Microbiome–Aging–Wrinkles Axis of Skin: Molecular Insights and Microbial Interventions
Abstract
1. Introduction
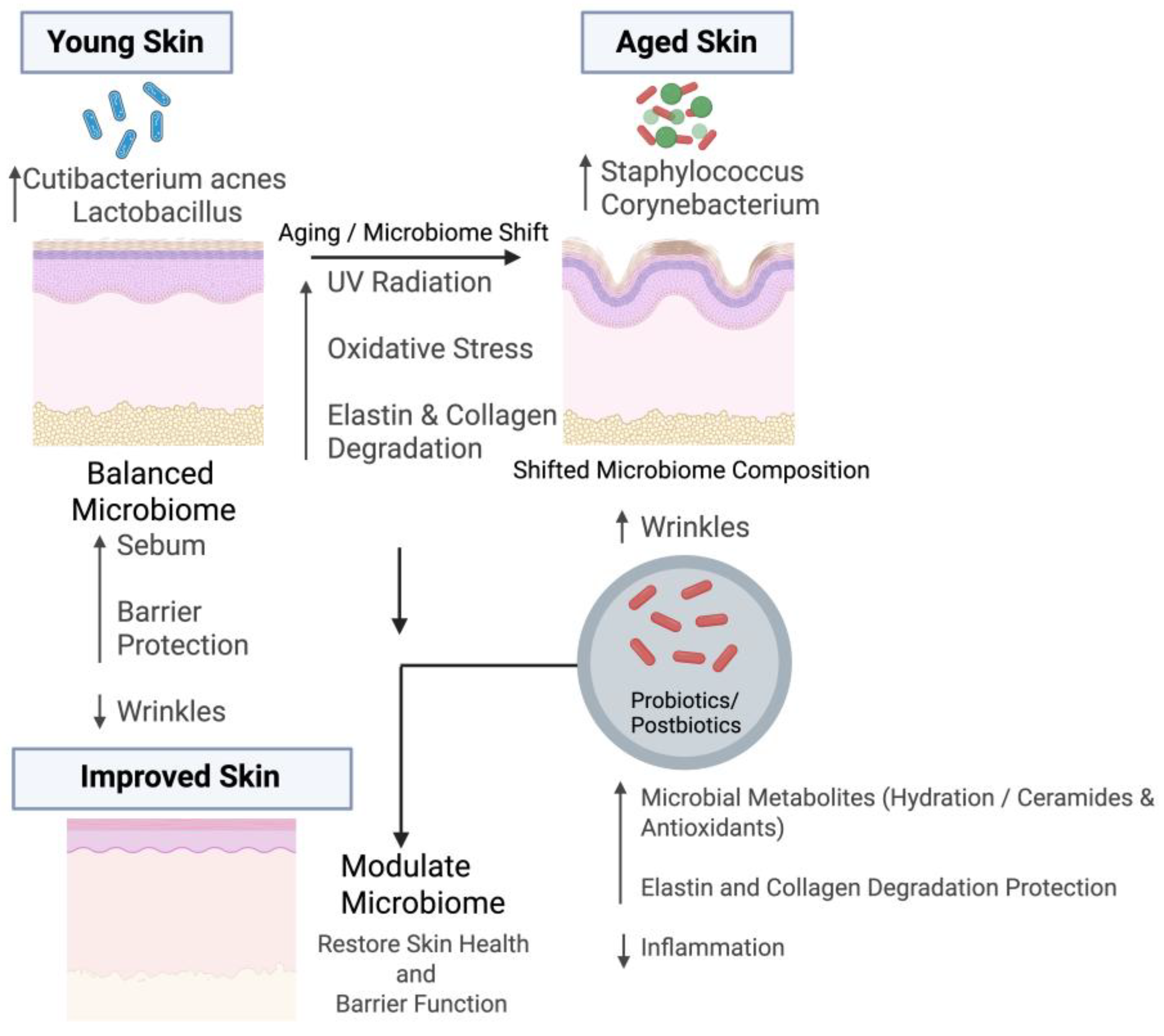
2. Microbiome–Skin Interaction During Aging
3. Microbiome Associated with Wrinkles
4. Understanding of Microbiome on Wrinkle Formation During Aging
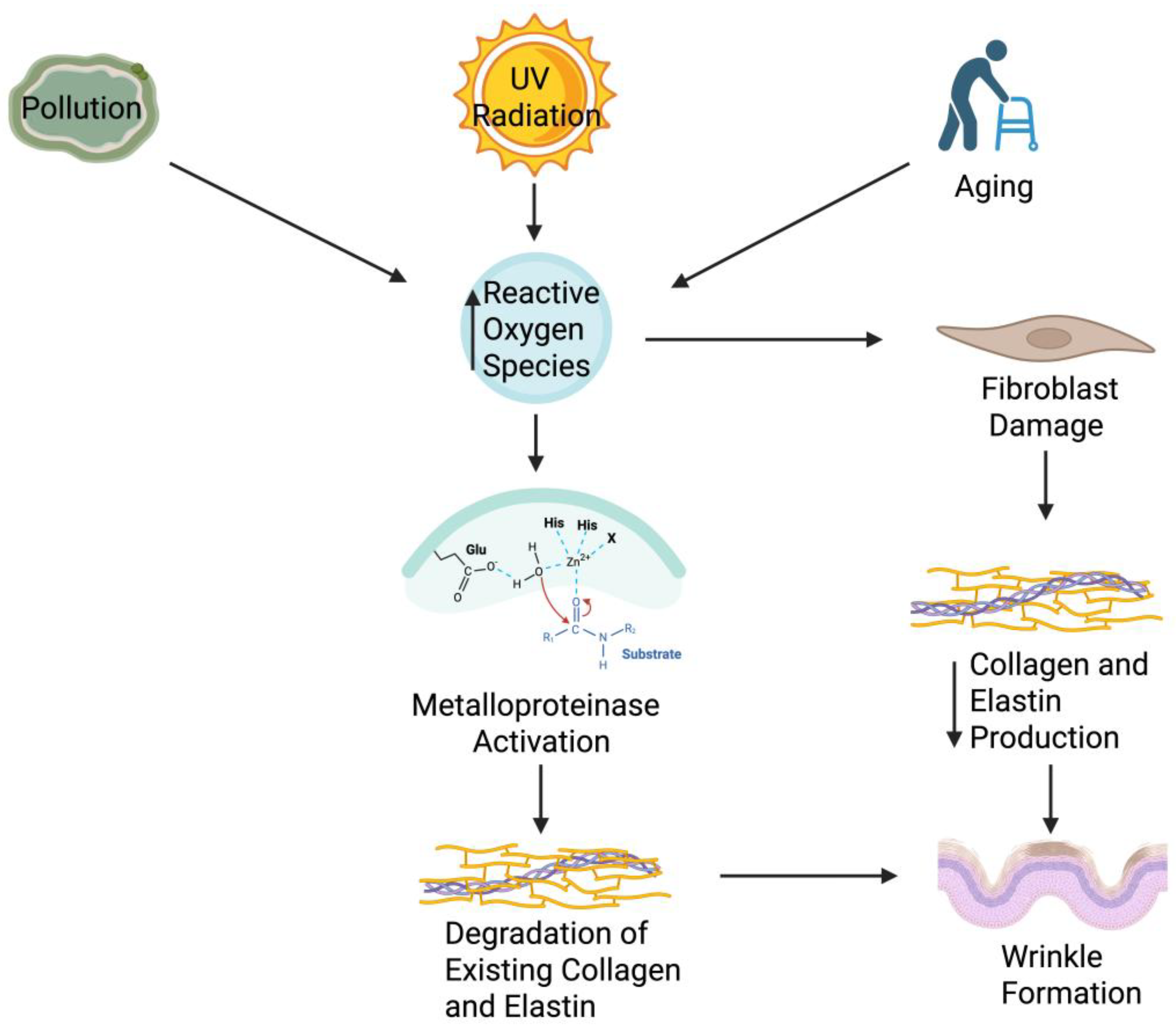
4.1. Mechanistic Overview of Wrinkle Formation in Aging Skin
4.2. Elastin Degradation and Microbiome Interactions
4.3. Role of MMP-1 and MMP-9 in Wrinkle Formation
4.4. Collagen and ECM Breakdown in Skin Aging: Microbial and Molecular Modulation
| Wrinkle Formation Contributor | Mechanism of Action | Microbiome Influence |
|---|---|---|
| Collagen Degradation | Matrix metalloproteinases break down collagen, and are activated by factors such as UV radiation, reactive oxygen factors, and inflammation. | The microbiome modulates inflammation. Good microbiota help suppress inflammation, keeping matrix metalloproteinases levels low. Certain microbes also produce antioxidants that neutralize oxidants [86] |
| Oxidative Stress | Reactive oxygen species cause wrinkles by damaging the dermal fibroblasts, activating matrix metalloproteinases, and leading to a reduction in skin elasticity. | Certain skin bacteria produce antioxidant enzymes that neutralize ROS [87] |
| Inflammation | Inflammation activates matrix metalloproteinases, damage the fibroblasts that produce collagen, and increase overall oxidative stress. | Healthy skin microbiota help suppress inflammation by inhibiting immune pathways and creating anti-inflammatory cytokines [88] |
| Loss of Skin Hydration | The stratum corneum contains high levels of water, maintaining the elasticity of the skin. | Commensal bacteria promote tight junction integrity and lipid production, which leads to less trans epidermal water loss and better moisture retention, keeping the skin elastic [89] |
