The Anti-Apoptotic Effect of C-Type Natriuretic Peptide and the Regulation of NPPC in Porcine Ovarian Granulosa Cells
Abstract
1. Introduction
2. Results
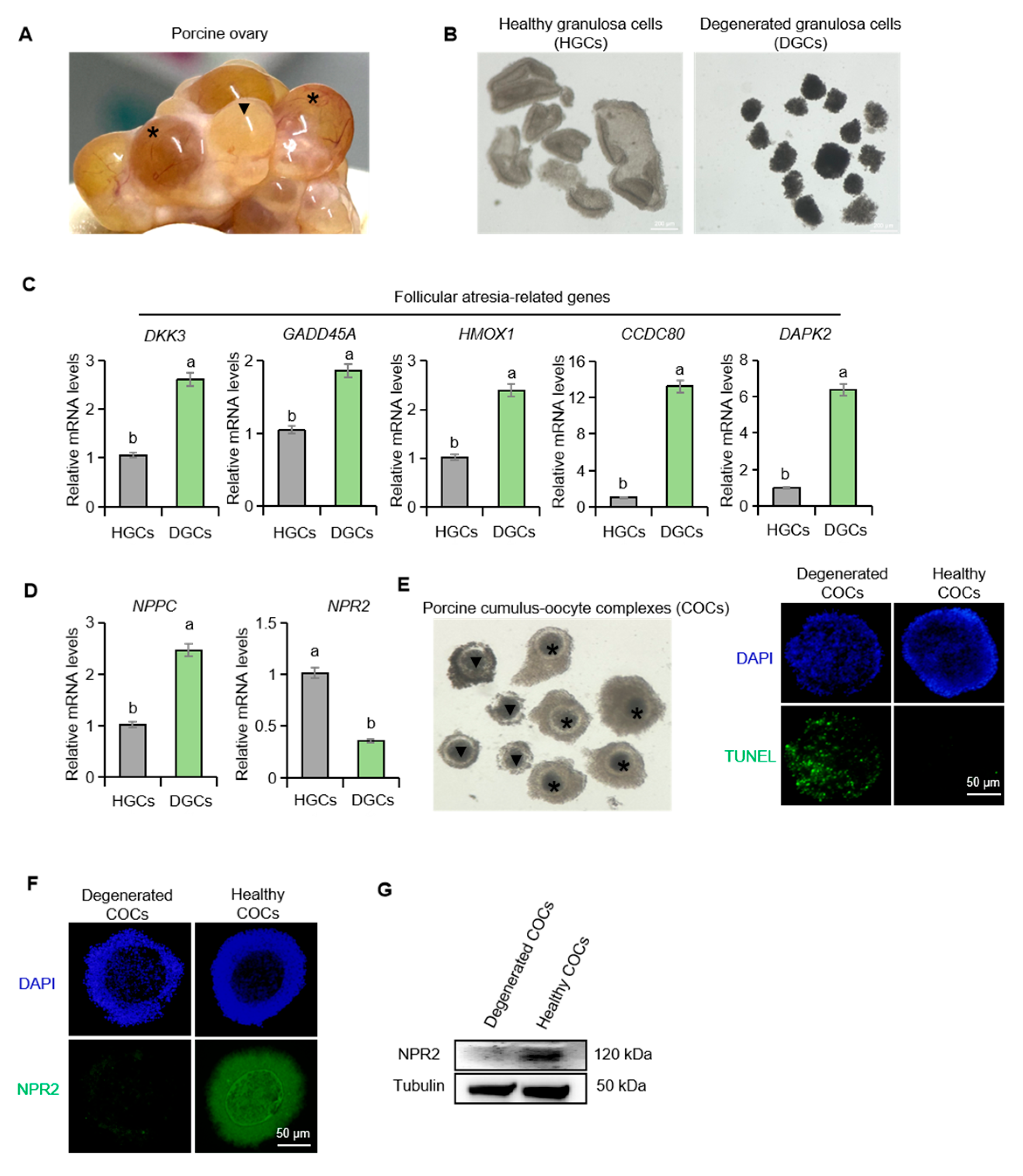
2.1. Differential Expression of NPPC and NPR2 in Healthy and Atretic Porcine Follicles
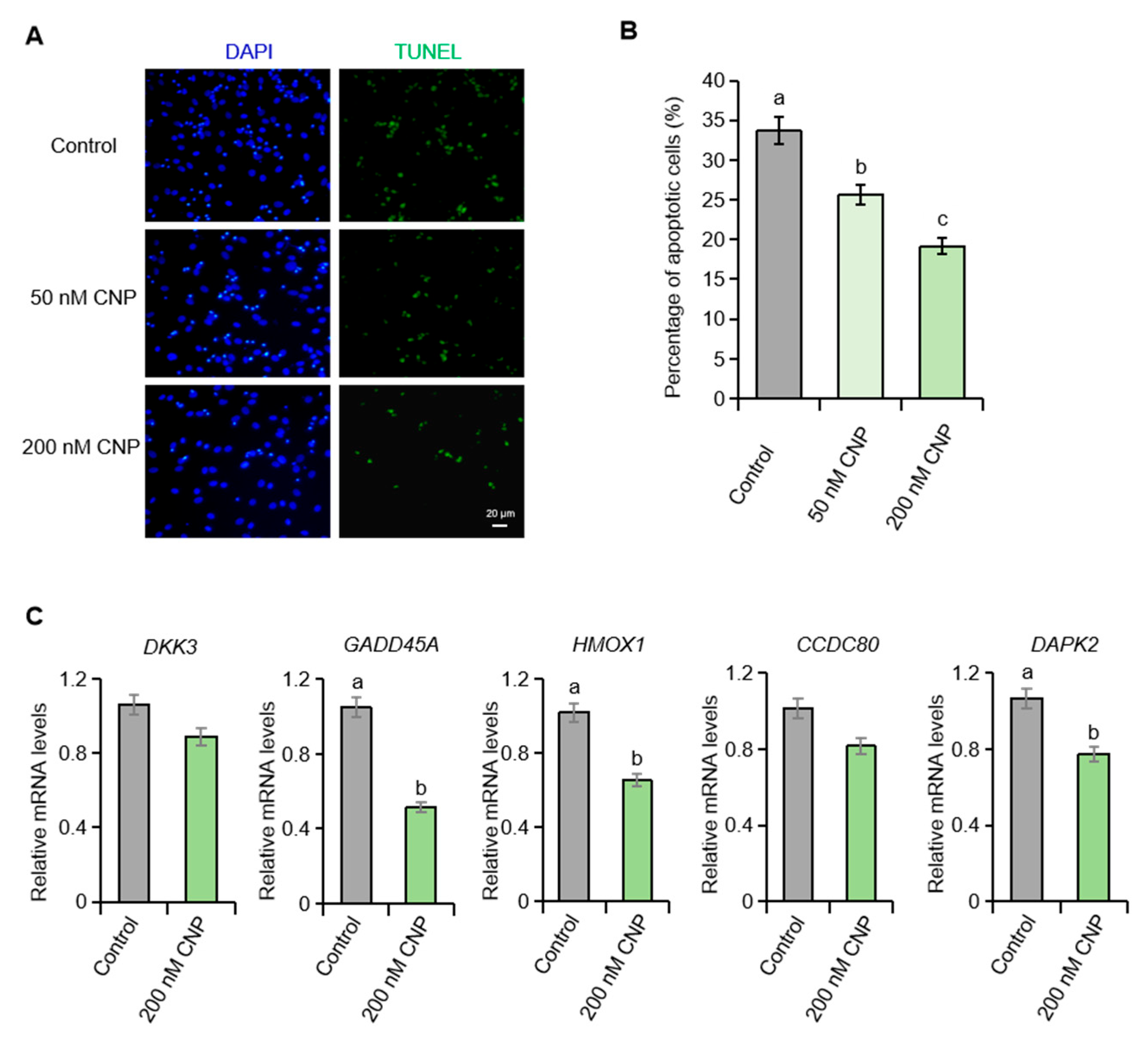
2.2. CNP Suppresses Apoptosis in Porcine Granulosa Cells Cultured In Vitro
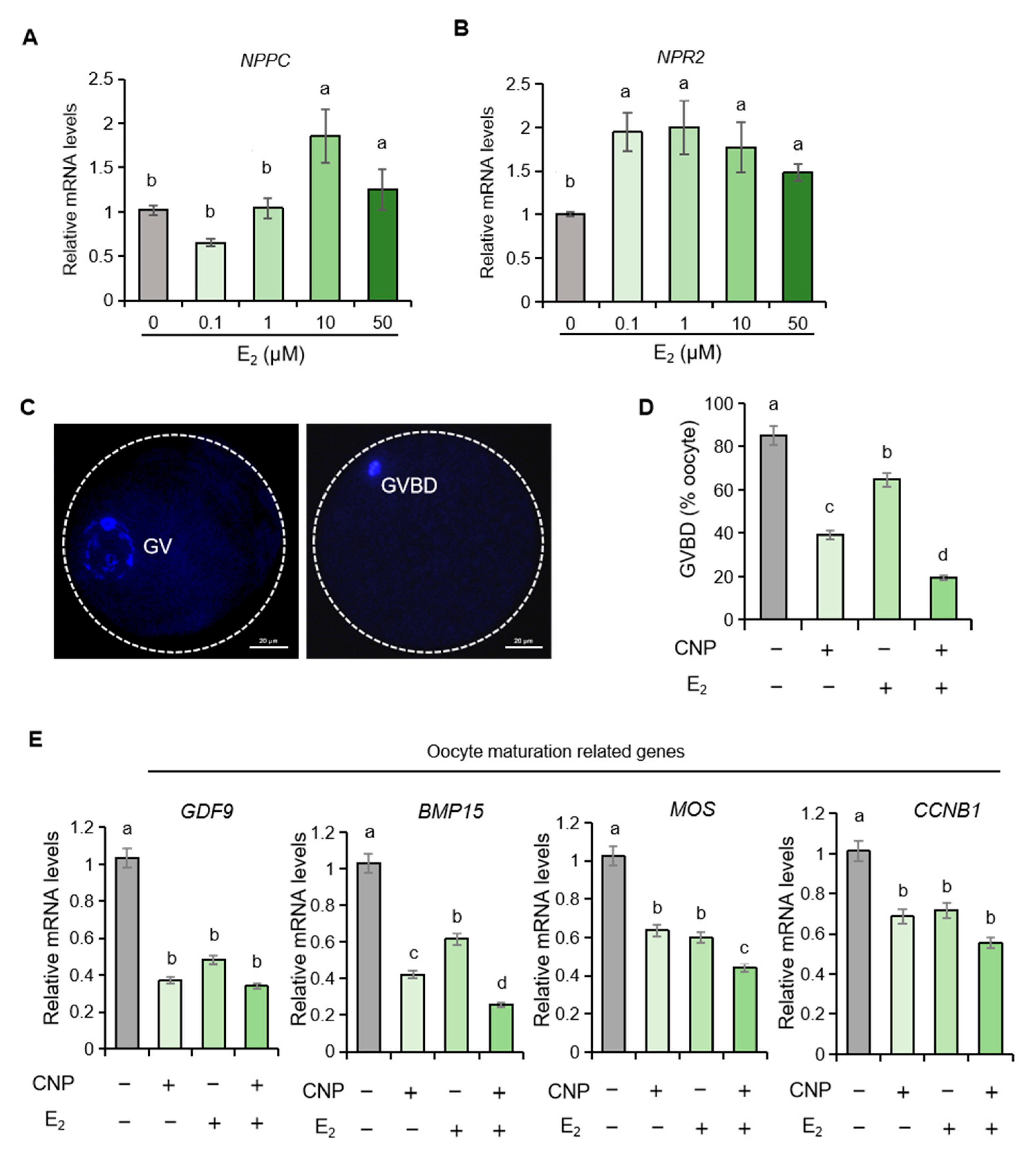
2.3. Estrogen Upregulates the mRNA Levels of NPPC and NPR2 in Porcine Granulosa Cells
2.4. Estrogen Synergizes with CNP to Orchestrate Meiotic Resumption in Porcine Oocytes
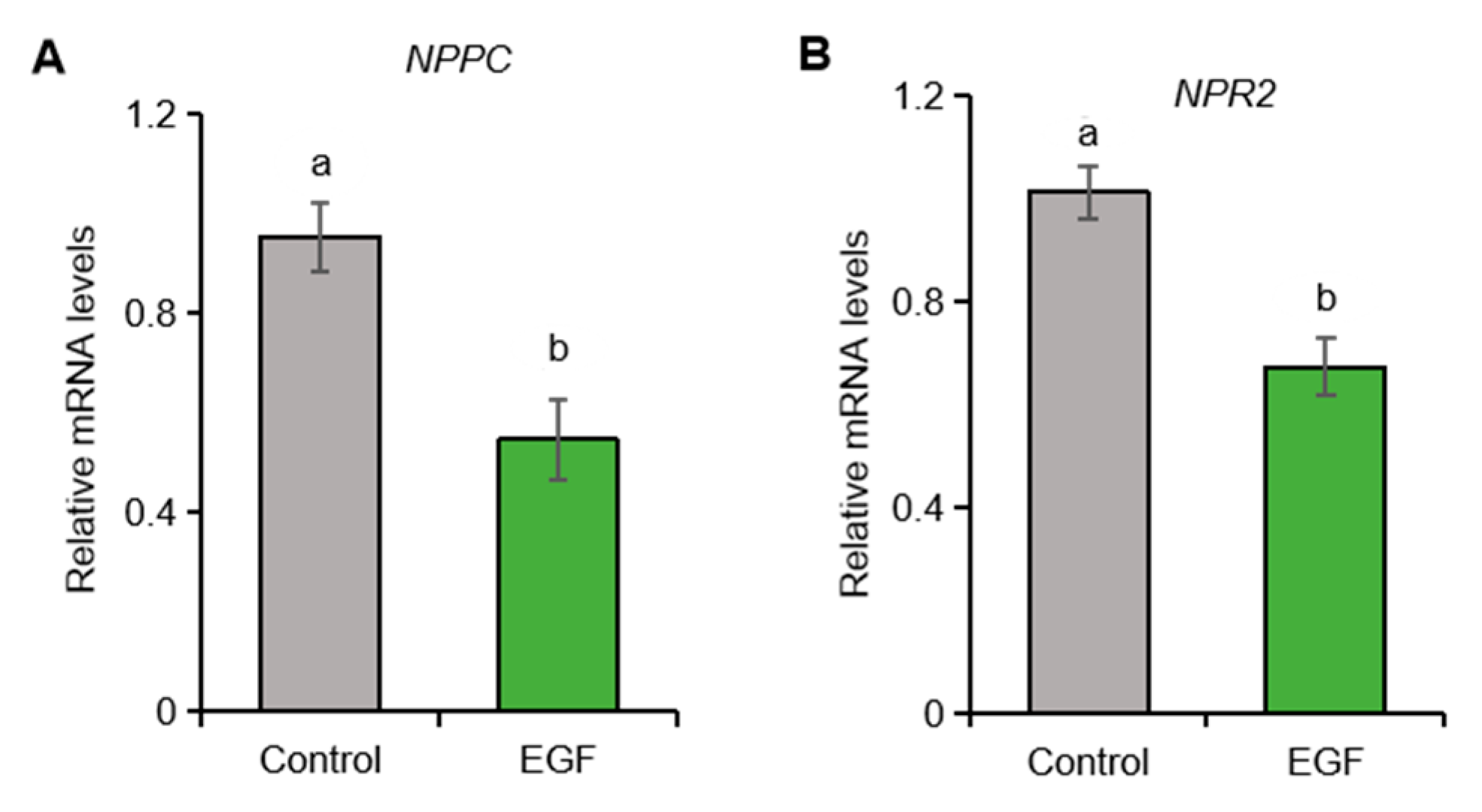
2.5. EGF Suppresses the Expression of NPPC and NPR2 in Porcine Granulosa Cells
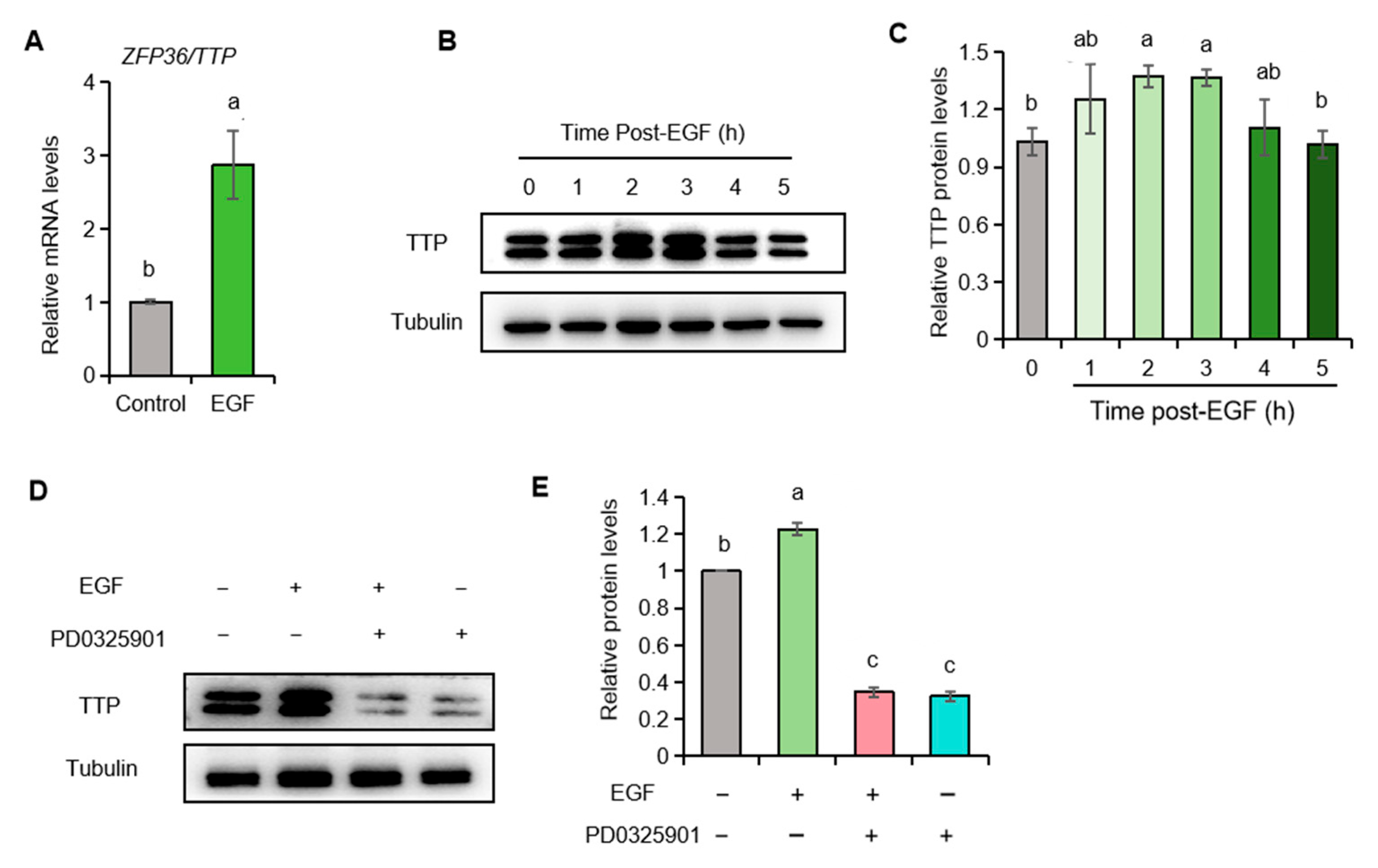
2.6. EGF Promotes ZFP36/TTP Expression in Porcine Granulosa Cells
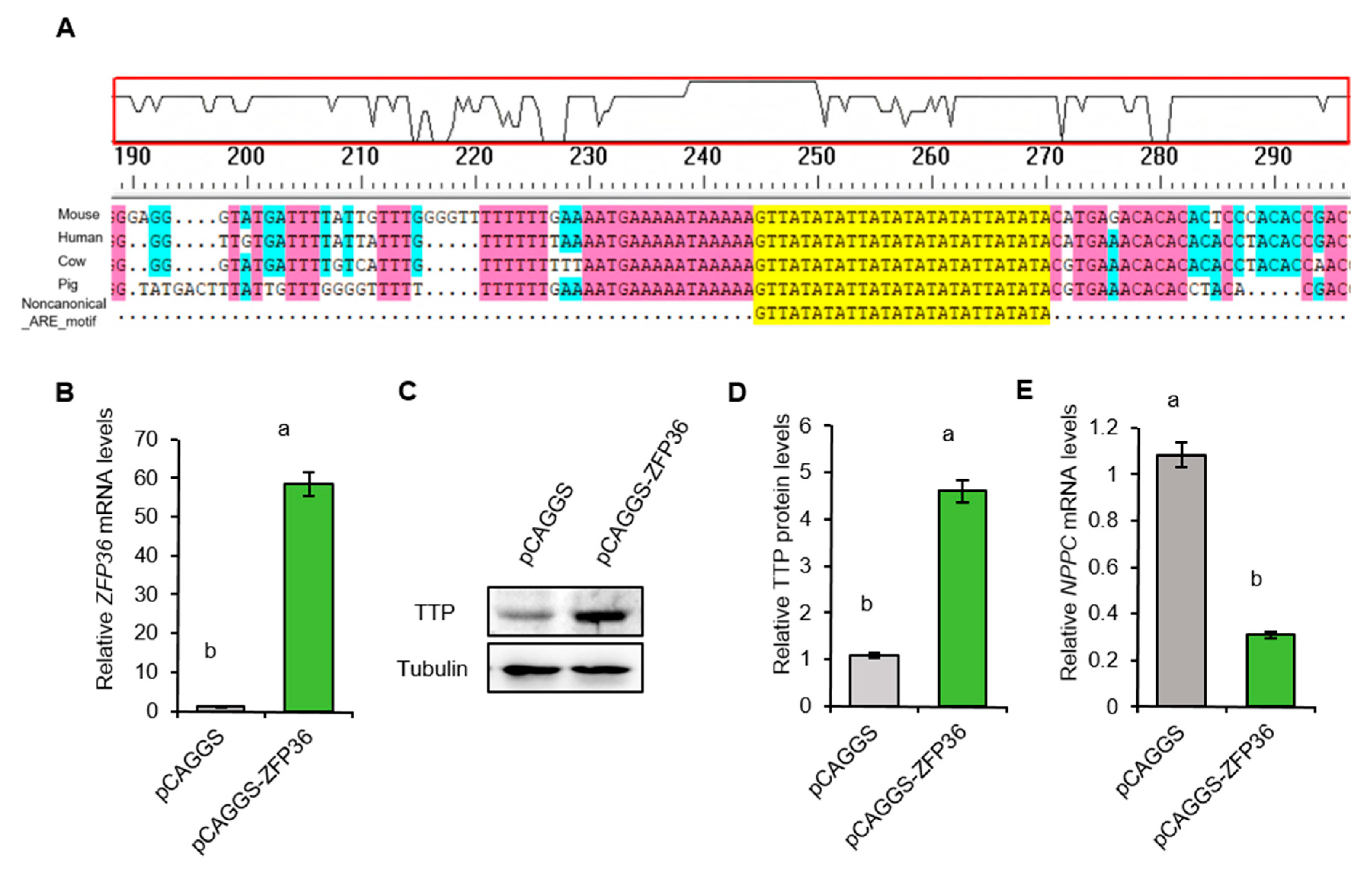
2.7. TTP Downregulates NPPC mRNA in Porcine Granulosa Cells
3. Discussion
4. Materials and Methods
4.1. Isolation and Cultivation of Porcine Granulosa Cells
4.2. Isolation and Cultivation of Porcine COCs
4.3. RNA Extraction and Quantitative Real-Time RT-PCR
4.4. Western Blot Analysis
4.5. Immunofluorescence
4.6. TdT-Mediated dUTP Nick-End Labeling (TUNEL) Assay
4.7. Analysis of Nuclear Stages of Oocytes
4.8. Construction and Transfection of Overexpression Vectors
4.9. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rintz, E.; Wegrzyn, G.; Fujii, T.; Tomatsu, S. Molecular Mechanism of Induction of Bone Growth by the C-Type Natriuretic Peptide. Int. J. Mol. Sci. 2022, 23, 5916. [Google Scholar] [CrossRef]
