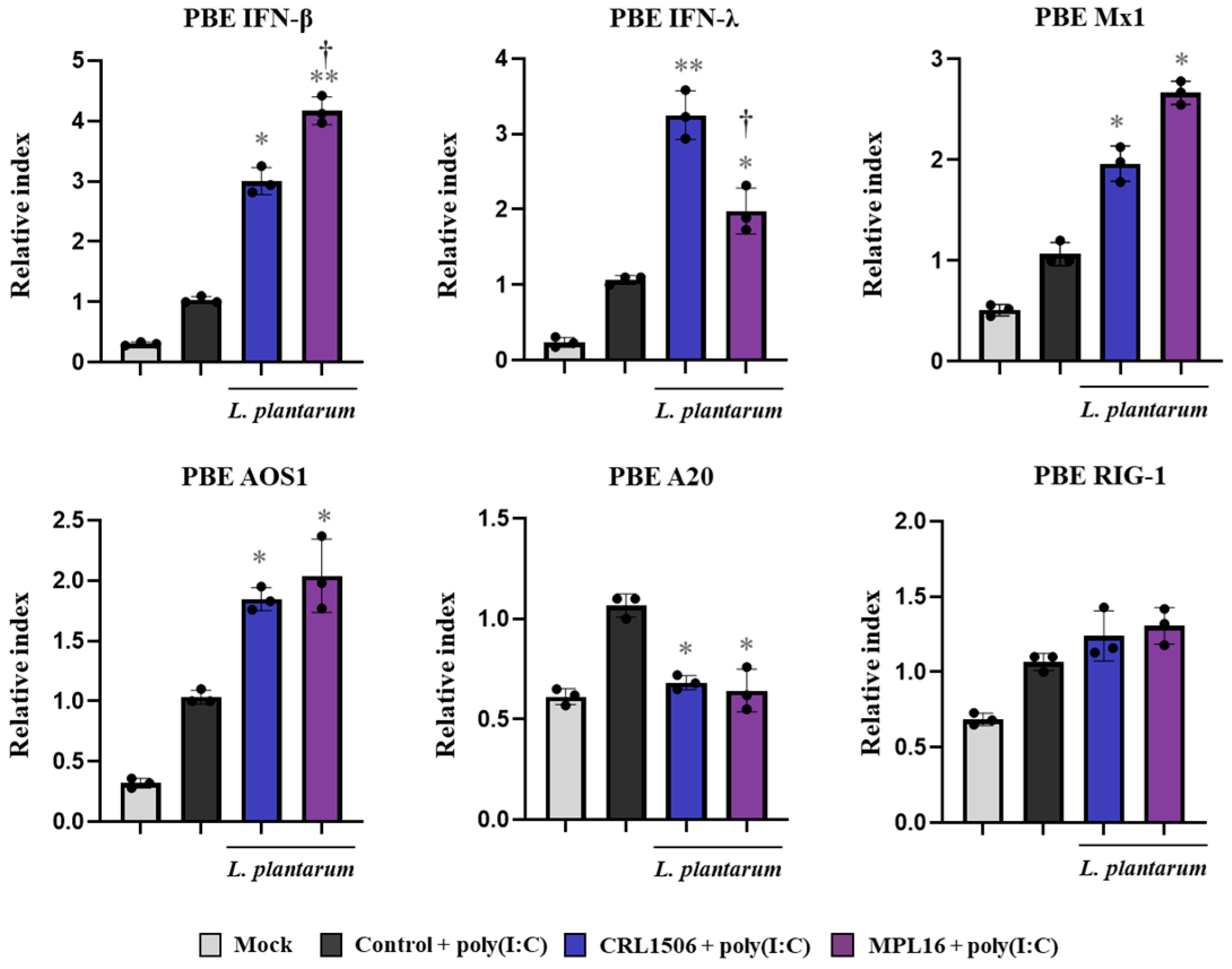
2.3. Lactiplantibacillus Plantarum Strains Improve TLR3-Mediated Innate Antiviral Immune Response in Porcine Alveolar Macrophages
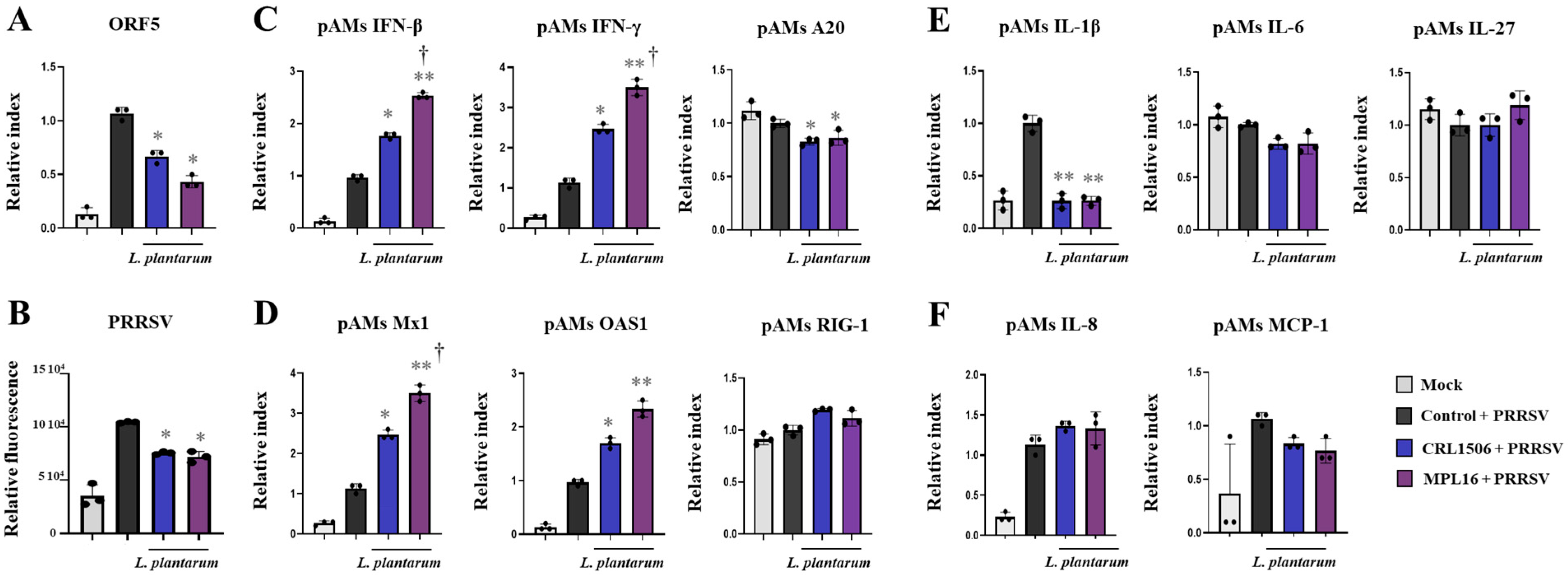
2.4. Lactiplantibacillus Plantarum Strains Improve Immunity Against PRRSV in Porcine Alveolar Macrophages
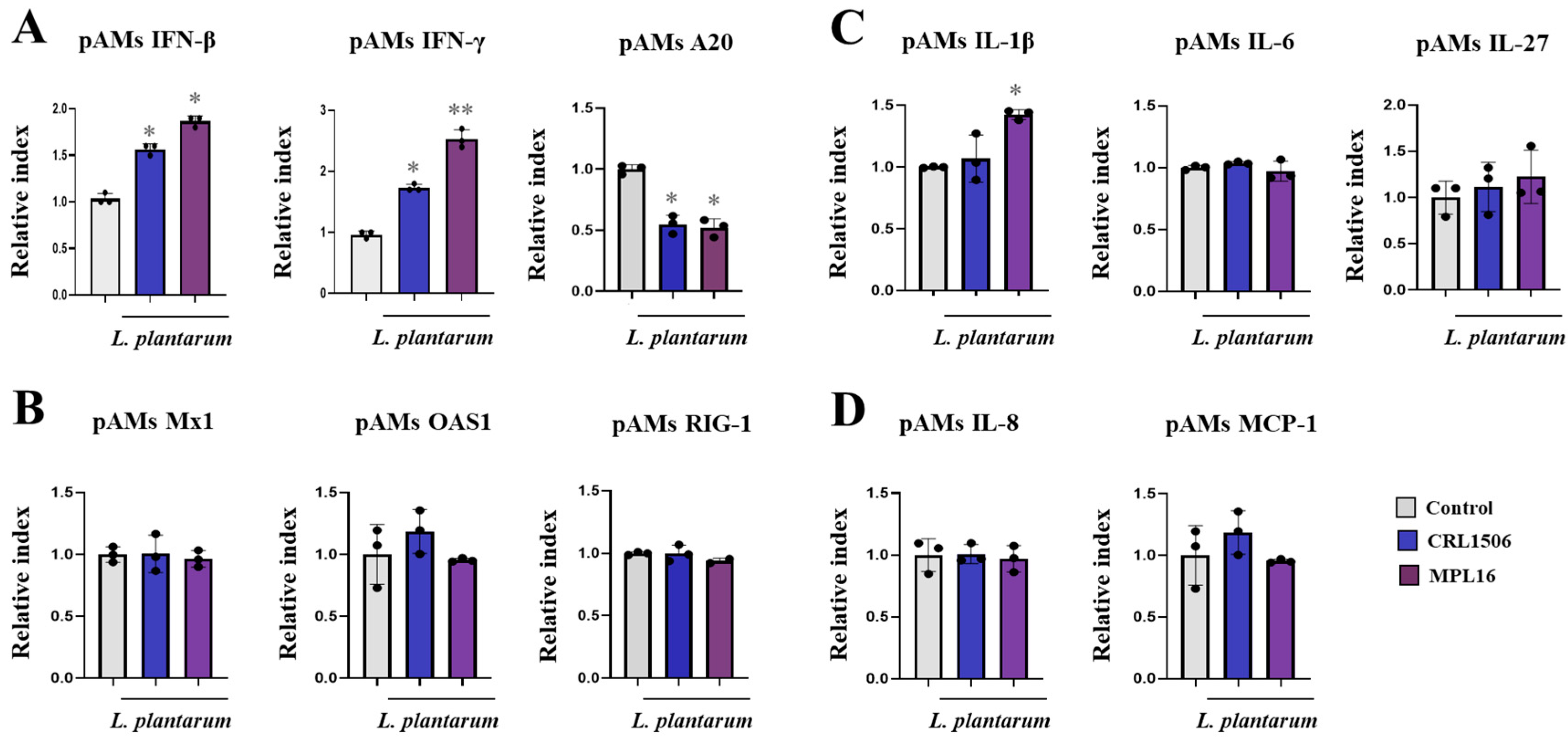
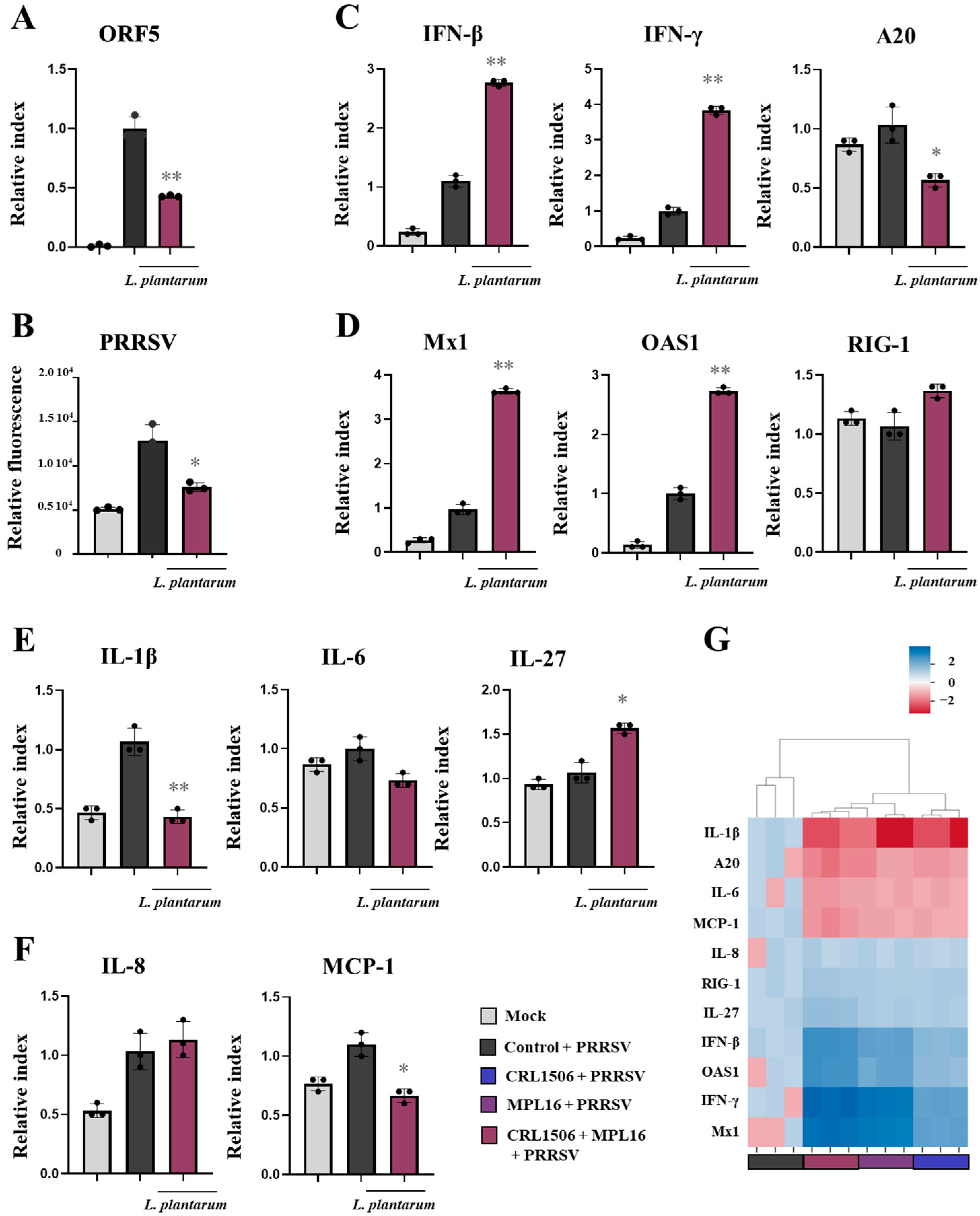
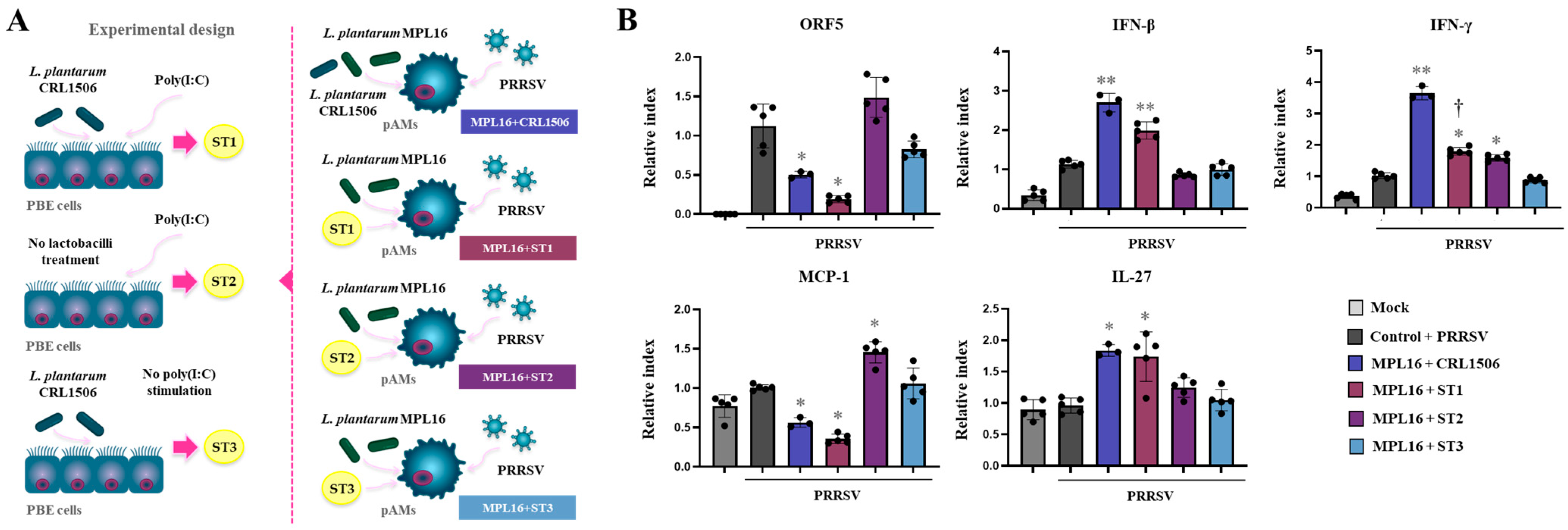
2.5. Combined Administration of Lactiplantibacillus plantarum Strains Is Not Better than Single Strains to Improve Antiviral Immunity in Porcine Alveolar Macrophages
2.6. Lactiplantibacillus plantarum CRL1506 Is Better than MPL16 to Improve IFN-λ in Porcine Respiratory Epithelial Cells
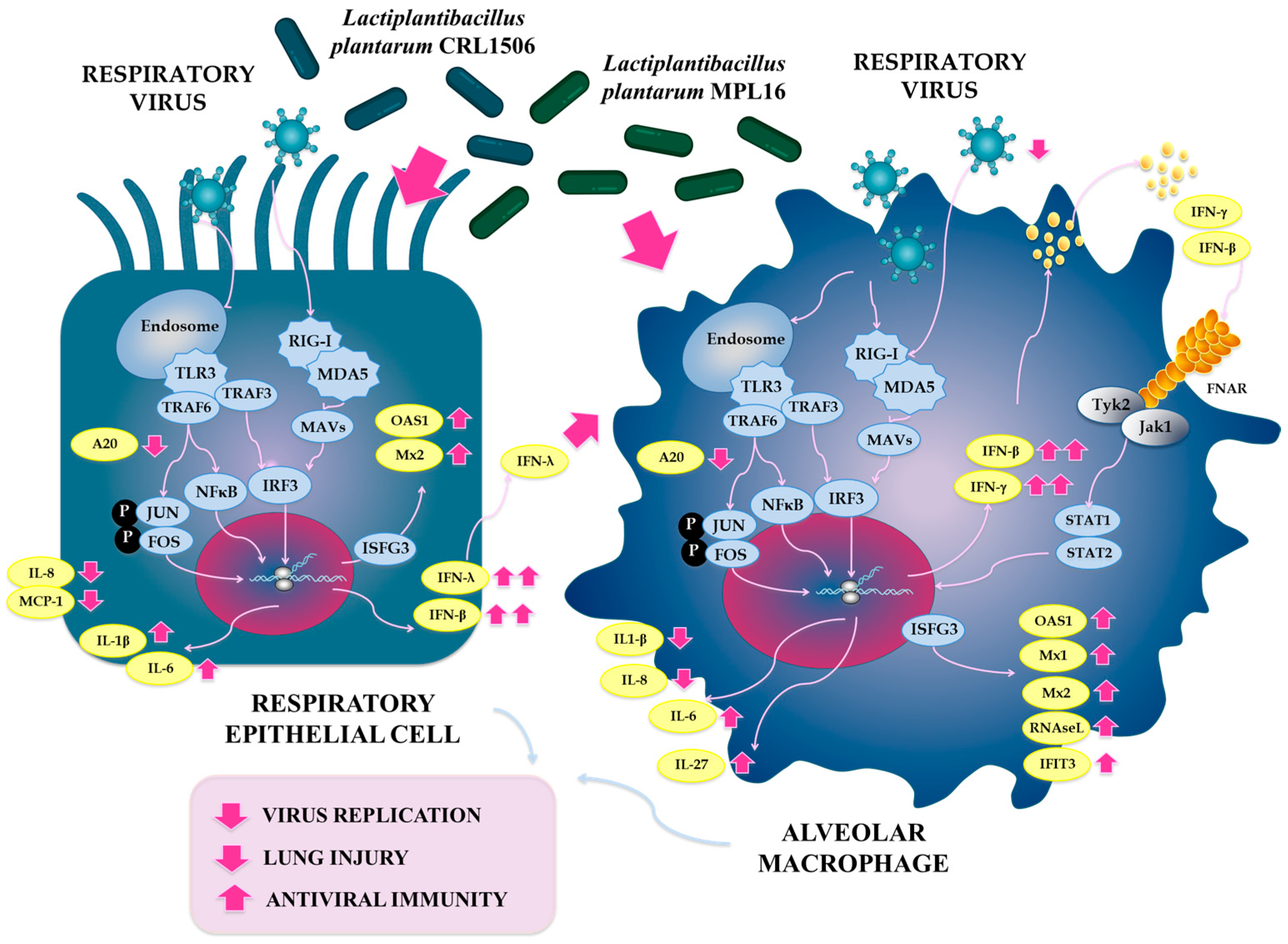
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. Animals and Feeding Procedures
4.3. Intranasal Administration of Poly(I:C) and RSV Infection
4.4. Lung Injury Parameters
4.5. Cytokine Concentrations in BAL
4.6. Alveolar Macrophage Primary Cultures
4.7. Porcine Bronchial Epithelial and Macrophages Cell Lines
4.8. Immunomodulatory Capacity of Lactobacilli in Porcine Cells
4.9. Immunofluorescence (IF)
4.10. RT-qPCR
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yu, X.; Wang, H.; Ma, S.; Chen, W.; Sun, L.; Zou, Z. Estimating the global and regional burden of lower respiratory infections attributable to leading pathogens and the protective effectiveness of immunization programs. Int. J. Infect. Dis. 2024, 149, 107268. [Google Scholar] [CrossRef]
- GBD 2021a. Upper Respiratory Infections Otitis Media Collaborators. Global, regional, and national burden of upper respiratory infections and otitis media, 1990–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2025, 25, 36–51, Correction in Lancet Infect. Dis. 2025, 25, E13. [Google Scholar] [CrossRef]
- GBD 2021b. Lower Respiratory Infections and Antimicrobial Resistance Collaborators. Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 974–1002. [Google Scholar] [CrossRef]
- Rosas-Salazar, C.; Shilts, M.H.; Tovchigrechko, A.; Chappell, J.D.; Larkin, E.K.; Nelson, K.E.; Moore, M.L.; Anderson, L.J.; Das, S.R.; Hartert, T.V. Nasopharyngeal microbiome in respiratory syncytial virus resembles profile associated with increased childhood asthma risk. Am. J. Respir. Crit. Care Med. 2016, 193, 1180–1183. [Google Scholar] [CrossRef]
