Fluoroquinolone-Induced Achilles Tendon Damage: Structural and Biochemical Insights into Collagen Type I Alterations
Abstract
1. Introduction
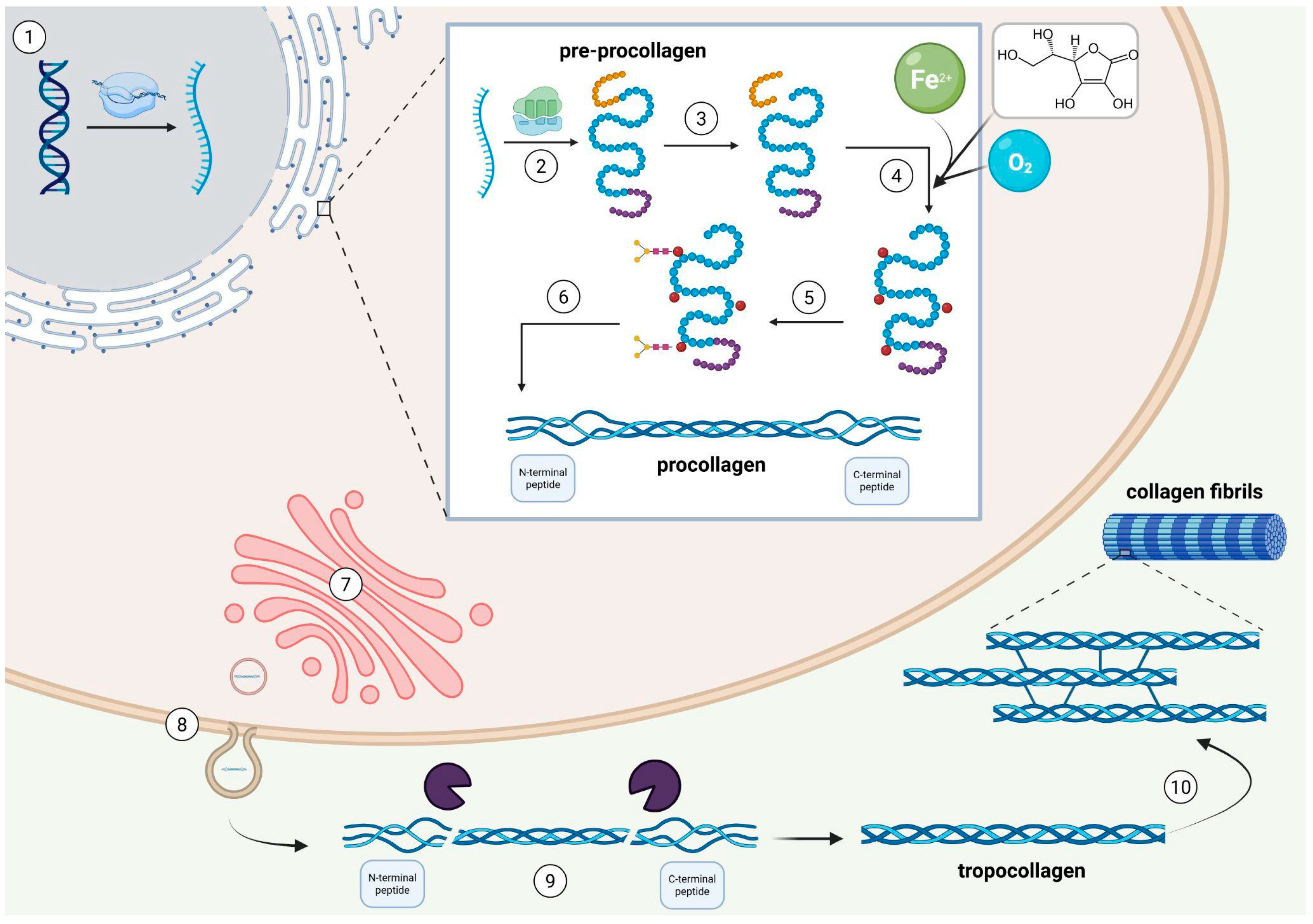
2. Collagen Type I
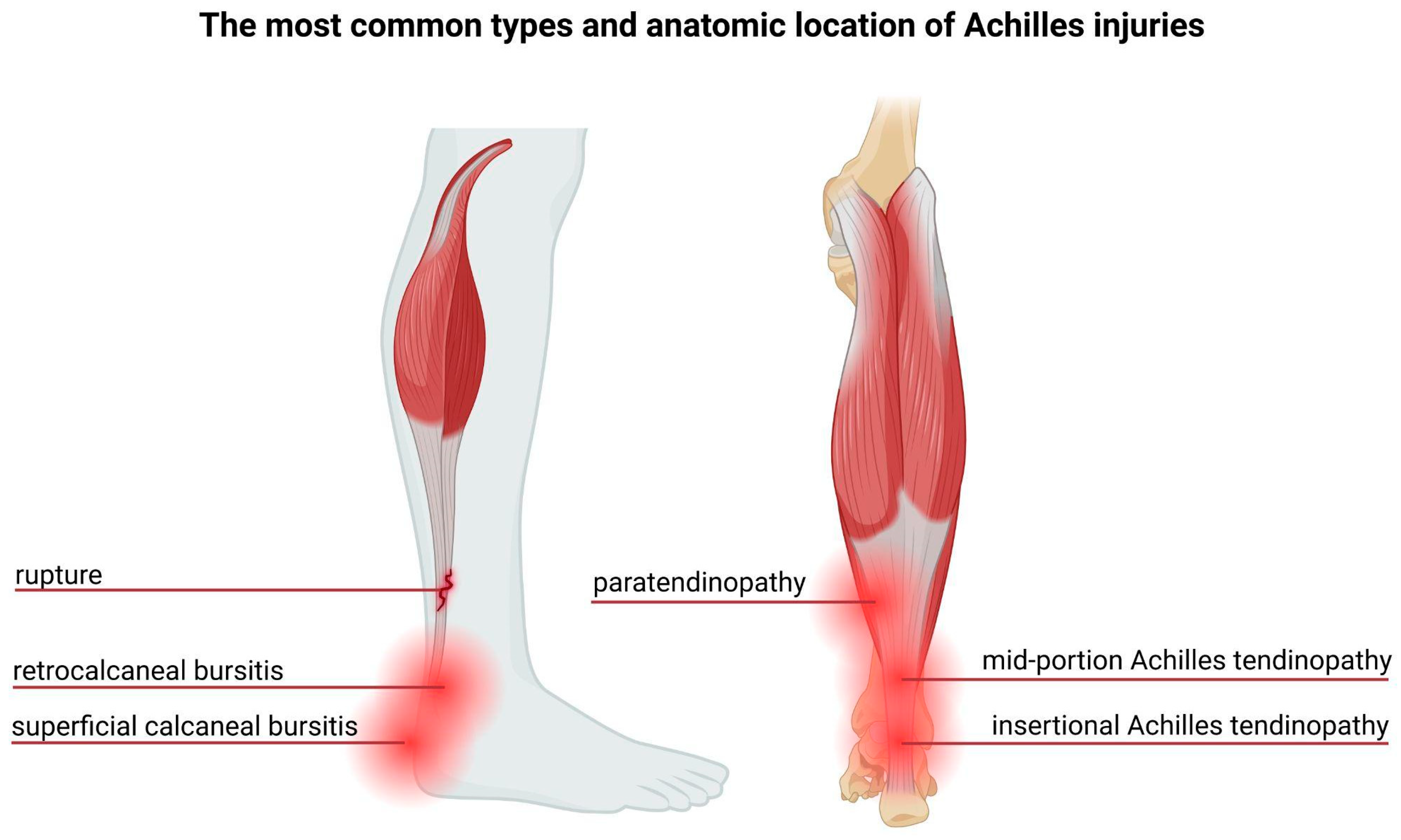
3. The Achilles Tendon—Structure and Injuries
4. Fluoroquinolones and How They Affect Collagen
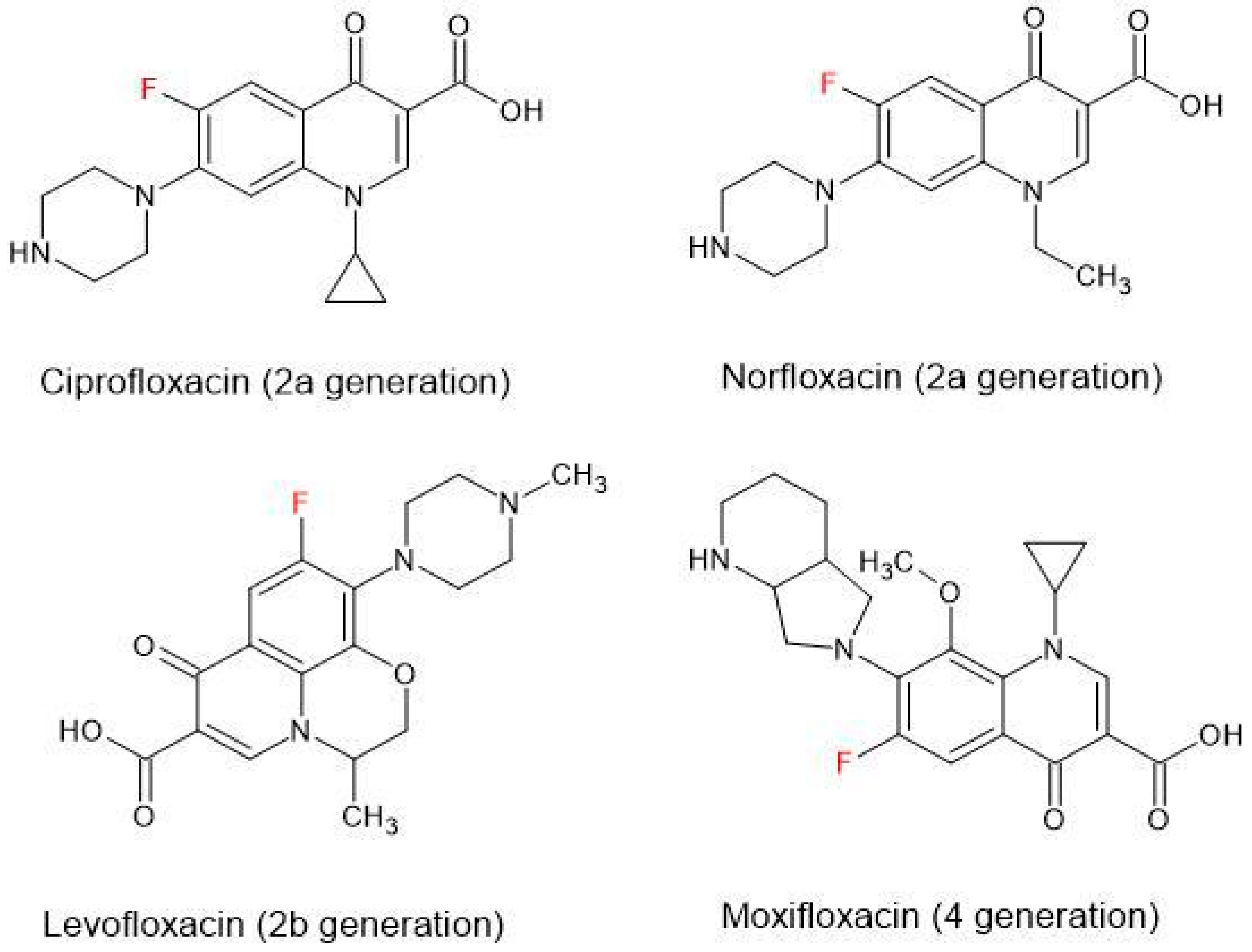
4.1. General Characteristics of Fluoroquinolones
4.2. Fluoroquinolone-Induced Tendinopathy—Epidemiology
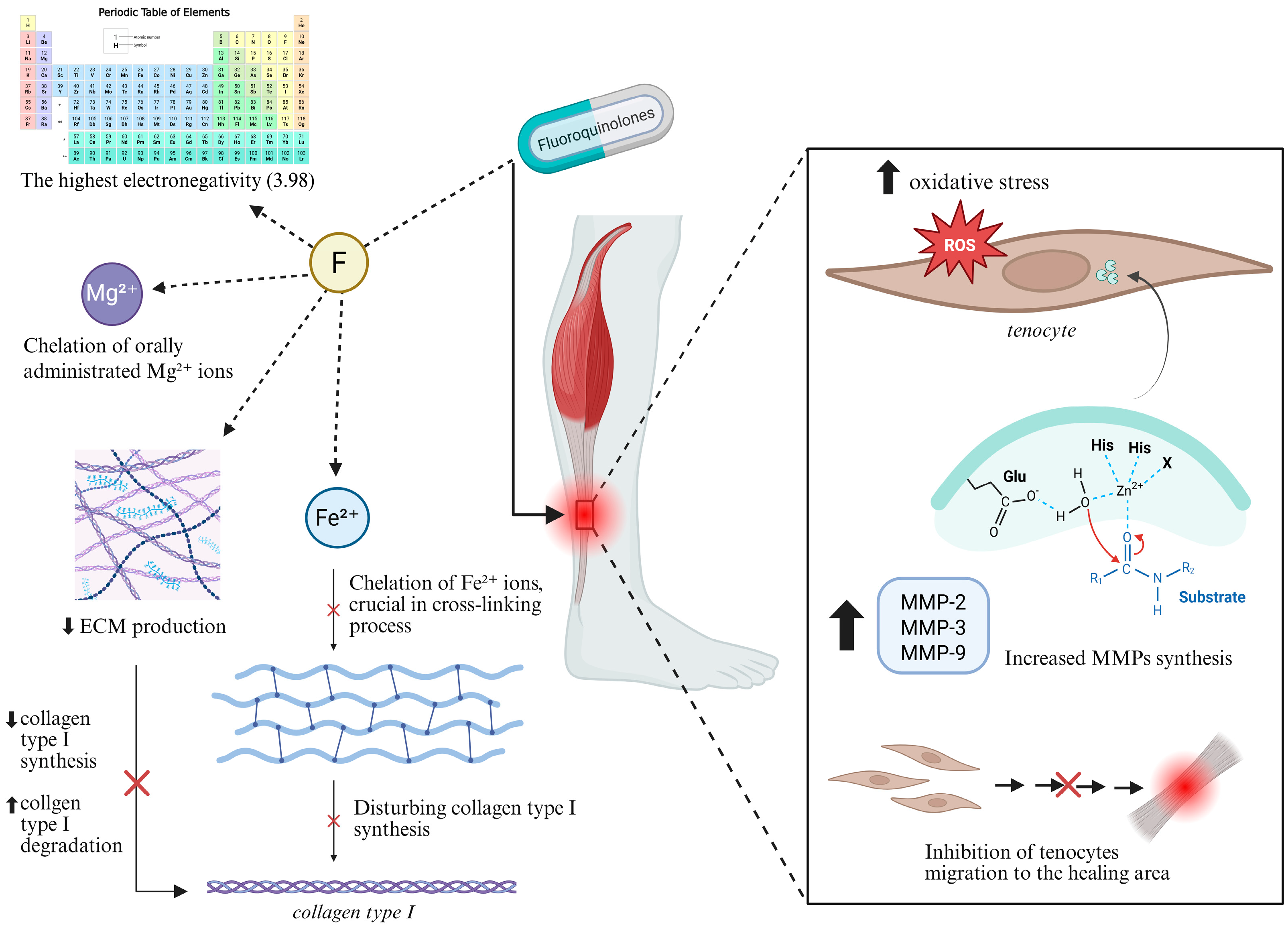
4.3. Fluoroquinolone-Induced Tendinopathy—Possible Pathogenesis
4.3.1. Chemical Characteristics of Fluorine
4.3.2. Disruption of Protein Synthesis
4.3.3. Increased Expression of Metalloproteinases
4.3.4. Oxidative Stress
5. The Cross-Section of Available Meta-Analyses
5.1. Influence of Age and Gender
5.2. Stratification by a Fluoroquinolone Type
5.3. Can the Dosage Alter the Risks?
5.4. Time Gap Between Exposure to Antibiotics and the Tendon Damage
5.5. Concomitant Use of Corticosteroids
5.6. Renal Failure or Hemodialysis in the Past
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ECM | extracellular matrix |
| MMP | matrix metalloproteinase |
| TIMP | tissue inhibitors of metalloproteinases |
| ROS | reactive oxygen species |
| PRDX5 | peroxiredoxin 5 |
| GPX3 | glutathione peroxidase 3 |
References
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Devlin, T.M. Textbook of Biochemistry: With Clinical Correlations, 7th ed.; Devlin, T.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Li, H.Y.; Hua, Y.H. Achilles Tendinopathy: Current Concepts about the Basic Science and Clinical Treatments. BioMed Res. Int. 2016, 2016, 6492597. [Google Scholar] [CrossRef]
- Egger, A.C.; Berkowitz, M.J. Achilles Tendon Injuries. Curr. Rev. Musculoskelet. Med. 2017, 10, 72–80. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, H.S.; Young, K.W.; Seo, S.G. Treatment of Acute Achilles Tendon Rupture. CiOS Clin. Orthop. Surg. 2020, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaleagasioglu, F.; Olcay, E. Fluoroquinolone-Induced Tendinopathy: Etiology and Preventive Measures. Tohoku J. Exp. Med. 2012, 226, 251–258. [Google Scholar] [CrossRef]
