Molecular Mechanisms of the Microbiota–Gut–Brain Axis in the Onset and Progression of Stroke
Abstract
1. Introduction
Role of the Microbiota in the Health-Disease Coupling
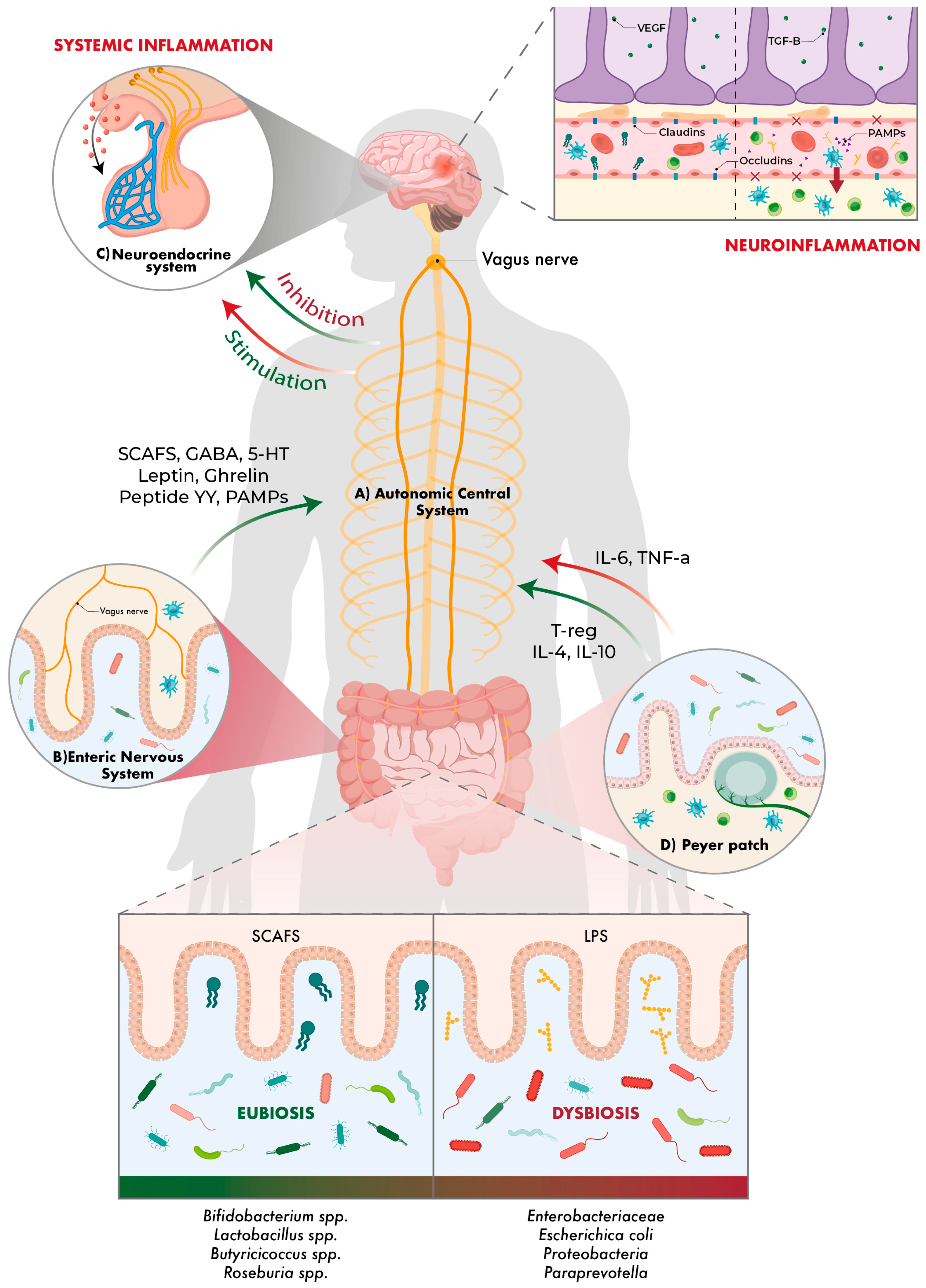
2. Gut–Brain Axis
3. Gut–Brain–Microbiota Axis Approach to Neurological Diseases
4. Gut–Brain–Microbiota Axis and Its Relationships with Stroke
- Small vessel disease mainly affects the penetrating arteries and often causes small deep strokes, known as lacunar infarcts. These arteries are more vulnerable to the effects of chronic hypertension, unlike the large vessels [72].
- Oral contraceptives and hormone replacement therapy also increase the risk of stroke through thromboembolic mechanisms. A recent meta-analysis has suggested that each 10 µg dose increase and each additional 5 years of use increases the risk of stroke by 20% [76].
- Alcohol and drug abuse. The risk of ischaemic stroke is related to the amount of alcohol consumed daily. However, any alcohol consumption increases the risk of haemorrhagic stroke. On the other hand, regular use of phenethylamines (such as cocaine) is associated with an increased risk of all stroke subtypes and is a predisposing factor in those under 35 years of age [76].
