Neurotransmitter Levels (Dopamine, Epinephrine, Norepinephrine, Serotonin) and Associations with Lipid Profiles in Patients with Prediabetes or Newly Diagnosed Type 2 Diabetes Mellitus
Abstract
1. Introduction
- Neurotransmitter serum levels differ significantly between PreDM and newly diagnosed T2DM.
- Neurochemical changes are linked to lipid and insulin resistance markers beyond traditional glycemic measures.
- Neurotransmitter profiles could have diagnostic or stratification value in early T2DM classification.
2. Results
2.1. Characteristics of Patients with PreDM and T2DM: Clinical and Demographic Overview at Baseline
2.2. Comparing the Neurotransmitter Values Between the PreDM and T2DM Groups
2.2.1. Comparing the Neurotransmitter Levels According to BMI Category in PreDM and T2DM Groups
2.2.2. Comparing Neurotransmitter Levels According to Gender in the PreDM and T2DM Groups
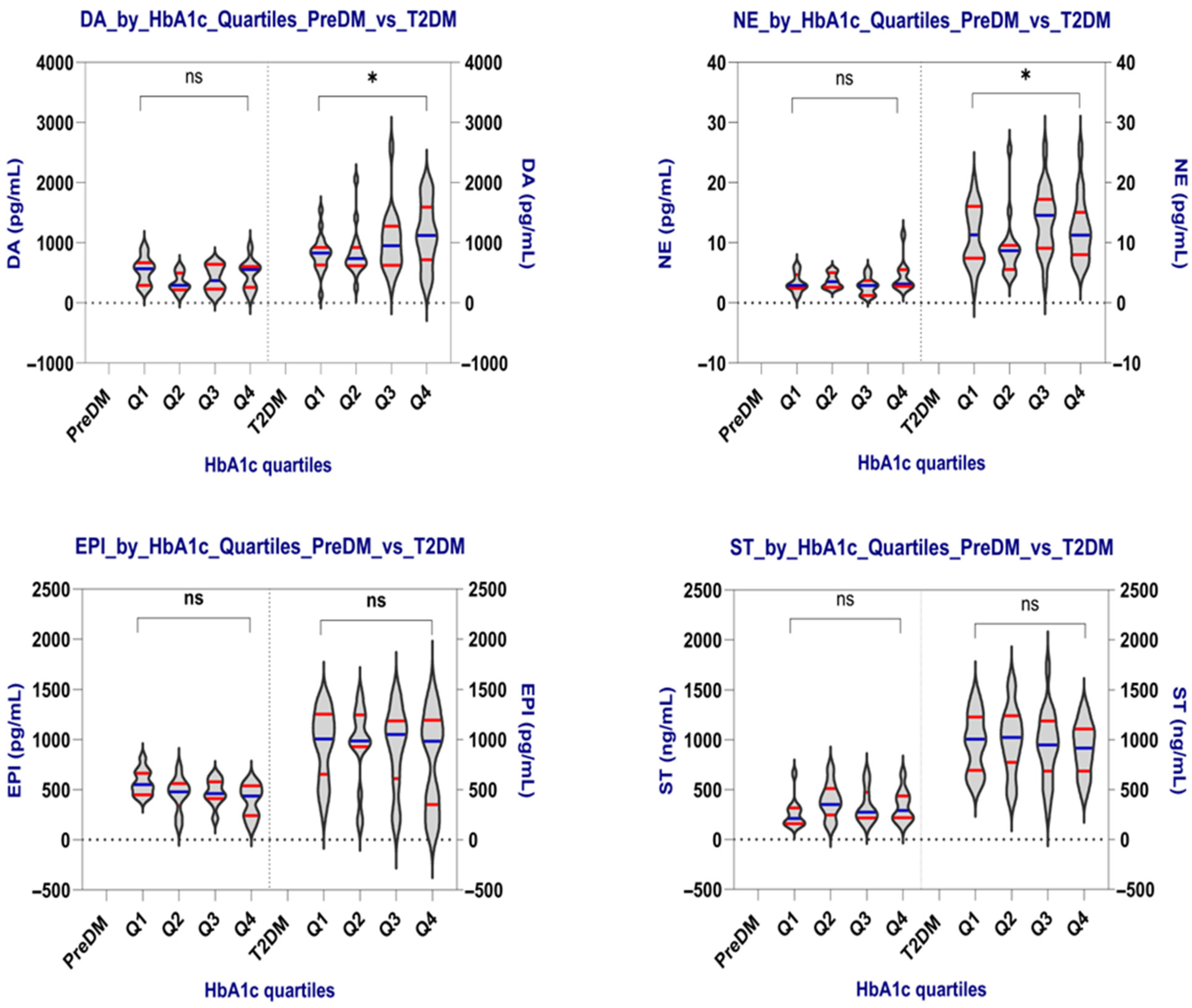
