Effect of Estrogen on Sirt1 Signaling in Human Macrophages
Abstract
1. Introduction
2. Results
2.1. Male and Female Human Primary M1 Macrophages Express Estrogen Receptors
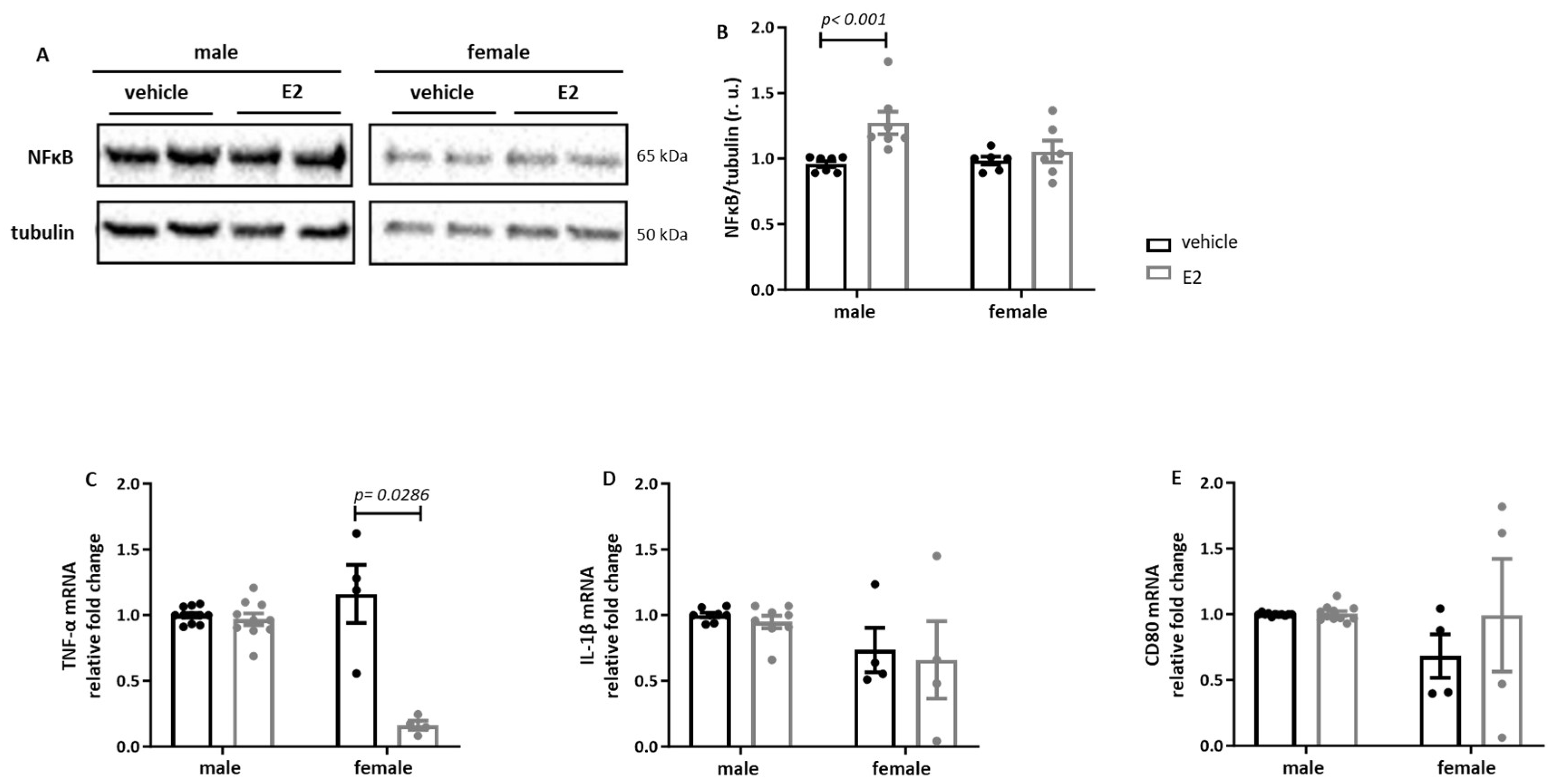
2.2. Sex Differences in the E2 Effects on Human Primary Pro-Inflammatory M1 Macrophages
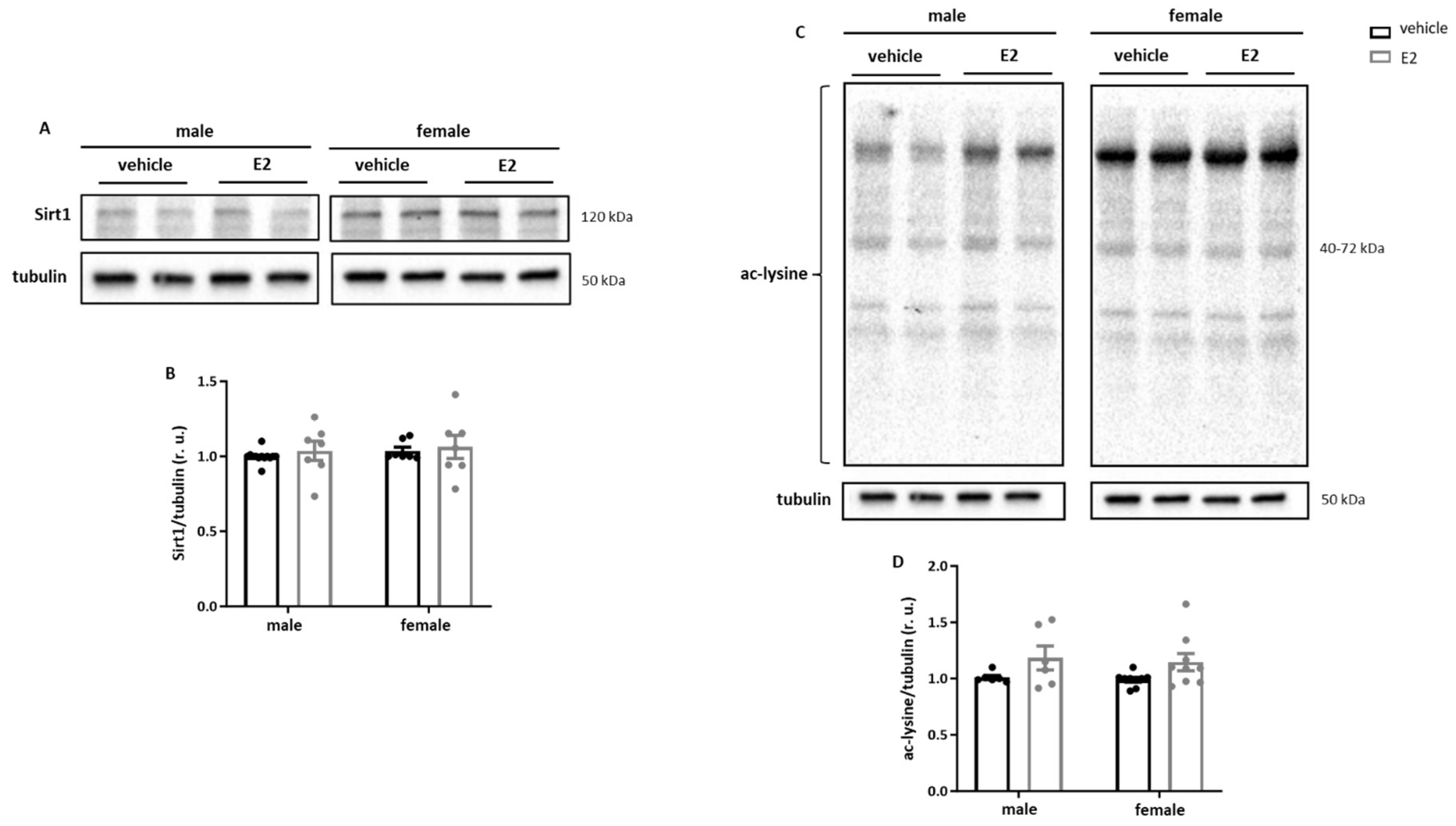
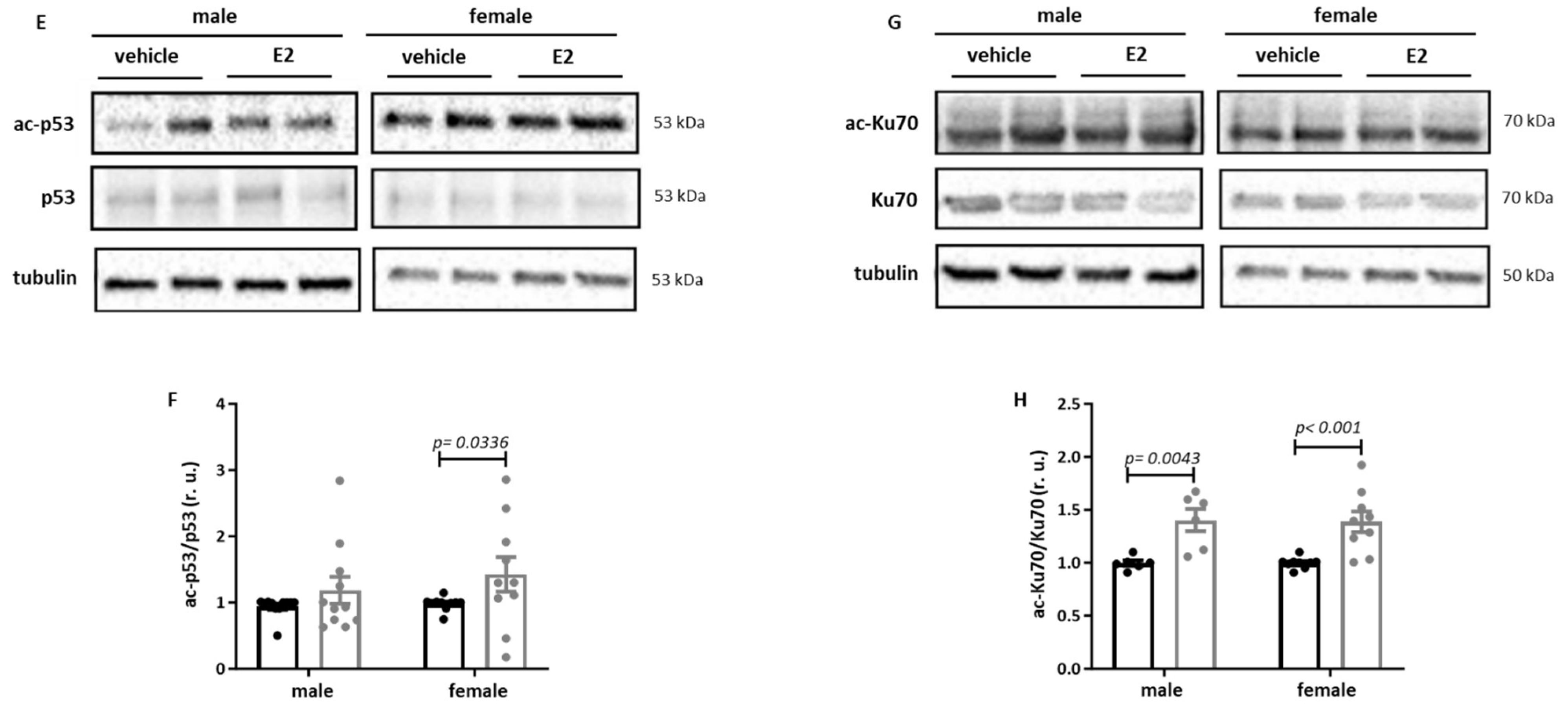
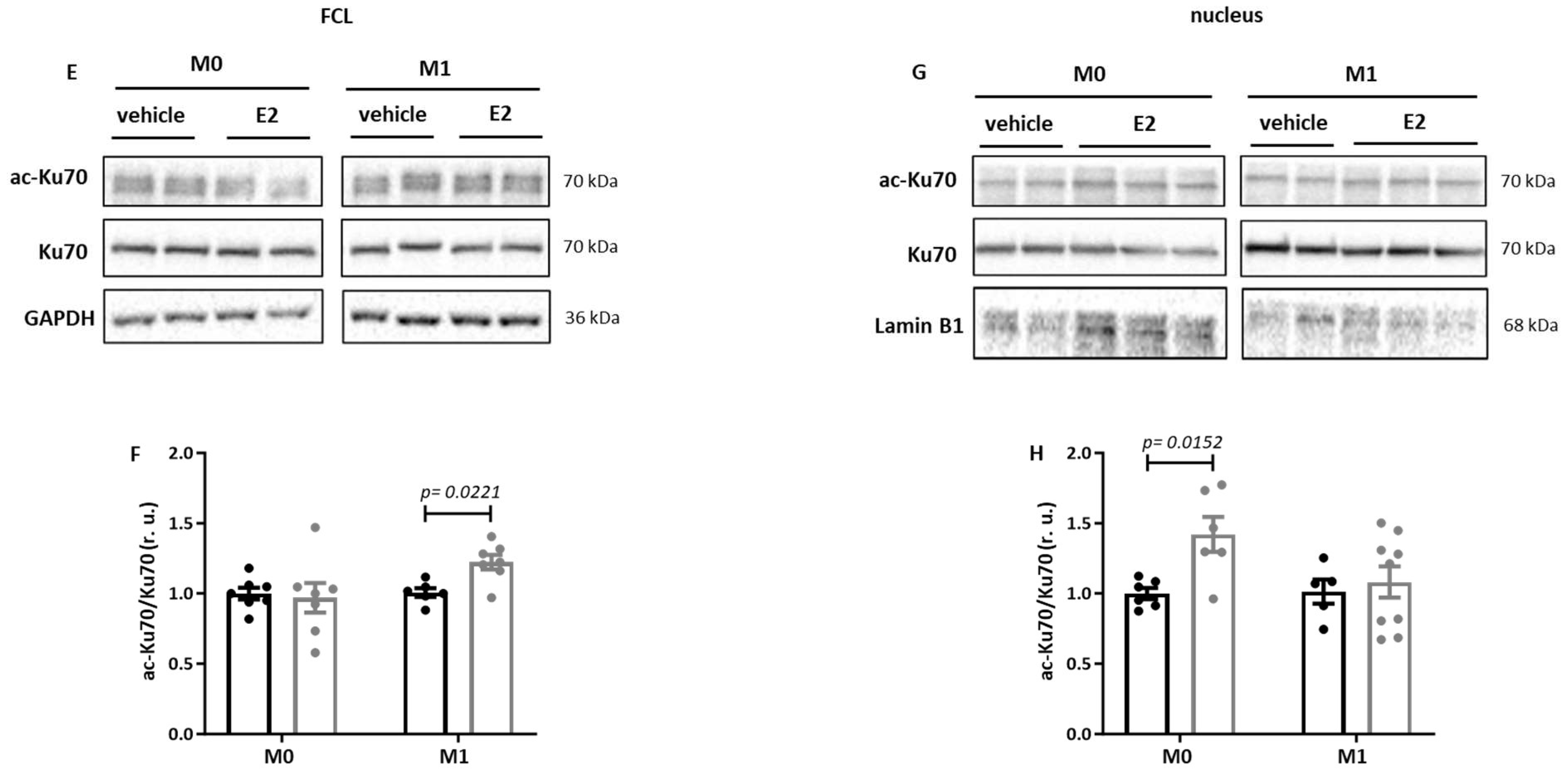
2.3. E2 Treatment Increases Acetylation of Sirt1 Targets in Primary Human M1 Macrophages
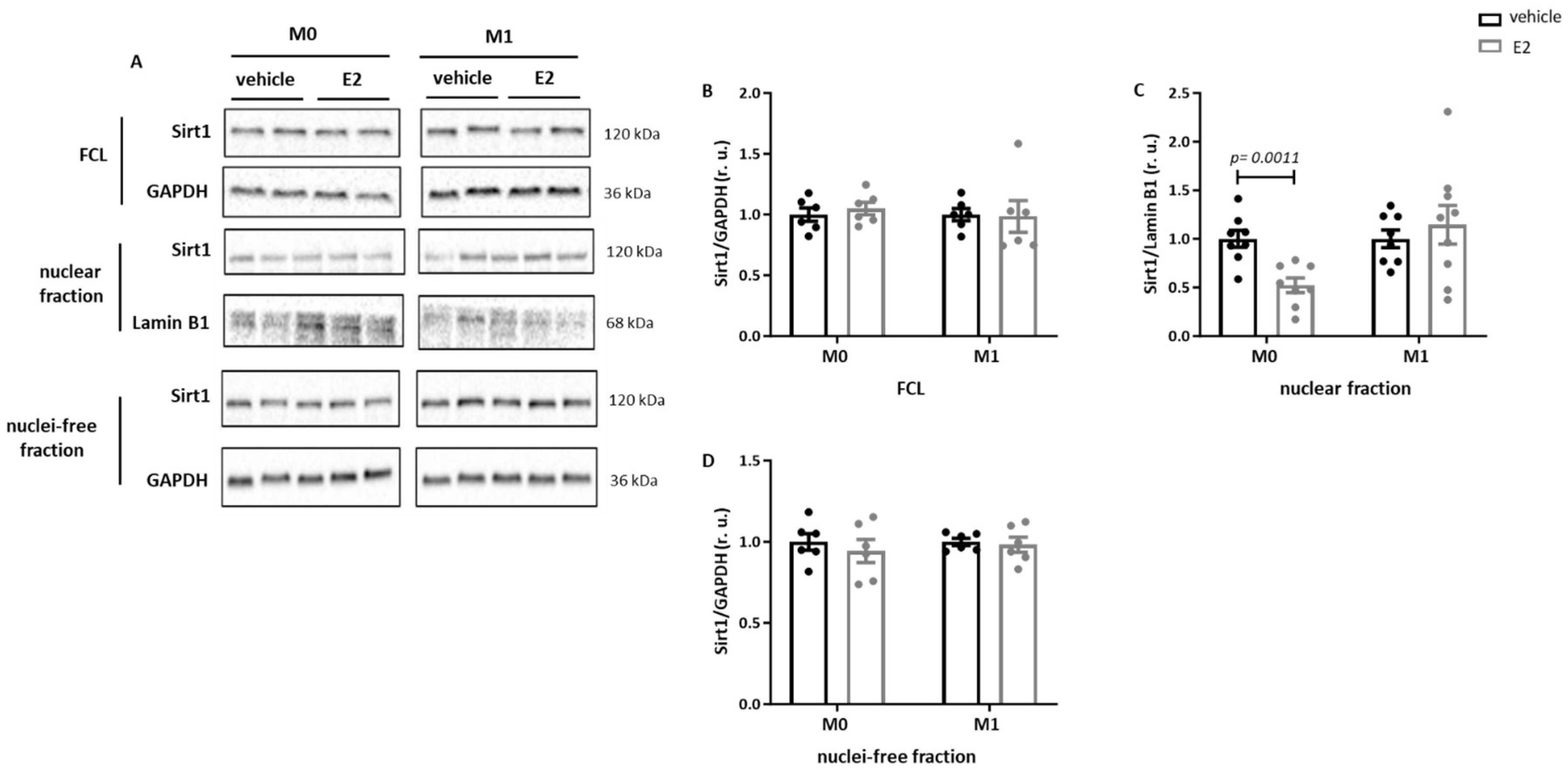
2.4. E2 Modulates the Nuclear Sirt1 Expression in Male Human M0 and M1 Monocyte-like Cells
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Isolation and Cultivation of Human Monocyte-Derived Macrophages
4.2. In Vitro Studies with Human Monocytic THP-1 Cells
4.3. Macrophage Polarization
4.4. Activation of ERs
4.5. RNA Isolation and Quantitative Real Time (RT)-PCR
4.6. Western Blot Analysis
4.7. Isolation of Pure Intact Nuclei
4.8. Flow Cytometry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef]
- Matthews, K.A.; Meilahn, E.; Kuller, L.H.; Kelsey, S.F.; Caggiula, A.W.; Wing, R.R. Menopause and risk factors for coronary heart disease. N. Engl. J. Med. 1989, 321, 641–646. [Google Scholar] [CrossRef]
- Huxley, R.; Barzi, F.; Woodward, M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. BMJ 2006, 332, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V. Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat. Rev. Drug Discov. 2006, 5, 425–438. [Google Scholar] [CrossRef]
- Fleury, M.A.; Annabi, M.S.; Voisine, M.; Hervault, M.; Boilard, A.J.; Shen, M.; Marette, A.; Cote, N.; Clavel, M.A. Impact of sex and sex hormones on pathophysiology and progression of aortic stenosis in a murine model. Physiol. Rep. 2022, 10, e15433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shoemaker, R.; Thatcher, S.E.; Batifoulier-Yiannikouris, F.; English, V.L.; Cassis, L.A. Administration of 17beta-estradiol to ovariectomized obese female mice reverses obesity-hypertension through an ACE2-dependent mechanism. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1066–E1075. [Google Scholar] [CrossRef]