- Ritter, J.R.; Flower, R.; Graeme, H.; Loke, Y.K.; MacEwan, D.; Rang, H.P. Rang & Dale’s Pharmacology, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Barberán, J.; De La Cuerda, A.; González, M.I.T.; Aparicio, A.L.; Vinuesa, C.M.; Sánchez, A.R.; Barberán, L.C. Safety of Fluoroquinolones. Rev. Esp. Quimioter. 2024, 37, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Selvaraj, V.; Sekaran, S.; Dhanasekaran, A.; Warrier, S. Type 1 Collagen: Synthesis, Structure and Key Functions in Bone Mineralization. Differentiation 2024, 136, 100757. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Józsa, L.; Jarvinnen, M. Basic Science of Tendons. In Principles and Practice of Orthopaedic Sports Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; pp. 21–37. [Google Scholar]
- Kirchgesner, T.; Larbi, A.; Omoumi, P.; Malghem, J.; Zamali, N.; Manelfe, J.; Lecouvet, F.; Vande Berg, B.; Djebbar, S.; Dallaudière, B. Drug-Induced Tendinopathy: From Physiology to Clinical Applications. Jt. Bone Spine 2014, 81, 485–492. [Google Scholar] [CrossRef]
- O’Brien, M. Structure and Metabolism of Tendons. Scand. J. Med. Sci. Sports 1997, 7, 55–61. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Biology of Tendon Injury: Healing, Modeling and Remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181. [Google Scholar] [PubMed]
- Kvist, M. Achilles Tendon Injuries in Athletes. Sports Med. 1994, 18, 173–201. [Google Scholar] [CrossRef]
- Manent, A.; López, L.; Coromina, H.; Santamaría, A.; Domínguez, A.; Llorens, N.; Sales, M.; Videla, S. Acute Achilles Tendon Ruptures: Efficacy of Conservative and Surgical (Percutaneous, Open) Treatment—A Randomized, Controlled, Clinical Trial. J. Foot Ankle Surg. 2019, 58, 1229–1234, Erratum in J. Foot Ankle Surg. 2020, 59, 874. [Google Scholar] [CrossRef]
- Aicale, R.; Tarantino, D.; Maffulli, N. Overuse Injuries in Sport: A Comprehensive Overview. J. Orthop. Surg. Res. 2018, 13, 309. [Google Scholar] [CrossRef]
- Knapik, J.J.; Pope, R. Achilles Tendinopathy: Pathophysiology, Epidemiology, Diagnosis, Treatment, Prevention, and Screening. J. Spec. Oper. Med. 2020, 20, 125–140. [Google Scholar] [CrossRef]
- Kannus, P.J.L. Histopathological Changes Preceding Spontaneous Rupture of a Tendon. A Controlled Study of 891 Patients. J. Bone Jt. Surg. 1991, 73, 1507–1525. [Google Scholar] [CrossRef]
