Kdm7aa Orchestrates an Immunomodulatory Cardiomyocyte Program to Enable Zebrafish Heart Regeneration
Abstract
1. Introduction
2. Results
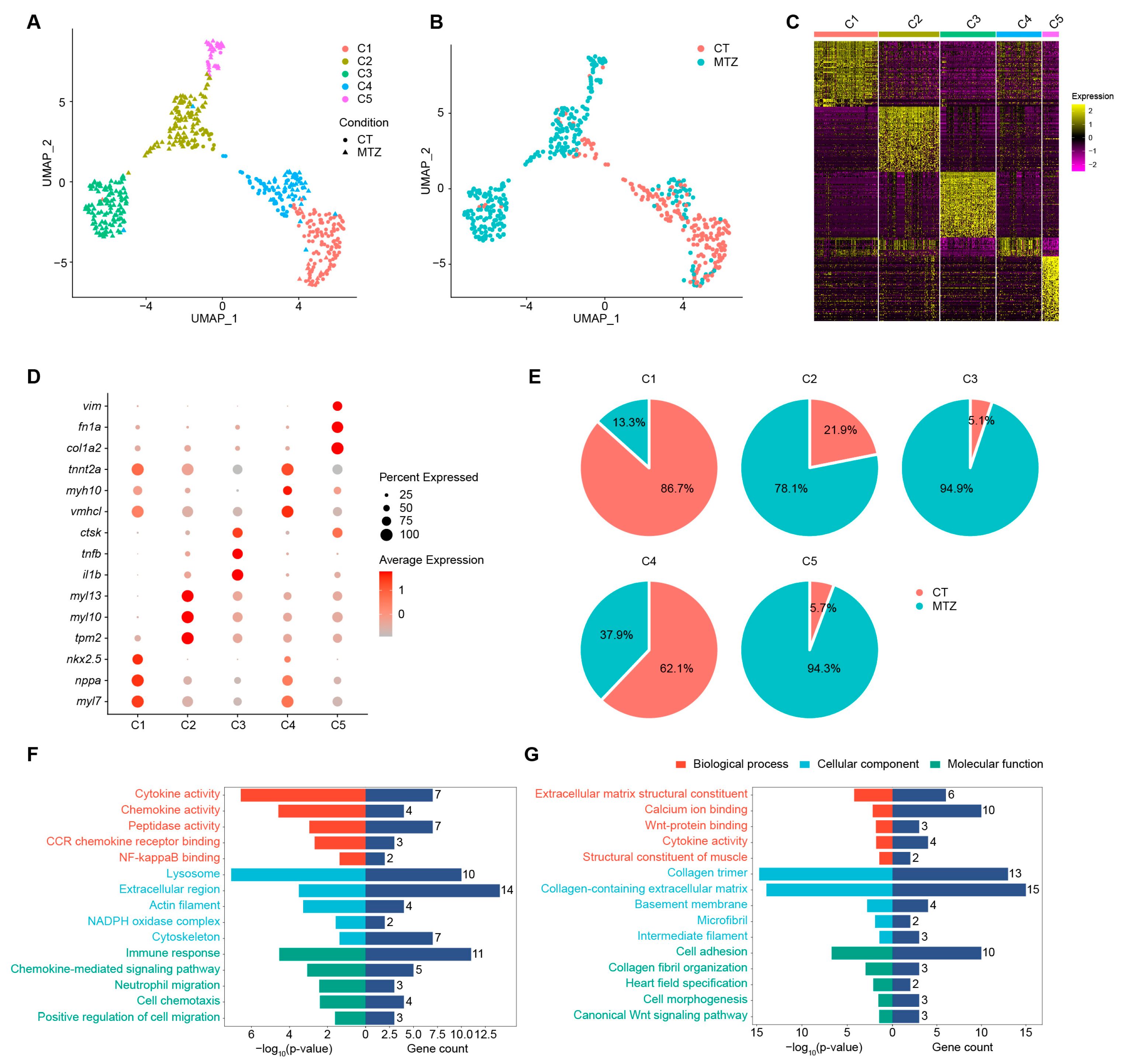
2.1. Single-Cell Transcriptomics Reveals Distinct Ventricular Cardiomyocyte Populations and Regeneration-Specific Subsets
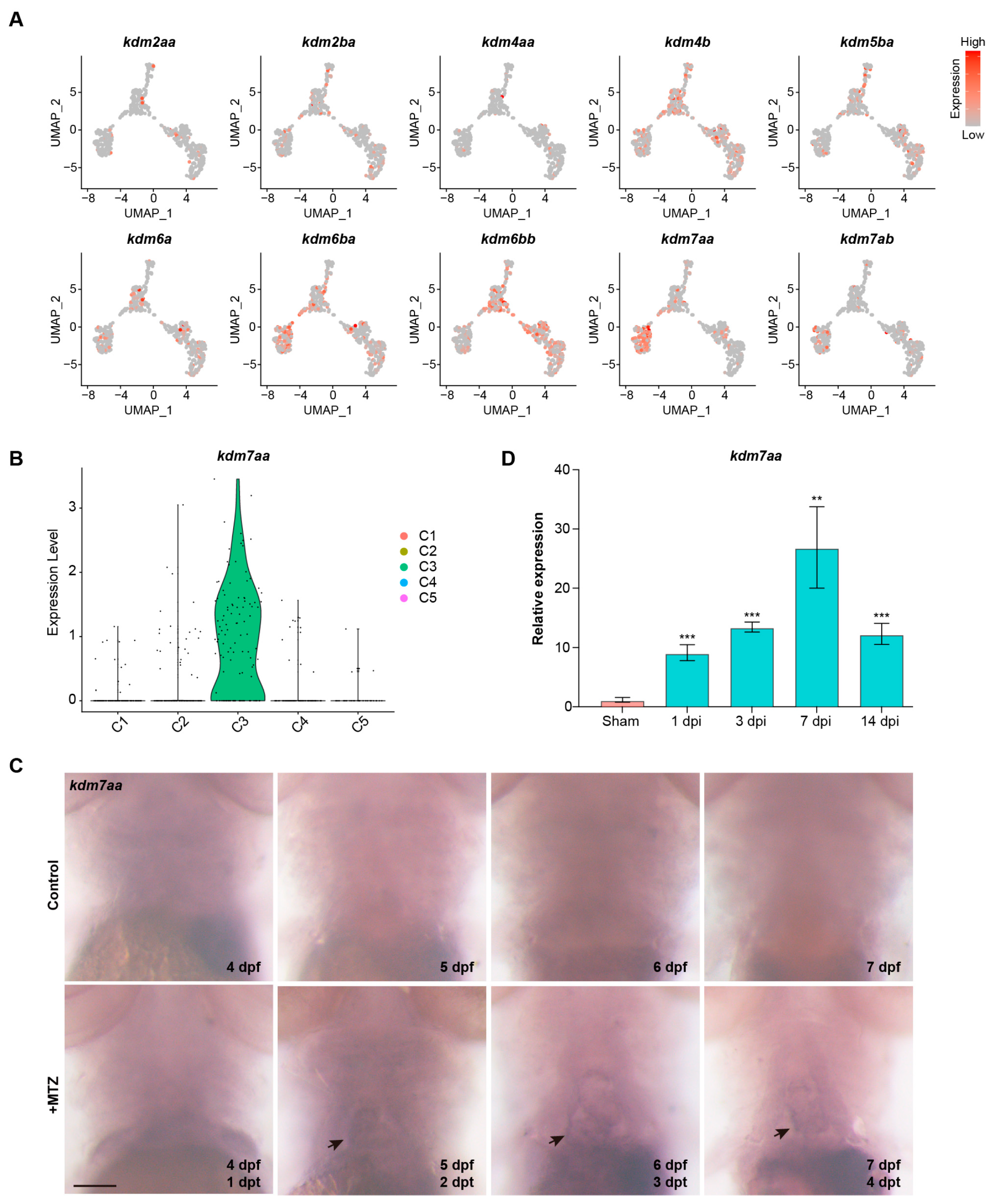
2.2. KDM7aa Is a Regeneration-Induced Epigenetic Regulator Expressed in the Immunomodulatory Cluster
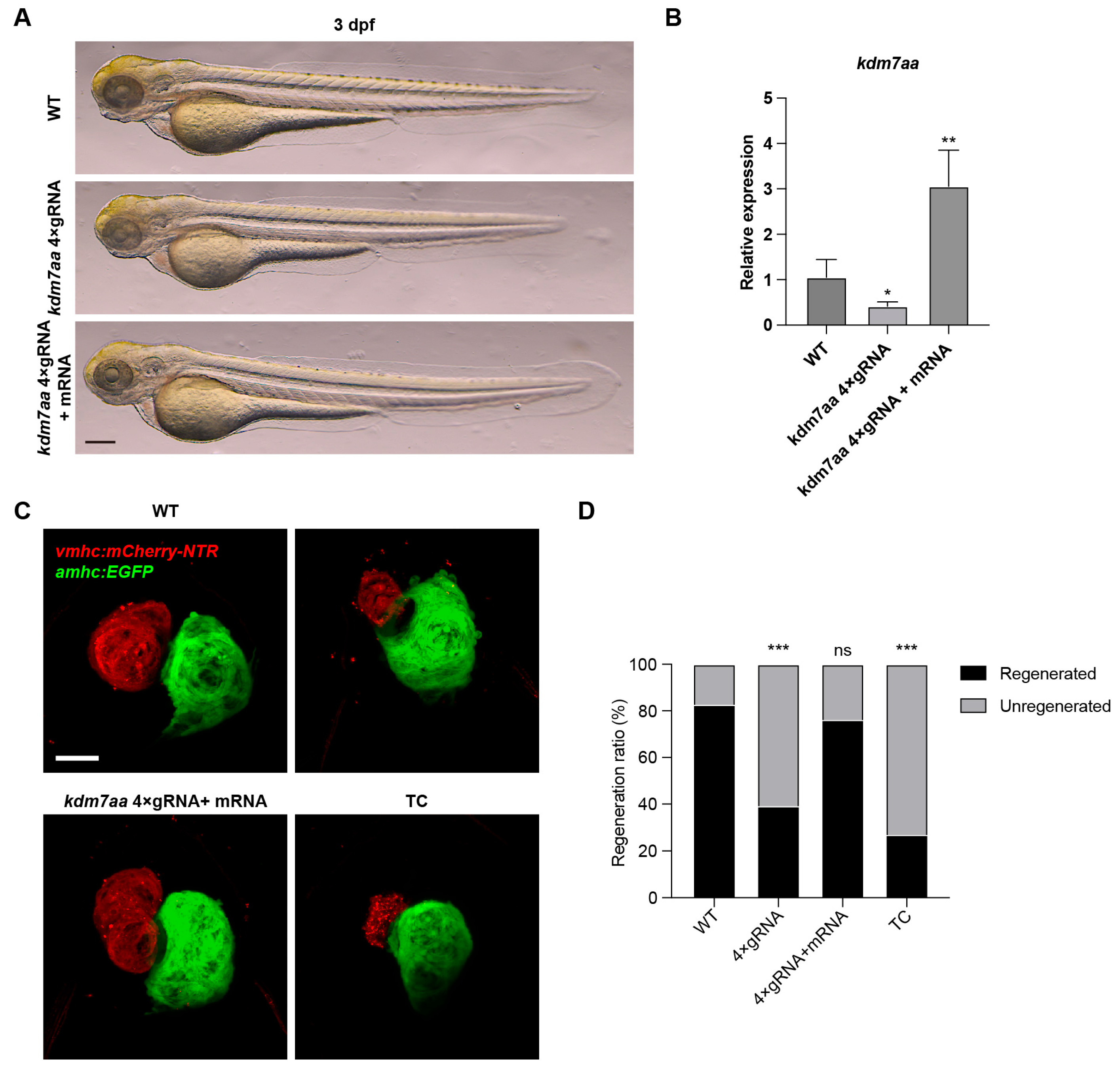
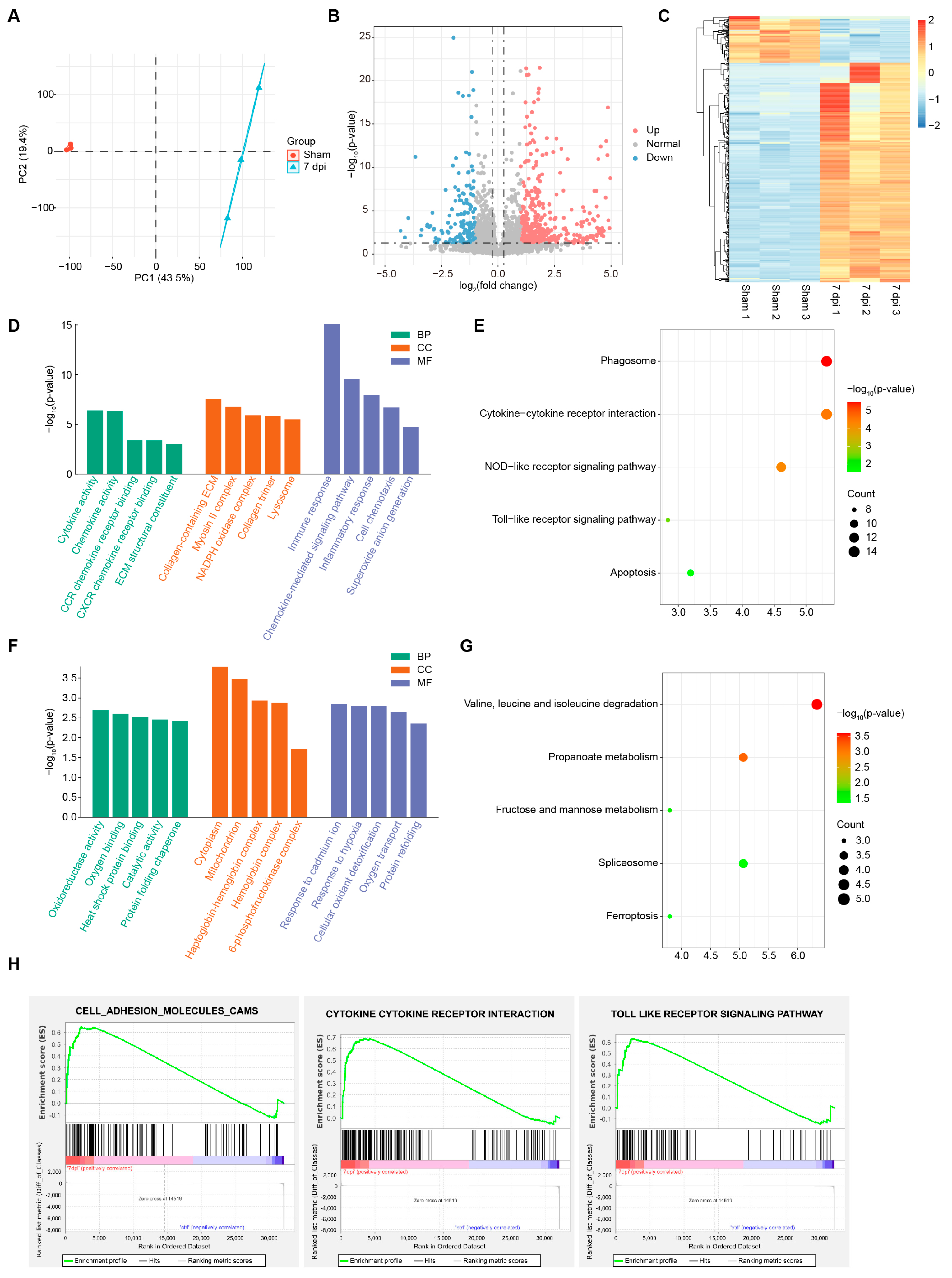
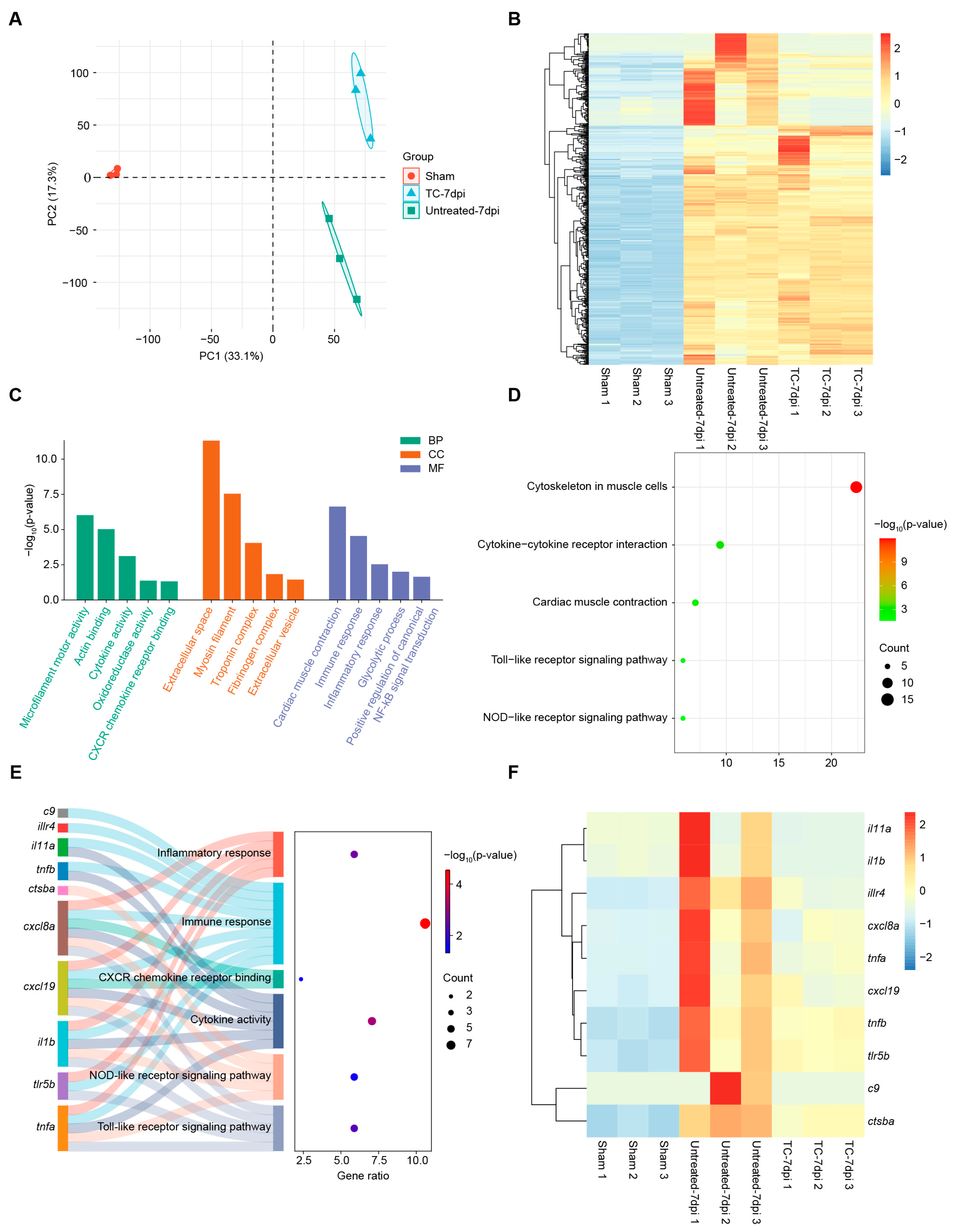
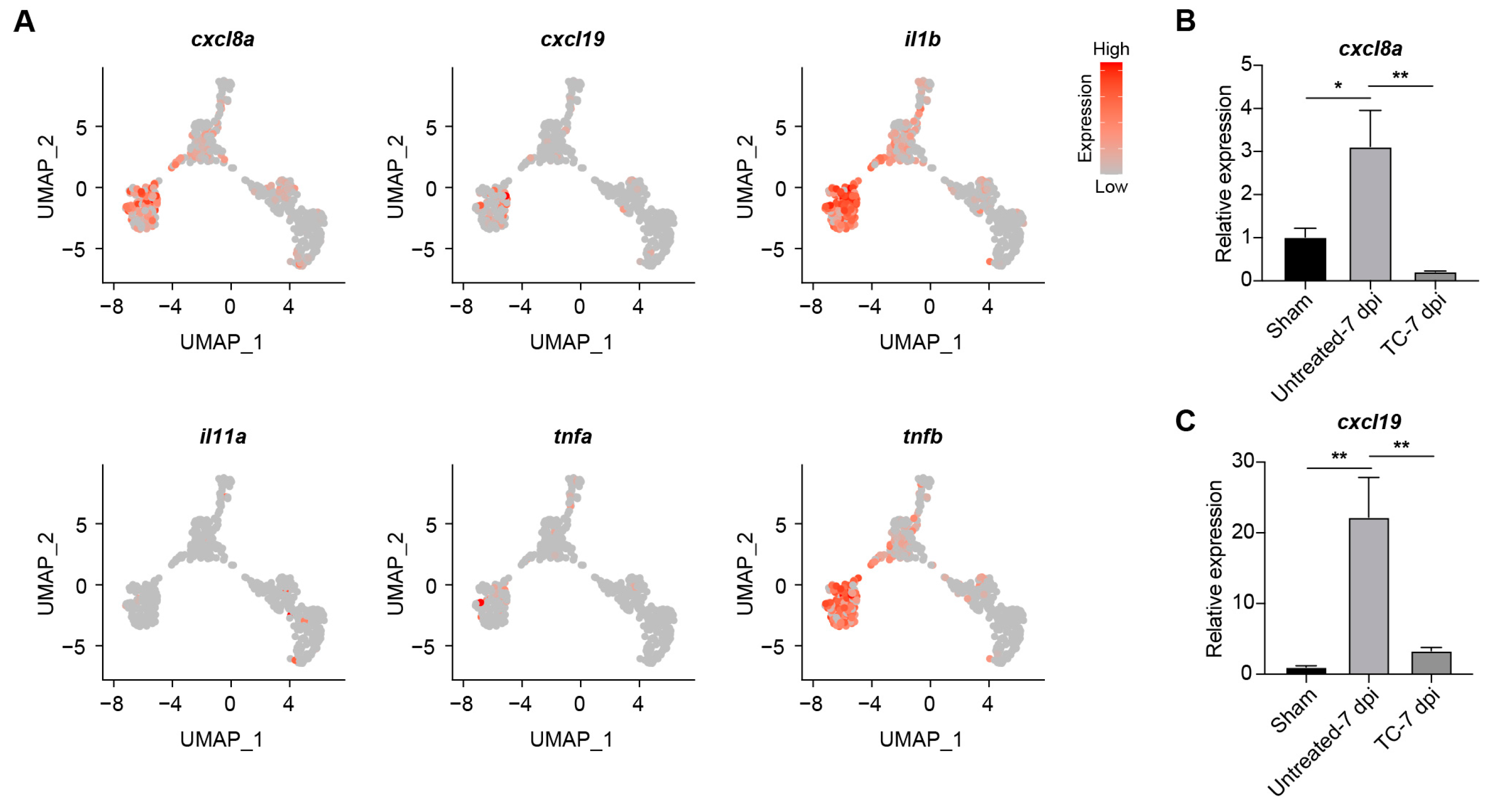
2.3. Transcriptomic Profiling Reveals kdm7aa-Dependent Regulation of Immune Pathways During Regeneration
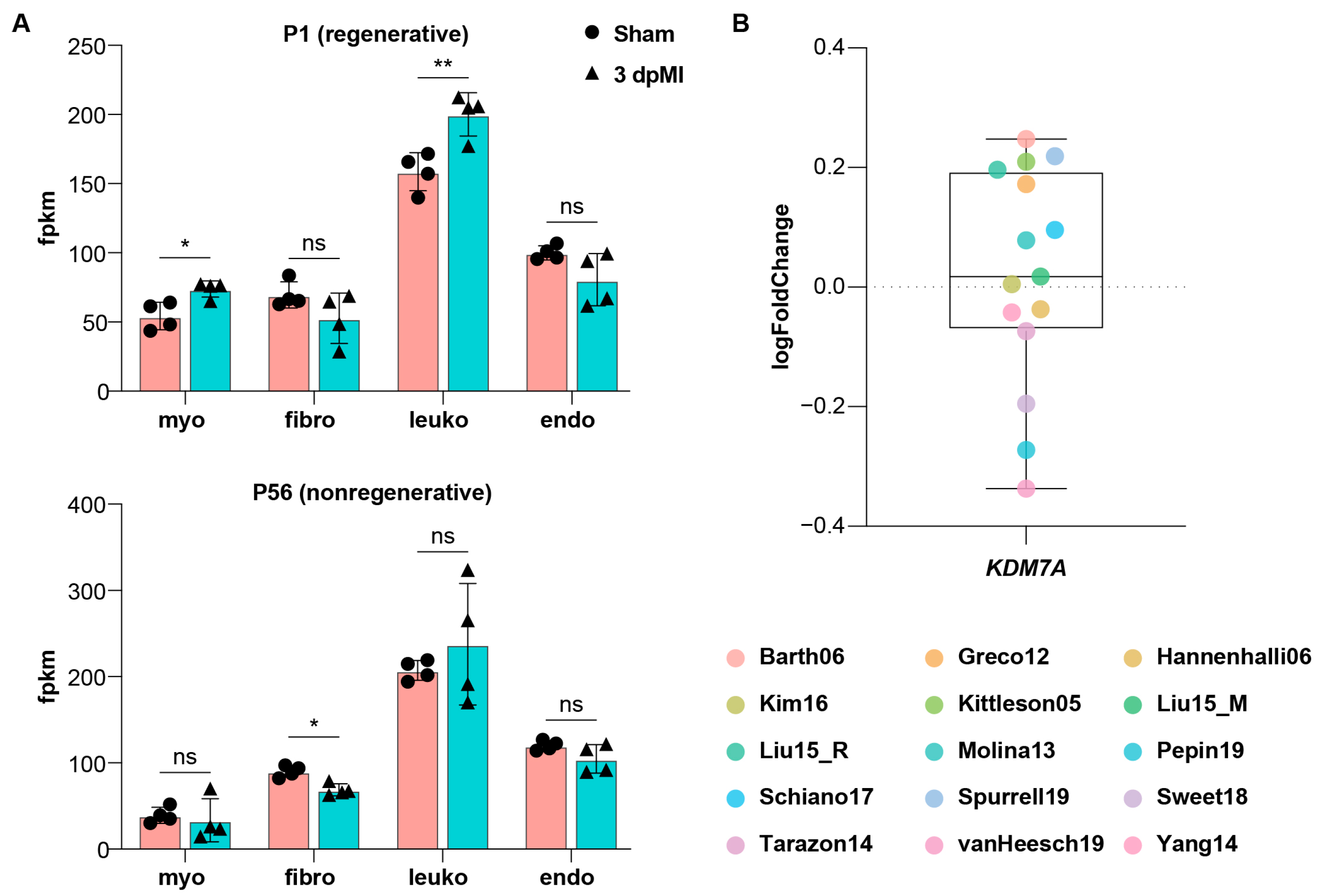
2.4. Kdm7a Upregulation Is Associated with Regenerative Capacity and Conserved in Mammalian Hearts
3. Discussion
4. Materials and Methods
4.1. Zebrafish Husbandry
4.2. Analysis of Single-Cell RNA-Seq Data
4.3. Chemical Treatment
4.4. Cryoinjury
4.5. Transcriptomic Analysis
4.6. In Situ Hybridization