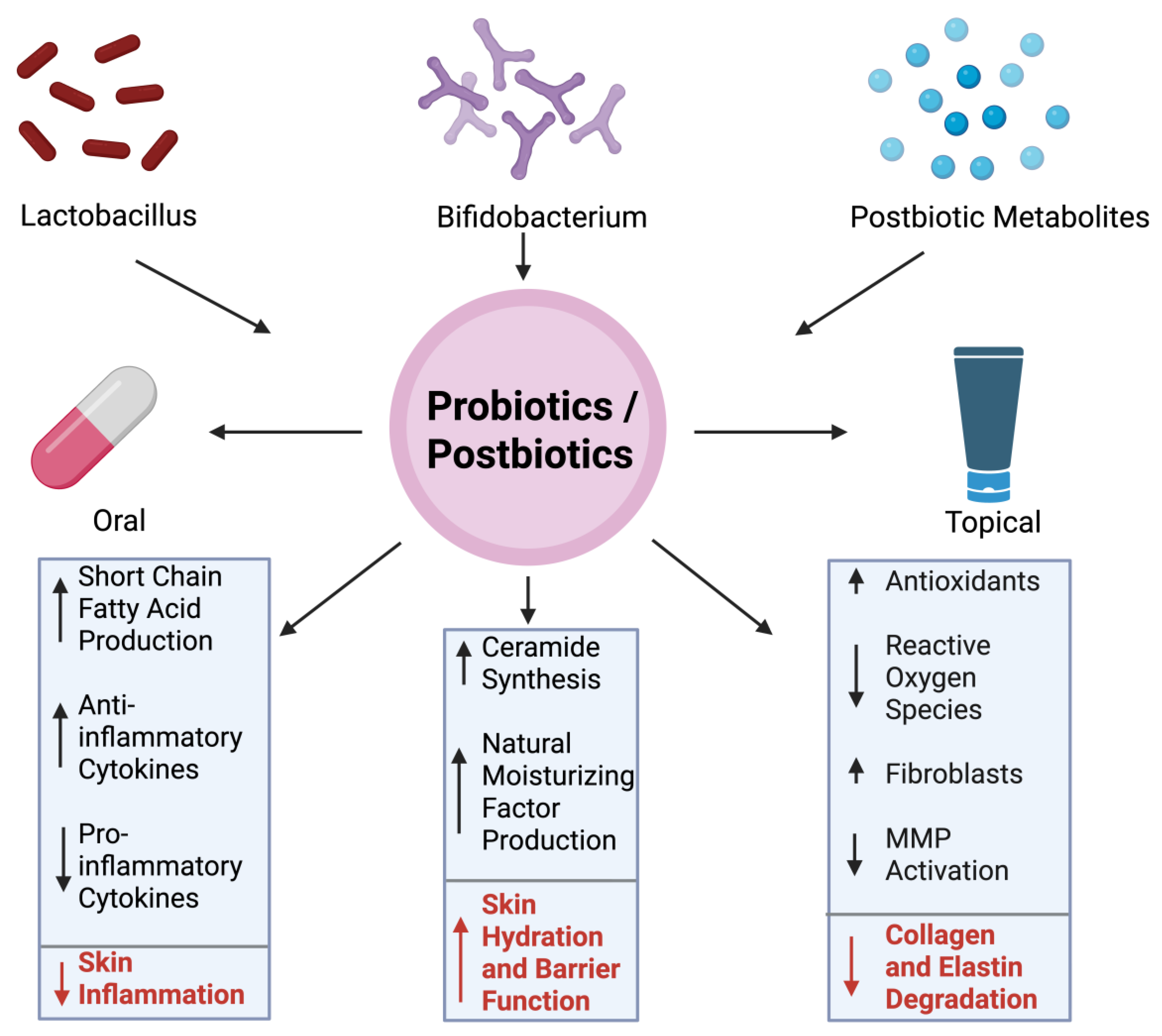
5. Therapeutic Approach
5.1. Probiotics/Postbiotics in Reducing Wrinkles
5.2. Clinical Evidence and Preventive Potential of Probiotics and Postbiotics in Skin Aging
6. Diagnostic Potential of Skin Microbiome Profiling
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fredricks, D.N. Microbial ecology of human skin in health and disease. J. Investig. Dermatol. Symp. Proc. 2001, 6, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Lekkala, L.; Yadav, D.; Jain, S.; Yadav, H. Microbiome and Postbiotics in Skin Health. Biomedicines 2025, 13, 791. [Google Scholar] [CrossRef]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Jain, S.; Yadav, H. Age-Related Cognitive Decline and Dementia: Interface of Microbiome-Immune-Neuronal Interactions. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2025, 80, glaf038. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Hussein, R.S.; Dayel, S.B.; Abahussein, O.; El-Sherbiny, A.A. Influences on Skin and Intrinsic Aging: Biological, Environmental, and Therapeutic Insights. J. Cosmet. Dermatol. 2024, 24, e16688. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef] [PubMed]
- Bossi, O.; Gartsbein, M.; Leitges, M.; Kuroki, T.; Grossman, S.; Tennenbaum, T. UV irradiation increases ROS production via PKCδ signaling in primary murine fibroblasts. J. Cell. Biochem. 2008, 105, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, X.; Peng, T.; Yi, X.; Luo, L.; Yang, J.; Liu, J.; Wang, Y.; He, T.; Wang, X. New insights into the skin microbial communities and skin aging. Front. Microbiol. 2020, 11, 565549. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, J.J.; Myeong, N.R.; Kim, T.; Kim, D.; An, S.; Kim, H.; Park, T.; Jang, S.I.; Yeon, J.H.; et al. Segregation of age-related skin microbiome characteristics by functionality. Sci. Rep. 2019, 9, 16748. [Google Scholar] [CrossRef]
