Application of Modulators of Ca2+-Activated Big-Conductance Potassium Channels Against Cd2+-Induced Cytotoxicity: A Study on Two Rat Cell Lines, PC12 and AS-30D
Abstract
1. Introduction
2. Results
2.1. Action of BK(Ca) Modulators on PC12 Cells in the Absence and in the Presence of Cd2+
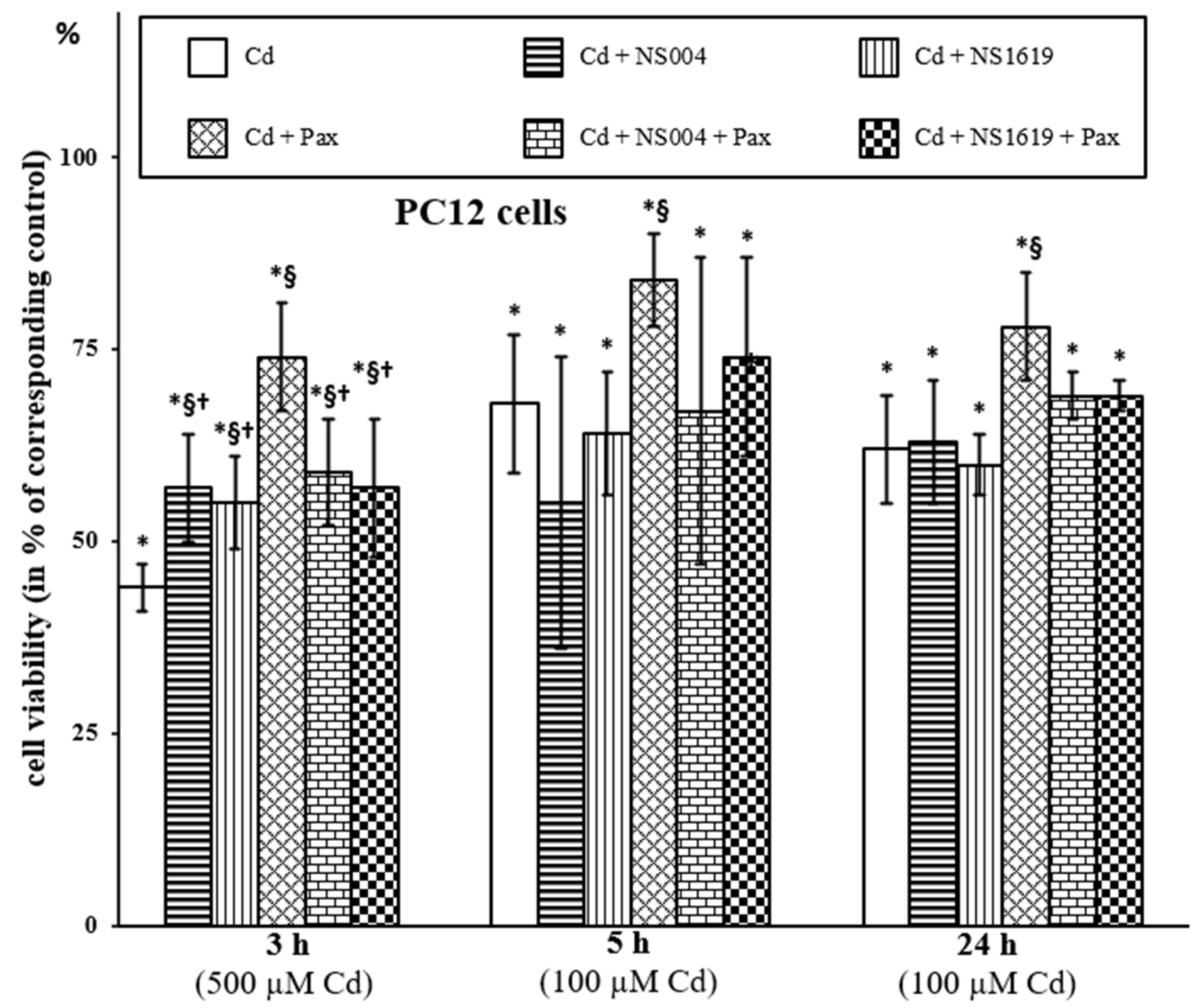
2.1.1. Effects of BK(Ca) Modulators on Viability of PC12 Cells
2.1.2. Effects of BK(Ca) Modulators on Respiration of PC12 Cells
2.1.3. Effects of BK(Ca) Modulators on ΔΨmito Changes in PC12 Cells
2.1.4. Effects of BK(Ca) Modulators on ROS Production in PC12 Cells
2.2. Action of BK(Ca) Modulators on AS-30D Cells in the Absence and in the Presence of Cd2+
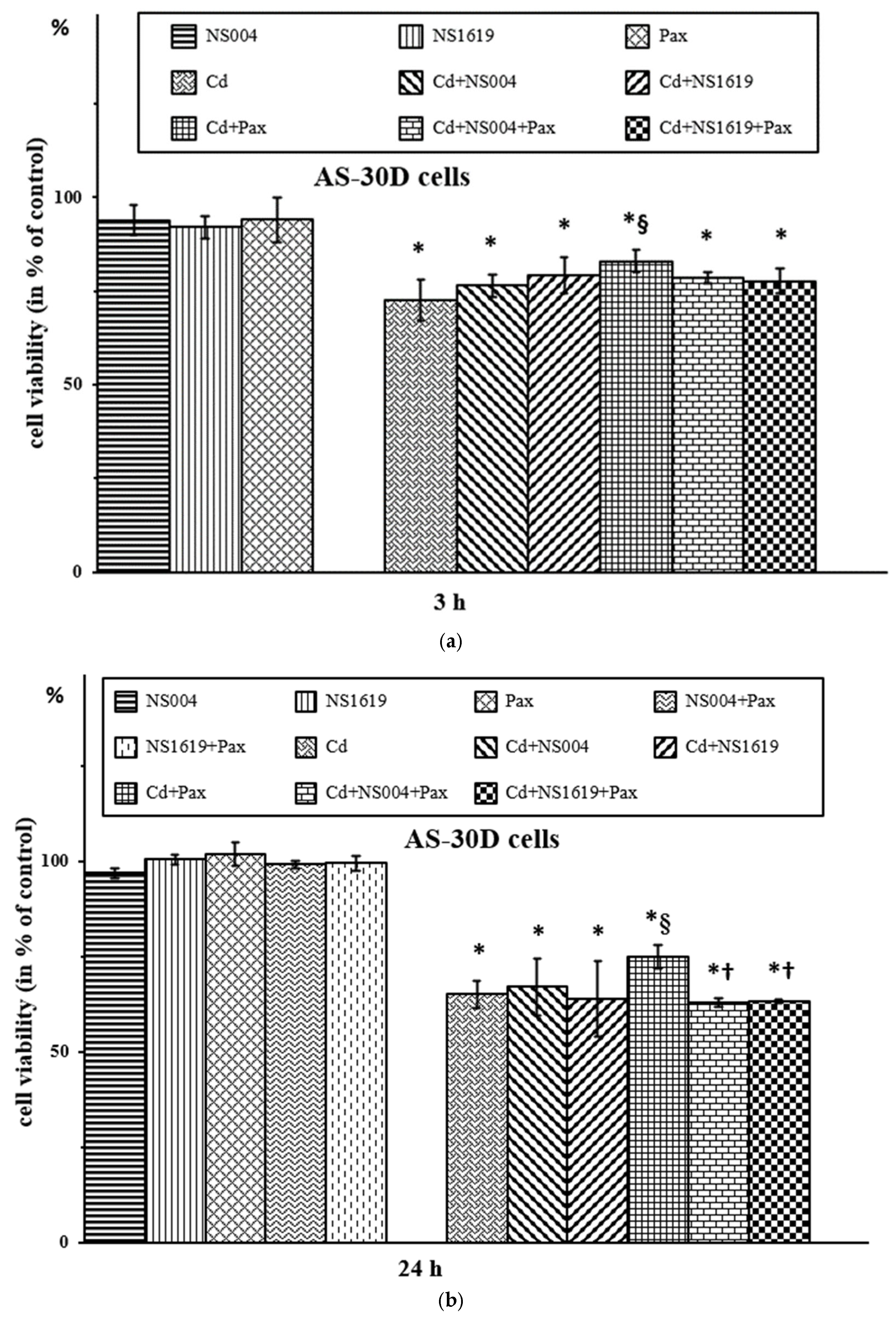
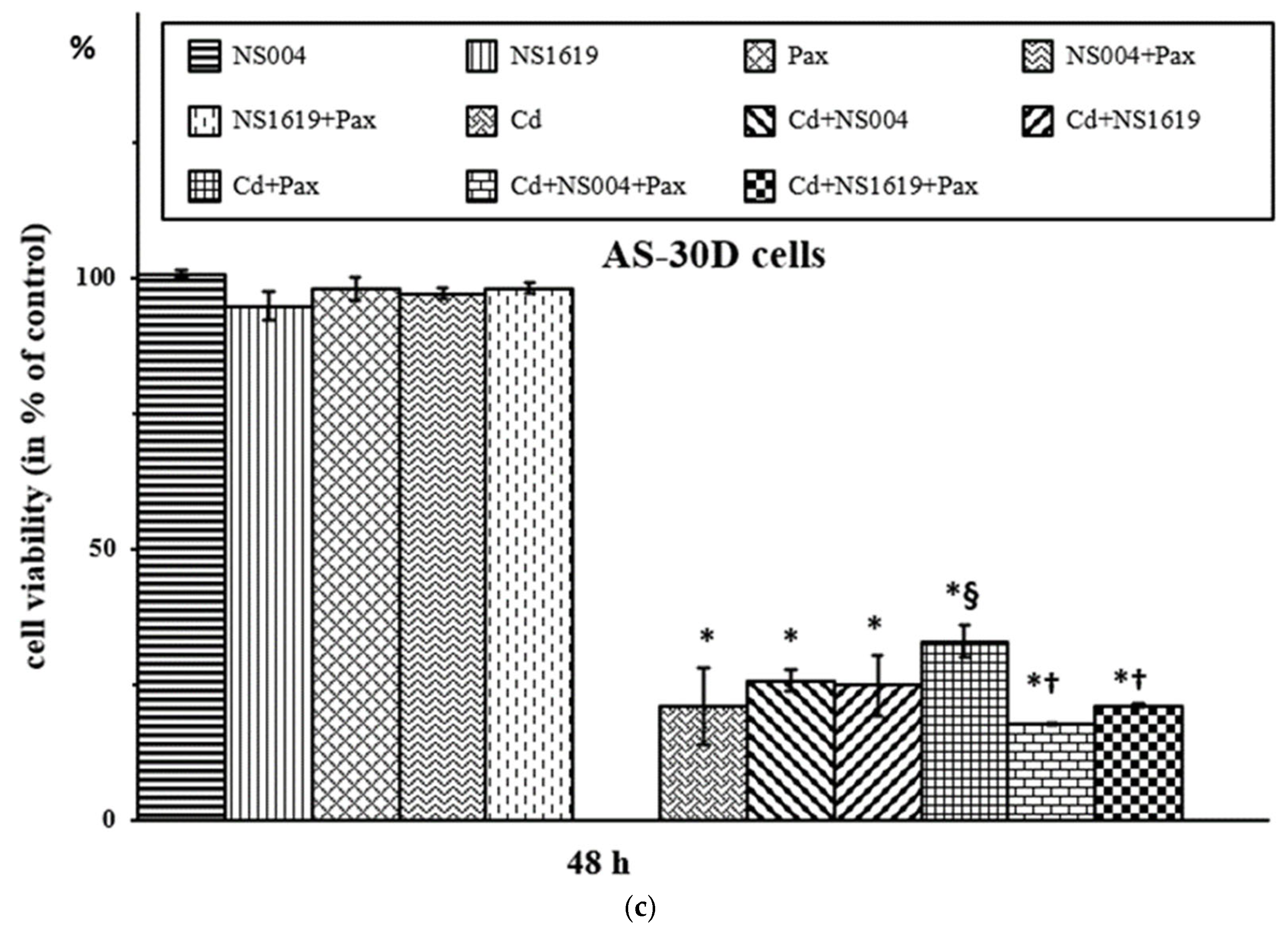
2.2.1. Effects of BK(Ca) Modulators on Viability of AS-30D Cells
2.2.2. Effects of BK(Ca) Modulators on Respiration of AS-30D Cells
2.2.3. Effects of BK(Ca) Modulators on ΔΨmito Changes in AS-30D Cells
2.2.4. Effects of BK(Ca) Modulators on ROS Production in AS-30D Cells
3. Discussion
3.1. Synopsis of the Results on the Effect of BK(Ca) Modulators on PC12 and AS-30D Cells Exposed to Cd2+
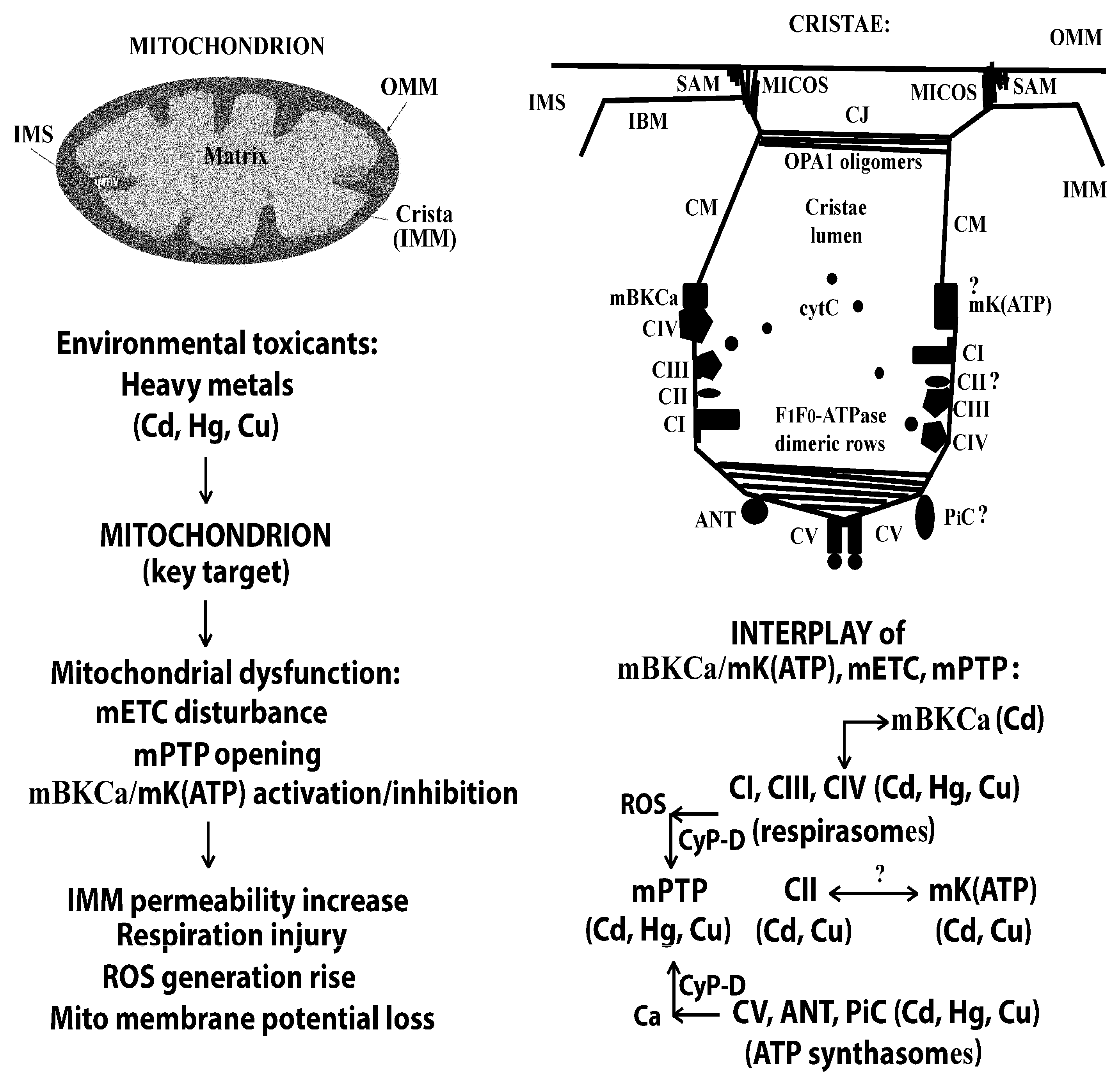
3.2. On the Relationship Between mBK(Ca), mETC, and mPTP in the Absence and in the Presence of Cd2+
4. Materials and Methods
4.1. Chemicals
4.2. PC12 Cells
4.3. AS-30D Cells
4.4. Statistical Analysis
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BK(Ca) | Ca2+-activated big-conductance potassium channel |
| pBK(Ca) | plasmatic BK(Ca) |
| mBK(Ca) | mitochondrial BK(Ca) |
| ROS | reactive oxygen species |
| RLM | rat liver mitochondria |
| mPTP | mitochondrial permeability transition pore |
| K(ATP) | ATP-sensitive potassium channel |
| mK(ATP) | mitochondrial K(ATP) |
| mETC | mitochondrial electron transport chain |
| IMM | inner mitochondrial membrane |
| Pax | paxilline |
| NS004 | 5-trifluoromethyl-1-(5-chloro-2-hydroxyphenyl)-1,3-dihydro-2H-benzimidazole-2-one |
| NS1619 | 1,3-dihydro-1- [2-hydroxy-5-(trifluoromethyl) phenyl]-5-(trifluoromethyl)-2H-benzimidazole-2-one |
| ΔΨmito | mitochondrial membrane potential |
| LDH | lactate dehydrogenase |
| OCR | oxygen consumption rate |
| FCCP | carbonyl cyanide p-trifluoromethoxyphenylhydrazone |
| CCCP | carbonyl cyanide 3-chlorophenylhydrazone |
| oligo | oligomycin |
| CI-CV | respiratory complexes I, II, III, IV, or V of mETC |
| F1F0-TPase | ATP synthase or CV of mETC |
| JC-1 | 5,5′,6,6′-tetrachloro-1,1′,3,3′- tetraethylbenzimidazolcarbocyanine iodide |
| DCFH2-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| TB | trypan blue |
| PI | propidium iodide |
| RET | reverse electron transport |
| FET | forward electron transport |
| mSCs | mitochondrial supercomplexes |
| OXPHOS | oxidative phosphorylation system |
| ANT | adenine nucleotide translocase |
| PiC | mitochondrial phosphate carrier |
| VDAC | voltage-dependent anion channel |
| IBM | inner boundary membrane |
| OMM | outer mitochondrial membrane |
| CMs | crista membranes |
| CJs | crista junctions |
| MICOS | mitochondrial cristae organizing system |
| SAM | OMM sorting and assembly machinery |
| OPA1 | optic atrophy 1 protein |
| VSD | voltage sensor domain |
| RCK1/2 | K+ conductance regulator domains 1 and 2 |
| Rot | rotenone |
| Stig | stigmatellin |
| CsA | cyclosporine A |
| DTT | dithiothreitol |
| RR | ruthenium red |
| CyP-D | cyclophilin D |
| CoQ, or Q | ubiquinone |
| EGTA | ethylenebis(oxyethylene-nitrilo)]tetraacetic acid |
| CL | cardiolipin |
| IF1 | ATPase inhibitory factor 1 |
| FB | ATP synthase coupling factor |
| PBS | phosphate-buffered saline |
| HBSS | Hank’s balanced salt solution |
References
- Nordberg, G.F.; Åkesson, A.; Nogawa, K.; Nordberg, M. Chapter 7—Cadmium. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 141–196. [Google Scholar] [CrossRef]
- Reddam, A.; McLarnan, S.; Kupsco, A. Environmental Chemical Exposures and Mitochondrial Dysfunction: A Review of Recent Literature. Curr. Environ. Health Rep. 2022, 9, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Kamogashira, T.; Yamasoba, T. Heavy Metal Exposure: Molecular Pathways, Clinical Implications, and Protective Strategies. Antioxidants 2024, 13, 76. [Google Scholar] [CrossRef]
- Davidova, S.; Milushev, V.; Satchanska, G. The Mechanisms of Cadmium Toxicity in Living Organisms. Toxics 2024, 12, 875. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Mandrioli, J.; Violi, F.; Bargellini, A.; Weuve, J.; Fini, N.; Grill, P.; Michalke, B. Lead, cadmium and mercury in cerebrospinal fluid and risk of amyotrophic lateral sclerosis: A case-control study. J. Trace Elem. Med. Biol. 2017, 43, 121–125. [Google Scholar] [CrossRef]
- Tassone, G.; Kola, A.; Valensin, D.; Pozzi, C. Dynamic Interplay Between Copper Toxicity and Mitochondrial Dysfunction in Alzheimer’s Disease. Life 2021, 11, 386. [Google Scholar] [CrossRef]