- Rosas-Salazar, C.; Shilts, M.H.; Tovchigrechko, A.; Schobel, S.; Chappell, J.D.; Larkin, E.K.; Gebretsadik, T.; Halpin, R.A.; Nelson, K.E.; Moore, M.L.; et al. Nasopharyngeal Lactobacillus is associated with a reduced risk of childhood wheezing illnesses following acute respiratory syncytial virus infection in infancy. J. Allergy Clin. Immunol. 2018, 142, 1447–1456.e1449. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, I.; van den Broek, M.F.L.; Allonsius, C.N.; Spacova, I.; Wittouck, S.; Martens, K.; Wuyts, S.; Cauwenberghs, E.; Jokicevic, K.; Vandenheuvel, D.; et al. Lactobacilli Have a Niche in the Human Nose. Cell Rep. 2020, 31, 107674. [Google Scholar] [CrossRef]
- Tomosada, Y.; Chiba, E.; Zelaya, H.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013, 14, 40. [Google Scholar] [CrossRef]
- Clua, P.; Tomokiyo, M.; Tonetti, F.R.; Islam, M.A.; Castillo, V.G.; Marcial, G.; Salva, S.; Alvarez, S.; Takahashi, H.; Kurata, S.; et al. The role of alveolar macrophages in the improved protection against respiratory syncytial virus and pneumococcal superinfection induced by the peptidoglycan of Lactobacillus rhamnosus CRL1505. Cells 2020, 9, 1653. [Google Scholar] [CrossRef] [PubMed]
- Gabryszewski, S.J.; Bachar, O.; Dyer, K.D.; Percopo, C.M.; Killoran, K.E.; Domachowske, J.B.; Rosenberg, H.F. Lactobacillus mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J. Immunol. 2011, 186, 1151–1161. [Google Scholar] [CrossRef]
- Percopo, C.M.; Rice, T.A.; Brenner, T.A.; Dyer, K.D.; Luo, J.L.; Kanakabandi, K.; Sturdevant, D.E.; Porcella, S.F.; Domachowske, J.B.; Keicher, J.D.; et al. Immunobiotic Lactobacillus administered post-exposure averts the lethal sequelae of respiratory virus infection. Antivir. Res. 2015, 121, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, H.; Tada, A.; Vizoso-Pinto, M.G.; Salva, S.; Kanmani, P.; Agüero, G.; Alvarez, S.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic Lactobacillus rhamnosus modulates inflammation-coagulation interactions and reduces influenza virus-associated pulmonary damage. Inflamm. Res. 2015, 64, 589–602. [Google Scholar] [CrossRef]
- Islam, M.A.; Albarracin, L.; Tomokiyo, M.; Valdez, J.C.; Sacur, J.; Vizoso-Pinto, M.G.; Andrade, B.G.N.; Cuadrat, R.R.C.; Kitazawa, H.; Villena, J. Immunobiotic Lactobacilli Improve Resistance of Respiratory Epithelial Cells to SARS-CoV-2 Infection. Pathogens 2021, 10, 1197. [Google Scholar] [CrossRef]