- Oliva, F.; Marsilio, E.; Asparago, G.; Frizziero, A.; Berardi, A.C.; Maffulli, N. The Impact of Hyaluronic Acid on Tendon Physiology and Its Clinical Application in Tendinopathies. Cells 2021, 10, 3081. [Google Scholar] [CrossRef]
- Matrisian, L.M. Metalloproteinases and Their Inhibitors in Matrix Remodeling. Trends Genet. 1990, 6, 121–125. [Google Scholar] [CrossRef]
- Riley, G.P.; Curry, V.; Degroot, J.; Van El, B.; Verzijl, N.; Hazleman, B.L.; Bank, R.A. Matrix Metalloproteinase Activities and Their Relationship with Collagen Remodelling in Tendon Pathology. Matrix Biol. 2002, 21, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, W.; Lou, J.; Xing, X.; Tu, Y.; Manske, P.R. Flexor Tendon Healing in the Rat: A Histologic and Gene Expression Study. J. Hand Surg. 2003, 28, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Oakes, B.W. Tissue Healing and Repair: Tendons and Ligaments. In Rehabilitation of Sports Injuries: Scientific Basis; Volume X of the Encyklopaedia of sports medicine, an IOC Medical Committee publication in collaboration with the International Federation of Sports Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 56–92. [Google Scholar]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone Resistance: Mechanisms, Impact on Bacteria, and Role in Evolutionary Success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Quinolone generations: Natural history or natural selection? J. Antimicrob. Chemother. 2000, 46 (Suppl. T1), 17–24. [Google Scholar] [CrossRef]
- Owens, R.C.; Ambrose, P.G. Antimicrobial Safety: Focus on Fluoroquinolones. Clin. Infect. Dis. 2005, 41, S144–S157. [Google Scholar] [CrossRef]
- Drlica, K.; Malik, M.; Kerns, R.J.; Zhao, X. Quinolone-Mediated Bacterial Death. Antimicrob. Agents Chemother. 2008, 52, 385–392. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone Antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, P.D.; Sturkenboom, M.C.; Herings, R.M.; Leufkens, H.G.; Stricker, B.H. Fluoroquinolones and Risk of Achilles Tendon Disorders: Case-Control Study. BMJ 2002, 324, 1306–1307. [Google Scholar] [CrossRef]
- Lewis, T.; Cook, J. Fluoroquinolones and Tendinopathy: A Guide for Athletes and Sports Clinicians and a Systematic Review of the Literature. J. Athl. Train. 2014, 49, 422–427. [Google Scholar] [CrossRef]