- Arruebarrena, M.A.; Hawe, C.T.; Lee, Y.M.; Branco, R.C. Mechanisms of Cadmium Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 16558. [Google Scholar] [CrossRef]
- Wei, R.; Wei, P.; Yuan, H.; Yi, X.; Aschner, M.; Jiang, Y.M.; Li, S.J. Inflammation in Metal-Induced Neurological Disorders and Neurodegenerative Diseases. Biol. Trace Elem. Res. 2024, 202, 4459–4481. [Google Scholar] [CrossRef]
- Fujie, T.; Ando, R.; Abe, M.; Ichida, N.; Ito, K.; Hara, T.; Yamamoto, C.; Kaji, T. Protection of cultured vascular endothelial cells against cadmium cytotoxicity by simultaneous treatment or pretreatment with manganese. J. Toxicol. Sci. 2024, 49, 349–358. [Google Scholar] [CrossRef]
- Queiroz, M.I.C.; Lazaro, C.M.; Dos Santos, L.M.B.; Rentz, T.; Virgilio-da-Silva, J.V.; Moraes-Vieira, P.M.M.; Cunha, F.A.S.; Santos, J.C.C.; Vercesi, A.E.; Leite, A.C.R.; et al. In vivo chronic exposure to inorganic mercury worsens hypercholesterolemia, oxidative stress and atherosclerosis in the LDL receptor knockout mice. Ecotoxicol. Environ. Saf. 2024, 275, 116254. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.A.; Dymkowska, D.; Wieckowski, M.R.; Wojtczak, L. Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol. Appl. Pharmacol. 2008, 231, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.A.; Korotkov, S.M.; Saris, N.E. In vitro modulation of heavy metal-induced rat liver mitochondria dysfunction: A comparison of copper and mercury with cadmium. J. Trace Elem. Med. Biol. 2011, 25 (Suppl. S1), S63–S73. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.A.; Sokolova, T.V.; Emelyanova, L.V.; Zakharova, I.O. Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: Effects of cadmium, mercury, and copper. Sci. World J. 2012, 2012, 136063. [Google Scholar] [CrossRef] [PubMed]
- Buha, A.; Matovic, V.; Antonijevic, B.; Bulat, Z.; Curcic, M.; Renieri, E.A.; Tsatsakis, A.M.; Schweitzer, A.; Wallace, D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. Int. J. Mol. Sci. 2018, 19, 1501. [Google Scholar] [CrossRef]
- Dumková, J.; Vaškovicová, N.; Kristeková, D.; Vrlíková, L.; Faruzelová, A.; Jedličková, A.; Tatíčková, M.; Alexa, L.; Coufalík, P.; Bahelková, M.; et al. Secondary target organs exhibit differential ability to clear cadmium after cadmium oxide nanoparticle inhalation. J. Hazard. Mater. 2025, 496, 139181. [Google Scholar] [CrossRef]
- Cordier, W.; Yousaf, M.; Nell, M.J.; Steenkamp, V. Underlying mechanisms of cytotoxicity in HepG2 hepatocarcinoma cells exposed to arsenic, cadmium and mercury individually and in combination. Toxicol. Vitr. 2021, 72, 105101. [Google Scholar] [CrossRef]
- Elmorsy, E.; Al-Ghafari, A.; Al Doghaither, H.; Ghulam, J. Effects of environmental metals on mitochondrial bioenergetics of the CD-1 mice pancreatic beta-cells. Toxicol. Vitr. 2021, 70, 105015. [Google Scholar] [CrossRef]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The mechanism of the cadmium-induced toxicity and cellular response in the liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Shi, L.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H. Heavy metals induced mitochondrial dysfunction in animals: Molecular mechanism of toxicity. Toxicology 2022, 469, 153136. [Google Scholar] [CrossRef]
- Korotkov, S.M. Mitochondrial Oxidative Stress Is the General Reason for Apoptosis Induced by Different-Valence Heavy Metals in Cells and Mitochondria. Int. J. Mol. Sci. 2023, 24, 14459. [Google Scholar] [CrossRef]
- Qu, F.; Zheng, W. Cadmium Exposure: Mechanisms and Pathways of Toxicity and Implications for Human Health. Toxics 2024, 12, 388. [Google Scholar] [CrossRef]
- Lee, W.K.; Thévenod, F. Cell organelles as targets of mammalian cadmium toxicity. Arch. Toxicol. 2020, 94, 1017–1049. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Glazunov, V.V.; Nikitina, E.R.; Korotkov, S.M. Bivalent metal ions modulate Cd2+ effects on isolated rat liver mitochondria. J. Bioenerg. Biomembr. 2001, 33, 303–318. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Glazunov, V.V.; Korotkov, S.M. Cyclosporin A-sensitive permeability transition pore is involved in Cd2+-induced dysfunction of isolated rat liver mitochondria: Doubts no more. Arch. Biochem. Biophys. 2002, 405, 252–264. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Korotkov, S.M. Mechanism of primary Cd2+-induced rat liver mitochondria dysfunction: Discrete modes of Cd2+ action on calcium and thiol-dependent domains. Toxicol. Appl. Pharmacol. 2003, 192, 56–68. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Glazunov, V.V.; Korotkov, S.M. Cd2+ versus Ca2+-produced mitochondrial membrane permeabilization: A proposed direct participation of respiratory complexes I and III. Chem. Biol. Interact. 2004, 150, 253–270. [Google Scholar] [CrossRef]
- Szabo, I.; Szewczyk, A. Mitochondrial Ion Channels. Annu. Rev. Biophys. 2023, 52, 229–254. [Google Scholar] [CrossRef]
