MicroRNAs in Tissue Regeneration: Lessons from Animal Models
Abstract
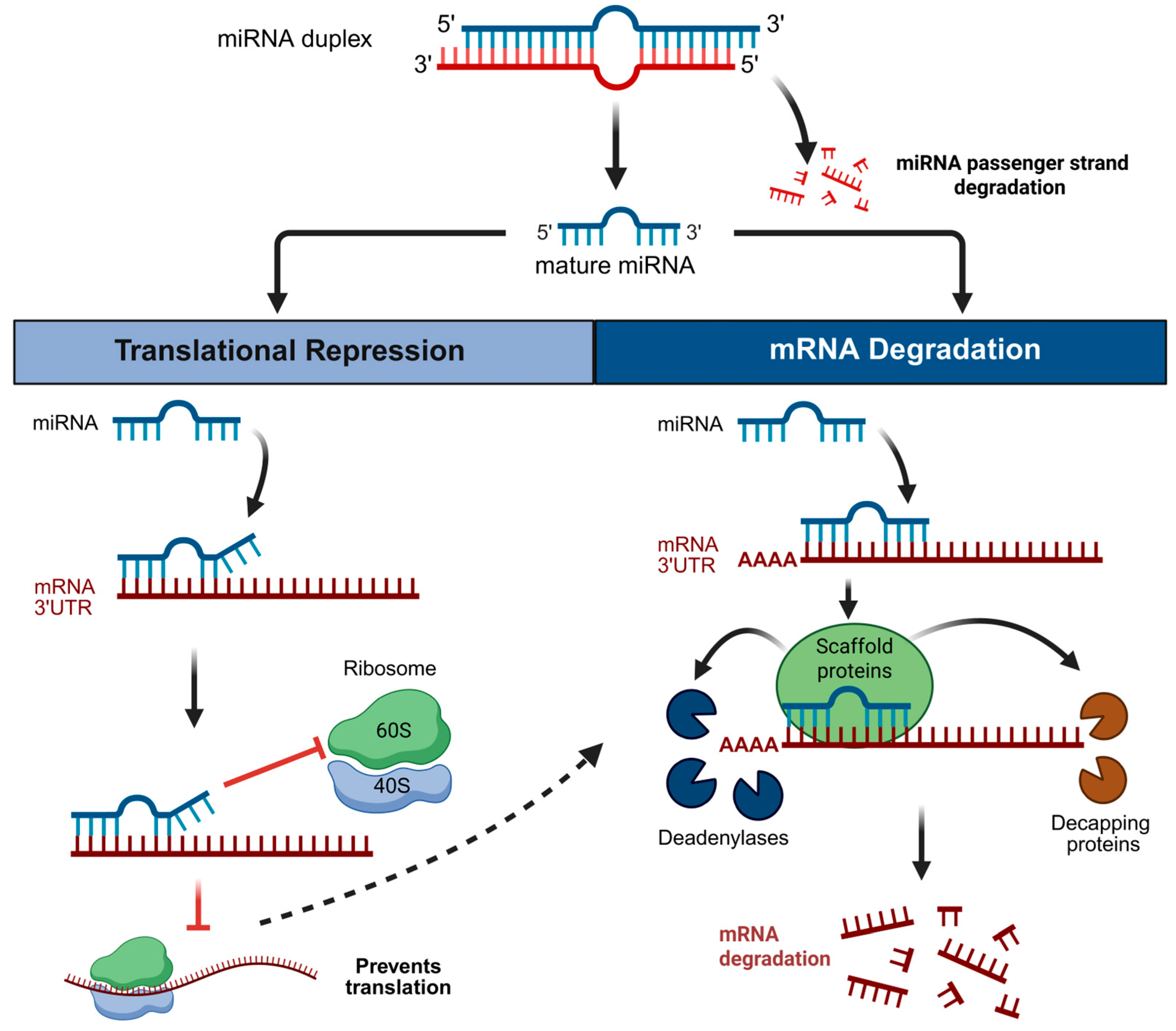
1. Introduction
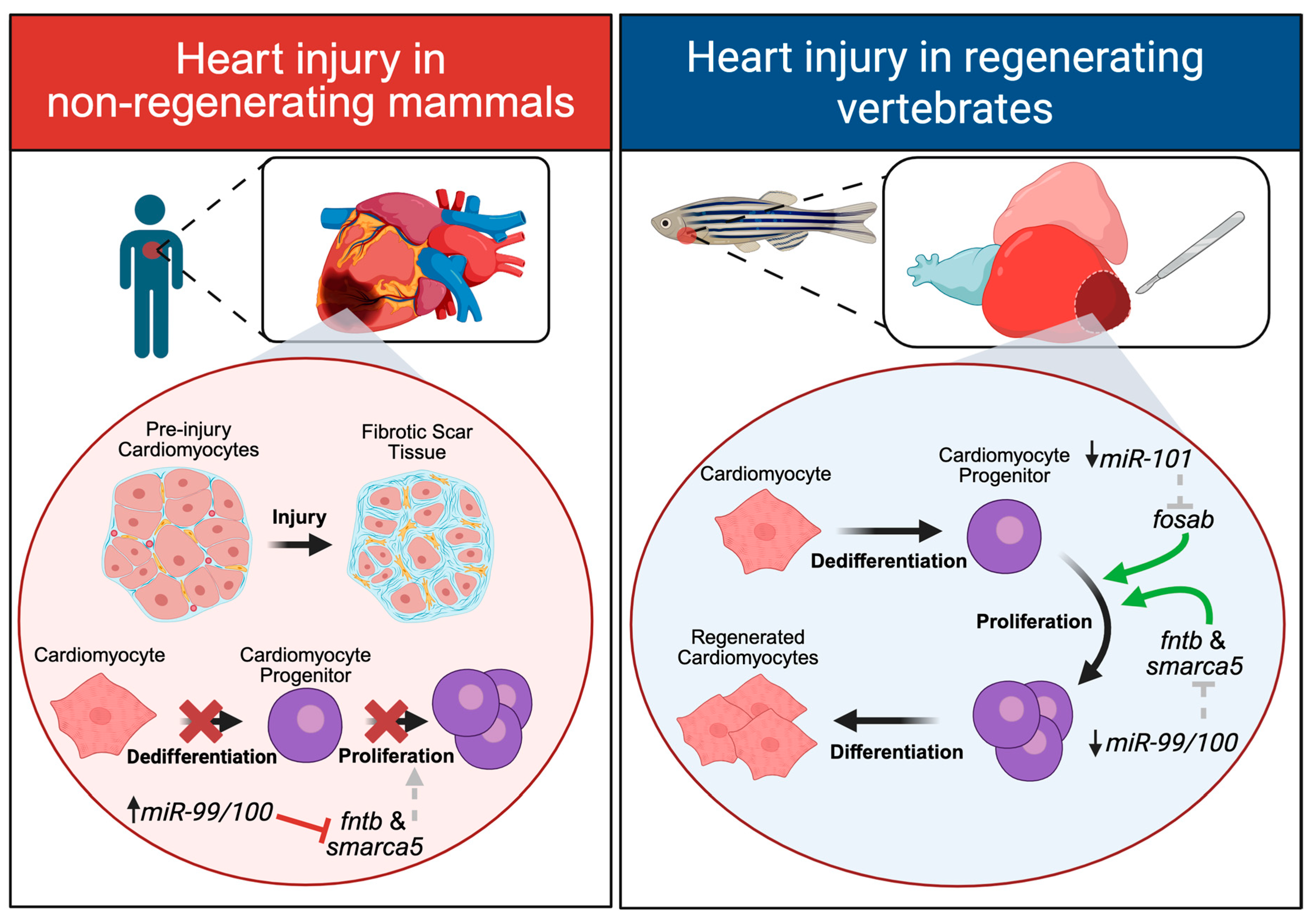
2. Heart Regeneration
microRNAs in Heart Regeneration
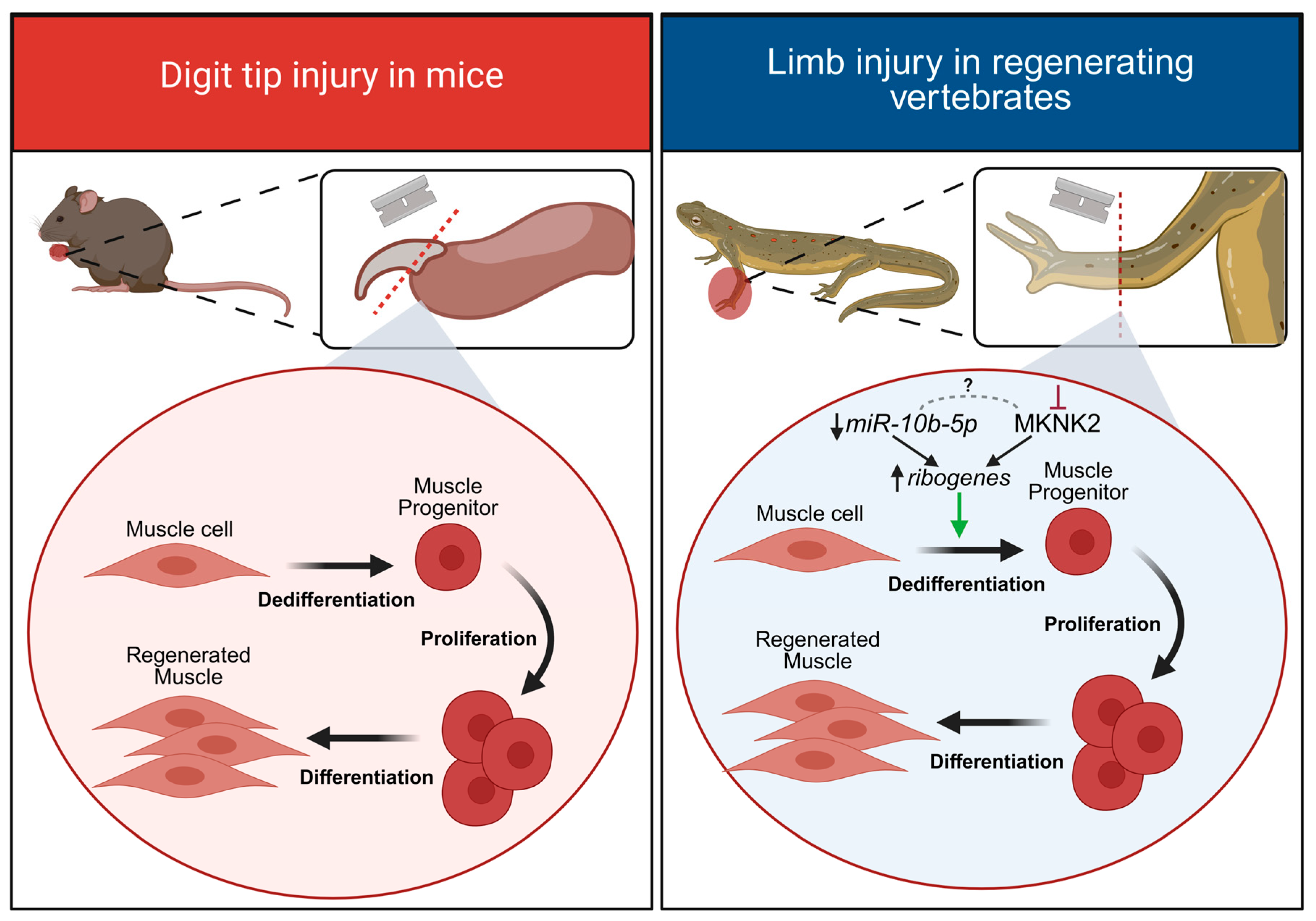
3. Limb Regeneration
microRNAs in Limb Regeneration
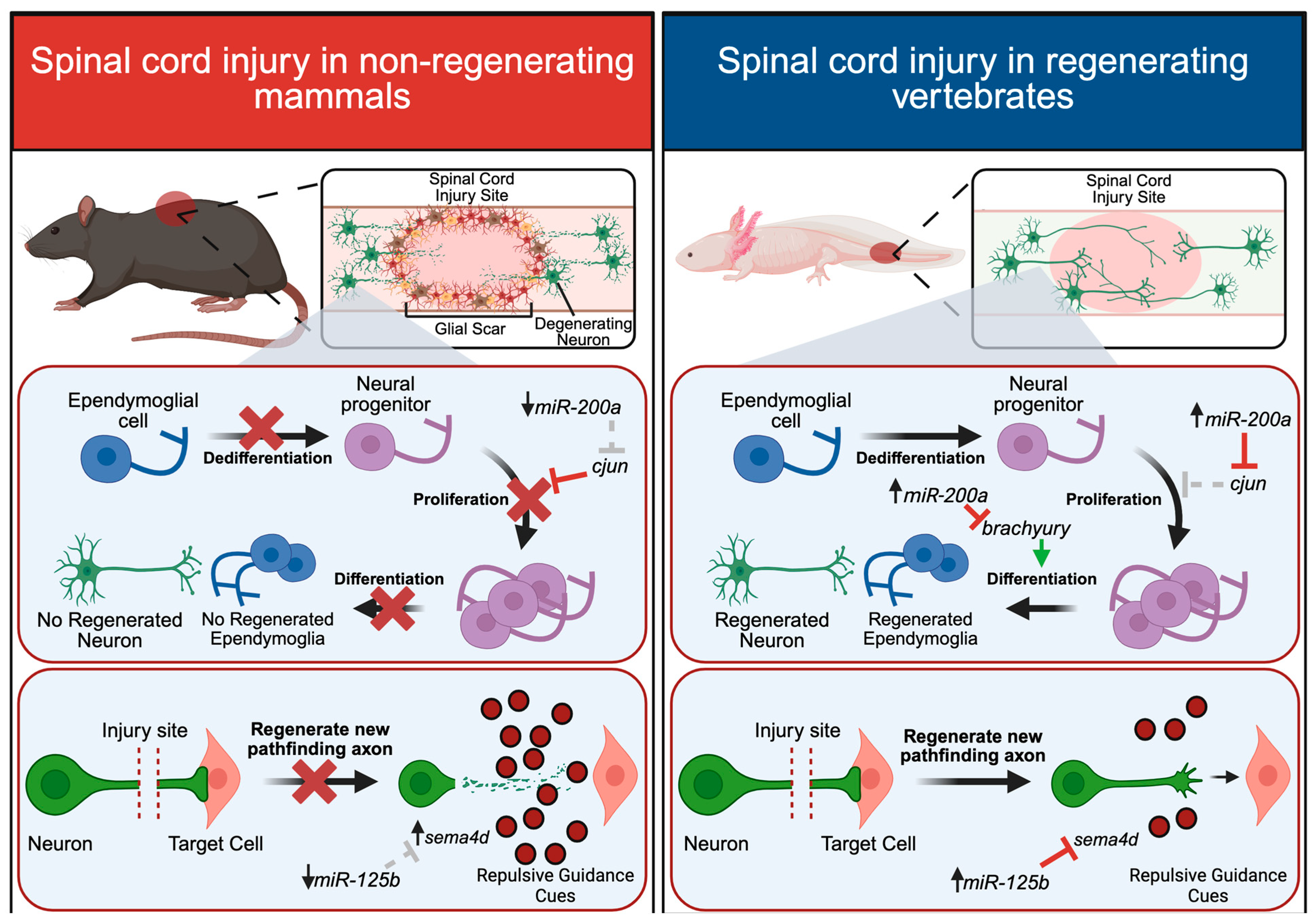
4. Spinal Cord Regeneration
4.1. microRNAs in Spinal Cord Regeneration
4.2. Neurite Outgrowth and Axon Guidance
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tremblay, A. Mémoires Pour Servir à L’histoire D’un Genre de Polypes D’eau Douce, à Bras en Forme de Cornes; Jean & Herman Verbeek: Leiden, The Netherlands, 1744. [Google Scholar]
- Galliot, B. Hydra, a fruitful model system for 270 years. Int. J. Dev. Biol. 2012, 56, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Bely, A.E.; Nyberg, K.G. Evolution of animal regeneration: Re-emergence of a field. Trends Ecol. Evol. 2010, 25, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.H. Experimental studies of the regeneration of planaria maculata. Arch. Für Entwickelungsmechanik Der Org. 1898, 7, 364–397. [Google Scholar]
- Steinböck, O. Regenerationsversuche mit Hofstenia giselae Steinb. (Turbellaria acoela). Wilhelm Roux’Archiv Entwicklungsmechanik Org. 1967, 158, 394–458. [Google Scholar] [CrossRef]
- Gemberling, M.; Bailey, T.J.; Hyde, D.R.; Poss, K.D. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2014, 29, 611–620. [Google Scholar] [CrossRef]
- Bölük, A.; Yavuz, M.; Demircan, T. Axolotl: A resourceful vertebrate model for regeneration and beyond. Dev. Dyn. 2022, 251, 1914–1933. [Google Scholar] [CrossRef]
- Aguirre, A.; Montserrat, N.; Zacchigna, S.; Nivet, E.; Hishida, T.; Krause, M.N.; Kurian, L.; Ocampo, A.; Vazquez-Ferrer, E.; Rodriguez-Esteban, C.; et al. In vivo activation of a conserved microRNA program induces mammalian heart regeneration. Cell Stem Cell 2014, 15, 589–604. [Google Scholar] [CrossRef]
- Diaz Quiroz, J.F.; Tsai, E.; Coyle, M.; Sehm, T.; Echeverri, K. Precise control of miR-125b levels is required to create a regeneration-permissive environment after spinal cord injury: A cross-species comparison between salamander and rat. Dis. Models Mech. 2014, 7, 601–611. [Google Scholar] [CrossRef]
- Beauchemin, M.; Smith, A.; Yin, V.P. Dynamic microRNA-101a and Fosab expression controls zebrafish heart regeneration. Development 2015, 142, 4026–4037. [Google Scholar] [CrossRef]
- Sen, C.K.; Ghatak, S. miRNA control of tissue repair and regeneration. Am. J. Pathol. 2015, 185, 2629–2640. [Google Scholar] [CrossRef] [PubMed]