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MI | Myocardial infarction |
| KMT | Lysine methyltransferase |
| KDM | Lysine demethylase |
| sgRNA | Single-guide RNA |
| PC | Principal component |
| PCA | Principal-component analysis |
| UMAP | Uniform manifold approximation and projection |
| MTZ | Metronidazole |
| dpf | Day post-fertilization |
| dpt | Day post-treatment |
| dpi | Day post-injury |
| PBS | Phosphate-buffered saline |
| DEG | Differentially expressed gene |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| GSEA | Gene-set enrichment analysis |
| PFA | Paraformaldehyde |
| DIG | Digoxigenin |
| qRT-PCR | Quantitative real-time PCR |
| CM-V | Ventricular cardiomyocyte |
| CM-A | Atrial cardiomyocyte |
| EC | Endothelial cell |
| EP | Epicardial cell |
| EPDC | Epicardial-derived cell |
| scRNA-seq | Single-cell RNA sequencing |
| ECM | Extracellular matrix |
References
- Garbern, J.C.; Lee, R.T. Heart regeneration: 20 years of progress and renewed optimism. Dev. Cell 2022, 57, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in health and disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar]
- Jopling, C.; Sleep, E.; Raya, M.; Marti, M.; Raya, A.; Izpisua Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef]
- Xu, S.; Webb, S.E.; Lau, T.C.K.; Cheng, S.H. Matrix metalloproteinases (MMPs) mediate leukocyte recruitment during the inflammatory phase of zebrafish heart regeneration. Sci. Rep. 2018, 8, 7199. [Google Scholar] [CrossRef]
- Lai, S.L.; Marin-Juez, R.; Moura, P.L.; Kuenne, C.; Lai, J.K.H.; Tsedeke, A.T.; Guenther, S.; Looso, M.; Stainier, D.Y. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. eLife 2017, 6, e25605. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, Y.; Cui, Y.; Xing, X.; Zhang, L.; Liu, D.; Zhang, Y.; Dong, J.; Jin, L.; Pang, M.; et al. Single-cell analysis reveals an Angpt4-initiated EPDC-EC-CM cellular coordination cascade during heart regeneration. Protein Cell 2023, 14, 350–368. [Google Scholar] [CrossRef]
- Kraus, L. Targeting epigenetic regulation of cardiomyocytes through development for therapeutic cardiac regeneration after heart failure. Int. J. Mol. Sci. 2022, 23, 11878. [Google Scholar] [CrossRef]