- Potter, L.R.; Abbey-Hosch, S.; Dickey, D.M. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev. 2006, 27, 47–72. [Google Scholar] [CrossRef]
- Straczynska, P.; Papis, K.; Morawiec, E.; Czerwinski, M.; Gajewski, Z.; Olejek, A.; Bednarska-Czerwinska, A. Signaling mechanisms and their regulation during in vivo or in vitro maturation of mammalian oocytes. Reprod. Biol. Endocrin. 2022, 20, 37. [Google Scholar] [CrossRef]
- Xi, G.; An, L.; Jia, Z.; Tan, K.; Zhang, J.; Wang, Z.; Zhang, C.; Miao, K.; Wu, Z.; Tian, J. Natriuretic peptide receptor 2 (NPR2) localized in bovine oocyte underlies a unique mechanism for C-type natriuretic peptide (CNP)-induced meiotic arrest. Theriogenology 2018, 106, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Pan, M.; Yin, L.; Zhang, Y.; Tang, Y.; Lu, S.; Gao, Y.; Wei, Q.; Han, B.; Ma, B. C-Type Natriuretic Peptide Pre-Treatment Improves Maturation Rate of Goat Oocytes by Maintaining Transzonal Projections, Spindle Morphology, and Mitochondrial Function. Animals 2023, 13, 3880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Fan, X.; Li, R.; Zhang, C.; Zhang, J. Effects of pre-incubation with C-type natriuretic peptide on nuclear maturation, mitochondrial behavior, and developmental competence of sheep oocytes. Biochem. Bioph. Res. Commun. 2018, 497, 200–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Liu, W.; Yang, Y.; Wang, X.; Zhang, Z.; Guo, Q.; Wang, C.; Xia, G. Natriuretic peptides improve the developmental competence of in vitro cultured porcine oocytes. Reprod. Biol. Endocrin. 2017, 15, 41. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Ho, T.M.; De Vos, M.; Sanchez, F.; Romero, S.; Ledger, W.L.; Anckaert, E.; Vuong, L.N.; Smitz, J. A fresh start for IVM: Capacitating the oocyte for development using pre-IVM. Hum. Reprod. Update 2024, 30, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Fakhar-I-Adil, M.; Angel-Velez, D.; Araftpoor, E.; Amin, Q.A.; Hedia, M.; Bühler, M.; Gevaert, K.; Menten, B.; Van Soom, A.; Chuva De Sousa Lopes, S.M.; et al. Biphasic CAPA-IVM Improves Equine Oocyte Quality and Subsequent Embryo Development Without Inducing Genetic Aberrations. Int. J. Mol. Sci. 2025, 26, 5495. [Google Scholar] [CrossRef]
- Zhong, Y.; Lin, J.; Liu, X.; Hou, J.; Zhang, Y.; Zhao, X. C-Type natriuretic peptide maintains domestic cat oocytes in meiotic arrest. Reprod. Fert. Develop. 2016, 28, 1553–1559. [Google Scholar] [CrossRef]
- Tang, Y.; Cui, J.; Wang, X.; Yang, Q.; Yue, Y.; Gao, C.; Wang, Y.; Wang, W.; Zhang, S.; Tian, J.; et al. “Meiosis arrester” C-natriuretic peptide directly stimulates oocyte mtDNA accumulation and is implicated in aging-associated oocyte mtDNA loss. Faseb J. 2023, 37, e23295. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Liu, Q.; Li, J.; Wu, H.; Xu, R.; Sun, Y.; Cheng, M.; Zhao, X.; Pan, M.; et al. C-type natriuretic peptide improves maternally aged oocytes quality by inhibiting excessive PINK1/Parkin-mediated mitophagy. Elife 2023, 12, RP88523. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.; Sugiura, K.; Wigglesworth, K.; Xia, G.; Eppig, J.J. Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology 2011, 152, 4377–4385. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Q.; Yang, Y.; Liu, W.; Zhang, M.; Xia, G.; Wang, C. Epidermal growth factor-network signaling mediates luteinizing hormone regulation of BNP and CNP and their receptor NPR2 during porcine oocyte meiotic resumption. Mol. Reprod. Dev. 2014, 81, 1030–1041. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Kawamura, N.; Takae, S.; Okada, A.; Kawagoe, Y.; Mulders, S.; Terada, Y.; Hsueh, A.J.W. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum. Reprod. 2011, 26, 3094–3101. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Xu, X.; Li, J.; Yuan, F.; Bo, S.; Qiao, J.; Xia, G.; Su, Y.; Zhang, M. Transforming growth factor-beta is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells. Cell Death Dis. 2019, 10, 558. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Liu, Z.; Li, B.; Teng, Y.; Zhang, J.; Tang, Y.; Sun, S.; Liu, H. Involvement of FoxO1 in the effects of follicle-stimulating hormone on inhibition of apoptosis in mouse granulosa cells. Cell Death Dis. 2014, 5, e1475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Baumgarten, S.C.; Wu, Y.; Bennett, J.; Winston, N.; Hirshfeld-Cytron, J.; Stocco, C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol. Endocrinol. 2013, 27, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Z.; Li, W.; Zhang, L.; Zhou, J.; Sun, M.; Zhou, J.; Yao, W.; Zhang, X.; Wang, H.; et al. The FSH-HIF-1alpha-VEGF Pathway Is Critical for Ovulation and Oocyte Health but Not Necessary for Follicular Growth in Mice. Endocrinology 2020, 161, bqaa038. [Google Scholar] [CrossRef]
