Expression of Neurotrophin Genes in the Hypothalamus of Stressed and Allopregnanolone-Infused Sheep
Abstract
1. Introduction
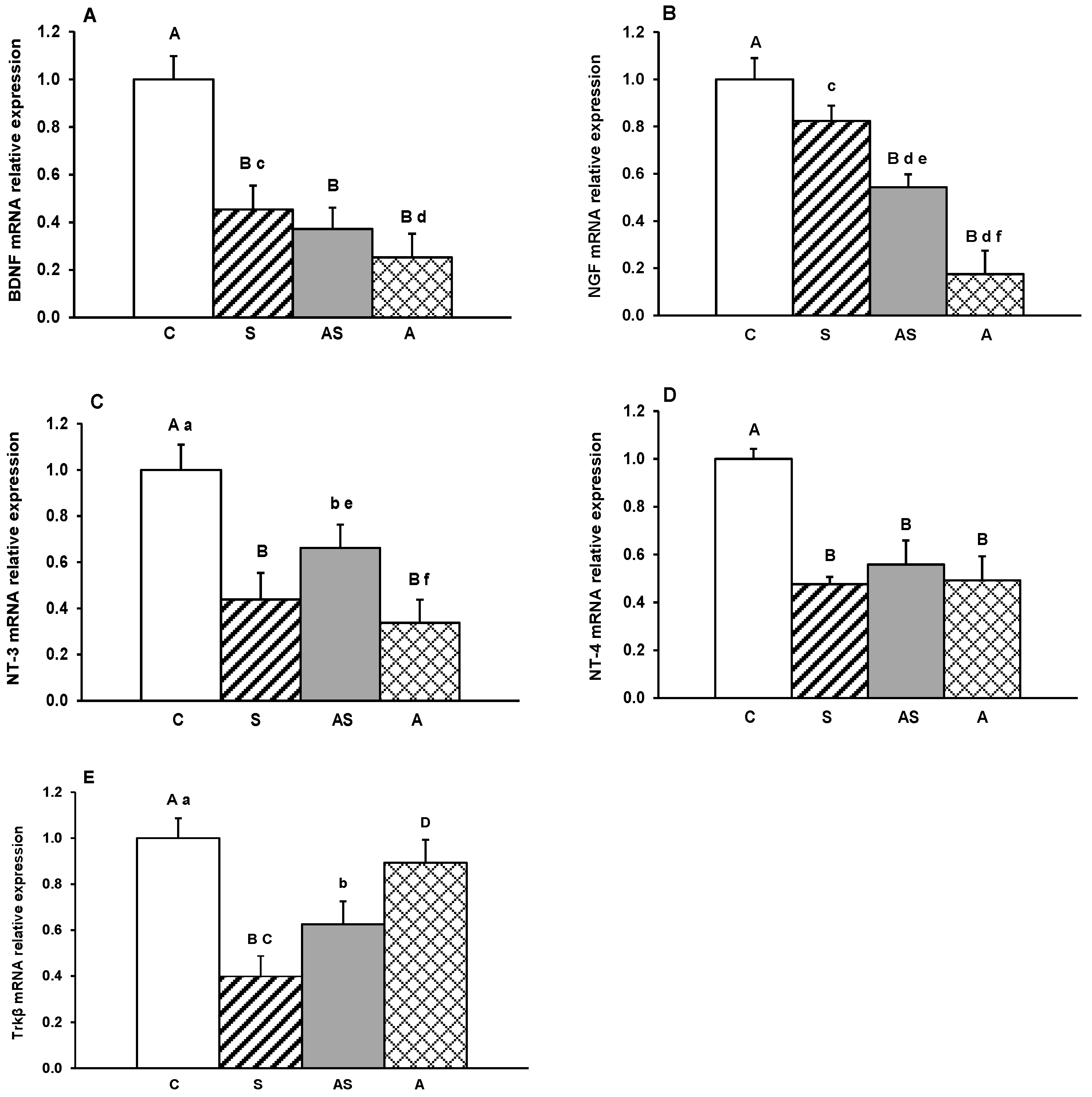
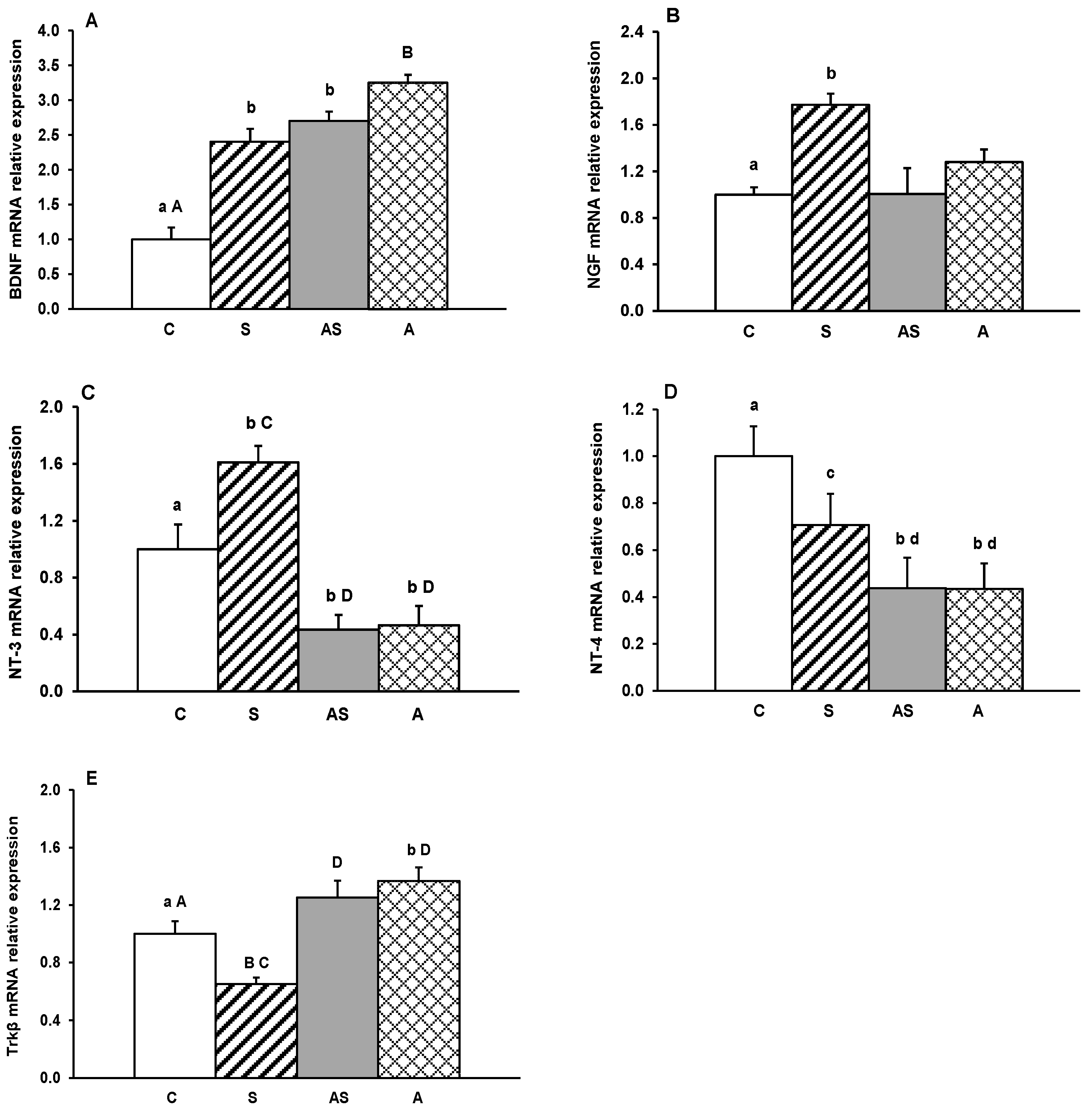
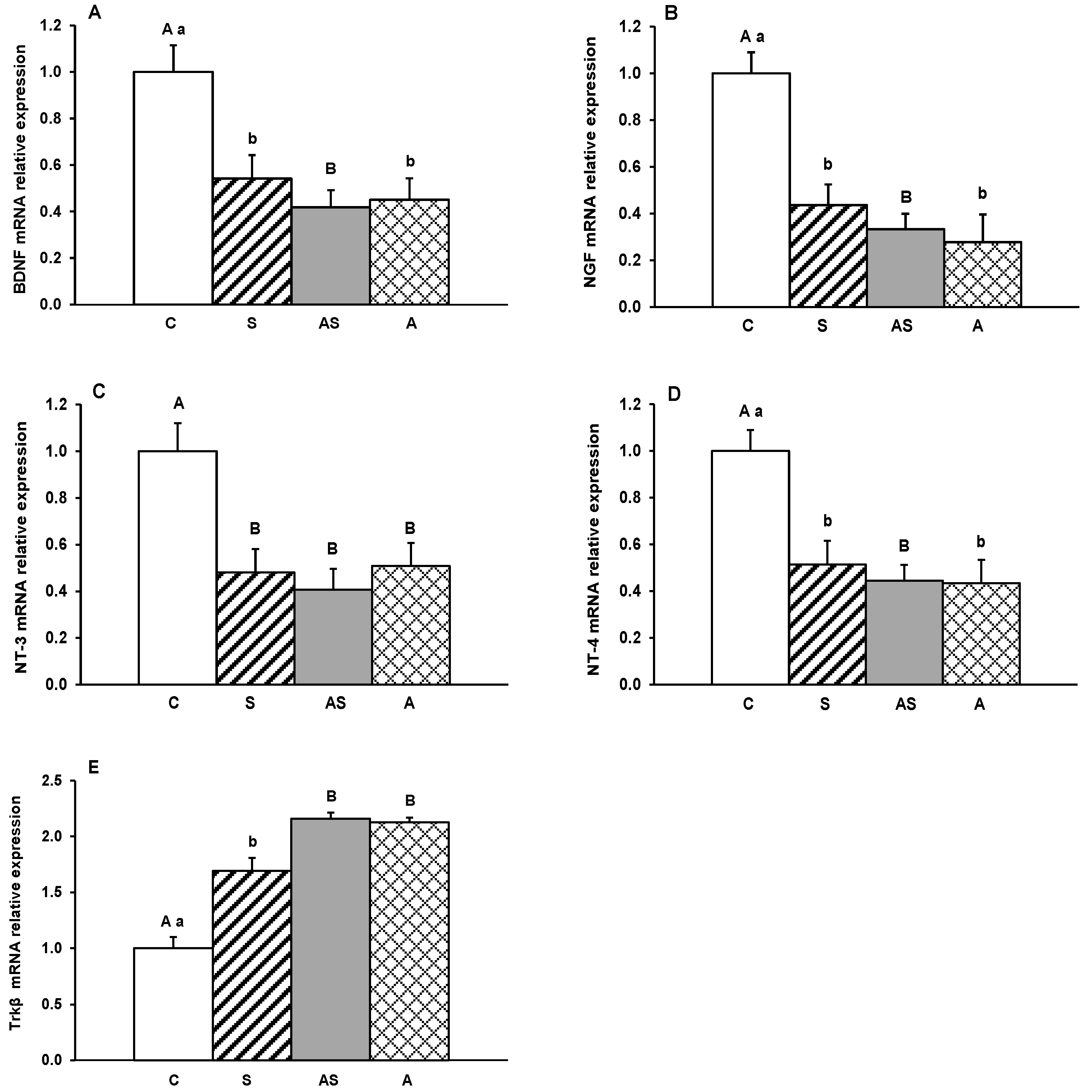
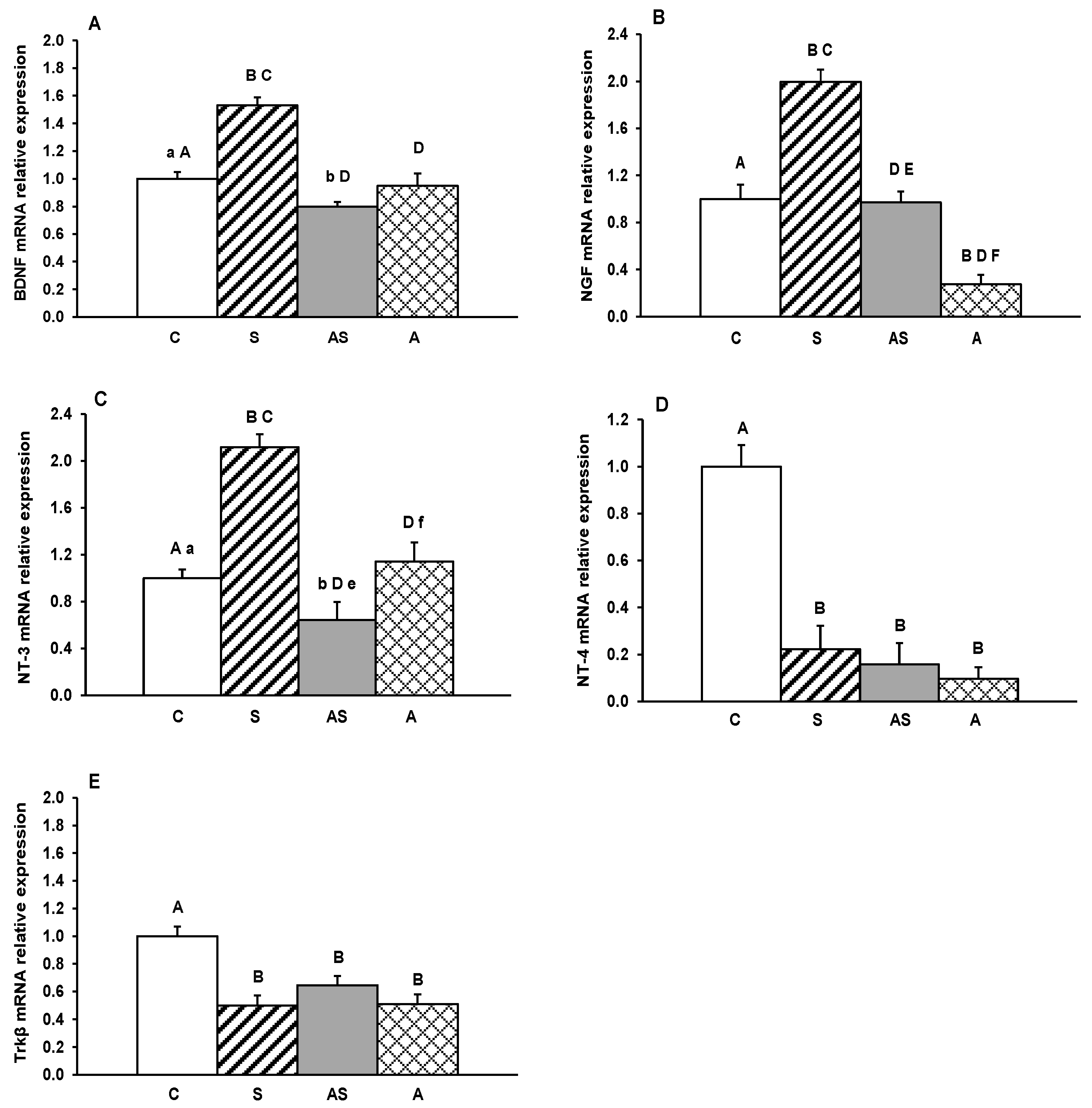
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Management
4.2. Brain Surgery and Experimental Design
4.3. Brain Tissue Collection
4.4. Total RNA Isolation, cDNA Synthesis and Quantitative Real-Time Polymerase Chain Reaction Analysis
4.5. Statistical Analyses
5. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartkowska, K.; Turlejski, K.; Djavadian, R.L. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol. Exp. 2010, 70, 454–467. [Google Scholar] [CrossRef]
- Hernández-del Caño, C.; Varela-Andrés, N.; Cebrián-León, A.; Deogracias, R. Neurotrophins and Their Receptors: BDNF’s Role in GABAergic Neurodevelopment and Disease. Int. J. Mol. Sci. 2024, 25, 8312. [Google Scholar] [CrossRef]
- Vilar, M.; Mira, H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front. Neurosci. 2016, 10, 26. [Google Scholar] [CrossRef]
- Accogli, A.; Addour-Boudrahem, N.; Srour, M. Neurogenesis, neuronal migration, and axon guidance. Handb. Clin. Neurol. 2020, 173, 25–42. [Google Scholar]
- Migaud, M.; Batailler, M.; Segura, S.; Duittoz, A.; Franceschini, I.; Pillon, D. Emerging new sites for adult neurogenesis in the mammalian brain: A comparative study between the hypothalamus and the classical neurogenic zones. Eur. J. Neurosci. 2010, 32, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Bedont, J.L.; Pak, T.; Wang, H.; Song, J.; Miranda-Angulo, A.; Takiar, V.; Charubhumi, V.; Balordi, F.; Takebayashi, H.; et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 2012, 15, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Blackshaw, S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol. 2018, 170, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Fitzsimons, C.P.; Lucassen, P.J. Neurogenesis in the adult hypothalamus: A distinct form of structural plasticity involved in metabolic and circadian regulation, with potential relevance for human pathophysiology. Handb. Clin. Neurol. 2021, 179, 125–140. [Google Scholar]
