Unusual Dual Brain Tumor Morphologies in an MEN1 Patient: A Case Report of Diagnostic Challenges and Methylation Insights
Abstract
1. Introduction
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakker, R.V. Multiple endocrine neoplasia type 1. Indian J. Endocrinol. Metab. 2012, 16, S272–S274. [Google Scholar] [CrossRef]
- Hu, X.; Guan, J.; Wang, Y.; Shi, S.; Song, C.; Li, Z.P.; Feng, S.T.; Chen, J.; Luo, Y. A narrative review of multiple endocrine neoplasia syndromes: Genetics, clinical features, imaging findings, and diagnosis. Ann. Transl. Med. 2021, 9, 944. [Google Scholar] [CrossRef]
- Caimari, F.; Korbonits, M. Novel genetic causes of pituitary adenomas. Clin. Cancer Res. 2016, 22, 5030–5042. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, H.B.; Frederiksen, A.L.; Pedersen, I.S.; Ettrup, M.S.; Wirenfeldt, M.; Boldt, H.; Nguyen, N.; Andersen, M.S.; Bjarkam, C.R.; Poulsen, F.R. PDP type brain tumor in association with multiple endocrine neoplasia type 1. Heliyon 2024, 10, e27418. [Google Scholar] [CrossRef] [PubMed]
- Syro, L.V.; Scheithauer, B.W.; Kovacs, K.; Toledo, R.A.; Londoño, F.J.; Ortiz, L.D.; Rotondo, F.; Horvath, E.; Uribe, H. Pituitary tumors in patients with MEN1 syndrome. Clinics 2012, 67, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Johann, P.D.; Erkek, S.; Zapatka, M.; Kerl, K.; Buchhalter, I.; Hovestadt, V.; Jones, D.T.W.; Sturm, D.; Hermann, C.; Segura Wang, M.; et al. Atypical Teratoid/Rhabdoid Tumors Are Comprised of Three Epigenetic Subgroups with Distinct Enhancer Landscapes. Cancer Cell 2016, 29, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Agarwal, S.K.; Perrier, N.D.; Lines, K.E.; Valk, G.D.; Thakker, R.V. Multiple Endocrine Neoplasia Type 1: Latest Insights. Endocr. Rev. 2020, 42, 133–170. [Google Scholar] [CrossRef] [PubMed]