- Cros, M.P.; Mir-Pedrol, J.; Toloza, L.; Knödlseder, N.; Maruotti, J.; Zouboulis, C.C.; Güell, M.; Fábrega, M.-J. New insights into the role of Cutibacterium acnes-derived extracellular vesicles in inflammatory skin disorders. Sci. Rep. 2023, 13, 16058, Corrected in Sci. Rep. 2024, 14, 3027. [Google Scholar]
- Szabó, K.; Erdei, L.; Bolla, B.S.; Tax, G.; Bíró, T.; Kemény, L. Factors shaping the composition of the cutaneous microbiota. Br. J. Dermatol. 2017, 176, 344–351. [Google Scholar] [CrossRef]
- Shibagaki, N.; Suda, W.; Clavaud, C.; Bastien, P.; Takayasu, L.; Iioka, E.; Kurokawa, R.; Yamashita, N.; Hattori, Y.; Shindo, C.; et al. Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci. Rep. 2017, 7, 10567. [Google Scholar] [CrossRef]
- Myers, T.; Bouslimani, A.; Huang, S.; Hansen, S.T.; Clavaud, C.; Azouaoui, A.; Ott, A.; Gueniche, A.; Bouez, C.; Zheng, Q.; et al. A multi-study analysis enables identification of potential microbial features associated with skin aging signs. Front. Aging 2024, 4, 1304705. [Google Scholar] [CrossRef]
- Ratanapokasatit, Y.; Laisuan, W.; Rattananukrom, T.; Petchlorlian, A.; Thaipisuttikul, I.; Sompornrattanaphan, M. How microbiomes affect skin aging: The updated evidence and current perspectives. Life 2022, 12, 936. [Google Scholar] [CrossRef]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical relevance of elastin in the structure and function of skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of age and regional differences on skin elasticity as measured by the Cutometer®. Skin Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.-Y. Solar Elastosis. 2022. Available online: https://dermnetnz.org/topics/solar-elastosis (accessed on 22 July 2025).
