Alpinetin Alleviates Cardiac Inflammation and Remodeling via TLR4/MyD88/NF-κB Signaling Pathway in Rats with Acute Myocardial Infarction
Abstract
1. Introduction
2. Results
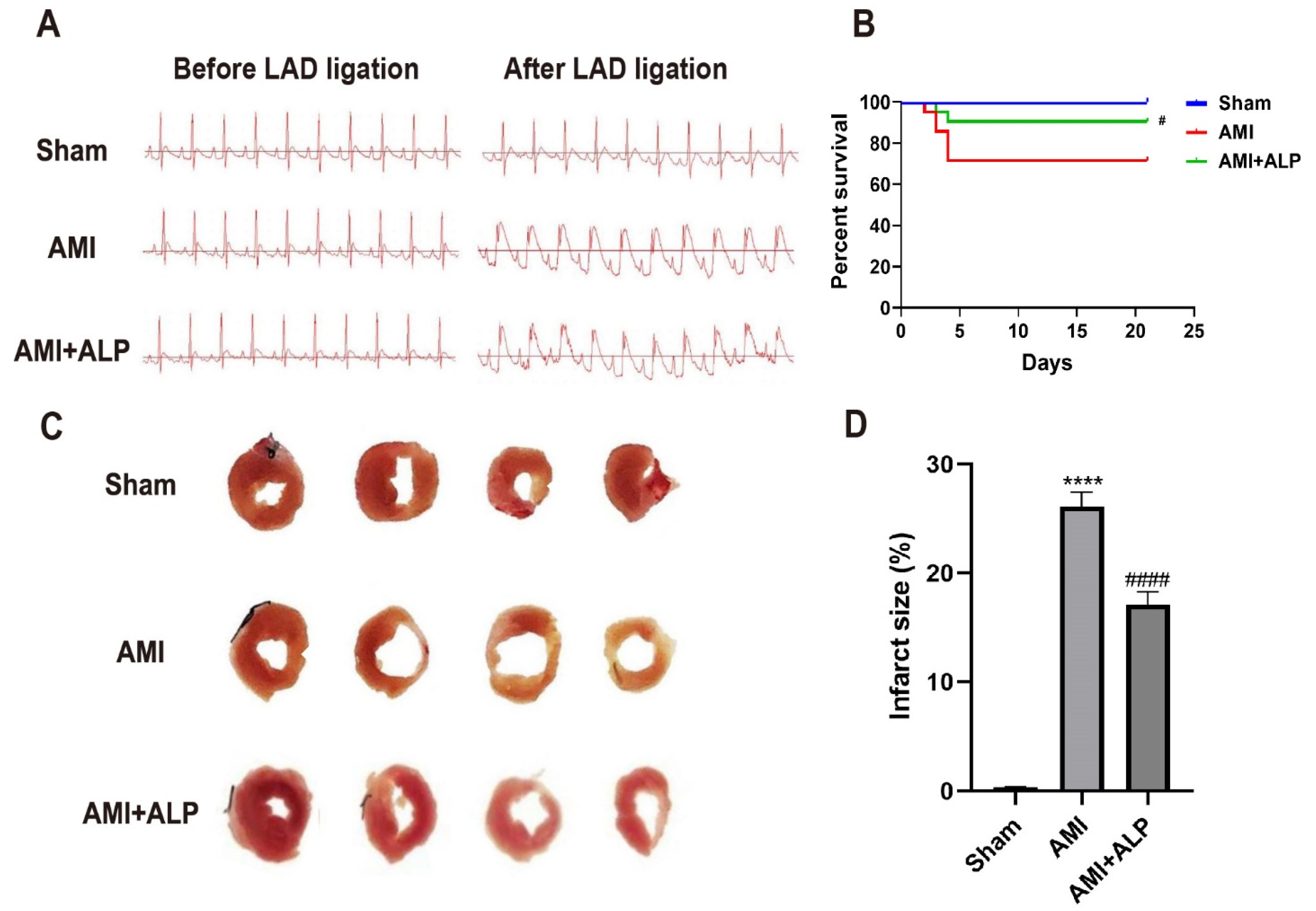
2.1. ALP Reduced Myocardial Injury in AMI Rats
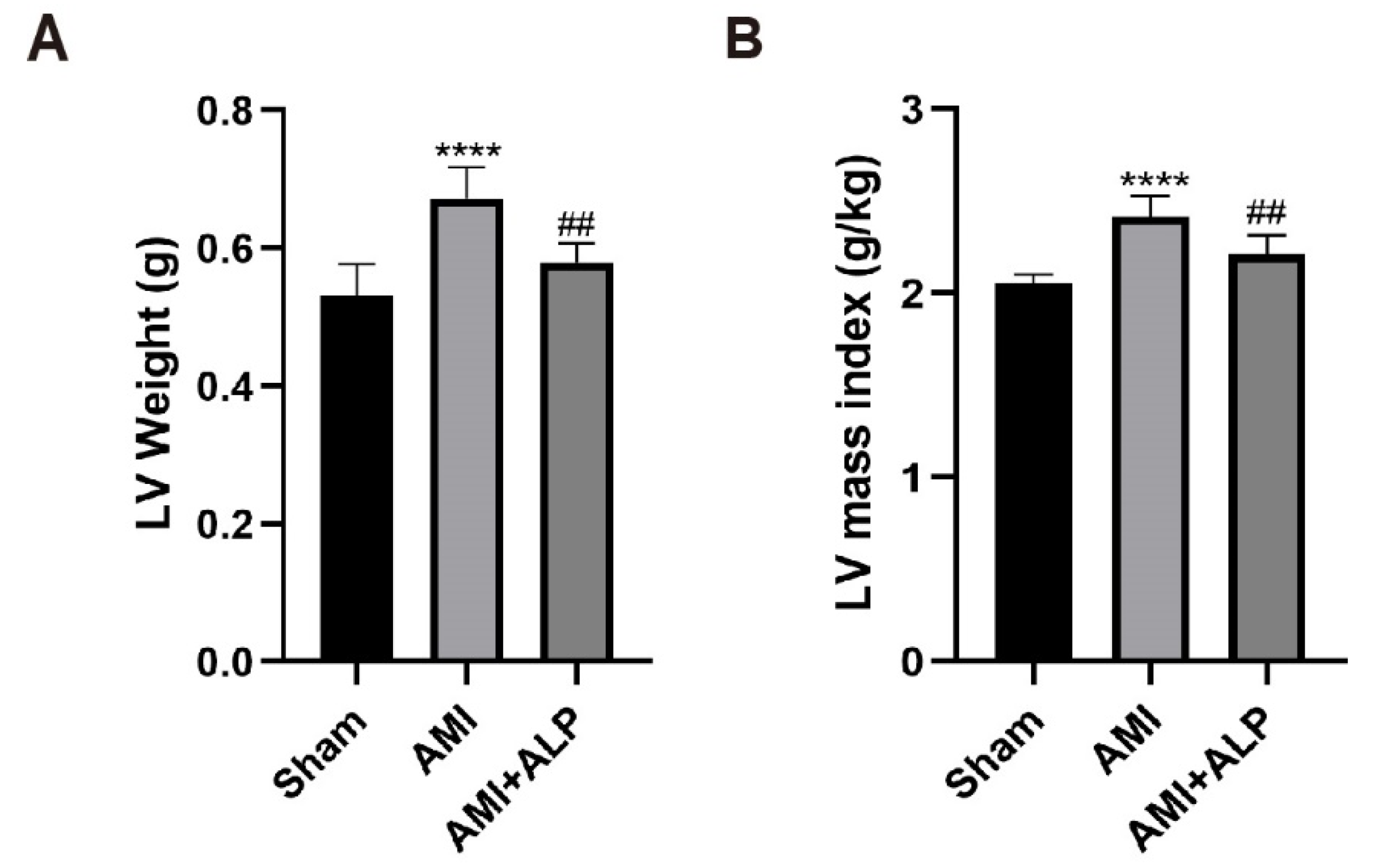
2.2. ALP Reduced Myocardial Remodeling in AMI Rats
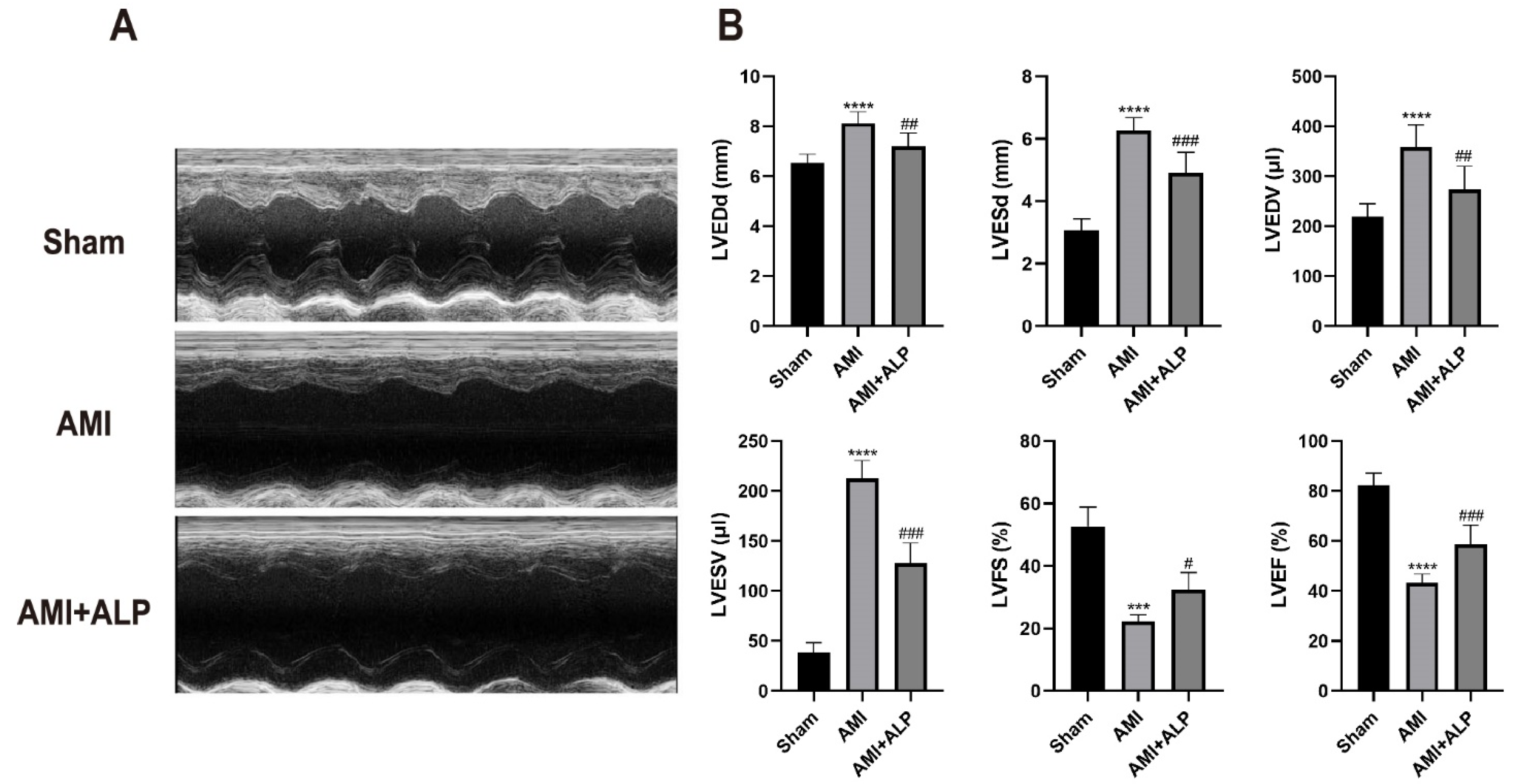
2.3. ALP Ameliorated Cardiac Dysfunction in AMI Rats
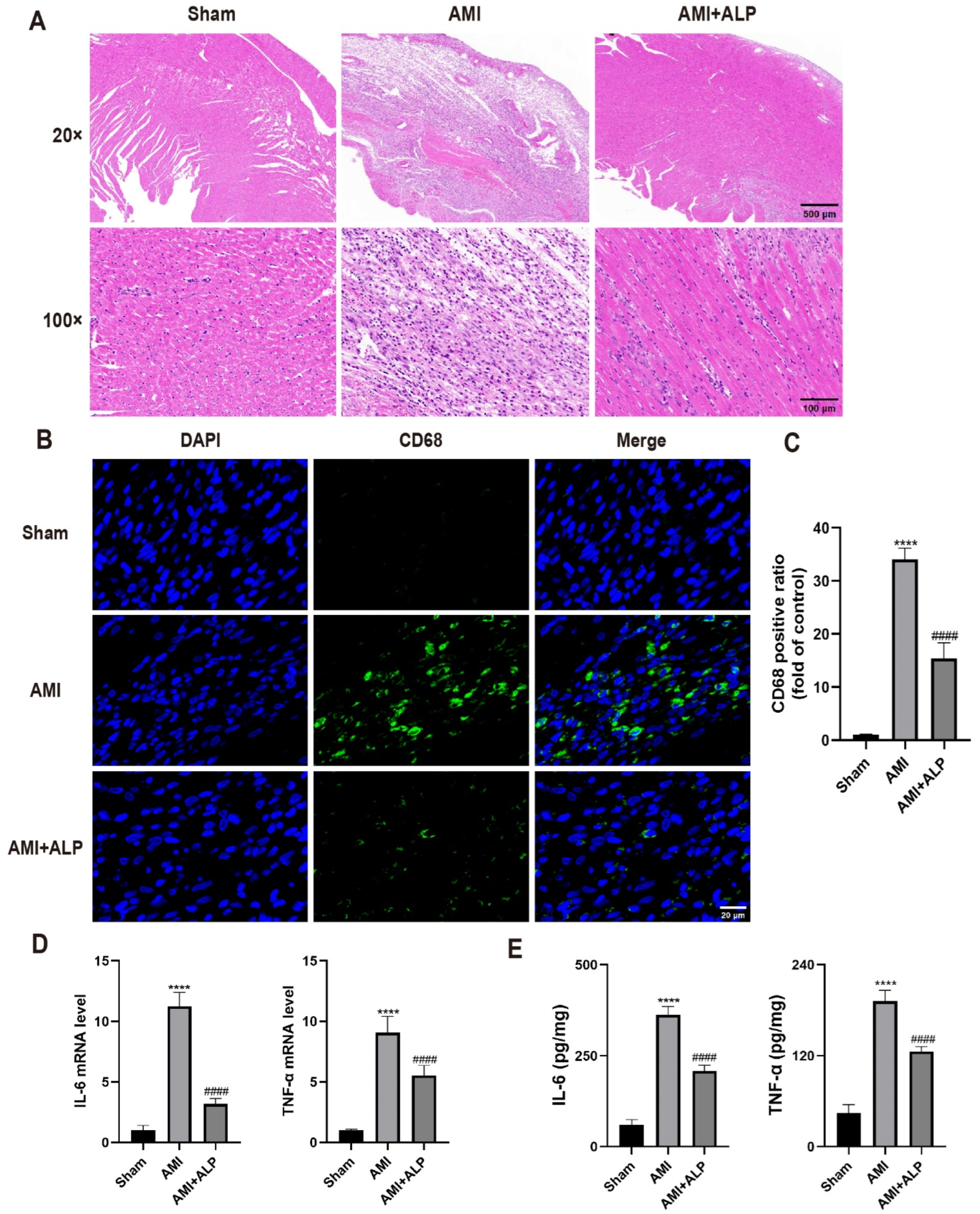
2.4. ALP Suppressed the Inflammatory Response in the Hearts of AMI Rats
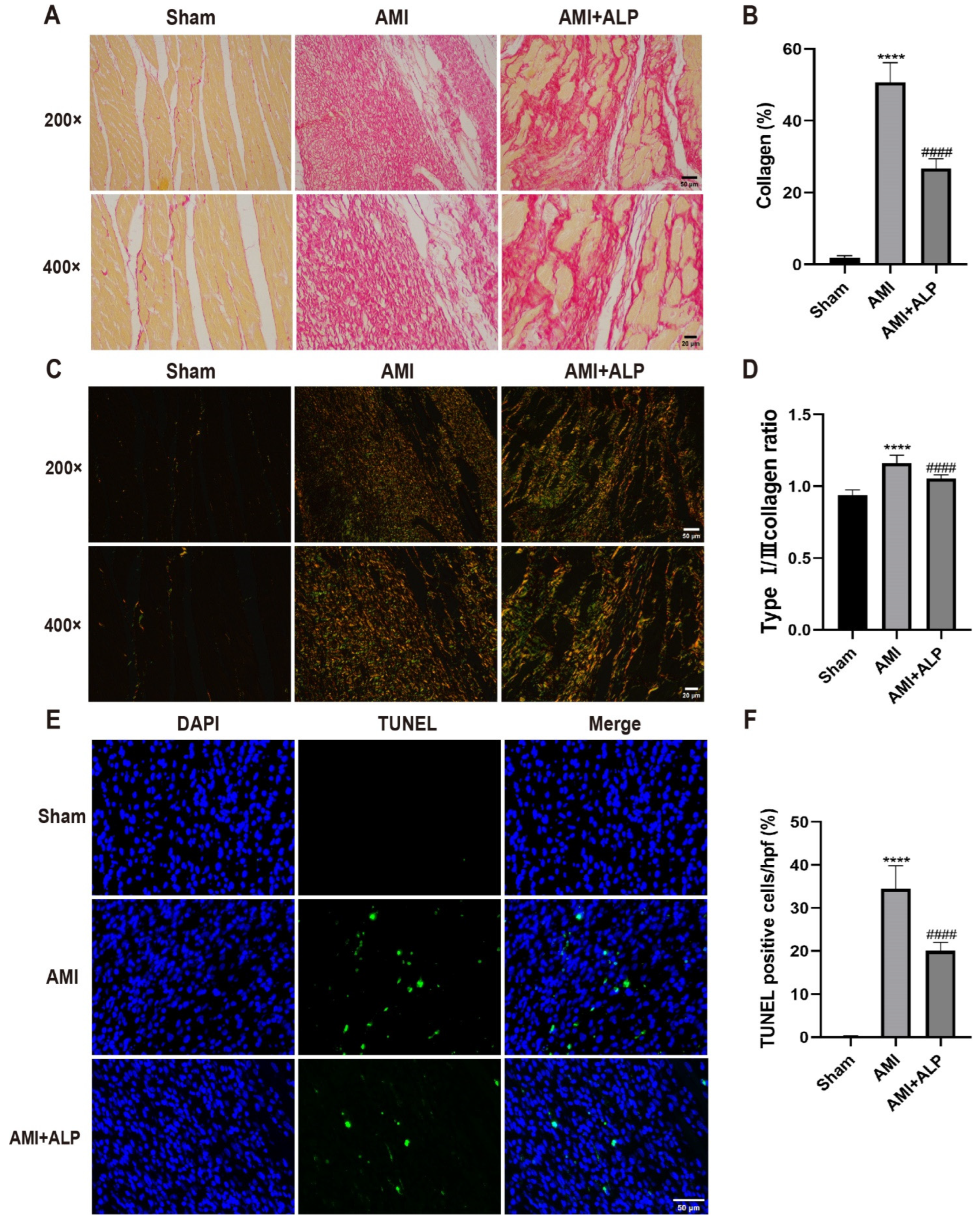
2.5. ALP Alleviated the Collagen Deposition in AMI Rats
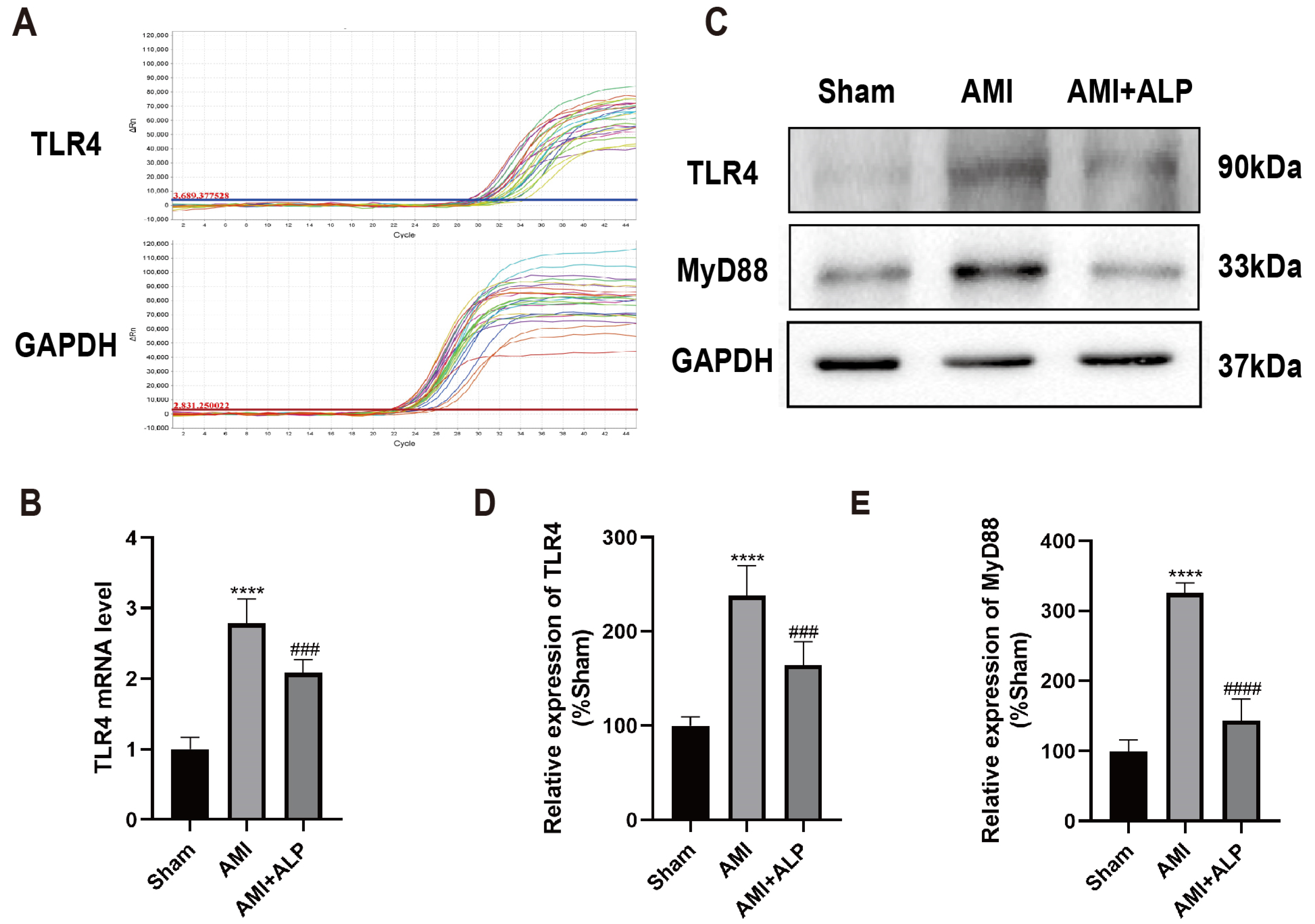
2.6. ALP Downregulated the Expressions of TLR4 and MyD88 in AMI Rats
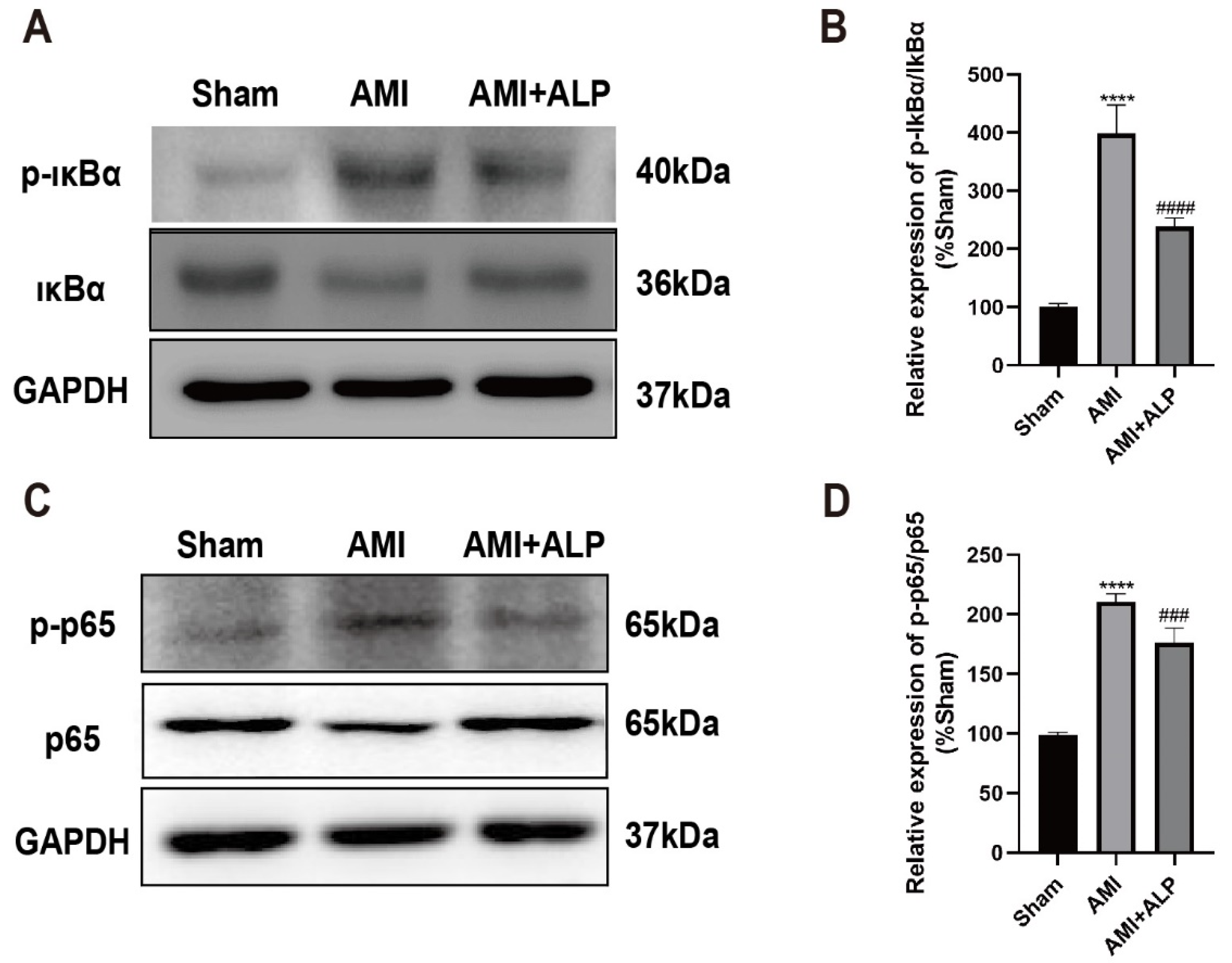
2.7. ALP Inhibited the Phosphorylation of IκBα and NF-κB p65 Protein in Myocardium of AMI Rats
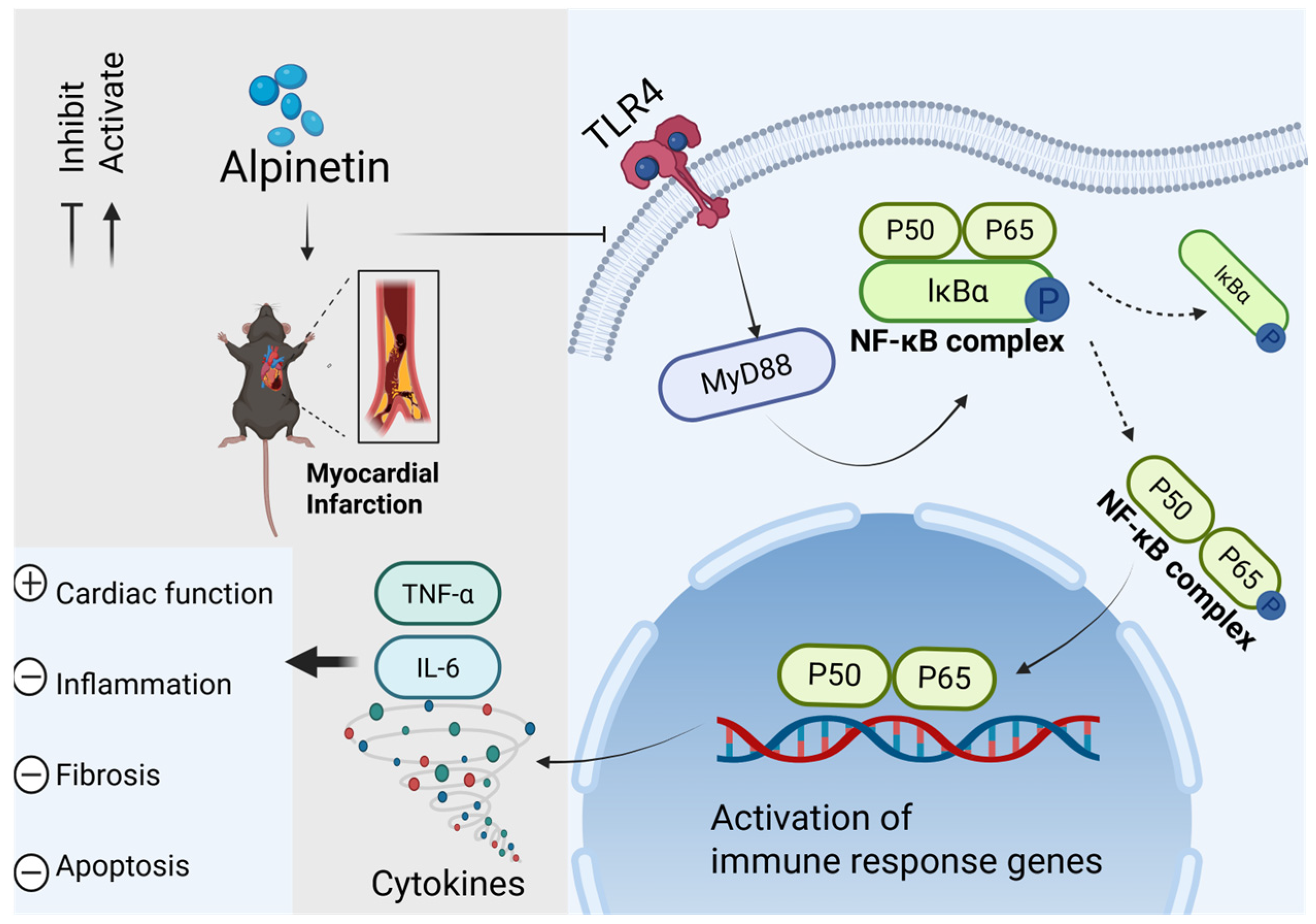
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Alpinetin Preparation
4.3. Animals and Experimental Protocols
4.4. Echocardiogram (UCG)
4.5. Cardiac Mass Index
4.6. Histopathological Examination
4.7. Quantitative Real-Time PCR (qRT-PCR) Detection
4.8. Western Blotting Analysis
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxidative Med. Cell Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Rana, J.N.; Gul, K.; Mumtaz, S. Isorhamnetin: Reviewing Recent Developments in Anticancer Mechanisms and Nanoformulation-Driven Delivery. Int. J. Mol. Sci. 2025, 26, 7381. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Xu, H.; Mai, J.N.; Liang, J.; Huang, C.Y.; Jin, Q.Q. Research progress in Spectrum-effect relationship of flavoniods. China Pharm. 2017, 28, 3882–3885. [Google Scholar]
