Study on the Effect of Bee Venom and Its Main Component Melittin in Delaying Skin Aging in Mice
Abstract
1. Introduction
2. Results
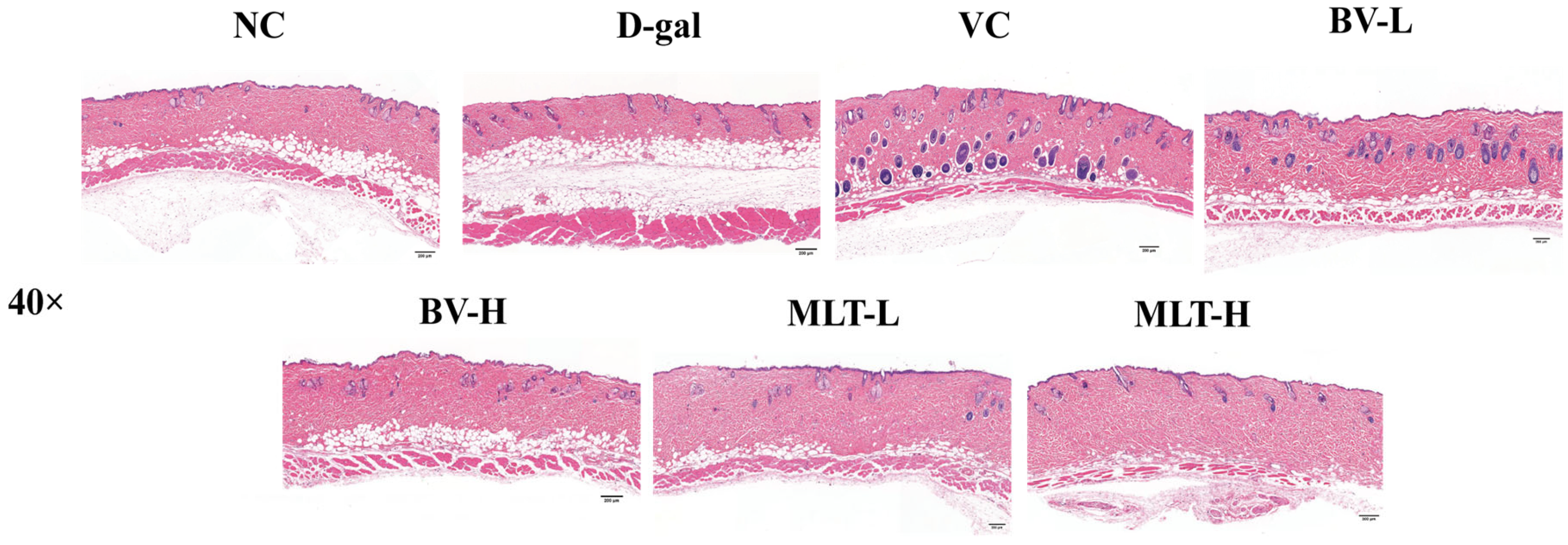
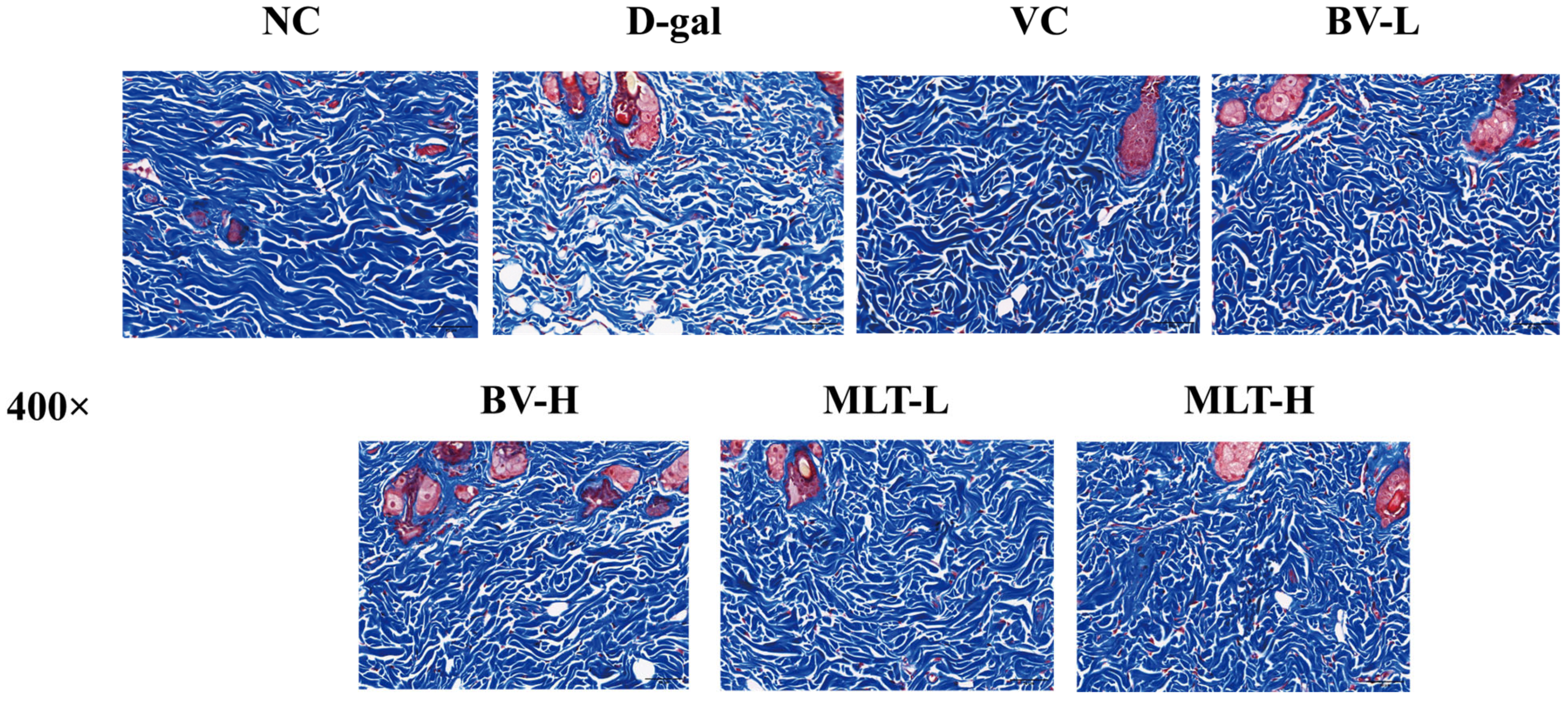
2.1. Effects of BV and MLT on Skin Morphology
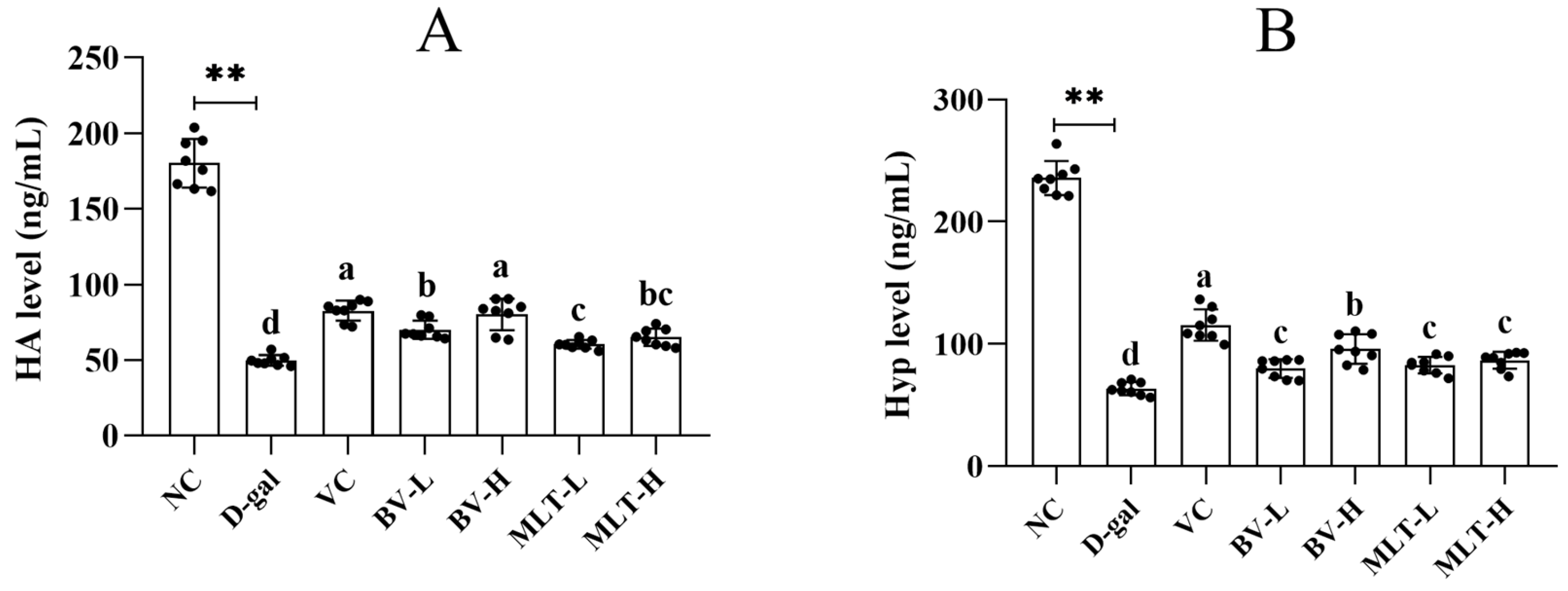
2.2. Effects of BV and MLT on Hyaluronic Acid and Hydroxyproline Content
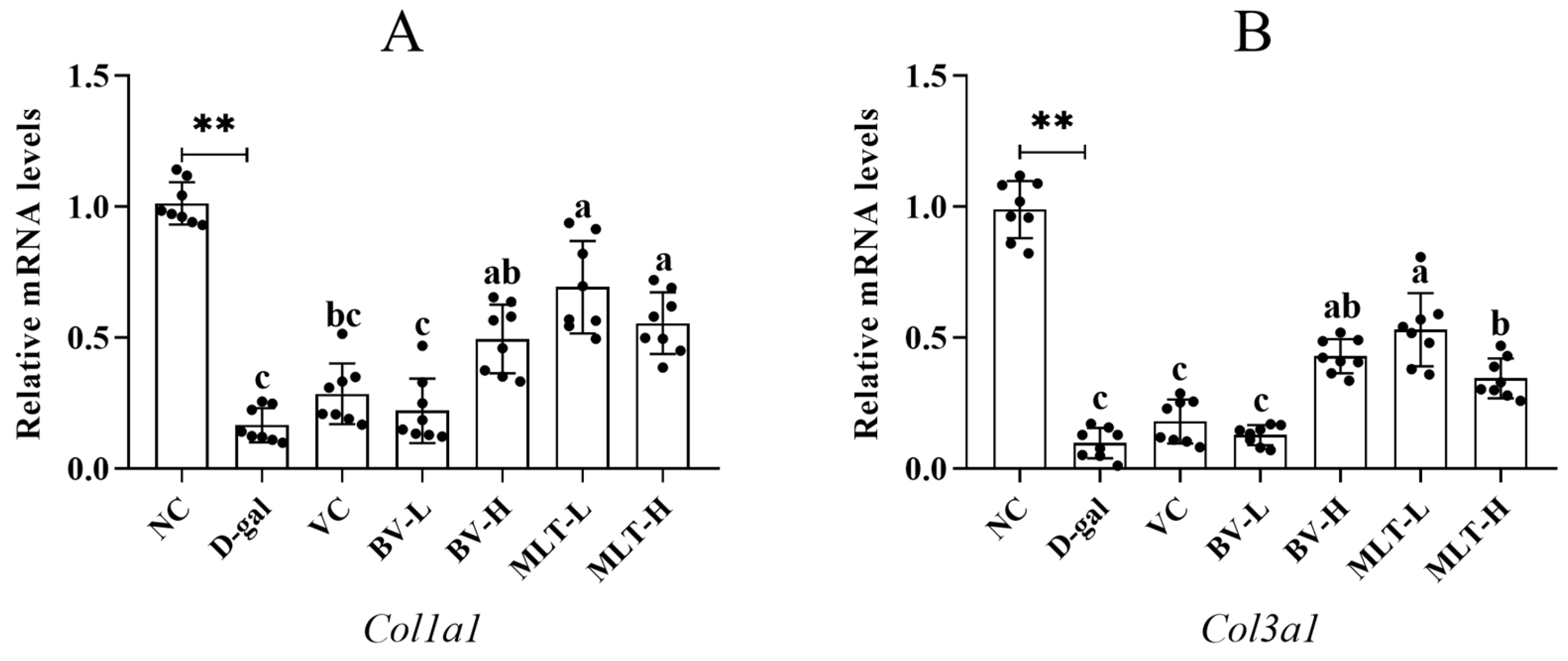
2.3. Effects of BV and MLT on the Expression of Collagen and Inflammatory Factors
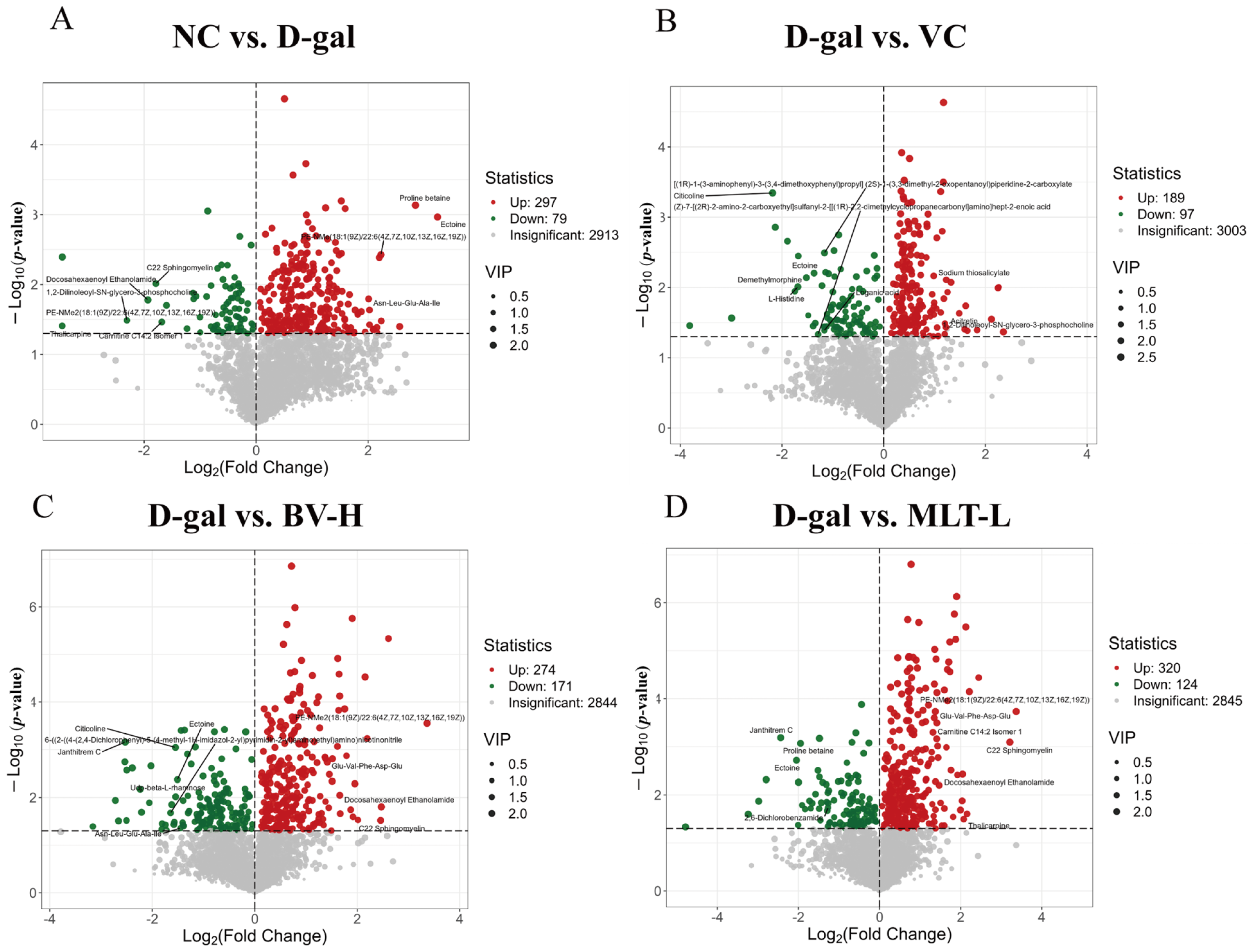
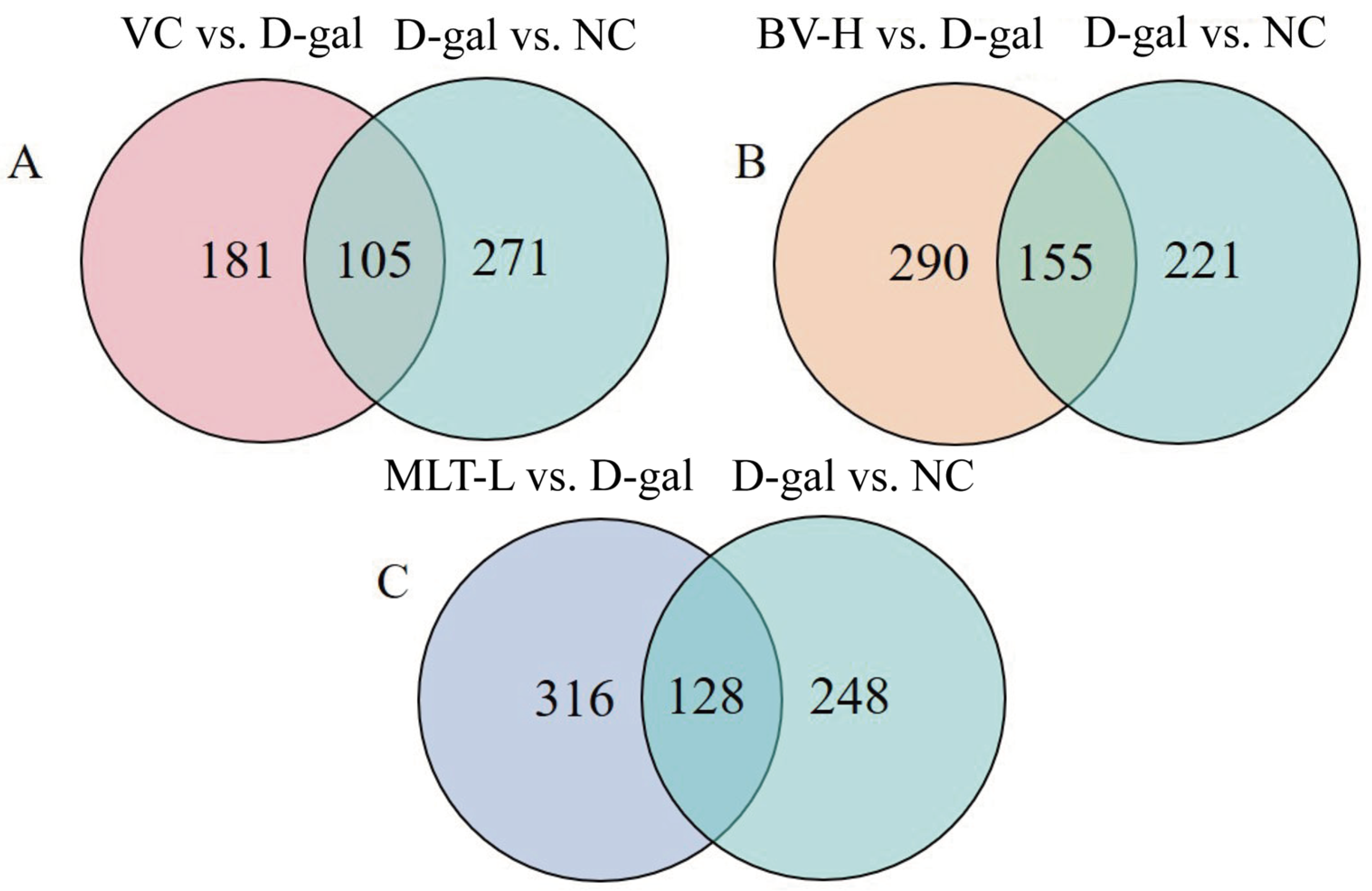
2.4. Metabolomic Analysis with BV and MLT Treatment
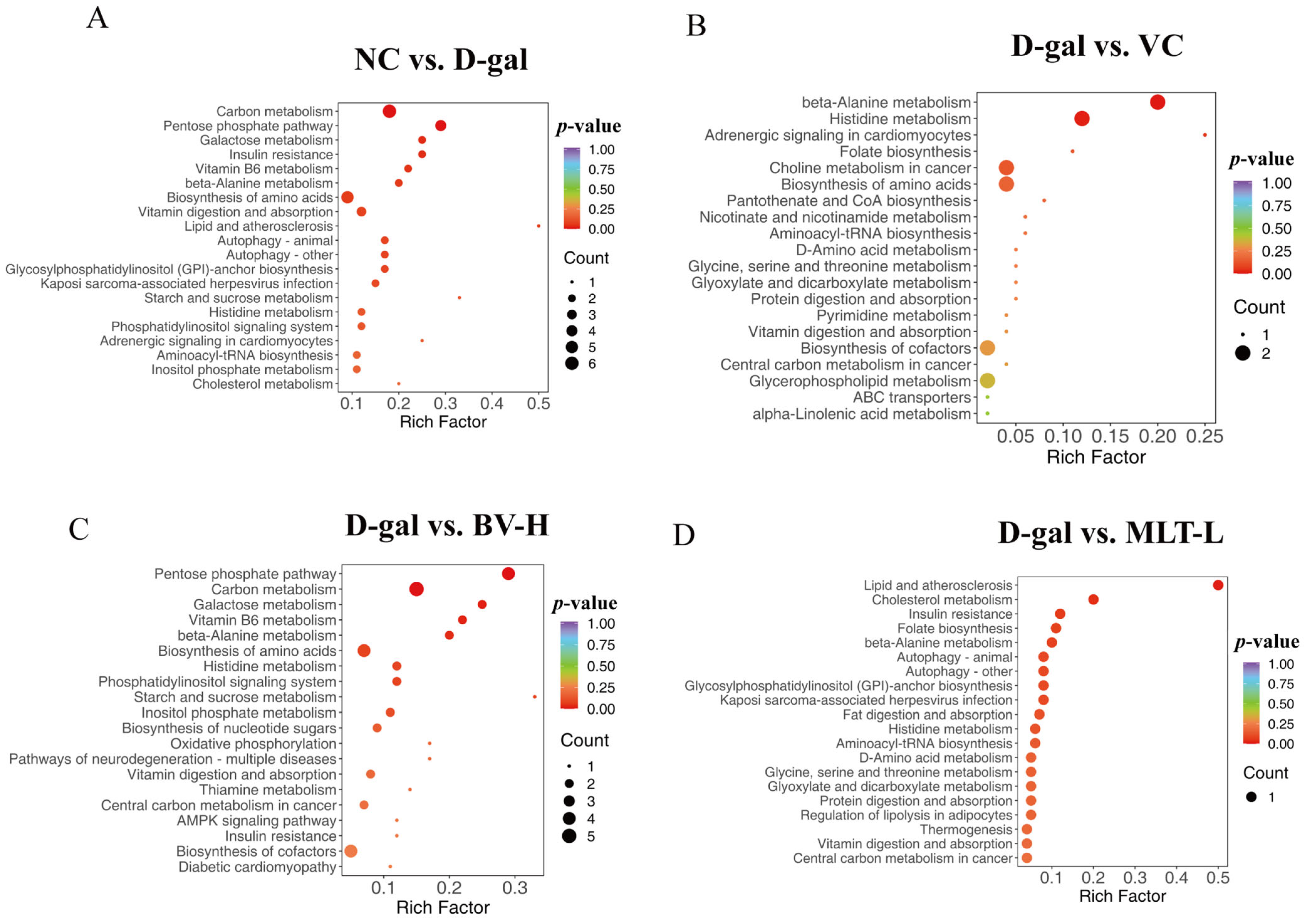
2.5. KEGG Enrichment Analysis of Differential Metabolites
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Experimental Design in Mice
4.3. Morphological Observation of Skin
4.4. Determination of the Content of Hyaluronic Acid and Hydroxyproline
4.5. Measurement of the Expression Levels of Collagen and Inflammatory Factors
4.6. Metabolomics Analysis
4.7. Metabolomics Data Processing