- McFarland, L.V. Efficacy of single-strain probiotics versus multi-strain mixtures: Systematic review of strain and disease specificity. Dig. Dis. Sci. 2021, 66, 694–704. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, W.C.; Su, C.H.; Lin, W.D.; Wu, W.T.; Yu, B.; Hsu, Y.M. Effects of multi-strain probiotics on immune responses and metabolic balance in Helicobacter pylori-infected mice. Nutrients 2020, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Rigassio-Radler, D.; Denmark, R.; Haley, T.; Touger-Decker, R. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br. J. Nutr. 2013, 109, 1999–2007. [Google Scholar] [CrossRef]
- Rautava, S.; Salminen, S.; Isolauri, E. Specific probiotics in reducing the risk of acute infections in infancy–a randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2009, 101, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Smallcombe, C.C.; Linfield, D.T.; Harford, T.J.; Bokun, V.; Ivanov, A.I.; Piedimonte, G.; Rezaee, F. Disruption of the airway epithelial barrier in a murine model of respiratory syncytial virus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L358–L368. [Google Scholar] [CrossRef]
- Schaunaman, N.; Dimasuay, K.G.; Cervantes, D.; Li, L.; Numata, M.; Kraft, M.; Chu, H.W. Tollip inhibits IL-33 release and inflammation in influenza a virus-infected mouse airways. J. Innate Immun. 2023, 15, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; Brewah, Y.A.; Delaney, T.; Welliver, T.; Burwell, T.; Benjamin, E.; Kuta, E.; Kozhich, A.; McKinney, L.; Suzich, J.; et al. Macrophage impairment underlies airway occlusion in primary respiratory syncytial virus bronchiolitis. J. Infect. Dis. 2008, 198, 1783–1793. [Google Scholar] [CrossRef]
- Goritzka, M.; Makris, S.; Kausar, F.; Durant, L.R.; Pereira, C.; Kumagai, Y.; Culley, F.J.; Mack, M.; Akira, S.; Johansson, C. Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J. Exp. Med. 2015, 212, 699–714. [Google Scholar] [CrossRef]
- Goubau, D.; Romieu-Mourez, R.; Solis, M.; Hernandez, E.; Mesplède, T.; Lin, R.; Hiscott, J. Transcriptional re-programming of primary macrophages reveals distinct apoptotic and anti-tumoral functions of IRF-3 and IRF-7. Eur. J. Immunol. 2009, 39, 527–540. [Google Scholar] [CrossRef]