- Wu, R.S.; Lam, I.I.; Clay, H.; Duong, D.N.; Deo, R.C.; Coughlin, S.R. A rapid method for directed gene knockout for screening in G0 zebrafish. Dev. Cell 2018, 46, 112–125. [Google Scholar] [CrossRef]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef]
- Quaife-Ryan, G.A.; Sim, C.B.; Ziemann, M.; Kaspi, A.; Rafehi, H.; Ramialison, M.; El-Osta, A.; Hudson, J.E.; Porrello, E.R. Multicellular transcriptional analysis of mammalian heart regeneration. Circulation 2017, 136, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, M.L.; Lee, R.T. Regeneration of the heart. EMBO Mol. Med. 2011, 3, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xiang, C.; Li, Y.; Nie, Y. Targeting immunoregulation for cardiac regeneration. J. Mol. Cell. Cardiol. 2023, 177, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goumenaki, P.; Gunther, S.; Kikhi, K.; Looso, M.; Marin-Juez, R.; Stainier, D.Y.R. The innate immune regulator MyD88 dampens fibrosis during zebrafish heart regeneration. Nat. Cardiovasc. Res. 2024, 3, 1158–1176. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.W.; Lee, S.K. UTX, a histone H3-lysine 27 demethylase, acts as a critical switch to activate the cardiac developmental program. Dev. Cell 2012, 22, 25–37. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Guo, C.; Lu, Q.; Wang, W.; Jia, Z.; Chen, P.; Ma, K.; Reinberg, D.; Zhou, C. ISL1 and JMJD3 synergistically control cardiac differentiation of embryonic stem cells. Nucleic Acids Res. 2016, 44, 6741–6755. [Google Scholar] [CrossRef]
- Dal-Pra, S.; Hodgkinson, C.P.; Mirotsou, M.; Kirste, I.; Dzau, V.J. Demethylation of H3K27 is essential for the induction of direct cardiac reprogramming by miR combo. Circ. Res. 2017, 120, 1403–1413. [Google Scholar] [CrossRef]
- Ben-Yair, R.; Butty, V.L.; Busby, M.; Qiu, Y.; Levine, S.S.; Goren, A.; Boyer, L.A.; Burns, C.G.; Burns, C.E. H3K27me3-mediated silencing of structural genes is required for zebrafish heart regeneration. Development 2019, 146, dev178632. [Google Scholar] [CrossRef]