4.1. Microbiota and Atherosclerosis
4.2. Microbiota and Immunosuppression
5. Microbiota and Stroke: Microbiota Profiles and Their Relationship to Stroke
5.1. Ischaemic Stroke
5.2. Haemorrhagic Stroke
5.3. Effects of Stroke on the Gut Microbiota
6. Links Between Microbiota and Blood Biomarkers Related to Increased Stroke Risk
6.1. Glutamate
6.2. Trimethylamine N-Oxide (TMAO)
6.3. Phenylacetylglnutamine (PAGIn)
6.4. Short-Chain Fatty Acids (SCFA)
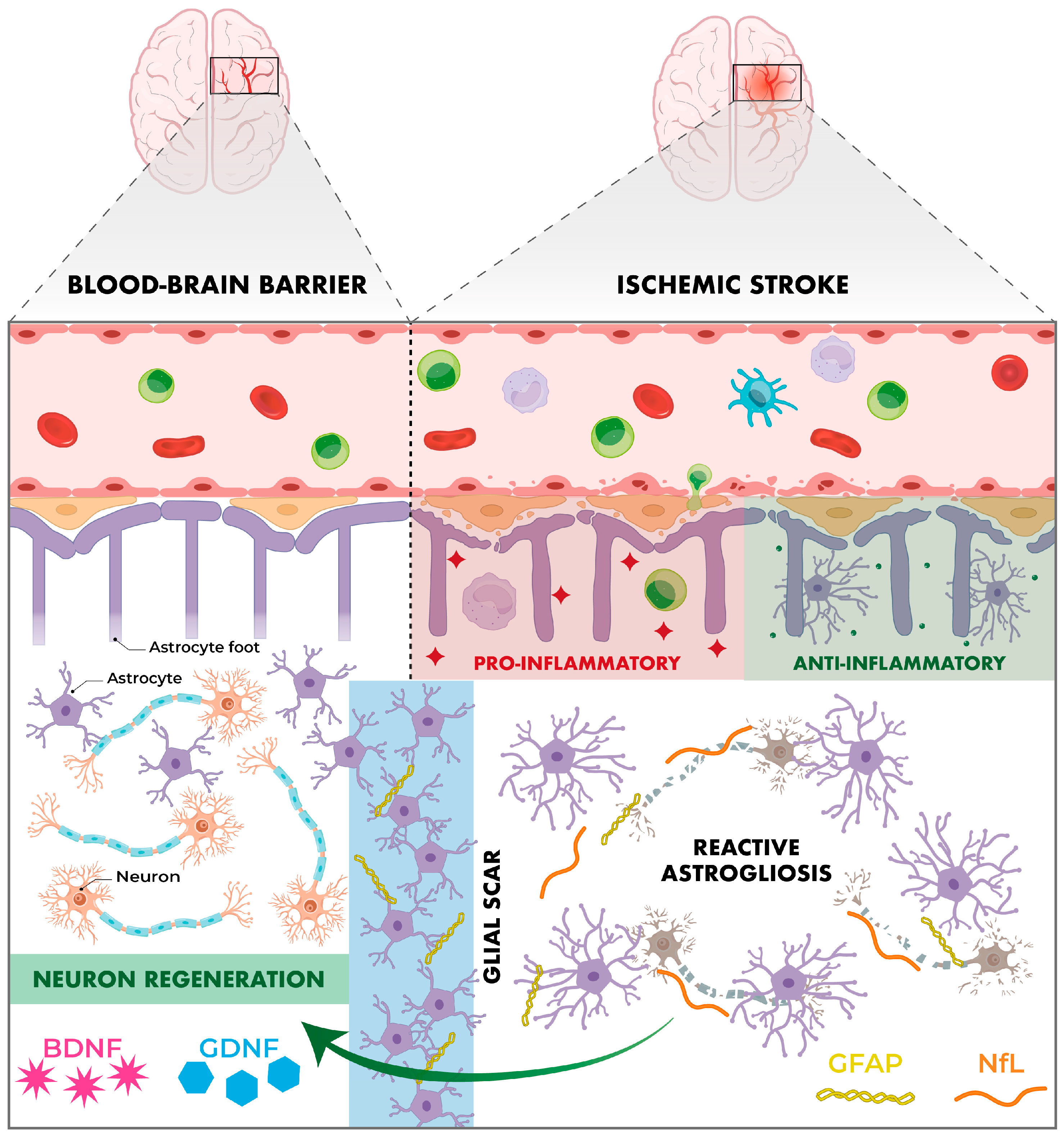
6.5. Neurochemical Biomarkers
6.5.1. Brain-Derived Neurotrophic Factor (BDNF)
6.5.2. Glial Cell-Derived Neurotrophic Factor (GDNF)
6.5.3. Glial Fibrillary Acidic Protein (GFAP)
6.5.4. Neurofilament Light Chain (NfL)
6.5.5. Neuregulin-1 (NRG-1)
6.5.6. Nerve Growth Factor (NGF)
6.5.7. Insulin-like Growth Factor-1 (IGF-1)
6.5.8. S100B
7. Conclusions and Perspectives
7.1. Dietary Interventions
7.2. Probiotics-Prebiotics-Synbiotics
7.3. Faecal Microbiota Transplant
7.4. Emerging Therapies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANS | Autonomic nervous system |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| DASH | Dietary Approaches to Stop Hypertension |
| ENS | Enteric nervous system |
| FMT | Faecal microbiota transplantation |
| GABA | Gamma-aminobutyric acid |
| GDNF | Glial cell-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| HHA | Hypothalamic–pituitary–adrenal axis |
| IGF-1 | Insulin-like growth factor |
| LPS | Lipopolysaccharide |
| MGBA | Microbiota–gut–brain axis |
| MIND | Mediterranean-DASH Intervention for Neurodegenerative Delay |
| Nfl | Neurofilament light chain |
| NGF | Nerve growth factor |
| NRGs | Neuregulins |
| PAGln | Phenylacetylglnutamine |
| PAMPs | Patterns associated with pathogens |
| PNS | Parasympathetic nervous system |
| PSCI | Post-stroke cognitive impairment |
| SAIs | Stroke-associated infections |
| SCFAs | Short-chain fatty acids |
| SNS | Sympathetic nervous system |
| TMAO | Trimethylamine-N-oxide |
References
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Cantón, R.; Ramos, P.D.L.; García-Botella, A.; García-Lledó, A.; Hernández-Sampelayo, T.; Gómez-Pavón, J.; del Castillo, J.G.; Martín-Delgado, M.C.; Sánchez, F.J.M.; Martínez-Sellés, M.; et al. Human intestinal microbiome: Role in health and disease. Rev. Esp. Quimioter. 2024, 37, 438–453. [Google Scholar] [CrossRef]
- Lu, R.; Li, D.; Guo, Y.; Cui, Z.; Wei, Z.; Fan, G.; Zhang, W.; Wang, Y.; Gu, Y.; Han, M.; et al. Comparative metagenomics highlights the habitat-related diversity in taxonomic composition and metabolic potential of deep-sea sediment microbiota. Heliyon 2024, 10, e39055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qi, H.; Li, M.; Wang, Z.; Jia, X.; Sun, T.; Du, S.; Su, C.; Zhi, M.; Du, W.; et al. Diet Mediate the Impact of Host Habitat on Gut Microbiome and Influence Clinical Indexes by Modulating Gut Microbes and Serum Metabolites. Adv. Sci. 2024, 11, 2310068. [Google Scholar] [CrossRef] [PubMed]
- Coyte, K.Z.; Rakoff-Nahoum, S. Understanding Competition and Cooperation within the Mammalian Gut Microbiome. Curr. Biol. 2019, 29, R538–R544. [Google Scholar] [CrossRef] [PubMed]
- Simonet, C.; McNally, L. Kin selection explains the evolution of cooperation in the gut microbiota. Proc. Natl. Acad. Sci. USA 2021, 118, e2016046118. [Google Scholar] [CrossRef]
- Tannock, G.W. Understanding the gut microbiota by considering human evolution: A story of fire, cereals, cooking, molecular ingenuity, and functional cooperation. Microbiol. Mol. Biol. Rev. 2024, 88, e0012722. [Google Scholar] [CrossRef]
- Week, B.; Russell, S.L.; Schulenburg, H.; Bohannan, B.J.M.; Bruijning, M. Applying evolutionary theory to understand host–microbiome evolution. Nat. Ecol. Evol. 2025, 9, 1769–1780. [Google Scholar] [CrossRef]
- Urban, J.; Kav, A.B.; Kindschuh, W.F.; Park, H.; Khan, R.R.; Watters, E.; Pe’er, I.; Uhlemann, A.-C.; Korem, T. Identification of Sample Processing Errors in Microbiome Studies Using Host Genetic Profiles. bioRxiv 2025. [Google Scholar] [CrossRef]
- Wilde, J.; Slack, E.; Foster, K.R. Host control of the microbiome: Mechanisms, evolution, and disease. Science 2024, 385, eadi3338. [Google Scholar] [CrossRef]
- Keshavarz, M.; Franz, M.; Xie, H.; Zanchi, C.; Mbedi, S.; Sparmann, S.; Rolff, J. Immune-mediated indirect interaction between gut microbiota and bacterial pathogens. BMC Biol. 2025, 23, 278. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.K.; Alfarttoosi, K.H.; Sanghvi, G.; Roopashree, R.; Kashyap, A.; Krithiga, T.; Taher, W.M.; Alwan, M.; Jawad, M.J.; Al-Nuaimi, A.M.A. The Role of Gut Microbiota in Modulating Immune Signaling Pathways in Autoimmune Diseases. NeuroMolecular Med. 2025, 27, 65. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Mazzola, M.; Jurjus, A.; Cappello, F.; Carini, F.; Damiani, P.; Geagea, A.G.; Zeenny, M.N.; Leone, A. The fingerprint of the human gastrointestinal tract microbiota: A hypothesis of molecular mapping. J. Biol. Regul. Homeost. Agents 2022, 31, 245–249. [Google Scholar]