- Casparian, J.M.; Luchi, M.; Moffat, R.E.; Hinthorn, D. Quinolones and Tendon Rupture. South. Med. J. 2000, 93, 488–491. [Google Scholar] [CrossRef]
- Gawor, A.; Gajewski, Z.; Paczek, L.; Konopka, A.; Wryk, G.; Bulska, E.; Czarkowska-Paczek, B. Fluorine-Containing Drug Administration in Rats Results in Fluorination of Selected Proteins in Liver and Brain Tissue. Int. J. Mol. Sci. 2022, 23, 4202. [Google Scholar] [CrossRef]
- Bidell, M.R.; Lodise, T.P. Fluoroquinolone-Associated Tendinopathy: Does Levofloxacin Pose the Greatest Risk? Pharmacotherapy 2016, 36, 679–693. [Google Scholar] [CrossRef]
- Monkovic, J.M.; Gibson, H.; Sun, J.W.; Montclare, J.K. Fluorinated Protein and Peptide Materials for Biomedical Applications. Pharmaceuticals 2022, 15, 1201. [Google Scholar] [CrossRef]
- Millanao, A.R.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Hidalgo, A.A. Biological Effects of Quinolones: A Family of Broad-Spectrum Antimicrobial Agents. Molecules 2021, 26, 7153. [Google Scholar] [CrossRef]
- Pitman, S.K.; Hoang, U.T.P.; Wi, C.H.; Alsheikh, M.; Hiner, D.A.; Percival, K.M. Revisiting Oral Fluoroquinolone and Multivalent Cation Drug-Drug Interactions: Are They Still Relevant? Antibiotics 2019, 8, 108. [Google Scholar] [CrossRef]
- Badal, S.; Her, Y.F.; James Maher, L. Nonantibiotic Effects of Fluoroquinolones in Mammalian Cells. J. Biol. Chem. 2015, 290, 22287–22297. [Google Scholar] [CrossRef] [PubMed]
- Bisaccia, D.R.; Aicale, R.; Tarantino, D.; Peretti, G.M.; Maffulli, N. Biological and Chemical Changes in Fluoroquinolone-Associated Tendinopathies: A Systematic Review. Br. Med. Bull. 2019, 130, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Karousou, E.; Ronga, M.; Vigetti, D.; Passi, A.; Maffulli, N. Collagens, Proteoglycans, MMP-2, MMP-9 and TIMPs in Human Achilles Tendon Rupture. In Clinical Orthopaedics and Related Research; Springer: New York, NY, USA, 2008; Volume 466, pp. 1577–1582. [Google Scholar]