2.2.3. Comparing Neurotransmitter Levels According to the HbA1c Quartiles in the PreDM and T2DM Groups
Comparison by HbA1c Quartiles
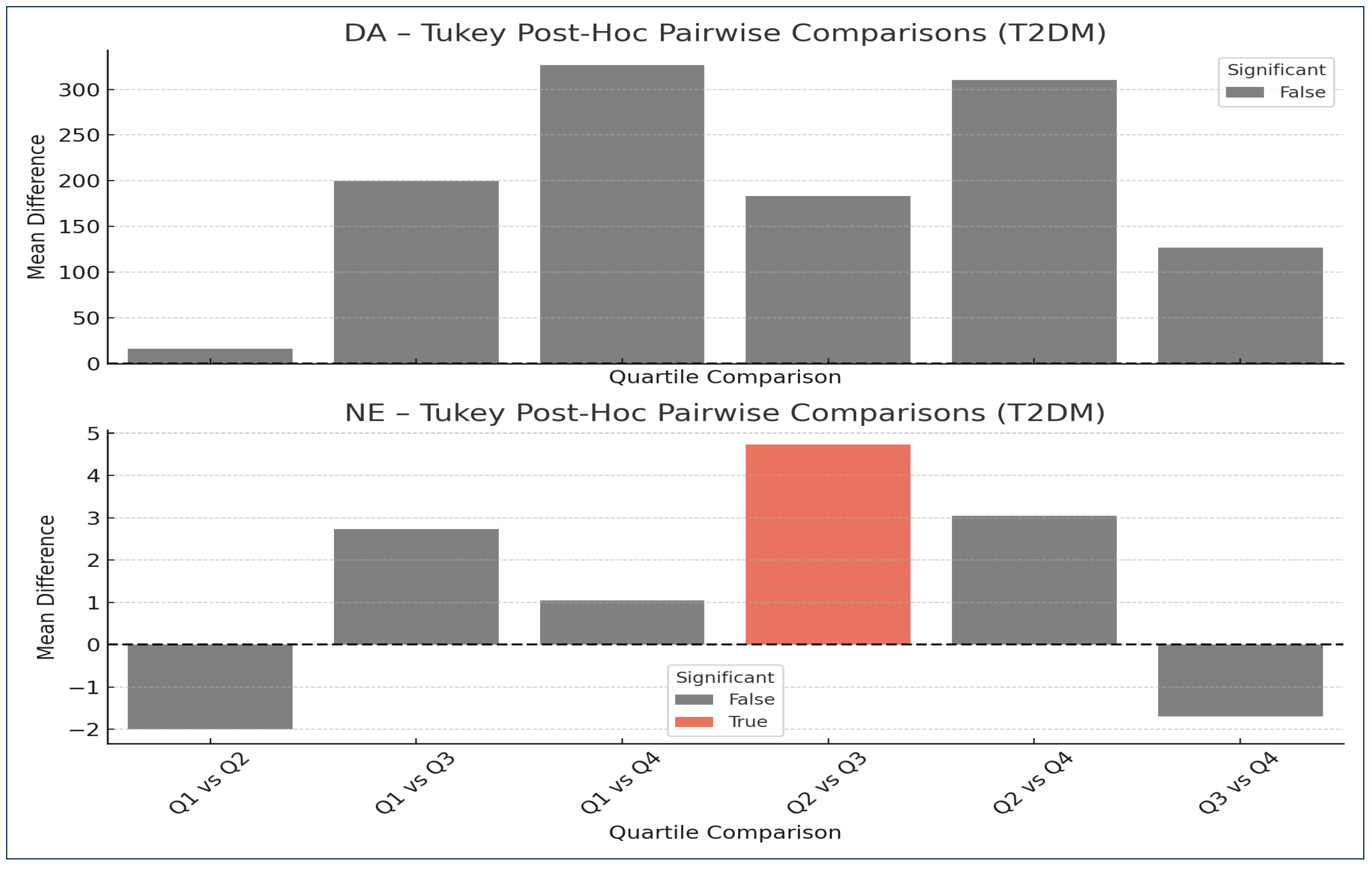
Post Hoc Analysis
2.3. Regression Analyses
2.3.1. Multiple Linear Regression (MLR)
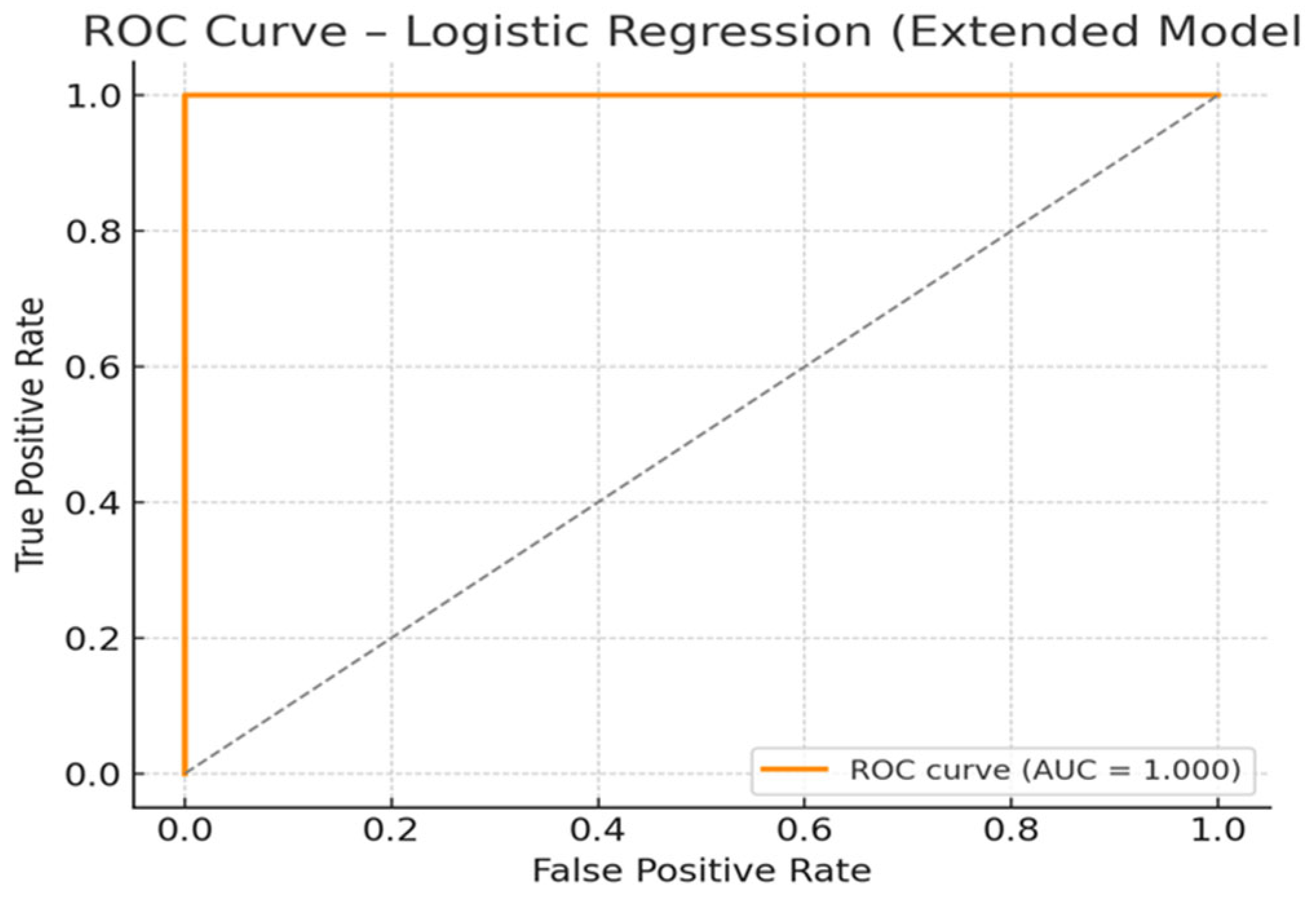
2.3.2. Logistic Regression
- -
- ST showed the most considerable effect with an OR of 8.70, indicating that each 1 SD increase in ST level was associated with an almost 9-fold increase in the odds of T2DM;
- -
- NE and HbA1c were also strong predictors, with ORs of 3.76 and 3.15, respectively, suggesting meaningful contributions of both neurochemical activation and glycemic control.
- -
- EPI and DA also increased T2DM odds (ORs: 2.61 and 1.38), supporting the idea that neurotransmitter elevations reflect disease severity;
- -
- Age contributed modestly (OR = 1.35), consistent with its established role as a risk factor for T2DM.