- Graillon, T.; Romanet, P.; Camilla, C.; Gélin, C.; Appay, R.; Roche, C.; Lagarde, A.; Mougel, G.; Farah, K.; Le Bras, M.; et al. A cohort study of CNS tumors in Multiple Endocrine Neoplasia Type 1. Clin. Cancer Res. 2024, 30, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Pierotti, L.; Pardi, E.; Dinoi, E.; Piaggi, P.; Borsari, S.; Della Valentina, S.; Sardella, C.; Michelucci, A.; Caligo, M.A.; Bogazzi, F.; et al. Cutaneous lesions and other non-endocrine manifestations of Multiple Endocrine Neoplasia type 1 syndrome. Front. Endocrinol. 2023, 14, 1191040. [Google Scholar] [CrossRef] [PubMed]
- Broggi, G.; Gianno, F.; Shemy, D.T.; Massimino, M.; Milanaccio, C.; Mastronuzzi, A.; Rossi, S.; Arcella, A.; Giangaspero, F.; Antonelli, M. Atypical teratoid/rhabdoid tumor in adults: A systematic review of the literature with meta-analysis and additional reports of 4 cases. J. Neuro-Oncol. 2022, 157, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Coronado, K.W.; Zohdy, Y.M.; Maldonado, J.; Pradilla, G.; Garzon-Muvdi, T. Sellar atypical teratoid/rhabdoid tumor in adults: Survival analysis of treatment strategies. Illustrative case. J. Neurosurg. Case Lessons 2023, 6, CASE23287. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rovira, M.A.; Connor, M.; Osorio, R.C.; Russler-Germain, E.; Schmidt, R.; Johnson, G.W.; Silverstein, J.; Dahiya, S.; Farrell, N.F.; Weiss, M.C.; et al. Case report: Molecular characterization of adult atypical teratoid rhabdoid tumor and review of the literature. Front. Oncol. 2025, 15, 1510439. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Al-Kofide, A.; Siddiqui, K.; Alhindi, H.; Alshaikh, N.; Alshail, E. Atypical teratoid rhabdoid tumors (ATRT): King Faisal specialist hospital and research centre experience. Int. J. Pediatr. Adolesc. Med. 2021, 8, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, H.; Huang, A. Atypical Teratoid Rhabdoid Tumors. In Oncology of CNS Tumors; Springer: Cham, Switzerland, 2019; pp. 615–629. [Google Scholar]
- Ostrom, Q.T.; Chen, Y.; M. de Blank, P.; Ondracek, A.; Farah, P.; Gittleman, H.; Wolinsky, Y.; Kruchko, C.; Cohen, M.L.; Brat, D.J.; et al. The descriptive epidemiology of atypical teratoid/rhabdoid tumors in the United States, 2001–2010. Neuro-Oncol. 2014, 16, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Calandrelli, R.; Massimi, L.; Pilato, F.; Verdolotti, T.; Ruggiero, A.; Attinà, G.; Gessi, M.; Colosimo, C. Atypical Teratoid Rhabdoid Tumor: Proposal of a Diagnostic Pathway Based on Clinical Features and Neuroimaging Findings. Diagnostics 2023, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Zin, F.; Cotter, J.A.; Haberler, C.; Dottermusch, M.; Neumann, J.; Schüller, U.; Schweizer, L.; Thomas, C.; Nemes, K.; Johann, P.D.; et al. Histopathological patterns in atypical teratoid/rhabdoid tumors are related to molecular subgroup. Brain Pathol. 2021, 31, e12967. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.; Johann, P.D.; Grabovska, Y.; De Dieu Andrianteranagna, M.J.; Yao, F.; Frühwald, M.; Hasselblatt, M.; Bourdeaut, F.; Williamson, D.; Huang, A.; et al. Molecular subgrouping of atypical teratoid/rhabdoid tumors-a reinvestigation and current consensus. Neuro-Oncol. 2020, 22, 613–624. [Google Scholar] [CrossRef] [PubMed]

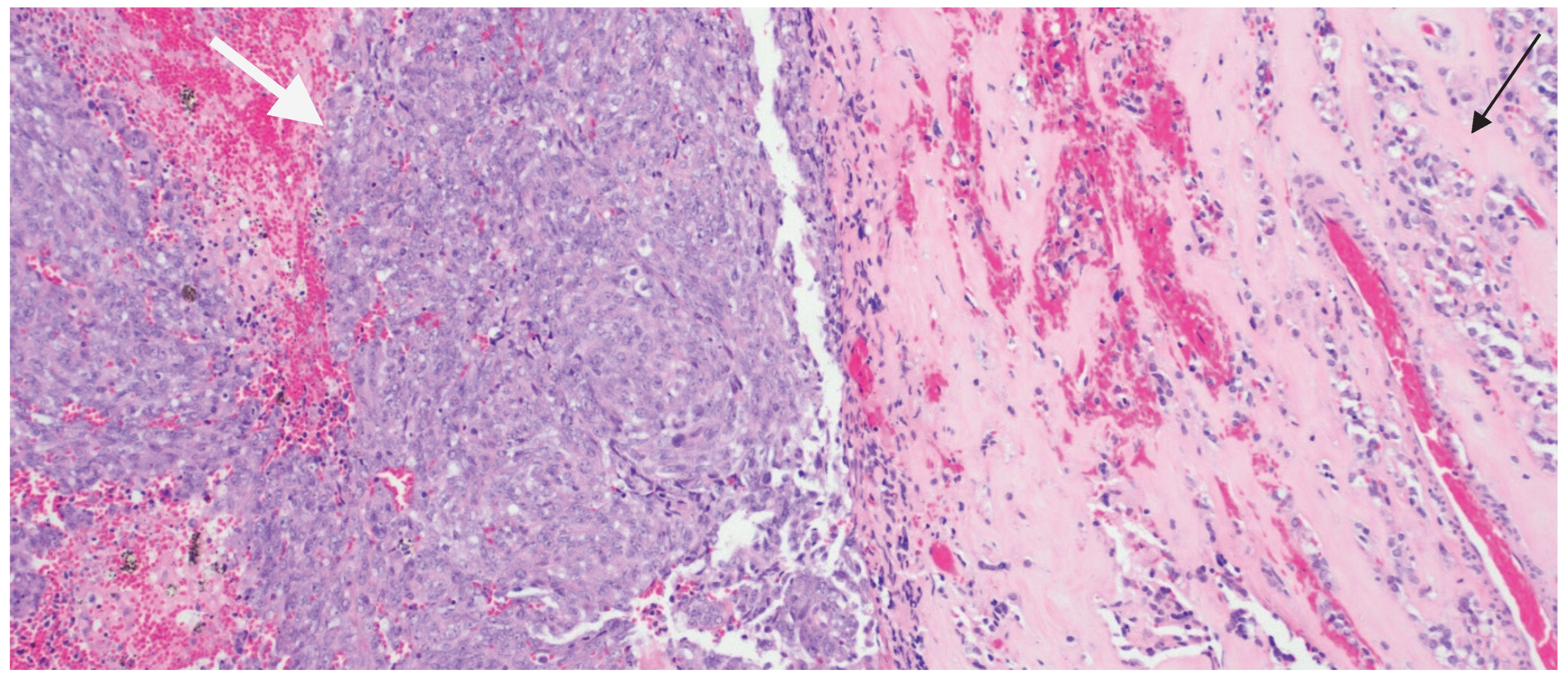

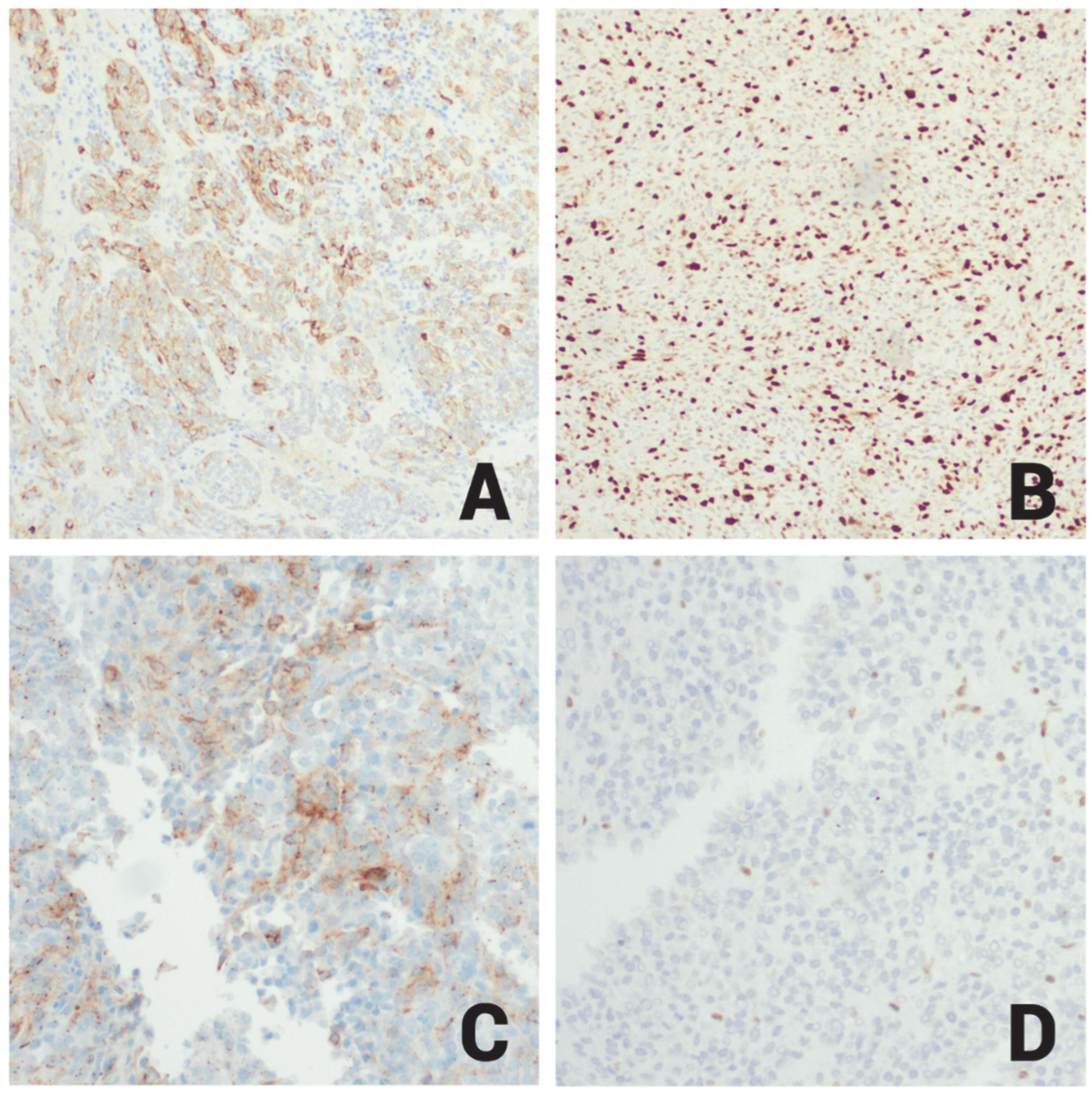
| Immunohistochemical Workup | Result | Notes |
|---|---|---|
| CK7, CK20, CK5/6, P40, D2-40, P63, BAP1, Desmin, Myogenin, STAT6, ER, PR, SOX10, S100, MiTF, Melan A, HMB45 | Negative | — |