- Simonin, M.-A.; Gegout-Pottie, P.; Minn, A.; Gillet, P.; Netter, P.; Terlain, B. Pefloxacin-Induced Achilles Tendon Toxicity in Rodents: Biochemical Changes in Proteoglycan Synthesis and Oxidative Damage to Collagen. Antimicrob. Agents Chemother. 2000, 44, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.-Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2020, 19, 1202–1224. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative Stress and Apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Yuan, J.; Murrell, G.A.C.; Trickett, A.; Wang, M.X. Involvement of Cytochrome c Release and Caspase-3 Activation in the Oxidative Stress-Induced Apoptosis in Human Tendon Fibroblasts. Biochim. Biophys. Acta Mol. Cell Res. 2003, 1641, 35–41. [Google Scholar] [CrossRef]
- Chang, H.N.; Pang, J.H.S.; Chen, C.P.C.; Ko, P.C.; Lin, M.S.; Tsai, W.C.; Yang, Y.M. The Effect of Aging on Migration, Proliferation, and Collagen Expression of Tenocytes in Response to Ciprofloxacin. J. Orthop. Res. 2012, 30, 764–768. [Google Scholar] [CrossRef]
- Tsai, W.C.; Hsu, C.C.; Chen, H.C.; Hsu, Y.H.; Lin, M.S.; Wu, C.W.; Pang, J.H.S. Ciprofloxacin-Mediated Inhibition of Tenocyte Migration and down-Regulation of Focal Adhesion Kinase Phosphorylation. Eur. J. Pharmacol. 2009, 607, 23–26. [Google Scholar] [CrossRef]
- Furuta, H.; Yamada, M.; Nagashima, T.; Matsuda, S.; Nagayasu, K.; Shirakawa, H.; Kaneko, S. Increased Expression of Glutathione Peroxidase 3 Prevents Tendinopathy by Suppressing Oxidative Stress. Front. Pharmacol. 2023, 14, 1137952. [Google Scholar] [CrossRef]
- Kato, M.; Takada, S.; Kashida, Y.; Nomura, M. Histological Examination on Achilles Tendon Lesions Induced by Quinolone Antibacterial Agents in Juvenile Rats. Toxicol. Pathol. 1995, 23, 385–392. [Google Scholar] [CrossRef]
- Wunderli, S.L.; Blache, U.; Beretta Piccoli, A.; Niederöst, B.; Holenstein, C.N.; Passini, F.S.; Silván, U.; Bundgaard, L.; auf dem Keller, U.; Snedeker, J.G. Tendon Response to Matrix Unloading Is Determined by the Patho-Physiological Niche. Matrix Biol. 2020, 89, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Beberok, A.; Wrześniok, D.; Szlachta, M.; Rok, J.; Rzepka, Z.; Respondek, M.; Buszman, E. Lomefloxacin Induces Oxidative Stress and Apoptosis in COLO829 Melanoma Cells. Int. J. Mol. Sci. 2017, 18, 2194. [Google Scholar] [CrossRef]
- Wang, M.X.; Wei, A.; Yuan, J.; Clippe, A.; Bernard, A.; Knoops, B.; Murrell, G.A.C. Antioxidant Enzyme Peroxiredoxin 5 Is Upregulated in Degenerative Human Tendon. Biochem. Biophys. Res. Commun. 2001, 284, 667–673. [Google Scholar] [CrossRef]