- Zhao, X.W. Research progress in structure-activity relationship of flavoniods. Chin. Tradit. Herb. Drugs 2015, 46, 3264–3271. [Google Scholar]
- Zhang, J.; Ma, B. Alpinetin alleviates LPS-induced lung epithelial cell injury by inhibiting p38 and ERK1/2 signaling via aquaporin-1. Tissue Cell 2024, 87, 102305. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; He, L.; Wang, C.; Yang, L. Alpinetin Protects Chondrocytes and Exhibits Anti-Inflammatory Effects via the NF-kappaB/ERK Pathway for Alleviating Osteoarthritis. Inflammation 2020, 43, 1742–1750. [Google Scholar] [CrossRef]
- Wu, D.; Li, S.; Liu, X.; Xu, J.; Jiang, A.; Zhang, Y.; Liu, Z.; Wang, J.; Zhou, E.; Wei, Z.; et al. Alpinetin prevents inflammatory responses in OVA-induced allergic asthma through modulating PI3K/AKT/NF-kappaB and HO-1 signaling pathways in mice. Int. Immunopharmacol. 2020, 89, 107073. [Google Scholar]
- Rodrigo, R.; Retamal, C.; Schupper, D.; Vergara-Hernández, D.; Saha, S.; Profumo, E.; Buttari, B.; Saso, L. Antioxidant Cardioprotection against Reperfusion Injury: Potential Therapeutic Roles of Resveratrol and Quercetin. Molecules 2022, 27, 2564. [Google Scholar] [CrossRef]
- Marniemi, J.; Alanen, E.; Impivaara, O.; Seppänen, R.; Hakala, P.; Rajala, T.; Rönnemaa, T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 188–197. [Google Scholar] [CrossRef]