- Sabin, K.Z.; Jiang, P.; Gearhart, M.D.; Stewart, R.; Echeverri, K. AP-1cFos/JunB/miR-200a regulate the pro-regenerative glial cell response during axolotl spinal cord regeneration. Commun. Biol. 2019, 2, 91. [Google Scholar] [CrossRef]
- Walker, S.E.; Sabin, K.Z.; Gearhart, M.D.; Yamamoto, K.; Echeverri, K. Regulation of stem cell identity by miR-200a during spinal cord regeneration. Development 2022, 149, dev200033. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Sehm, T.; Sachse, C.; Frenzel, C.; Echeverri, K. miR-196 is an essential early-stage regulator of tail regeneration, upstream of key spinal cord patterning events. Dev. Biol. 2009, 334, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Torrini, C.; John Cubero, R.; Dirkx, E.; Braga, L.; Ali, H.; Prosdocimo, G.; Ines Gutierrez, M.; Collesi, C.; Licastro, D.; Zentilin, L.; et al. Common regulatory pathways mediate activity of microRNAs inducing cardiomyocyte proliferation. Cell Rep. 2019, 27, 2759–2771. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, E.; Elewa, A.; Brito, G.; Kumar, A.; Segerstolpe, A.; Karampelias, C.; Bjorklund, A.; Sandberg, R.; Echeverri, K.; Lui, W.O.; et al. A small noncoding RNA links ribosome recovery and translation control to dedifferentiation during salamander limb regeneration. Dev. Cell 2023, 58, 450–460.e6. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Chang, K.W.; Kao, S.Y.; Wu, Y.H.; Tsai, M.M.; Tu, H.F.; Liu, C.J.; Lui, M.T.; Lin, S.C. Passenger strand miRNA miR-31* regulates the phenotypes of oral cancer cells by targeting RhoA. Oral Oncol. 2013, 49, 27–33. [Google Scholar] [CrossRef]
- Uchino, K.; Takeshita, F.; Takahashi, R.U.; Kosaka, N.; Fujiwara, K.; Naruoka, H.; Sonoke, S.; Yano, J.; Sasaki, H.; Nozawa, S.; et al. Therapeutic effects of microRNA-582-5p and -3p on the inhibition of bladder cancer progression. Mol. Ther. 2013, 21, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, W.W.; Li, H.; Liu, F.; Khorshidi, A.; Rutnam, Z.J.; Yang, B.B. Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Res. 2013, 41, 9688–9704. [Google Scholar] [CrossRef]
- Bazzini, A.A.; Lee, M.T.; Giraldez, A.J. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 2012, 336, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Béthune, J.; Artus-Revel, C.G.; Filipowicz, W. Kinetic analysis reveals successive steps leading to miRNA-mediated silencing in mammalian cells. EMBO Rep. 2012, 13, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Djuranovic, S.; Nahvi, A.; Green, R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 2012, 336, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Mathonnet, G.; Fabian, M.R.; Svitkin, Y.V.; Parsyan, A.; Huck, L.; Murata, T.; Biffo, S.; Merrick, W.C.; Darzynkiewicz, E.; Pillai, R.S.; et al. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science 2007, 317, 1764–1767. [Google Scholar] [CrossRef]