- Seghinsara, A.M.; Abedelahi, A.; Salimnejad, R. Effect of Vitamin E and Selenium on Oxidative Stress and Tissue Damages Induced by Electromagnetic Fields in Immature Mice Ovarian. Crescent J. Med. Biol. Sci. 2017, 4, 120–125. [Google Scholar]
- Mișcă, O.M.; Mișcă, L.C.; Huzum, B.; Neamţu, A.A.; Cerbu, S.; Chioibaș, D.R.; Crăiniceanu, P.Z.; Motoc, A.G.M. A Prospective Randomized Pilot Study on the Efficacy of a Dietary Supplementation Regimen of Vitamin E and Selenium for the Prevention of Fluoroquinolone-Induced Tendinopathy. Pharmaceuticals 2025, 18, 575. [Google Scholar] [CrossRef] [PubMed]
- Duman, E.; Müller-Deubert, S.; Pattappa, G.; Stratos, I.; Sieber, S.A.; Clausen-Schaumann, H.; Sarafian, V.; Shukunami, C.; Rudert, M.; Docheva, D. Fluoroquinolone-Mediated Tendinopathy and Tendon Rupture. Pharmaceuticals 2025, 18, 184. [Google Scholar] [CrossRef]
- Alves, C.; Mendes, D.; Marques, F.B. Fluoroquinolones and the Risk of Tendon Injury: A Systematic Review and Meta-Analysis. Eur. J. Clin. Pharmacol. 2019, 75, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Arabyat, R.M.; Raisch, D.W.; McKoy, J.M.; Bennett, C.L. Fluoroquinolone-Associated Tendon-Rupture: A Summary of Reports in the Food and Drug Administrations Adverse Event Reporting System. Expert. Opin. Drug Saf. 2015, 14, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Persson, R.; Jick, S. Clinical Implications of the Association between Fluoroquinolones and Tendon Rupture: The Magnitude of the Effect with and without Corticosteroids. Br. J. Clin. Pharmacol. 2019, 85, 949–959. [Google Scholar] [CrossRef]
- Van Der Vlist, A.C.; Breda, S.J.; Oei, E.H.G.; Verhaar, J.A.N.; De Vos, R.J. Clinical Risk Factors for Achilles Tendinopathy: A Systematic Review. Br. J. Sports Med. 2019, 53, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgio, A.; Sirone, M.; Adravanti, F.M.; Testa, E.A.; Riegger, M.; Filardo, G. Achilles Tendon Complications of Fluoroquinolone Treatment: A Molecule-Stratified Systematic Review and Meta-Analysis. EFORT Open Rev. 2024, 9, 581–588. [Google Scholar] [CrossRef]
- Khaliq, Y.; Zhanel, G.G. Fluoroquinolone-Associated Tendinopathy: A Critical Review of the Literature. Clin. Infect. Dis. 2003, 36, 1404–1410. [Google Scholar] [CrossRef]