- Tommasin, L.; Carrer, A.; Nata, F.B.; Frigo, E.; Fogolari, F.; Lippe, G.; Carraro, M.; Bernardi, P. Adenine nucleotide translocator and ATP synthase cooperate in mediating the mitochondrial permeability transition. J. Physiol. 2025. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Glazunov, V.V.; Korotkov, S.M. Cd2+-promoted mitochondrial permeability transition: A comparison with other heavy metals. Acta Biochim. Pol. 2004, 51, 545–551. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Emelyanova, L.V.; Korotkov, S.M.; Brailovskaya, I.V.; Savina, M.V. On the mechanism(s) of membrane permeability transition in liver mitochondria of lamprey, Lampetra fluviatilis L.: Insights from cadmium. Biomed. Res. Int. 2014, 2014, 691724. [Google Scholar] [CrossRef]
- Belyaeva, E.A. Mechanism(s) of Membrane Permeability Transition in Liver Mitochondria of Lamprey, Lampetra fluviatilis L. during Pre-spawning Starvation. In Recent Developments in Medicine and Medical Research; Elshimali, J.Y.I., Ed.; B P International: West Bengal, India, 2021; Volume 16, pp. 105–129. [Google Scholar] [CrossRef]
- Belyaeva, E.A. Effect of diazoxide on AS-30D rat ascites hepatoma cells treated by Cd2+. J. Evol. Biochem. Physiol. 2013, 49, 489–497. [Google Scholar] [CrossRef]
- Belyaeva, E.A. Modulators of mitochondrial ATP-sensitive potassium channel affect cytotoxicity of heavy metals: Action on isolated rat liver mitochondria and AS-30D ascites hepatoma cells. Ecotoxicol. Environ. Saf. 2023, 256, 114829. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Sokolova, T.V. Mitigating effect of paxilline against injury produced by Cd2+ in rat pheochromocytoma PC12 and ascites hepatoma AS-30D cells. Ecotoxicol. Environ. Saf. 2020, 196, 110519. [Google Scholar] [CrossRef]
- Sancho, M.; Kyle, B.D. The Large-Conductance, Calcium-Activated Potassium Channel: A Big Key Regulator of Cell Physiology. Front. Physiol. 2021, 12, 750615. [Google Scholar] [CrossRef]
- Zahra, A.; Liu, R.; Han, W.; Meng, H.; Wang, Q.; Wang, Y.; Campbell, S.L.; Wu, J. KCa-Related Neurological Disorders: Phenotypic Spectrum and Therapeutic Indications. Curr. Neuropharmacol. 2023, 21, 1504–1518. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Dymkowska, D.; Wieckowski, M.R.; Wojtczak, L. Reactive oxygen species produced by the mitochondrial respiratory chain are involved in Cd2+-induced injury of rat ascites hepatoma AS-30D cells. Biochim. Biophys. Acta 2006, 1757, 1568–1574. [Google Scholar] [CrossRef]
- Belyaeva, E.A. Stigmatellin as a modulator of metal-induced inner mitochondrial membrane permeabilization. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 79. [Google Scholar] [CrossRef]
- Belyaeva, E.A. Mitochondrial respiratory chain inhibitors modulate the metal-induced inner mitochondrial membrane permeabilization. Acta Biochim. Pol. 2010, 57, 435–441. [Google Scholar] [CrossRef]
- Belyaeva, E.A. Respiratory complex II in mitochondrial dysfunction-mediated cytotoxicity: Insight from cadmium. J. Trace Elem. Med. Biol. 2018, 50, 80–92. [Google Scholar] [CrossRef]
- Okoye, C.N.; MacDonald-Jay, N.; Kamunde, C. Effects of bioenergetics, temperature and cadmium on liver mitochondria reactive oxygen species production and consumption. Aquat. Toxicol. 2019, 214, 105264. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Pacini, A.; Gulisano, M.; Taddei, N.; Fiorillo, C.; Becatti, M. Cadmium-induced cytotoxicity: Effects on mitochondrial electron transport chain. Front. Cell Dev. Biol. 2020, 8, 604377. [Google Scholar] [CrossRef]
- Belyaeva, E.A. Role of Mitochondrial Respiratory Chain in Neurotoxic Action of Heavy Metals: Comparison of Cd2+, Hg2+ and Cu2+. Cut. Edge Res. Biol. 2022, 2, 144–174. [Google Scholar] [CrossRef]
- de Lira-Sánchez, J.A.; Esparza-Perusquía, M.; Martínez, F.; Pardo, J.P.; Flores-Herrera, O. Heavy metals do not induce ROS production by mitochondrial respirasome. Biochim. Biophys. Acta Bioenerg. 2023, 1864, 148999. [Google Scholar] [CrossRef]
- Romanova, N.; Sule, K.; Issler, T.; Hebrok, D.; Persicke, M.; Thévenod, F.; Prenner, E.J.; Lee, W.K. Cadmium-cardiolipin disruption of respirasome assembly and redox balance through mitochondrial membrane rigidification. J. Lipid Res. 2025, 66, 100750. [Google Scholar] [CrossRef]
- González-Sanabria, N.; Echeverría, F.; Segura, I.; Alvarado-Sánchez, R.; Latorre, R. BK in Double-Membrane Organelles: A Biophysical, Pharmacological, and Functional Survey. Front. Physiol. 2021, 12, 761474. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Sokolova, T.V. Cd(II)-induced cytotoxicity is attenuated by K+ channels modulators. In Metal Ions in Biology and Medicine; Pele, L., Powell, J.J., Kinrade, S., Jugdaohsingh, R., Collery, P., Maymard, I., Badawi, A., Eds.; John Libbey Eurotext: Paris, France, 2011; Volume 11, pp. 133–139. ISBN 978-2-7420-0809-4. [Google Scholar]