- Sun, S.J.; Wu, X.P.; Song, H.L.; Li, G.Q. Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation, oxidative stress and P38MAPK pathway in rat. Int. J. Clin. Exp. Med. 2015, 8, 22063–22072. [Google Scholar]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs. Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef]
- Maruyama, K.; Imanaka-Yoshida, K. The Pathogenesis of Cardiac Fibrosis: A Review of Recent Progress. Int. J. Mol. Sci. 2022, 23, 2617. [Google Scholar] [CrossRef]
- Gu, Y.; Hu, X.; Ge, P.B.; Chen, Y.; Wu, S.; Zhang, X.W. CTRP1 Aggravates Cardiac Dysfunction Post Myocardial Infarction by Modulating TLR4 in Macrophages. Fron.t Immunol. 2021, 12, 635267. [Google Scholar]
- Bayer, A.L.; Alcaide, P. MyD88: At the heart of inflammatory signaling and cardiovascular disease. J. Mol. Cell Cardiol. 2021, 161, 75–85. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar]
- Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules 2025, 30, 1184. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y.; Liu, Z.; Li, A.; Tong, W.; Xiong, X.; Nie, J.; Zhong, N.; Zhu, G.; Liu, J.; et al. Alpinetin inhibits neuroinflammation and neuronal apoptosis via targeting the JAK2/STAT3 signaling pathway in spinal cord injury. CNS Neurosci. Ther. 2023, 9, 1094–1108. [Google Scholar] [CrossRef]