- -
- TC was the only variable with an OR clearly <1 (OR = 0.897), suggesting a potential inverse association, though the effect size was modest;
- -
- Variables like BMI, TG, TyG, and sex (male) were near OR = 1.00, implying minimal or no contribution to classification in this model when other variables are considered.
2.4. Exploratory Correlation Analysis—Neurotransmitters and Metabolic Profile
2.4.1. Exploratory Correlation Analysis in PreDM Group
- -
- EPI—HbA1c (rho = −0.348, p-value = 0.028, q-value (FDR) = 0.928): Suggests a potential inverse relationship between epinephrine and glycemic burden. This may reflect reduced adrenergic activity as glucose regulation worsens.
- -
- ST—TyG-BMI (rho = 0.312, p-value = 0.050, q-value (FDR) = 0.935): Indicates a possible link between serotonergic activation and worsening insulin resistance or visceral adiposity.
2.4.2. Exploratory Correlation Analysis in the T2DM Group
- -
- EPI—TyG-WC (rho = −0.365, p-value = 0.002, q-value (FDR) = 0.076): Suggests an inverse relationship between epinephrine levels and central insulin resistance markers.
- -
- EPI—TyG-WHtR (rho = −0.311, p-value = 0.009, q-value (FDR) = 0.158): Reinforces the above, linking epinephrine suppression with visceral metabolic burden.
- -
- DA—LDL-C (rho = 0.288, p-value = 0.016, q-value (FDR) = 0.159): Indicates potential coupling between dopaminergic activity and atherogenic lipid profiles.
- -
- DA—TC (rho = 0.290, p-value = 0.015, q-value (FDR) = 0.176): Indicates potential coupling between dopaminergic activity and atherogenic lipid profiles.
- -
- DA—HbA1c (rho = 0.269, p-value = 0.024, q-value (FDR) = 0.199): Dopamine increases in line with glycemic deterioration.
3. Discussion
- Biomarker development: ST, NE, and DA may function as supplementary biomarkers for early disease detection, risk assessment, or tracking disease progression, especially when used alongside glycemic and lipid measurements.
- Refining risk models: Incorporating neurochemical markers into multivariable risk prediction could improve accuracy in identifying individuals transitioning from PreDM to T2DM or at risk for complications.
- Therapeutic targeting: If further validated, monoamine pathways could become treatment targets. For example, dopaminergic modulation (such as in bromocriptine) or serotonergic modulation might affect metabolic outcomes beyond just glycemia.
- Patient stratification: Patients with unusually high neurotransmitter levels may constitute a distinct “neuro-metabolic phenotype” of T2DM, justifying customized preventive or therapeutic strategies.
- Cross-sectional design: Causality cannot be determined. Longitudinal studies are necessary to establish the directionality (i.e., whether changes in neurotransmitters precede or follow metabolic deterioration).
- Measurement context: Circulating levels of neurotransmitters may not accurately reflect tissue-specific or central concentrations; peripheral factors (such as clearance, transporter function, and degradation) can influence serum levels.
- Sample size and power: Although sufficient for primary comparisons, limited power may explain the lack of significance in some adjusted models or correlations.
- Assay specificity and stability: Quantifying neurotransmitters in serum or plasma can be affected by preanalytical variables such as stability and degradation, requiring rigorous standardization.
- Potential confounding factors such as diet, medications, or stress exposure were not fully controlled, which could affect neurotransmitter levels.
- Future research should incorporate prospective cohorts, intervention studies targeting monoamine pathways, simultaneous measurement of tissue and CSF levels, and integration with imaging or genetic biomarkers to map the neuro-metabolic axis thoroughly.
4. Materials and Methods
4.1. Patient Selection
4.2. Assessment of Diabetes and Prediabetes
4.3. Medical History, Biometric Parameter Evaluation, and Demographic Information
4.4. Laboratory Investigations
4.4.1. Sample Collection
- -
- Two additive-free tubes (Becton Dickinson Vacutainer, Franklin Lakes, NJ, USA) with approximately 5 mL of venous blood from each patient. In line with standard processing protocols, blood samples were allowed to clot and then centrifuged within 4 h of collection at 3000× g for 10 min using a Hermle centrifuge (Hermle AG, Gosheim, Baden-Württemberg, Germany). The resulting serum from one tube was aliquoted into pre-labeled vials, sealed tightly to prevent contamination, and stored at controlled temperatures between −20 °C and −80 °C to ensure sample preservation. To maintain specimen integrity, freeze–thaw cycles were strictly avoided. Before analysis, frozen serum samples were passively thawed to reach room temperature. These aliquots were used for immunological investigations. The serum from the second tube was used for biochemical investigations.
- -
- And peripheral venous blood collected in ethylene-diamine-tetra-acetic acid (EDTA) vacutainer tubes (Becton Dickinson Vacutainer, Franklin Lakes, NJ, USA) was used to perform a complete blood count (CBC). Utilizing flow cytometry under Coulter’s principle, we successfully established an extended leukocyte differential by analyzing five distinct parameters using the MINDRAY BC-6800 (Mindray, Shenzhen, China). This approach enabled us to effectively identify and characterize a range of hemoleucogram markers.
4.4.2. Biochemical Investigations
- FPG and 2hPG were determined using an enzymatic hexokinase/glucose-6-phosphate dehydrogenase (G6PDH) method. The reaction produces NADPH, which is measured photometrically at 340 nm.
- TC was measured by an enzymatic colorimetric method involving cholesterol esterase and cholesterol oxidase, with quantification based on the generation of hydrogen peroxide (H2O2) and subsequent chromogenic reaction.
- TG were assessed enzymatically using lipoprotein lipase, glycerol kinase, and glycerol-3-phosphate oxidase, resulting in H2O2 formation, which was detected photometrically.
- HDL-C and LDL-C were measured using direct enzymatic methods with selective inhibition or solubilization, allowing specific quantification of HDL-C or LDL-C, respectively, without prior sample pretreatment.
- CREA was determined using a specific enzymatic method, based on the conversion of creatinine to sarcosine, followed by oxidation and H2O2 generation, providing improved accuracy over the traditional Jaffe method.
- HbA1c was measured using an enzymatic photometric assay. Hemoglobin was lysed, and the N-terminal valine of HbA1c was selectively cleaved and oxidized by fructosyl-peptide oxidase, forming H2O2, which was then quantified. HbA1c results were expressed as a percentage (%) of total hemoglobin.
4.5. Determination of TyG and TyG-Related Indices
4.6. Immunological Assessment
4.6.1. Assays and Specificity
4.6.2. Pre-Analytical Handling
4.6.3. Analytical Limitations and Quality Controls
4.6.4. Test Principle
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation (IDF). IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 25 September 2025).