- Belyaeva, E.A. The effect of modulators of large-conductance Ca2+-modulated K+ channels on rat AS-30D ascites hepatoma cells and isolated liver mitochondria treated with Cd2+. J. Evol. Biochem. Physiol. 2015, 51, 259–270. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.; Reilly, S.; Xin, H.; Guo, N.; Zhang, X. Role of organellar Ca2+-activated K+ channels in disease development. Life Sci. 2023, 316, 121433. [Google Scholar] [CrossRef]
- Han, X.; Xi, L.; Wang, H.; Huang, X.; Ma, X.; Han, Z.; Wu, P.; Ma, X.; Lu, Y.; Wang, G.; et al. The potassium ion channel opener NS1619 inhibits proliferation and induces apoptosis in A2780 ovarian cancer cells. Biochem. Biophys. Res. Commun. 2008, 375, 205–209. [Google Scholar] [CrossRef]
- Debska-Vielhaber, G.; Godlewski, M.M.; Kicinska, A.; Skalska, J.; Kulawiak, B.; Piwonska, M.; Zablocki, K.; Kunz, W.S.; Szewczyk, A.; Motyl, T. Large-conductance K+ channel openers induce death of human glioma cells. J. Physiol. Pharmacol. 2009, 60, 27–36. [Google Scholar] [PubMed]
- Chen, M.; Sun, H.Y.; Hu, P.; Wang, C.F.; Li, B.X.; Li, S.J.; Li, J.J.; Tan, H.Y.; Gao, T.M. Activation of BKca channels mediates hippocampal neuronal death after reoxygenation and reperfusion. Mol. Neurobiol. 2013, 48, 794–807. [Google Scholar] [CrossRef]
- Ge, L.; Hoa, N.T.; Wilson, Z.; Arismendi-Morillo, G.; Kong, X.T.; Tajhya, R.B.; Beeton, C.; Jadus, M.R. Big Potassium (BK) ion channels in biology, disease and possible targets for cancer immunotherapy. Int. Immunopharmacol. 2014, 22, 427–443. [Google Scholar] [CrossRef]
- Augustynek, B.; Koprowski, P.; Rotko, D.; Kunz, W.S.; Szewczyk, A.; Kulawiak, B. Mitochondrial BK Channel Openers CGS7181 and CGS7184 Exhibit Cytotoxic Properties. Int. J. Mol. Sci. 2018, 19, 353. [Google Scholar] [CrossRef]
- Van, N.T.H.; Kim, W.K.; Nam, J.H. Challenges in the Therapeutic Targeting of KCa Channels: From Basic Physiology to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 2965. [Google Scholar] [CrossRef]
- Wrzosek, A.; Gałecka, S.; Żochowska, M.; Olszewska, A.; Kulawiak, B. Alternative Targets for Modulators of Mitochondrial Potassium Channels. Molecules 2022, 27, 299. [Google Scholar] [CrossRef]
- Kulawiak, B.; Szewczyk, A. Glutamate-induced cell death in HT22 mouse hippocampal cells is attenuated by paxilline, a BK channel inhibitor. Mitochondrion 2012, 12, 169–172. [Google Scholar] [CrossRef]
- Belyaeva, E.A. Cd2+-induced injury of rat ascites hepatoma AS-30D cells: A possible involvement of Ca2+-activated large-conductance potassium channels. Mitochondrion 2013, 13, 927. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Sokolova, T.V. Mechanism(s) of Modulation of Cd2+-Induced Cytotoxicity by Paxilline and NS1619/NS004: An Involvement of Ca2+-Activated Big-Conductance Potassium Channel and/or Respiratory Chain of Mitochondria? Zh. Evol. Biokhim. Fiziol. 2020, 56, 737. [Google Scholar] [CrossRef]
- Chen, L.; Xu, B.; Liu, L.; Luo, Y.; Zhou, H.; Chen, W.; Shen, T.; Han, X.; Kontos, C.D.; Huang, S. Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic. Biol. Med. 2011, 50, 624–632. [Google Scholar] [CrossRef]
- Jiang, C.; Yuan, Y.; Hu, F.; Wang, Q.; Zhang, K.; Wang, Y.; Gu, J.; Liu, X.; Bian, J.; Liu, Z. Cadmium induces PC12 cells apoptosis via an extracellular signal-regulated kinase and c-Jun N-terminal kinase-mediated mitochondrial apoptotic pathway. Biol. Trace Elem. Res. 2014, 158, 249–258. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ukiana, J.; Uson-Lopez, R.; Sikder, M.T.; Saito, T.; Kurasaki, M. Cytotoxic effects of cadmium and zinc co-exposure in PC12 cells and the underlying mechanism. Chem. Biol. Interact. 2017, 269, 41–49. [Google Scholar] [CrossRef]
- Belyaeva, E.A. Impact of Zn2+ and selenite on rat ascites hepatoma AS-30D cells and rat liver mitochondria: A comparison with Cd2+, Hg2+, Cu2+, and Ca2+. In Recent Developments in Chemistry and Biochemistry Research; Turkmen, A., Ed.; B P International: West Bengal, India, 2025; Volume 10, pp. 130–159. [Google Scholar] [CrossRef]
- Shah, N.; Saxena, B.; Gupta, R. Mitochondria: Key Mediator for Environmental Toxicant-Induced Neurodegeneration. Int. J. Toxicol. 2025, 10915818251369414. [Google Scholar] [CrossRef]
- Szewczyk, A.; Bednarczyk, P.; Kulawiak, B.; Żochowska, M.; Kalenik, B.; Lewandowska, J.; Pytlak, K.; Gałecka, S.; Wrzosek, A.; Koprowski, P. Mitochondrial potassium channels: New properties and functions. Biochim. Biophys. Acta Bioenerg. 2025, 1866, 149546. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, S.Z.; Cao, C.M.; Bruce, I.C.; Xia, Q. The mitochondrial permeability transition pore and the Ca2+-activated K+ channel contribute to the cardioprotection conferred by tumor necrosis factor-alpha. Cytokine 2005, 32, 199–205. [Google Scholar] [CrossRef]
- Stowe, D.F.; Aldakkak, M.; Camara, A.K.; Riess, M.L.; Heinen, A.; Varadarajan, S.G.; Jiang, M.T. Cardiac mitochondrial preconditioning by Big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H434–H440. [Google Scholar] [CrossRef]