- Lee, D.E.; Huh, C.-S.; Ra, J.; Choi, I.-D.; Jeong, J.-W.; Kim, S.-H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.-H.; et al. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef]
- Teng, Y.; Huang, Y.; Danfeng, X.; Tao, X.; Fan, Y. The role of probiotics in skin photoaging and related mechanisms: A review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2455–2464. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and their health modulatory biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef]
- Huuskonen, L.; Anglenius, H.; Ahonen, I.; Tiihonen, K. Effects of bacterial lysates and metabolites on collagen homeostasis in TNF-α-challenged human dermal fibroblasts. Microorganisms 2023, 11, 1465. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Yadav, D.; Katiyar, S.; Jain, S.; Yadav, H. Postbiotics as Mitochondrial Modulators in Inflammatory Bowel Disease: Mechanistic Insights and Therapeutic Potential. Biomolecules 2025, 15, 954. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Claesson, M.J. Gut microbiota: Changes throughout the lifespan from infancy to elderly. Int. Dairy J. 2010, 20, 281–291. [Google Scholar] [CrossRef]
- Gottlieb, M.G.V.; Closs, V.E.; Junges, V.M.; Schwanke, C.H.A. Impact of human aging and modern lifestyle on gut microbiota. Crit. Rev. Food Sci. Nutr. 2018, 58, 1557–1564. [Google Scholar] [CrossRef]
- Thakur, R.; Batheja, P.; Kaushik, D.; Michniak, B. Structural and biochemical changes in aging skin and their impact on skin permeability barrier. Ski. Aging Handb. 2009, 55–90. [Google Scholar] [CrossRef]
- Pagac, M.P.; Davient, B.; Plado, L.A.; Lam, H.Y.I.; Lee, S.M.; Ravikrishnan, A.; Chua, W.L.E.; Muralidharan, S.; Sridharan, A.; Irudayaswamy, A.S.; et al. Life stage impact on the human skin ecosystem: Lipids and the microbial community. npj Biofilms Microbiomes 2025, 11, 13. [Google Scholar] [CrossRef]
- Kreouzi, M.; Theodorakis, N.; Nikolaou, M.; Feretzakis, G.; Anastasiou, A.; Kalodanis, K.; Sakagianni, A. Skin microbiota: Mediator of interactions between metabolic disorders and cutaneous health and disease. Microorganisms 2025, 13, 161. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3509–3534. [Google Scholar] [CrossRef]
- Pająk, J.; Nowicka, D.; Szepietowski, J.C. Inflammaging and immunosenescence as part of skin aging—A narrative review. Int. J. Mol. Sci. 2023, 24, 7784. [Google Scholar] [CrossRef]
- Huang, S.; Haiminen, N.; Carrieri, A.-P.; Hu, R.; Jiang, L.; Parida, L.; Russell, B.; Allaband, C.; Zarrinpar, A.; Vázquez-Baeza, Y.; et al. Human skin, oral, and gut microbiomes predict chronological age. Msystems 2020, 5, e00630-19. [Google Scholar] [CrossRef]
- Dimarzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.; Giuliani, M. Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharmacol. 2008, 21, 137–143. [Google Scholar] [CrossRef]
- Souak, D.; Barreau, M.; Courtois, A.; André, V.; Duclairoir Poc, C.D.; Feuilloley, M.G.; Gault, M. Challenging cosmetic innovation: The skin microbiota and probiotics protect the skin from UV-induced damage. Microorganisms 2021, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Qu, Y.; Zheng, Y.; Ouyang, M.; Zhang, Y.; Lai, W.; Xu, Q. UVA-induced photoaging inhibits autophagic degradation by impairing lysosomal function in dermal fibroblasts. Biochem. Biophys. Res. Commun. 2019, 518, 611–618. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing skin health: By oral administration of natural compounds and minerals with implications to the dermal microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef]
- Meunier, M.; Scandolera, A.; Chapuis, E.; Lambert, C.; Jarrin, C.; Robe, P.; Chajra, H.; Auriol, D.; Reynaud, R. From stem cells protection to skin microbiota balance: Orobanche rapum extract, a new natural strategy. J. Cosmet. Dermatol. 2019, 18, 1140–1154. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B.; Johnstone, D.M.; Gordon, L.C.; Kiat, H.; Hamblin, M.R. “Photobiomics”: Can light, including photobiomodulation, alter the microbiome? Photobiomodulation Photomed. Laser Surg. 2019, 37, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Woolery-Lloyd, H.; Andriessen, A.; Day, D.; Gonzalez, N.; Green, L.; Grice, E.; Henry, M. Review of the microbiome in skin aging and the effect of a topical prebiotic containing thermal spring water. J. Cosmet. Dermatol. 2023, 22, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Hu, G.; Yi, L.; Ge, W.; Yang, Q.; Yang, X.; He, Y.; Liu, Z.; Chen, W.-H. Integrated analysis of facial microbiome and skin physio-optical properties unveils cutotype-dependent aging effects. Microbiome 2024, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.; Bascom, C.C.; Hu, P.; Binder, R.L.; Fadayel, G.; Huggins, T.G.; Jarrold, B.B.; Osborne, R.; Rocchetta, H.L.; Swift, D.; et al. Aging-associated changes in the adult human skin microbiome and the host factors that affect skin microbiome composition. J. Investig. Dermatol. 2022, 142, 1934–1946.e21. [Google Scholar] [CrossRef] [PubMed]