- She, P.; Zhang, H.; Peng, X.; Sun, J.; Gao, B.; Zhou, Y.; Zhu, X.; Hu, X.; Lai, K.S.; Wong, J.; et al. The Gridlock transcriptional repressor impedes vertebrate heart regeneration by restricting expression of lysine methyltransferase. Development 2020, 147, dev190678. [Google Scholar] [CrossRef]
- Xiao, C.; Hou, J.; Wang, F.; Song, Y.; Zheng, J.; Luo, L.; Wang, J.; Ding, W.; Zhu, X.; Xiong, J.W. Endothelial Brg1 fine-tunes Notch signaling during zebrafish heart regeneration. NPJ Regen. Med. 2023, 8, 21. [Google Scholar] [CrossRef]
- Gonzalez-Rosa, J.M.; Burns, C.E.; Burns, C.G. Zebrafish heart regeneration: 15 years of discoveries. Regeneration 2017, 4, 105–123. [Google Scholar] [CrossRef] [PubMed]
- El-Sammak, H.; Yang, B.; Guenther, S.; Chen, W.; Marin-Juez, R.; Stainier, D.Y.R. A vegfc-emilin2a-cxcl8a signaling axis required for zebrafish cardiac regeneration. Circ. Res. 2022, 130, 1014–1029. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xie, F.; Tian, L.; Manno, S.H.; Manno, F.A.M., 3rd; Cheng, S.H. Prolonged neutrophil retention in the wound impairs zebrafish heart regeneration after cryoinjury. Fish Shellfish Immunol. 