- Yang, S.; Penna, V.; Lavine, K.J. Functional diversity of cardiac macrophages in health and disease. Nat. Rev. Cardiol. 2025, 22, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Park, M.D.; Silvin, A.; Ginhoux, F.; Merad, M. Macrophages in health and disease. Cell 2022, 185, 4259–4279. [Google Scholar] [CrossRef]
- Shiratori, H.; Feinweber, C.; Luckhardt, S.; Linke, B.; Resch, E.; Geisslinger, G.; Weigert, A.; Parnham, M.J. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol. Immunol. 2017, 88, 58–68. [Google Scholar] [CrossRef]
- Jaguin, M.; Houlbert, N.; Fardel, O.; Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell. Immunol. 2013, 281, 51–61. [Google Scholar] [CrossRef]
- Tang, Y.; Pan, W.; Ding, W.; Pan, X.; Zhu, J.; Chen, H.; Zhu, X.; Chen, J.; Cheng, Z.; Zhang, Y.; et al. Prostaglandin E2 alleviates inflammatory response and lung injury through EP4/cAMP/IKK/NF-kappaB pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167801. [Google Scholar] [CrossRef]
- Xu, X.J.; Reichner, J.S.; Mastrofrancesco, B.; Henry, W.L., Jr.; Albina, J.E. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J. Immunol. 2008, 180, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Guyre, P.M.; Pioli, P.A. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J. Immunol. 2010, 184, 5029–5037. [Google Scholar] [CrossRef]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Koditz, R.; Pfohl, M.; Schatz, H. Changes in proinflammatory cytokine activity after menopause. Endocr. Rev. 2002, 23, 90–119. [Google Scholar] [CrossRef] [PubMed]
- Pelekanou, V.; Kampa, M.; Kiagiadaki, F.; Deli, A.; Theodoropoulos, P.; Agrogiannis, G.; Patsouris, E.; Tsapis, A.; Castanas, E.; Notas, G. Estrogen anti-inflammatory activity on human monocytes is mediated through cross-talk between estrogen receptor ERalpha36 and GPR30/GPER1. J. Leukoc. Biol. 2016, 99, 333–347. [Google Scholar] [CrossRef]
