Idiopathic Abdominal Wall Endometrioma: Case Report with Investigation of the Pathological, Molecular Cytogenetic and Cell Growth Features In Vitro
Abstract
1. Introduction
2. Results
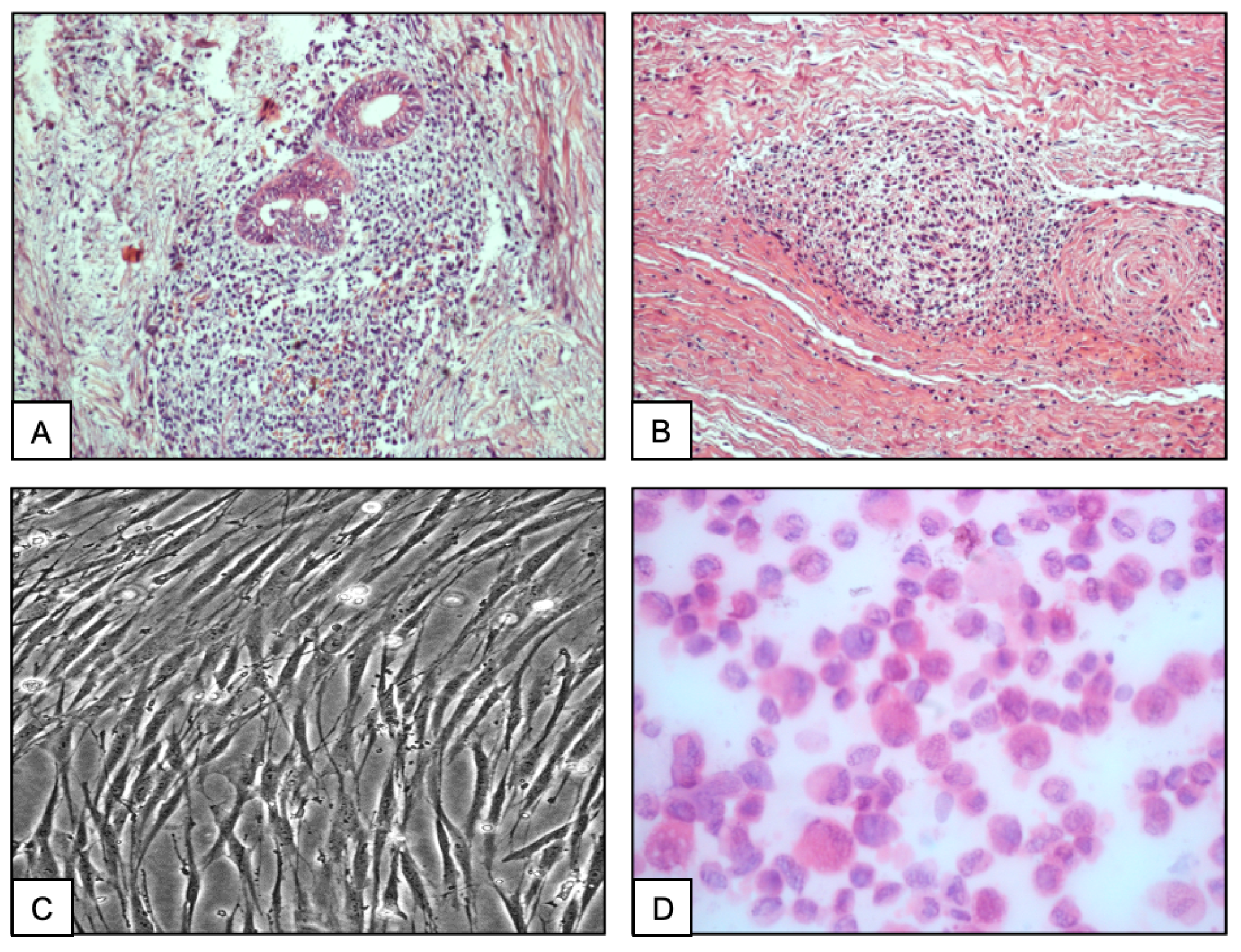
2.1. Histopathological Analysis
2.2. Stromal Cell Culture
2.3. Immunohistochemistry
2.4. Immunocytochemistry
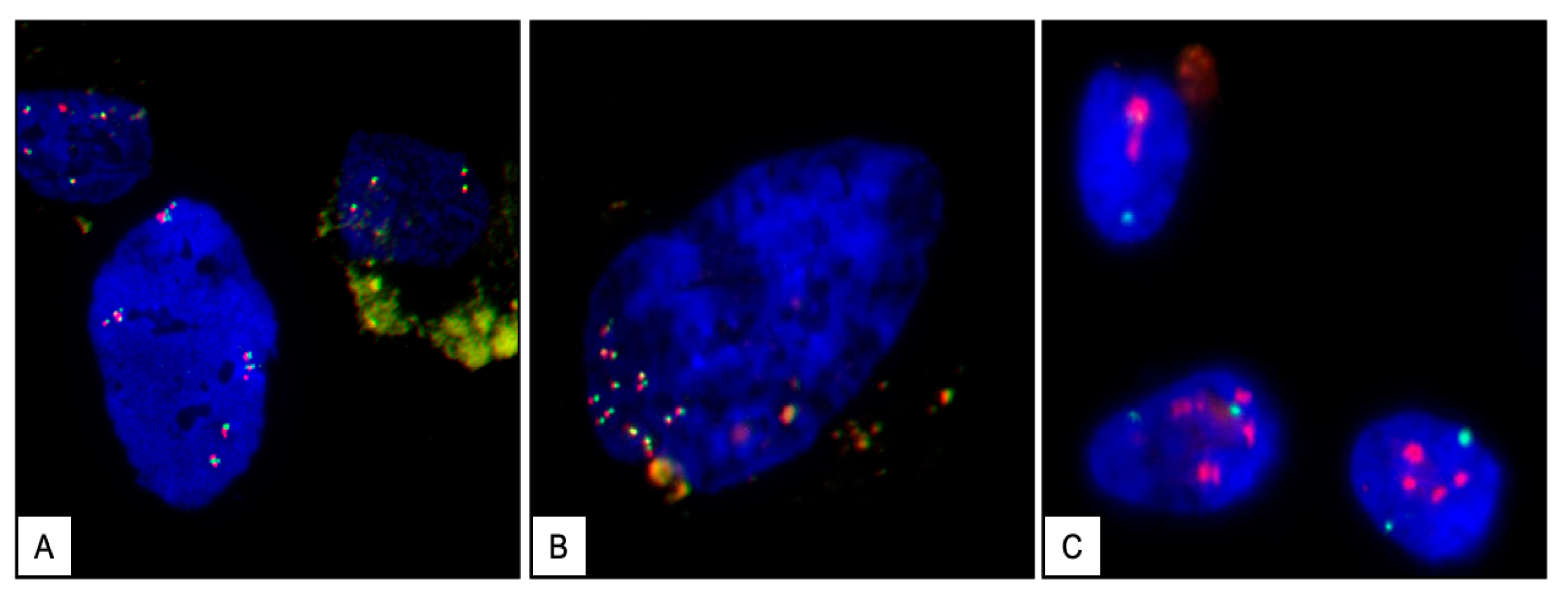
2.5. Cytogenetic Analysis of the Stromal Cells
2.6. Assessment of the c-MYC Gene Structure by Fluorescence In Situ Hybridization (FISH)
3. Discussion
4. Materials and Methods
4.1. Patient
4.2. Tissue Processing and Cell Culture
4.3. Immuno-Histochemistry
4.4. Immuno-Cytochemistry
4.5. Conventional Cytogenetics Analysis
4.6. Assessment of the c-MYC Gene Structure by Fluorescence In Situ Hybridization
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanco, R.G.; Parithivel, V.S.; Shah, A.K.; Gumbs, M.A.; Schein, M.; Gerst, P.H. Abdominal wall endometriomas. Am. J. Surg. 2003, 185, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Kyamidis, K.; Lora, V.; Kanitakis, J. Spontaneous cutaneous umbilical endometriosis: Report of a new case with immunohistochemical study and literature review. Dermatol. Online J. 2011, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.E.; Ayers, P.G.; Lee, T.T. Abdominal Wall Endometriosis. Obstet. Gynecol. Clin. N. Am. 2022, 49, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Latcher, J.W. Endometriosis of the umbilicus. Am. J. Obstet. Gynecol. 1953, 66, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Das, S. Primary umbilical endometriosis: A case report and review of literature. Arch. Gynecol. Obstet. 2014, 290, 807–809. [Google Scholar] [CrossRef]
- Boesgaard-Kjer, D.; Boesgaard-Kjer, D.; Kjer, J.J. Primary umbilical endometriosis (PUE). Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 44–45. [Google Scholar] [CrossRef]
- Horton, J.D.; Dezee, K.J.; Ahnfeldt, E.P.; Wagner, M. Abdominal wall endometriosis: A surgeon’s perspective and review of 445 cases. Am. J. Surg. 2008, 196, 207–212. [Google Scholar] [CrossRef]
- Efremidou, E.I.; Kouklakis, G.; Mitrakas, A.; Liratzopoulos, N.; Polychronidis, A.C. Primary umbilical endometrioma: A rare case of spontaneous abdominal wall endometriosis. Int. J. Gen. Med. 2012, 5, 999–1002. [Google Scholar] [CrossRef][Green Version]