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. 1), S19–S40, Erratum in Diabetes Care 2023, 46, 1106. https://doi.org/10.2337/dc23-er05. [Google Scholar] [CrossRef] [PubMed]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayyar, A.; Hammad, M.M.; Williams, M.R.; Al-Onaizi, M.; Abubaker, J.; Alzaid, F. Neurotransmitters in Type 2 Diabetes and the Control of Systemic and Central Energy Balance. Metabolites 2023, 13, 384. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef]
- Rubí, B.; Maechler, P. Minireview: New roles for peripheral dopamine on metabolic control and tumor growth: Let’s seek the balance. Endocrinology 2010, 151, 5570–5581. [Google Scholar] [CrossRef]
- Volkow, N.D.; Morales, M. The brain on drugs: From reward to addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Holmes, C. Neuronal source of plasma dopamine. Clin. Chem. 2008, 54, 1864–1871. [Google Scholar] [CrossRef]
- Cryer, P.E. Mechanisms of sympathoadrenal failure and hypoglycemia in diabetes. J. Clin. Investig. 2006, 116, 1470–1473. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Tecott, L.H. Serotonin and the orchestration of energy balance. Cell Metab. 2007, 6, 352–361. [Google Scholar] [CrossRef]
- Lam, D.D.; Garfield, A.S.; Marston, O.J.; Shaw, J.; Heisler, L.K. Brain serotonin system in the coordination of food intake and body weight. Pharmacol. Biochem. Behav. 2010, 97, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, X.; Zhou, H.; Zhou, J. The Serotonergic System Dysfunction in Diabetes Mellitus: Roles in Glucose Regulation and Neuropathy. Front. Cell. Neurosci. 2022, 16, 899069. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; De Tullio, A.; Iovino, M.; Disoteo, O.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. Dopamine in the Regulation of Glucose Homeostasis, Pathogenesis of Type 2 Diabetes, and Chronic Conditions of Impaired Dopamine Activity/Metabolism: Implication for Pathophysiological and Therapeutic Purposes. Biomedicines 2023, 11, 2993. [Google Scholar] [CrossRef] [PubMed]
- Freyberg, Z.; Gittes, G.K. Roles of Pancreatic Islet Catecholamine Neurotransmitters in Glycemic Control and in Antipsychotic Drug-Induced Dysglycemia. Diabetes 2023, 72, 3–15. [Google Scholar] [CrossRef]
- Caldwell, B.; Ustione, A.; Piston, D.W. Dopamine Receptor Signaling in MIN6 β-Cells Revealed by Fluorescence Fluctuation Spectroscopy. Biophys. J. 2016, 111, 609–618. [Google Scholar] [CrossRef]
- Nonogaki, K. The Regulatory Role of the Central and Peripheral Serotonin Network on Feeding Signals in Metabolic Diseases. Int. J. Mol. Sci. 2022, 23, 1600. [Google Scholar] [CrossRef]
- Serlie, M.J. Peripheral and central serotonin in the regulation of glucose metabolism. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 893–900. [Google Scholar]
- Defronzo, R.A. Bromocriptine: A sympatholytic, d2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care 2011, 34, 789–794, Erratum in Diabetes Care 2011, 34, 1442. [Google Scholar] [CrossRef]
- Ahrițculesei, R.-V.; Boldeanu, L.; Dijmărescu, A.L.; Assani, M.-Z.; Boldeanu, M.V.; Siloși, I.; Vere, C.C. Neurotransmitter Alterations in Prediabetes and Type 2 Diabetes Mellitus: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 7847. [Google Scholar] [CrossRef]
- Mangoulia, P.; Milionis, C.; Vlachou, E.; Ilias, I. The Interrelationship between Diabetes Mellitus and Emotional Well-Being: Current Concepts and Future Prospects. Healthcare 2024, 12, 1457. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. 1), S1–S200. [Google Scholar] [CrossRef]
- MDRD GFR Equation. Available online: https://www.mdcalc.com/calc/76/mdrd-gfr-equation (accessed on 25 September 2025).
- Mitroi Sakizlian, D.D.; Boldeanu, L.; Mitrea, A.; Clenciu, D.; Vladu, I.M.; Ciobanu Plasiciuc, A.E.; Șarla, A.V.; Siloși, I.; Boldeanu, M.V.; Assani, M.Z.; et al. The Interplay of Cardiometabolic Syndrome Phenotypes and Cardiovascular Risk Indices in Patients Diagnosed with Diabetes Mellitus. Int. J. Mol. Sci. 2025, 26, 6227. [Google Scholar] [CrossRef]
- Dang, K.; Wang, X.; Hu, J.; Zhang, Y.; Cheng, L.; Qi, X.; Liu, L.; Ming, Z.; Tao, X.; Li, Y. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003-2018. Cardiovasc. Diabetol. 2024, 23, 8. [Google Scholar] [CrossRef]
- Kim, J.; Jeon, M.; Paeng, K.J.; Paeng, I.R. Competitive enzyme-linked immunosorbent assay for the determination of catecholamine, dopamine in serum. Anal. Chim. Acta 2008, 619, 87–93. [Google Scholar] [CrossRef]
- Available online: https://weldonbiotech.com/wp-content/uploads/2018/04/CAT-E-75e2.pdf (accessed on 25 September 2025).