- Barcena, M.L.; Niehues, M.H.; Christiansen, C.; Estepa, M.; Haritonow, N.; Sadighi, A.H.; Muller-Werdan, U.; Ladilov, Y.; Regitz-Zagrosek, V. Male Macrophages and Fibroblasts from C57/BL6J Mice Are More Susceptible to Inflammatory Stimuli. Front. Immunol. 2021, 12, 758767. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xin, Z.; Gao, S.; Li, Y.; Guo, S.; Fu, Y.; Xu, R.; Wang, D.; Cheng, J.; Liu, L.; et al. SIRT6-regulated macrophage efferocytosis epigenetically controls inflammation resolution of diabetic periodontitis. Theranostics 2023, 13, 231–249. [Google Scholar] [CrossRef]
- Smulan, L.J.; Martinez, N.; Kiritsy, M.C.; Kativhu, C.; Cavallo, K.; Sassetti, C.M.; Singhal, A.; Remold, H.G.; Kornfeld, H. Sirtuin 3 Downregulation in Mycobacterium tuberculosis-Infected Macrophages Reprograms Mitochondrial Metabolism and Promotes Cell Death. mBio 2021, 12, 1. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.W.; Lee, S.Y.; Hong, K.W.; Bae, S.S.; Kim, K.; Kim, C.D. SIRT1/Adenosine Monophosphate-Activated Protein Kinase alpha Signaling Enhances Macrophage Polarization to an Anti-inflammatory Phenotype in Rheumatoid Arthritis. Front. Immunol. 2017, 8, 1135. [Google Scholar] [CrossRef]
- Elangovan, S.; Ramachandran, S.; Venkatesan, N.; Ananth, S.; Gnana-Prakasam, J.P.; Martin, P.M.; Browning, D.D.; Schoenlein, P.V.; Prasad, P.D.; Ganapathy, V.; et al. SIRT1 is essential for oncogenic signaling by estrogen/estrogen receptor alpha in breast cancer. Cancer Res. 2011, 71, 6654–6664. [Google Scholar] [CrossRef]
- Yao, Y.; Li, H.; Gu, Y.; Davidson, N.E.; Zhou, Q. Inhibition of SIRT1 deacetylase suppresses estrogen receptor signaling. Carcinogenesis 2010, 31, 382–387. [Google Scholar] [CrossRef]