- Heinen, A.; Aldakkak, M.; Stowe, D.F.; Rhodes, S.S.; Riess, M.L.; Varadarajan, S.G.; Camara, A.K. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1400–H1407. [Google Scholar] [CrossRef]
- Cheng, Y.; Gu, X.Q.; Bednarczyk, P.; Wiedemann, F.R.; Haddad, G.G.; Siemen, D. Hypoxia increases activity of the BK-channel in the inner mitochondrial membrane and reduces activity of the permeability transition pore. Cell. Physiol. Biochem. 2008, 22, 127–136. [Google Scholar] [CrossRef]
- Kulawiak, B.; Kudin, A.P.; Szewczyk, A.; Kunz, W.S. BK channel openers inhibit ROS production of isolated rat brain mitochondria. Exp. Neurol. 2008, 212, 543–547. [Google Scholar] [CrossRef]
- Cheng, Y.; Debska-Vielhaber, G.; Siemen, D. Interaction of mitochondrial potassium channels with the permeability transition pore. FEBS Lett. 2010, 584, 2005–2012. [Google Scholar] [CrossRef]
- Cheng, Y.; Gulbins, E.; Siemen, D. Activation of the permeability transition pore by Bax via inhibition of the mitochondrial BK channel. Cell. Physiol. Biochem. 2011, 27, 191–200. [Google Scholar] [CrossRef]
- Stowe, D.F.; Yang, M.; Heisner, J.S.; Camara, A.K.S. Endogenous and Agonist-induced Opening of Mitochondrial Big Versus Small Ca2+-sensitive K+ Channels on Cardiac Cell and Mitochondrial Protection. J. Cardiovasc. Pharmacol. 2017, 70, 314–328. [Google Scholar] [CrossRef]
- Goswami, S.K.; Ponnalagu, D.; Hussain, A.T.; Shah, K.; Karekar, P.; Gururaja Rao, S.; Meredith, A.L.; Khan, M.; Singh, H. Expression and Activation of BKCa Channels in Mice Protects Against Ischemia-Reperfusion Injury of Isolated Hearts by Modulating Mitochondrial Function. Front. Cardiovasc. Med. 2019, 5, 194. [Google Scholar] [CrossRef]
- Du, X.; Carvalho-de-Souza, J.L.; Wei, C.; Carrasquel-Ursulaez, W.; Lorenzo, Y.; Gonzalez, N.; Kubota, T.; Staisch, J.; Hain, T.; Petrossian, N.; et al. Loss-of-function BK channel mutation causes impaired mitochondria and progressive cerebellar ataxia. Proc. Natl. Acad. Sci. USA 2020, 117, 6023–6034. [Google Scholar] [CrossRef]
- Cordeiro, B.; Terentyev, D.; Clements, R.T. BKCa channel activation increases cardiac contractile recovery following hypothermic ischemia/reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H625–H633. [Google Scholar] [CrossRef]
- Borchert, G.H.; Hlaváčková, M.; Kolář, F. Pharmacological activation of mitochondrial BKCa channels protects isolated cardiomyocytes against simulated reperfusion-induced injury. Exp. Biol. Med. 2013, 238, 233–241. [Google Scholar] [CrossRef]
- Booth, D.M.; Enyedi, B.; Geiszt, M.; Várnai, P.; Hajnóczky, G. Redox Nanodomains Are Induced by and Control Calcium Signaling at the ER-Mitochondrial Interface. Mol. Cell 2016, 63, 240–248. [Google Scholar] [CrossRef]
- Krabbendam, I.E.; Honrath, B.; Culmsee, C.; Dolga, A.M. Mitochondrial Ca2+-activated K+ channels and their role in cell life and death pathways. Cell Calcium 2018, 69, 101–111. [Google Scholar] [CrossRef]
- Debska, G.; Kicinska, A.; Dobrucki, J.; Dworakowska, B.; Nurowska, E.; Skalska, J.; Dolowy, K.; Szewczyk, A. Large-conductance K+ channel openers NS1619 and NS004 as inhibitors of mitochondrial function in glioma cells. Biochem. Pharmacol. 2003, 65, 1827–1834. [Google Scholar] [CrossRef]
- Kicinska, A.; Szewczyk, A. Large-Conductance Potassium Cation Channel Opener NS1619 Inhibits Cardiac Mitochondria Respiratory Chain. Toxicol. Mech. Methods 2004, 14, 59–61. [Google Scholar] [CrossRef]
- Gáspár, T.; Katakam, P.; Snipes, J.A.; Kis, B.; Domoki, F.; Bari, F.; Busija, D.W. Delayed neuronal preconditioning by NS1619 is independent of calcium activated potassium channels. J. Neurochem. 2008, 105, 1115–1128. [Google Scholar] [CrossRef]
- Łukasiak, A.; Skup, A.; Chlopicki, S.; Łomnicka, M.; Kaczara, P.; Proniewski, B.; Szewczyk, A.; Wrzosek, A. SERCA, complex I of the respiratory chain and ATP-synthase inhibition are involved in pleiotropic effects of NS1619 on endothelial cells. Eur. J. Pharmacol. 2016, 786, 137–147. [Google Scholar] [CrossRef]
- Cordeiro, B.; Clements, R.T. Activation of large-conductance Ca2+-activated K+ channels (BK) during ischemic cardioplegic arrest (CP) promotes formation of mitochondrial supercomplexes and enhanced mitochondrial respiration. Circulation 2014, 130 (Suppl. 2), A20427. [Google Scholar]
- Piwońska, M.; Szewczyk, A.; Schröder, U.H.; Reymann, K.G.; Bednarczyk, I. Effectors of large-conductance calcium-activated potassium channel modulate glutamate excitotoxicity in organotypic hippocampal slice cultures. Acta Neurobiol. Exp. 2016, 76, 20–31. [Google Scholar] [CrossRef]