- Casarini, L.; Riccetti, L.; De Pascali, F.; Gilioli, L.; Marino, M.; Vecchi, E.; Morini, D.; Nicoli, A.; La Sala, G.B.; Simoni, M. Estrogen Modulates Specific Life and Death Signals Induced by LH and hCG in Human Primary Granulosa Cells In Vitro. Int. J. Mol. Sci. 2017, 18, 926. [Google Scholar] [CrossRef]
- Regan, S.L.P.; Knight, P.G.; Yovich, J.L.; Leung, Y.; Arfuso, F.; Dharmarajan, A. Granulosa Cell Apoptosis in the Ovarian Follicle-A Changing View. Front. Endocrinol. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Billig, H.; Furuta, I.; Hsueh, A.J. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology 1993, 133, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Celestino, J.J.H.; Chaves, R.N.; Matos, M.H.T.; Saraiva, M.V.A.; Bruno, J.B.; Maia-Júnior, J.E.; Silva, J.R.V.; Figueiredo, J.R. Mechanisms of atresia in ovarian follicles. Anim. Reprod. 2009, 6, 495–508. [Google Scholar]
- Xi, H.; Chen, X.; Wang, X.; Jiang, F.; Niu, D. Role of programmed cell death in mammalian ovarian follicular atresia. J. Steroid Biochem. 2025, 247, 106667. [Google Scholar] [CrossRef]
- Lu, J.; Guo, M.; Wang, X.; Wang, R.; Xi, G.; An, L.; Tian, J.; Chu, M. A Redesigned Method for CNP-Synchronized In Vitro Maturation Inhibits Oxidative Stress and Apoptosis in Cumulus-Oocyte Complexes and Improves the Developmental Potential of Porcine Oocytes. Genes 2023, 14, 1885. [Google Scholar] [CrossRef]
- Tsuji, T.; Kiyosu, C.; Akiyama, K.; Kunieda, T. CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol. Reprod. Dev. 2012, 79, 795–802. [Google Scholar] [CrossRef]