- Yao, Y.; Brodie, A.M.; Davidson, N.E.; Kensler, T.W.; Zhou, Q. Inhibition of estrogen signaling activates the NRF2 pathway in breast cancer. Breast Cancer Res. Treat. 2010, 124, 585–591. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ikeda, Y.; Miyauchi, T.; Uchikado, Y.; Akasaki, Y.; Ohishi, M. Estrogen-SIRT1 Axis Plays a Pivotal Role in Protecting Arteries Against Menopause-Induced Senescence and Atherosclerosis. J. Atheroscler. Thromb. 2020, 27, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Juhn, K.; Lee, H.; Kim, S.H.; Min, B.H.; Lee, K.M.; Cho, M.H.; Park, G.H.; Lee, K.H. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp. Mol. Med. 2007, 39, 8–13. [Google Scholar] [CrossRef]
- Barcena, M.L.; Christiansen-Mensch, C.; Aslam, M.; Haritonow, N.; Ladilov, Y.; Regitz-Zagrosek, V. Upregulation of Mitochondrial Sirt3 and Alleviation of the Inflammatory Phenotype in Macrophages by Estrogen. Cells 2024, 13, 1420. [Google Scholar] [CrossRef]
- Kim, S.C.; Boese, A.C.; Moore, M.H.; Cleland, R.M.; Chang, L.; Delafontaine, P.; Yin, K.J.; Lee, J.P.; Hamblin, M.H. Rapid estrogen receptor-alpha signaling mediated by ERK activation regulates vascular tone in male and ovary-intact female mice. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H330–H342. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef]
- Majeed, Y.; Halabi, N.; Madani, A.Y.; Engelke, R.; Bhagwat, A.M.; Abdesselem, H.; Agha, M.V.; Vakayil, M.; Courjaret, R.; Goswami, N.; et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci. Rep. 2021, 11, 8177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, Y.; Lu, Z.; Wang, B.; Li, L.; You, M.; Wang, L.; Cao, T.; Zhao, Y.; Li, Q.; et al. Mitochondrial dysfunction caused by SIRT3 inhibition drives proinflammatory macrophage polarization in obesity. Obesity 2023, 31, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Yan, W.; Chen, C.; Chen, H. Estrogen Downregulates miR-21 Expression and Induces Inflammatory Infiltration of Macrophages in Polymyositis: Role of CXCL10. Mol. Neurobiol. 2017, 54, 1631–1641. [Google Scholar] [CrossRef]