- Sotnikov, D.V.; Zherdev, A.V.; Zvereva, E.A.; Eremin, S.A.; Dzantiev, B.B. Changing Cross-Reactivity for Different Immunoassays Using the Same Antibodies: Theoretical Description and Experimental Confirmation. Appl. Sci. 2021, 11, 6581. [Google Scholar] [CrossRef]
| Parameters | PreDM Group (n = 40) | T2DM Group (n = 70) | p-Value |
|---|---|---|---|
| Age (yrs) (mean ± SD) | 56.18 ± 12.09 | 63.87 ± 11.02 | 0.002 |
| Gender, Female/Male (n) | 18/22 | 33/37 | 1.000 |
| Area of residence, Rural/Urban (n) | 19/21 | 30/40 | 0.786 |
| Hypertension, n (%) | 34 (85.00%) | 47 (67.14%) | 0.069 |
| Dyslipidemia, n (%) | 31 (77.50%) | 47 (67.14%) | 0.351 |
| Hepatosteatosis, n (%) | 26 (65.00%) | 51 (72.86%) | 0.516 |
| SBP (mmHg) (mean ± SD) | 134.32 ± 16.50 | 135.00 ± 16.12 | 0.792 |
| DBP (mmHg) (mean ± SD) | 79.51 ± 13.04 | 78.91 ± 13.10 | 0.812 |
| Anthropometric characteristics | |||
| BMI (kg/m2) (mean ± SD) | 30.57 ± 8.41 | 29.32 ± 4.47 | 0.384 |
| BMI category (n) | |||
| Normal (18.5–24.9 kg/m2) | 14 | 18 | |
| Overweight (25–29.9 kg/m2) | 11 | 25 | |
| Obese (≥30 kg/m2) | 15 | 27 | |
| Height (m) [median (range)] | 1.60 (1.5–1.88) | 1.65 (1.48–1.89) | 0.222 |
| Weight (kg) [median (range)] | 73.25 (50.0–140.5) | 79.00 (62.0–137.0) | 0.285 |
| WC (cm) (mean ± SD) | 97.25 ± 14.35 | 96.11 ± 15.54 | 0.576 |
| HC (cm) [median (range)] | 105.50 (82.0–147.0) | 106.00 (89.0–141.0) | 0.893 |
| WHR (mean ± SD) | 0.90 ± 0.08 | 0.90 ± 0.12 | 0.791 |
| WHtR (mean ± SD) | 0.61 ± 0.09 | 0.58 ± 0.09 | 0.188 |
| Glycemic metabolism | |||
| FPG (mg/dL) [median (range)] | 103.50 (56.0–121.0) | 143.00 (106.0–273.0) | <0.0001 |
| 2hPG (mg/dL) [median (range)] | 151.50 (141.0–196.0) | 248.00 (129.0–475.0) | <0.0001 |
| HbA1c (%) (mean ± SD) | 5.97 ± 0.22 | 9.04 ± 2.16 | <0.0001 |
| Lipid profile and derived indices | |||
| TC (mg/dL) [median (range)] | 177.50 (94.0–377.0) | 199.00 (84.0–464.57) | 0.213 |
| TG (mg/dL) [median (range)] | 118.00 (44.0–387.0) | 118.50 (45.0–345.0) | 0.986 |
| TyG index [median (range)] | 8.64 (7.73–9.89) | 8.97 (7.84–10.77) | 0.001 |
| TyG-BMI (mean ± SD) | 266.40 ± 77.56 | 268.60 ± 49.31 | 0.128 |
| TyG-WC (mean ± SD) | 840.80± 123.00 | 878.10 ± 153.40 | 0.038 |
| TyG-WHtR (mean ± SD) | 5.23 ± 0.76 | 5.30 ± 0.92 | 0.102 |
| TG/HDL-c [median (range)] | 2.42 (0.59–5.56) | 2.88 (0.65–15.22) | 0.117 |
| LDL-C (mg/dL) [median (range)] | 113.10 (35.60–368.00) | 96.80 (41.00–296.00) | 0.434 |
| HDL-C (mg/dL) (mean ± SD) | 51.22 ± 12.42 | 45.03 ± 14.978 | 0.022 |
| Renal and hematological function | |||
| eGFR (mL/min/1.73 m2) MDRD (mean ± SD) | 70.96 ± 26.08 | 93.52 ± 30.43 | <0.0001 |
| CREA [median (range)] | 0.87 (0.56–1.75) | 0.74 (0.42–2.35) | 0.0557 * |
| Hb (g/dL) (mean ± SD) | 13.38 ± 2.05 | 13.18 ± 1.92 | 0.520 |
| WBC (×103/μL) [median (range)] | 7.21 (5.27–12.66) | 8.62 (4.21–25.36) | 0.0577 * |
| Parameter | PreDM (n = 40) | T2DM (n = 70) | p-Value |
|---|---|---|---|
| DA (pg/mL) mean ± SD median (range) | 445.17 ± 222.45 461.74 (110.07–930.39) | 955.10 ± 458.32 865.20 (135.68–2587.00) | <0.0001 |
| ST (ng/mL) mean ± SD median (range) | 327.24 ± 164.73 285.70 (142.90–713.40) | 957.86 ± 305.84 987.30 (297.40–1720.00) | <0.0001 |
| NE (pg/mL) mean ± SD median (range) | 3.56 ± 1.87 2.93 (1.07–11.33) | 11.70 ± 5.32 10.88 (2.73–25.53) | <0.0001 |
| EPI (pg/mL) mean ± SD median (range) | 467.17 ± 157.43 476.90 (127.60–816.10) | 910.23 ± 400.43 991.00 (121.40–1482.00) | <0.0001 |
| Parameters | PreDM | T2DM | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal (n = 14) | Overweight (n = 11) | Obese (n = 15) | p | Normal (n = 18) | Overweight (n = 25) | Obese (n = 27) | p | |
| DA (pg/mL) mean ± SD median (range) | 497.30 ± 226.66 | 429.88 ± 238.90 | 407.72 ± 212.06 | 0.387 | 956.84 ± 458.29 | 902.18 ± 457.69 | 1002.90 ± 470.84 | 0.814 |
| 557.84 121.97–20.12 | 456.80 110.07–930.39 | 295.62 129.25–671.60 | 900.28 469.61–2587.14 | 765.65 135.68–1977.70 | 985.21 262.47–2059.50 | |||
| NE (pg/mL) mean ± SD median (range) | 3.70 ± 1.53 | 3.89 ± 2.81 | 3.20 ± 1.30 | 0.797 | 12.45 ± 5.24 | 10.67 ± 4.23 | 12.03 ± 6.26 | 0.481 |
| 3.12 1.07–6.22 | 2.867 1.14–11.33 | 2.91 1.21–5.45 | 11.30 4.85–25.51 | 10.80 4.35–20.00 | 9.62 2.73–25.53 | |||
| EPI (pg/mL) mean ± SD median (range) | 453.33 ± 158.77 | 459.05 ± 204.37 | 510.05 ± 117.53 | 0.406 | 947.04 ± 412.02 | 898.41 ± 441.75 | 896.64 ± 364.88 | 0.792 |