- Autry, A.E. Function of brain-derived neurotrophic factor in the hypothalamus: Implications for depression pathology. Front. Mol. Neurosci. 2022, 15, 1028223. [Google Scholar] [CrossRef]
- Casarotto, P.; Umemori, J.; Castrén, E. BDNF receptor TrkB as the mediator of the antidepressant drug action. Front. Mol. Neurosci. 2022, 15, 1032224. [Google Scholar] [CrossRef]
- Nicholson, E.L.; Garry, M.I.; Ney, L.J.; Hsu, C.-M.K.; Zuj, D.V.; Felmingham, K.L. The influence of the BDNF Val66Met genotype on emotional recognition memory in post-traumatic stress disorder. Sci. Rep. 2023, 13, 5033. [Google Scholar] [CrossRef]
- Suliman, S.; Hemmings, S.M.J.; Seedat, S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: Systematic review and meta-regression analysis. Front. Integr. Neurosci. 2013, 7, 55. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions. Pharmaceutics 2023, 15, 2081. [Google Scholar] [CrossRef]
- Mondal, A.C.; Fatima, M. Direct and indirect evidences of BDNF and NGF as key modulators in depression: Role of antidepressants treatment. Int. J. Neurosci. 2019, 129, 283–296. [Google Scholar] [CrossRef]
- Overstreet, D.H.; Fredericks, K.; Knapp, D.; Breese, G.; McMichael, J. Nerve growth factor (NGF) has novel antidepressant-like properties in rats. Pharmacol. Biochem. Behav. 2010, 94, 553–560. [Google Scholar] [CrossRef]
- McGeary, J.E.; Gurel, V.; Knopik, V.S.; Spaulding, J.; McMichael, J. Effects of nerve growth factor (NGF), fluoxetine, and amitriptyline on gene expression profiles in rat brain. Neuropeptides 2011, 45, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Monteggia, L.M. Brain-Derived neurotrophic factor signaling in depression and antidepressant action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Guilloux, J.P.; Douillard-Guilloux, G.; Kota, R.; Wang, X.; Gardier, A.M.; Martinowich, K.; Tseng, G.C.; Lewis, D.A.; Sibille, E. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 2012, 17, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y. Involvement of brain-derived neurotrophic factor in late-life depression. Am. J. Geriatr. Psychiatry 2013, 21, 433–449. [Google Scholar] [CrossRef]
- Ray, M.T.; Shannon Weickert, C.; Webster, M.J. Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Transl. Psychiatry 2014, 4, e389. [Google Scholar] [CrossRef]