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turnheim, K. When drug therapy gets old: Pharmacokinetics and pharmacodynamics in the elderly. Exp. Gerontol. 2003, 38, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Krętowski, R.; Kalinowska, M.; Świderski, G.; Cechowska-Pasko, M.; Lewandowski, W. Possible mechanisms of the prevention of doxorubicin toxicity by cichoric acid-antioxidant nutrient. Nutrients 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-induced (extrinsic) skin aging: Exposomal factors and underlying mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef]
- Krutmann, J.; Schalka, S.; Watson, R.E.B.; Wei, L.; Morita, A. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.; Hay, R.J.; Griffiths, C.E. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future Options. Gerontologist 2016, 56 (Suppl. S2), S230–S242. [Google Scholar] [CrossRef]
- Gómez-Linton, D.R.; Alavez, S.; Alarcón-Aguilar, A.; López-Diazguerrero, N.E.; Konigsberg, M.; Pérez-Flores, L.J. Some naturally occurring compounds that increase longevity and stress resistance in model organisms of aging. Biogerontology 2019, 20, 583–603. [Google Scholar] [CrossRef]
- Moreno, M.; Giralt, E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: Melittin, apamin and mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee Venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Han, S.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Park, K.K. Evaluation of the skin phototoxicity and photosensitivity of honeybee venom. J. Cosmet. Dermatol. 2017, 16, e68–e75. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.A.; Shaden AM Khalifa, S.A.M.; Elashal, M.H.; Musharraf, S.G.; Saeed, A.; Khatib, A.; Tahir, H.E.; Zou, X.; Naggar, Y.A.; Arshad Mehmood, A.; et al. Cosmetic Applications of Bee Venom. Toxins 2021, 13, 810. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Kweon, H.Y.; Woo, S.O.; Lee, M.Y.; Baek, H.; Park, K.K. Inhibitory effect of bee venom against ultraviolet B induced MMP-1 and MMP-3 in human dermal fibroblasts. J. Apicult. Res. 2017, 46, 94–98. [Google Scholar] [CrossRef]