- Kawulok, J.; Kawulok, M.; Deorowicz, S. Environmental metagenome classification for constructing a microbiome fingerprint. Biol. Direct 2019, 14, 20. [Google Scholar] [CrossRef]
- Yılmaz, S.S.; Kuşkucu, M.A.; Çakan, H.; Aygün, G. Effective use of skin microbiome signatures for fingerprint identification. Ski. Res. Technol. 2024, 30, e70052. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Mahowald, M.A.; Rey, F.E.; Seedorf, H.; Turnbaugh, P.J.; Fulton, R.S.; Wollam, A.; Shah, N.; Wang, C.; Magrini, V.; Wilson, R.K.; et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA 2009, 106, 5859–5864. [Google Scholar] [CrossRef]
- Koliada, A.; Moseiko, V.; Romanenko, M.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. Sex differences in the phylum-level human gut microbiota composition. BMC Microbiol. 2021, 21, 131. [Google Scholar] [CrossRef]
- Spindler, M.P.; Siu, S.; Mogno, I.; Li, Z.; Yang, C.; Mehandru, S.; Britton, G.J.; Faith, J.J. Human gut microbiota stimulate defined innate immune responses that vary from phylum to strain. Cell Host Microbe 2022, 30, 1481–1498.e5. [Google Scholar] [CrossRef]
- Giles, K.; Pluvinage, B.; Boraston, A.B. Structure of a glycoside hydrolase family 50 enzyme from a subfamily that is enriched in human gut microbiome bacteroidetes. Proteins 2017, 85, 182–187. [Google Scholar] [CrossRef]
- Indiani, C.M.D.S.P.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child. Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut microbiota and BMI throughout childhood: The role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Tai, W.-C.; Liang, C.-M.; Wu, C.-K.; Tsai, M.-C.; Hu, W.-H.; Huang, P.-Y.; Chen, C.-H.; Kuo, Y.-H.; Yao, C.-C.; et al. Alternations of the gut microbiota and the Firmicutes/Bacteroidetes ratio after biologic treatment in inflammatory bowel disease. J. Microbiol. Immunol. Infect. 2025, 58, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bensch, H.M.; Tolf, C.; Waldenström, J.; Lundin, D.; Zöttl, M. Bacteroidetes to Firmicutes: Captivity changes the gut microbiota composition and diversity in a social subterranean rodent. Anim. Microbiome 2023, 5, 9. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Yang, Y.; Zhong, W.; He, F.; Li, J. The mycobiome as integral part of the gut microbiome: Crucial role of symbiotic fungi in health and disease. Gut Microbes 2024, 16, 2440111. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Li, S.; Ogamune, K.J.; Ahmed, A.A.; Kim, I.H.; Zhang, Y.; Cai, D. Fungi in the Gut Microbiota: Interactions, Homeostasis, and Host Physiology. Microorganisms 2025, 13, 70. [Google Scholar] [CrossRef]
- Nieves-Ramírez, M.E.; Partida-Rodríguez, O.; Laforest-Lapointe, I.; Reynolds, L.A.; Brown, E.M.; Valdez-Salazar, A.; Morán-Silva, P.; Rojas-Velázquez, L.; Morien, E.; Parfrey, L.W.; et al. Asymptomatic Intestinal Colonization with Protist Blastocystis Is Strongly Associated with Distinct Microbiome Ecological Patterns. mSystems 2018, 3, e00007-18. [Google Scholar] [CrossRef]
- Aguilera-Campos, K.I.; Boisard, J.; Törnblom, V.; Jerlström-Hultqvist, J.; Behncké-Serra, A.; Cotillas, E.A.; Stairs, C.W. Anaerobic breviate protist survival in microcosms depends on microbiome metabolic function. ISME J. 2025, 19, wraf171. [Google Scholar] [CrossRef]
- Guerra, A. Human associated Archaea: A neglected microbiome worth investigating. World J. Microbiol. Biotechnol. 2024, 40, 60. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Mahnert, A.; Shinde, T.; Kumpitsch, C.; Weinberger, V.; Schmidt, H.; Moissl-Eichinger, C. Age-related dynamics of predominant methanogenic archaea in the human gut microbiome. BMC Microbiol. 2025, 25, 193. [Google Scholar] [CrossRef]
- Lv, Z.; Xiong, D.; Shi, J.; Long, M.; Chen, Z. The Interaction Between Viruses and Intestinal Microbiota: A Review. Curr. Microbiol. 2021, 78, 3597–3608. [Google Scholar] [CrossRef]
- SAndreu-Sánchez, S.; Ripoll-Cladellas, A.; Culinscaia, A.; Bulut, O.; Bourgonje, A.R.; Netea, M.G.; Lansdorp, P.; Aubert, G.; Bonder, M.J.; Franke, L.; et al. Antibody signatures against viruses and microbiome reflect past and chronic exposures and associate with aging and inflammation. iScience 2024, 27, 109981. [Google Scholar] [CrossRef]
- King, A. Hidden players: The bacteria-killing viruses of the gut microbiome. Nature 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Dallari, S.; Heaney, T.; Rosas-Villegas, A.; Neil, J.A.; Wong, S.-Y.; Brown, J.J.; Urbanek, K.; Herrmann, C.; Depledge, D.P.; Dermody, T.S.; et al. Enteric viruses evoke broad host immune responses resembling those elicited by the bacterial microbiome. Cell Host Microbe 2021, 29, 1014–1029.e8. [Google Scholar] [CrossRef]