- Victory, R.; Diamond, M.P.; Johns, D.A. Villar’s nodule: A case report and systematic literature review of endometriosis externa of the umbilicus. J. Minim. Invasive Gynecol. 2007, 14, 23–32. [Google Scholar] [CrossRef]
- Rindos, N.B.; Mansuria, S. Diagnosis and Management of Abdominal Wall Endometriosis: A Systematic Review and Clinical Recommendations. Obstet. Gynecol. Surv. 2017, 72, 116–122. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Witz, C.A. Pathogenesis of endometriosis. Gynecol. Obstet. Investig. 2002, 53 (Suppl. 1), 52–62. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Sakamoto, Y.; Ochiai, H.; Maeshima, A. Umbilical endometriosis with urachal remnant. Arch. Dermatol. 2012, 148, 1331–1332. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Mogami, H.; Bou Nemer, L.; Word, L.; Rogers, D.; Miller, R.; Word, R.A. Endometrial stromal cell attachment and matrix homeostasis in abdominal wall endometriomas. Hum. Reprod. 2018, 33, 280–291. [Google Scholar] [CrossRef]
- Malutan, A.; Drugan, T.; Georgescu, C.; Ciortea, R.; Bucuri, C.; Bobric, A.; Rada, M.P.; Mihu, D. Vascular endothelial growth factor serum levels in women with advanced endometriosis. Acta Endocrinol. 2016, 12, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Borghese, B.; Zondervan, K.T.; Abrao, M.S.; Chapron, C.; Vaiman, D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 2017, 91, 254–264. [Google Scholar] [CrossRef]
- Rooney, D.E. Human Cytogenetics: A Practical Approach, 1st ed.; IRL Press: Oxford, UK, 1987; ISBN 9780199632893. [Google Scholar]
- Bektaş, H.; Bilsel, Y.; Sari, Y.S.; Ersöz, F.; Koç, O.; Deniz, M.; Boran, B.; Huq, G.E. Abdominal wall endometrioma; A 10-year experience and brief review of the literature. J. Surg. Res. 2010, 164, e77–e81. [Google Scholar] [CrossRef]
- Goumenou, A.G.; Matalliotakis, I.M.; Tzardi, M.; Fragouli, I.G.; Mahutte, N.G.; Arici, A. p16, retinoblastoma (pRb), and cyclin D1 protein expression in human endometriotic and adenomyotic lesions. Fertil. Steril. 2006, 85 (Suppl. 1), 1204–1207. [Google Scholar] [CrossRef]
- Sang, L.; Fang, Q.-J.; Zhao, X.-B. A research on the protein expression of p53, p16, and MDM2 in endometriosis. Medicine 2019, 98, e14776. [Google Scholar] [CrossRef]