- Garlet, A.; Andre-Frei, V.; Del Bene, N.; Cameron, H.J.; Samuga, A.; Rawat, V.; Ternes, P.; Leoty-Okombi, S. Facial skin microbiome composition and functional shift with aging. Microorganisms 2024, 12, 1021. [Google Scholar] [CrossRef]
- Blaise, G.; Nikkels, A.F.; Hermanns-Lê, T.; Nikkels-Tassoudji, N.; Piérard, G.E. Corynebacterium-associated skin infections. Int. J. Dermatol. 2008, 47, 884–890. [Google Scholar] [CrossRef]
- Bouilly-Gauthier, D.; Jeannes, C.; Maubert, Y.; Duteil, L.; Queille-Roussel, C.; Piccardi, N.; Montastier, C.; Manissier, P.; Pierard, G.; Ortonne, J.P. Clinical evidence of benefits of a dietary supplement containing probiotic and carotenoids on ultraviolet-induced skin damage. Br. J. Dermatol. 2010, 163, 536–543. [Google Scholar] [CrossRef]
- Rong, J.; Shan, C.; Liu, S.; Zheng, H.; Liu, C.; Liu, M.; Jin, F.; Wang, L. Skin resistance to UVB-induced oxidative stress and hyperpigmentation by the topical use of Lactobacillus helveticus NS8-fermented milk supernatant. J. Appl. Microbiol. 2017, 123, 511–523. [Google Scholar] [CrossRef]
- Moloney, S.J.; Edmonds, S.H.; Giddens, L.D.; Learn, D.B. The hairless mouse model of photoaging: Evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochem. Photobiol. 1992, 56, 505–511. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, J.; Andersen, M.; Katta, R.; Zouboulis, C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Mechanism of UVB-induced wrinkling of the skin: Paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase. J. Investig. Dermatol. Symp. Proc. 2009, 14, 36–43. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Mills, S.J.; Ashworth, J.J. Ageing and wound healing. Biogerontology 2002, 3, 337–345. [Google Scholar] [CrossRef]
- Lähteenvuo, J.; Rosenzweig, A. Effects of aging on angiogenesis. Circ. Res. 2012, 110, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Berard, F.; Marty, J.-P.; Nicolas, J.-F. Allergen penetration through the skin. Eur. J. Dermatol. 2003, 13, 324–330. [Google Scholar]
- Shin, S.H.; Lee, Y.H.; Rho, N.-K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Alkawar, A.M.; Castellanos, A.J.; Carpenter, M.A.; Hutcherson, R.J.; Madkhali, M.A.; Johnson, R.M.; Bottomley, M.; Kemp, M.G. Insulin-like Growth Factor-1 Impacts p53 Target Gene Induction in UVB-irradiated Keratinocytes and Human Skin. Photochem. Photobiol. 2020, 96, 1332–1341. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet radiation-induced skin aging: The role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhang, J.-Q.; Li, L.; Guo, M.-M.; He, Y.-F.; Dong, Y.-M.; Meng, H.; Yi, F. Advanced glycation end products in the skin: Molecular mechanisms, methods of measurement, and inhibitory pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef] [PubMed]
- Sellheyer, K. Pathogenesis of solar elastosis: Synthesis or degradation? J. Cutan. Cutan. Pathol. 2003, 30, 123–127. [Google Scholar] [CrossRef]
- Cheung, C.T.; Lancien, U.; Corvec, S.; Mengeaud, V.; Mias, C.; Véziers, J.; Khammari, A.; Dréno, B. Pro-inflammatory activity of Cutibacterium acnes phylotype IA1 and extracellular vesicles: An in vitro study. Exp. Dermatol. 2024, 33, e15150. [Google Scholar] [CrossRef] [PubMed]
- Shirzad, M.; Hamedi, J.; Motevaseli, E.; Modarressi, M.H. Anti-elastase and anti-collagenase potential of Lactobacilli exopolysaccharides on human fibroblast. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S1), 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Age-related changes in the fibroblastic differon of the dermis: Role in skin aging. Int. J. Mol. Sci. 2022, 23, 6135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, H.; Go, Y.; Chan, X.H.; Huang, Q.; Wu, J. Research advances on the damage mechanism of skin glycation and related inhibitors. Nutrients 2022, 14, 4588. [Google Scholar] [CrossRef]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of collagen I and collagen III in tissue injury and regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44, 224–231. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Xie, X.; Pang, R.; Huang, S.; Ying, H.; Chen, M.; Xue, L.; Zhang, J.; Ding, Y.; et al. Skin microbiome profiling reveals the crucial role of microbial metabolites in anti-photoaging. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12987. [Google Scholar] [CrossRef]