- Miao, Y.; Lv, Q.; Qiao, S.; Yang, L.; Tao, Y.; Yan, W.; Wang, P.; Cao, N.; Dai, Y.; Wei, Z. Alpinetin improves intestinal barrier homeostasis via regulating AhR/suv39h1/TSC2/mTORC1/autophagy pathway. Toxicol. Appl. Pharmacol. 2019, 384, 114772. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, T.; Ming, Q.; Han, G.; Zhang, Y.; Liang, J.; Zhu, D. Alpinetin ameliorates inflammatory response in LPS-induced endometritis in mice. Int. Immunopharmacol. 2018, 62, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Kongsui, R.; Thongrong, S.; Jittiwat, J. In Vivo Neuroprotective Effects of Alpinetin Against Experimental Ischemic Stroke Damage Through Antioxidant and Anti-Inflammatory Mechanisms. Int. J. Mol. Sci. 2025, 26, 5093. [Google Scholar] [CrossRef]
- Oliveira, J.B.; Soares, A.; Sposito, A.C. Inflammatory Response During Myocardial Infarction. Adv. Clin. Chem. 2018, 84, 39–79. [Google Scholar]
- Cheng, X.J.; Li, L.; Xin, B.Q. MiR-124 Regulates the Inflammation and Apoptosis in Myocardial Infarction Rats by Targeting STAT3. Cardiovasc. Toxicol. 2021, 21, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Huang, S.; Frangogiannis, N.G. Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. Br. J. Pharmacol. 2018, 175, 1377–1400. [Google Scholar] [CrossRef]
- Westman, P.C.; Lipinski, M.J.; Luger, D.; Waksman, R.; Bonow, R.O.; Wu, E.; Epstein, S.E. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2050–2060. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Wang, Y.; Abarbanell, A.M.; Herrmann, J.L.; Weil, B.R.; Poynter, J.; Manukyan, M.C.; Crisostomo, P.R.; Meldrum, D.R. Toll-like receptor signaling pathways and the evidence linking toll-like receptor signaling to cardiac ischemia/reperfusion injury. Shock 2010, 34, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, B.; Yang, H.; Dai, C.; Fang, J.; Qin, T.; Huang, H. Key Player in Cardiac Hypertrophy, Emphasizing the Role of Toll-Like Receptor 4. Front. Cardiovasc. Med. 2020, 7, 579036. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Ikegawa, M.; Ori, D.; Akira, S. Decoding Toll-like receptors: Recent insights and perspectives in innate immunity. Immunity 2024, 57, 649–673. [Google Scholar] [CrossRef]
- Malik, J.A.; Kaur, G.; Agrewala, J.N. Revolutionizing medicine with toll-like receptors: A path to strengthening cellular immunity. Int. J. Biol. Macromol. 2023, 253 Pt 7, 127252. [Google Scholar] [CrossRef]
- Hasan, R.; Siregar, G.A.; Lindarto, D. Syzygium Polyanthum Reduced TNF-α and ADAM17 Protein Expression in Myocardial Infarction Rat Model. Med. Arch. 2020, 74, 416–420. [Google Scholar] [CrossRef]
- Jing, R.; Long, T.Y.; Pan, W.; Li, F.; Xie, Q.Y. IL-6 knockout ameliorates myocardial remodeling after myocardial infarction by regulating activation of M2 macrophages and fibroblast cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6283–6291. [Google Scholar]
- Newby, L.K. Inflammation as a Treatment Target after Acute Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2562–2563. [Google Scholar] [CrossRef]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 133–144, Erratum in Nat. Rev. Cardiol. 2017, 14, 314. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and fibrosis. Matrix. Biol. 2018, 68, 106–121. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.Y.; Liang, X.P.; Guo, C.X.; Lu, P.P.; Ma, L.H. Xinfuli Granule improves post-myocardial infarction ventricular remodeling and myocardial fibrosis in rats by regulating TGF-β/Smads signaling pathway. J. Geriatr. Cardiol. 2017, 14, 301–307. [Google Scholar]