- Wang, B.; Yanez, A.; Novina, C.D. MicroRNA-repressed mRNAs contain 40S but not 60S components. Proc. Natl. Acad. Sci. USA 2008, 105, 5343–5348. [Google Scholar] [CrossRef]
- Fukaya, T.; Tomari, Y. MicroRNAs mediate gene silencing via multiple different pathways in drosophila. Mol. Cell 2012, 48, 825–836. [Google Scholar] [CrossRef]
- Mishima, Y.; Fukao, A.; Kishimoto, T.; Sakamoto, H.; Fujiwara, T.; Inoue, K. Translational inhibition by deadenylation-independent mechanisms is central to microRNA-mediated silencing in zebrafish. Proc. Natl. Acad. Sci. USA 2012, 109, 1104–1109. [Google Scholar] [CrossRef]
- Subtelny, A.O.; Eichhorn, S.W.; Chen, G.R.; Sive, H.; Bartel, D.P. Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature 2014, 508, 66–71. [Google Scholar] [CrossRef]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The intricate balance between microRNA-induced mRNA decay and translational repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef]
- Xu, W.; San Lucas, A.; Wang, Z.; Liu, Y. Identifying microRNA targets in different gene regions. BMC Bioinform. 2014, 15 (Suppl. S7), S4. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, W.; Liu, Y.; Liu, T.; Li, C.; Wang, L. Oncogenic role of microRNA-532-5p in human colorectal cancer via targeting of the 5′UTR of RUNX3. Oncol. Lett. 2018, 15, 7215–7220. [Google Scholar] [CrossRef]
- Shakyawar, S.; Southekal, S.; Guda, C. mintRULS: Prediction of miRNA-mRNA Target Site Interactions Using Regularized Least Square Method. Genes 2022, 13, 1528. [Google Scholar] [CrossRef] [PubMed]
- Hausser, J.; Syed, A.P.; Bilen, B.; Zavolan, M. Analysis of CDS-located miRNA target sites suggests that they can effectively inhibit translation. Genome Res. 2013, 23, 604–615. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Ghosh, D.; Mitra, R.; Zhao, Z. MBSTAR: Multiple instance learning for predicting specific functional binding sites in microRNA targets. Sci. Rep. 2015, 5, 8004. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Wen, M.; Cong, P.; Zhang, Z.; Lu, H.; Li, T. DeepMirTar: A deep-learning approach for predicting human miRNA targets. Bioinformatics 2018, 34, 3781–3787. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Behm-Ansmant, I.; Rehwinkel, J.; Doerks, T.; Stark, A.; Bork, P.; Izaurralde, E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006, 20, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Stark, A.; Brennecke, J.; Bushati, N.; Russell, R.B.; Cohen, S.M. Animal microRNAs confer robustness to gene expression and have a significant impact on 3′UTR evolution. Cell 2005, 123, 1133–1146. [Google Scholar] [CrossRef]