- Yu, J.R.; Navarro, J.; Coburn, J.C.; Mahadik, B.; Molnar, J.; Holmes, J.H., 4th; Nam, A.J.; Fisher, J.P. Current and future perspectives on skin tissue engineering: Key features of biomedical research, translational assessment, and clinical application. Adv. Healthc. Mater. 2019, 8, e1801471. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Su, X.H.; Liu, J.Z.; Zhao, Y.; Li, Z.; Xu, X.; Li, H.; Nashun, B. Comparison of naturally aging and D-galactose induced aging model in beagle dogs. Exp. Ther. Med. 2017, 14, 5881–5888. [Google Scholar] [CrossRef][Green Version]
- Sun, K.; Yang, P.; Zhao, R.; Bai, Y.; Guo, Z. Matrine Attenuates D-Galactose-Induced Aging-Related Behavior in Mice via Inhibition of Cellular Senescence and Oxidative Stress. Oxid. Med. Cell Longev. 2018, 2018, 7108604. [Google Scholar] [CrossRef]
- Rajendran, P.; Al-Saeedi, F.J.; Ammar, R.B.; Abdallah, B.M.; Ali, E.M.; Al Abdulsalam, N.K.; Tejavat, S.; Althumairy, D.; Veeraraghavan, V.P.; Alamer, S.A.; et al. Geraniol attenuates oxidative stress and neuroinflammation-mediated cognitive impairment in D galactose-induced mouse aging model. Aging 2024, 16, 5000–5026. [Google Scholar] [CrossRef]