- González, A.; López, B.; Ravassa, S.; San José, G.; Díez, J. The complex dynamics of myocardial interstitial fibrosis in heart failure. Focus on collagen cross-linking. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1421–1432. [Google Scholar]
- Kurose, H. Cardiac Fibrosis and Fibroblasts. Cells 2021, 10, 1716. [Google Scholar] [CrossRef]
- Uchinaka, A.; Yoshida, M.; Tanaka, K.; Hamada, Y.; Mori, S.; Maeno, Y.; Miyagawa, S.; Sawa, Y.; Nagata, K.; Yamamoto, H.; et al. Overexpression of collagen type III in injured myocardium prevents cardiac systolic dysfunction by changing the balance of collagen distribution. J. Thorac. Cardiovasc. Surg. 2018, 156, 217–226.e213. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Ambrosy, A.P.; Velazquez, E.J. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Curr. Cardiol. Rep. 2017, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Liu, C.Y.; Zhan, Y.; Wang, Y.; Hu, Q.J.; Zeng, Z.L. Alpinetin ameliorates bleomycin-induced pulmonary fibrosis by repressing fibroblast differentiation and proliferation. Biomed. Pharmacother. 2024, 171, 116101. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lin, J.Z.; Wang, Y.R. Effect of Alpinetin on Heart Function, Blood Lipid and Vascular Endothelial Function in Rats with Coronary Heart Disease. Chin. J. Clin. Pharmacol. 2021, 37, 2813–2817. [Google Scholar]