- Lim, L.P.; Glasner, M.E.; Yekta, S.; Burge, C.B.; Bartel, D.P. Vertebrate microRNA genes. Science 2003, 299, 1540. [Google Scholar] [CrossRef]
- Yuva-Aydemir, Y.; Simkin, A.; Gascon, E.; Gao, F.B. MicroRNA-9. RNA Biol. 2011, 8, 557–564. [Google Scholar] [CrossRef]
- Morgan, T.H.; Moszkowski, M. Regeneration; Macmillan: New York, NY, USA, 1901; Volume 7. [Google Scholar]
- Oberpriller, J.O.; Oberpriller, J.C. Response of the adult newt ventricle to injury. J. Exp. Zool. 1974, 187, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Flink, I.L. Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, Amblystoma mexicanum: Confocal microscopic immunofluorescent image analysis of bromodeoxyuridine-labeled nuclei. Anat. Embryol. 2002, 205, 235–244. [Google Scholar] [CrossRef]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, N.; Li, M.; Calvert, J.W.; Tejada, T.; Lambert, J.P.; Wu, J.; Kesteven, S.H.; Holman, S.R.; Matsuda, T.; Lovelock, J.D.; et al. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell 2014, 157, 795–807. [Google Scholar] [CrossRef]
- Garbern, J.C.; Lee, R.T. Heart regeneration: 20 years of progress and renewed optimism. Dev. Cell 2022, 57, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, J.B.; Leinbach, R.; Gold, H. The relationship of myocardial infarct size and prognosis. Circulation 1976, 53 (Suppl. S3), I141–I144. [Google Scholar]
- Jopling, C.; Sleep, E.; Raya, M.; Marti, M.; Raya, A.; Carlos Izpisua Belmonte, J. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef]
- Richardson, W.J.; Clarke, S.A.; Quinn, T.A.; Holmes, J.W. Physiological implications of myocardial scar structure. Compr. Physiol. 2015, 5, 1877–1909. [Google Scholar] [CrossRef]
- Borgens, R.B. Mice regrow the tips of their foretoes. Science 1982, 217, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, C.M. Trapped fingers and amputated finger tips in children. J. Pediatr. Surg. 1974, 9, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, L.J.; Reiner, M.A.; Bleicher, M.A. Nonoperative management of distal fingertip amputations in children. Pediatrics 1979, 64, 1–3. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, D.; Gu, S.; Xiao, S.; Song, F. The global burden of traumatic amputation in 204 countries and territories. Front. Public Health 2023, 11, 1258853. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234, Erratum in Lancet 2023, 402, 1132. [Google Scholar] [CrossRef]