- Tirka, P.S.W.; Praharsini, I.; IGAAD, R.L.K.; Suryawati, N.; Vibriyantikarna, N. High serum level of matrix metalloproteinase-1 (MMP-1) correlates positively with The severity of photoaging facial wrinkles. Int. J. Sci. Adv. 2023, 4, 122–129. [Google Scholar] [CrossRef]
- Hong, Y.-F.; Lee, H.Y.; Jung, B.J.; Jang, S.; Chung, D.K.; Kim, H. Lipoteichoic acid isolated from Lactobacillus plantarum down-regulates UV-induced MMP-1 expression and up-regulates type I procollagen through the inhibition of reactive oxygen species generation. Mol. Immunol. 2015, 67, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.-T.; Kim, Y.J.; Jeong, J.W.; Jang, S.S.; Ahn, Y.-T.; Sim, J.-H.; Huh, C.-S.; et al. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Zhao, J.; Mo, Q.; Wang, C.; Wang, D.; Li, M. Weizmannia coagulans extracellular proteins reduce skin acne by inhibiting pathogenic bacteria and regulating TLR2/TRAF6-mediated NF-κB and MAPKs signaling pathways. Probiotics Antimicrob. Proteins 2025, 17, 705–720. [Google Scholar] [CrossRef]
- Daly, C.H. Biomechanical properties of dermis. J. Investig. Dermatol. 1982, 79, 17–20. [Google Scholar] [CrossRef]
- Ciornei, B.; Vaduva, A.; David, V.L.; Popescu, D.; Vulcanescu, D.D.; Adam, O.; Avram, C.R.; Pacurari, A.C.; Boia, E.S. Comparison of type I and type III collagen concentration between Oreochromis mossambicus and Oreochromis niloticus in relation to skin scaffolding. Medicina 2023, 59, 1002. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Russo, E.; Di Gloria, L.; Cerboneschi, M.; Smeazzetto, S.; Baruzzi, G.P.; Romano, F.; Ramazzotti, M.; Amedei, A. Facial skin microbiome: Aging-related changes and exploratory functional associations with host genetic factors, a pilot study. Biomedicines 2023, 11, 684. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kang, C.-H. Lactic acid bacteria improve the photoprotective effect via MAPK/AP-1/MMP signaling pathway on skin fibroblasts. Microorganisms 2022, 10, 2481. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Cho, S.; Chung, J.H.; Hammerberg, C.; Fisher, G.J.; Voorhees, J.J. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-κB and activator protein-1 in inflammatory acne lesions in vivo. Am. J. Pathol. 2005, 166, 1691–1699. [Google Scholar] [CrossRef]
- Grinnell, F. Fibronectin and wound healing. J. Cell. Biochem. 1984, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, N.; Maciejczyk, M. Hyaluronic Acid and Skin: Its Role in Aging and Wound-Healing Processes. Gels 2025, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Zhang, P.; Li, Q.; Han, Z. Oxidative stress and gut microbiome in inflammatory skin diseases. Front. Cell Dev. Biol. 2022, 10, 849985. [Google Scholar] [CrossRef]
- Sharma, M.R.; Mitrani, R.; Werth, V.P. Effect of TNFα blockade on UVB-induced inflammatory cell migration and collagen loss in mice. J. Photochem. Photobiol. B Biol. 2020, 213, 112072. [Google Scholar]
- Bai, G.-L.; Wang, P.; Huang, X.; Wang, Z.-Y.; Cao, D.; Liu, C.; Liu, Y.-Y.; Li, R.-L.; Chen, A.-J. Rapamycin protects skin fibroblasts from UVA-induced photoaging by inhibition of p53 and phosphorylated HSP27. Front. Cell Dev. Biol. 2021, 9, 633331. [Google Scholar] [CrossRef]
- Etherington, D.J. Collagen degradation. Ann. Rheum. Dis. 1977, 36 (Suppl. S2), 14. [Google Scholar] [CrossRef]
- Wenk, J.; Brenneisen, P.; Meewes, C.; Wlaschek, M.; Peters, T.; Blaudschun, R.; Ma, W.; Kuhr, L.; Schneider, L.; Scharffetter-Kochanek, K. UV-induced oxidative stress and photoaging. Curr. Probl. Dermatol. 2001, 29, 83–94. [Google Scholar]
- Pessa, J.E.; Nguyen, H.; John, G.B.; Scherer, P.E. The anatomical basis for wrinkles. Aesthetic Surg. J. 2014, 34, 227–234. [Google Scholar] [CrossRef]
- Choi, J.W.; Kwon, S.H.; Huh, C.H.; Park, K.C.; Youn, S.W. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: A comprehensive and objective approach. Ski. Res. Technol. 2013, 19, e349–e355. [Google Scholar]
- Papaccio, F.; Caputo, S.; Bellei, B. Focus on the contribution of oxidative stress in skin aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Tarapatzi, G.; Filidou, E.; Kandilogiannakis, L.; Spathakis, M.; Gaitanidou, M.; Arvanitidis, K.; Drygiannakis, I.; Valatas, V.; Kotzampassi, K.; Manolopoulos, V.G.; et al. The Probiotic Strains Bifidοbacterium lactis, Lactobacillus acidophilus, Lactiplantibacillus plantarum and Saccharomyces boulardii Regulate Wound Healing and Chemokine Responses in Human Intestinal Subepithelial Myofibroblasts. Pharmaceuticals 2022, 15, 1293. [Google Scholar] [CrossRef] [PubMed]