- Shrum, S.; Rusch, N.J.; MacMillan-Crow, L.A. Specific BK Channel Activator NS11021 Protects Rat Renal Proximal Tubular Cells from Cold Storage-Induced Mitochondrial Injury In Vitro. Biomolecules 2019, 9, 825. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; Wei, A.C.; Grunnet, M.; O’Rourke, B. Energetic performance is improved by specific activation of K+ fluxes through KCa channels in heart mitochondria. Biochim. Biophys. Acta 2010, 1797, 71–80. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Igoshkina, A.D.; Semenova, A.A.; Mikina, N.V.; Khoroshavina, E.I.; Belosludtsev, K.N. Benzimidazole Derivative NS1619 Inhibits Functioning of Mitochondria Isolated from Mouse Skeletal Muscle. Biochem. Moscow Suppl. Ser. A 2023, 17, 127–135. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Starinets, V.S.; Belosludtseva, N.V.; Mikheeva, I.B.; Chelyadnikova, Y.A.; Igoshkina, A.D.; Vafina, A.B.; Vedernikov, A.A.; Belosludtsev, K.N. BKCa Activator NS1619 Improves the Structure and Function of Skeletal Muscle Mitochondria in Duchenne Dystrophy. Pharmaceutics 2022, 14, 2336. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Starinets, V.S.; Chelyadnikova, Y.A.; Belosludtseva, N.V.; Mikheeva, I.B.; Penkina, D.K.; Igoshkina, A.D.; Talanov, E.Y.; Kireev, I.I.; Zorov, D.B.; et al. Effect of Large-Conductance Calcium-Dependent K+ Channel Activator NS1619 on Function of Mitochondria in the Heart of Dystrophin-Deficient Mice. Biochemistry 2023, 88, 189–201. [Google Scholar] [CrossRef]
- Ohya, S.; Kuwata, Y.; Sakamoto, K.; Muraki, K.; Imaizumi, Y. Cardioprotective effects of estradiol include the activation of large-conductance Ca2+-activated K+ channels in cardiac mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, 1635–1642. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Wieckowski, M.R.; Broszkiewicz, M.; Skowronek, K.; Siemen, D.; Szewczyk, A. Putative Structural and Functional Coupling of the Mitochondrial BKCa Channel to the Respiratory Chain. PLoS ONE 2013, 8, e68125. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zhang, Z.; Zhu, R.; Olcese, R.; Stefani, E.; Toro, L. The mitochondrial BKCa channel cardiac interactome reveals BKCa association with the mitochondrial import receptor subunit Tom22, and the adenine nucleotide translocator. Mitochondrion 2017, 33, 84–101. [Google Scholar] [CrossRef]
- Ježek, P.; Jabůrek, M.; Holendová, B.; Engstová, H.; Dlasková, A. Mitochondrial Cristae Morphology Reflecting Metabolism, Superoxide Formation, Redox Homeostasis, and Pathology. Antioxid. Redox Signal. 2023, 39, 635–683. [Google Scholar] [CrossRef]
- Cogliati, S.; Frezza, C.; Soriano, M.E.; Varanita, T.; Quintana-Cabrera, R.; Corrado, M.; Cipolat, S.; Costa, V.; Casarin, A.; Gomes, L.C.; et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 2013, 155, 160–171. [Google Scholar] [CrossRef]
- Zanna, C.; Ghelli, A.; Porcelli, A.M.; Karbowski, M.; Youle, R.J.; Schimpf, S.; Wissinger, B.; Pinti, M.; Cossarizza, A.; Vidoni, S.; et al. OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain 2008, 131 Pt 2, 352–367. [Google Scholar] [CrossRef]
- Veress, R.; Terentyeva, R.; Belevych, A.E.; Perger, F.; Nichtova, Z.; Pokrass, A.; Cheng, Y.; Chorna, S.; Deschenes, I.; Gyorke, S.; et al. Pharmacological Enhancement of Small Conductance Ca2+-Activated K+ Channels Suppresses Cardiac Arrhythmias in a Mouse Model of Catecholaminergic Polymorphic Ventricular Tachycardia. Circ. Res. 2025, 137, 386–399. [Google Scholar] [CrossRef]
- Han, X.C.; Liu, C.; Jiang, Z.J.; Wang, Z.L.; Li, Q.; Yuan, Y.C.; Wang, H.; Yang, J.K. KCNH6 Potassium Channel Regulates Assembling of Mitochondrial Complex I and Promotes Insulin Secretion in Islet Beta Cells. FASEB J. 2025, 39, e70776. [Google Scholar] [CrossRef]
- Cui, J. BK Channel Gating Mechanisms: Progresses Toward a Better Understanding of Variants Linked Neurological Diseases. Front. Physiol. 2021, 12, 762175. [Google Scholar] [CrossRef]
- Schreiber, M.; Salkoff, L. A novel calcium-sensing domain in the BK channel. Biophys. J. 1997, 73, 1355–1363. [Google Scholar] [CrossRef]
- Xia, X.M.; Zeng, X.; Lingle, C.J. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature 2002, 418, 880–884. [Google Scholar] [CrossRef]
- Zeng, X.H.; Xia, X.M.; Lingle, C.J. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J. Gen. Physiol. 2005, 125, 273–286. [Google Scholar] [CrossRef]
- Ramírez-Camacho, I.; García-Niño, W.R.; Flores-García, M.; Pedraza-Chaverri, J.; Zazueta, C. Alteration of mitochondrial supercomplexes assembly in metabolic diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165935. [Google Scholar] [CrossRef]
- Belyaeva, E.A. Regulated mitochondrial permeability transition: A possible involvement of mitochondrial respiratory complexes I and III. Mitochondrion 2004, 4, 71. [Google Scholar]
- Zhang, Y.; Li, J.H.; Liu, X.R.; Jiang, F.L.; Tian, F.F.; Liu, Y. Spectroscopic and microscopic studies on the mechanisms of mitochondrial toxicity induced by different concentrations of cadmium. J. Membr. Biol. 2011, 241, 39–49. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, W.; Zhao, Z.; Li, N.; Mou, Z.; Sun, D.; Cai, Y.; Wang, W.; Lin, Y. Quercetin loading CdSe/ZnS nanoparticles as efficient antibacterial and anticancer materials. J. Inorg. Biochem. 2017, 167, 36–48. [Google Scholar] [CrossRef]