| 463.98 127.61–64.29 | 446.69 142.89–816.07 | 507.10 212.08–706.63 | 1010.20 121.38–1437.20 | 987.26 127.21–1482.40 | 962.78 132.84–1447.20 | |||
| ST (ng/mL) mean ± SD median (range) | 279.46 ± 147.26 | 296.95 ± 176.25 | 394.06 ± 159.63 | 0.078 | 939.11 ± 338.42 | 949.73 ± 334.26 | 977.87 ± 263.63 | 0.772 |
| 225.30 142.89–53.57 | 229.30 149.12–713.38 | 344.64 226.05–664.29 | 968.15 444.64–1719.80 | 967.62 297.41–1573.30 | 994.97 444.64–1377.70 | |||
| Parameters | PreDM | T2DM | |||||
|---|---|---|---|---|---|---|---|
| Male (n = 18) | Female (n = 22) | p-Value | Male (n = 37) | Female (n = 33) | p-Value | ||
| DA (pg/mL) | mean ± SD | 412.09 ± 207.05 | 472.23 ± 235.56 | 0.487 | 927.11 ± 518.71 | 986.49 ± 385.11 | 0.749 |
| median (range) | 295.62 134.47–834.31 | 501.47 110.07–930.39 | 839.22 135.68–2587.40 | 893.49 469.61–1977.10 | |||
| NE (pg/mL) | mean ± SD | 3.36 ± 1.44 | 3.73 ± 2.18 | 0.768 | 11.98 ± 5.20 | 11.37 ± 5.51 | 0.496 |
| median (range) | 2.92 1.07–6.22 | 2.95 1.21–11.33 | 11.27 4.35–25.53 | 9.00 2.73–25.53 | |||
| EPI (pg/mL) | mean ± SD | 490.76 ± 112.41 | 464.23 ± 188.32 | 0.579 | 826.17 ± 419.83 | 1004.48 ± 360.72 | 0.072 |
| median (range) | 491.35 199.60–706.63 | 474.07 127.61–816.07 | 984.92 121.38–1467.30 | 1070.40 132.84–1482.40 | |||
| ST (ng/mL) | mean ± SD | 364.55 ± 183.49 | 296.72 ± 144.82 | 0.156 | 958.27 ± 305.04 | 957.39 ± 311.48 | 0.862 |
| median (range) | 330.76 145.90–713.38 | 238.52 142.89–664.29 | 994.97 444.64–1522.30 | 949.04 297.41–1719.80 | |||
| PreDM Patients | ||||
|---|---|---|---|---|
| HbA1c Quartiles | Parameters | |||
| DA (pg/mL) | NE (pg/mL) | EPI (pg/mL) | ST (ng/mL) | |
| Q1 (5.70–5.80) (n = 11) mean ± SD median (range) | 543.20 ± 230.31 | 3.30 ± 1.60 | 572.20 ± 126.40 | 261.54 ± 151.04 |
| 556.67 227.49–930.39 | 2.89 1.07–6.22 | 550.86 417.87–816.07 | 212.08 145.90–662.42 | |
| Q2 (5.81–5.90) (n = 9) mean ± SD median (range) | 335.93 ± 160.76 | 3.69 ± 1.22 | 457.48 ± 173.09 | 390.62 ± 179.44 |
| 294.40 121.97–600.59 | 3.54 2.48–5.84 | 478.39 142.89–770.38 | 351.80 153.56–713.38 | |
| Q3 (5.91–6.15) (n = 10) mean ± SD median (range) | 406.38 ± 215.58 | 2.80 ± 1.44 | 474.48 ± 129.50 | 333.13 ± 170.90 |
| 373.39 129.25–671.60 | 2.88 1.14–5.45 | 461.82 212.08–636.02 | 273.70 158.96–664.29 | |
| Q4 (6.16–6.45) (n = 10) mean ± SD median (range) | 474.43 ± 244.42 | 4.50 ± 2.69 | 388.94 ± 161.75 | 336.58 ± 158.07 |
| 557.84 110.07–920.12 | 3.14 2.65–11.33 | 435.88 127.61–597.98 | 291.58 149.12–653.57 | |
| p-value | 0.240 | 0.398 | 0.125 | 0.250 |
| T2DM patients | ||||
| Q1 (6.51–7.20) (n = 19) mean ± SD median (range) | 820.65 ± 302.52 | 11.20 ± 4.86 | 952.70 ± 349.93 | 972.89 ± 295.44 |
| 829.41 135.68–1547.30 | 11.33 2.73–20.00 | 1006.40 273.95–1412.10 | 942.21 535.50–1477.70 | |
| Q2 (7.21–8.65) (n = 16) mean ± SD median (range) | 837.22 ± 412.00 | 9.19 ± 5.12 | 961.40 ± 381.57 | 1000.30 ± 336.12 |
| 739.45 135.68–1547.30 | 8.67 2.73–20.00 | 987.26 273.95–1412.10 | 1023.00 535.50–1477.70 | |
| Q3 (8.66–10.73) (n = 18) mean ± SD median (range) | 1020.30 ± 509.67 | 13.93 ± 5.32 | 922.21 ± 405.60 | 946.24 ± 346.67 |
| 952.67 323.91–2587.40 | 14.58 3.73–25.51 | 1050.40 139.07–1447.20 | 949.04 297.41–1719.80 | |
| Q4 (10.74–15.50) (n = 17) mean ± SD median (range) | 1147.30 ± 537.01 | 12.24 ± 5.29 | 801.92 ± 474.38 | 913.42 ± 259.38 |
| 1124.30 270.38–1977.70 | 11.27 6.10–25.53 | 984.92 121.38–1482.40 | 917.20 474.38–1344.00 | |
| p-value | 0.047 | 0.032 | 0.796 | 0.877 |
| Neurotransmitter (pg/mL) | Group 1 | Group 2 | Mean Difference | p-Value | 95% CI Lower | 95% CI Upper | Significant |
|---|---|---|---|---|---|---|---|
| DA | Q1 | Q2 | 16.568 | 0.999 | −383.742 | 416.878 | FALSE |
| DA | Q1 | Q3 | 199.639 | 0.531 | −188.411 | 587.688 | FALSE |
| DA | Q1 | Q4 | 326.635 | 0.138 | −67.232 | 720.502 | FALSE |
| DA | Q2 | Q3 | 183.071 | 0.635 | −222.291 | 588.432 | FALSE |
| DA | Q2 | Q4 | 310.067 | 0.203 | −100.867 | 721.001 | FALSE |
| DA | Q3 | Q4 | 126.996 | 0.836 | −272.004 | 525.996 | FALSE |
| NE | Q1 | Q2 | −2.003 | 0.662 | −6.607 | 2.601 | FALSE |
| NE | Q1 | Q3 | 2.735 | 0.378 | −1.729 | 7.198 | FALSE |
| NE | Q1 | Q4 | 1.046 | 0.929 | −3.484 | 5.576 | FALSE |
| NE | Q2 | Q3 | 4.737 | 0.045 | 0.075 | 9.400 | TRUE |
| NE | Q2 | Q4 | 3.049 | 0.332 | −1.678 | 7.775 | FALSE |
| NE | Q3 | Q4 | −1.689 | 0.767 | −6.278 | 2.901 | FALSE |
| Neurotransmitter | Predictor | Coefficient β | Standard Error | t-Value | p-Value |