- Stephenson, A.L.; Wu, W.; Cortes, D.; Rochon, P.A. Tendon Injury and Fluoroquinolone Use: A Systematic Review. Drug Saf. 2013, 36, 709–721. [Google Scholar] [CrossRef]
| Mechanism | Takeaway Information |
|---|---|
| Direct tenocyte toxicity | Dose-dependent tenocyte apoptosis or necrosis is demonstrated in preclinical models. |
| Oxidative stress (ROS/NO) | FQs induce ROS/NO in tendon cells leading to matrix damage; a component of multifactorial injury. |
| MMP and collagen synthesis | Upregulated MMPs and reduced type-I collagen synthesis leading to ECM weakening. |
| Mg2+chelation and integrin signalling | Chelation impairs integrin/MAPK signalling and cell–ECM adhesion leading to impaired repair. |
| Structure-toxicity link (C7) | Higher tendon toxicity with C7 methyl-piperazinyl (oflox/levo) vs. piperazinyl (cipro/nor) in animal or toxicologic data. |
| Mitochondrial or topoisomerase II (speculative) | Possible host topoisomerase II or mitochondrial involvement; supportive but limited direct tendon data. |
| Fluoroquinolone | Takeaway Information |
|---|---|
| Ofloxacin | Highest documented AT/ATR incidence among common FQs: ≈1.40% (95% CI, 0.88%–2.03%; SE 2.51); significantly greater than levo or cipro (≈0.17% each; p < 0.0001). |
| Levofloxacin | Expected risk ≈ 0.17% (similar to cipro in pooled estimates); most FAERS rupture reports and strongest disproportionality signal among FQs. |
| Ciprofloxacin | Expected risk ≈ 0.17%; ATR not significantly increased in some pooled analyses, but class risk still applies. |
| Norfloxacin | Meta-analytic stratification shows increased ATR risk for norfloxacin (agent-specific signal). |
| Moxifloxacin/others | Tendon events reported but smaller pharmacovigilance signals vs. levo; pooled “other molecules” group ≈ 0.31%. |
| Finding | Additional Information | References |
|---|---|---|
| Incidence & Warning | Tendinopathy is uncommon in general use (~0.14–0.4%); class carries boxed warning for tendinitis/rupture. | [62] |
| Risk vs. Non-use (meta-analysis) | FQ exposure increases risk: ATR OR ≈ 2.5; AT OR ≈ 4.0; any tendon disorder OR ≈ 2.0 (all significant). | [56] |
| Predominant Site | ~90% of reported cases involve the Achilles; other tendons less frequent. | [61] |
| Often Bilateral | Bilateral involvement is common in Achilles cases (~40–50%). | [61] |