- Dwivedi, Y. Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsychiatr. Dis. Treat. 2009, 5, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Tripp, A.; Oh, H.; Guilloux, J.P.; Martinowich, K.; Lewis, D.A.; Sibille, E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am. J. Psychiatry 2012, 169, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.M.; Underwood, M.D.; Huang, Y.-Y.; Hsiung, S.-C.; Liu, Y.; Simpson, N.R.; Bakalian, M.J.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; et al. Association of BDNF Val66Met polymorphism and brain BDNF levels with major depression and suicide. Int. J. Neuropsychopharmacol. 2018, 21, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Badowska-Szalewska, E.; Ludkiewicz, B.; Krawczyk, R.; Moryś, J. Exposure to mild stress and brain derived neurotrophin factor (BDNF) immunoreactivity in the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei: Comparison between aged and adult rats. J. Chem. Neuroanat. 2016, 78, 57–64. [Google Scholar] [CrossRef]
- Jeanneteau, F.D.; Lambert, W.M.; Ismaili, N.; Bath, K.G.; Lee, F.S.; Garabedian, M.J.; Chao, M.V. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc. Natl. Acad. Sci. USA 2012, 109, 1305–1310. [Google Scholar] [CrossRef]
- Naert, G.; Maurice, T.; Tapia-Arancibia, L.; Givalois, L. Neuroactive steroids modulate HPA axis activity and cerebral brain-derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology 2007, 32, 1062–1078. [Google Scholar] [CrossRef]
- Badowska-Szalewska, E.; Krawczyk, R.; Ludkiewicz, B.; Moryś, J. The effect of mild stress stimulation on the nerve growth factor (NGF) and tyrosine kinase receptor A (TrkA) immunoreactivity in the paraventricular nucleus (PVN) of the hypothalamus and hippocampus in aged vs. adult rats. Neuroscience 2015, 290, 346–356. [Google Scholar] [CrossRef]
- Diviccaro, S.; Cioffi, L.; Falvo, E.; Giatti, S.; Melcangi, R.C. Allopregnanolone: An overview on its synthesis and effects. J. Neuroendocrinol. 2022, 34, e12996. [Google Scholar] [CrossRef]
- Almeida, F.B.; Nin, M.S.; Barros, H.M.T. The role of allopregnanolone in depressive-like behaviors: Focus on neurotrophic proteins. Neurobiol. Stress 2020, 12, 100218. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Panzica, G.C. Allopregnanolone: State of the art. Prog. Neurobiol. 2014, 113, 1–5. [Google Scholar] [CrossRef]