- Flake, N.M.; Bonebreak, D.B.; Gold, M.S. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J. Neurophysiol. 2005, 93, 1585–1597. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Q.; Chen, Q.; Lin, Y.; Sun, X.; Zhang, H.; Zhu, M.; Dong, S. The inflammation and estrogen metabolism impacts of polychlorinated biphenyls on endometrial cancer cells. Toxicol. Vitr. 2015, 29, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, J.; Miao, J.; Zhang, X.; Yan, Y.; Bai, L.; Chang, J.; Wang, Y.; Wang, L.; Bian, Y.; et al. Anti-inflammatory activity of the Tongmai Yangxin pill in the treatment of coronary heart disease is associated with estrogen receptor and NF-kappaB signaling pathway. J. Ethnopharmacol. 2021, 276, 114106. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Wang, R.; Tang, H.; Dong, Y.; Chan, A.; Sareddy, G.R.; Vadlamudi, R.K.; Brann, D.W. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol. Cell. Endocrinol. 2014, 389, 84–91. [Google Scholar] [CrossRef]
- Bjorling, D.E.; Wang, Z.Y. Estrogen and neuroinflammation. Urology 2001, 57 (Suppl. 1), 40–46. [Google Scholar] [CrossRef]
- Mattsson, C.; Olsson, T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr. Med. Chem. 2007, 14, 2918–2924. [Google Scholar] [CrossRef] [PubMed]
- Santolla, M.F.; Avino, S.; Pellegrino, M.; De Francesco, E.M.; De Marco, P.; Lappano, R.; Vivacqua, A.; Cirillo, F.; Rigiracciolo, D.C.; Scarpelli, A.; et al. SIRT1 is involved in oncogenic signaling mediated by GPER in breast cancer. Cell Death Dis. 2015, 6, e1834. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Zhan, Y.; Liu, Z.; Ding, T.; Li, M.; Yu, H.; Zhang, L.; Li, H.; Luo, A.; Zhang, D.; et al. SIRT1-mediated ERbeta suppression in the endothelium contributes to vascular aging. Aging Cell 2016, 15, 1092–1102. [Google Scholar] [CrossRef]
- Lannigan, D.A. ERK1/2-RSK2 Signaling in Regulation of ERalpha-Mediated Responses. Endocrinology 2022, 163, bqac106. [Google Scholar] [CrossRef]
- Liao, Z.H.; Huang, T.; Xiao, J.W.; Gu, R.C.; Ouyang, J.; Wu, G.; Liao, H. Estrogen signaling effects on muscle-specific immune responses through controlling the recruitment and function of macrophages and T cells. Skelet. Muscle 2019, 9, 20, Correction in Skelet. Muscle 2022, 12, 15. [Google Scholar] [CrossRef]