- Darwich, L.; Abelic, R.; Manicassamy, B.; Aitchison, J.D.; Aderem, A.; Elliott, R.M.; García-Sastre, A.; Racaniello, V.; Snijder, E.J.; Yokoyama, W.M.; et al. Immunology of porcine reproductive and respiratory syndrome virus: State-of-the-art and research challenges. Virus Res. 2010, 154, 1653. [Google Scholar] [CrossRef]
- Ibsen, M.S.; Gad, H.H.; Thavachelvam, K.; Boesen, T.; Despres, P.; Hartmann, R. The 2-5-oligoadenylate synthetase 3 enzyme potently synthesizes the 2′-5′-oligoadenylates required for RNase L activation. J. Virol. 2014, 88, 14222–14231. [Google Scholar] [CrossRef]
- Behera, A.K.; Kumar, M.; Lockey, R.F.; Mohapatra, S.S. 2′-5′ oligoadenylate synthetase plays a critical role in interferon-γ inhibition of respiratory syncytial virus infection of human epithelial cells. J. Biol. Chem. 2002, 277, 25601–25608. [Google Scholar] [CrossRef]
- Wen, X.; Mo, S.; Chen, S.; Yu, G.; Gao, L.; Chen, S.; Deng, Y.; Xie, X.; Zang, N.; Ren, L.; et al. Pathogenic difference of respiratory syncytial virus infection in cotton rats of different ages. Microb. Pathog. 2019, 137, 103749. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Isolauri, E.; Kirjavainen, P.V.; Tölkko, S.; Salminen, S.J. The mucus binding of Bifidobacterium lactis Bb12 is enhanced in the presence of Lactobacillus GG and Lact. delbrueckii subsp. bulgaricus. Lett. Appl. Microbiol. 2000, 30, 10–13. [Google Scholar] [CrossRef]
- Bisson, J.F.; Hidalgo, S.; Rozan, P.; Messaoudi, M. Preventive effects of different probiotic formulations on travelers’ diarrhea model in Wistar rats: Preventive effects of probiotics on TD. Dig. Dis. Sci. 2010, 55, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, S.; Li, Y.; Sun, F.; Wang, Q.; Zhao, Q.; Yu, J.; Tian, F.; Wu, J.; Zhu, R.; et al. A porcine alveolar macrophage cell line stably expressing CD163 demonstrates virus replication and cytokine secretion characteristics similar to primary alveolar macrophages following PRRSV infection. Vet. Microbiol. 2020, 244, 108690. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Ji, G.; Yang, X.; Lv, X.; Zhang, Y.; Ge, M.; Pan, Y.; Li, Q.; Wang, H.; Zeng, F. Investigation on host susceptibility of Tibetan pig to infection of porcine reproductive and respiratory syndrome virus through viral challenge study. Vet. Microbiol. 2016, 183, 62–68. [Google Scholar] [CrossRef]