- Blazheva, D.; Mihaylova, D.; Averina, O.; Slavchev, A.; Brazkova, M.; Poluektova, E.; Danilenko, V.; Krastanov, A. Antioxidant potential of probiotics and postbiotics: A biotechnological approach to improving their stability. Russ. J. Genet. 2022, 58, 1036–1050. [Google Scholar] [CrossRef]
- Theodorou, I.M.; Kapoukranidou, D.; Theodorou, M.; Tsetis, J.K.; Menni, A.E.; Tzikos, G.; Bareka, S.; Shrewsbury, A.; Stavrou, G.; Kotzampassi, K. Cosmeceuticals: A review of clinical studies claiming to contain specific, well-characterized strains of probiotics or postbiotics. Nutrients 2024, 16, 2526. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Shi, D.; Ingrassia, M.; Gedeon, H.; Chu, T.; Zhang, J.; Wang, C. Skincare Benefits of a Postbiotic Ferment Produced Through Djon Djon Mushroom Fermentation by Saccharomyces. J. Cosmet. Dermatol. 2025, 24, e70067. [Google Scholar] [CrossRef] [PubMed]
- Colletti, A.; Pellizzato, M.; Cicero, A.F. The possible role of probiotic supplementation in inflammation: A narrative review. Microorganisms 2023, 11, 2160. [Google Scholar] [CrossRef] [PubMed]
- França, K. Topical probiotics in dermatological therapy and skincare: A concise review. Dermatol. Ther. 2021, 11, 71–77. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, H.; Zhang, Y.; Li, M.; Wang, D.; Zhao, D.; Zhang, J.; Wang, C. Protective effects of Lactobacillus reuteri SJ-47 strain exopolysaccharides on human skin fibroblasts damaged by UVA radiation. Bioresour. Bioprocess. 2022, 9, 127. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Man, M.Q.; Hu, L. Aging in the dermis: Fibroblast senescence and its significance. Aging Cell 2024, 23, e14054. [Google Scholar] [CrossRef]
- Gruber, F.; Ornelas, C.M.; Karner, S.; Narzt, M.-S.; Nagelreiter, I.M.; Gschwandtner, M.; Bochkov, V.; Tschachler, E. Nrf2 deficiency causes lipid oxidation, inflammation, and matrix-protease expression in DHA-supplemented and UVA-irradiated skin fibroblasts. Free Radic. Biol. Med. 2015, 88, 439–451. [Google Scholar] [CrossRef]
- Kanak, S.; Krzemińska, B. The Active compounds in plants of the genus Alchemilla with proven skin care and therapeutic formulations in the treatment of skin diseases. Prospect. Pharm. Sci. 2024, 22, 188–198. [Google Scholar] [CrossRef]
- Melnyk, N.; Nyczka, A.; Piwowarski, J.P.; Granica, S. Traditional Use of Chamomile Flowers (Matricariae flos) in Inflammatory-Associated Skin Disorders. Prospect. Pharm. Sci. 2024, 22, 59–73. [Google Scholar] [CrossRef]
- Mao, Z. Frontiers in Skin Rejuvenation: Recent Advances in Anti-Aging Skincare Technologies Based on Proteins, Peptides, and Peptide Derivatives. Mod. Health Sci. 2025, 8, 69. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, Y.; Cai, F.; Cao, D.; Wang, L.; Qiao, Z.; Hong, Q.; Li, N.; Zheng, Y.; Su, M.; et al. Next-generation probiotics: Microflora intervention to human diseases. BioMed Res. Int. 2022, 2022, 5633403. [Google Scholar] [CrossRef] [PubMed]
| Study | Changes | Mechanism |
|---|---|---|
| Randomized, double blind, placebo-controlled trial | Decreased transepidermal water loss and wrinkle depth, with an increase in skin gloss and skin elasticity. | HY7714 modulates the expression of enzymes involved in ceramide synthesis and protects against ultraviolet radiation. HY7714 also inhibits the activity of metalloproteinases, which degrade collagen and elastin in the skin [44]. |
| Multi Center and Deep Sequencing Survey | Aging, skin physio-optical conditions, and facial microbiome. | Aging influences skin microbiomes. The Facial Aging Index can see changes in the skin microbiome to assess aging [45]. |
| Observational Study | Significant changes were observed in aging at three body sites examined (face, buttocks, and arm). Decreased sebum and increased lipids/natural moisturizing factors (NMFs)/antimicrobial peptides (AMPs). | Aging reduces the amount of sebum through hormonal changes and alterations in the sebaceous glands. Changes in the lipids or NMFs and AMPs cause changes in the skin microbiome [46]. |