- Pellegrini, C.; Gori, I.; Achtari, C.; Hornung, D.; Chardonnens, E.; Wunder, D.; Fiche, M.; Canny, G.O. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil. Steril. 2012, 98, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Jimenez, E.; Rodriguez, F.; del Tanago, J.G. c-myc, c-erb-B2, nm23 and p53 expression in human endometriosis. Oncol. Rep. 1998, 5, 49–52. [Google Scholar] [CrossRef]
- Meola, J.; e Silva, J.C.R.; Dentillo, D.B.; da Silva, W.A.; Veiga-Castelli, L.C.; de Souza Bernardes, L.A.; Ferriani, R.A.; de Paz, C.C.P.; Giuliatti, S.; Martelli, L. Differentially expressed genes in eutopic and ectopic endometrium of women with endometriosis. Fertil. Steril. 2010, 93, 1750–1773. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Velasco, J.A.; Arici, A. Apoptosis and the pathogenesis of endometriosis. Semin. Reprod. Med. 2003, 21, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Kaponis, A.; Iwabe, T.; Taniguchi, F.; Makrydimas, G.; Sofikitis, N.; Paschopoulos, M.; Paraskevaidis, E.; Terakawa, N. Apoptosis in human endometrium and endometriosis. Hum. Reprod. Update 2004, 10, 29–38. [Google Scholar] [CrossRef]
- Reis, F.M.; Petraglia, F.; Taylor, R.N. Endometriosis: Hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update 2013, 19, 406–418. [Google Scholar] [CrossRef]
- Proestling, K.; Birner, P.; Gamperl, S.; Nirtl, N.; Marton, E.; Yerlikaya, G.; Wenzl, R.; Streubel, B.; Husslein, H. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 75. [Google Scholar] [CrossRef]
- Kumari, P.; Sharma, I.; Saha, S.C.; Srinivasan, R.; Sharma, A. Promoter methylation status of key genes and its implications in the pathogenesis of endometriosis, endometrioid carcinoma of ovary and endometrioid endometrial cancer. J. Cancer Res. Ther. 2022, 18, S328–S334. [Google Scholar] [CrossRef]
- Nezhat, F.; Cohen, C.; Rahaman, J.; Gretz, H.; Cole, P.; Kalir, T. Comparative immunohistochemical studies of bcl-2 and p53 proteins in benign and malignant ovarian endometriotic cysts. Cancer 2002, 94, 2935–2940. [Google Scholar] [CrossRef]
- Li, F.; Alderman, M.H.; Tal, A.; Mamillapalli, R.; Coolidge, A.; Hufnagel, D.; Wang, Z.; Neisani, E.; Gidicsin, S.; Krikun, G.; et al. Hematogenous Dissemination of Mesenchymal Stem Cells from Endometriosis. Stem Cells 2018, 36, 881–890. [Google Scholar] [CrossRef]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418, 41–49. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Channabasavaiah, A.D.; Joseph, J.V. Thoracic endometriosis: Revisiting the association between clinical presentation and thoracic pathology based on thoracoscopic findings in 110 patients. Medicine 2010, 89, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Wu, J.; Cheng, J.; Di, W. Serous adenocarcinoma arising from endometriosis in cesarean section abdominal wall scar: A case report and literature review. Int. J. Clin. Exp. Pathol. 2017, 10, 7534–7541. [Google Scholar] [PubMed]
- Obata, K.; Ikoma, N.; Oomura, G.; Inoue, Y. Clear cell adenocarcinoma arising from umbilical endometriosis. J. Obstet. Gynaecol. Res. 2013, 39, 455–461. [Google Scholar] [CrossRef]
- Lauslahti, K. Malignant external endometriosis. A case of adenocarcinoma of umbilical endometriosis. Acta Pathol. Microbiol. Scand. Suppl. 1972, 233, 98–102. [Google Scholar] [PubMed]
- Bulun, S.E.; Wan, Y.; Matei, D. Epithelial Mutations in Endometriosis: Link to Ovarian Cancer. Endocrinology 2019, 160, 626–638. [Google Scholar] [CrossRef]
- Tso, S.; Brockley, J.; Recica, H.; Ilchyshyn, A. Sister Mary Joseph’s nodule: An unusual but important physical finding characteristic of widespread internal malignancy. Br. J. Gen. Pract. 2013, 63, 551–552. [Google Scholar] [CrossRef]
- Gogusev, J.; Lepelletier, Y.; El Khattabi, L.; Grigoroiu, M.; Validire, P. Establishment and Characterization of a Stromal Cell Line Derived from a Patient with Thoracic Endometriosis. Reprod. Sci. 2020, 27, 1627–1636. [Google Scholar] [CrossRef]
- McCarty, K.S.; Miller, L.S.; Cox, E.B.; Konrath, J.; McCarty, K.S. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch. Pathol. Lab. Med. 1985, 109, 716–721. [Google Scholar] [PubMed]
- Knoll, J.H.M.; Lichter, P. In situ hybridization to metaphase chromosomes and interphase nuclei. Curr. Protoc. Hum. Genet. 2005, 45, 4.3.1–4.3.31. [Google Scholar] [CrossRef]



| Antibody Used | Specificity | Source | Abdominal Wall Endometriosis | Mid Term Cell Culture |
|---|---|---|---|---|
| Cytokeratin (cl. AE1/AE3) | Epithelial cells | Dako-Agilent | Weak/moderate Gl. | 2–3% |
| Vimentin (cl. V9) | Mesenchymal cells | Dako-Agilent | Negatif Gl., Moderate St. | 94% |
| Ki67 (cl. Mib-1) | Proliferating cells | Dako-Agilent | Weak (5% of St.), Gl. = 0 | 15% |
| ER (cl. EP1) | Estrogen receptor | Dako-Agilent | Moderate/Strong Gl., St. = 0 | 45% |
| PR (cl. PgR 1294) | Progesterone receptor | Dako-Agilent | Strong St. | 65% |