- Liang, W.; Li, Z.; Wang, P.; Fan, P.; Zhang, Y.; Zhang, Q.; Wang, Y.; Xu, X.; Liu, B. Differences of immune responses between Tongcheng (Chinese local breed) and Large White pigs after artificial infection with highly pathogenic porcine reproductive and respiratory syndrome virus. Virus Res. 2016, 215, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Meng, X.; Zhen, Y.; Zhang, Y.; Hu, X.; Zhang, Q.; Zhou, X.; Liu, B. Integration of transcriptome and proteome in lymph nodes reveal the different immune responses to PRRSV between PRRSV-resistant Tongcheng pigs and PRRSV-susceptible large white pigs. Front. Genet. 2022, 13, 800178. [Google Scholar] [CrossRef] [PubMed]
- Overend, C.; Mitchell, R.; He, D.; Rompato, G.; Grubman, M.J.; Garmendia, A.E. Recombinant swine beta interferon protects swine alveolar macrophages and MARC-145 cells from infection with porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2007, 88, 925–931. [Google Scholar] [CrossRef]
- Chen, X.X.; Qiao, S.; Li, R.; Wang, J.; Li, X.; Zhang, G. Evasion strategies of porcine reproductive and respiratory syndrome virus. Front. Microbiol. 2023, 14, 1140449. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, N.; Li, Z.; Wang, P.; Qi, Z.; Liang, W.; Zhou, X.; Xu, X.; Liu, B. 2′,5′-Oligoadenylate synthetase 1(OAS1) inhibits PRRSV replication in Marc-145 cells. Antivir. Res. 2016, 132, 268–273. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, X.; Tian, Y.; Jiang, D.; Ren, J.; Li, Z.; Ding, X.; Zhang, Q.; Yoo, D.; Miller, L.C.; et al. GTPase activity of porcine Mx1 plays a dominant role in inhibiting the N-Nsp9 interaction and thus inhibiting PRRSV replication. J. Virol. 2024, 98, e01844–e01923. [Google Scholar] [CrossRef]
- Thanawongnuwech, R.; Thacker, B.; Halbur, P.; Thacker, E.L. Increased production of proinflammatory cytokines following infection with porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Clin. Diagn. Lab Immunol. 2004, 11, 901–908. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Zhang, X.; Zheng, X.; Zhu, Y.; Han, H.; Feng, W. Highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) induces IL-6 production through TAK-1/JNK/AP-1 and TAK-1/NF-κB signaling pathways. Vet. Microbiol. 2021, 256, 109061. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Li, C.; Wang, C.; Liu, Y.; Wang, G.; He, X.; Hu, L.; Liu, Y.; Cui, M.; et al. Secondary Haemophilus parasuis infection enhances highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection-mediated inflammatory responses. Vet. Microbiol. 2014, 204, 35–42. [Google Scholar] [CrossRef] [PubMed]