- Iten, L.E.; Bryant, S.V. Forelimb regeneration from different levels of amputation in the newt, Notophthalmus viridescens: Length, rate, and stages. Wilhelm Roux’Archiv Entwicklungsmechanik Org. 1973, 173, 263–282. [Google Scholar] [CrossRef]
- McCusker, C.; Bryant, S.V.; Gardiner, D.M. The axolotl limb blastema: Cellular and molecular mechanisms driving blastema formation and limb regeneration in tetrapods. Regeneration 2015, 2, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Kragl, M.; Knapp, D.; Nacu, E.; Khattak, S.; Maden, M.; Henning Epperlein, H.; Tanaka, E.M. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 2009, 460, 60–65. [Google Scholar] [CrossRef]
- Sandoval-Guzman, T.; Wang, H.; Khattak, S.; Schuez, M.; Roensch, K.; Nacu, E.; Tazaki, A.; Joven, A.; Tanaka, E.M.; Simon, A. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell 2014, 14, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Lehoczky, J.A.; Robert, B.; Tabin, C.J. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc. Natl. Acad. Sci. USA 2011, 108, 20609–20614. [Google Scholar] [CrossRef]
- Holman, E.C.; Campbell, L.J.; Hines, J.; Crews, C.M. Microarray analysis of microRNA expression during axolotl limb regeneration. PLoS ONE 2012, 7, e41804. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, B. The mRNA and microRNA Landscape of the Blastema Niche in Regenerating Newt Limbs. Int. J. Mol. Sci. 2024, 25, 9225. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, J.; Su, J.; Cui, J.; Xie, X.; Chen, F. Integrative Analysis of MicroRNAome, Transcriptome, and Proteome during the Limb Regeneration of Cynops orientalis. J. Proteome Res. 2019, 18, 1088–1098. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.P.; Dutta, A.; Ghosh, S. Cellular response after crush injury in adult zebrafish spinal cord. Dev. Dyn. 2010, 239, 2962–2979. [Google Scholar] [CrossRef]
- Zukor, K.A.; Kent, D.T.; Odelberg, S.J. Meningeal cells and glia establish a permissive environment for axon regeneration after spinal cord injury in newts. Neural Dev. 2011, 6, 1. [Google Scholar] [CrossRef]
- Edwards-Faret, G.; Gonzalez-Pinto, K.; Cebrian-Silla, A.; Penailillo, J.; Garcia-Verdugo, J.M.; Larrain, J. Cellular response to spinal cord injury in regenerative and non-regenerative stages in Xenopus laevis. Neural Dev. 2021, 16, 2. [Google Scholar] [CrossRef]
- Walker, S.E.; Echeverri, K. Spinal cord regeneration- the origins of progenitor cells for functional rebuilding. Curr. Opin. Genet. Dev. 2022, 75, 101917. [Google Scholar] [CrossRef]
- Gargioli, C.; Slack, J.M.W. Cell lineage tracing during Xenopus tail regeneration. Development 2004, 131, 2669–2679. [Google Scholar] [CrossRef]