- Sze, Y.; Brunton, P.J. Neurosteroids and early-life programming: An updated perspective. Curr. Opin. Endocr. Metab. Res. 2022, 25, 100367. [Google Scholar] [CrossRef]
- Infantes-López, M.I.; Nieto-Quero, A.; Chaves-Peña, P.; Zambrana-Infantes, E.; Cifuentes, M.; Márquez, J.; Pedraza, C.; Pérez-Martín, M. New insights into hypothalamic neurogenesis disruption after acute and intense stress: Implications for microglia and inflammation. Front. Neurosci. 2023, 17, 1190418, Erratum in Front. Neurosci. 2023, 17, 1335034. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Brody, S.; Kanes, S.J. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol. Stress 2020, 12, 100212. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.B.; van den Pol, A.N. Neurotrophin-3 potentiates excitatory GABAergic synaptic transmission in cultured developing hypothalamic neurones of the rat. J. Physiol. 1999, 518, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, Y.; Yi, C.X.; Tong, Q.; Cai, D. The hypothalamus for whole-body physiology: From metabolism to aging. Protein Cell 2022, 13, 394–421. [Google Scholar] [CrossRef] [PubMed]
- Lévy, F.; Batailler, M.; Meurisse, M.; Migaud, M. Adult Neurogenesis in Sheep: Characterization and Contribution to Reproduction and Behavior. Front. Neurosci. 2017, 11, 570. [Google Scholar] [CrossRef]
- Clarke, I.J. Hypothalamus as an endocrine organ. Compr. Physiol. 2015, 5, 217–253. [Google Scholar] [CrossRef]
- Batailler, M.; Droguerre, M.; Baroncini, M.; Fontaine, C.; Prevot, V.; Migaud, M. DCX-expressing cells in the vicinity of the hypothalamic neurogenic niche: A comparative study between mouse, sheep, and human tissues. J. Comp. Neurol. 2014, 522, 1966–1985. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Tapia-Arancibia, L.; Rage, F.; Givalois, L.; Arancibia, S. Physiology of BDNF: Focus on hypothalamic function. Front. Neuroendocrinol. 2004, 25, 77–107. [Google Scholar] [CrossRef]
- Roszkowicz-Ostrowska, K.; Młotkowska, P.; Marciniak, E.; Szlis, M.; Barszcz, M.; Misztal, T. Activation of BDNF-TrkB Signaling in Specific Structures of the Sheep Brain by Kynurenic Acid. Cells 2024, 13, 1928. [Google Scholar] [PubMed]