- Nayfach, S.; Páez-Espino, D.; Call, L.; Low, S.J.; Sberro, H.; Ivanova, N.N.; Proal, A.D.; Fischbach, M.A.; Bhatt, A.S.; Hugenholtz, P.; et al. Metagenomic compendium of 189,680 DNA viruses from the human gut microbiome. Nat. Microbiol. 2021, 6, 960–970. [Google Scholar] [CrossRef]
- Leonard, J.M.; Del Toro, D. Defining the Microbiome Components (Bacteria, Viruses, Fungi) and Microbiome Geodiversity. Surg. Infect. 2023, 24, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, D.; Ostrov, B.E. The Impact of the Microbiome on Immunosenescence. Immunol. Investig. 2018, 47, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Hohman, L.S.; Osborne, L.C. A gut-centric view of aging: Do intestinal epithelial cells contribute to age-associated microbiota changes, inflammaging, and immunosenescence? Aging Cell 2022, 21, e13700. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ortega, E.F.; Meydani, S.N.; Adkins, Y.; Stephensen, C.B.; Thompson, B.; Zwickey, H. Nutrition, Immunosenescence, and Infectious Disease: An Overview of the Scientific Evidence on Micronutrients and on Modulation of the Gut Microbiota. Adv. Nutr. Int. Rev. J. 2022, 13, S1–S26. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, N. Roles of Sex Hormones and Gender in the Gut Microbiota. J. Neurogastroenterol. Motil. 2021, 27, 314–325. [Google Scholar] [CrossRef]
- Gancz, N.; Levinson, J.; Callaghan, B. Sex and gender as critical and distinct contributors to the human brain-gut-microbiome axis. Brain Res. Bull. 2023, 199, 110665. [Google Scholar] [CrossRef]
- Kreznar, J.H.; Keller, M.P.; Traeger, L.L.; Rabaglia, M.E.; Schueler, K.L.; Stapleton, D.S.; Zhao, W.; Vivas, E.I.; Yandell, B.S.; Broman, A.T.; et al. Host Genotype and Gut Microbiome Modulate Insulin Secretion and Diet-Induced Metabolic Phenotypes. Cell Rep. 2017, 18, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.A.; Piecyk, A.; Jorge, F.; Cerrato, R.; Kalbe, M.; Dheilly, N.M. Host phenotype and microbiome vary with infection status, parasite genotype, and parasite microbiome composition. Mol. Ecol. 2022, 31, 1577–1594. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.V.; Vranken, S.; Coleman, M.A.; Wernberg, T.; Steinberg, P.D.; Marzinelli, E.M. Host genotype and microbiome associations in co-occurring clonal and non-clonal kelp, Ecklonia radiata. Mol. Ecol. 2023, 32, 4584–4598. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Y.; Mao, C.; Xiang, X.; Chen, J. Combined metagenomic and metabolomic analyses reveal gut microbiota dysbiosis and metabolic dysfunction in pediatric neurodevelopmental disorders. Front. Immunol. 2025, 16, 1645137. [Google Scholar] [CrossRef]
- Cortés, M.; Olate, P.; Rodriguez, R.; Diaz, R.; Martínez, A.; Hernández, G.; Sepulveda, N.; Paz, E.A.; Quiñones, J. Human Microbiome as an Immunoregulatory Axis: Mechanisms, Dysbiosis, and Therapeutic Modulation. Microorganisms 2025, 13, 2147. [Google Scholar] [CrossRef]
- Easwaran, M.; Abdelrahman, F.; El-Shibiny, A.; Venkidasamy, B.; Thiruvengadam, M.; Sivalingam, P.; Ganapathy, D.; Ahn, J.; Shin, H.J. Exploring bacteriophages to combat gut dysbiosis: A promising new frontier in microbiome therapy. Microb. Pathog. 2025, 208, 108008. [Google Scholar] [CrossRef]
- Pandit, S.S.; Meganathan, P.; Vedagiri, H. Harmonizing gut microbiota dysbiosis: Unveiling the influence of diet and lifestyle interventions. Metab. Open 2025, 27, 100384. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Bertin, L.; Bonazzi, E.; Facchin, S.; Lorenzon, G.; Maniero, D.; DE Barba, C.; Tomasulo, A.; Fortuna, A.; Zingone, F.; Barberio, B.; et al. The microbiota-brain connection in neurological diseases: The ubiquitous short-chain fatty acids. Minerva Gastroenterol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate role of gut microbiota in vitamin B nutrition and its influences on human health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef]
- Grant, E.T.; Parrish, A.; Boudaud, M.; Hunewald, O.; Hirayama, A.; Ollert, M.; Fukuda, S.; Desai, M.S. Dietary fibers boost gut microbiota-produced B vitamin pool and alter host immune landscape. Microbiome 2024, 12, 179. [Google Scholar] [CrossRef]
- Li, G.; Dong, S.; Liu, C.; Yang, J.; Rensen, P.C.N.; Wang, Y. Serotonin signaling to regulate energy metabolism: A gut microbiota perspective. Life Metab. 2024, 4, loae039. [Google Scholar] [CrossRef]