- Al-Hasawi, N.A.; Amine, S.A.; Novotny, L. The In Vitro Anti-Proliferative Interaction of Flavonoid Quercetin and Toxic Metal Cadmium in the 1321N1 Human Astrocytoma Cell Line. Sci. Pharm. 2018, 86, 36. [Google Scholar] [CrossRef]
- Pingili, R.B.; Challa, S.R.; Pawar, A.K.; Toleti, V.; Kodali, T.; Koppula, S. A systematic review on hepatoprotective activity of quercetin against various drugs and toxic agents: Evidence from preclinical studies. Phytother. Res. 2020, 34, 5–32. [Google Scholar] [CrossRef]
- Capriglione, F.; Maiuolo, J.; Celano, M.; Damante, G.; Russo, D.; Bulotta, S.; Maggisano, V. Quercetin Protects Human Thyroid Cells against Cadmium Toxicity. Int. J. Mol. Sci. 2021, 22, 6849. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Ding, L.; Zhao, P.; Zhang, C.; Wang, H.; Yang, Z.; Liu, Z. Alleviating effect of quercetin on cadmium-induced oxidative damage and apoptosis by activating the Nrf2-keap1 pathway in BRL-3A cells. Front. Pharmacol. 2022, 13, 969892. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhu, H.; Huang, R.; Yan, B.; Xu, B.; Shi, Y.; Mao, J.; Liu, Z.; Wang, J. Roles of Cyt-c/Caspase-9/Caspase-3/Bax/Bcl-2 pathway in Cd-induced testicular injury in rats and the protective effect of quercetin. Toxicon 2024, 237, 107561. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Ju, W.S.; Kim, K.; Kim, J.; Yu, J.O.; Ryu, J.S.; Kim, J.S.; Lee, H.A.; Koo, D.B.; Choo, Y.K. Quercetin Induces Mitochondrial Apoptosis and Downregulates Ganglioside GD3 Expression in Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 5146. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yan, T.; Zhang, X.; Yan, T.; Wang, Z. Tumor microenvironment-responsive diagnosis and treatment of integrated drug-loaded CdTe quantum dots for treatment tumors. Nanotechnology 2025, 36, 235101. [Google Scholar] [CrossRef]
- Silva-Pinto, P.A.; de Pontes, J.T.C.; Aguilar-Morón, B.; Canales, C.S.C.; Pavan, F.R.; Roque-Borda, C.A. Phytochemical insights into flavonoids in cancer: Mechanisms, therapeutic potential, and the case of quercetin. Heliyon 2025, 1, e42682. [Google Scholar] [CrossRef]
- De Marchi, U.; Biasutto, L.; Garbisa, S.; Toninello, A.; Zoratti, M. Quercetin can act either as an inhibitor or an inducer of the mitochondrial permeability transition pore: A demonstration of the ambivalent redox character of polyphenols. Biochim. Biophys. Acta 2009, 1787, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ramirez, V.D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 2000, 130, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Graziani, I.; Lopez-Sanchez, C.; Garcia-Martinez, V.; Gutierrez-Merino, C. Complex I and cytochrome C are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim. Biophys. Acta 2011, 1807, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Acuña, C.; Lopez-Alarcón, C.; Aliaga, M.E.; Speisky, H. Inhibition of mitochondrial complex I by various non-steroidal anti-inflammatory drugs and its protection by quercetin via a coenzyme Q-like action. Chem. Biol. Interact. 2012, 199, 18–28. [Google Scholar] [CrossRef]
- Dabrowska, A.; Zajac, M.; Bednarczyk, P.; Lukasiak, A. Effect of Quercetin on mitoBKCa Channel and Mitochondrial Function in Human Bronchial Epithelial Cells Exposed to Particulate Matter. Int. J. Mol. Sci. 2022, 24, 638. [Google Scholar] [CrossRef]
- Kampa, R.P.; Gliździńska, A.; Szewczyk, A.; Bednarczyk, P.; Filipek, S. Flavonoid quercetin abolish paxilline inhibition of the mitochondrial BKCa channel. Mitochondrion 2022, 65, 23–32. [Google Scholar] [CrossRef]
- Sanchez, M.; McManus, O.B. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 1996, 35, 963–968. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, X.M.; Lingle, C.J. The functionally relevant site for paxilline inhibition of BK channels. Proc. Natl. Acad. Sci. USA 2020, 117, 1021–1026. [Google Scholar] [CrossRef]
- Longland, C.L.; Dyer, J.L.; Michelangeli, F. The mycotoxin paxilline inhibits the cerebellar inositol 1,4,5-trisphosphate receptor. Eur. J. Pharmacol. 2000, 408, 219–225. [Google Scholar] [CrossRef]
- Bilmen, J.G.; Wootton, L.L.; Michelangeli, F. The mechanism of inhibition of the sarco/endoplasmic reticulum Ca2+-ATPase by paxilline. Arch. Biochem. Biophys. 2002, 406, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kamitsuka, P.J.; Ghanem, M.M.; Ziar, R.; McDonald, S.E.; Thomas, M.G.; Kwakye, G.F. Defective Mitochondrial Dynamics and Protein Degradation Pathways Underlie Cadmium-Induced Neurotoxicity and Cell Death in Huntington’s Disease Striatal Cells. Int. J. Mol. Sci. 2023, 24, 7178. [Google Scholar] [CrossRef]