- López-Fuertes, L.; Campos, E.; Doménech, N.; Ezquerra, A.; Castro, J.M.; Domínguez, J.; Alonso, F. Porcine reproductive and respiratory syndrome (PRRS) virus down-modulates TNF-α production in infected macrophages. Virus Res. 2000, 69, 41–46. [Google Scholar] [CrossRef]
- Bi, J.; Song, S.; Fang, L.; Wang, D.; Jing, H.; Gao, L.; Xiao, S. Porcine reproductive and respiratory syndrome virus induces IL-1β production depending on TLR4/MyD88 pathway and NLRP3 inflammasome in primary porcine alveolar macrophages. Mediat. Inflamm. 2014, 2014, 403515. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kleiboeker, S.B. Porcine reproductive and respiratory syndrome virus induces apoptosis through a mitochondria-mediated pathway. Virology 2007, 365, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Alex Pasternak, J.; MacPhee, D.J.; Harding, J.C.S. Fetal cytokine response to porcine reproductive and respiratory syndrome virus-2 infection. Cytokine 2020, 126, 154883. [Google Scholar] [CrossRef]
- Amarilla, S.P.; Gómez-Laguna, J.; Carrasco, L.; Rodríguez-Gómez, I.M.; Caridad y Ocerín, J.M.; Morgan, S.B.; Graham, S.P.; Frossard, J.-P.; Drew, T.W.; Salguero, F.J. A comparative study of the local cytokine response in the lungs of pigs experimentally infected with different PRRSV-1 strains: Upregulation of IL-1α in highly pathogenic strain induced lesions. Vet. Immunol. Immunopathol. 2015, 164, 137–147. [Google Scholar] [CrossRef]
- Fukuyama, K.; Zhuang, T.; Toyoshi, E.; Raya Tonetti, F.; Saha, S.; Zhou, B.; Ikeda-Ohtsubo, W.; Nishiyama, K.; Aso, H.; Villena, J.; et al. Establishment of a porcine bronchial epithelial cell line and its application to study innate immunity in the respiratory epithelium. Front. Immunol. 2023, 14, 1117102. [Google Scholar] [CrossRef]
- Yarlagadda, T.; Stedman, J.; Maresco-Pennisi, D.; Coleman, A.; Cervin, A.; Spann, K. Lactobacillus rhamnosus D3189 modulates antiviral and inflammatory responses in primary nasal epithelial cells, reducing respiratory syncytial virus shedding. Front. Cell Infect. Microbiol. 2025, 15, 1625517. [Google Scholar] [CrossRef]