- Yoshimura, E.; Hamada, Y.; Hatamoto, Y.; Nakagata, T.; Nanri, H.; Nakayama, Y.; Iwasaka, C.; Hayashi, T.; Suzuki, I.; Ando, T.; et al. Effect of short-term dietary intervention on fecal serotonin, gut microbiome-derived tryptophanase, and energy absorption in a randomized crossover trial: An exploratory analysis. Gut Microbes 2025, 17, 2514137. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Wang, L.; Xu, X.; Chen, Y.; Wang, H.; Wang, G.; Zhao, J.; Chen, W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients 2022, 14, 3704. [Google Scholar] [CrossRef] [PubMed]
- Peña-Durán, E.; García-Galindo, J.J.; López-Murillo, L.D.; Huerta-Huerta, A.; Balleza-Alejandri, L.R.; Beltrán-Ramírez, A.; Anaya-Ambriz, E.J.; Suárez-Rico, D.O. Microbiota and Inflammatory Markers: A Review of Their Interplay, Clinical Implications, and Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 1773. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Jain, S.; Agadzi, B.; Yadav, H. A Cascade of Microbiota-Leaky Gut-Inflammation- Is it a Key Player in Metabolic Disorders? Curr. Obes. Rep. 2025, 14, 32. [Google Scholar] [CrossRef]
- Aznou, A.; Drenth, J.P.H.; Nieuwdorp, M.; Meijnikman, A.S. Exploring the Role of the Gut Microbiome Across the Lifespan: Implications for Aging and Metabolic Disorders. J. Endocr. Soc. 2025, 9, bvaf130. [Google Scholar] [CrossRef]
- Caballero-Villarraso, J.; Galvan, A.; Escribano, B.M.; Tunez, I. Interrelationships Among Gut Microbiota and Host: Paradigms, Role in Neurodegenerative Diseases and Future Prospects. CNS Neurol. Disord. Drug Targets 2017, 16, 945–964. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Ma, B.D.Y.; Chan, T.Y.H.; Lo, B.W.Y. Unveiling the hidden culprit: How the brain-gut axis fuels neuroinflammation in ischemic stroke. Surg. Neurol. Int. 2024, 15, 394. [Google Scholar] [CrossRef]
- Dandamudi, B.J.; Dimaano, K.A.M.; Shah, N.; AlQassab, O.; Al-Sulaitti, Z.; Nelakuditi, B.; Mohammed, L. Neurodegenerative Disorders and the Gut-Microbiome-Brain Axis: A Literature Review. Cureus 2024, 16, e72427. [Google Scholar] [CrossRef]
- Parkinson Disease—ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/1-s2.0-S0002838X20304196 (accessed on 21 August 2025).
- Christovich, A.; Luo, X.M. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29, Erratum in Int. J. Stroke 2022, 17, 478. [Google Scholar] [CrossRef] [PubMed]
- Thayabaranathan, T.; Kim, J.; Cadilhac, D.A.; Thrift, A.G.; A Donnan, G.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; et al. Global stroke statistics 2022. Int. J. Stroke 2022, 17, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef]
- Montaño, A.; Hanley, D.F.; Hemphill, J.C. Hemorrhagic stroke. Handb. Clin. Neurol. 2021, 176, 229–248. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. The Role of Gut Microbiota in an Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 915. [Google Scholar] [CrossRef] [PubMed]
- Bangad, A.; Abbasi, M.; de Havenon, A. Secondary Ischemic Stroke Prevention. Neurotherapeutics 2023, 20, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Potter, T.B.H.; Tannous, J.; Vahidy, F.S. A Contemporary Review of Epidemiology, Risk Factors, Etiology, and Outcomes of Premature Stroke. Curr. Atheroscler. Rep. 2022, 24, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Rathipriya, A.G.; Mahalakshmi, A.M.; Sharma, S.; Hediyal, T.A.; Ray, B.; Sunanda, T.; Rungratanawanich, W.; Kashyap, R.S.; Qoronfleh, M.W.; et al. The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke. Cells 2022, 11, 1239. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, C.; Lv, B.; He, S.; Zheng, Y.; Yang, W.; Wang, B.; Li, D.; Lin, J. Two-sample Mendelian randomization to study the causal association between gut microbiota and atherosclerosis. Front. Immunol. 2023, 14, 1282072. [Google Scholar] [CrossRef]
- Hu, W.; Kong, X.; Wang, H.; Li, Y.; Luo, Y. Ischemic stroke and intestinal flora: An insight into brain–gut axis. Eur. J. Med. Res. 2022, 27, 73. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, Z.; Liu, H.; Lim, J.Y.; Li, D.W.H.; Zhang, S.; Luo, F.; Wang, X.; Sun, C.; Tang, R.; et al. Research development on gut microbiota and vulnerable atherosclerotic plaque. Heliyon 2024, 10, e25186. [Google Scholar] [CrossRef]
- Granados-Martinez, C.; Alfageme-Lopez, N.; Navarro-Oviedo, M.; Nieto-Vaquero, C.; Cuartero, M.I.; Diaz-Benito, B.; Moro, M.A.; Lizasoain, I.; Hernandez-Jimenez, M.; Pradillo, J.M. Gut Microbiota, Bacterial Translocation, and Stroke: Current Knowledge and Future Directions. Biomedicines 2024, 12, 2781. [Google Scholar] [CrossRef]
- Shuai, H.; Wang, Z.; Xiao, Y.; Ge, Y.; Mao, H.; Gao, J. Genetically supported causality between gut microbiota, immune cells, and ischemic stroke: A two-sample Mendelian randomization study. Front. Microbiol. 2024, 15, 1402718. [Google Scholar] [CrossRef]