- Gaete, M.; Munoz, R.; Sanchez, N.; Tampe, R.; Moreno, M.; Contreras, E.G.; Lee-Liu, D.; Larrain, J. Spinal cord regeneration in Xenopus tadpoles proceeds through activation of Sox2-positive cells. Neural Dev. 2012, 7, 13. [Google Scholar] [CrossRef]
- Reimer, M.M.; Sorensen, I.; Kuscha, V.; Frank, R.E.; Liu, C.; Becker, C.G.; Becker, T. Motor neuron regeneration in adult zebrafish. J. Neurosci. 2008, 28, 8510–8516. [Google Scholar] [CrossRef]
- Benraiss, A.; Arsanto, J.P.; Coulon, J.; Thouveny, Y. Neurogenesis during caudal spinal cord regeneration in adult newts. Dev. Genes Evol. 1999, 209, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Echeverri, K.; Tanaka, E.M. Ectoderm to mesoderm lineage switching during axolotl tail regeneration. Science 2002, 298, 1993–1996. [Google Scholar] [CrossRef] [PubMed]
- Berberoglu, M.A.; Gallagher, T.L.; Morrow, Z.T.; Talbot, J.C.; Hromowyk, K.J.; Tenente, I.M.; Langenau, D.M.; Amacher, S.L. Satellite-like cells contribute to pax7-dependent skeletal muscle repair in adult zebrafish. Dev. Biol. 2017, 424, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, L.; Yi, C.; Zhou, J.; Yong, Z.; Hu, Y.; Pan, X.; Qiao, N.; Cai, H.; Zhao, W.; et al. Divergent stem cell mechanisms governing the primary body axis and appendage regeneration in the axolotl. bioRxiv 2025. [Google Scholar] [CrossRef]
- Walker, S.E.; Senatore, A.; Carlone, R.L.; Spencer, G.E. Context-dependent role of miR-124 in retinoic acid-induced growth cone attraction of regenerating motorneurons. Cell. Mol. Neurobiol. 2022, 42, 847–869. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, X.; Song, Z.; Yao, H.; Han, A.; Zhang, Y.; Cai, Y.; Hu, B. MicroRNA-9 promotes axon regeneration of mauthner-cell in zebrafish via her6/calcium activity pathway. Cell. Mol. Life Sci. 2024, 81, 104. [Google Scholar] [CrossRef]
- Santos Da Silva, J.; Medina, M.; Zuliani, C.; Di Nardo, A.; Witke, W.; Dotti, C.G. RhoA/ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. J. Cell Biol. 2003, 162, 1267–1279. [Google Scholar] [CrossRef]
- Zhang, X.F.; Schaefer, A.W.; Burnette, D.T.; Schoonderwoert, V.T.; Forscher, P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron 2003, 40, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Roloff, F.; Scheiblich, H.; Dewitz, C.; Dempewolf, S.; Stern, M.; Bicker, G. Enhanced neurite outgrowth of human model (NT2) neurons by small-molecule inhibitors of Rho/ROCK signaling. PLoS ONE 2015, 10, e0118536. [Google Scholar] [CrossRef] [PubMed]
- Witman, N.; Heigwer, J.; Thaler, B.; Lui, W.O.; Morrison, J.I. miR-128 regulates non-myocyte hyperplasia, deposition of extracellular matrix and Islet1 expression during newt cardiac regeneration. Dev. Biol. 2013, 383, 253–263. [Google Scholar] [CrossRef]