- Misztal, T.; Młotkowska, P.; Marciniak, E.; Misztal, A. Allopregnanolone reduces neuroendocrine response to acute stressful stimuli in sheep. J. Endocrinol. 2020, 244, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Rage, F.; Givalois, L.; Marmigère, F.; Tapia-Arancibia, L.; Arancibia, S. Immobilization stress rapidly modulates BDNF mRNA expression in the hypothalamus of adult male rats. Neuroscience 2002, 112, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Marmigere, F.; Givalois, L.; Rage, F.; Arancibia, S.; Tapia-Arancibia, L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus 2003, 13, 646–655. [Google Scholar] [CrossRef]
- Givalois, L.; Naert, G.; Rage, F.; Ixart, G.; Arancibia, S.; Tapia-Arancibia, L. A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Mol. Cell. Neurosci. 2004, 27, 280–295. [Google Scholar] [CrossRef]
- Naert, G.; Ixart, G.; Maurice, T.; Tapia-Arancibia, L.; Givalois, L. Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol. Cell. Neurosci. 2011, 46, 55–66. [Google Scholar] [CrossRef]
- Misztal, T.; Młotkowska, P.; Marciniak, E.; Barszcz, M.; Osuch, B.; Gajewska, A.; Misztal, A. Effects of Stress and Allopregnanolone on the Expression of Neurotrophins and TrkB Receptor in the Sheep Hippocampus. Int. J. Mol. Sci. 2025, 26, 6190. [Google Scholar] [CrossRef]
- Shieh, P.B.; Hu, S.C.; Bobb, K.; Timmusk, T.; Ghosh, A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 1998, 20, 727–740. [Google Scholar] [CrossRef]
- Pruunsild, P.; Sepp, M.; Orav, E.; Koppel, I.; Timmusk, T. Identification of cis-Elements and Transcription Factors Regulating Neuronal Activity-Dependent Transcription of Human BDNF Gene. J. Neurosci. 2011, 31, 3295–3308. [Google Scholar] [CrossRef]
- Ortiz, J.B.; Anglin, J.M.; Daas, E.J.; Paode, P.R.; Nishimura, K.; Conrad, C.D. BDNF and TrkB Mediate the Improvement from Chronic Stress-induced Spatial Memory Deficits and CA3 Dendritic Retraction. Neuroscience 2018, 388, 330–346. [Google Scholar]
- Robinson, S.; Mogul, A.S.; Taylor-Yeremeeva, E.M.; Khan, A.; Tirabassi, A.D.; Wang, H.Y. Stress Diminishes BDNF-stimulated TrkB Signaling, TrkB-NMDA Receptor Linkage and Neuronal Activity in the Rat Brain. Neuroscience 2021, 473, 142–158, Erratum in Neuroscience 2022, 490, 316–319. [Google Scholar] [CrossRef]