- Zhang, R.; Han, L.; Pu, L.; Jiang, G.; Guan, Q.; Fan, W.; Liu, H. Investigating causal associations of gut microbiota and blood metabolites on stroke and its subtypes: A Mendelian randomization study. J. Stroke Cerebrovasc. Dis. 2025, 34, 108233. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shi, J.; Yu, G.; Xu, J.; Dong, Y.; Lin, Y.; Xie, H.; Liu, J.; Sun, J. Specific gut microbiome signatures predict the risk of acute ischemic stroke. Front. Aging Neurosci. 2024, 16, 1451968. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Jiang, C.; Ni, J.; Zhang, X.; Wu, Z.; Chen, G.; Huang, J.; Dai, Z.; Ji, W.; Li, L.; et al. Identifying gut microbiota with high specificity for ischemic stroke with large vessel occlusion. Sci. Rep. 2024, 14, 14086. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Sun, Y.; Wang, J.; Wei, S.; Mao, L.; Zheng, J.; Liu, P.; Yang, X.; Chen, Z. Relationship Between the Gut Microbiota and Neurological Deficits in Patients With Cerebral Ischemic Stroke. Neurorehabilit. Neural Repair 2024, 38, 527–538. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Hua, W.; Liu, H.; Zhang, B.; Dong, H.; Liu, J.; Zhou, Y.; Yang, P.; Jing, M. Causal relationship between gut microbiota and functional outcomes after ischemic stroke: A comprehensive Mendelian randomization study. J. Stroke Cerebrovasc. Dis. 2024, 33, 107814. [Google Scholar] [CrossRef]
- Singh, A.A.; Kharwar, A.; Dandekar, M.P. A Review on Preclinical Models of Ischemic Stroke: Insights Into the Pathomechanisms and New Treatment Strategies. Curr. Neuropharmacol. 2022, 20, 1667–1686. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, A.; Pu, J.; Chen, D.; Zhong, Y.; Bai, D.; Yang, L. Post-stroke cognitive impairment: Exploring molecular mechanisms and omics biomarkers for early identification and intervention. Front. Mol. Neurosci. 2024, 17, 1375973. [Google Scholar] [CrossRef]
- Gruenbaum, B.F.; Merchant, K.S.; Zlotnik, A.; Boyko, M. Gut Microbiome Modulation of Glutamate Dynamics: Implications for Brain Health and Neurotoxicity. Nutrients 2024, 16, 4405. [Google Scholar] [CrossRef]
- Li, Z.; He, X.; Fang, Q.; Yin, X. Gut Microbe-Generated Metabolite Trimethylamine-N-Oxide and Ischemic Stroke. Biomolecules 2024, 14, 1463. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, X.; Zhang, J.; Niu, S.; Xiao, H.C.; Chen, H.; Qu, C. Association between plasma trimethylamine N-oxide and cerebral white matter hyperintensity: A cross-sectional study. Front. Aging Neurosci. 2024, 16, 1498502. [Google Scholar] [CrossRef]
- Cai, X.; Cai, X.; Xie, Q.; Xiao, X.; Li, T.; Zhou, T.; Sun, H. NLRP3 inflammasome and gut microbiota–brain axis: A new perspective on white matter injury after intracerebral hemorrhage. Neural Regen. Res. 2025, 21, 62–80. [Google Scholar] [CrossRef]
- Demarquoy, J. Revisiting the Role of Carnitine in Heart Disease Through the Lens of the Gut Microbiota. Nutrients 2024, 16, 4244. [Google Scholar] [CrossRef]
- Xu, X.; Jia, L.; Qiao, B.; Gong, Y.; Gao, S.; Wang, Y.; Du, J. Changes in Phenylacetylglutamine Levels Provide Add-On Value in Risk Stratification of Hypertensive Patients: A Longitudinal Cohort Study. Metabolites 2025, 15, 64. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.K.; Kalyan, M.; Hediyal, T.A.; Anand, N.; Kendaganna, P.H.; Pendyala, G.; Yelamanchili, S.V.; Yang, J.; Chidambaram, S.B.; Sakharkar, M.K.; et al. Role of the Gut Bacteria-Derived Metabolite Phenylacetylglutamine in Health and Diseases. ACS Omega 2023, 9, 3164–3172. [Google Scholar] [CrossRef]
- Alhasan, M.M.; Landa, M.S.; García, S.I.; Gerlach, R.G.; Harb, H.; Fahlbusch, F.B.; Conrad, M.L.; Barrientos, G. Distinct gut microbial signature and altered short chain fatty acid metabolism at disease onset in a rat preclinical model of superimposed preeclampsia. Sci. Rep. 2024, 14, 32137. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, J.; Li, S.; Zhang, D.; Bai, X. Progress of research on short-chain fatty acids, metabolites of gut microbiota, and acute ischemic stroke. Clin. Neurol. Neurosurg. 2025, 249, 108725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, L.; Huang, T.; Jia, Y.; Yang, P.; Zhang, Q.; Fang, C.; Shi, M.; Guo, D.; Peng, Y.; et al. High Serum Brain-Derived Neurotrophic Factor Is Associated With Decreased Risks of Poor Prognosis After Ischemic Stroke. Stroke 2023, 54, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.-K.; Wilken-Resman, B.; Smith, C.J.; Mitchell, A.; McGonegal, L.; Sims-Robinson, C. Brain-Derived Neurotrophic Factor and Nerve Growth Factor Therapeutics for Brain Injury: The Current Translational Challenges in Preclinical and Clinical Research. Neural Plast. 2022, 2022, 3889300. [Google Scholar] [CrossRef]
- Molska, M.; Mruczyk, K.; Cisek-Woźniak, A.; Prokopowicz, W.; Szydełko, P.; Jakuszewska, Z.; Marzec, K.; Trocholepsza, M. The Influence of Intestinal Microbiota on BDNF Levels. Nutrients 2024, 16, 2891. [Google Scholar] [CrossRef]