- Jewell, N.A.; Cline, T.; Mertz, S.E.; Smirnov, S.V.; Flaño, E.; Schindler, C.; Grieves, J.L.; Durbin, R.K.; Kotenko, S.V.; Durbin, J.E. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J. Virol. 2010, 84, 11515–11522. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, T.; Kojima, T.; Masaki, T.; Yokota, S.; Imaizumi, T.; Tsutsumi, H.; Himi, T.; Fujii, N.; Sawada, N. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res. 2011, 160, 360–366. [Google Scholar] [CrossRef]
- Kalinowski, A.; Galen, B.T.; Ueki, I.F.; Sun, Y.; Mulenos, A.; Osafo-Addo, A.; Clark, B.; Joerns, J.; Liu, W.; Nadel, J.A.; et al. Respiratory syncytial virus activates epidermal growth factor receptor to suppress interferon regulatory factor 1-dependent interferon-lambda and antiviral defense in airway epithelium. Mucosal Immunol. 2018, 11, 958–967. [Google Scholar] [CrossRef]
- Su, R.; Shereen, M.A.; Zeng, X.; Liang, Y.; Li, W.; Ruan, Z.; Li, Y.; Liu, W.; Liu, Y.; Wu, K.; et al. The TLR3/IRF1/type III IFN axis facilitates antiviral responses against enterovirus infections in the intestine. mBio 2020, 11, e02540–e02620. [Google Scholar] [CrossRef]
- Mallampalli, R.K.; Adair, J.; Elhance, A.; Farkas, D.; Chafin, L.; Long, M.E.; De, M.; Mora, A.L.; Rojas, M.; Peters, V.; et al. Interferon lambda signaling in macrophages is necessary for the antiviral response to influenza. Front. Immunol. 2021, 12, 735576. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.B.; McAllister, T.A.; Topp, E.; Wright, A.D.; Alexander, T.W. The nasopharyngeal microbiota of feedlot cattle that develop bovine respiratory disease. Vet. Microbiol. 2015, 180, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Timsit, E.; Workentine, M.; van der Meer, F.; Alexander, T. Distinct bacterial metacommunities inhabit the upper and lower respiratory tracts of healthy feedlot cattle and those diagnosed with bronchopneumonia. Vet. Microbiol. 2018, 221, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Amat, S.; Timsit, E.; Baines, D.; Yanke, J.; Alexander, T.W. Development of bacterial therapeutics against the bovine respiratory pathogen Mannheimia haemolytica. Appl. Environ. Microbiol. 2019, 85, e01359-19. [Google Scholar] [CrossRef]
- Amat, S.; Timsit, E.; Workentine, M.; Schwinghamer, T.; van der Meer, F.; Guo, Y.; Alexander, T.W. A single intranasal dose of bacterial therapeutics to calves confers longitudinal modulation of the nasopharyngeal microbiota: A pilot study. mSystems 2023, 8, e0101622. [Google Scholar] [CrossRef]