- Berry, A.; Bindocci, E.; Alleva, E. NGF, brain and behavioral plasticity. Neural Plast. 2012, 2012, 784040. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Aloe, L.; Alleva, E.; De Simone, R.; Goedert, M.; Levi-Montalcini, R. Nerve growth factor mRNA and protein increase in hypothalamus in a mouse model of aggression. Proc. Natl. Acad. Sci. USA 1989, 86, 8555–8559. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995, 15 Pt 1, 1768–1777. [Google Scholar] [CrossRef]
- Saruta, J.; Iida, M.; Kondo, Y.; To, M.; Hayashi, T.; Hori, M.; Sato, S.; Tsukinoki, K. Chronic stress induces neurotrophin-3 in rat submandibular gland. Yonsei Med. J. 2012, 53, 1085–1092. [Google Scholar] [CrossRef]
- Yu, H.; Wang, D.D.; Wang, Y.; Liu, T.; Lee, F.S.; Chen, Z.Y. Variant BDNF Val66Met polymorphism affects stress response and vulnerability to anxiety and depression. Mol. Psychiatry 2012, 17, 396–408. [Google Scholar]
- Saruta, J.; Sato, S.; Tsukinoki, K. The role of neurotrophins related to stress in saliva and salivary glands. Histol. Histopathol. 2010, 25, 1317–1330. [Google Scholar]
- Giuliani, F.A.; Escudero, C.; Casas, S.; Bazzocchini, V.; Yunes, R.; Laconi, M.R.; Cabrera, R. Allopregnanolone and puberty: Modulatory effect on glutamate and GABA release and expression of 3α-hydroxysteroid oxidoreductase in the hypothalamus of female rats. Neuroscience 2013, 243, 64–75. [Google Scholar] [CrossRef]
- Almeida, F.B.; Pinna, G.; Barros, H.M.T. The Role of HPA Axis and Allopregnanolone on the Neurobiology of Major Depressive Disorders and PTSD. Int. J. Mol. Sci. 2021, 22, 5495. [Google Scholar] [CrossRef]
- Bali, A.; Jaggi, A.S. Multifunctional aspects of allopregnanolone in stress and related disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 64–78. [Google Scholar] [CrossRef]
- Purdy, R.H.; Morrow, A.L.; Moore, P.H.; Paul, S.M. Stress-Induced Elevations of Gamma-Aminobutyric Acid Type A Receptor-Active Steroids in the Rat Brain. Proc. Natl. Acad. Sci. USA 1991, 88, 4553. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Sun, Y.; McGregor, A.; Connor, B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology 2012, 63, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.S.; Shao, S.H.; Yuan, B.P.; Pan, F.; Li, Z.L. Acute stress and chronic stress change brain-derived neurotrophic factor (BDNF) and tyrosine kinase-coupled receptor (TrkB) expression in both young and aged rat hippocampus. Yonsei Med. J. 2010, 51, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.P.; Poddighe, L.; Boi, M.; Sanna, F.; Piludu, M.A.; Sanna, F.; Corda, M.G.; Giorgi, O.; Quartu, M. Effect of Acute Stress on the Expression of BDNF, TrkB, and PSA-NCAM in the Hippocampus of the Roman Rats: A Genetic Model of Vulnerability/Resistance to Stress-Induced Depression. Int. J. Mol. Sci. 2018, 19, 3745. [Google Scholar] [CrossRef]
- Serra, M.P.; Sanna, F.; Boi, M.; Poddighe, L.; Secci, L.; Trucas, M.; Fernández-Teruel, A.; Corda, M.G.; Giorgi, O.; Quartu, M. Acute Stress Induces Different Changes on the Expression of BDNF and TrkB in the Mesocorticolimbic System of Two Lines of Rats Differing in Their Response to Stressors. Int. J. Mol. Sci. 2022, 23, 14995. [Google Scholar] [CrossRef]
- Shirayama, Y.; Fujita, Y.; Oda, Y.; Iwata, M.; Muneoka, K.; Hashimoto, K. Allopregnanolone induces antidepressant-like effects through BDNF-TrkB signaling independent from AMPA receptor activation in a rat learned helplessness model of depression. Behav. Brain Res. 2020, 390, 112670. [Google Scholar] [CrossRef]
- Strzetelski, J. IZ PIB–INRA Feeding Recommendations for Ruminants and Feed Tables; IZ PIB-INRA: Krakow, Poland, 2014. (In Polish) [Google Scholar]
- Welento, J.; Szteyn, S.; Milart, Z. Observations on the stereotaxic configuration of the hypothalamus nuclei in the sheep. Anat. Anz. 1969, 124, 1–27. [Google Scholar]
- Traczyk, W.; Przekop, F. Methods of investigation of the function of the hypothalamus and hypophysis in chronic experiments in sheep. Acta Physiol. Pol. 1963, 14, 217–226. [Google Scholar]
- Młotkowska, P.; Marciniak, E.; Roszkowicz-Ostrowska, K.; Misztal, T. Effects of allopregnanolone on central reproductive functions in sheep under natural and stressful conditions. Theriogenology 2020, 158, 138–147. [Google Scholar] [CrossRef]