- Mojtabavi, H.; Shaka, Z.; Momtazmanesh, S.; Ajdari, A.; Rezaei, N. Circulating brain-derived neurotrophic factor as a potential biomarker in stroke: A systematic review and meta-analysis. J. Transl. Med. 2022, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, G.Y.; Ding, S. Glial Cell Line-Derived Neurotrophic Factor and Focal Ischemic Stroke. Neurochem. Res. 2021, 46, 2638–2650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Z.; He, R.; Li, H.; Ding, S. GLAST-CreERT2 mediated deletion of GDNF increases brain damage and exacerbates long-term stroke outcomes after focal ischemic stroke in mouse model. Glia 2020, 68, 2395–2414. [Google Scholar] [CrossRef] [PubMed]
- Forró, T.; Manu, D.R.; Băjenaru, O.-L.; Bălașa, R. GFAP as Astrocyte-Derived Extracellular Vesicle Cargo in Acute Ischemic Stroke Patients—A Pilot Study. Int. J. Mol. Sci. 2024, 25, 5726. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Xu, M.; Yu, B.; Guo, M.; Wang, X.; Shi, G.; Zhou, R. Glial Fibrillary Acidic Protein as a Potential Indicator for Symptomatic Intracranial Hemorrhage in Acute Ischemic Patients Undergoing Endovascular Thrombectomy. Clin. Interv. Aging 2024, 19, 123–132. [Google Scholar] [CrossRef]
- Bamodu, O.A.; Chan, L.; Wu, C.-H.; Yu, S.-F.; Chung, C.-C. Beyond diagnosis: Leveraging routine blood and urine biomarkers to predict severity and functional outcome in acute ischemic stroke. Heliyon 2024, 10, e26199. [Google Scholar] [CrossRef]
- Vollmuth, C.; Fiessler, C.; Montellano, F.A.; Kollikowski, A.M.; Essig, F.; Oeckl, P.; Barba, L.; Steinacker, P.; Schulz, C.; Ungethüm, K.; et al. Incremental value of serum neurofilament light chain and glial fibrillary acidic protein as blood-based biomarkers for predicting functional outcome in severe acute ischemic stroke. Eur. Stroke J. 2024, 9, 751–762. [Google Scholar] [CrossRef]
- Noll, J.M.; Sherafat, A.A.; Ford, G.D.; Ford, B.D. The case for neuregulin-1 as a clinical treatment for stroke. Front. Cell. Neurosci. 2024, 18, 1325630. [Google Scholar] [CrossRef]
- Cescon, M.; Gambarotta, G.; Calabrò, S.; Cicconetti, C.; Anselmi, F.; Kankowski, S.; Lang, L.; Basic, M.; Bleich, A.; Bolsega, S.; et al. Gut microbiota depletion delays somatic peripheral nerve development and impairs neuromuscular junction maturation. Gut Microbes 2024, 16, 2363015. [Google Scholar] [CrossRef]
- Gu, C.-L.; Zhang, L.; Zhu, Y.; Bao, T.-Y.; Zhu, Y.-T.; Chen, Y.-T.; Pang, H.-Q. Exploring the cellular and molecular basis of nerve growth factor in cerebral ischemia recovery. Neuroscience 2025, 566, 190–197. [Google Scholar] [CrossRef]
- Hayes, C.A.; Valcarcel-Ares, M.N.; Ashpole, N.M. Preclinical and clinical evidence of IGF-1 as a prognostic marker and acute intervention with ischemic stroke. J. Cereb. Blood Flow Metab. 2021, 41, 2475–2491. [Google Scholar] [CrossRef]
- Castro-Vidal, Z.A.; Mathew, F.; Ibrahim, A.A.; Shubhangi, F.N.U.; Cherian, R.R.; Choi, H.K.; Begum, A.; Ravula, H.K.; Giri, H. The Role of Gastrointestinal Dysbiosis and Fecal Transplantation in Various Neurocognitive Disorders. Cureus 2024, 16, e72451. [Google Scholar] [CrossRef] [PubMed]
- Devranis, P.; Vassilopoulou, Ε.; Tsironis, V.; Sotiriadis, P.M.; Chourdakis, M.; Aivaliotis, M.; Tsolaki, M. Mediterranean Diet, Ketogenic Diet or MIND Diet for Aging Populations with Cognitive Decline: A Systematic Review. Life 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Akbar, Z.; Fituri, S.; Ouagueni, A.; Alalwani, J.; Sukik, A.; Al-Jayyousi, G.F.; Bassil, M.; Tayyem, R. Associations of the MIND Diet with Cardiometabolic Diseases and Their Risk Factors: A Systematic Review. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 3353–3371. [Google Scholar] [CrossRef]
- Salari-Moghaddam, A.; Nouri-Majd, S.; Shakeri, F.; Keshteli, A.H.; Benisi-Kohansal, S.; Saadatnia, M.; Esmaillzadeh, A. The association between adherence to the MIND diet and stroke: A case–control study. Nutr. Neurosci. 2022, 25, 1956–1961. [Google Scholar] [CrossRef]
- Cherian, L.; Wang, Y.; Fakuda, K.; Leurgans, S.; Aggarwal, N.; Morris, M. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) Diet Slows Cognitive Decline After Stroke. J. Prev. Alzheimer’s Dis. 2019, 6, 267–273. [Google Scholar] [CrossRef]
- Hediyal, T.A.; Vichitra, C.; Anand, N.; Bhaskaran, M.; Essa, S.M.; Kumar, P.; Qoronfleh, M.W.; Akbar, M.; Kaul-Ghanekar, R.; Mahalakshmi, A.M.; et al. Protective effects of fecal microbiota transplantation against ischemic stroke and other neurological disorders: An update. Front. Immunol. 2024, 15, 1324018. [Google Scholar] [CrossRef]
- Chen, D.; Xie, J.; Chen, X.; Qin, B.; Kong, D.; Luo, J. Fecal microbiota transplantation alleviates neuronal Apoptosis, necroptosis and reactive microglia activation after ischemic stroke. Neuroscience 2025, 564, 299–305. [Google Scholar] [CrossRef]