| BRAF V600E, Synaptophysin, INSM1, TPIT, SF-1, LH, FSH, TSH, GH, ACTH | Negative | |
| AE1/AE3, CAM5.2, H3K27ME3 | Negative | |
| PIT1, Prolactin | Positive | PIT1 shown in Figure 3B |
| EMA | Focal Positive | EMA shown in Figure 4A |
| P53 | Positive (20%) | — |
| Ki-67 | High (60%) | High proliferative index (Figure 4B) |
| SSTR2 | Patchy Positive | Shown in Figure 4C |
| INI-1 | Positive | Loss of nuclear INI1 expression in ATRT cells (mutant); Figure 4D |
| E-cadherin | Retained | — |
| Reticulin stain | Positive | Highlights loss of reticulin investment in adenoma cells; |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, V.; Aboud, O.; Barakat, A. Unusual Dual Brain Tumor Morphologies in an MEN1 Patient: A Case Report of Diagnostic Challenges and Methylation Insights. Int. J. Mol. Sci. 2025, 26, 10065. https://doi.org/10.3390/ijms262010065
Patel V, Aboud O, Barakat A. Unusual Dual Brain Tumor Morphologies in an MEN1 Patient: A Case Report of Diagnostic Challenges and Methylation Insights. International Journal of Molecular Sciences. 2025; 26(20):10065. https://doi.org/10.3390/ijms262010065
Chicago/Turabian StylePatel, Viharkumar, Orwa Aboud, and Abdelrahman Barakat. 2025. "Unusual Dual Brain Tumor Morphologies in an MEN1 Patient: A Case Report of Diagnostic Challenges and Methylation Insights" International Journal of Molecular Sciences 26, no. 20: 10065. https://doi.org/10.3390/ijms262010065
APA StylePatel, V., Aboud, O., & Barakat, A. (2025). Unusual Dual Brain Tumor Morphologies in an MEN1 Patient: A Case Report of Diagnostic Challenges and Methylation Insights. International Journal of Molecular Sciences, 26(20), 10065. https://doi.org/10.3390/ijms262010065