- Wang, Z.; Lau, C.; Chan, F.; Yao, X.; He, Z.; Huang, Y. Vasorelaxant effects of cardamonin and alpinetin from Alpinia henryi K. Schum. J. Cardiovasc. Pharmacol. 2001, 37, 596–606. [Google Scholar] [CrossRef]
- Peng, H.; Chen, L.; Deng, Y. Ginsenoside Rh2 mitigates myocardial damage in acute myocardial infarction by regulating pyroptosis of cardiomyocytes. Clin. Exp. Hypertens. 2023, 45, 2229536. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhang, L.; Li, Z. Metoprolol protects against myocardial infarction by inhibiting miR-1 expression in rats. J. Pharm. Pharmacol. 2020, 72, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Xiang, J.Z.; Ming, Z.Y.; Fu, Q.; Ma, R.; Zhang, Q.F.; Dun, Y.Y.; Yang, L.; Liu, H. Activation of epidermal growth factor receptor mediates reperfusion arrhythmias in anaesthetized rats. Cardiovasc. Res. 2012, 93, 60–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rao, T.; Tong, H.; Li, J.; Huang, J.; Yin, Y.; Zhang, J. Exploring the role and mechanism of hyperoside against cardiomyocyte injury in mice with myocardial infarction based on JAK2/STAT3 signaling pathway. Phytomedicine 2024, 128, 155319. [Google Scholar] [CrossRef] [PubMed]
| Sham Group | AMI Group | AMI + ALP Group | |
|---|---|---|---|
| Body weight (kg) | 0.26 ± 0.02 | 0.27 ± 0.01 | 0.25 ± 0.01 |
| Heart weight (g) | 0.84 ± 0.08 | 0.94 ± 0.13 | 0.87 ± 0.05 |
| Cardiac mass index (g/kg) | 3.22 ± 0.14 | 3.56 ± 0.33 | 3.41 ± 0.20 |
| LV weight (g) | 0.53 ± 0.04 | 0.66 ± 0.06 **** | 0.56 ± 0.02 ## |