- Wu, C.; Hou, Q.; Hu, F.; Zhang, F. Effect of Codonopsis pilosula var. modesta on anti-oxidation of skin tissue of aged mice induced by D-galactose. Pharmacol. Clin. Chin. Mat. Med. 2014, 30, 92–96. [Google Scholar]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Asai, T.T.; Oikawa, F.; Yoshikawa, K.; Inoue, N.; Sato, K. Food-derived collagen peptides, Prolyl-Hydroxyproline (Pro-Hyp), and Hydroxyprolyl Glycine (Hyp-Gly) enhance growth of primary cultured mouse skin fibroblast using fetal bovine serum free from hydroxyprolyl peptide. Int. J. Mol. Sci. 2019, 21, 229. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef]
- Zhao, H.T.; Huang, L.; Zhang, M.A.; Liu, M.M.; Zhao, S.G.; Chen, S.Y. Comparative on the anti-inflammatory effects to ear swelling in mice of bee venom between Apis cerana cerana and Apis mellifera ligustica. J. Shanxi Univ. 2022, 42, 110. [Google Scholar]
- Liu, Z.; Mi, J.; Wu, H. Relationships between circulating metabolites and facial skin aging: A Mendelian randomization study. Hum. Genom. 2023, 17, 23. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Xie, W. Research Progress in Skin Aging, Metabolism, and Related Products. Int. J. Mol. Sci. 2023, 24, 15930. [Google Scholar] [CrossRef]
- Umbayev, B.; Askarova, S.; Almabayeva, A.; Saliev, T.; Masoud, A.R.; Bulanin, D. Galactose-Induced Skin Aging: The Role of Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 7145656. [Google Scholar] [CrossRef]
- Avogaro, A.; de Kreutzenberg, S.V.; Fadini, G.P. Insulin signaling and life span. Pflugers Arch. 2010, 459, 301–314. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Keast, D. Energy metabolism and the skin. Int. J. Biochem. 1991, 23, 1175–1183. [Google Scholar] [CrossRef]
- Chen, J.H.; Lin, X.; Bu, C.; Zhang, X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Volpi, E. Amino acid metabolism and regulatory effects in aging. Curr. Opin. Clin. Nutr. Metab. Care. 2008, 11, 45–49. [Google Scholar] [CrossRef]