2019, 94, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Itou, J.; Oishi, I.; Kawakami, H.; Glass, T.J.; Richter, J.; Johnson, A.; Lund, T.C.; Kawakami, Y. Migration of cardiomyocytes is essential for heart regeneration in zebrafish. Development 2012, 139, 4133–4142. [Google Scholar] [CrossRef]
- Kanneganti, T.D.; Lamkanfi, M.; Nunez, G. Intracellular NOD-like receptors in host defense and disease. Immunity 2007, 27, 549–559. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, J.S.; Nahm, M.H. NOD-like receptors in infection, immunity, and diseases. Yonsei Med. J. 2016, 57, 5–14. [Google Scholar] [CrossRef]
- Zhang, R.; Han, P.; Yang, H.; Ouyang, K.; Lee, D.; Lin, Y.F.; Ocorr, K.; Kang, G.; Chen, J.; Stainier, D.Y.; et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature 2013, 498, 497–501. [Google Scholar] [CrossRef]
- Tian, J.; Zhuang, Y.; Liu, Y.; Zheng, Y.; Liu, X.; Lin, S.; Zheng, C.; Wu, Z. ROS-mediated unfolded protein response activation drives hepatocyte apoptosis in mesaconitine-induced liver injury. Toxics 2025, 13, 155. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Shi, Y.; Tian, J.; Liu, X.; Weng, F.; Wu, Z. Kdm7aa Orchestrates an Immunomodulatory Cardiomyocyte Program to Enable Zebrafish Heart Regeneration. Int. J. Mol. Sci. 2025, 26, 10044. https://doi.org/10.3390/ijms262010044
Lin W, Shi Y, Tian J, Liu X, Weng F, Wu Z. Kdm7aa Orchestrates an Immunomodulatory Cardiomyocyte Program to Enable Zebrafish Heart Regeneration. International Journal of Molecular Sciences. 2025; 26(20):10044. https://doi.org/10.3390/ijms262010044
Chicago/Turabian StyleLin, Weibin, Yuan Shi, Jin Tian, Xinru Liu, Fubin Weng, and Zekai Wu. 2025. "Kdm7aa Orchestrates an Immunomodulatory Cardiomyocyte Program to Enable Zebrafish Heart Regeneration" International Journal of Molecular Sciences 26, no. 20: 10044. https://doi.org/10.3390/ijms262010044
APA StyleLin, W., Shi, Y., Tian, J., Liu, X., Weng, F., & Wu, Z. (2025). Kdm7aa Orchestrates an Immunomodulatory Cardiomyocyte Program to Enable Zebrafish Heart Regeneration. International Journal of Molecular Sciences, 26(20), 10044. https://doi.org/10.3390/ijms262010044