- Misztal, T.; Hasiec, M.; Szlis, M.; Tomaszewska-Zaremba, D.; Marciniak, E. Stimulatory effect of dopamine derivative, salsolinol, on pulsatile luteinizing hormone secretion in seasonally anestrous sheep: Focus on dopamine, kisspeptin and gonadotropin-releasing hormone. Anim. Reprod. Sci. 2019, 208, 106102. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempflfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Murray, S.J.; Mitchell, N.L. The translational benefits of sheep as large animal models of human neurological disorders. Front. Vet. Sci. 2022, 9, 831838. [Google Scholar] [CrossRef]

| Gene | Primers (5′–3′) | Genbank Acc. No. | Amplicon Size |
|---|---|---|---|
| BDNF | F: CGTTGGCTGACACTTTTGAA R: CGCAGCATCCAGGTAATTTT | XM_012143442.1 | 188 |
| NGF | F: CAGTCCAAGGGGCTGGAT R: AGTGTGGCCAGGACAGAAAG | XM_004002369.5 | 101 |
| NT-3 | F: TGCCACGATCTTACAGGTGA R: TGCCTGGATCAGCTTGATTA | XM_004006944.5 | 151 |
| NT-4 | F: CCTGAGATGTCACGAAGGAC R: TGAACACCTGTCAGCACCTC | XM_027978595.3 | 112 |
| TRKβ | F: TGTCTGAGCTGATCCTGGTG R: TATCTGCAGGTTTGCCAGTG | XM_012117231.2 | 155 |
| GAPDH | F: GGGTCATCATCTCTGCACCT R: GGTCATAAGTCCCTCCACGA | NM_001190390.1 | 131 |
| PPIC | F: TGGAAAAGTCGTGCCCAAGA R: TGCTTATACCACCAGTGCCA | XM_004008676.1 | 158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młotkowska, P.; Osuch, B.; Marciniak, E.; Roszkowicz-Ostrowska, K.; Misztal, T. Expression of Neurotrophin Genes in the Hypothalamus of Stressed and Allopregnanolone-Infused Sheep. Int. J. Mol. Sci. 2025, 26, 10062. https://doi.org/10.3390/ijms262010062
Młotkowska P, Osuch B, Marciniak E, Roszkowicz-Ostrowska K, Misztal T. Expression of Neurotrophin Genes in the Hypothalamus of Stressed and Allopregnanolone-Infused Sheep. International Journal of Molecular Sciences. 2025; 26(20):10062. https://doi.org/10.3390/ijms262010062
Chicago/Turabian StyleMłotkowska, Patrycja, Bartosz Osuch, Elżbieta Marciniak, Katarzyna Roszkowicz-Ostrowska, and Tomasz Misztal. 2025. "Expression of Neurotrophin Genes in the Hypothalamus of Stressed and Allopregnanolone-Infused Sheep" International Journal of Molecular Sciences 26, no. 20: 10062. https://doi.org/10.3390/ijms262010062
APA StyleMłotkowska, P., Osuch, B., Marciniak, E., Roszkowicz-Ostrowska, K., & Misztal, T. (2025). Expression of Neurotrophin Genes in the Hypothalamus of Stressed and Allopregnanolone-Infused Sheep. International Journal of Molecular Sciences, 26(20), 10062. https://doi.org/10.3390/ijms262010062