- Gou, Y.; Li, X.; Li, P.; Zhang, H.; Xu, T.; Wang, H.; Wang, B.; Ma, X.; Jiang, X.; Zhang, Z. Estrogen receptor beta upregulates CCL2 via NF-kappaB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Hum. Reprod. 2019, 34, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Solomon, J.M.; Pasupuleti, R.; Xu, L.; McDonagh, T.; Curtis, R.; DiStefano, P.S.; Huber, L.J. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol. Cell. Biol. 2006, 26, 28–38. [Google Scholar] [CrossRef]
- Sivakumar, K.K.; Stanley, J.A.; Behlen, J.C.; Wuri, L.; Dutta, S.; Wu, J.; Arosh, J.A.; Banu, S.K. Inhibition of Sirtuin-1 hyperacetylates p53 and abrogates Sirtuin-1-p53 interaction in Cr(VI)-induced apoptosis in the ovary. Reprod. Toxicol. 2022, 109, 121–134. [Google Scholar] [CrossRef]
- Khan, M.; Ullah, R.; Rehman, S.U.; Shah, S.A.; Saeed, K.; Muhammad, T.; Park, H.Y.; Jo, M.H.; Choe, K.; Rutten, B.P.F.; et al. 17beta-Estradiol Modulates SIRT1 and Halts Oxidative Stress-Mediated Cognitive Impairment in a Male Aging Mouse Model. Cells 2019, 8, 928. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, R.; Hao, S.; Luo, P.; Wang, P.; Almatari, Y.; Guo, L.; Guo, L. Up-regulation of SIRT1 induced by 17beta-estradiol promotes autophagy and inhibits apoptosis in osteoblasts. Aging (Albany NY) 2021, 13, 23652–23671. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Su, S.C.; Chiang, C.F.; Chien, C.Y.; Hsu, C.C.; Yu, T.Y.; Huang, S.M.; Shieh, Y.S.; Kao, H.W.; Tsai, C.S.; et al. Estrogen modulates vascular smooth muscle cell function through downregulation of SIRT1. Oncotarget 2017, 8, 110039–110051. [Google Scholar] [CrossRef]
- Khan, M.; Shah, S.A.; Kim, M.O. 17beta-Estradiol via SIRT1/Acetyl-p53/NF-kB Signaling Pathway Rescued Postnatal Rat Brain Against Acute Ethanol Intoxication. Mol. Neurobiol. 2018, 55, 3067–3078. [Google Scholar] [CrossRef]