| Cross-sectional, observational microbiome | Significant decrease in Actinomycetes and an increase in Corynebacterium kroppenstedtii in the older group (>55 years old). Significantly higher proportion of Cutibacterium acnes and Lactobacillus crispatus in the younger group (18–35 years old). | Younger skin produces more sebum, creating an oily environment that promotes the development of bacteria like Lactobacillus crispatus. Older skin has reduced sebum production, leading to the promotion of the development of Corynebacterium kroppenstedtii [47]. |
| Study/Product | Model/Population | Microbial Strain/Component | Mechanism | Observed Effects on Skin/Wrinkles |
|---|---|---|---|---|
| Blaise et al. | Human, aged skin | Corynebacterium spp. | Skin microbiome profiling | Increased keratolytic activity; associated with wrinkle formation [48]. |
| Lee et al. | Clinical (oral supplementation) | Lactobacillus plantarum HY7714 | Oral probiotic | Improved skin hydration and reduced wrinkle depth [23]. |
| Bouilly-Gauthier et al. | Human, clinical trial | Lactobacillus johnsonii + carotenoids (synbiotic) | Oral | Increased resistance to UV-A and sunlight-induced damage [49]. |
| Rong et al. | In vitro/animal model | Lactobacillus helveticus supernatant | Topical/supernatant exposure | Reduced UVB-induced oxidative stress and pigmentation [50]. |
| Product Type | Name of Product | Content | Mechanism |
|---|---|---|---|
| Topical Serum | Gallinée Youthful Serum | Prebiotics, probiotics, and postbiotics | Improves the skin barrier, hydrates the skin, and reduces overall inflammation, all of which lead to healthier and younger looking skin. [102] |
| Oral capsule | Pendulum Skin probiotic | Probiotics strains (Akkermansia muciniphila and Clostridium butyricum) and prebiotics (Chicory insulin) | Supports gut microbiome balance, reducing inflammation and stress, leading to healthier skin. [103] |
| Topical Cream | Aurelia London probiotic B-Hydrated Moisturizer | Probiotic strains (Bifidobacterium) and prebiotics (inulin) | Suppress the skin immune response, preventing inflammation. Moisturizes the skin. https://www.aurelialondon.com/collections/moisturiser (accessed on 28 March 2025) |
| Topical Serum | Esse Probiotic Serum | Probiotics (Lactobacillus and Bifidobacterium) and prebiotics (inulin) | Probiotics outcompete harmful bacteria, stimulate collagen production, all of which increase the strength of the skin barrier. https://us.esseskincare.com/product/probiotic-serum/?srsltid=AfmBOopJFB37shr1IpubtR5yi92y2ZN-uLqk0k2_dXz0J7L8h8aXbfiX (accessed on 28 March 2025) |
| Topical Skincare | Lavera Barrier Balance Skincare Range | Prebiotics (Inulin and Lactobacillus ferment) | Nourish the beneficial skin bacteria, strengthening the skin’s natural barrier. https://www.lavera.com/products/care-series/barrier-balance-skin-care-series (accessed on 28 March 2025) |
| Oral Synbiotic Supplement | Patients with mild atopic dermatitis | Bifidobacterium animalis subsp., Lactis BS01, Lactiplantibacillus Plantarum LP-14 and Lacticaesibacillus Rhamnosus LR05 with Fructooligosaccharides and riboflavin | Significant improvements in itching and redness as well as a reduction in lesion severity. https://www.nutraingredients-usa.com/Article/2024/04/25/Probiotical-study-links-synbiotic-with-improvements-in-skin-conditions/ (accessed on 28 March 2025) |
| Oral Synbiotic Supplement | Adults with melasma | Lactococcus lactis, Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium bifidum | https://onlinelibrary.wiley.com/doi/10.1111/jocd.13955? (accessed on 28 March 2025) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Challa, V.; Prajapati, S.K.; Gangani, S.; Yadav, D.; Lekkala, L.; Jain, S.; Yadav, H. Microbiome–Aging–Wrinkles Axis of Skin: Molecular Insights and Microbial Interventions. Int. J. Mol. Sci. 2025, 26, 10022. https://doi.org/10.3390/ijms262010022
Challa V, Prajapati SK, Gangani S, Yadav D, Lekkala L, Jain S, Yadav H. Microbiome–Aging–Wrinkles Axis of Skin: Molecular Insights and Microbial Interventions. International Journal of Molecular Sciences. 2025; 26(20):10022. https://doi.org/10.3390/ijms262010022
Chicago/Turabian StyleChalla, Varun, Santosh Kumar Prajapati, Surabhi Gangani, Dhananjay Yadav, Lalitha Lekkala, Shalini Jain, and Hariom Yadav. 2025. "Microbiome–Aging–Wrinkles Axis of Skin: Molecular Insights and Microbial Interventions" International Journal of Molecular Sciences 26, no. 20: 10022. https://doi.org/10.3390/ijms262010022
APA StyleChalla, V., Prajapati, S. K., Gangani, S., Yadav, D., Lekkala, L., Jain, S., & Yadav, H. (2025). Microbiome–Aging–Wrinkles Axis of Skin: Molecular Insights and Microbial Interventions. International Journal of Molecular Sciences, 26(20), 10022. https://doi.org/10.3390/ijms262010022