- Xi, G.; An, L.; Wang, W.; Hao, J.; Yang, Q.; Ma, L.; Lu, J.; Wang, Y.; Wang, W.; Zhao, W.; et al. The mRNA-destabilizing protein Tristetraprolin targets “meiosis arrester” Nppc mRNA in mammalian preovulatory follicles. Proc. Natl. Acad. Sci. USA 2021, 118, e2018345118. [Google Scholar] [CrossRef]
- Fortune, J.E.; Rivera, G.M.; Yang, M.Y. Follicular development: The role of the follicular microenvironment in selection of the dominant follicle. Anim. Reprod. Sci. 2004, 82–83, 109–126. [Google Scholar] [CrossRef]
- Shokrollahi, B.; Zheng, H.Y.; Ma, X.Y. The effects of apelin on IGF1/FSH-induced steroidogenesis, proliferation, Bax expression, and total antioxidant capacity in granulosa cells of buffalo ovarian follicles. Vet. Res. Commun. 2023, 47, 1523–1533. [Google Scholar] [CrossRef]
- Shokrollahi, B.; Shang, J.; Saadati, N.; Ahmad, H.I.; Yang, C. Reproductive roles of novel adipokines apelin, visfatin, and irisin in farm animals. Theriogenology 2021, 82, 172–178. [Google Scholar] [CrossRef]
- Kiyosu, C.; Tsuji, T.; Yamada, K.; Kajita, S.; Kunieda, T. NPPC/NPR2 signaling is essential for oocyte meiotic arrest and cumulus oophorus formation during follicular development in the mouse ovary. Reproduction 2012, 144, 187–193. [Google Scholar] [CrossRef]
- McGee, E.; Spears, N.; Minami, S.; Hsu, S.Y.; Chun, S.Y.; Billig, H.; Hsueh, A.J. Preantral ovarian follicles in serum-free culture: Suppression of apoptosis after activation of the cyclic guanosine 3′,5′-monophosphate pathway and stimulation of growth and differentiation by follicle-stimulating hormone. Endocrinology 1997, 138, 2417–2424. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Wang, W.; Fazlani, S.A.; Yao, F.; Yang, M.; Hao, J.; An, L.; Tian, J. C-type natriuretic peptide enhances mouse preantral follicle growth. Reproduction 2019, 157, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.; Itoh, H.; Saito, T.; Yamahara, K.; Doi, K.; Mori, Y.; Ogawa, Y.; Yamashita, J.; Tanaka, T.; Inoue, M. Oxidative stress augments secretion of endothelium-derived relaxing peptides, C-type natriuretic peptide and adrenomedullin. J. Hypertens. 2000, 18, 575–580. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, K.; Wang, C.C.; Tao, J.; Wu, Z.; Teerds, K.; Zhang, S. Characterization of Long Non-Coding RNA Profiles in Porcine Granulosa Cells of Healthy and Atretic Antral Follicles: Implications for a Potential Role in Apoptosis. Int. J. Mol. Sci. 2021, 22, 2677. [Google Scholar] [CrossRef]
- Ozay, A.C.; Ozay, O.E.; Edebal, O.H. Role of C-type natriuretic peptide in polycystic ovary syndrome. Int. J. Gynecol. Obstet. 2023, 161, 601–606. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Liu, W.; Zhang, Z.; Zhang, Y.; Zhang, W.; Chen, Z.; Xia, G.; Wang, C. High level of C-type natriuretic peptide induced by hyperandrogen-mediated anovulation in polycystic ovary syndrome mice. Clin. Sci. 2018, 132, 759–776. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Y.; Liu, W.; Chen, Q.; Wang, H.; Wang, X.; Zhang, Y.; Zhang, M.; Xia, G. Brain natriuretic peptide and C-type natriuretic peptide maintain porcine oocyte meiotic arrest. J. Cell. Physiol. 2015, 230, 71–81. [Google Scholar] [CrossRef]
- Herbison, A.E. The gonadotropin-releasing hormone pulse generator. Endocrinology 2018, 159, 3723–3736. [Google Scholar] [CrossRef] [PubMed]