- Elbaz, A.; Rivas, D.; Duque, G. Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology 2009, 10, 747–755. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Narayanan, R.; Dalton, J.T.; McKinsey, T.A.; Levin, E.R. Estrogen regulates histone deacetylases to prevent cardiac hypertrophy. Mol. Biol. Cell 2013, 24, 3805–3818. [Google Scholar] [CrossRef]
- Collins, M.K.; McCutcheon, C.R.; Petroff, M.G. Impact of Estrogen and Progesterone on Immune Cells and Host-Pathogen Interactions in the Lower Female Reproductive Tract. J. Immunol. 2022, 209, 1437–1449. [Google Scholar] [CrossRef]
- Gilliver, S.C. Sex steroids as inflammatory regulators. J. Steroid Biochem. Mol. Biol. 2010, 120, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Vural, P.; Akgul, C.; Canbaz, M. Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacol. Res. 2006, 54, 298–302. [Google Scholar] [CrossRef]
- Diedrich, M.; Tadic, J.; Mao, L.; Wacker, M.A.; Nebrich, G.; Hetzer, R.; Regitz-Zagrosek, V.; Klose, J. Heart protein expression related to age and sex in mice and humans. Int. J. Mol. Med. 2007, 20, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Barcena de Arellano, M.L.; Pozdniakova, S.; Kuhl, A.A.; Baczko, I.; Ladilov, Y.; Regitz-Zagrosek, V. Sex differences in the aging human heart: Decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense. Aging (Albany NY) 2019, 11, 1918–1933. [Google Scholar] [CrossRef]
- Schanton, M.; Maymo, J.; Camisay, M.F.; Perez-Perez, A.; Casale, R.; Sanchez-Margalet, V.; Erlejman, A.; Varone, C. Crosstalk between estradiol and NFkappaB signaling pathways on placental leptin expression. Reproduction 2020, 160, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Oparil, S.; Yu, H.; Gong, K.; Feng, W.; Black, J.; Chen, Y.F.; Nozell, S. Estrogen modulates NFkappaB signaling by enhancing IkappaBalpha levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-beta. PLoS ONE 2012, 7, e36890. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Eckert, A.; Lai, K.; Adelman, S.J.; Harnish, D.C. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ. Res. 2001, 89, 823–830. [Google Scholar] [CrossRef]