| CD9 (cl. 4H7B9) | Tetraspanin Cell adhesion molecule | ThermoFisher | ND | 55% |
| CD10 (cl. DAK-CD10) | Common acute leukemia antigen | Dako-Agilent | Strong St. | 85% |
| CD13 (cl. 3D8) | Aminopeptidase | Santa Cruz | ND | 80% |
| CD73 (cl. EPR6114) | Mesenchymal stromal/stem cells * | Abcam | Weak Gl., St. | 75% |
| CD90 (cl. 7E1B11) | Mesenchymal stromal/stem cells * | Abcam | Weak Gl., St. | 68% |
| CD105 (cl. EPR10145-12) | Mesenchymal stromal/stem cells * | Abcam | Weak Gl., St. | 61% |
| HLA-DR (cl. L243) | Class II MHC | Dako-Agilent | Weak Gl., St. | 0 |
| c-Myc (cl. C-33) | Proto oncogene | Santa cruz | Strong St., Weak Gl. | 60% |
| P16 (INK4a) (cl. 1E12E10) | Cyclin-dependent kinase inhibitor | ThermoFisher | Strong St. | 40% |
| P53 Wt-Mt (cl. DO-7) | P53 tumor suppressor | Dako-Agilent | Weak Gl., St. | 32% |
| Rb (cl. 1F8) | Retinoblastoma protein | ThermoFisher | Weak Gl., St. | 35% |
| WT1 (cl. 6F-H2) | Wilms tumor protein I | Dako-Agilent | Moderate Gl., St. | 45–50% |
| CDL1 (cl. h-CALD) | Caldesmon | Santa Cruz | Moderate Gl., St. | 65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogusev, J.; Lepelletier, Y.; Cohen, H.; Ami, O.; Validire, P. Idiopathic Abdominal Wall Endometrioma: Case Report with Investigation of the Pathological, Molecular Cytogenetic and Cell Growth Features In Vitro. Int. J. Mol. Sci. 2025, 26, 775. https://doi.org/10.3390/ijms26020775
Gogusev J, Lepelletier Y, Cohen H, Ami O, Validire P. Idiopathic Abdominal Wall Endometrioma: Case Report with Investigation of the Pathological, Molecular Cytogenetic and Cell Growth Features In Vitro. International Journal of Molecular Sciences. 2025; 26(2):775. https://doi.org/10.3390/ijms26020775
Chicago/Turabian StyleGogusev, Jean, Yves Lepelletier, Henri Cohen, Olivier Ami, and Pierre Validire. 2025. "Idiopathic Abdominal Wall Endometrioma: Case Report with Investigation of the Pathological, Molecular Cytogenetic and Cell Growth Features In Vitro" International Journal of Molecular Sciences 26, no. 2: 775. https://doi.org/10.3390/ijms26020775
APA StyleGogusev, J., Lepelletier, Y., Cohen, H., Ami, O., & Validire, P. (2025). Idiopathic Abdominal Wall Endometrioma: Case Report with Investigation of the Pathological, Molecular Cytogenetic and Cell Growth Features In Vitro. International Journal of Molecular Sciences, 26(2), 775. https://doi.org/10.3390/ijms26020775