- Lepp, A.C.; Carlone, R.L. MicroRNA dysregulation in response to RARβ2 inhibition reveals a negative feedback loop between microRNAs 1, 133a, and RARβ2 during tail and spinal cord regeneration in the adult newt. Dev. Dyn. 2014, 244, 1519–1537. [Google Scholar] [CrossRef]
- Lepp, A.C.; Carlone, R.L. RARβ2 expression is induced by the down-regulation of microRNA 133a during caudal spinal cord regeneration in the adult newt. Dev. Dyn. 2015, 243, 1581–1590. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Croce, C.M. MicroRNA: Trends in clinical trials of cancer diagnosis and therapy strategies. Exp. Mol. Med. 2023, 55, 1314–1321. [Google Scholar] [CrossRef]
- King, B.L.; Yin, V.P. A Conserved MicroRNA Regulatory Circuit Is Differentially Controlled during Limb/Appendage Regeneration. PLoS ONE 2016, 11, e0157106. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Sempere, L.F.; Azmi, A.S.; Moore, A. microRNA-based diagnostic and therapeutic applications in cancer medicine. Wiley Interdiscip. Rev. RNA 2021, 12, e1662. [Google Scholar] [CrossRef]
- Lee, J.; Li, Z.; Brower-Sinning, R.; John, B. Regulatory circuit of human microRNA biogenesis. PLoS Comput. Biol. 2007, 3, e67. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, D.; Zhu, H.M.; Zhao, Y.L.; Iraqi, F.A.; Morahan, G.; Xiao, Z.C. Upstream regulation of microRNA-9 through a complex cellular machinery during neurogenesis. Brain Res. 2025, 1848, 149328. [Google Scholar] [CrossRef]
- López-Urrutia, E.; Bustamante Montes, L.P.; Ladrón de Guevara Cervantes, D.; Pérez-Plasencia, C.; Campos-Parra, A.D. Crosstalk between long non-coding RNAs, micro-RNAs and mRNAs: Deciphering molecular mechanisms of master regulators in cancer. Front. Oncol. 2019, 9, 669. [Google Scholar] [CrossRef]
- Sebastian-delaCruz, M.; Gonzalez-Moro, I.; Olazagoitia-Garmendia, A.; Castellanos-Rubio, A.; Santin, I. The role of lncRNAs in gene expression regulation through mRNA stabilization. Noncoding RNA 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wen, Z.; Lynn, R.C.; Baudet, M.L.; Holt, C.E.; Sasaki, Y.; Bassell, G.J.; Zheng, J.Q. Regulation of chemotropic guidance of nerve growth cones by microRNA. Mol. Brain 2011, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Bellon, A.; Iyer, A.; Bridi, S.; Lee, F.C.Y.; Ovando-Vazquez, C.; Corradi, E.; Longhi, S.; Roccuzzo, M.; Strohbuecker, S.; Naik, S.; et al. miR-182 regulates Slit2-mediated axon guidance by modulation the local translation of a specific mRNA. Cell Rep. 2017, 18, 1171–1186. [Google Scholar] [CrossRef]
| Heart Regeneration | ||||||
| miRNA | Role | Target(s) | Animal | Reference | Conserved target in mammals | Predicted Target(s) |