- Geng, J.; Dong, Y.; Huang, H.; Wen, X.; Xu, T.; Zhao, Y.; Liu, Y. Role of nasal microbiota in regulating host anti-influenza immunity in dogs. Microbiome 2025, 13, 27. [Google Scholar] [CrossRef]
- Takenouchi, T.; Masujin, K.; Miyazaki, A.; Suzuki, S.; Takagi, M.; Kokuho, T.; Uenishi, H. Isolation and immortalization of macrophages derived from fetal porcine small intestine and their susceptibility to porcine viral pathogen infections. Front. Vet. Sci. 2022, 9, 919077. [Google Scholar] [CrossRef]
- Lipinski, J.H.; Ranjan, P.; Dickson, R.P.; O’Dwyer, D.N. The lung microbiome. J. Immunol. 2024, 212, 1269–1275. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arellano-Arriagada, L.; Albarracin, L.; Fukuyama, K.; Cisterna-Vergara, S.; Gong, W.; Namai, F.; Nishiyama, K.; Suda, Y.; Kitazawa, H.; Villena, J. Combined Lactiplantibacillus plantarum CRL1506 and MPL16 Nasal Priming More Effectively Modulates Respiratory Antiviral Innate Immunity than Single Strains. Int. J. Mol. Sci. 2025, 26, 10079. https://doi.org/10.3390/ijms262010079
Arellano-Arriagada L, Albarracin L, Fukuyama K, Cisterna-Vergara S, Gong W, Namai F, Nishiyama K, Suda Y, Kitazawa H, Villena J. Combined Lactiplantibacillus plantarum CRL1506 and MPL16 Nasal Priming More Effectively Modulates Respiratory Antiviral Innate Immunity than Single Strains. International Journal of Molecular Sciences. 2025; 26(20):10079. https://doi.org/10.3390/ijms262010079
Chicago/Turabian StyleArellano-Arriagada, Luciano, Leonardo Albarracin, Kohtaro Fukuyama, Solange Cisterna-Vergara, Weichen Gong, Fu Namai, Keita Nishiyama, Yoshihito Suda, Haruki Kitazawa, and Julio Villena. 2025. "Combined Lactiplantibacillus plantarum CRL1506 and MPL16 Nasal Priming More Effectively Modulates Respiratory Antiviral Innate Immunity than Single Strains" International Journal of Molecular Sciences 26, no. 20: 10079. https://doi.org/10.3390/ijms262010079
APA StyleArellano-Arriagada, L., Albarracin, L., Fukuyama, K., Cisterna-Vergara, S., Gong, W., Namai, F., Nishiyama, K., Suda, Y., Kitazawa, H., & Villena, J. (2025). Combined Lactiplantibacillus plantarum CRL1506 and MPL16 Nasal Priming More Effectively Modulates Respiratory Antiviral Innate Immunity than Single Strains. International Journal of Molecular Sciences, 26(20), 10079. https://doi.org/10.3390/ijms262010079