- Barcena, M.L.; Estepa, M.; Marx, L.; Breiter, A.; Haritonow, N.; Stawowy, P. The impact of the PCSK-9/VLDL-Receptor axis on inflammatory cell polarization. Cytokine 2023, 161, 156077. [Google Scholar] [CrossRef]
- Barcena, M.L.; Jeuthe, S.; Niehues, M.H.; Pozdniakova, S.; Haritonow, N.; Kuhl, A.A.; Messroghli, D.R.; Regitz-Zagrosek, V. Sex-Specific Differences of the Inflammatory State in Experimental Autoimmune Myocarditis. Front. Immunol. 2021, 12, 686384. [Google Scholar] [CrossRef] [PubMed]
- Barcena, M.L.; Tonini, G.; Haritonow, N.; Breiter, P.; Milting, H.; Baczko, I.; Muller-Werdan, U.; Ladilov, Y.; Regitz-Zagrosek, V. Sex and age differences in AMPK phosphorylation, mitochondrial homeostasis, and inflammation in hearts from inflammatory cardiomyopathy patients. Aging Cell 2023, 22, e13894. [Google Scholar] [CrossRef]
- Rosner, M.; Schipany, K.; Hengstschlager, M. Merging high-quality biochemical fractionation with a refined flow cytometry approach to monitor nucleocytoplasmic protein expression throughout the unperturbed mammalian cell cycle. Nat. Protoc. 2013, 8, 602–626. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcena, M.L.; Breiter, A.; Temp, J.; Ladilov, Y.; Regitz-Zagrosek, V. Effect of Estrogen on Sirt1 Signaling in Human Macrophages. Int. J. Mol. Sci. 2025, 26, 8670. https://doi.org/10.3390/ijms26178670
Barcena ML, Breiter A, Temp J, Ladilov Y, Regitz-Zagrosek V. Effect of Estrogen on Sirt1 Signaling in Human Macrophages. International Journal of Molecular Sciences. 2025; 26(17):8670. https://doi.org/10.3390/ijms26178670
Chicago/Turabian StyleBarcena, Maria Luisa, Anne Breiter, Julia Temp, Yury Ladilov, and Vera Regitz-Zagrosek. 2025. "Effect of Estrogen on Sirt1 Signaling in Human Macrophages" International Journal of Molecular Sciences 26, no. 17: 8670. https://doi.org/10.3390/ijms26178670
APA StyleBarcena, M. L., Breiter, A., Temp, J., Ladilov, Y., & Regitz-Zagrosek, V. (2025). Effect of Estrogen on Sirt1 Signaling in Human Macrophages. International Journal of Molecular Sciences, 26(17), 8670. https://doi.org/10.3390/ijms26178670