| miR-99-5p/100-5p | Cardiomyocyte proliferation and dedifferentiation | Smarca5 fntb | Zebrafish and Mice | [8] | Yes | epdr1 znf197 thap2 |
| miR-101a-3p | Cardiomyocyte proliferation Prevents scar formation | fosab (fos) | Zebrafish | [10] | Unknown | cpeb1a stmn1a mkp1 |
| miR-128-3p | Deposition of extracellular matrix Non-myocyte hyperplasia | islet1 | Red Spotted Newt | [86] | Unknown | aff4 szrd1 kdm7a |
| Limb Regeneration | ||||||
| miRNA | Role | Target | Animal | Reference | Conserved target in mammals | Predicted Target(s) |
| miR-10b-5p | Protein synthesis | rpS29 rpL30 rpL4 | Pleurodeles waltl and Notophthalmus viridescens | [17] | Unknown | mkn2 cadm2 tfap2c |
| miR-21-5p | jagged1 | Axolotl | [66] | Unknown | Rhou Gpd2 prdm11 | |
| Spinal Cord & Motorneuron Regeneration | ||||||
| miRNA | Role | Target | Animal | Reference | Conserved target in mammals | Predicted Target(s) |
| miR-1-3p | Tail growth | rarb2 | Notophthalmus viridescens | [87] | Unknown | mmd slc44a1 hacd3 |
| miR-133a-3p | Tail growth | rarb2 | Notophthalmus viridescens | [88] | Unknown | ptbp1 maml1 bicc1 |
| miR-9 | Axon growth | her6 | Zebrafish | [82] | Unknown | cdk8 arrdc3 vps13c |
| miR-124-3p | Axon pathfinding | rock | Lymnaea stagnalis | [81] | Unknown | pxdnl fbxo36 nes |
| miR-125b | Axon growth | Sema4D | Axolotl and Rat, Zebrafish | [9] | Yes | adamts4 atoh8 azi2 |
| miR-200a | Axon growth Glial scar formation Cell proliferation | c-jun | Axolotl | [12] | Unknown | gfap vimentin cspg4/5 |
| Stem cell fate decisions | brachyury | Axolotl | [13] | Unknown | ||
| miR-196-3p | Cell proliferation | pax7 bmp4 | Axolotl | [15] | Unknown | zmynd11 nr6a1 slc9a6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, S.E.; Piazza, A.; Carlone, R.L.; Spencer, G.E. MicroRNAs in Tissue Regeneration: Lessons from Animal Models. Int. J. Mol. Sci. 2025, 26, 10043. https://doi.org/10.3390/ijms262010043
Walker SE, Piazza A, Carlone RL, Spencer GE. MicroRNAs in Tissue Regeneration: Lessons from Animal Models. International Journal of Molecular Sciences. 2025; 26(20):10043. https://doi.org/10.3390/ijms262010043
Chicago/Turabian StyleWalker, Sarah E., Alicia Piazza, Robert L. Carlone, and Gaynor E. Spencer. 2025. "MicroRNAs in Tissue Regeneration: Lessons from Animal Models" International Journal of Molecular Sciences 26, no. 20: 10043. https://doi.org/10.3390/ijms262010043
APA StyleWalker, S. E., Piazza, A., Carlone, R. L., & Spencer, G. E. (2025). MicroRNAs in Tissue Regeneration: Lessons from Animal Models. International Journal of Molecular Sciences, 26(20), 10043. https://doi.org/10.3390/ijms262010043