|---|---|---|---|---|---|
| DA | C (Sex) [T.Male] | −24.44 | 76.644 | −0.319 | 0.7505 |
| DA | C (Status) [T.T2DM] | 335.09 | 111.618 | 3.002 | 0.0034 * |
| DA | HbA1c | 48.55 | 30.832 | 1.575 | 0.1185 |
| DA | BMI | 2.10 | 6.204 | 0.338 | 0.7363 |
| DA | Age | 4.77 | 3.386 | 1.409 | 0.1618 |
| DA | TC | 0.90 | 0.594 | 1.517 | 0.1324 |
| DA | TG | 0.08 | 1.423 | 0.057 | 0.9544 |
| DA | TyG | 4.76 | 197.190 | 0.024 | 0.9808 |
| EPI | C (Sex) [T.Male] | −127.11 | 67.453 | −1.884 | 0.0624 ** |
| EPI | C (Status) [T.T2DM] | 502.08 | 98.234 | 5.111 | <0.001 * |
| EPI | HbA1c | −26.68 | 27.135 | −0.983 | 0.3278 |
| EPI | BMI | 1.05 | 5.460 | 0.193 | 0.8473 |
| EPI | Age | 1.76 | 2.980 | 0.592 | 0.5552 |
| EPI | TC | −0.21 | 0.523 | −0.399 | 0.6904 |
| EPI | TG | 0.47 | 1.253 | 0.373 | 0.7100 |
| EPI | TyG | −24.72 | 173.544 | −0.142 | 0.8870 |
| NE | C (Sex) [T.Male] | 0.38 | 0.895 | 0.426 | 0.6707 |
| NE | C (Status) [T.T2DM] | 6.51 | 1.303 | 4.997 | <0.001 * |
| NE | HbA1c | 0.58 | 0.360 | 1.610 | 0.1106 |
| NE | BMI | −0.01 | 0.072 | −0.109 | 0.9136 |
| NE | Age | 0.03 | 0.040 | 0.868 | 0.3876 |
| NE | TC | 0.00 | 0.007 | 0.583 | 0.5609 |
| NE | TG | −0.01 | 0.017 | −0.416 | 0.6780 |
| NE | TyG | −0.54 | 2.302 | −0.234 | 0.8152 |
| ST | C (Sex) [T.Male] | −15.82 | 54.256 | −0.291 | 0.7713 |
| ST | C (Status) [T.T2DM] | 699.18 | 79.014 | 8.849 | <0.001 * |
| ST | HbA1c | −12.76 | 21.826 | −0.585 | 0.5601 |
| ST | BMI | 4.57 | 4.392 | 1.042 | 0.3001 |
| ST | Age | −1.32 | 2.397 | −0.551 | 0.5831 |
| ST | TC | 0.16 | 0.420 | 0.381 | 0.7042 |
| ST | TG | 0.05 | 1.008 | 0.051 | 0.9594 |
| ST | TyG | −27.03 | 139.589 | −0.194 | 0.8469 |
| Predictor | Coefficient (β) | OR Per 1 SD | Direction of Effect |
|---|---|---|---|
| z_ST | 2.164 | 8.703 | Increased odds |
| z_NE | 1.325 | 3.763 | Increased odds |
| z_HbA1c | 1.146 | 3.145 | Increased odds |
| z_EPI | 0.958 | 2.607 | Increased odds |
| z_DA | 0.322 | 1.380 | Increased odds |
| z_Age | 0.301 | 1.351 | Increased odds |
| z_BMI | <0.001 | 1.000 | No effect |
| z_TG | <0.001 | 1.000 | No effect |
| z_TyG | <0.001 | 1.000 | No effect |
| Sex_Male | <0.001 | 1.000 | No effect |
| z_TC | −0.108 | 0.897 | Decreased odds |
| Neurotransmitter | Variable | Spearman-Rho | p-Value | N | q-Value |
|---|---|---|---|---|---|
| DA | HbA1c | −0.134 | 0.409 | 40 | 0.999 |
| DA | TC | −0.091 | 0.575 | 40 | 1.000 |
| DA | TG | 0.100 | 0.538 | 40 | 1.000 |
| DA | TyG | 0.080 | 0.622 | 40 | 1.000 |
| DA | TyG-BMI | −0.048 | 0.768 | 40 | 1.000 |
| DA | TyG-WC | 0.000 | 0.999 | 40 | 1.000 |
| DA | TyG-WHtR | 0.092 | 0.570 | 40 | 1.000 |
| DA | TG/HDL-C | 0.138 | 0.396 | 40 | 0.997 |
| DA | LDL-C | −0.055 | 0.736 | 40 | 1.000 |
| DA | HDL-C | 0.062 | 0.702 | 40 | 1.000 |
| NE | HbA1c | 0.137 | 0.398 | 40 | 0.999 |
| NE | TC | −0.024 | 0.882 | 40 | 1.000 |
| NE | TG | −0.139 | 0.393 | 40 | 0.995 |
| NE | TyG | −0.005 | 0.974 | 40 | 1.000 |
| NE | TyG-BMI | −0.129 | 0.428 | 40 | 1.000 |
| NE | TyG-WC | 0.141 | 0.386 | 40 | 0.970 |
| NE | TyG-WHtR | 0.085 | 0.602 | 40 | 1.000 |
| NE | TG/HDL-C | −0.065 | 0.692 | 40 | 1.000 |
| NE | LDL-C | 0.006 | 0.971 | 40 | 1.000 |
| NE | HDL-C | 0.021 | 0.898 | 40 | 1.000 |
| EPI | HbA1c | −0.348 | 0.028 | 40 | 0.928 |
| EPI | TC | −0.036 | 0.825 | 40 | 1.000 |
| EPI | TG | −0.166 | 0.307 | 40 | 0.957 |
| EPI | TyG | −0.173 | 0.286 | 40 | 0.954 |
| EPI | TyG-BMI | 0.174 | 0.282 | 40 | 0.949 |
| EPI | TyG-WC | −0.110 | 0.498 | 40 | 1.000 |
| EPI | TyG-WHtR | −0.047 | 0.771 | 40 | 1.000 |
| EPI | TG/HDL-C | −0.074 | 0.650 | 40 | 1.000 |
| EPI | LDL-C | 0.014 | 0.930 | 40 | 1.000 |
| EPI | HDL-C | −0.236 | 0.143 | 40 | 0.936 |
| ST | HbA1c | 0.078 | 0.632 | 40 | 1.000 |
| ST | TC | 0.162 | 0.317 | 40 | 0.960 |
| ST | TG | 0.092 | 0.572 | 40 | 1.000 |
| ST | TyG | 0.106 | 0.516 | 40 | 1.000 |
| ST | TyG-BMI | 0.312 | 0.050 | 40 | 0.935 |
| ST | TyG-WC | −0.149 | 0.360 | 40 | 0.963 |
| ST | TyG-WHtR | −0.163 | 0.315 | 40 | 0.958 |
| ST | TG/HDL-C | −0.103 | 0.529 | 40 | 1.000 |
| ST | LDL-C | 0.128 | 0.432 | 40 | 1.000 |
| ST | HDL-C | 0.179 | 0.268 | 40 | 0.936 |
| Neurotransmitter | Variable | Spearman-ho | p-Value | N | q-Value |
|---|---|---|---|---|---|
| DA | HbA1c | 0.269 | 0.024 | 70 | 0.199 |
| DA | TC | 0.290 | 0.015 | 70 | 0.176 |
| DA | TG | 0.187 | 0.121 | 70 | 0.689 |
| DA | TyG | 0.180 | 0.136 | 70 | 0.758 |
| DA | TyG-BMI | 0.135 | 0.265 | 70 | 0.884 |