- Ott, P.; Clemmesen, O.; Larsen, F.S. Cerebral metabolic disturbances in the brain during acute liver failure: From hyperammonemia to energy failure and proteolysis. Neurochem. Int. 2005, 47, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, A.; Hildebrand, J.; Soehle, J.; Wenck, H.; Terstegen, L.; Gallinat, S.; Knott, A.; Winnefeld, M.; Zamboni, N. An integrative metabolomics and transcriptomics study to identify metabolic alterations in aged skin of humans in vivo. BMC Genom. 2017, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, Q.G.; Yin, Z.T.; Zhou, Y.M.; Zhou, X.L.; Hu, Z.Z. Metabonomics Study on Anti-aging Effect of Procyanidin B2 in D-Galactose-Induced Aging in Mice. Food Sci. 2018, 39, 134–139. [Google Scholar]
- Khan, A.; Bai, H.; Khan, A.; Bai, Z. Neferine prevents ultraviolet radiation-induced skin photoaging. Exp. Ther. Med. 2020, 19, 3189–3196. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tian, S.H.; Zhao, H.T.; Liu, J.J.; Ma, X.M.; Zheng, L.F.; Guo, H.R.; Jiang, Y.S. Metabolomics reveals that alcohol extract of propolis alleviates D-gal-induced skeletal muscle senescence in mice. Food Biosci. 2022, 49, 101885. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Liu, M.; Chen, L.; Gong, Y.; Ma, W.; Jiang, Y. Study on the Effect of Bee Venom and Its Main Component Melittin in Delaying Skin Aging in Mice. Int. J. Mol. Sci. 2025, 26, 742. https://doi.org/10.3390/ijms26020742
Zhao H, Liu M, Chen L, Gong Y, Ma W, Jiang Y. Study on the Effect of Bee Venom and Its Main Component Melittin in Delaying Skin Aging in Mice. International Journal of Molecular Sciences. 2025; 26(2):742. https://doi.org/10.3390/ijms26020742
Chicago/Turabian StyleZhao, Huiting, Miaomiao Liu, Longlong Chen, Yu Gong, Weihua Ma, and Yusuo Jiang. 2025. "Study on the Effect of Bee Venom and Its Main Component Melittin in Delaying Skin Aging in Mice" International Journal of Molecular Sciences 26, no. 2: 742. https://doi.org/10.3390/ijms26020742
APA StyleZhao, H., Liu, M., Chen, L., Gong, Y., Ma, W., & Jiang, Y. (2025). Study on the Effect of Bee Venom and Its Main Component Melittin in Delaying Skin Aging in Mice. International Journal of Molecular Sciences, 26(2), 742. https://doi.org/10.3390/ijms26020742