| LV mass index (g/kg) | 2.06 ± 0.04 | 2.42 ± 0.11 **** | 2.21 ± 0.09 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, M.; Chen, X.; Huang, F.; Chen, L.; Liu, C.; Li, W.; Li, Y.; Chen, S.; Deng, Z.; Wei, Z.; et al. Alpinetin Alleviates Cardiac Inflammation and Remodeling via TLR4/MyD88/NF-κB Signaling Pathway in Rats with Acute Myocardial Infarction. Int. J. Mol. Sci. 2025, 26, 10073. https://doi.org/10.3390/ijms262010073
Feng M, Chen X, Huang F, Chen L, Liu C, Li W, Li Y, Chen S, Deng Z, Wei Z, et al. Alpinetin Alleviates Cardiac Inflammation and Remodeling via TLR4/MyD88/NF-κB Signaling Pathway in Rats with Acute Myocardial Infarction. International Journal of Molecular Sciences. 2025; 26(20):10073. https://doi.org/10.3390/ijms262010073
Chicago/Turabian StyleFeng, Mei, Xinxiang Chen, Fan Huang, Lin Chen, Can Liu, Wei Li, Yinyan Li, Shaobin Chen, Zhen Deng, Zhengyi Wei, and et al. 2025. "Alpinetin Alleviates Cardiac Inflammation and Remodeling via TLR4/MyD88/NF-κB Signaling Pathway in Rats with Acute Myocardial Infarction" International Journal of Molecular Sciences 26, no. 20: 10073. https://doi.org/10.3390/ijms262010073
APA StyleFeng, M., Chen, X., Huang, F., Chen, L., Liu, C., Li, W., Li, Y., Chen, S., Deng, Z., Wei, Z., Luo, Y., Yu, X., & Qin, A. (2025). Alpinetin Alleviates Cardiac Inflammation and Remodeling via TLR4/MyD88/NF-κB Signaling Pathway in Rats with Acute Myocardial Infarction. International Journal of Molecular Sciences, 26(20), 10073. https://doi.org/10.3390/ijms262010073