| Onset Window | Onset typically early: median ~8 days; 50% ≤6 days; range 2 h to 6 months; peak within first ~30 days. | [61,62] |
| Progression to Rupture | A large share of events progress to rupture (~40% of collated cases). | [61] |
| Absolute Excess Risk | Any tendon: +3.73/10,000 PY; Achilles: +2.91/10,000 PY; FQ and steroid vs steroid alone: +21.2/10,000 PY. | [58] |
| High-risk: Age ≥ 60 | Older adults have materially higher odds of tendon injury/rupture on FQs. | [56,58,62] |
| High-risk: Corticosteroids | Concomitant systemic steroids markedly amplify risk (largest single clinical co-factor). | [56,58,62] |
| High-risk: Renal Impairment & Transplant | CKD/dialysis and solid-organ transplant recipients show higher rates; dose adjustment/avoidance advised where possible. | [59,61,62] |
| Sex Signal | Mixed: one large analysis found higher odds in women on FQs; early case series skewed older men. | [58,61] |
| Drug-specific Risk Factor | Ofloxacin use identified as a clinical risk factor for Achilles tendinopathy in cohort-level evidence. | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowska, M.J.; Adamus, J.P.; Struzik, S.; Paczek, L. Fluoroquinolone-Induced Achilles Tendon Damage: Structural and Biochemical Insights into Collagen Type I Alterations. Int. J. Mol. Sci. 2025, 26, 10028. https://doi.org/10.3390/ijms262010028
Romanowska MJ, Adamus JP, Struzik S, Paczek L. Fluoroquinolone-Induced Achilles Tendon Damage: Structural and Biochemical Insights into Collagen Type I Alterations. International Journal of Molecular Sciences. 2025; 26(20):10028. https://doi.org/10.3390/ijms262010028
Chicago/Turabian StyleRomanowska, Magdalena J., Jakub P. Adamus, Sławomir Struzik, and Leszek Paczek. 2025. "Fluoroquinolone-Induced Achilles Tendon Damage: Structural and Biochemical Insights into Collagen Type I Alterations" International Journal of Molecular Sciences 26, no. 20: 10028. https://doi.org/10.3390/ijms262010028
APA StyleRomanowska, M. J., Adamus, J. P., Struzik, S., & Paczek, L. (2025). Fluoroquinolone-Induced Achilles Tendon Damage: Structural and Biochemical Insights into Collagen Type I Alterations. International Journal of Molecular Sciences, 26(20), 10028. https://doi.org/10.3390/ijms262010028