- Constantin, S. Progress and challenges in the search for the mechanisms of pulsatile gonadotropin-releasing hormone secretion. Front. Endocrinol. 2017, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; He, Y.; Wang, W.; Chu, Y.; Lin, Q.; Rui, R.; Li, Q.; Ju, S. PAK1 Is Involved in the Spindle Assembly during the First Meiotic Division in Porcine Oocytes. Int. J. Mol. Sci. 2023, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lu, J.; Zeng, J.; An, L.; Tian, J.; Xi, G. The Anti-Apoptotic Effect of C-Type Natriuretic Peptide and the Regulation of NPPC in Porcine Ovarian Granulosa Cells. Int. J. Mol. Sci. 2025, 26, 10046. https://doi.org/10.3390/ijms262010046
Liu X, Lu J, Zeng J, An L, Tian J, Xi G. The Anti-Apoptotic Effect of C-Type Natriuretic Peptide and the Regulation of NPPC in Porcine Ovarian Granulosa Cells. International Journal of Molecular Sciences. 2025; 26(20):10046. https://doi.org/10.3390/ijms262010046
Chicago/Turabian StyleLiu, Xingyuan, Jinlun Lu, Junyi Zeng, Lei An, Jianhui Tian, and Guangyin Xi. 2025. "The Anti-Apoptotic Effect of C-Type Natriuretic Peptide and the Regulation of NPPC in Porcine Ovarian Granulosa Cells" International Journal of Molecular Sciences 26, no. 20: 10046. https://doi.org/10.3390/ijms262010046
APA StyleLiu, X., Lu, J., Zeng, J., An, L., Tian, J., & Xi, G. (2025). The Anti-Apoptotic Effect of C-Type Natriuretic Peptide and the Regulation of NPPC in Porcine Ovarian Granulosa Cells. International Journal of Molecular Sciences, 26(20), 10046. https://doi.org/10.3390/ijms262010046