- Vijiyakumar, N.; Prince, S.E. A comprehensive review of cadmium-induced toxicity, signalling pathways, and potential mitigation strategies. Toxicol. Environ. Health Sci. 2025, 17, 79–94. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tokumoto, M.; Satoh, M. Molecular Mechanisms of Cadmium-Induced Toxicity and Its Modification. Int. J. Mol. Sci. 2025, 26, 7515. [Google Scholar] [CrossRef]
- Dymkowska, D.; Szczepanowska, J.; Wieckowski, M.R.; Wojtczak, L. Short-term and long-term effects of fatty acids in rat hepatoma AS-30D cells: The way to apoptosis. Biochim. Biophys. Acta 2006, 1763, 152–163. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]



| Time | Respiration Status | Control | NS004 | NS1619 |
|---|---|---|---|---|
| 3 h | Steady state | 35 ± 5 | 41 ± 3 | 43 ± 3 |
| + Oligo (St4o) | 16 ± 2 | 20 ± 1 * | 23 ± 2 * | |
| + FCCP (St3u) | 100 | 100 | 102 ± 2 | |
| 5 h | Steady state | 40 ± 4 | 38 ± 2 | 33 ± 3 |
| + Oligo (St4o) | 19 ± 1 | 14 ± 4 | 19 ± 1 | |
| + FCCP (St3u) | 100 | 80 ± 3 * | 92 ± 5 * | |
| 24 h | Steady state | 53 ± 7 | 39 ± 9 | 52 ± 14 |
| + Oligo (St4o) | 22 ± 3 | 21 ± 5 | 23 ± 8 | |
| + FCCP (St3u) | 100 | 82 ± 4 * | 83 ± 4 * |
| Time | [Cd], µM | (None) | NS004 (10 µM) | NS1619 (10 µM) | NS004 (30 µM) | NS1619 (30 µM) |
|---|---|---|---|---|---|---|
| 3 h | 0 | 100 | 97 ± 3 | 95 ± 5 | 92 ± 8 | 93 ± 7 |
| 10 | 97 ± 4 | n.d. | n.d. | n.d. | n.d. | |
| 30 | 95 ± 5 | n.d. | n.d. | n.d. | n.d. | |
| 50 | 94 ± 6 | 94 ± 7 | 98 ± 10 | 98 ± 8 | 90 ± 11 | |
| 100 | 93 ± 7 | 93 ± 9 | 95 ± 5 | 94 ± 14 | 91 ± 16 | |
| 500 | 31 ± 5 * | 30 ± 6 * | 35 ± 11 * | 35 ± 7 * | 30 ± 6 * | |
| 5 h | 0 | 100 | 93 ± 7 | 99 ± 6 | 94 ± 6 | 94 ± 5 |
| 50 | 109 ± 11 | 107 ± 8 | 104 ± 5 | 101 ± 9 | 96 ± 5 | |
| 100 | 114 ± 15 | 99 ± 5 | 95 ± 5 | 86 ± 7 *§ | 85 ± 6 *§ | |
| 16 h | 0 | 100 | 100 ± 3 | 106 ± 6 | 95 ± 5 | 97 ± 8 |
| 10 | 97 ± 4 | n.d. | n.d. | n.d. | n.d. | |
| 30 | 95 ± 5 | n.d. | n.d. | n.d. | n.d. | |
| 50 | 59 ± 7 * | 69 ± 11 * | 67 ± 9 * | 61 ± 6 * | 62 ± 9 * | |
| 100 | 25 ± 2 * | n.d. | n.d. | n.d. | n.d. | |
| 24 h | 0 | 100 | 98 ± 4 | 99 ± 5 | 85 ± 5 * | 92 ± 1 * |
| 10 | 105 ± 5 | n.d. | n.d. | n.d. | n.d. | |
| 30 | 89 ± 5 * | n.d. | n.d. | n.d. | n.d. | |
| 50 | 47 ± 6 * | 54 ± 4 * | 51 ± 6 * | 26 ± 1 *§ | 17 ± 1 *§ | |
| 100 | 16 ± 4 * | 17 ± 7 * | 19 ± 7 * | 13 ± 5 * | 13 ± 4 * |
| Time | Additions | ROS Production (a.u.) |
|---|---|---|
| 30 min | None | 3.0913 ± 0.0743 |
| NS1619 | 3.2022 ± 0.2396 | |
| NS1619 + Pax | 2.798 ± 0.66 | |
| NS004 | 3.0538 ± 0.1218 | |
| NS004 + Pax | 2.868 ± 0.154 | |
| Pax | 3.2765 ± 0.1765 | |
| Cd | 3.649 ± 0.1388 * | |
| Cd + Pax | 3.3155 ± 0.1792 § | |
| Cd + NS1619 | 3.3842 ± 0.2488 | |
| Cd + NS1619 + Pax | 2.684 ± 0.1004 *§† | |
| Cd + NS004 | 3.4575 ± 0.4532 | |
| Cd + NS004 + Pax | 2.702 ± 0.2435 *§† | |
| 3 h | None | 6.355 ± 0.347 |
| NS1619 | 9.4865 ± 0.6135 * | |
| NS1619 + Pax | 7.052 ± 0.268 # | |
| Pax | 6.445 ± 0.345 | |
| Cd | 9.969 ± 0.602 * | |
| Cd + Pax | 8.6005 ± 0.5005 *§ | |
| Cd + NS1619 | 11.363 ± 0.063 *§# | |
| Cd + NS1619 + Pax | 8.412 ± 0.597 *§$ |
| Time | Additions | ROS Production (%) |
|---|---|---|
| 50 min | None | 100 |
| NS004 | 68 ± 5 * | |
| NS1619 | 60 ± 3 * | |
| Pax | 120 ± 22 | |
| Cd | 121 ± 3 * | |
| Cd + NS004 | 84 ± 10 § | |
| Cd + NS1619 | 111 ± 5 § | |
| Cd + Pax | 120 ± 9 * | |
| 3 h | None | 100 |
| NS004 | 149 ± 15 * | |
| NS1619 | 151 ± 15 * | |
| Pax | 165 ± 29 * | |
| Cd | 129 ± 18 * | |
| Cd + NS004 | 146 ± 10 * | |
| Cd + NS1619 | 163 ± 16 * | |
| Cd + Pax | 147 ± 20 * | |
| 24 h | None | 100 |
| NS004 | 118 ± 25 | |
| NS1619 | 116 ± 3 * | |
| Pax | 149 ± 20 * | |
| Cd | 105 ± 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belyaeva, E.A.; Sokolova, T.V. Application of Modulators of Ca2+-Activated Big-Conductance Potassium Channels Against Cd2+-Induced Cytotoxicity: A Study on Two Rat Cell Lines, PC12 and AS-30D. Int. J. Mol. Sci. 2025, 26, 10048. https://doi.org/10.3390/ijms262010048
Belyaeva EA, Sokolova TV. Application of Modulators of Ca2+-Activated Big-Conductance Potassium Channels Against Cd2+-Induced Cytotoxicity: A Study on Two Rat Cell Lines, PC12 and AS-30D. International Journal of Molecular Sciences. 2025; 26(20):10048. https://doi.org/10.3390/ijms262010048
Chicago/Turabian StyleBelyaeva, Elena A., and Tatyana V. Sokolova. 2025. "Application of Modulators of Ca2+-Activated Big-Conductance Potassium Channels Against Cd2+-Induced Cytotoxicity: A Study on Two Rat Cell Lines, PC12 and AS-30D" International Journal of Molecular Sciences 26, no. 20: 10048. https://doi.org/10.3390/ijms262010048
APA StyleBelyaeva, E. A., & Sokolova, T. V. (2025). Application of Modulators of Ca2+-Activated Big-Conductance Potassium Channels Against Cd2+-Induced Cytotoxicity: A Study on Two Rat Cell Lines, PC12 and AS-30D. International Journal of Molecular Sciences, 26(20), 10048. https://doi.org/10.3390/ijms262010048