| DA | TyG-WC | 0.035 | 0.772 | 70 | 0.997 |
| DA | TyG-WHtR | 0.051 | 0.675 | 70 | 0.996 |
| DA | TG/HDL-C | 0.191 | 0.114 | 70 | 0.681 |
| DA | LDL-C | 0.288 | 0.016 | 70 | 0.195 |
| DA | HDL-C | −0.060 | 0.622 | 70 | 0.987 |
| NE | HbA1c | 0.152 | 0.209 | 70 | 0.772 |
| NE | TC | 0.067 | 0.581 | 70 | 0.984 |
| NE | TG | −0.055 | 0.652 | 70 | 0.993 |
| NE | TyG | 0.019 | 0.873 | 70 | 1.000 |
| NE | TyG-BMI | −0.008 | 0.946 | 70 | 1.000 |
| NE | TyG-WC | 0.031 | 0.798 | 70 | 1.000 |
| NE | TyG-WHtR | 0.025 | 0.837 | 70 | 1.000 |
| NE | TG/HDL-C | −0.032 | 0.795 | 70 | 0.998 |
| NE | LDL-C | 0.091 | 0.454 | 70 | 0.964 |
| NE | HDL-C | 0.035 | 0.771 | 70 | 0.996 |
| EPI | HbA1c | −0.123 | 0.310 | 70 | 0.936 |
| EPI | TC | −0.085 | 0.482 | 70 | 0.970 |
| EPI | TG | −0.014 | 0.907 | 70 | 1.000 |
| EPI | TyG | −0.028 | 0.816 | 70 | 1.000 |
| EPI | TyG-BMI | −0.157 | 0.193 | 70 | 0.772 |
| EPI | TyG-WC | −0.365 | 0.002 | 70 | 0.076 |
| EPI | TyG-WHtR | −0.311 | 0.009 | 70 | 0.158 |
| EPI | TG/HDL-C | 0.084 | 0.491 | 70 | 0.976 |
| EPI | LDL-C | −0.086 | 0.479 | 70 | 0.967 |
| EPI | HDL-C | −0.164 | 0.174 | 70 | 0.760 |
| ST | HbA1c | −0.067 | 0.582 | 70 | 0.985 |
| ST | TC | −0.017 | 0.886 | 70 | 1.000 |
| ST | TG | −0.096 | 0.428 | 70 | 0.946 |
| ST | TyG | −0.091 | 0.454 | 70 | 0.964 |
| ST | TyG-BMI | 0.004 | 0.976 | 70 | 1.000 |
| ST | TyG-WC | 0.078 | 0.524 | 70 | 0.980 |
| ST | TyG-WHtR | 0.056 | 0.646 | 70 | 0.989 |
| ST | TG/HDL-C | −0.124 | 0.308 | 70 | 0.884 |
| ST | LDL-C | 0.006 | 0.963 | 70 | 1.000 |
| ST | HDL-C | 0.095 | 0.433 | 70 | 0.952 |
| Analyte | Catalog No. | Detection Range | Sensitivity | Intra-/Inter-Assay CV (%) | Reported Cross-Reactivity |
|---|---|---|---|---|---|
| Dopamine | E-EL-0046 | 31.25–2000 pg/mL | 18.75 pg/mL | <10 | No significant |
| Norepinephrine | E-EL-0047 | 0.31–20 ng/mL | 0.19 ng/mL | <10 | No significant |
| Epinephrine | E-EL-0045 | 31.25–2000 pg/mL | 18.75 pg/mL | <10 | No significant |
| Serotonin | E-EL-0033 | 15.63–1000 ng/mL | 9.38 ng/mL | <10 | No significant |
| Calibration Curves | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DA | EPI | ST | NE | ||||||
| pg/mL | OD | OD | ng/mL | OD | pg/mL | OD | |||
| STD 1 | 2000 | 0.169 | 0.126 | STD 1 | 1000 | 0.107 | STD 1 | 20 | 0.096 |
| STD 2 | 1000 | 0.338 | 0.299 | STD 2 | 500 | 0.280 | STD 2 | 10 | 0.195 |
| STD 3 | 500 | 0.510 | 0.647 | STD 3 | 250 | 0.501 | STD 3 | 5 | 0.333 |
| STD 4 | 250 | 0.822 | 0.932 | STD 4 | 125 | 0.968 | STD 4 | 2.5 | 0.756 |
| STD 5 | 125 | 1.430 | 1.984 | STD 5 | 62.5 | 1.817 | STD 5 | 1.25 | 1.316 |
| STD 6 | 62.5 | 2.062 | 2.272 | STD 6 | 31.25 | 2.370 | STD 6 | 0.63 | 2.557 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahrițculesei, R.V.; Boldeanu, L.; Assani, M.-Z.; Mitrea, A.; Obleaga, C.V.; Vladu, I.M.; Clenciu, D.; Boldeanu, M.V.; Vere, C.C. Neurotransmitter Levels (Dopamine, Epinephrine, Norepinephrine, Serotonin) and Associations with Lipid Profiles in Patients with Prediabetes or Newly Diagnosed Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2025, 26, 10068. https://doi.org/10.3390/ijms262010068
Ahrițculesei RV, Boldeanu L, Assani M-Z, Mitrea A, Obleaga CV, Vladu IM, Clenciu D, Boldeanu MV, Vere CC. Neurotransmitter Levels (Dopamine, Epinephrine, Norepinephrine, Serotonin) and Associations with Lipid Profiles in Patients with Prediabetes or Newly Diagnosed Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2025; 26(20):10068. https://doi.org/10.3390/ijms262010068
Chicago/Turabian StyleAhrițculesei, Roxana Viorela, Lidia Boldeanu, Mohamed-Zakaria Assani, Adina Mitrea, Cosmin Vasile Obleaga, Ionela Mihaela Vladu, Diana Clenciu, Mihail Virgil Boldeanu, and Cristin Constantin Vere. 2025. "Neurotransmitter Levels (Dopamine, Epinephrine, Norepinephrine, Serotonin) and Associations with Lipid Profiles in Patients with Prediabetes or Newly Diagnosed Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 26, no. 20: 10068. https://doi.org/10.3390/ijms262010068
APA StyleAhrițculesei, R. V., Boldeanu, L., Assani, M.-Z., Mitrea, A., Obleaga, C. V., Vladu, I. M., Clenciu, D., Boldeanu, M. V., & Vere, C. C. (2025). Neurotransmitter Levels (Dopamine, Epinephrine, Norepinephrine, Serotonin) and Associations with Lipid Profiles in Patients with Prediabetes or Newly Diagnosed Type 2 Diabetes Mellitus. International Journal of Molecular Sciences, 26(20), 10068. https://doi.org/10.3390/ijms262010068