- Yang, X.; Hong, C.; Guan, T.; Zhang, C.; Xiao, P.; Yang, Y.; Xiao, H.; He, Z. Investigation of the effects of Periplaneta americana (L.) extract on ischemic stroke based on combined multi-omics of gut microbiota. Front. Pharmacol. 2024, 15, 1429960. [Google Scholar] [CrossRef]
- Huan, P.; Sun, L.; Chen, S.; Zhong, Y.; Zhuang, Y. A peptide from Boletus griseus-Hypomyces chrysospermus protects against hypertension and associated cardiac and renal damage through modulating RAAS and intestinal microbiota. J. Food Sci. 2025, 90, e17617. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, Z.; Li, S.; Zhang, H.; Zeng, S.; Zuo, X.; Xiao, Y.; Zhang, L.; Li, Z.; Zhu, Q.; et al. An ultrasonic degraded polysaccharide extracted from Pueraria lobata ameliorate ischemic brain injury in mice by regulating the gut microbiota and LPS-TLR4 pathway. Ultrason. Sonochem. 2025, 112, 107200. [Google Scholar] [CrossRef]
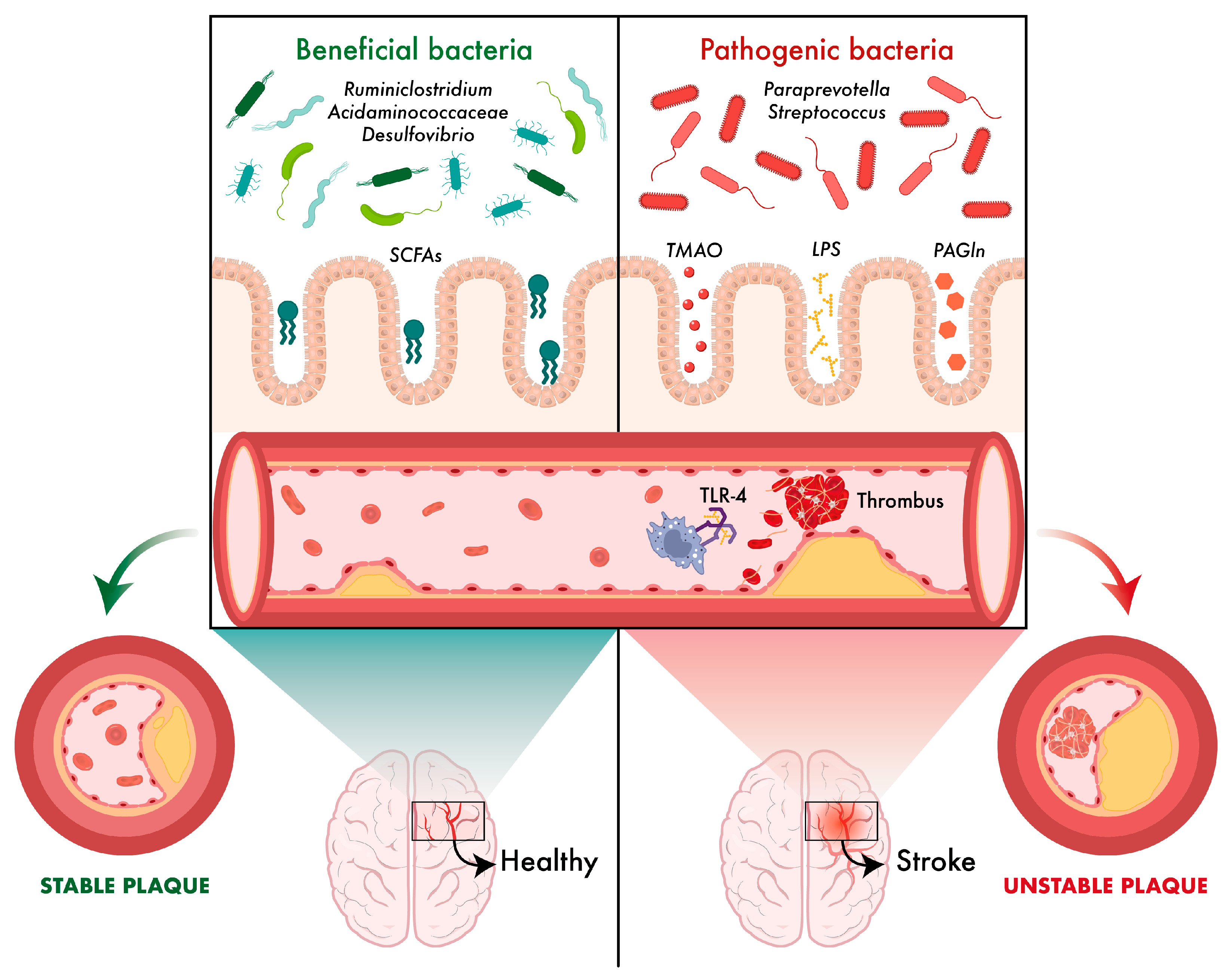
| Microorganism or Groups of Microorganisms | Overall Effect | Actions Related to Stroke |
|---|---|---|
| Ruminiclostridium | Beneficial | Protective effect against cerebral atherosclerosis; contributes to plaque stability and inflammatory reduction |
| Rikenellaceae, Streptococcaceae, Paraprevotella, Streptococcus | Detrimental | Associated with increased risk of atherosclerosis and thrombosis; promote vascular inflammation |
| Prevotella, Ruminococcus | Beneficial | Producers of SCFAs (butyrate); reduce inflammation and maintain the integrity of the intestinal and blood–brain barriers |
| Enterobacteriaceae, Firmicutes | Detrimental (if excessive) | Proinflammatory; increase intestinal permeability and immune activation via TLR4; worsen brain damage |
| Streptococcus pneumoniae, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, Enterococcus faecalis | Detrimental | Opportunistic bacteria proliferate, leading to dysbiosis and an increase in post-stroke infections |
| Butyricicoccaceae, Barnesiella, Clostridiaceae, Lachnospiraceae | Beneficial | Producers of SCFAs (butyrate, propionate); modulate inflammation and protect the BBB |
| Bifidobacterium, Blautia, Butyricimonas, Dorea | Beneficial | Related to improved lipid metabolism and reduced inflammation; greater abundance correlates with improved prognosis |
| Proteobacteria | Detrimental | Increased in severe stroke; induces systemic inflammation and neuroinflammation |
| Collinsella, Akkermansia, Eubacterium oxidoreducens, Verrucomicrobiaceae | Detrimental | Associated with poorer functional recovery and greater neuroinflammation |
| Escherichia-Shigella, Bacteroides, Agathobacter, Lactococcus, Ruminococcaceae 1, Peptostreptococcaceae, Odoribacter | Beneficial | Related to better neurological prognosis and immune balance |
| Desulfovibrio, Acidaminococcaceae | Beneficial | Involved in lipid metabolism protective against atherosclerosis |
| Porphyromonadaceae | Detrimental | Endothelial damage and increased risk of cerebral haemorrhage |
| Enterococcaceae, Clostridiales, Peptoniphilaceae | Detrimental | Increase intestinal inflammation and worsen neurological prognosis |
| Lachnospiraceae, Ruminococcaceae 1 | Beneficial | Predictors of better recovery; produce butyrate and reduce neuroinflammation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero-Villarraso, J.; Pons-Villarta, S.; Cruces-Párraga, J.; Navarrete-Pérez, A.; Camargo, A.; Moreno, J.A.; Túnez, I.; Agüera-Morales, E. Molecular Mechanisms of the Microbiota–Gut–Brain Axis in the Onset and Progression of Stroke. Int. J. Mol. Sci. 2025, 26, 10071. https://doi.org/10.3390/ijms262010071
Caballero-Villarraso J, Pons-Villarta S, Cruces-Párraga J, Navarrete-Pérez A, Camargo A, Moreno JA, Túnez I, Agüera-Morales E. Molecular Mechanisms of the Microbiota–Gut–Brain Axis in the Onset and Progression of Stroke. International Journal of Molecular Sciences. 2025; 26(20):10071. https://doi.org/10.3390/ijms262010071
Chicago/Turabian StyleCaballero-Villarraso, Javier, Sara Pons-Villarta, Jerónimo Cruces-Párraga, Ainoa Navarrete-Pérez, Antonio Camargo, Juan Antonio Moreno, Isaac Túnez, and Eduardo Agüera-Morales. 2025. "Molecular Mechanisms of the Microbiota–Gut–Brain Axis in the Onset and Progression of Stroke" International Journal of Molecular Sciences 26, no. 20: 10071. https://doi.org/10.3390/ijms262010071
APA StyleCaballero-Villarraso, J., Pons-Villarta, S., Cruces-Párraga, J., Navarrete-Pérez, A., Camargo, A., Moreno, J. A., Túnez, I., & Agüera-Morales, E. (2025). Molecular Mechanisms of the Microbiota–Gut–Brain Axis in the Onset and Progression of Stroke. International Journal of Molecular Sciences, 26(20), 10071. https://doi.org/10.3390/ijms262010071








